 Endocrine disrupting chemicals (EDCs) have been implicated in a broad spectrum of health problems related to reproduction, thyroid function, neurodevelopment, and metabolism.
Endocrine disrupting chemicals (EDCs) have been implicated in a broad spectrum of health problems related to reproduction, thyroid function, neurodevelopment, and metabolism.
Abstract
Endocrine disrupting chemicals (EDCs) have been implicated in a broad spectrum of health problems related to reproduction, thyroid function, neurodevelopment, and metabolism. In many cases, EDCs in the environment are at extremely low concentrations which rarely induce health problems alone, however, a mixture of these EDCs may interact and induce potential additive and synergistic effects. Many mixture studies on EDCs were conducted in terms of high doses with the direct effect addition method, which didn't comply with the dose–response relationship of toxicants in the “S” or “U” shaped curves. In the present study, the thyroid disrupting effects of a mixture of three EDCs, propylthiouracil (PTU), polychlorinated biphenyls (PCBs), and ammonium perchlorate (AP), were measured in an ovariectomized rat model. Sixty female SD rats were ovariectomized bilaterally and randomly assigned to ovariectomization (OVX) control, PTU + PCBs, PTU + AP, PCBs + AP and PTU + PCBs + AP groups treated with doses at lowest observed adverse effect levels (LOAELs) or benchmark dose lower limits (BMDLs) obtained from our previous dose–response relationship studies. OVX control animals were treated with vehicle control while all other animals were treated with different combinations of EDCs by gavage for 8 days. The body weight change, serum total thyroxine (tT4), triiodothyroxine (tT3), the thyroid/body weight ratio, and thyroid histopathological endpoints were measured and analyzed using factorial analysis and dose addition. All EDC treated groups showed a marked change compared to vehicle control in serum tT4, the thyroid/body weight ratio, and the thyroid epithelium/colloid ratio. Both factorial analysis and dose addition analysis showed a synergistic effect on thyroid function by PTU, PCBs and AP together, but the modes of interaction varied when either two were mixed at LOAELs. To conclude, a mixture of PTU, PCBs, and AP mainly acted synergistically on thyroid function and induced a significant health effect.
Introduction
Endocrine disrupting chemicals (EDCs) are defined as exogenous agents that can interfere with the synthesis, secretion, transport, metabolism, binding action, or elimination of natural blood borne hormones in the body, which are responsible for homeostasis, reproduction, and developmental processes.1 A WHO report indicates that about 800 chemicals being used in daily life possess endocrine disrupting properties.2 With extensive application and long-time bio-accumulation and bio-magnification, EDCs are not only restricted to environmental presence such as in water, soil, air, and food, but routinely discovered in human and animal tissues.3 These chemicals are from very heterogeneous groups that function as synthetic chemicals, plasticizers, pesticides, fungicides, and even some drugs,4 causing health problems such as diabetes, obesity, reproductive abnormalities, neoplasm, cardiovascular disease, and thyroid malfunction.1
Traditional toxicological studies are single-chemical oriented; statistics shows that only 5% are focused on the toxicity of chemical mixtures.5 Such scarcity of mixture studies also applies to EDCs; a PubMed search of “EDCs mixture” consists of only 5.6% of all results searched in the keyword “EDCs”. EDCs presented in the environment are in the form of low-dose mixtures, and thus toxicology research studies on single EDCs of high-dose exposure cannot reflect the real environmental exposure scenario.6 Besides, the mixture of EDCs in the environment may act additively or synergistically to induce greater toxicity than single EDC exposure due to their similarities in the mode of action and targets.7 Buha and colleagues found that co-exposure to cadmium and polychlorinated biphenyls (PCBs) for 28 days induced synergistic effects on thyroid function in male Wistar rats.8 Crofton discovered that exposure to a mixture of TCDD-like PCBs, dioxins, and PBDEs resulted in additive effects on thyroid function at low doses, while acting synergistically when exposed to these chemicals at high levels.9 Another study introduced a zebrafish model to research on a mixture of EDCs, finding that the mixture acts either synergistically or additively on thyroid function and the concentration addition method can be extended to chemicals with different modes of action.10 But little was known about how these EDCs with different modes of action would behave together in a mammalian model.
Most EDC mixture studies used the direct effect addition method by simply adding the effect of several individual chemicals to obtain a combined toxicity. However, the method was problematic, since it failed to consider the non-monotonic feature of “S” or “U”-shaped dose–response curves.11,12 Taking these features into consideration, mixture studies have been carried out for decades based on more sound methods, such as factorial analysis and the dose/concentration addition method.13 The factorial design is a classical statistical tool to study the interaction effect between factors using analysis of variance (ANOVA) and it is an efficient way to investigate several factors with adequate power.14 Dose addition indicated that chemicals can induce the same toxicity only with differences in their potency; thus, the dose of chemical A can be transferred into chemical B and the cumulative effect will be that of the summed dose of B.15 By assuming that EDCs of interest have the same mode of action, the expected effect can be computed and compared with the observed effect to determine whether the mode of the combined effect is synergistic, antagonistic, or additive. One popular example is the toxic equivalent factor (TEF) method of transferring doses of polychlorinated biphenyls (PCBs), tetrachlorodibenzo-p-dioxins (TCDDs) and polybrominated diphenyl ethers (PBDEs) into doses of one reference chemical and determining the combined toxicity based on the summed dose of the reference chemical.16
Here, we will present a case study of using both factorial analysis and dose addition to examine the modes of interaction of co-exposure of animals to three EDCs at relatively low doses with minimum thyroid effects. Propylthiouracil (PTU), PCBs, and ammonium perchlorates (APs) were used as modeled chemicals. Though not regularly found in the environment, PTU was a clinical medicine17 and was widely used as a proven thyroidal function inhibitor in experimental settings.18 PCBs were industrial chemicals persistently existing in the environment and not biologically degradable.4,19 AP was an industrial material applied in rockets and missiles and there were reasonable concerns on occupational exposure.14 All three chemicals were well-established EDCs that possess the ability to disrupt normal thyroid function.17,19,20
Previous studies in our lab had laid ground to further carry out a mixture study of these EDCs. Several thyroid-function related endpoints were screened and appropriate exposure times were confirmed in an ovariectomized (OVX) rat model.21 The dose–response relationships of all three chemicals had been established and their toxicological reference doses such as benchmark dose lower limits (BMDLs) and lowest observed adverse effect levels (LOAELs) were obtained22,23 (ESI Table 1†). Both factorial analysis and the dose addition method would be applied to determine the mixture effect mode and the merits of each method would be compared based on the results.
Materials and methods
Procurement and maintenance of animals
Sixty female Sprague Dawley (SD) albino rats (body weight: 230–250 g) free of specific pathogens were purchased from Beijing Vital River Co. Ltd (Beijing, China). The animals were housed singly in cages under conditions of controlled temperature (20–26 °C) and relative humidity (50%–65%), a 12 hour light/12 hour dark cycle and air change of 10 times per h. All animals were fed a soy- and alfalfa-free diet (Hua Fu Kang Bioscience, Beijing, China) and provided with unlimited purified water throughout the study. All experiments are carried out in accordance with the Guide for the Care and Use of the Animals Management Rules of the Ministry Health of the People's Republic of China (Documentation No. 55, 2001, China). The study was approved by the Institutional Animal Care and Use Committee of China National Center for Food Safety Risk Assessment. During the experiment, all animals were treated humanely and maximum care was taken to minimize animal sufferings.
Chemicals
PTU (≥99.0% purity) was purchased from Sigma-Aldrich (St Louis, MO, USA). PCB (Aroclor 1254; ≥99.0% purity) was obtained from AccuStandard Inc. (New Haven, CT, USA). AP (≥99.0% purity) was purchased from Xiya Reagent (Chengdu, China). Non-transgenic corn oil was purchased from COFCO Ltd (Beijing, China).
Experimental design
After acclimation to the housing environment for 7 days, animals were randomly assigned to 6 groups (n = 10 per group) and received bilateral ovariectomy. Following a 12-day recovery phase, all animals received EDC exposure based on the dosimetry in Table 1 via gavage for 8 consecutive days. As indicated earlier, the doses used in the present study were obtained from our previous dose–response studies on PCBs, PTU, and AP22,23 (ESI Table 1†). All the previous studies used the same experimental model, methods, and conditions. Briefly, LOAELs were determined based on the lowest dose at which a significant change was observed for each chemical respectively and the lowest doses among different endpoints for each EDC would be the chosen ones. Thus LOAELs for PCBs, PTU, and AP were 0.1, 0.1, and 50 mg per kg bw respectively (ESI Table 1†). While BMDLs were obtained based on the dose–response models using “Benchmark Dose Software (BMDS), version 2.4” (Environmental Protection Agency, Washington D.C., USA). Please refer to supplementary data† and previous studies22,23 for detailed information on model selection and BMDL data on each parameter of individual chemicals. To maintain the consistency of using the same model for all three chemicals, the Hill model proved suitable for all tT4 datasets and the BMDLs for tT4 are 0.02, 0.02, and 28 mg per kg bw for PCBs, PTU, and AP respectively.
Table 1. Co-treatment designs for PTU, PCBs, and AP.
| Group | n | Mixture doses (mg per kg bw) | |
| OVX control | 10 | Corn oil + water | |
| PTU + PCBs | 10 | 0.1 + 0.1 | |
| PTU + AP | 10 | 0.1 + 50.0 | |
| LOAEL | PCBs + AP | 10 | 0.1 + 50.0 |
| PTU + PCBs + AP | 10 | 0.1 + 0.1 + 50.0 | |
| BMDL | PTU + PCBs + AP | 10 | 0.02 + 0.02 + 28.0 |
Solutions of PTU and PCBs were made by dissolving in corn oil while AP solution was prepared in water. To maintain consistent exposure levels of vehicle control, animals in AP treatment groups received corn oil based on their weight. All rats were observed daily for clinical signs of impairment and recorded for body weight every two days. After the last treatment, rats were fasted overnight and euthanized by overdose of sodium pentobarbital (50 mg per kg bw) anesthesia. Blood from abdomen aorta was collected in pro-coagulation tubes and centrifuged (4 °C, 3000 rpm) for 15 min, followed by serum collection and stored at –80 °C until use. Thyroids of individual animals were dissected and immediately weighed before being preserved in 4% formaldehyde solution.
Determination of serum thyroid hormone concentrations
Serum tT3 and tT4 concentrations were measured using commercially available radioimmunoassay kits (Beijing North Biotechnology Institute, Beijing, China). All experiments were carried out according to manufacturer's instructions. The concentrations were calculated from the calibration curves established by standard T3 and T4. The lowest concentration detectable (sensitivity) was 0.2 ng ml–1 and 3 ng ml–1 respectively for T3 and T4. For both kits, the mean intra-assay and inter-assay variations were 10% and 15% accordingly.
Histopathology and morphometry
Thyroid tissues were paraffin embedded and cut into 4–6 μm transverse sections. The sections were stained with hematoxylin and eosin (HE) and were described with abnormalities/lesions. H&E stained histological sections were analyzed by the Leica Q500MC image analysis and pictures were taken. After transformation of the binary image, areas of the epithelium and colloid were measured and calculated for the ratio of the follicular epithelium area versus the colloid area as described before.22
Statistical analysis
The “Statistical Product and Service Solutions (SPSS), version 20.0” (IBM Inc., Chicago, IL, USA) software was employed to compare the statistical differences between groups and conduct factorial analysis.
Factorial analysis
If the equal variance (β = 0.10) was assumed for the dataset, factorial analysis can be applied; if not, equal variance must be achieved by transforming data before factorial analysis. For three EDCs, please refer Table 2 for dosimetry design. Analysis of variance (ANOVA) was employed to test the statistically significant difference for each effect (p < 0.05). For the main effect, p < 0.05 indicated that the effect of individual chemicals was statistically significant; each chemical can significantly induce the thyroid disrupting effect in our case. For interactions, p < 0.05 indicated that there were interactions among tested EDCs and the chemicals cast their effect dependently; the thyroid disrupting effect for one chemical is different based on the level of the other. After interaction, the addition of the effects (change from the control of the thyroid disrupting endpoints) of single chemicals (expected effect) was compared with the effect of the mixture exposure (observed effect). If the expected effect was greater than the observed one, the interaction would be categorized as antagonistic; if the expected effect was smaller than the observed one, the interaction would be synergistic.
Table 2. Factorial design of 3 factors with 2 levels.
| B1 |
B2 |
|||
| C1 | C2 | C1 | C2 | |
| A1 | A1 + B1 + C1 | A1 + B1 + C2 | A1 + B2 + C1 | A1 + B2 + C2 |
| A2 | A2 + B1 + C1 | A2 + B1 + C2 | A2 + B2 + C1 | A2 + B2 + C2 |
Dose addition analysis
Based on the results from the previous studies22,23 (ESI†), dose–response curves for serum tT4 datasets for each EDC can be modeled with the Hill equation. By using the same model, we assume that the shapes are similar, indicating that the three EDCs can induce the same effect on thyroid function but only with different potency. With this assumption, the dose addition method can be applied to calculate the expected effect of the mixture of EDCs.
The Hill model for the parameter serum tT4 from BMD analysis was the most suitable according to previous experiments. The dose–response equations generated by the Hill model were obtained for all three chemicals. Generally, the equation for the Hill model was
| μ(d) = γ + V·dn/(kn + dn) | 1 |
Here “d” indicated the dose of the chemical, “μ(d)” indicated the effect induced by the chemical at “d” dose; “γ” indicated the intercept, which represented the background effect. “V” was the maximum effect; “k” represented the dose that 50% of the test subjects produced a significant effect; n was the power exponent (when n was restricted, 1 ≤ n ≤ 18).24
In our study, we used PTU as the indicating chemical. Thus, to complete the Hill equation with all parameters, we used parameters obtained from BMD analysis on serum tT4 of PTU. The Hill equation for PTU was presented in eqn (2). By transferring eqn (2), we can calculate PTU doses corresponding to a given effect, as shown in eqn (3). Thus, we used eqn (3) to calculate “PTU” doses respectively for PCBs and AP based on their serum tT4 effect. So the total “PTU” dose present in the organisms was calculated as shown in eqn (4). Now with the mixed dose (dmix), we can calculate the expected effects of the mixture dose of PTU based on eqn (2).
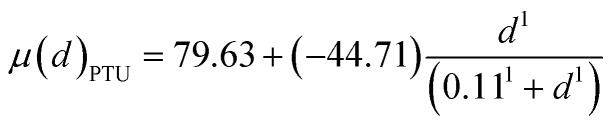 |
2 |
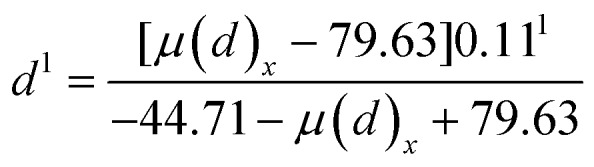 |
3 |
| dmix = dPTU + dPCBs→PTU + dAP→PTU | 4 |
After the calculation, serum tT4 levels in the mixture exposure (observed effect) were compared with the calculated expected effect. We conducted a single sample t-test to compare if there was significant difference between the observed effect and expected effect, and the significant level was set at α = 0.05. When no statistically significant difference was found, it indicated that there was no interaction among the three EDCs on serum tT4 levels and the mode of interaction was dose addition; when there was a significantly different result, the effect would be antagonistic when the expected effect was greater than the observed effect, otherwise it would be synergistic.
Results
Clinical observation and body weight
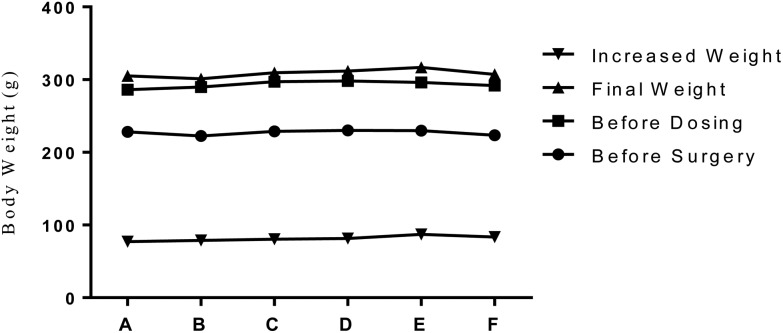
After ovariectomy, several rats in different groups showed redness of wounds and lethargy. The symptoms disappeared with the injection of 20 000 units of penicillin for 3 consecutive days. No other clinical changes were observed in the overall appearance, body position, co-ordination or gait, activity, lacrimation and vocalization. The average body weight before surgery, during the dose, and before sacrifice showed no significant difference among different groups, as indicated in Fig. 1.
Fig. 1. PTU, PCBs, and AP on the body weight change in ovariectomized rats. Note: A: OVX. Control; B: PTU + PCBs (LOAEL); C: PTU + AP (LOAEL); D: PCBs + AP (LOAEL); E: PTU + PCBs + AP (LOAEL); F: PTU + PCBs + AP (BMDL). Mean body weight and weight change were graphed. One-way ANOVA was used to test the significant differences (p < 0.05) of each weight parameter among different groups.
Serum tT4 and tT3 levels
The serum tT3 levels of the mixture groups were lower than that in the OVX control, but the differences were significant except for the PCB + AP group (P < 0.05). The serum tT4 levels of all mixture groups were significantly lower than those of the OVX control (P < 0.05). See Table 3.
Table 3. Single exposure or mixture exposure to PTU, PCBs, and AP on thyroidal function parameters in ovariectomized rats.
| Groups | N | Serum tT3 (ng ml–1) | Serum tT4 (ng ml–1) | Thyroid/body weight ratio (1/105) | Follicular epithelium/colloid ratio | |
| OVX control | 10 | 0.73 ± 0.19 | 60.34 ± 27.93 | 6.47 ± 1.21 | 0.61 ± 0.12 | |
| LOAEL | PTU | 10 | 0.66 ± 0.11 | 55.09 ± 6.64 | 7.76 ± 2.08 | 1.06 ± 0.23 |
| PCBs | 10 | 0.95 ± 0.16 | 51.54 ± 15.52 | 6.52 ± 1.15 | 0.64 ± 0.14 | |
| AP | 10 | 0.69 ± 0.14 | 62.11 ± 16.78 | 7.58 ± 1.13 | 1.48 ± 0.46 | |
| PTU + PCBs | 10 | 0.51 ± 0.06a | 29.14 ± 7.89a | 9.04 ± 0.94a | 0.85 ± 0.19a | |
| PTU + AP | 10 | 0.49 ± 0.06a | 33.29 ± 7.03a | 11.33 ± 1.89a | 1.09 ± 0.42a | |
| PCBs + AP | 10 | 0.53 ± 0.07 | 33.34 ± 5.82a | 9.38 ± 0.86a | 0.98 ± 0.20a | |
| PTU + PCBs + AP | 10 | 0.48 ± 0.06a | 26.44 ± 4.31a | 13.23 ± 2.88a | 1.70 ± 0.44a | |
| BMDL | PTU + PCBs + AP | 10 | 0.50 ± 0.08a | 29.28 ± 9.50a | 10.24 ± 3.09a | 1.34 ± 0.35a |
Histopathology and morphometry
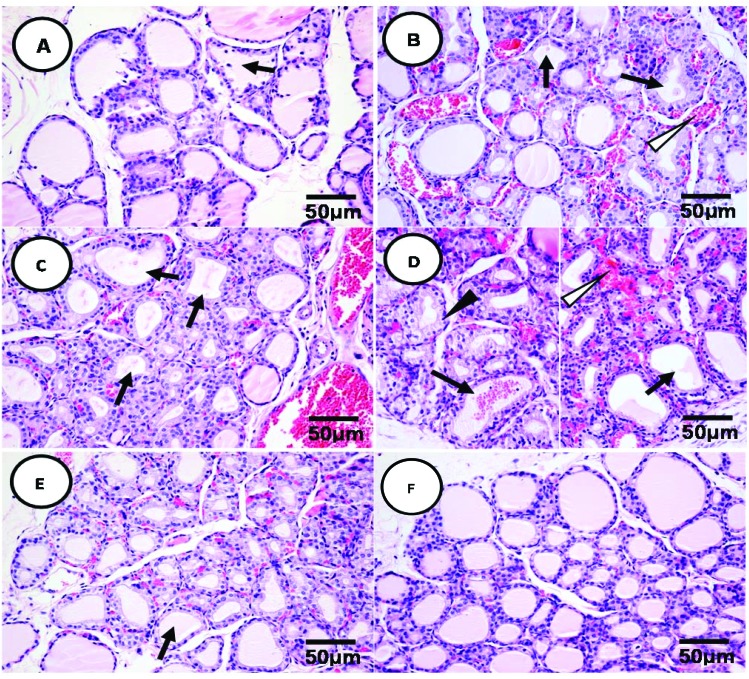
The histopathological changes of thyroid tissue by different mixtures of EDCs were observed at the microscopic level. Among OVX control animals, thyroid follicular cells were round in shape with basophilic cytoplasm and the follicles were relatively similar in size and shape (mostly spherical or oval) and filled with gel-like colloids with light pink color (Fig. 2F). Co-exposure of PTU (0.1 mg per kg bw) and PCBs (0.1 mg per kg bw) causes hypertrophy of follicular epithelial cells and deciduous epithelium in the follicles (Fig. 2A). Co-exposure of PTU (0.1 mg per kg bw) and AP (50 mg per kg bw) induced hypertrophy of the epithelial cells, reduced volume and faded color in the colloid, and congestion in the capillary vessels (Fig. 2B). Animals exposed to PCBs (0.1 mg per kg bw) and AP (50 mg per kg bw) showed obvious hypertrophy of epithelium and decreased the colloid volume (Fig. 2C). With all PTU (0.1 mg per kg bw), PCBs (0.1 mg per kg bw), and AP (50 mg per kg bw) co-exposure, animals showed more severe histological damage such as hypertrophy of follicular epithelial cells accompanying the deformed and deciduous cells; the colloid volume was reduced to a great extent, and even disappeared. In addition, severe congestion of capillary vessels was also observed with infiltration of red blood cells (Fig. 2D). Animals exposed to PTU, PCBs, and AP at BMDLs showed hypertrophy of epithelial cells, decreased colloid volume, and congestion in the capillary vessels to a small extent (Fig. 2E).
Fig. 2. The combined effect of PTU, PCBs, and AP on thyroid histopathology of OVX rats. Note:  : reduced or disappeared colloids in thyroid follicles.
: reduced or disappeared colloids in thyroid follicles.  : congestion of capillary vessels between thyroid follicles.
: congestion of capillary vessels between thyroid follicles.  : deformed, deciduous and necrotic epithelial cells of the thyroid follicles. (A) The thyroid section of animals treated with PTU (0.1 mg per kg bw) + PCBs (0.1 mg per kg bw). (B) The thyroid section of animals treated with PTU (0.1 mg per kg bw) + AP (50.0 mg per kg bw). (C) The thyroid section of animals treated with PCBs (0.1 mg per kg bw) + AP (50.0 mg per kg bw). (D) The thyroid section of animals treated with PTU (0.1 mg per kg bw) + PCBs (0.1 mg per kg bw) + AP (50.0 mg per kg bw). (E) The thyroid section of animals treated with PTU (0.02 mg per kg bw) + PCBs (0.02 mg per kg bw) + AP (28.0 mg per kg bw). (OVX CTL.) The thyroid section of animals from the control group.
: deformed, deciduous and necrotic epithelial cells of the thyroid follicles. (A) The thyroid section of animals treated with PTU (0.1 mg per kg bw) + PCBs (0.1 mg per kg bw). (B) The thyroid section of animals treated with PTU (0.1 mg per kg bw) + AP (50.0 mg per kg bw). (C) The thyroid section of animals treated with PCBs (0.1 mg per kg bw) + AP (50.0 mg per kg bw). (D) The thyroid section of animals treated with PTU (0.1 mg per kg bw) + PCBs (0.1 mg per kg bw) + AP (50.0 mg per kg bw). (E) The thyroid section of animals treated with PTU (0.02 mg per kg bw) + PCBs (0.02 mg per kg bw) + AP (28.0 mg per kg bw). (OVX CTL.) The thyroid section of animals from the control group.
Quantitative measurements for histopathological changes indicated that all mixture exposures of EDCs led to an increased thyroid/body weight ratio and an epithelium/colloid ratio compared with OVX control rats. These differences were statistically significant (P < 0.05), as shown in Table 3.
Factorial analysis results
In the present study, datasets of serum tT3 and tT4, the thyroid/body weight ratio, and the thyroid follicular epithelium/colloid ratio for individual EDC exposures (from previous studies) and mixture exposures (current studies) are summarized in Table 3. Equality of variance tests indicated that all four parameters required data transformation. After logarithm transformation, all datasets reached the homogeneity of variances, see Table 4.
Table 4. Test results of homogeneity of variances (P values) for each endpoint.
| |
Serum tT3 | Serum tT4 | Thyroid/body weight ratio | Follicular epithelium/colloid ratio | |
| Homogeneity of variance | Original data | <0.001a | <0.001a | 0.008a | 0.001a |
| Log-transformed data | 0.49 | 0.17 | 0.27 | 0.859 | |
The main effect tests indicated significant changes of serum tT3 and tT4, the thyroid/body weight ratio, and the thyroid follicular epithelium/colloid ratio caused by PTU and AP (P < 0.001). But PCBs only induced significant changes in serum tT4 and the thyroid/body weight ratio (P < 0.001), which was consistent with our analysis in the published paper.22 For interactions, PTU and PCBs synergistically increased the serum tT3 level (P = 0.001) and antagonistically increased the thyroid follicular/epithelium ratio (P = 0.008). No interaction between PTU and PCBs was found on serum tT4 and the thyroid/body weight ratio (P > 0.05). No interaction between PTU and AP on serum tT3 and tT4, and the thyroid/body weight ratio (P > 0.0.5) was found, and the absence of interaction indicated that the mode of mixture toxicity was addition. PTU and AP antagonistically affected the thyroid follicular epithelium/colloid ratio (P = 0.007). PCBs and AP synergistically decreased serum tT3 (P < 0.001), but additively decreased serum tT4, the thyroid/body weight ratio, and the thyroid follicular epithelium/colloid ratio (P > 0.05). The mixture exposure of PTU, PCBs, and AP induced a synergistical effect on serum tT3 (P < 0.001) and tT4 (P = 0.005) and an antagonistic effect on the thyroid follicular epithelium/colloid ratio (P < 0.001), but no effect on the thyroid/body weight ratio, see Table 5.
Table 5. Results of factorial analysis for thyroid endpoints of OVX rat treated with PTU, PCBs, and AP.
| Variance source |
Serum tT3 |
Serum tT4 |
Thyroid/body weight ratio |
Follicular epithelium/colloid ratio |
|||||
| F | P | F | P | F | P | F | P | ||
| Main effect | PTU | 44.93 | <0.001a | 54.30 | <0.001a | 65.21 | <0.001a | 23.59 | <0.001a |
| PCBs | 0.15 | 0.70 | 67.71 | <0.001a | 13.17 | <0.001a | 0.28 | 0.60 | |
| AP | 31.76 | <0.001a | 26.82 | <0.001a | 72.82 | <0.001a | 81.94 | <0.001a | |
| Interaction | PTU*PCBs | 12.99 | 0.001b | 1.12 | 0.295 | 0.38 | 0.54 | 7.45 | 0.008b |
| PTU*AP | 0.14 | 0.71 | 0.33 | 0.57 | 2.12 | 0.15 | 7.62 | 0.007b | |
| PCBs*AP | 12.95 | 0.001b | 2.56 | 0.11 | 1.33 | 0.25 | 1.21 | 0.27 | |
| PTU*PCBs*AP | 44.52 | <0.001b | 8.50 | 0.005 | 2.48 | 0.12 | 26.46 | <0.001b | |
Dose addition analysis results
By applying eqn (3), LOAELs and BMDLs of PCBs and AP were transformed to the doses of PTU correspondingly. After calculation, LOAELs of PCBs and AP were 0.19 and 0.07 mg per kg bw as the doses of PTU respectively. BMDLs of PCBs and AP were both 0.02 mg per kg bw as the dose of PTU. See Table 6.
Table 6. The doses of PCBs and AP based on the RPF method and Hill equation.
| Combined doses | PCBs (mg per kg bw) |
AP (mg per kg bw) |
||
| Pre-transformation | Post- transformation (as in PTU) | Pre- transformation | Post- transformation (as in PTU) | |
| LOAEL | 0.1 | 0.19 | 50.0 | 0.07 |
| BMDL | 0.02 | 0.02 | 28.0 | 0.02 |
By using eqn (4), we calculated the total dose in the form of PTU for each combination of EDCs. Applying the new combined PTU doses to eqn (2), we calculated the expected serum tT4 levels (Table 7). The t-test indicated a significant difference between the expected value and the observed values of serum tT4, and since all the expected effects (difference from OVX control) were less than those of the observed effects, the interaction modes among PTU, PCBs, and AP were synergistic, see Table 7.
Table 7. Comparisons between predicted observed effects on the serum tT4 level induced by PTU, PCBs, and AP.
| Combined doses | Groups | Combined doses as in PTU (mg per kg bw) | Expected serum tT4 levels (ng ml–1) | Observed serum tT4 levels (ng ml–1) |
| LOAEL | PTU + PCBs | 0.29 | 51.46 | 29.14 ± 7.89a |
| PTU + AP | 0.17 | 63.12 | 33.29 ± 7.03a | |
| PCBs + AP | 0.26 | 54.38 | 33.34 ± 5.82a | |
| PTU + PCBs + AP | 0.36 | 44.66 | 26.44 ± 4.31a | |
| BMDL | PTU + PCBs + AP | 0.06 | 73.80 | 29.28 ± 9.50a |
Discussion
Though the health concerns of mixture toxicants have been recognized for decades, studies on the mixture still remain a big challenge.7 Chemical mixtures possess two characteristics: producing a different toxicity profile compared with those induced by a single high-dose exposure in most experimental scenarios; mixtures at low doses in the environment may induce toxicity and adverse health effects when individual exposure at the same low dose may not cause any effect.7 Moreover, a complex cocktail of chemicals may induce greater toxicity compared with single chemicals due to interactions, especially synergism.12 Thus, studying mixture toxicity requires consideration of co-exposure to multiple chemicals at relatively low doses. Our current study tested the mode of interaction among three EDCs based on the low doses derived from their dose–response relationship in an OVX rat model. To the best of our knowledge, this study is the first to examine the mixture effects on the thyroid function of PTU, PCBs, and AP in vivo by using LOAELs or BMDLs, which mimic the mixture toxicity at low exposure with minimal biological effects.
To reduce the possibility of interference from fluctuated estrogen hormones, the OVX model was introduced to study thyroid hormone disruptors and was recommended to be included into the first-tier of screening tests.25 OVX rats lost the ability to produce sufficient estrogen, which could bind estrogen receptors present ubiquitously in the hypothalamus-pituitary-thyroid (HPT) axis and induce feedback effects on thyroids.26 The model had been used consistently in our studies and proven effective.21–23 Due to the disrupted metabolism after the inhibition of estrogen hormones, rats underwent ovariectomy and showed fast increase of body weight in a short period of time. The overall body weight of OVX rats in the present study increased to 75–78 g, which was consistent with our previous studies.21–23
Factorial design was a classic statistical method which not only can distinguish differences among predicting factors, but can also prove if there was any interaction between different factors.14 Factorial design had several advantages such as quick designing, easy application, and independent of the toxicological points such as ED50 and BMDLs.14 Besides, the results of factorial analysis were easy to read and interpret; the change of one factor would induce the effect of change on the related factor. However, factorial analysis only roughly estimated the interaction between variables and it would be hard to decide the sources of the main effects and interactions when multiple factors were present.14 In factorial analysis, the sample size, dose, and sensitivity of endpoints may affect the interpretation of interaction modes; these may explain the inconsistent interaction results in our findings. The inconsistency of interaction from factorial analysis of EDCs was also found in other studies. Zhan et al. applied factorial analysis to study the anti-androgen toxicity of phoxim and fenvalerate on rats and showed additive interactions at low doses but antagonistic effects at high doses.27 Şekeroğlu et al., by using a factorial study, also found that the cocktail exposure of deltamethrin and thiacloprid synergistically affected T3 and T4, but additively induced a change in the TSH level.28
Direct effect addition was widely employed to investigate the mixture toxicity of chemicals, but paradoxical in toxicology as indicated earlier.29 Dose addition, which took the dose–response relationship into consideration, was a more sound method based on the assumption that chemicals had similar mechanisms and targets to induce toxic effects with only difference in potency.30 The additive toxicity was due to similar chemical structures, properties, or the same toxicological modes of action. For example, these groups of chemicals, pesticides, TCDDs, PBDEs, and PCBs, respectively act on similar molecular targets and their effective dose can be transcribed to similar chemicals in the same group.10,16,31
However, problems remained that most mixtures tested were with similar modes of action, but the low-level presence of various kinds of chemicals with different mechanisms were more likely the case in the environment. Will dose addition also be applicable in our case? The modeled chemicals here had slightly different mechanisms of inducing thyroid disruption, but all with the same effect targets – thyroid hormones. PTU can affect thyroid function by inhibiting the activity of thyroid peroxidase (TPO) which downregulates the synthesis of thyroglobulin, an essential component of T4.17. PCBs can impair thyroid function either by mimicking T4 to bind to thyroid hormone receptors due to their similarity in chemical structures32 or by involving with aromatic receptors (AhR)16 and inhibiting liver chromosome enzymes.33 AP possessed strong oxidative ability and disrupting thyroid function through inhibiting the function of a sodium-iodide symporter, which are essential to transport iodide ions into thyroid follicles to synthesize T4.20 All EDCs will finally target the HPT axis to induce increased secretion of TSH and histological changes of thyroid tissues.34 Besides, application of the dose addition method largely relied on the shapes of the dose–response relationship for each individual chemical. The dose–response relationship curves for serum tT4 from the Hill model for each individual chemical indicated similar shapes.22,23 All interaction results were consistent with different combinations of EDCs (synergism) from the dose addition method, which seemed more reliable than the results from factorial analysis. However, other studies also indicated that these differences in assessing interactions between chemicals in the mixture largely depended on the concepts and methods used.35
Conclusions
Our present study examined the mixture toxicity of different combined exposures of PTU, PCBs, and AP on the thyroid function of OVX rats by using both factorial analysis and dose addition methods. The results indicated by using factorial analysis, PTU, PCBs, and AP at LOAELs induced different profiles of interaction modes. Difficulties remained to infer how these three chemicals interact with each other by using factorial design. Dose addition analysis showed consistent results of mode of interaction, as at both LOAELs and BMDLs of doses, the three EDCs synergistically posed their effect on thyroid function related parameters. However, further research studies are required to improve the dose addition method used in our case. First, the exposure levels of these EDCs in the environment can be used in the study to mimic the real case scenario of low dose exposure. Second, using a more profound dose addition method for the design, such as the isobole method.
Conflicts of interest
There are no conflicts of interest to declare.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation of China [81273081 to Z. Liu] and we express our gratitude to the National Scientific Fund Committee of China for financial support.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tx00193a
References
- Diamanti-Kandarakis E., Bourguignon J. P., Giudice L. C., Hauser R., Prins G. S., Soto A. M., Zoeller R. T., Gore A. C. Endocr. Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO-UNEP, State of the Science of Endocrine Disrupting Chemicals – 2012 Summary for Decision-Makers, ed. Bergman A., Heindel J. J., Jobling S., Kidd K. A. and Zoeller R. T., Available at: http://www.who.int/ceh/publications/endocrine/en/index.html.
- Nakai K., Suzuki K., Oka T., Murata K., Sakamoto M., Okamura K., Hosokawa T., Sakai T., Nakamura T., Saito Y. Tohoku J. Exp. Med. 2004;202:227–237. doi: 10.1620/tjem.202.227. [DOI] [PubMed] [Google Scholar]
- Duntas L. H. Endocrine. 2015;48:53–64. doi: 10.1007/s12020-014-0442-4. [DOI] [PubMed] [Google Scholar]
- Yang R. S., Toxicology of chemical mixtures: case studies, mechanisms, and novel approaches, Elsevier, 2013. [Google Scholar]
- Frye C. A., Bo E., Calamandrei G., Calza L., Dessi-Fulgheri F., Fernandez M., Fusani L., Kah O., Kajta M., Le Page Y., Patisaul H. B., Venerosi A., Wojtowicz A. K., Panzica G. C. J. Neuroendocrinol. 2012;24:144–159. doi: 10.1111/j.1365-2826.2011.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A. Int. J. Androl. 2008;31:233–240. doi: 10.1111/j.1365-2605.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- Buha A., Antonijevic B., Bulat Z., Jacevic V., Milovanovic V., Matovic V. Toxicol. Lett. 2013;221:83–90. doi: 10.1016/j.toxlet.2013.06.216. [DOI] [PubMed] [Google Scholar]
- Crofton K. M., Craft E. S., Hedge J. M., Gennings C., Simmons J. E., Carchman R. A., Carter W. H., DeVito Jr. M. J. Environ. Health Perspect. 2005;113:1549–1554. doi: 10.1289/ehp.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thienpont B., Barata C., Raldua D. Toxicol. Appl. Pharmacol. 2013;269:169–175. doi: 10.1016/j.taap.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. C. Pharmacol. Rev. 1989;41:93–141. [PubMed] [Google Scholar]
- Kortenkamp A., Altenburger R. Sci. Total Environ. 1998;221:59–73. doi: 10.1016/s0048-9697(98)00261-7. [DOI] [PubMed] [Google Scholar]
- Greco W. R., Bravo G., Parsons J. C. Pharmacol. Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- Collins L. M., Dziak J. J., Kugler K. C., Trail J. B. Am. J. Prev. Med. 2014;47:498–504. doi: 10.1016/j.amepre.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosgra S., van Eijkeren J. C., Slob W. Crit. Rev. Toxicol. 2009;39:418–426. doi: 10.1080/10408440902787592. [DOI] [PubMed] [Google Scholar]
- Van den Berg M., Birnbaum L., Bosveld A. T., Brunstrom B., Cook P., Feeley M., Giesy J. P., Hanberg A., Hasegawa R., Kennedy S. W., Kubiak T., Larsen J. C., van Leeuwen F. X., Liem A. K., Nolt C., Peterson R. E., Poellinger L., Safe S., Schrenk D., Tillitt D., Tysklind M., Younes M., Waern F., Zacharewski T. Environ. Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurog A. Endocrinology. 1976;98:1031–1046. doi: 10.1210/endo-98-4-1031. [DOI] [PubMed] [Google Scholar]
- Mellert W., Deckardt K., Walter J., Gfatter S., van Ravenzwaay B. Regul. Toxicol. Pharmacol. 2003;38:368–377. doi: 10.1016/j.yrtph.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Ulbrich B., Stahlmann R. Arch. Toxicol. 2004;78:252–268. doi: 10.1007/s00204-003-0519-y. [DOI] [PubMed] [Google Scholar]
- De Groef B., Decallonne B. R., Van der Geyten S., Darras V. M., Bouillon R. Eur. J. Endocrinol. 2006;155:17–25. doi: 10.1530/eje.1.02190. [DOI] [PubMed] [Google Scholar]
- Chen H., Zhang X., Li Q., Su Q., Jia X., Liu Z. Chin. J. Food Hyg. 2014;26:5. [Google Scholar]
- Chen H., Zhang X., Jia X., Li Q., Su Q., Wang W., Liu Z. Environ. Toxicol. Pharmacol. 2015;40:733–740. doi: 10.1016/j.etap.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Mao W., Chen H., Bao H., Song Y., Sui H., Liu Z. J. Toxicol. 2016;30:91–95. [Google Scholar]
- Gutting B. W., Rukhin A., Marchette D., Mackie R. S., Thran B., Risk Anal., 2016. 10.1111/risa.12564 , , n/a-n/a . [DOI] [PubMed] [Google Scholar]
- O'Connor J. C., Frame S. R., Davis L. G., Cook J. C. Toxicol. Sci. 1999;51:54–70. doi: 10.1093/toxsci/51.1.54. [DOI] [PubMed] [Google Scholar]
- Arain S. A., Shah M. H., Meo S. A., Jamal Q. Saudi Med. J. 2003;24:174–178. [PubMed] [Google Scholar]
- Zhan N., Wang X., Wang S. Chin. J. Ind. Hyg. Occup. Dis. 2001;4:261–264. [Google Scholar]
- Şekeroğlu V., Şekeroğlu Z. A., Demirhan E. Toxicol. Ind. Health. 2014;30:40–46. doi: 10.1177/0748233712448114. [DOI] [PubMed] [Google Scholar]
- Tallarida R. J. Genes Cancer. 2011;2:1003–1008. doi: 10.1177/1947601912440575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewe S. Arch. Exp. Pathol. Pharmakol. 1926;114:313–326. [Google Scholar]
- Soto A. M., Chung K. L., Sonnenschein C. Environ. Health Perspect. 1994;102:380. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan K. R., Kodavanti P. R., McKinney J. D. Toxicol. Appl. Pharmacol. 2000;162:10–21. doi: 10.1006/taap.1999.8826. [DOI] [PubMed] [Google Scholar]
- Kato Y., Ikushiro S., Haraguchi K., Yamazaki T., Ito Y., Suzuki H., Kimura R., Yamada S., Inoue T., Degawa M. Toxicol. Sci. 2004;81:309–315. doi: 10.1093/toxsci/kfh225. [DOI] [PubMed] [Google Scholar]
- O'Connor J. C., Frame S. R., Ladics G. S. Toxicol. Sci. 2002;69:79–91. doi: 10.1093/toxsci/69.1.79. [DOI] [PubMed] [Google Scholar]
- Curcic M., Buha A., Stankovic S., Milovanovic V., Bulat Z., Đukić-Ćosic D., Antonijević E., Vučinić S., Matović V., Antonijevic B. Toxicology. 2016 doi: 10.1016/j.tox.2016.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.