Abstract
Background and Objective
Schizophrenia and Alzheimer’s disease are characterised by abnormalities in glutamatergic pathways related to N-methyl-d-aspartate receptor hypofunction. Glycine is an N-methyl-d-aspartate receptor co-agonist; inhibition of glycine transporter 1 may improve N-methyl-d-aspartate receptor function. This phase I, randomised, two-part study evaluated the safety, tolerability and pharmacokinetic profile of BI 425809, a novel glycine transporter 1 inhibitor, in healthy male and female volunteers.
Methods
Part 1 evaluated BI 425809 10, 25, 50 or 75 mg once daily or 75 mg twice daily in young subjects, and 25 mg or 50 mg once daily in elderly subjects. Each dose group comprised 12 subjects who received BI 425809 (n = 9) or placebo (n = 3) for 14 days (day 1: single dose; days 4–14: multiple dosing). Part 2 compared pharmacokinetic profiles in 12 subjects who received a single dose of BI 425809 25 mg in the morning and evening.
Results
Pharmacokinetic profiles were similarly shaped for all dose groups. Median time to maximum plasma concentration was 3.0–4.5 h with steady state being reached between days 6 and 10. Pharmacokinetic parameters demonstrated dose linearity at the predicted therapeutic exposure range of BI 425809 ≤ 25 mg once daily, but increased less than dose proportionally for ≥ 50 mg once daily. All reported adverse events were of mild-to-moderate intensity, 51/84 (61%; part 1) subjects had one or more treatment-related adverse event, no serious adverse events occurred and no dose dependency was observed.
Conclusions
Pharmacokinetic properties support both morning and evening dosing. BI 425809 was generally well tolerated at all tested doses.
Clinicaltrials.gov identifier
Electronic supplementary material
The online version of this article (10.1007/s40261-018-0660-2) contains supplementary material, which is available to authorized users.
Key Points
| BI 425809 was well tolerated within the tested dose range for all subjects (healthy young and elderly), with reported adverse events being of mild-to-moderate intensity. |
| Within the predicted therapeutic exposure range of BI 425809 ≤ 25 mg once daily, pharmacokinetic results demonstrated dose linearity and supported both morning and evening dosing regimens. |
Introduction
Cognitive impairment is a significant feature of neurodegenerative conditions, such as Alzheimer’s disease (AD), and psychiatric conditions, including schizophrenia [1–4]. These conditions are underpinned by abnormalities in glutamatergic pathways related to N-methyl-d-aspartate (NMDA) receptor hypofunction in cortical and hippocampal brain areas. Such abnormalities have been associated with the cognitive impairment experienced by these patients [5, 6]. Glycine is an NMDA receptor co-agonist; inhibition of glycine transporter 1 (GlyT1), which regulates synaptic glycine levels, may therefore improve NMDA receptor function by raising glycine levels in the synaptic cleft [7]. Animal data indicate that improved NMDA receptor signalling leads to an increase in long-term potentiation and synaptic plasticity [8], which improves cognitive function and memory [9].
BI 425809, a potent and selective GlyT1 inhibitor, is a new chemical entity hypothesised to improve cognitive function and memory in patients with schizophrenia and AD. A phase I, first-in-human, single rising dose study found single doses of BI 425809 up to 100 mg, administered as an oral solution, to be generally well tolerated in young healthy male volunteers [10].
A pharmacokinetic analysis of BI 425809 supported a once-daily (QD) dosing regimen for future multiple-dose studies [10]. Plasma concentration–time profiles were similarly shaped at all dose levels (0.5–150 mg) and were characterised by rapid absorption (median time to maximum plasma concentration of ~ 45 min) followed by a biphasic distribution phase. Dose proportionality was observed in all exposure parameters [area under the concentration–time curve over the time interval from 0 to the last quantifiable data point (AUC0–tz), area under the concentration–time curve over the time interval from 0 extrapolated to infinity (AUC0–∞), maximum plasma concentration (Cmax)] across the entire dose range. The geometric mean (gMean) terminal half-life was not dose dependent, ranging between 32.5 and 47.0 h [10]. The bioavailability of BI 425809 administered as a tablet was lower than the bioavailability of BI 425809 administered as an oral solution. Adjusted gMean ratios for AUC0–tz and Cmax for BI 425809 administered as a tablet fasted vs. oral solution fasted were 80.5% [90% confidence interval (CI) 74.0–87.6) and 50.0% (90% CI 45.1–55.4), respectively. Furthermore, the single rising dose study identified a moderate increase in BI 425809 bioavailability following administration as a tablet under fed conditions (high-fat/high-calorie breakfast of ~ 1000 kcal) vs. strongly fasted conditions [adjusted gMean ratios: AUC0–tz 125.9% (90% CI 115.7–137.0); Cmax 142.1% [90% CI 128.3–157.4)] [10]. The current study extends this investigation to include less extreme meal conditions (standard-fat/standard-calorie meal of ~ 500 kcal) that are more representative of daily life. A recent drug–drug interaction study confirmed in-vitro data indicating that cytochrome P450 (CYP) 3A4 is a major component of the BI 425809 metabolic pathway [11].
The primary objective of this study was to evaluate the safety and tolerability of multiple rising doses of BI 425809, compared with placebo, in healthy young and elderly subjects. Furthermore, BI 425809 pharmacokinetic parameters were determined to explore dose proportionality, attainment of steady state and the effect of a standardised light breakfast on BI 425809 steady-state exposure. Finally, this study additionally investigated differences in the pharmacokinetics, safety and tolerability of BI 425809 when administered in the morning vs. evening, to evaluate whether an evening dosing regimen, which may attenuate sedative or visual adverse events (AEs), would be beneficial for subjects in phase II trials.
Subjects and Methods
Ethical Conduct
All study procedures, protocols and documents met with institutional approval following review by the study centre’s independent ethics committee. The study was conducted in accordance with the clinical study protocol, the International Conference for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice guidelines [12] and local legislation, in accordance with the principles of the Declaration of Helsinki [13]. All subjects provided signed and dated written informed consent prior to study procedures.
Study Design
This was a phase I study conducted at the Human Pharmacology Unit CRS Clinical Research Services in Mannheim, Germany (Clinicaltrials.gov identifier: NCT02337283), consisting of two parts. The first part was randomised, double blinded, and placebo controlled within parallel-dose groups, and aimed to assess the safety, tolerability and pharmacokinetics following multiple doses of BI 425809 10–75 mg, in healthy young and healthy elderly subjects compared with placebo. The second part of this study was randomised and open-label, with a two-treatment, two-sequence, two-period crossover, and aimed to assess the pharmacokinetics, safety and tolerability of single BI 425809 25-mg doses given in the morning or evening in healthy young subjects. An overview of the study design is given in Fig. 1.
Fig. 1.
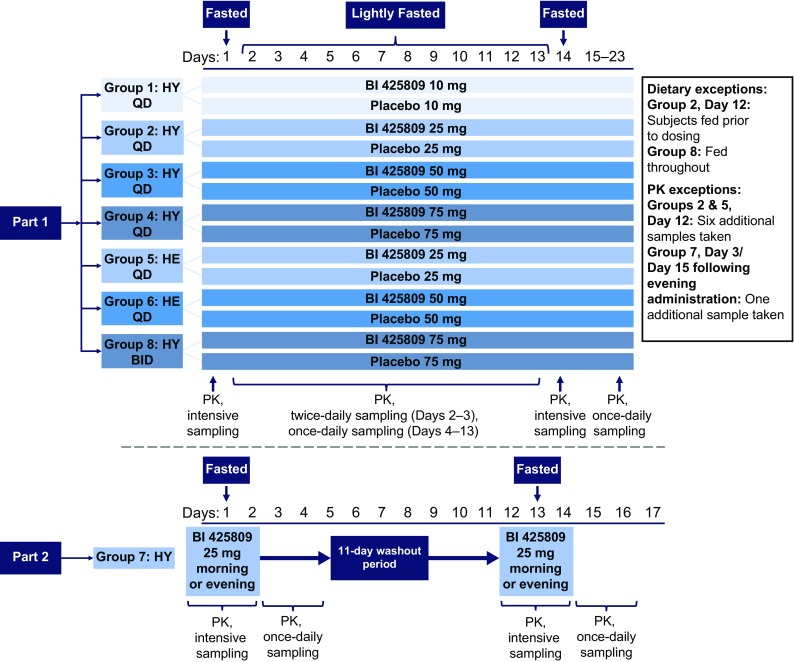
Study design diagram. BID twice daily, HE healthy elderly, HY healthy young, PK pharmacokinetics, QD once daily
Part 1: Multiple-Dose Component
Subjects were allocated to seven sequential dose groups; five groups comprised healthy young subjects [BI 425809 QD at 10, 25, 50 or 75 mg, or BI 425809 twice daily (BID) at 75 mg], and two comprised healthy elderly subjects (BI 425809 QD at 25 or 50 mg). Within each dose group, subjects were randomised to receive the active drug or placebo at a ratio of 3:1, such that nine subjects received the active drug and three received placebo. Each subject received a single dose of BI 425809 or placebo on day 1, followed by QD (dose groups 1–6, 10–75 mg) or BID (dose group 8, 75 mg) dosing for 10 days from day 4 onwards. On day 14, all dose groups received one single dose of BI 425809 or placebo. Dose groups were investigated consecutively in ascending order and a documented safety review was undertaken prior to each dose escalation. Healthy elderly subjects were dosed after a respective dose of BI 425809 had been well tolerated in healthy young subjects. Blood samples for pharmacokinetic assessments were taken following the first and last doses, and at regular intervals between days 1 and 14, after which daily samples were collected until day 23. Urine was collected for pharmacokinetic analysis during pre-specified periods following the first and last doses in the QD dose groups only. The effects of different types of food intake on BI 425809 pharmacokinetics were analysed in dose groups 2 and 5 (BI 425809 25 mg in young and elderly subjects, respectively) under light-fed (standardised light breakfast [~ 500 kcal] served 30 min before BI 425809 administration; day 12, dose group 2) [Table 1 of the Electronic Supplementary Material (ESM)], and light-fasted (no food for ≥ 10 h before, until 1 h after BI 425809 administration; day 12, dose group 5) conditions. In both dose groups, additional pharmacokinetic profiles were taken on day 12 and compared with the respective pharmacokinetic profiles on day 14. Fasted conditions (no food for ≥ 10 h before, until 3.5 h after BI 425809 administration) were employed on days 1 and 14, with light-fasted conditions used for the remaining treatment days in all groups except group 8 (Fig. 1). Twice-daily dosing and administration under light-fed conditions were applied to dose group 8 to increase the bioavailability of BI 425809.
Part 2: Morning vs. Evening Dosing
Each subject (dose group 7) received two single doses of BI 425809 25 mg separated by a washout period of at least 11 days. The dose of BI 425809 was administered either in the morning or in the evening, per the randomisation scheme. All subjects were in a fasted state for the administration of BI 425809. Blood samples for pharmacokinetic assessment were taken prior to and up to 96 h after administration of the active drug (Fig. 1).
Subjects
Inclusion Criteria
A total of 96 subjects were entered into the study: 84 in part 1, of whom 24 were elderly subjects, and 12 in part 2. Eligible subjects were healthy male or female (of non-childbearing potential) volunteers, 18–50 years of age for young subjects, or 65–80 years of age for elderly subjects, with a body mass index of 18.5–29.9 kg/m2.
Exclusion Criteria
Subjects were excluded if they exhibited any evidence of a concomitant disease judged as clinically relevant upon physical examination, vital-sign assessment, electrocardiogram (ECG) or laboratory test (haematology, clinical chemistry, infectious serology and urinalysis). Other key exclusion criteria included: gastrointestinal, hepatic, renal, respiratory, cardiovascular, metabolic, immunological or hormonal disorders; diseases of the central nervous system, other neurological disorders, or psychiatric disorders; history of suicidal behaviour, macular degeneration or relevant orthostatic hypotension, fainting spells or blackouts; chronic or relevant acute infections; history of relevant allergy/hypersensitivity (including allergy to the study medication or its excipients); drug abuse, excessive alcohol intake (> 20 g/day for female individuals and > 30 g/day for male individuals), use of tobacco (more than five cigarettes, one cigar or one pipe per day) or inability to refrain from smoking on study days.
Treatments
BI 425809 and placebo tablets were manufactured by Boehringer Ingelheim International GmbH, Germany. Subjects received either placebo or BI 425809 as 5- or 25-mg tablets according to the dose strength required by the dosing group assigned. To maintain blinding, placebo tablets were matched in size, colour and shape to BI 425809 tablets (white to off-white; round; 5 mg, ~ 6 mm in diameter; 25 mg, ~ 10 mm in diameter). The tablet formulation was considered appropriate based on the planned dosing, similarity to the intended market formulation and the expectation that sufficient exposure would be obtained to meet the study aims.
Study Endpoints
Safety Assessments
The primary endpoint of part 1 and the secondary endpoint of part 2 was the number (N [%]) of subjects with drug-related AEs. Adverse events were defined as any untoward medical occurrence and included any clinically relevant findings from physical examination, laboratory tests, ECG, vital-sign measurement and the exacerbation of any pre-existing condition. Adverse events were classified according to the following criteria: mild, awareness of signs or symptoms that were easily tolerated; moderate, sufficient discomfort to interfere with usual activities; and severe, incapacitating or causing an inability to work or perform usual activities. Further criteria of interest were: neurological examination; safety laboratory tests (clinical chemistry, haematology and urinalysis); 12-lead ECGs; visual tests (Ishihara test, Jaeger Eye Chart and Amsler grid); ophthalmological examination, including fundus photography, (part 1 only); vital signs [blood pressure, pulse rate and respiratory rate (BI 425809 75-mg BID group only)]; oxygen saturation (BI 425809 75-mg BID group only); suicidality monitoring; and assessment of visual analogue scales (VASs) for possible central nervous system effects (subjective feelings) using the scales developed by Bond and Lader [14] and Bowdle et al. [15].
Pharmacokinetic Analysis
The primary pharmacokinetic endpoints (assessed in part 2) were AUC0–tz and Cmax of the analyte. Secondary endpoints assessed in part 1 included AUC of the analyte from 0 to 24 h (AUC0–24) and Cmax after the first dose; and AUC and Cmax at steady state over a uniform dosing interval τ (AUCτ,ss and Cmax,ss) following the last dose. Additional pharmacokinetic parameters assessed during this study are described within the ESM. The pharmacokinetic software used in this study was Phoenix WinNonlin Version 6.3 (Certara L.P. [Pharsight], St. Louis, MO; for the non-compartmental analysis) using the linear-up log-down trapezoidal method. Statistical analysis software, SAS® Version 9.4 (SAS Institute, Cary, NC, USA), was used to produce graphs and tables.
Bioanalytical Assay
BI 425809 plasma and urine concentrations were determined by a validated liquid chromatography tandem mass spectrometry assay. The analyses were performed at the Department of Pharmacokinetics and Drug Metabolism, BI Pharmaceuticals Inc., Ridgefield, CT, USA. Linear calibration standard curves (seven concentrations) with 1/× 2 weighting were used to determine BI 425809 concentrations over 1.00–1000 nm. Concentrations below the lower limit of quantification were not replaced by zero at any timepoint (including the lag phase and pre-dose values). For the non-compartmental analysis, concentration values identified as below the lower limit of quantification in the lag phase (period between time zero and the first time point with a concentration above the quantification limit) were set to zero. Back-calculation of calibration standards, tabulation of the standard curve fit parameters and measurement of quality-control samples were used to assess assay performance. The quality-control plasma samples were prepared in K3-ethylenediaminetetraacetic acid human plasma, and those for the urine samples were prepared in 0.05% Tween 20 human urine. Three concentrations were used for both. Quality-control samples were then analysed to assess the accuracy and precision of pharmacokinetic measurements.
Statistical Analyses
Descriptive statistics were calculated for all safety and pharmacokinetic endpoints. Safety was assessed in the treated set, which included all randomised subjects who received one or more doses of the study drug. Pharmacokinetic parameters were assessed in subjects from the treated set who provided one or more evaluable secondary pharmacokinetic endpoint values (pharmacokinetic set). A more detailed description of the statistical analyses can be found in the ESM.
Part 1: Multiple-Dose Component
Based on the commonly used dose group size of 12 subjects, it was planned to include 84 subjects in the multiple-dose component of the study. The size of 12 subjects per dose is often used in multiple rising dose trials of the present type, and is generally considered sufficient for the exploratory evaluation of multiple-dose safety and pharmacokinetics. The pharmacokinetic endpoints AUC0–24, Cmax, AUCτ,ss and Cmax,ss were assessed for dose proportionality (QD treatment groups only; assessed separately for healthy young and healthy elderly subjects). The linearity index was calculated by building the ratio of AUCτ,ss, over AUC0–∞ and attainment of steady state was assessed based on trough concentrations of BI 425809. Safety endpoints were examined over time and for any differences from baseline values; baseline was defined as the last measurement before the first study-drug administration.
Part 2: Morning vs. Evening Dosing
The second part of this study included 12 subjects in order to reach a certain precision in estimating the ratio of gMean with 95% probability. The effect of evening dosing was determined based on AUC0–tz and Cmax, and the related CI. Safety endpoints were examined over time and for any differences from baseline values. Baseline value was considered to be the last measurement before study-drug administration in the morning and the last measurement before study-drug administration in the evening.
Results
Study Population and Disposition
Ninety-six subjects entered into the study with 93 completing the planned observation time (Fig. 2). Demographic characteristics were similar across all treatment groups (Table 2 of the ESM). Briefly, part 1 of this study included 57 male and 27 female subjects (young and elderly); all except two were Caucasian. Part 2 of the study consisted of ten male and two female healthy young subjects (all Caucasian). Mean (standard deviation) age was 40.5 (8.3) years for young subjects and 71.0 (4.1) years for elderly subjects. During part 1, two subjects discontinued, one young subject after the sixth dose of placebo and one elderly subject after the first dose of BI 425809 50 mg. During part 2, all except one subject (a healthy young subject discontinued following administration of the single morning dose) completed the planned observation time per protocol.
Fig. 2.
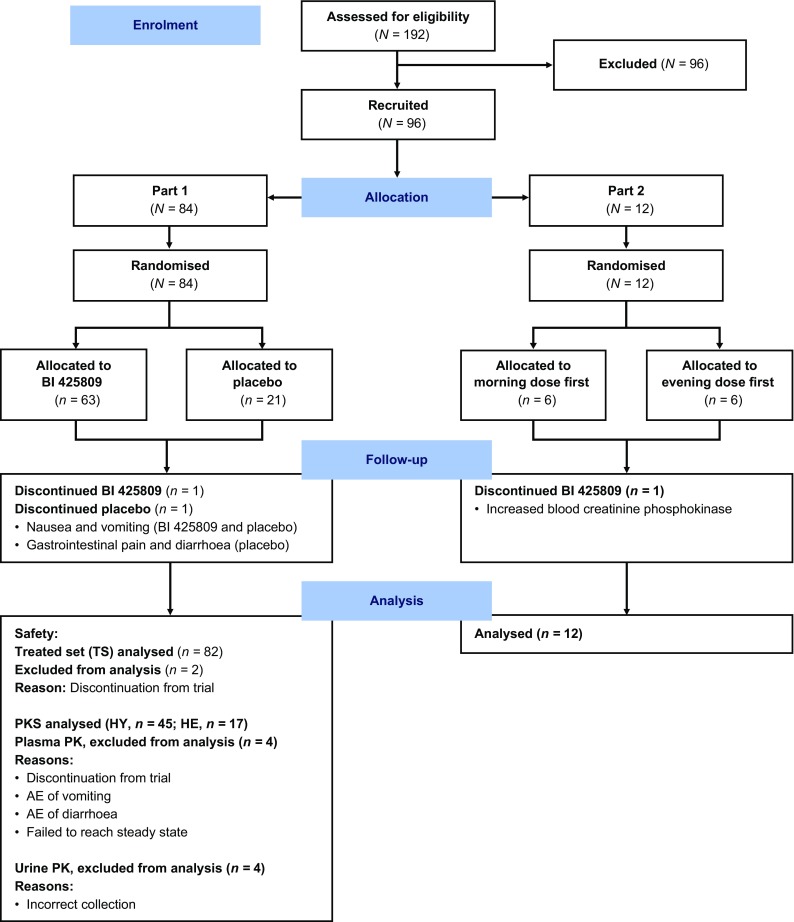
CONSORT diagram. AE adverse event, HE healthy elderly, HY healthy young, PK pharmacokinetics, PKS all subjects from the Treated set who received BI 425809, participated in part 1, and provided one or more PK endpoints judged as evaluable, Treated set all subjects who received at least one dose of study drug/placebo
Safety and Tolerability
A summary of all AEs by system organ class and preferred term (≥ 5% incidence in either part 1 or part 2) is provided in Table 1.
Table 1.
Summary of adverse events by system organ class and preferred term [≥ 5% total incidence (part 1 or part 2 where applicable)] for young subjects and elderly subjects
| AE by SOC, n (%) Preferred term, n (%) |
Young subjects | Elderly subjects | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Part 1 | Part 2a | Parts 1 and 2 | ||||||||||||||
| Placebo n = 15 |
BI | Total N = 60 |
BI | BI n = 57 |
Total N = 72 |
Placebo n = 6 |
BI | Total n = 24 |
||||||||
| 10 mg QD n = 9 |
25 mg QD n = 9 |
50 mg QD n = 9 |
75 mg QD n = 9 |
75 mg BID n = 9 |
Total BI n = 45 |
25 mg QD n =12 |
25 mg QD n = 9 |
50 mg QD n = 9 |
Total BI n = 18 |
|||||||
| Total with AEs | 8 (53.3) | 5 (55.6) | 7 (77.8) | 6 (66.7) | 6 (66.7) | 6 (66.7) | 30 (66.7) | 38 (63.3) | 2 (16.7) | 32 (56.1) | 40 (55.6) | 3 (50.0) | 8 (88.9) | 8 (88.9) | 16 (88.9) | 19 (79.2) |
| Subjects with investigator-defined drug-related AEs | 7 (46.7) | 4 (44.4) | 6 (66.7) | 6 (66.7) | 5 (55.6) | 6 (66.7) | 27 (60.0) | 34 (56.7) | 0 (–) | 27 (47.4) | 34 (47.2) | 3 (50.0) | 6 (66.7) | 8 (88.9) | 14 (77.8) | 17 (70.8) |
| Subjects with AEs leading to study discontinuation | 1 (6.7) | 0 (–) | 0 (–) | 0 (–) | 0 (–) | 0 (–) | 0 (–) | 1 (1.7) | 0 (–) | 0 (–) | 1 (1.4) | 0 (–) | 0 (–) | 1 (11.1) | 1 (5.6) | 1 (4.2) |
| Infections and infestations | 4 (26.7) | 1 (11.1) | 2 (22.2) | 1 (11.1) | 1 (11.1) | 0 (–) | 5 (11.1) | 9 (15.0) | 1 (8.3) | 6 (10.5) | 10 (13.9) | 1 (16.7) | 3 (33.3) | 3 (33.3) | 6 (33.3) | 7 (29.2) |
| Nasopharyngitis | 2 (13.3) | 0 (–) | 2 (22.2) | 0 (–) | 1 (11.1) | 0 (–) | 3 (6.7) | 5 (8.3) | 1 (8.3) | – | – | 0 (–) | 1 (11.1) | 1 (11.1) | 2 (11.1) | 2 (8.3) |
| Folliculitis | – | – | – | – | – | – | – | – | – | – | – | 0 (–) | 2 (22.2) | 1 (11.1) | 3 (16.7) | 3 (12.5) |
| Psychiatric disorders | 1 (6.7) | 0 (–) | 1 (11.1) | 0 (–) | 0 (–) | 3 (33.3) | 4 (8.9) | 5 (8.3) | 0 (–) | 4 (7.0) | 5 (6.9) | 0 (–) | 2 (22.2) | 2 (22.2) | 4 (22.2) | 4 (16.7) |
| Insomnia | 1 (6.7) | 0 (–) | 0 (–) | 0 (–) | 0 (–) | 3 (33.3) | 3 (6.7) | 4 (6.7) | 0 (–) | – | – | – | – | – | – | – |
| Nervous system disorder | 3 (20.0) | 4 (44.4) | 3 (33.3) | 3 (33.3) | 5 (55.6) | 2 (22.2) | 17 (37.8) | 20 (33.3) | 1 (8.3) | 18 (31.6) | 21 (29.2) | 1 (16.7) | 2 (22.2) | 3 (33.3) | 5 (27.8) | 6 (25.0) |
| Dizziness | 1 (6.7) | 1 (11.1) | 0 (–) | 1 (11.1) | 3 (33.3) | 2 (22.2) | 7 (15.6) | 8 (13.3) | 0 (–) | – | – | 1 (16.7) | 1 (11.1) | 2 (22.2) | 3 (16.7) | 4 (16.7) |
| Headache | 3 (20.0) | 3 (33.3) | 3 (33.3) | 3 (33.3) | 4 (44.4) | 0 (–) | 13 (28.9) | 16 (26.7) | 1 (8.3) | – | – | 0 (–) | 1 (11.1) | 1 (11.1) | 2 (11.1) | 2 (8.3) |
| Eye disorders | 2 (13.3) | 0 (–) | 2 (22.2) | 3 (33.3) | 0 (–) | 2 (22.2) | 7 (15.6) | 9 (15.0) | 0 (–) | 7 (12.3) | 9 (12.5) | 1 (16.7) | 1 (11.1) | 1 (11.1) | 2 (11.1) | 3 (12.5) |
| Ocular discomfort | 1 (6.7) | 0 (–) | 0 (–) | 0 (–) | 0 (–) | 2 (22.2) | 2 (4.4) | 3 (5.0) | 0 (–) | – | – | – | – | – | – | – |
| Vision blurred | 0 (–) | 0 (–) | 2 (22.2) | 1 (11.1) | 0 (–) | 0 (–) | 3 (6.7) | 3 (5.0) | 0 (–) | – | – | – | – | – | – | – |
| Gastrointestinal disorders | 1 (6.7) | 0 (–) | 3 (33.3) | 0 (–) | 2 (22.2) | 0 (–) | 5 (11.1) | 6 (10.0) | 0 (–) | 5 (8.8) | 6 (8.3) | 2 (33.3) | 6 (66.7) | 5 (55.6) | 11 (61.1) | 13 (54.2) |
| Constipation | – | – | – | – | – | – | – | – | – | – | – | 0 (–) | 0 (–) | 4 (44.4) | 4 (22.2) | 4 (16.7) |
| Hard faeces | – | – | – | – | – | – | – | – | – | – | – | 2 (33.3) | 2 (22.2) | 0 (–) | 2 (11.1) | 4 (16.7) |
| Nausea | – | – | – | – | – | – | – | – | – | – | – | 0 (–) | 1 (11.1) | 1 (11.1) | 2 (11.1) | 2 (8.3) |
| Vomiting | – | – | – | – | – | – | – | – | – | – | – | 0 (–) | 1 (11.1) | 1 (11.1) | 2 (11.1) | 2 (8.3) |
| Musculoskeletal and connective tissue disorders | 0 (–) | 1 (11.1) | 0 (–) | 1 (11.1) | 1 (11.1) | 0 (–) | 3 (6.7) | 3 (5.0) | 0 (–) | 3 (5.3) | 3 (4.2) | 0 (–) | 1 (11.1) | 2 (22.2) | 3 (16.7) | 3 (12.5) |
| Back pain | – | – | – | – | – | – | – | – | – | – | – | 0 (–) | 1 (11.1) | 1 (11.1) | 2 (11.1) | 2 (8.3) |
| General disorders and administration site conditions | 2 (13.3) | 0 (–) | 0 (–) | 2 (22.2) | 1 (11.1) | 1 (11.1) | 4 (8.9) | 6 (10.0) | 0 | 4 (7.0) | 6 (8.3) | 1 (16.7) | 2 (22.2) | 4 (44.4) | 6 (33.3) | 7 (29.2) |
| Fatigue | 2 (13.3) | 0 (–) | 0 (–) | 0 (–) | 1 (11.1) | 0 (–) | 1 (2.2) | 3 (5.0) | 0 (–) | – | – | 1 (6.7) | 0 (–) | – | – | – |
| Venepuncture site haematoma | – | – | – | – | – | – | – | – | – | – | – | 0 (–) | 1 (11.1) | 4 (44.4) | 4 (22.2) | 5 (20.8) |
| Injury, poisoning and procedural complications | – | – | – | – | – | – | – | – | – | – | – | 1 (16.7) | 2 (22.2) | 0 (–) | 2 (11.1) | 3 (12.5) |
AE adverse event, BI BI 425809, BID twice daily, QD once daily, SOC system organ class
aSubjects in part 2 received two single doses of BI 425809 or placebo separated by a ≥ 11-d washout period, while subjects in part 1 received daily doses of BI 425809 or placebo over 12 d
Part 1: Multiple-Dose Component
In total, 63.3% of young subjects experienced one or more AEs: 66.7% in the active-treatment arm and 53.3% in the placebo arm. Drug-related AEs were recorded for 60.0% of subjects receiving BI 425809 and 46.7% receiving placebo. A greater number of elderly subjects (79.2%) than young subjects reported one or more AEs during the study; the incidence was higher for subjects receiving the active drug compared with those receiving placebo (88.9 vs. 50.0%, respectively). As with young subjects, drug-related AEs were reported to a greater extent in elderly subjects who received BI 425809 (77.8%) than those who received placebo (50.0%). The overall frequency of subjects with one or more drug-related AE was similar across all treatment groups, indicating that AEs were not dose dependent (Table 1).
All treatment-emergent AEs were of mild or moderate intensity and no serious AEs were reported. Within parts 1 and 2, the most commonly reported AEs for young subjects in both the active and placebo groups were nervous system disorders, and infections and infestations (system organ class), and headache, dizziness, nasopharyngitis and insomnia (preferred term) (Table 1). In elderly subjects, the most commonly reported AEs in the active-treatment and placebo groups were gastrointestinal disorders (system organ class), and fatigue, constipation, hard faeces, dizziness and venepuncture-site haematoma (preferred term) (Table 1). There were no relevant differences between the treatment groups in the profile of reported AEs.
Both age groups reported eye disorders; all were classified as mild and subjects recovered within a short period of time. Specific events reported by young subjects included ocular discomfort and increased lacrimation (placebo group), visual blurring and impairment, blepharospasm, ocular discomfort, reduced visual acuity and transient reduced visual acuity (active treatment). Elderly subjects reported ocular discomfort (placebo group), blurred vision, visual impairment and reduced visual acuity (active treatment).
No clinically relevant changes in laboratory parameters, vital signs, 12-lead ECG, neurological examinations, ophthalmological examinations, or oxygen saturation monitoring were observed. No suicidal behaviours were noted. Neither VAS revealed clinically relevant dose-related changes in mood state or perception.
Part 2: Morning vs. Evening Dosing
Adverse events were reported by two subjects [nasopharyngitis (n = 1; duration, days 2–7) and headache (n = 1; duration, days 1–2)] following a single morning dose of BI 425809 25 mg. In each case, the AEs were mild or moderate in intensity and considered not to be drug related. No serious AEs were reported. No clinically relevant changes in laboratory parameters, vital signs, 12-lead ECG, neurological examinations, ophthalmological examinations, suicidality monitoring, oxygen saturation monitoring or either VAS were noted.
Pharmacokinetic Results
A full list of secondary pharmacokinetic parameters (part 1) is detailed in Table 3 of the ESM.
Part 1: Multiple-Dose Component
BI 425809 was absorbed with median time to maximum plasma concentration values of 3.0–4.5 h after single as well as multiple doses (Table 2); plasma concentrations subsequently decreased in a biphasic manner. Steady-state conditions were reached between day 6 and day 10 for all treatment groups (i.e. 2–6 days after the start of multiple dosing). Plasma concentration–time profiles were similarly shaped across all dose groups (Fig. 3a) and both age groups (Fig. 3b). Systemic exposure (Cmax, Cmax,ss, AUC0–24 and AUCτ,ss) demonstrated a trend towards a less-than-dose-proportional increase following both single and multiple doses at 50 mg and above (QD) (Table 2). The BI 425809 gMean terminal half-life after the first dose and the terminal half-life at steady state were similar across treatment groups (QD and BID, 34.2–59.3 h), and the fraction eliminated from urine ranged from 1.77 to 2.71% (after single-dose administration) and from 3.90 to 7.10% (after multiple dosing). The linearity index, assessed separately for each dose group, was generally close to 1 for BI 425809 QD groups but clearly decreased (0.553) in the 75-mg BID group (Table 3 of the ESM).
Table 2.
Pharmacokinetic parameters after single and multiple doses of BI 425809 during part 1a
| Pharmacokinetic parameter | BI 425809 | ||||||
|---|---|---|---|---|---|---|---|
| Young subjects | Elderly subjects | ||||||
| 10 mg QD n = 9 |
25 mg QD n = 9 |
50 mg QD n = 9 |
75 mg QD n = 9 |
75 mg BIDb n = 9 |
25 mg QD n = 8 |
50 mg QD n = 8 |
|
| Single-dose administration | |||||||
| AUC0–24, nmol·h/L | 1410 (20.1) | 3720 (18.2) | 6020 (24.4) | 7170 (17.9) | 12,000 (13.7) | 3460 (18.5) | 5620 (17.9) |
| AUC0–24,norm, nmol·h/L/mg | 141 (20.1) | 149 (18.2) | 120 (24.4) | 95.6 (17.9) | 160 (13.7) | 139 (18.5) | 112 (17.9) |
| Cmax, nmol/L | 109 (19.0) | 278 (19.7) | 397 (28.7) | 451 (17.9) | 884 (8.72) | 235 (18.7) | 371 (25.9) |
| Cmax,norm, nmol/L/mg | 10.9 (19.0) | 11.1 (19.7) | 7.95 (28.7) | 6.01 (17.9) | 11.8 (8.72) | 9.4 (18.7) | 7.43 (25.9) |
| tmaxc, h | 4 (3.5, 5) | 4.5 (2, 5) | 4.5 (3.5, 12) | 4 (2, 5) | 4.02 (3, 5) | 3.5 (2, 5) | 4.5 (2, 8) |
| Multiple-dose administration | |||||||
| AUCτ,ssb, nmol·h/L | 3870 (22.2) | 10,600 (31.7) | 14,200 (29.4) | 16,500 (29.6) | 18,800 (20.2) | 11,000 (11.6) | 13,900 (30.8) |
| AUCτ,ss,normb, nmol·h/L/mg | 387 (22.2) | 425 (31.7) | 285 (29.4) | 220 (29.6) | 251 (20.2) | 439 (11.6) | 278 (30.8) |
| Cmax,ssb, nmol/L | 221 (19.5) | 582 (27.6) | 800 (26.3) | 1020 (23.1) | 1930 (17.2) | 618 (10.9) | 802 (25.0) |
| Cmax,ss,normb, nmol/L/mg | 22.1 (19.5) | 23.3 (27.6) | 16.0 (26.3) | 13.6 (23.1) | 25.8 (17.2) | 24.7 (10.9) | 16.0 (25.0) |
| tmax,ssb,c, h | 4.5 (2, 5) | 3.75 (2, 6) | 3.5 (2, 6) | 4.5 (2, 6) | 3.5 (2, 4.5) | 3 (2, 4.52) | 4.5 (3.5, 6) |
AUC0–24 area under the concentration–time curve from 0 to 24 h, AUCτ,ss area under the concentration–time curve at steady state over a uniform dosing interval, BID twice daily, Cmax maximum plasma concentration, Cmax,ss, maximum plasma concentration at steady state over a uniform dosing interval, CV coefficient of variation, norm dose normalised, QD once daily, tmax, time to Cmax, tmax,ss time from last dosing to Cmax at steady state over a uniform dosing interval
aGeometric mean (%CV), unless otherwise stated
bFor dose group BI 425809 75 mg BID, AUCτ,22, AUCτ,22,norm, Cmax,22, Cmax,22,norm, and tmax,22 are given whereby 22 relates to 22 h
cFor tmax and tmax,ss the median and range (minimum, maximum) are given
Fig. 3.
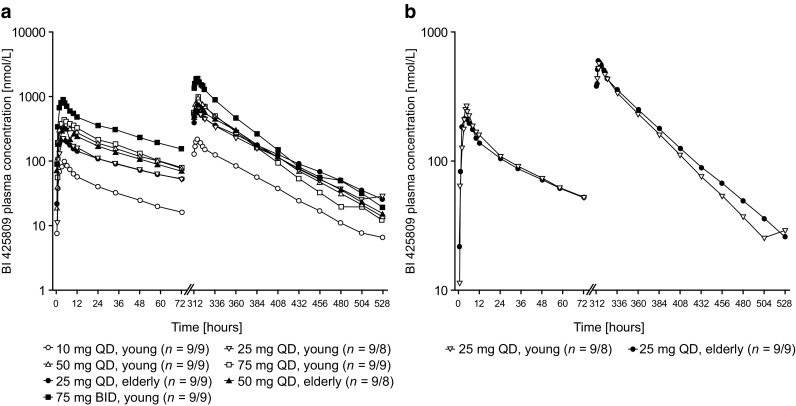
Geometric mean drug plasma concentration–time profiles of BI 425809 after single and multiple oral administration of BI 425809 tablets: a once daily (QD) or twice daily (BID) in young and elderly subjects and b QD in young vs. elderly subjects (semi-log scale)
Subjects receiving BI 425809 25 mg following a standard breakfast (light fed) demonstrated a 23% increase in steady-state Cmax compared with fasted conditions [707 vs. 574 nmol/L; gMean ratio (light fed:fasted), 123.2%] along with a 14% increase in steady-state AUC [12,200 vs. 10,700 nmol·h/L; gMean ratio (light fed:fasted), 114.0%] and a delayed time to maximum plasma concentration. As expected, subjects showed similar steady-state exposure under light-fasted (Cmax, 662 nmol/L; AUC 11,600 nmol·h/L) and fasted conditions (Cmax, 611 nmol/L; AUC, 11,100 nmol·h/L; gMean ratio [light fasted:fasted] Cmax, 108.3%; AUC, 104.5%) [Fig. 1 and Table 4 of the ESM]. Additionally, only slight differences in BI 425809 exposure were identified between light-fed and light-fasted conditions at steady state [gMean ratio (light fed:light fasted) Cmax, 106.8%; AUC, 105.2%]. At higher doses (75 mg), single administration of BI 425809 with food (subjects in the BI 425809 BID group) at day 1 led to increased absorption of BI 425809 compared with fasted conditions (subjects in the BI 425809 QD group). Indeed, Cmax and AUC0–24 values approximately doubled [884 nmol/L and 12,000 nmol·h/L (fed); 451 nmol/L and 7170 nmol·h/L (fasted), respectively; Table 2].
Part 2: Morning vs. Evening Dosing
Overall, gMean pharmacokinetic parameters suggested a trend towards slightly increased BI 425809 exposure following the evening administration, as suggested by a 15% higher gMean Cmax (90% CI 98.1–136) and 20% higher AUC0–tz (90% CI 107–135) compared with morning administration (Table 3). However, when comparing individual AUC0–tz and Cmax values, there appeared to be no systematic trend, with a somewhat higher variability for the morning dosing (Fig. 4).
Table 3.
Bioavailability of BI 425809 administered as a 25-mg single morning or evening dose (part 2)
| Pharmacokinetic parameter | BI 425809 25 mg QD N = 12 |
|||
|---|---|---|---|---|
| Adjusted gMean evening dose | Adjusted gMean morning dose | Adjusted gMean ratio evening/morning (90% CI [%]) |
Intraindividual gCV, % | |
| AUC0–tz, nmol·h/L | 8410 | 7000 | 120 (107–135) | 14.6 |
| Cmax, nmol/L | 278 | 241 | 115 (98.1–136) | 21.2 |
| AUC0–∞, nmol·h/L | 10,400 | 8770 | 118 (104–134) | 16.1 |
| tmaxa, h | 4.50 (2.00, 8.00) | 4.00 (1.00, 4.50) | – | – |
AUC0–tz area under the concentration–time curve over the time interval from 0 to the last quantifiable data point, AUC0–∞ area under the concentration–time curve over the time interval from 0 extrapolated to infinity, Cmax maximum plasma concentration, gCV geometric coefficient of variation, gMean geometric mean, tmax time to Cmax
aFor tmax, the median and range (minimum, maximum) are given
Fig. 4.
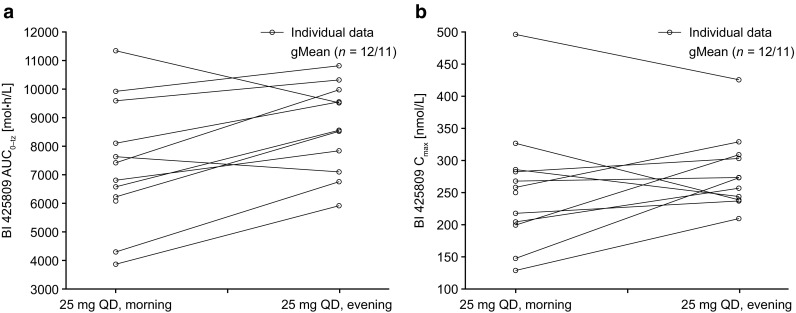
Intra-individual comparison of a area under the concentration–time curve over the time interval from 0 to the last quantifiable data point (AUC0–tz) and b maximum plasma concentration (Cmax) values of BI 425809 after single oral administration of 25 mg in the morning or in the evening in healthy young subjects. gMean geometric mean, QD once daily
Discussion
This study demonstrated that BI 425809 administered orally as multiple rising doses of 10, 25, 50 and 75 mg QD, or 75 mg BID in healthy young subjects, or as multiple doses of 25 or 50 mg QD in elderly subjects, over 11 days, was generally well tolerated. All treatment-related AEs were considered of mild or moderate intensity and were in general alignment with recent reports on GlyT1 inhibitors [16–18].
Selective GlyT1 inhibition results in increased synaptic glycine levels, which may be favourable for the treatment of cognitive impairment associated with schizophrenia and AD [5–7]. Increases in glycine levels, however, have been associated with visual disturbances and electroretinogram alterations [19, 20]. Inhibition of the GlyT1 transporter in the rat retina leads to electroretinogram alterations, which may explain the visual disturbances reported in clinical trials with GlyT1 inhibitors [20]. As such, the visual effects of active BI 425809 administration were extensively monitored throughout this study. Additionally, ophthalmological examination, including fundus photography, was performed. Visual AEs were reported by nine subjects across the treatment groups; all were mild, quickly reversible and did not appear to be dose dependent. Visual analogue scales to monitor possible central nervous system effects (subjective feelings) by evaluating external and internal perception, alertness, mood and calmness were employed during the multiple-dose component of this study and did not reveal any clinically relevant differences between the treatment groups. Overall, the frequency of subjects with one or more drug-related AE was similar across all treatment groups indicating a lack of dose dependency. Contrary to this, however, administration of BI 425809 as a solution during the previous single rising dose study demonstrated that higher doses were associated with an increased incidence of AEs, the most common being central nervous system and visual effects. This difference may be explained by a greater speed of absorption following administration of BI 425809 as a solution, compared with the tablet formulation [10].
There were no clinically relevant differences in the pharmacokinetics, safety and tolerability between healthy young and healthy elderly subjects during this study. Given that one of the key indications for this therapy will largely consist of elderly patients with AD, the good tolerability of BI 425809 observed within the elderly subjects included here is of particular importance.
Plasma concentration–time profiles of BI 425809 were shaped similarly across all dose groups, and dose proportionality was observed in exposure parameters (Cmax, AUC0–24, Cmax,ss and AUCτ,ss) for BI 425809 10 and 25 mg. At doses of ≥ 50 mg, the increase in exposure was clearly less than dose proportional. By contrast, dose-linear pharmacokinetics following single administration of BI 425809 as an oral solution from 0.5 to 150 mg has been demonstrated [10], suggesting that the less-than-dose-proportional increase in plasma exposure in the current study can be attributed to the tablet formulation, likely owing to impaired dissolution of the drug from its tablet form rather than effects on absorption or disposition of the drug. Indeed, BI 425809 demonstrates low intrinsic solubility.
Given that only a slight increase in plasma exposure was seen following administration of BI 425809 75 mg compared with 50 mg (tablet formulation), it was anticipated that higher QD doses would not achieve further significant increases in plasma exposure. Consequently, a 75-mg BID dosing regimen under fed conditions was introduced to maximise plasma exposure, and thereby the exposure multiplies, in comparison with the maximum anticipated therapeutic dose of 25 mg QD. This approach successfully counteracted the impaired bioavailability of BI 425809, increasing plasma exposure by ~ 70%, based on AUC0–24 and Cmax after the first dose, compared with the 75-mg QD regimen, an effect that may be attributed to administration of the tablets with food. Nevertheless, such results must be treated with caution given that the comparison was between subject groups rather than intra-individually.
Interestingly, AUCτ,ss increased by just 14%, while Cmax,ss increased by 90% following administration of BI 425809 75 mg BID vs. 75 mg QD, indicating that after multiple dosing an additional mechanism is impacting plasma exposure. This is further illustrated by a linearity index below 1 for the 75-mg BID dose group, compared with a value close to 1 for the QD treatment groups. Autoinduction of BI 425809 metabolism may be the underlying mechanism behind such observations, as in-vitro and clinical studies have indicated that BI 425809 is primarily metabolised by CYP3A4, and has the potential to induce CYP3A4 activity [11, 21]. Given that this phenomenon was only apparent for the 75-mg BID dose group, it can be concluded that autoinduction of CYP3A4 at dosing regimens leading to lower exposure is of minor importance.
In summary, these data suggest that decreased bioavailability observed at higher tablet dosing regimens is caused by two factors; low intrinsic solubility and autoinduction. Neither, however, appear to be relevant to the anticipated therapeutic dose range.
An additional factor found to impact upon BI 425809 plasma exposure level is food. A recent investigation demonstrated a 42% increase in Cmax, and a 26% increase in AUC, following a single dose of BI 425809 25 mg administered as a tablet with a high-fat/high-calorie meal vs. under fasted conditions [10]. This is in line with the low intrinsic solubility of BI 425809 as it has recently been demonstrated that increased bile secretion, known to accompany high-fat meal consumption, plays an important role in drug accessibility, possibly owing to improved solubility [22]. In the present study, additional exploratory data were generated to characterise the administration of BI 425809 with food.
Administration of BI 425809 25 mg in young subjects, in the presence of a standardised light breakfast, led to only a 14 and 23% increase in steady-state AUC and Cmax, respectively, compared with fasted conditions. As mentioned above, the food effect seems to be increased (~ 70%) at higher doses, e.g. when comparing single-dose data of the 75-mg dosing regimens. Considering this dose dependency, it may be reasonable to speculate that at doses below 25 mg such a food effect may be reduced or even completely eradicated.
It must be noted that the food-effect investigations employed during this study are clearly exploratory in nature; however, they might be closer to the conditions apparent in clinical practice. Because evaluations were partly conducted after multiple dosings with differing food conditions, the preceding doses and conditions might have impacted the food effect readout. Furthermore, the above-mentioned factors such as dose dependency of tablet bioavailability and autoinduction at higher doses require a differential consideration of the influence of food on BI 425809 exposure.
Exposure to a BI 425809 25-mg tablet was approximately 15–20% higher [15% higher gMean Cmax (90% CI 98.1–136) and 20% higher AUC0–tz (90% CI 107–135)] when administered in the evening compared with the morning. Considering an exposure multiple of 3.2-fold when comparing AUC0–24 and Cmax between the 25-mg dose group and the well tolerated 75-mg BID dose group, a potential exposure increase by 20% for the evening dosing is considered not clinically relevant at least with regard to safety. It might be speculated that there is a slight circadian rhythm for the absorption, distribution, metabolism and excretion mechanisms of BI 425809, alternatively this may be owing to procedural differences for the evening vs. morning dosing. Overall, based on current data, the exposure increase of 15–20% (if a true effect) is not considered clinically relevant, suggesting that either morning or evening doses could be considered for future studies.
Conclusions
In this phase I, multiple rising-dose study, BI 425809 was well tolerated within the tested dose range in healthy young and elderly subjects. Within the predicted therapeutic exposure range of BI 425809 ≤ 25 mg QD, pharmacokinetic parameters demonstrated no obvious deviation from dose linearity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Jakub Pekarek and Marion Schmid for their technical support of the pharmacokinetic analyses, and Jasmin Link for her review of the manuscript.
Funding
The work presented here, including the conduct of the study, data analysis and interpretation, was funded by Boehringer Ingelheim. The sponsor was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. Editorial support in the form of initial preparation of the outline based on input from all authors, and collation and incorporation of author feedback to develop subsequent drafts, assembling tables and figures, copy editing and referencing was provided by Louisa Pettinger, PhD, and Sam Halliwell, PhD, of Fishawack Communications, and was funded by Boehringer Ingelheim International GmbH.
Conflict of interest
The authors met the criteria for authorship as recommended by the International Committee of Medical Journal Editors. All authors (except Armin Schultz, who is an employee of CRS Clinical Research Services, Mannheim) are employees of Boehringer Ingelheim, but received no direct compensation related to the development of this manuscript. Armin Schultz has no disclosures to declare.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
References
- 1.Evans JD, Bond GR, Meyer PS, et al. Cognitive and clinical predictors of success in vocational rehabilitation in schizophrenia. Schizophr Res. 2004;70(2–3):331–342. doi: 10.1016/j.schres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz MM, Wexler BE, Fujimoto M, et al. Symptoms versus neurocognition as predictors of change in life skills in schizophrenia after outpatient rehabilitation. Schizophr Res. 2008;102(1–3):303–311. doi: 10.1016/j.schres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velligan DI, Mahurin RK, Diamond PL, et al. The functional significance of symptomatology and cognitive function in schizophrenia. Schizophr Res. 1997;25(1):21–31. doi: 10.1016/S0920-9964(97)00010-8. [DOI] [PubMed] [Google Scholar]
- 4.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 5.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158(9):1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 6.Danysz W, Parsons CG. Alzheimer’s disease, beta-amyloid, glutamate, NMDA receptors and memantine: searching for the connections. Br J Pharmacol. 2012;167(2):324–352. doi: 10.1111/j.1476-5381.2012.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto K. Glycine transport inhibitors for the treatment of schizophrenia. Open Med Chem J. 2010;4:10–19. doi: 10.2174/1874104501004010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooke SF, Bliss TV. Long-term potentiation and cognitive drug discovery. Curr Opin Investig Drugs. 2005;6(1):25–34. [PubMed] [Google Scholar]
- 9.Collingridge GL, Volianskis A, Bannister N, et al. The NMDA receptor as a target for cognitive enhancement. Neuropharmacology. 2013;64:13–26. doi: 10.1016/j.neuropharm.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moschetti V, Desch M, Goetz S, et al. Safety, tolerability and pharmacokinetics or oral BI 425809, a glycine transporter 1 inhibitor, in healthy male volunteers: a partially randomised, single-blind, placebo-controlled, first-in-human study. Eur J Drug Metab Pharmacokinet. 2018;43(2):239–249. doi: 10.1007/s13318-017-0440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desch M, Goettel M, Goetz S, et al. Effects of the potent cytochrome p450 3A4 inhibitor, itraconazole, on the pharmacokinetics of BI 425809, a new glycine transporter 1 (GlyT1) inhibitor. Clin Pharmacol Ther. 2017;101(S1):S52. [Google Scholar]
- 12.International Council For Harmonisation Of Technical Requirements For Pharmaceuticals For Human Use (ICH). ICH harmonised guideline. Integrated addendum to ICH E6 (R1): guideline for good clinical practice E6 (R2). Current Step 4 version; 9 Nov 2016.
- 13.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 14.Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47(3):211–218. doi: 10.1111/j.2044-8341.1974.tb02285.x. [DOI] [Google Scholar]
- 15.Bowdle TA, Radant AD, Cowley DS, et al. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology. 1998;88(1):82–88. doi: 10.1097/00000542-199801000-00015. [DOI] [PubMed] [Google Scholar]
- 16.D’Souza DC, Singh N, Elander J, et al. Glycine transporter inhibitor attenuates the psychotomimetic effects of ketamine in healthy males: preliminary evidence. Neuropsychopharmacology. 2012;37(4):1036–1046. doi: 10.1038/npp.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirayasu Y, Sato S-I, Takahashi H, et al. A double-blind randomized study assessing safety and efficacy following one-year adjunctive treatment with bitopertin, a glycine reuptake inhibitor, in Japanese patients with schizophrenia. BMC Psychiatry. 2016;16(1):1. doi: 10.1186/s12888-016-0778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umbricht D, Alberati D, Martin-Facklam M, et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry. 2014;71(6):637–646. doi: 10.1001/jamapsychiatry.2014.163. [DOI] [PubMed] [Google Scholar]
- 19.Liem-Moolenaar M, Peeters P, Kamerling IMC, et al. Early stage development of the glycine-1 re-uptake inhibitor SCH 900435: central nervous system effects compared with placebo in healthy men. Br J Clin Pharmacol. 2013;75(6):1455–1467. doi: 10.1111/bcp.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CN, Pettersen B, Seitis G, et al. GlyT1 inhibitor reduces oscillatory potentials of the electroretinogram in rats. Cutan Ocul Toxicol. 2014;33(3):206–211. doi: 10.3109/15569527.2013.833937. [DOI] [PubMed] [Google Scholar]
- 21.Desch M, Schmitt H, Hohl K, et al. Pharmacokinetic interaction of BI 425809, a new glycine transporter 1 (GlyT1) inhibitor, with cytochrome p450 (CYP) isoenzymes and p-glycoprotein (P-gp) probe drug. Clin Pharmacol Ther. 2017;101(S1):S52. [Google Scholar]
- 22.Lyng E, Havenaar R, Shastri P, et al. Increased bioavailability of celecoxib under fed versus fasted conditions is determined by postprandial bile secretion as demonstrated in a dynamic gastrointestinal model. Drug Dev Ind Pharm. 2016;42(8):1334–1339. doi: 10.3109/03639045.2015.1135935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


