Abstract
Background
Bioimpedance spectroscopy (BIS) with a whole-body model to distinguish excess fluid from major body tissue hydration can provide objective assessment of fluid status. BIS is integrated into the Body Composition Monitor (BCM) and is validated in adults, but not children. This study aimed to (1) assess agreement between BCM-measured total body water (TBW) and a gold standard technique in healthy children, (2) compare TBW_BCM with TBW from Urea Kinetic Modelling (UKM) in haemodialysis children and (3) investigate systematic deviation from zero in measured excess fluid in healthy children across paediatric age range.
Methods
TBW_BCM and excess fluid was determined from standard wrist-to-ankle BCM measurement. TBW_D2O was determined from deuterium concentration decline in serial urine samples over 5 days in healthy children. UKM was used to measure body water in children receiving haemodialysis. Agreement between methods was analysed using paired t test and Bland-Altman method comparison.
Results
In 61 healthy children (6–14 years, 32 male), mean TBW_BCM and TBW_D2O were 21.1 ± 5.6 and 20.5 ± 5.8 L respectively. There was good agreement between TBW_BCM and TBW_D2O (R2 = 0.97). In six haemodialysis children (4–13 years, 4 male), 45 concomitant measurements over 8 months showed good TBW_BCM and TBW_UKM agreement (mean difference − 0.4 L, 2SD = ± 3.0 L). In 634 healthy children (2–17 years, 300 male), BCM-measured overhydration was − 0.1 ± 0.7 L (10–90th percentile − 0.8 to + 0.6 L). There was no correlation between age and OH (p = 0.28).
Conclusions
These results suggest BCM can be used in children as young as 2 years to measure normally hydrated weight and assess fluid status.
Electronic supplementary material
The online version of this article (10.1007/s00467-018-3971-x) contains supplementary material, which is available to authorized users.
Keywords: Fluid volume, Bioimpedance, Chronic kidney disease, Overhydration, Total body water, Children, Haemodialysis
Introduction
Fluid management in haemodialysis impacts patient experience, morbidity and mortality [1, 2]. While inadequate fluid removal can lead to oedema and may precipitate heart failure, volume depletion can cause hypotension, dizziness, cramps, abdominal symptoms, prolonged recovery time following haemodialysis and accelerated loss of residual renal function. An increased risk of morbidity and mortality has been associated both with chronic fluid overload [3–5] and with intradialytic hypotension and loss of residual function [6–9].
Determination of optimal fluid status in a dialysis patient is challenging [10]. Conventionally, the assessment of fluid status is based on clinical symptoms and signs. Progressive reduction of target weight until the patient becomes symptomatic is described as ‘probing for dry weight’. Assessment of fluid status is even more challenging in children who often have rapid changes in flesh weight. A number of technologies are now available to aid fluid management in renal patients, of which bioimpedance spectroscopy (BIS) is one of the most widely studied.
Whole-body (wrist-to-ankle) BIS measurements can be used to determine extracellular, intracellular and total body water (ECW, ICW and TBW) volumes [11]. The addition of a 3-compartment body model to distinguish excess extracellular fluid from lean and adipose tissue allows an objective assessment of excess fluid or ‘overhydration’ (OH) [12]. The BIS fluid volume model and the body model are integrated into the Body Composition Monitor (BCM, Fresenius AG, Bad Homburg), which has been well validated [13] and shown to be associated with better survival in adult dialysis patients, [3, 5]. The BCM is increasingly being used to assist the management of adult dialysis patients [14, 15]. For paediatric patients to benefit from this technology, evidence of the applicability of the underlying models in children is required.
The extensive validation of the BCM models against gold standards that has been carried out in adults is impractical in children. However, it is possible to measure TBW in both healthy subjects and haemodialysis patients using child-friendly techniques, against which the TBW estimated by the BCM fluid volume model can be assessed. While there is no gold standard for quantifying excess extracellular fluid, it should be absent in healthy subjects. BCM OH measurements that are close to zero, and without systematic variation with age, in healthy children would provide further confirmation of the validity of the fluid volume model and indicate that the 3-compartment body model developed for adults can be applied to help manage fluid status in children on dialysis.
In this study, we investigated the agreement of BIS-derived TBW (i) with fluid volume obtained by deuterium dilution in healthy children and (ii) with the urea distribution volume (equivalent to TBW) derived from urea kinetic modelling (UKM) in children receiving haemodialysis. A further objective was to check for systematic deviation of the BCM-measured OH from zero in healthy children across the paediatric age range.
Methods
Study participants
For the method comparisons, healthy children aged 6 to 14 years related to hospital staff and children receiving haemodialysis at the Paediatric Nephrology Department of Leeds Teaching Hospitals NHS Trust UK were recruited. For the investigation of age-related changes in BCM-measured OH, a large cohort of healthy children aged 2 to 17 years from Germany and Sweden was studied. Children with limb amputations, cardiac or other chronic diseases were excluded.
Measurement of TBW_BCM and OH
BCM measurements to obtain TBW (TBW_BCM) and OH were made using standard whole-body electrode configuration after the child had been lying down for at least 5 min. TBW_BCM in healthy children was measured once on the dominant side. In children on haemodialysis, measurements were performed on the dominant or non-fistula side before the start of dialysis once a month for up to 8 months.
The BCM measures whole-body impedance over 50 frequencies (from 5 kHz to 1 MHz) and determines extracellular and total body resistance by Cole modelling [16] in order to estimate ECW and ICW using the fluid volume model [11]. The 3-compartment body composition model uses these volumes to separate the body weight into normally hydrated lean tissue mass (LTM), normally hydrated adipose tissue mass (ATM) and excess fluid (or overhydration, OH) [12]. As OH is simply the discrepancy between the actual body weight and the normally hydrated weight (LTM + ATM), it can be positive or negative. OH is typically within a range of − 1.1 to + 1.1 L (10th to 90th percentile) in healthy adults without cardiac or renal complications [14]. However, in both healthy subjects and dialysis patients, the deviation from normal hydration represented by a BCM-measured OH of 1.1L scales with the size of the individual. To compare individuals of different size, OH is normalised to ECW as excess fluid primarily accumulates in the extracellular space.
Measurement of TBW_D2O
Deuterium oxide (or ‘heavy water’, D2O) dilution can provide an accurate measure of TBW [17]. Deuterium is a naturally occurring, stable isotope of hydrogen that is safe for use in children. To avoid the need for infusions or blood samples, the D2O was taken orally in a drink containing 1 mL of 7% D2O per/kg/body weight (after emptying the bladder) and the children were asked to provide a small (7 mL) urine sample every evening for 6 days starting from the day before taking the D2O drink (the baseline). The D2O concentration in the urine samples was analysed by isotope ratio mass spectrometry (IRMS) [18] in the Medical Research Council Human Nutrition Research Laboratory in Cambridge, UK. D2O distributes throughout the total body water and the initial distribution volume (TBW_D2O) was determined from the mass of D2O administered and the decline in the concentration of deuterium in the urine samples [19]. TBW_D2O was calculated using both the multi-point back-extrapolation method and the two-point plateau method for quality control. The volume obtained using the multi-point method was used for analysis.
Measurement of TBW_UKM
In the haemodialysis cohort, TBW was determined using a modified version of the formal UKM procedure developed by Sargent and Gotch [20]. For each monitored dialysis session, pre- and post-dialysis serum urea samples were taken. The average urea clearance rate for the session was calculated for the dialyser, flow rates and current haematocrit. Urea generation was assumed to be constant and residual renal function was neglected. The urea distribution volume, the ‘kinetic’ V, required to give the measured change in serum urea level (after correction for rebound) with the recorded treatment time and calculated clearance was found by iteration. There were no problems with dialysis delivery such as access recirculation or clotting that would have led to an exaggerated kinetic V in any of the monitored sessions. Like D2O, urea distributes throughout the total body water (giving TBW_UKM).
Funding and ethical approval
The method comparison was funded by a grant from the British Renal Society and the work of one of the main investigator was supported by the National Institute of Health Research (NIHR) Devices for Dignity Healthcare Technology Consortium, UK. The Leeds East Local Research Ethics Committee approved the study protocol and healthy participants were recruited through the hospital’s on-line bulletin board. Parents provided informed consent for the study and children ‘assented’ to take part in line with local recommendations. Data collection for the extended cohort in Germany and Sweden was supported by Fresenius Medical Care, Bad Homburg, by providing the BCM device. Local ethics approval was sought at each site.
Data analysis
Agreement between methods was assessed using mean and SD (adjusted for multiple measurements when appropriate) of paired differences (Bland-Altman analysis), and R2 in an x/y graph. For overhydration, the 10th and 90th percentiles were also reported. Paired t tests were used, and a p value of < 0.05 was considered to indicate significance.
Results
Table 1 details age, gender, height, weight and BMI with standard deviation scores of the different groups of healthy children participating in this analysis.
Table 1.
Subject characteristics stratified by gender and origin [mean ± SD]. BMI is provided in both kg/m2 and standard deviation score
| Patient cohort | N | Age [years] | Height [cm] | BMI [kg/m2] | BMI_SDS |
|---|---|---|---|---|---|
| Heidelberg | 0.1 ± 1.1 | ||||
| Female | 180 | 9.1 ± 3.8 | 134.4 ± 21.6 | 17.7 ± 3.7 | |
| Male | 168 | 9.9 ± 4.5 | 141.4 ± 28.2 | 18.4 ± 3.9 | |
| Kiel | 0.0 ± 1.0 | ||||
| Female | 65 | 10.5 ± 2.3 | 144.7 ± 13.4 | 18.2 ± 3.3 | |
| Male | 65 | 10.5 ± 2.8 | 146.5 ± 18.1 | 17.6 ± 3.4 | |
| Gothenburg | 0.0 ± 1.0 | ||||
| Female | 23 | 12.0 ± 0.8 | 158.1 ± 8.0 | 19.4 ± 4.5 | |
| Male | 36 | 11.8 ± 0.8 | 155.1 ± 10.5 | 17.9 ± 2.4 | |
| Fresenius | −0.1 ± 0.2 | ||||
| Female | 6 | 12.8 ± 6.6 | 146.3 ± 29.8 | 18.7 ± 2.9 | |
| Male | 1 | 5.0 | 123 | 15.3 | |
| Leeds | 0.0 ± 0.9 | ||||
| Female | 29 | 10.1 ± 2.5 | 140.3 ± 14.4 | 17.8 ± 2.7 | |
| Male | 31 | 10.7 ± 2.3 | 143.4 ± 15.3 | 17.1 ± 2.2 | |
BMI body mass index, SD standard deviation
Agreement between D2O- and BCM-derived total body water
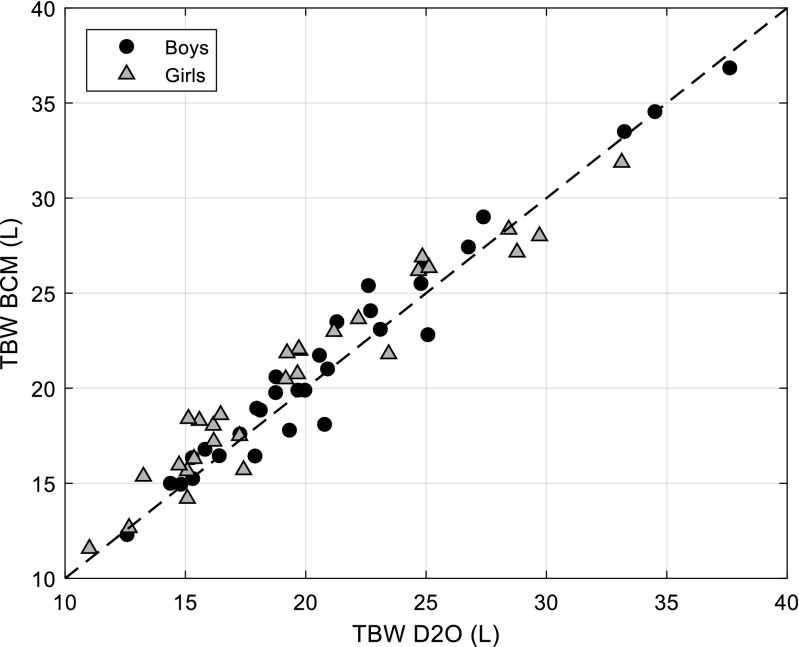
Sixty-one healthy children received TBW assessments by D2O dilution. Sixty children (28 female, median age 10.3) were able to provide sufficient urine samples for analysis. TBW_BCM was calculated using unadjusted model as provided by BCM Version 3.2 and above. The mean TBW_BCM (±SD) was 21.1 ± 5.6 L and that by deuterium dilution was 20.5 ± 5.8 L. There was good agreement between TBW_BCM and TBW_D2O (R2 = 0.97, Fig. 1) with a bias of + 0.6 L and 95% limits of − 2.0 to +3.2 L (Fig. S1).
Fig. 1.
TBW BCM vs. TBW D20 in 60 healthy children aged between 6 and 14 years. Corresponding Bland-Altman plot is shown in Supplementary Fig. S1. All subjects R2 = 0.97 (p < 0.001), Girls R2 = 0.97 (p < 0.001) and Boys: R2 = 0.98 (p < 0.001). BCM Body Composition Monitor, D2O deuterium oxide, TBW total body water
Agreement between BCM- and UKM-derived total body water in children receiving haemodialysis
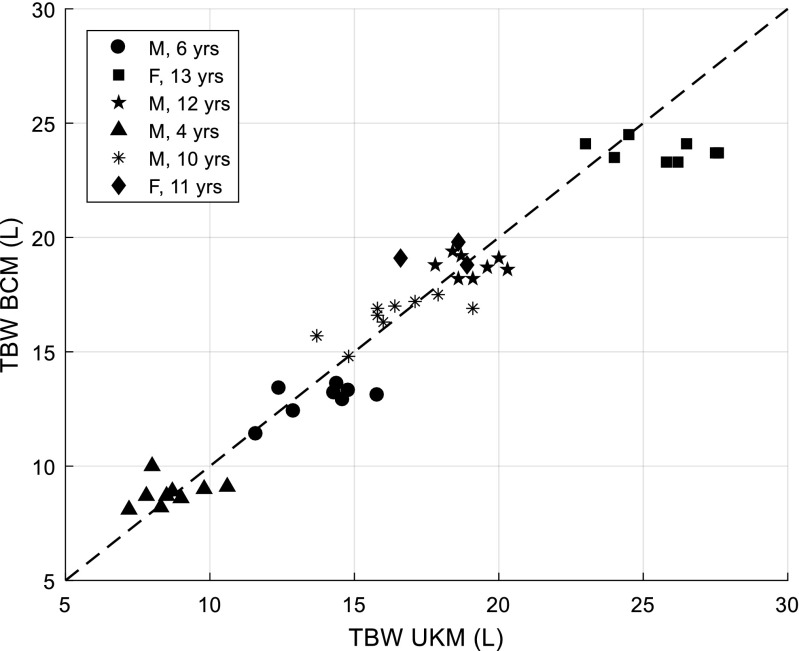
Six children between the ages of 4 and 13 years (2 female, median age 10.5 years) were receiving regular haemodialysis at the time of the study. Table 2 details age, gender, primary renal diagnosis, months on dialysis, height, BMI, requirement for ultrafiltration and months to transplantation for children on haemodialysis participating in the study. Forty-five concomitant measurements of TBW were taken by BCM and UKM over an 8-month period. There was good correlation between TBW_BCM and TBW_UKM (Fig. 2). The mean difference between the methods was − 0.4 L, 2SD = ± 3.0 L (Fig. S2). The mean difference between individually averaged data (N = 6) was 0.2 L, range (min to max) = − 1.2 to 1.9 L (Figs. S3 and S4). TBW_BCM showed better precision of the individual monthly measurements with a mean coefficient of variation (CV=SD/mean) of 3.5% compared to 7.7% for TBW_UKM.
Table 2.
Characteristics of haemodialysis cohort
| Patient | Gender | Primary renal diagnosis | Age [years] |
Months on HD |
Height [cm] |
BMI [kg/m2] | UF required on HD | Months to tx |
|---|---|---|---|---|---|---|---|---|
| 1 | M | Congenital renal dysplasia | 4 | 38 | 89 | 18.9 | Yes | 8 |
| 2 | M | Congenital renal dysplasia | 6 | 6 | 107 | 20.5 | No | 14 |
| 3 | M | Atypical haemolytic uraemic syndrome | 10 | 3 | 137 | 14.6 | Yes | 6 |
| 4 | F | Congenital renal dysplasia | 11 | 1 | 139 | 20.5 | No | 4 |
| 5 | M | Nephronophthisis | 12 | 18 | 140 | 16.7 | Yes | 15 |
| 6 | F | Unknown cause | 13 | 9 | 154 | 20.3 | Yes | 6 |
HD haemodialysis, BMI body mass index, UF ultrafiltration, Tx kidney transplantation
Please note the age, height, BMI and time on HD are at the start of the study
Fig. 2.
TBW_BCM vs. TBW_UKM in 6 children on haemodialysis. Measurements were made over 8 months. Corresponding Bland-Altman plot is shown in Supplementary Fig. S2. The same data but individually averaged is shown in Supplementary Figs. S3 and S4. Dashed line indicates line of identity. TBW total body water, BCM Body Composition Monitor, UKM Urea Kinetic Modelling
Investigation of age-related trends in BCM-measured OH in healthy children
For all healthy children (n = 634) who took part in the method comparison described above, the average BCM-measured OH was − 0.1 ± 0.7 L (10th to 90th percentile − 0.8 to + 0.6 L). The OH normalised to extracellular water (OH/ECW, mean ± SD) was − 1.0 ± 6.3% (10th to 90th percentile − 8.5 to + 6.4%). There was no correlation between age and OH (p = 0.28), (Fig. 3). The age distribution of all children under investigation is shown in Fig. S5.
Fig. 3.
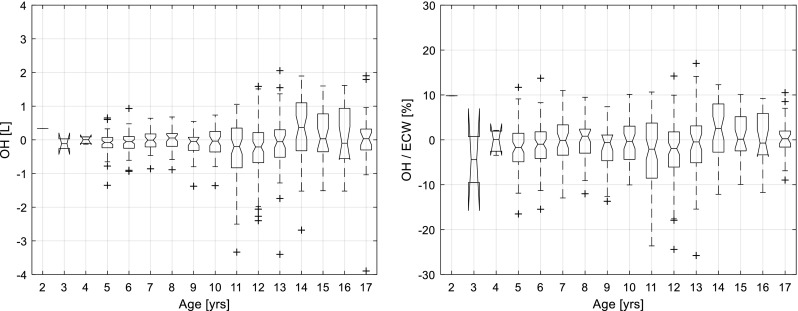
BCM-measured OH (a) and OH/ ECW (b) in all healthy children (n = 634). Boxes indicate interquartile range from 25th to 75th percentile, line is the median, notches indicate 95% confidence interval for median, whiskers are 1.5*interquartile range (covering 99.3% of data assuming normal distribution), crosses = outliers. BCM Body Composition Monitor, OH over hydration, ECW extra cellular water
For the subset of healthy children from Leeds (n = 60), the BCM-measured OH (mean ± SD) was 0.2 ± 0.5 L (10th to 90th percentile − 0.4 to + 0.9 L). The OH/ECW (mean ± SD) was + 2.3% ± 5.8 (10th to 90th percentile − 5.0 to 9.9%). There was no correlation between age and OH in this subset (p = 0.25).
The cohort from Germany and Sweden included 574 healthy children (349 from Heidelberg, 130 from Kiel, 34 from Bad Homburg and 61 from Gothenburg) with a median age of 11 years. Two hundred eighty-six (49.8%) were female. The OH (mean ± SD) was − 0.1 ± 0.7 L which corresponded to − 1.4 ± 6.2% relative to ECW (10th to 90th percentile − 8.6 to 5.9%). Again, there was no systematic variation in OH measured by BCM with age (p = 0.28) (Fig. S6).
Discussion
Bioimpedance measurements are very sensitive to the amount and distribution of fluid in tissue, allowing objective assessment of fluid status. Several bioimpedance-based techniques have been used in studies of children on haemodialysis. Changes in whole-body impedance at 50 kHz reflect changes in body water volume during dialysis [21]. The reactance component of this measurement (Xc) can help identify dry weight [22] and the change in the resistance component (R) with fluid removal correlates with intradialytic hypotension and left ventricular mass index [23]. The variability in blood pressure and heart rate during dialysis can be partially explained by vector analysis [24].
Despite its obvious application potential in dialysis, the use of bioimpedance has been largely restricted to research studies due to difficulties in interpreting the data and problems applying predictive equations derived in healthy subjects in patients with abnormal fluid status. The combination of BIS with the 3-compartment body model in the BCM can provide users with a straight-forward assessment of how far a patient is from their normally hydrated weight.
The fluid volume model used to derive ECW and ICW from BIS data includes parameters related to the resistivity of intracellular and extracellular water and body shape, while the 3-compartment model relies on ‘hydration parameters’ (i.e. the proportion of ICW and ECW per kg of lean and adipose tissue). For the BCM to be used in subjects that differ significantly from the reference population, the assumption that the hydration parameters do not require modification needs to be tested [25]. Dialysed children are a particularly critical population in this regard; differences in model parameters in growing and maturing children would be expected to impact the agreement between BCM-measured parameters and measurements using other techniques.
To assess the validity of the fluid model, we compared the TBW estimates by BCM with D2O dilution-derived measurements in a cohort of healthy children whose BMI distribution was consistent with that of a large German cohort reported previously [26]. There was good agreement between BCM- and D2O-derived TBW readings without any systematic differences related to age, suggesting these findings are likely to be generalisable.
The deuterium dilution method used was not suitable for children on haemodialysis for it required serial urine sampling. Urea kinetic modelling was used to calculate the urea distribution volume as an equivalent to TBW in a cohort of children undergoing haemodialysis. Again, we saw good agreement between the BCM- and UKM-derived TBW estimates without systematic differences across an age range of 4 to 13 years. The repeated measurements showed superior reproducibility of BCM-derived TBW estimates as compared to UKM-derived values, which is not surprising considering the simplicity of bioimpedance measurements as compared to the substantial biological and technical variation associated with a methodology requiring repeated blood sampling and laboratory measurements. The advantage of simplicity was also emphasised in a recent study comparing fluid status in children with nephrotic syndrome by bioimpedance and echocardiography [27].
The validity of the 3-compartment model for fluid overload was assessed in healthy children with an assumed normal state of hydration, i.e. only small differences between actual body weight and the normally hydrated weight (the sum of the normally hydrated lean and adipose tissue) reported by BCM. Such differences will be reported by BCM as ‘overhydration’ since the device is primarily designed to detect and quantify excess fluid in the extracellular compartment. The apparent OH reported by BCM in healthy children was small, without any systematic age-related variation from age 2 to 17 years. Furthermore, the relative overhydration observed in the cohort of healthy children (OH/ECW, mean ± SD) was − 1.0 ± 6.3% (10th to 90th percentile − 8.5 to + 6.4%), showing similar variation to adult healthy controls (10th to 90th percentile − 8 to 8%) [28].
While this study does not provide a rigorous validation of the models used by the BCM to determine body water volumes and normally hydrated tissue mass in children, our findings indicate that the models developed for use in adults can also be applied in children. Further justification for implementing the BCM to help the management of fluid status in paediatric dialysis patients will come with practical experience.
It is important to note that the BCM provides an estimate of a patient’s normally hydrated weight, which is not necessarily identical with the actual target weight due to several confounding factors, including variations in the length of limbs relative to the trunk, body temperature, electrolyte levels and the fluid and electrolyte content of the gut, that vary between measurements. Whereas the use of BCM cannot replace good clinical judgement, it can help prevent potentially harmful adjustments in target weight based on deceptive signs and symptoms. In addition, serial BCM readings allow changes in fluid status to be monitored accurately, provided a consistent measurement protocol is used [29].
The added value of BCM to blood pressure monitoring was impressively documented in a study of 463 dialysis sessions in 23 haemodialysed children [30]. Hypertension was present in 39% of dialysis sessions, of which only 31% were associated with moderate to severe BCM-measured OH. The authors concluded that hypertension is not always related to overhydration, and that use of BCM could avoid inappropriate ‘dry weight probing’ in patients with volume-independent hypertension. Furthermore, a recent study in children attempted to determine the clinical utility of BCM-measured fluid status by comparing it to clinical assessment and cardiovascular indicators. Assessment of fluid status based on clinical assessment was shown to be misleading; BCM measurements correlated with established biomarkers and cardiovascular measures [31].
Conclusion
Our study suggests that the BCM can be used in children as young as 2 years, at least for measurements of normally hydrated weight and fluid overload. The measurements are non-invasive, well tolerated, inexpensive and easy to perform. If carried out according to a standardised protocol [29], BCM measurements are highly reproducible, allowing changes in lean body mass to be distinguished from changes in fluid status and adjustments in target weight to be made before symptoms occur. Further studies are required to demonstrate the benefits of using BCM in guiding fluid management and improving clinical outcomes in this vulnerable population.
Electronic supplementary material
(DOCX 105 kb)
Acknowledgements
We are grateful to Antony Wright and Les Bluck (who sadly died in 2014) for their advice on measuring total body water in children and their independent analysis of the data, which was made possible by MRC Programme Funding (Physiological Modelling and Metabolic Risk: MC_UP_A090_1005).
We are also grateful to Fresenius Medical Care for providing the equipment, consumables and technical support for the study.
Compliance with ethical standards
Conflict of interest
EL received honoraria from Fresenius Medical Care for providing training in the use of bioimpedance spectroscopy in renal care.
PC, PW and UM are employees of Fresenius Medical Care.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00467-018-3971-x) contains supplementary material, which is available to authorized users.
The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Hecking M, Karaboyas A, Antlanger M, Saran R, Wizemann V, Chazot C, Rayner H, Hörl WH, Pisoni RL, Robinson BM, Sunder-Plassmann G, Moissl U. Significance of interdialytic weight gain versus chronic volume overload: consensus opinion. Am J Nephrol. 2013;38:78–90. doi: 10.1159/000353104. [DOI] [PubMed] [Google Scholar]
- 2.Weiner DE, Brunelli SM, Hunt A, Schiller B, Glassock R, Maddux FW, Johnson D, Parker T, Nissenson A. Improving clinical outcomes among hemodialysis patients: a proposal for a “volume first” approach from the chief medical officers of US dialysis providers. Am J Kidney Dis. 2014;64:685–695. doi: 10.1053/j.ajkd.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, Malecka-Masalska T, Marcelli D. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24:1574–1579. doi: 10.1093/ndt/gfn707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal R. Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension. 2010;56:512–517. doi: 10.1161/HYPERTENSIONAHA.110.154815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chazot C, Wabel P, Chamney P, Moissl U, Wieskotten S, Wizemann V. Importance of normohydration for the long-term survival of haemodialysis patients. Nephrol Dial Transplant. 2012;27:2404–2410. doi: 10.1093/ndt/gfr678. [DOI] [PubMed] [Google Scholar]
- 6.Stefánsson BV, Brunelli SM, Cabrera C, Rosenbaum D, Anum E, Ramakrishnan K, Jensen DE, Stålhammar NO. Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol. 2014;9:2124–2132. doi: 10.2215/CJN.02680314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26:724–734. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT, NECOSAD Study Group Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15:1061–1070. doi: 10.1097/01.ASN.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 9.Vilar E, Farrington K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial. 2011;24:487–494. doi: 10.1111/j.1525-139X.2011.00968.x. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Weir MR. Dry-weight: a concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1255–1260. doi: 10.2215/CJN.01760210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moissl UM, Wabel P, Chamney PW Bosaeus I, Levin NW, Bosy-Westphal A, Korth O, Müller MJ, Ellegård L, Malmros V, Kaitwatcharachai C, Kuhlmann MK, Zhu F, Fuller NJ. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006;27:921–933. doi: 10.1088/0967-3334/27/9/012. [DOI] [PubMed] [Google Scholar]
- 12.Chamney PW, Wabel P, Moissl UM Müller MJ, Bosy-Westphal A, Korth O, Fuller NJ. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr. 2007;85:80–89. doi: 10.1093/ajcn/85.1.80. [DOI] [PubMed] [Google Scholar]
- 13.Wabel P, Chamney P, Moissl U, Jirka T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009;27:75–80. doi: 10.1159/000167013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passauer J, Petrov H, Schleser A, Leicht J, Pucalka K. Evaluation of clinical dry weight assessment in haemodialysis patients using bioimpedance spectroscopy: a cross-sectional study. Nephrol Dial Transplant. 2010;25:545–551. doi: 10.1093/ndt/gfp517. [DOI] [PubMed] [Google Scholar]
- 15.Davies SJ, Davenport A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int. 2014;86:489–496. doi: 10.1038/ki.2014.207. [DOI] [PubMed] [Google Scholar]
- 16.Cole KS, Cole RH. Dispersion and absorption in dielectrics: 1. Alternating current characteristics. J Chem Phys. 1941;9:341–351. doi: 10.1063/1.1750906. [DOI] [Google Scholar]
- 17.van Marken Lichtenbelt WD, Westerterp KR, Wouters L. Deuterium dilution as a method for determining total body water: effect of test protocol and sampling time. Br J Nutr. 1994;72:491–497. doi: 10.1079/BJN19940053. [DOI] [PubMed] [Google Scholar]
- 18.Hilkert AW, Douthitt CB, Schlüter HJ, Brand WA. Isotope ratio monitoring gas chromatography/mass spectrometry of D/H by high temperature conversion isotope ratio mass spectrometry. Rapid Commun Mass Spectrom. 1999;13:1226–1230. doi: 10.1002/(SICI)1097-0231(19990715)13:13<1226::AID-RCM575>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Coward WA. Calculation of pool sizes and flux rates. In: Prentice AM, eds. The doubly-labelled water method for measuring energy expenditure. Technical recommendations for use in humans. Vienna: International Dietary Energy (http://archive.unu.edu/unupress/food2/UID05E/UID05E00.HTM, Table of Contents - Chapter 4. Accessed 27 Mar 2018)
- 20.Sargent JA, Gotch FA. Mathematic modeling of dialysis therapy. Kidney Int. 1980;18(Suppl. 10):S2–S10. [PubMed] [Google Scholar]
- 21.Bradbury MG, Smye SW, Brocklebank JT. Assessment of the sensitivity of bioimpedance to volume changes in body water. Pediatr Nephrol. 1995;9:337–340. doi: 10.1007/BF02254204. [DOI] [PubMed] [Google Scholar]
- 22.Paglialonga F, Ardissino G, Galli MA, Scarfia RV, Testa S, Edefonti A. Bioimpedance analysis and cardiovascular status in pediatric patients on chronic hemodialysis. Hemodial Int. 2012;16(Suppl 1):S20–S25. doi: 10.1111/j.1542-4758.2012.00743.x. [DOI] [PubMed] [Google Scholar]
- 23.Oh G, Wong C, Begin B, Salsbery K, Sutherland S, Chaudhuri A. Whole-body single-frequency bioimpedance analysis in pediatric hemodialysis patients. Pediatr Nephrol. 2014;29:1417–1423. doi: 10.1007/s00467-014-2778-7. [DOI] [PubMed] [Google Scholar]
- 24.Brooks ER, Fatallah-Shaykh SA, Langman CB, Wolf KM, Price HE. Bioelectric impedance predicts total body water, blood pressure, and heart rate during hemodialysis in children and adolescents. J Ren Nutr. 2008;18:304–311. doi: 10.1053/j.jrn.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Lindley EJ, Lopot F. The use of bioimpedance to aid volume assessment in dialysis patients. Kidney Int. 2015;87:240. doi: 10.1038/ki.2014.310. [DOI] [PubMed] [Google Scholar]
- 26.Kromeyer-Hauschild K, Wabitsch M, Kunze D, Geller F, Geiß HC, Hesse V, von Hippel A, Jaeger U, Johnsen D, Korte W, Menner K, Müller G, Müller JM, Niemann-Pilatus A, Remer T, Schaefer F, Wittchen HU, Zabransky S, Zellner K, Ziegler A, Hebebrand J (2001) [Percentile for the body mass index for children and adolescents using various German samples]. Monatsschr Kinderheilkd 149:807–818
- 27.Özdemir K, Mir MS, Dinçel N, Bozabali S, Kaplan Bulut İ, Yilmaz E, Sözeri B. Bioimpedance for assessing volume status in children with nephrotic syndrome. Turk J Med Sci. 2015;45:339–344. doi: 10.3906/sag-1312-132. [DOI] [PubMed] [Google Scholar]
- 28.http://www.bcm-fresenius.de/files/information_on_reference_ranges.pdf (Accessed 18 Feb 2018)
- 29.Lindley EJ, Keane DF, Schneditz D. Comparison of intradialytic changes in weight and fluid status. Nephrology. 2016;21:632. doi: 10.1111/nep.12671. [DOI] [PubMed] [Google Scholar]
- 30.Zaloszyc A, Schaefer B, Schaefer F, Krid S, Salomon R, Niaudet P, Schmitt CP, Fischbach M. Hydration measurement by bioimpedance spectroscopy and blood pressure management in children on hemodialysis. Pediatr Nephrol. 2013;28:2169–2177. doi: 10.1007/s00467-013-2540-6. [DOI] [PubMed] [Google Scholar]
- 31.Eng CSY, Bhowruth D, Mayes M, Stronach L, Blaauw M, Barber A, Rees L, Shroff RC (2017) Assessing the hydration status of children with chronic kidney disease and on dialysis: a comparison of techniques. Nephrol Dial Transplant. 10.1093/ndt/gfx287 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 105 kb)