Abstract
Patient: Male, 68
Final Diagnosis: Mesothelioma
Symptoms: Dyspnea
Medication: —
Clinical Procedure: —
Specialty: Pulmonology
Objective:
Rare disease
Background:
Malignant pleural mesothelioma (MPM) is a highly lethal cancer with a median survival of ∼12 months even with aggressive intervention. Frontline therapy relies on systemic cisplatin and pemetrexed chemotherapy and has a response rate of ∼35–41%; currently, there are no US Food and Drug Administration approved second-line therapies for MPM. Herein, we present a patient with MPM who experienced rapid disease progression after standard therapy but who had an exceptional and sustained response to immune checkpoint inhibition with single agent nivolumab.
Case Report:
A 68-year-old male with a history of work-related asbestos exposure was diagnosed with MPM. He was treated with primary resection followed by systemic chemotherapy with cisplatin and pemetrexed. When chemotherapy failed, he was switched to immunotherapy with nivolumab and achieved an exceptional response.
Conclusions:
We report the first case of a patient with MPM who experienced rapid disease progression after standard therapy but had an exceptional and sustained response to immune checkpoint inhibition with single agent nivolumab. As outcomes with traditional chemotherapy regimens remain disappointing, there is a substantial need for new approaches to MPM; our case highlights a new therapeutic opportunity even in the face of aggressive disease. Indeed, a new era of investigation utilizing immunotherapy for mesothelioma is beginning, with much anticipation.
MeSH Keywords: Immunotherapy, Lung Neoplasms, Mesothelioma, Programmed Cell Death 1 Receptor
Background
Malignant pleural mesothelioma (MPM) is a highly lethal cancer with a median survival of ∼12 months even with aggressive intervention. Frontline therapy relies on systemic cisplatin and pemetrexed chemotherapy and has a response rate of ∼35–41%; currently, there are no US Food and Drug Administration (FDA) approved second-line therapies for MPM. Immune checkpoint blockade is a relatively new treatment modality with promising outcomes in several forms of cancer, including renal cell carcinoma, melanoma, non-small cell lung cancer, and ovarian cancer, and has shown promise in in vitro and early clinical trials with MPM. Herein, we present a case of a patient with MPM who experienced rapid disease progression after standard therapy but who had an exceptional and sustained response to immune checkpoint inhibition with single agent nivolumab.
Case Report
In November 2014, a 68-year-old male with a history of previously resected prostate cancer (T2N0M0) 9 years prior and work-related asbestos exposure 30 years prior to initial presentation underwent a chest computed tomography (CT) scan for acute dyspnea. He was found to have a pulmonary embolism, moderate left-sided pleural effusion with mild thickening, and enhancement of the left pleura. There was no enhancement or lesion identified in the right hemithorax at that time. A left-sided thoracentesis was performed from which cytological analysis was inconclusive for the etiology. Two weeks later, a left mini thoracotomy was performed to obtain pleural biopsies, which showed poorly differentiated epithelioid type malignant mesothelioma. He subsequently underwent an extrapleural pneumonectomy and left hemidiaphragmatic stripping with heated intraoperative cisplatin lavage of the left hemithorax; mediastinoscopy at that time was positive for 2 out of 10 thoracic lymph nodes as well as 4 out of 4 sampled left peribronchial lymph nodes. The maximal depth of parietal pleural invasion was 3 mm and his pathologic staging at that time was T3N2M0; he tolerated the procedure well and had a Karnofsky performance status of 90% postoperatively.
Nine weeks following pneumonectomy, he was started on adjuvant chemotherapy with cisplatin and pemetrexed, following National Comprehensive Cancer Network guidelines. He received cisplatin and pemetrexed every 3 weeks for 6 cycles before switching to maintenance therapy with pemetrexed monotherapy every 3 weeks. The patient completed 3 cycles of maintenance pemetrexed before developing anasarca from capillary leak syndrome 10 months after his pneumonectomy. A staging CT scan demonstrated a local recurrence of multifocal nodular disease in the left hemithorax (Figure 1) as well as the accumulation of a large right pleural effusion and a new nodular density in the middle lobe of the right lung. He underwent a right hemithorax thoracentesis, which confirmed progression of MPM.
Figure 1.
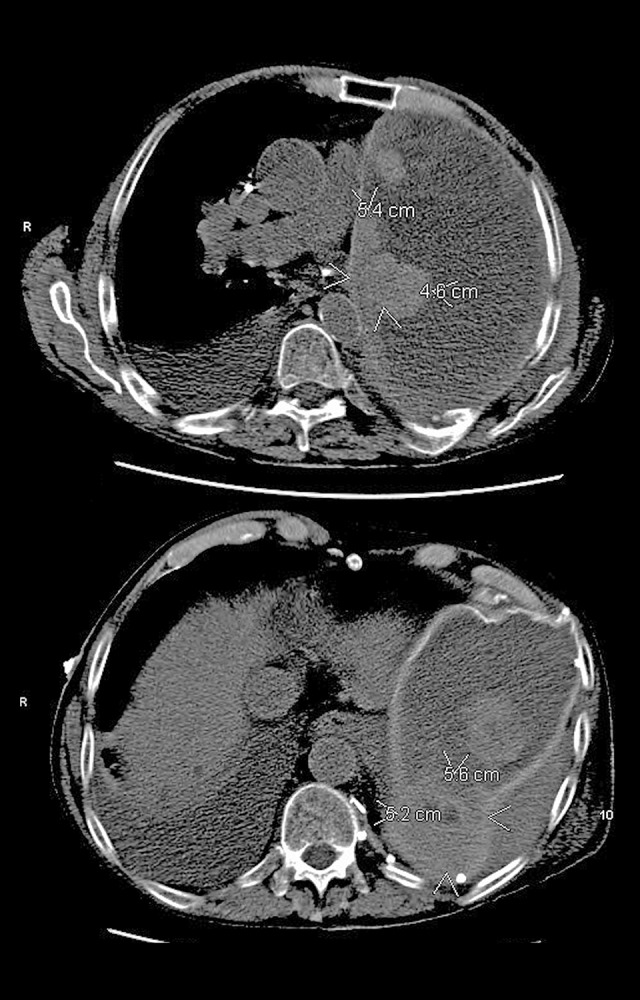
Computed tomography scan demonstrating multifocal recurrence of disease in the left hemithorax occurring after undergoing left pneumonectomy and 10 months of cisplatin-pemetrexed chemotherapy.
His disease progressed rapidly, and he became significantly debilitated requiring continuous supplemental oxygen. His Karnofsky performance status was 50% at that time. Given his deconditioned state, low response rate, and the side effect profile of second-line therapy with combination gemcitabine and vinorelbine, the decision was made to pursue immunotherapy based on the results of immunohistochemical and tumor molecular profiling, which demonstrated 1% tumor cell staining for PD-1 receptor at a 2+ intensity score, 10% elevated PDL-1 staining on tumor infiltrating T-cells, and a high tumor mutational burden with 13 mutations per megabase. Based on these results, he was treated with nivolumab 3 mg/kg infusions every 2 weeks through a compassionate access support program.
Five weeks following the initiation of nivolumab, the patient experienced a substantial clinical improvement with resolution of his dyspnea and cough, and a return to a Karnofsky performance status of 80%. Within 8 weeks of initiating nivolumab, he was independent of oxygen supplementation, and was able to return to work with a Karnofsky performance status of 90% at 4 months of nivolumab therapy. In addition to his clinical improvement, dramatic radiographic improvements were also observed with resolution of radiographic evidence of disease on chest CT scan 6 months after starting immunotherapy (Figure 2) with confirmation by positron emission tomography (PET) imaging at 9 months (Figure 3). Whilst the patient enjoyed a significant improvement in his disease, he did develop mild hypothyroidism requiring low dose levothyroxine supplementation as well as a low-grade elevation of his serum lipase, but no evidence of hepatitis or pancreatitis could be found and required no intervention. At the time of this writing, the patient has been maintained on 3 mg/kg nivolumab infusions every other week; he is free of clinically or radiologically apparent disease and he remains active and well more than 24 months after malignant disease was diagnosed.
Figure 2.
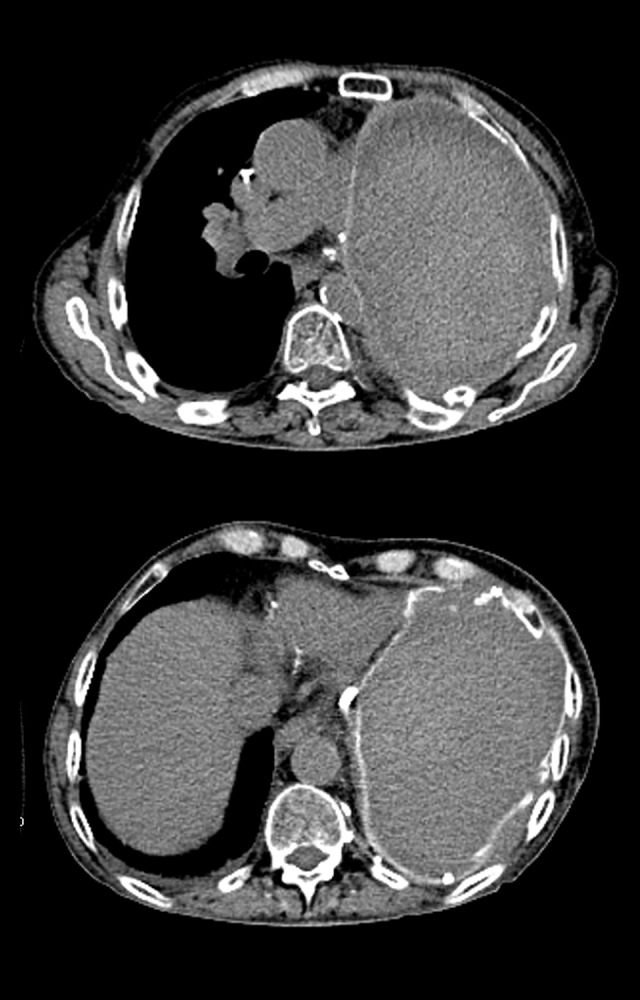
Computed tomography scan demonstrating complete resolution of recurrent disease in the left hemithorax after undergoing 6 months of nivolumab immunotherapy.
Figure 3.
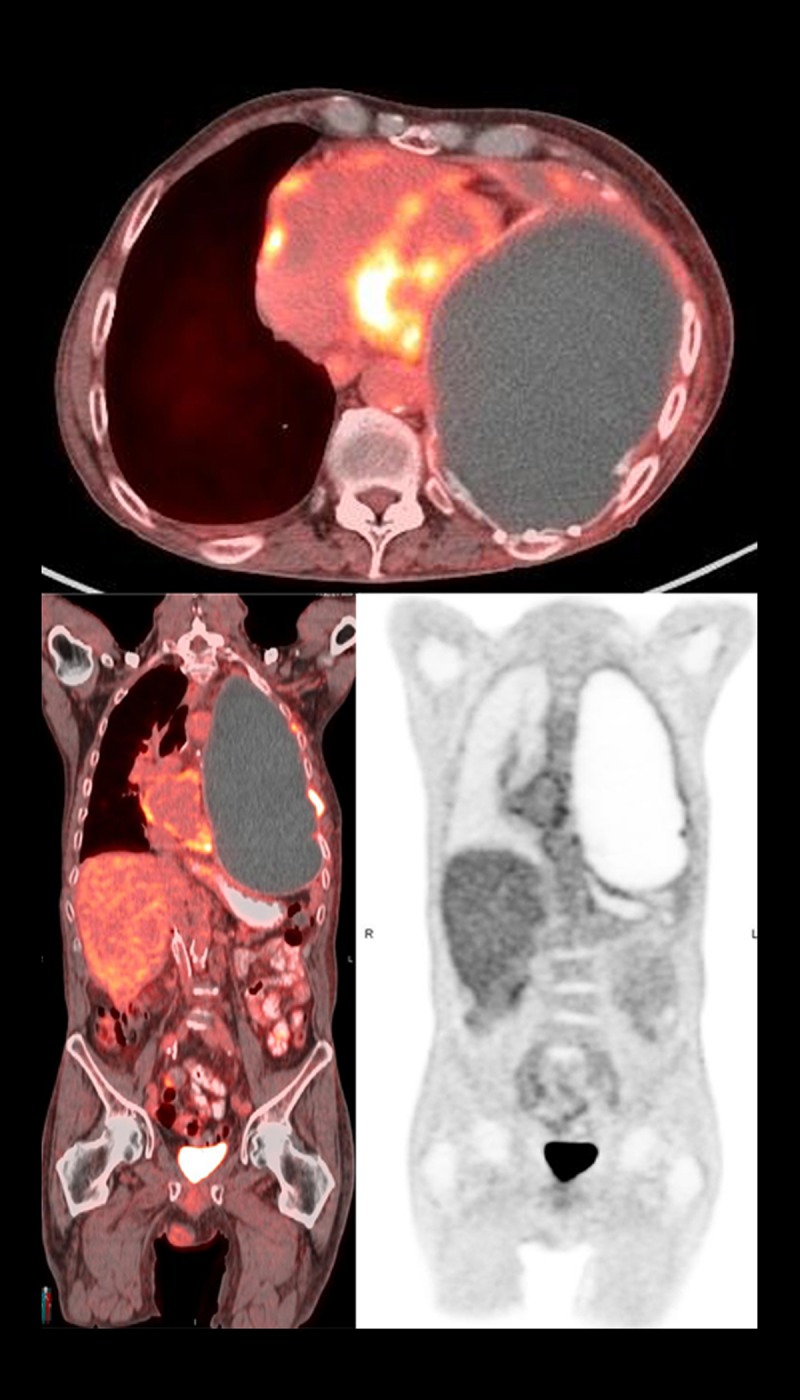
Positron emission tomography scan demonstrating absence of hypermetabolic activity after 9 months of nivolumab immunotherapy suggestive of complete remission of disease.
Discussion
Malignant pleural mesothelioma (MPM) is a rare, highly lethal form of cancer with an association to asbestos exposure arising from malignant transformation of pleural mesothelioma tumors. Pleural mesothelioma has a latency period of approximately 30 to 40 years, and even as long as 60 years, for tumor development, with an unpredictable time to malignant transformation to MPM. Despite legislative interventions beginning in the 1970s to curtail asbestos exposure, its manufacture and use is still commonplace in the developing world with an estimated 125 million people still exposed in the work-place globally, and the incidence of mesothelioma is projected to continue increasing for the next decade according to the World Health Organization [1].
Malignant transformation to MPM carries a very poor prognosis with a median survival time after MPM diagnosis of ∼345 days [2]. Treatment options remain limited with unclear surgical benefits and the most recent systemic therapy recommendations are informed by the 2003 EMPHACIS trial [3,4]. Current management relies on surgical resection and radiotherapy in addition to a foundation of systemic therapy. Frontline chemotherapy utilizes a combination of cisplatin and pemetrexed, which has a medial overall survival of approximately 12 months and provides a median time to progression of disease of 5.7 months [4,5]; response rates to frontline therapy are only 35–41% [5]. Presently, there are no FDA-approved second-line treatments available in the event of failure with cisplatin-pemetrexed. Salvage therapy with a combination of gemcitabine and vinorelbine is often attempted but has a response rate of ∼10% with 2.2 months of progression free survival, and overall survival of 10.9 months after diagnosis [6].
Anti-angiogenic immunotherapy has recently been attempted as both a combination and an adjuvant therapy to standard cisplatin-pemetrexed based regimens with mixed success. Early studies with epidermal growth factor receptor (EGFR) inhibitors, such as erlotinib and gefitinib, were unsuccessful despite evidence of EGFR overexpression in MPM and an established direct interaction between asbestos fibers and the EGFR receptor leading to upregulated EGFR mRNA and protein expression [7–9]. Despite these failures, other EGFR targeting therapies, such as cetuximab in combination with cisplatin-pemetrexed, are still currently under investigation (NCT00996567). Immunotherapies with anti-angiogenic mechanisms, particularly against the vascular-derived endothelial growth factor (VEGFR), are also under investigation due to promising results from preclinical survival data in mice [10,11]. These results have already been realized clinically in the phase III MAPS trial of bevacizumab, wherein the addition of bevacizumab to a regimen of cisplatin-pemetrexed improved overall survival by an average of 2.7 months compared to standard cisplatin-pemetrexed therapy alone [12]. The MAPS trial results were complicated, however, by a significant increase in thrombotic events or development of severe hypertension in the bevacizumab arm compared to the standard therapy arm (24% vs. 6%, respectively) which has significant implications for patient selection [12]. Other immunotherapy targets have been investigated, some of which target other aspects of MPM biology such as anti-mesothelin, anti-CD13, anti-FAK, and others have achieved mixed pre-clinical successes [13].
More recently, immune checkpoint blockade has emerged as a substantial therapeutic advance and opens for consideration new classes of immunotherapies that unmask tumor cells from immune evasion. Specifically, the cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), programmed death-1 (PD-1), and programmed death-1 ligands are receptors which many tumor types employ to evade detection through autoimmunity. CTLA-4 is an immunosuppressive receptor expressed on CD4+ lymphocytes, antigen presenting cells, and granulocytes in lymphoid tissues which prevents the activation and amplification of T-lymphocytes against self-antigens [14]. The PD-1 receptor is downstream from the CTLA-4 receptor immune cascade which directly inhibits effector T-cell functions to further downregulate immune responses. PD-1 receptors are primarily expressed on activated T and B lymphocytes, natural killer cells, monocytes, and tumor-infiltrating lymphocytes [15]. PD-1 receptors bind to immunosuppressive PD-1 ligands (PDL-1) located on the surface of leukocytes, and in some cases tumor cells themselves, which further serves to directly prevent immune activation resulting in sustained T-cell anergy and an immunosuppressive tumor microenvironment [16]. Antibody mediated blockade of either the PD-1 or PD-L1 receptor prevents T-cell anergy and leads to T-cell activation.
The first studies in MPM checkpoint blockade used anti-CTLA-4 antibodies were based on in vitro and in vivo murine MPM evidence demonstrating substantial tumor growth inhibition and greater tumor T-lymphocyte infiltration, especially when used in combination with cisplatin [17]. CTLA-4 blockade also resulted in long-term immunologic memory with 90% of mice that achieved complete tumor rejection remaining completely resistant to relapse following re-inoculation with tumor cells [18]. Clinical trials in MPM with CTLA-4 inhibition using tremelimumab were initially promising but ultimately failed in phase IIb trials to improve overall survival in MPM patients who failed cisplatin-pemetrexed therapy [19]. Despite this setback, CTLA-4 inhibitors are still under study in combination with other checkpoint inhibitors, namely the PD-L1 receptor antibodies nivolumab (MAPS-2 trial, NCT02716272) or durvalumab (NCT02588131) with the former already showing promising preliminary results [20].
Checkpoint inhibition in MPM has remained an area of intense interest due to the overall weight of preclinical data, their utility in other highly aggressive malignancies, the many therapeutic targets within the immune cascade, and the repeated implication of checkpoint inhibition in MPM pathophysiology. Downstream from CTLA-4, PD-L1 is often overexpressed in MPM and the magnitude of its expression has been inversely correlated with histological anti-tumor immune responses [21,22]. Higher expression of PD-L1 has also been correlated with shorter patient survival times [21,23]. It is important to note that PD-1 or PD-L1 expression does not always correlate to a clinical response with immune checkpoint blockade. This phenomenon may be in part due to the more complex activity of PD-L1 inhibition via co-inhibition with a second inhibitory receptor, PD-L2, and the use of a battery of tumor markers, which predate newer prognostic tools that further stratify PD-1/PD-L1 positive MPM tumors [24,25]. The picture is also complicated by a lack of consensus guidelines for histological assessment of PD-1 or PD-L1 expression; cutoff points for positive results are not well defined and values used as “increased” have ranged from 1% to 50% [13,23,26]. These uncertainties notwithstanding, blockade of either PD-1 or PD-L1 receptor has already shown promising results in inducing lasting clinical response and prolonged stability of disease in advanced malignancies, such as renal cancer, melanoma, non-small cell lung cancer, and ovarian cancer even after failure of their respective frontline systemic therapies [27–29].
Additional biomarkers are currently under investigation to predict successful response to PD-1 receptor blockade. Microsatellite instability (MSI) and tumor mutational burden (TMB) have recently been identified by Le and colleagues as contributors to immune checkpoint responses within the microenvironment of colon cancer tumors [25]. Tumors with a high helper T-cell and cytotoxic T-cell infiltration have been correlated with defects in tumor DNA mismatch repair. Defective mismatch repair results in increased micro-mutations which give rise to neo-antigens that, although leading to increased T-cell recruitment, result in increased tumor PD-L1 expression. Thus, increased MSI and TMB results in overall PD-1/PDL1 mediated tumor cell survival despite elevated T-cell tumor invasion [16]. The impact of MSI and TMB on response to checkpoint blockade has been evaluated in a sample of 41 colorectal cancer patients with and without mismatch repair defects leading to MSI and increased TMB, who were treated with PD-1 checkpoint blockade using pembrolizumab [25]. Positive responses to checkpoint blockade were observed with defective mismatch repair, increased MSI, and elevated TMB, resulting in longer progression-free survival compared to tumors with intact mismatch repair mechanisms without an elevated MSI or TMB (78% vs. 40%, respectively). Additionally, an increase in overall survival of 2.2 months was achieved in patients with elevated MSI and TMB treated with PD-1 blockade. These results have helped to improve the understanding of the mixed record of PD-1 blockade response and also to establish the utility of MSI and TMB as promising predictive markers for response to PD-1 blockade; further investigation in this regard is presently underway, however, this remains an area for further clarification, particularly in the case of MPM [30]. At the time of this writing, pembrolizumab is not an established therapy in MPM, however, a phase II trial is currently in recruitment (NCT02399371); it is important to note that MSI and TMB are not listed as outcome measures in this study and pose interesting questions for further studies.
The promising results from checkpoint blockade in other tumors have prompted further study with nivolumab and other PD-1 targeting immunotherapies. As with pembrolizumab, nivolumab is another PD-1 receptor blocking antibody which activates T-cells through check point blockade; it was first studied in advanced solid (non-MPM) tumors (NCT00441337) and has been associated with success in cancers with a strong mutational component [31]. Indeed, nivolumab has already been approved for use in melanoma, non-small cell lung cancer, and renal cell carcinoma and, more recently, has demonstrated improved outcomes in recurrent, cisplatin-resistant small cell lung cancer in the CheckMate-032 clinical trial [32]. PD-1 inhibitors such as nivolumab and pembrolizumab have a hypothetical, although not yet definitively proven, advantage over more upstream checkpoint blockade with anti-CTLA-4 antibodies in that PD-1 blockade activity occurs on peripheral T-cells and tumor infiltrating T-cells (TIL) compared to the more central, i.e., lymph node, action of CTLA-4 blockade [29]. Although a clear association with improved survival has not yet been definitively established, increased infiltration of MPM tumors with TILs have been widely reported and have been correlated with improved survival in some studies [33,34]. Despite the growing body of preclinical data supporting PD-1 blockade in MPM and the analogous mechanism to other PD-1 inhibitors, clinical trials with nivolumab are only now in the beginning phases (NCT02497508, NCT02716272).
Presently, there are no FDA-approved second-line therapies for MPM. Vinorelbine with gemcitabine is typically used as salvage therapy after failure of cisplatin-pemetrexed based therapy, but response rates are low (∼10%) and have a treatment limiting side effect profile for many patients [6]. Our patient experienced aggressive disease progression after chemotherapy with cisplatin-pemetrexed and had a rapid, early decline which was likely to be life-threatening within a few weeks. After failure of cisplatin-pemetrexed, his case was reviewed before an institutional tumor board where treatment decisions were guided by the unlikely utility of vinorelbine and gemcitabine given the advanced state of his disease. His tumor profile was reviewed and the potential for immunotherapy was discussed. Bevacizumab was considered based on early reports available at the time suggesting activity in MPM, but this therapy was ruled out due to risk given his previous history of pulmonary embolism coupled with presence of only one remaining lung following pneumonectomy. Given the recent successes of checkpoint blockade with PD-1 inhibitors in other aggressive cancers such as melanoma, PD-1 targeted checkpoint blockade was put forth based on the results of his tumor molecular profile which demonstrated elevated PD-1, PD-L1, and TMB and the body of pre-clinical evidence supporting checkpoint blockade in MPM. Based on these factors, nivolumab was initiated through a compassionate access protocol; pembrolizumab was materially unavailable in our clinic at the time.
Our patient’s clinical response to nivolumab was rapid and accompanied by the wholly unexpected resolution of radio-graphically apparent disease. Despite initial indications of demise well before the median 12.-month survival, our patient remains well more than 36 months after diagnosis.
Conclusions
The arrival of immunotherapy, and specifically checkpoint blockade, to cancer treatment has brought renewed optimism and hope for diseases where treatments and cures have remained elusive. There remains a substantial need for alternative approaches to MPM therapy as outcomes with traditional chemotherapy regimens remain disappointing at best, with high failure rates and significant side effect profiles. Here, we report a case of a patient with MPM who experienced rapid disease progression after standard chemotherapy but had an exceptional and sustained response to immune checkpoint inhibition with single agent nivolumab. Based on the response achieved in our patient and the growing body of pre-clinical in vitro studies, nivolumab may have promise as a future therapy in MPM, although further study in this regard is needed. As our patient’s case highlights, a new era of investigation and hope for checkpoint immunotherapy for metastatic mesothelioma is beginning with much anticipation indeed.
Acknowledgments
Special thanks are owed to (listed alphabetically) Sunil Dama, Vishal Jivan, Kiranmayee Lanka, and Philip Leming who provided technical assistance in the management of this patient.
Footnotes
Conflicts of interest
None.
References:
- 1.Delgermaa V, Takahashi K, Park EK, et al. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ. 2011;89:716–24. doi: 10.2471/BLT.11.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall AD, Bayes HK, Bardgett J, et al. Survival from malignant mesothelioma: Where are we now? J R Coll Physicians Edinb. 2015;45:123–26. doi: 10.4997/JRCPE.2015.207. [DOI] [PubMed] [Google Scholar]
- 3.Treasure T. What is the best approach for surgery of malignant pleural mesothelioma? It is to put our efforts into obtaining trustworthy evidence for practice. J Thorac Cardiovasc Surg. 2016;151:307–9. doi: 10.1016/j.jtcvs.2015.09.086. [DOI] [PubMed] [Google Scholar]
- 4.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin verses cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: malignant pleural mesothelioma. 2016. v.2.2016. Available at: https://www.nccn.org.
- 6.Zucali PA, Ceresoli GL, Garassino I, et al. Gemcitabine and vinorelbine in pemetrexed-pretreated patients with malignant pleural mesothelioma. Cancer. 2008;112:1555–61. doi: 10.1002/cncr.23337. [DOI] [PubMed] [Google Scholar]
- 7.Garland LL, Rankin C, Gandara DR, et al. Phase II study of erlotinib in patients with malignant pleural mesothelioma: A Southwest Oncology Group Study. J Clin Oncol. 2007;25:2406–13. doi: 10.1200/JCO.2006.09.7634. [DOI] [PubMed] [Google Scholar]
- 8.Govindan R, Kratzke RA, Herndon JE, et al. Gefitinib in patients with malignant mesothelioma: A phase II study by the Cancer and Leukemia Group B. Clin Cancer Res. 2005;11:2300–4. doi: 10.1158/1078-0432.CCR-04-1940. [DOI] [PubMed] [Google Scholar]
- 9.Zanella CL, Posada J, Tritton TR, et al. Asbestos causes stimulation of the extracellular signal-related kinase 1 mitogen-activated protein kinase cascade after phosphorylation of the epidermal growth factor receptor. Cancer Res. 1996;56:5334–38. [PubMed] [Google Scholar]
- 10.Li Q, Yano S, Ogino H, et al. The therapeutic efficacy of anti vascular endothelial growth factor antibody, bevacizumab, and pemetrexed against orthotopically implanted human pleural mesothelioma cells in severe combined immunodeficient mice. Clin Cancer Res. 2007;13:5918–25. doi: 10.1158/1078-0432.CCR-07-0501. [DOI] [PubMed] [Google Scholar]
- 11.Laszlo V, Ozsvar J, Klikovits T, et al. Preclinical investigation of the therapeutic potential of nintedanib in malignant pleural mesothelioma.. 13th Intl Conference iMig Abstract Book; 2016. Abs PP02.61. [Google Scholar]
- 12.Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomized, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405–14. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 13.Bonelli MA, Fumarola C, La Monica S, et al. New therapeutic strategies for malignant pleural mesothelioma. Biochem Pharmacol. 2017;123:8–18. doi: 10.1016/j.bcp.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Marcq E, Pauwels P, van Meerbeeck JP, et al. Targeting immune checkpoints: New opportunity for mesothelioma treatment? Cancer Treat Rev. 2015;41:914–24. doi: 10.1016/j.ctrv.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudley JC, Lin MT, Le DT, et al. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Rev. 2016;22:813–20. doi: 10.1158/1078-0432.CCR-15-1678. [DOI] [PubMed] [Google Scholar]
- 17.Wu L, Yun Z, Tagawa T, et al. CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma. Mol Cancer Ther. 2012;11:1809–19. doi: 10.1158/1535-7163.MCT-11-1014. [DOI] [PubMed] [Google Scholar]
- 18.Lesterhuis WJ, Salmons J, Nowak AK, et al. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PLoS One. 2013;8:e61895. doi: 10.1371/journal.pone.0061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AstraZeneca reports top-line result of tremelimumab monotherapy trial in mesothelioma. 2016 Feb 29; Available at: www.astrazeneca.com. [Google Scholar]
- 20.Ceresoli GL, Bonomi M, Sauta MG. Immune checkpoint inhibitors in malignant pleural mesothelioma: promises and challenges. Expert Rev Anticancer Ther. 2016;16:673–75. doi: 10.1080/14737140.2016.1191951. [DOI] [PubMed] [Google Scholar]
- 21.Mansfield AS, Roden AC, Peikert T, et al. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol. 2014;9:1036–40. doi: 10.1097/JTO.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–66. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 23.Combaz-Lair C, Galateau-Salle F, McLeer-Florin A, et al. Immune biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas. Hum Pathol. 2016;52:9–18. doi: 10.1016/j.humpath.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Powley IR, Sun XM, Chernova T, et al. Evaluation of sensitivity to PI3K/MTOR and FAK inhibition in pre-clinical models of malignant mesothelioma.. 13th Intl Conference iMig Abstract Book; 2016. Abs MS11.03. [Google Scholar]
- 25.Le DT, Uram JN, Want H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–56. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 27.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hause RJ, Pritchard CC, Shendure J, et al. Classification and characterization of microsatellite instability across 18 cancer types. Nature Med. 2016;22:1342–50. doi: 10.1038/nm.4191. [DOI] [PubMed] [Google Scholar]
- 31.Brahmer JR, Drake CC, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-lable, phase 1/2 trial. Lancet Oncol. 2016;17:883–95. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 33.Yamada N, Oizumi S, Kikuchi E, et al. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol Immunother. 2010;59:1543–49. doi: 10.1007/s00262-010-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–67. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]


