Parkinson’s disease has long been considered idiopathic, with most cases having no apparent genetic cause. However, Klein and Mazzulli argue that recent data instead indicate that Parkinson’s disease is a complex genetic disorder, involving a combinatorial code made up of partially penetrant mutations that converge on lysosomal cellular clearance pathways.
Keywords: neurodegeneration, alpha-synuclein, lysosomal storage disease, protein trafficking, autophagy
Abstract
Common forms of Parkinson’s disease have long been described as idiopathic, with no single penetrant genetic factor capable of influencing disease aetiology. Recent genetic studies indicate a clear association of variants within several lysosomal genes as risk factors for idiopathic Parkinson’s disease. The emergence of novel variants suggest that the aetiology of idiopathic Parkinson’s disease may be explained by the interaction of several partially penetrant mutations that, while seemingly complex, all appear to converge on cellular clearance pathways. These newly evolving data are consistent with mechanistic studies linking α-synuclein toxicity to lysosomal abnormalities, and indicate that idiopathic Parkinson’s disease resembles features of Mendelian lysosomal storage disorders at a genetic and biochemical level. These findings offer novel pathways to exploit for the development of disease-altering therapies for idiopathic Parkinson’s disease that target specific components of the lysosomal system.
Introduction
Lysosomal storage disorders (LSDs) are caused by loss-of-function variants in genes that encode lysosomal proteins, leading to lysosomal dysfunction and intra-lysosomal build-up (so-called ‘storage’) of non-degraded metabolites. The LSD family is composed of over 55 distinct diseases with an incidence of ∼1:7000 live births. The majority of LSDs are autosomal recessive and are caused by severe loss-of-function mutations in hydrolytic enzymes, trafficking components, or integral membrane transporters (Futerman and van Meer, 2004). Although clinical symptoms are usually manifested in childhood, adult-onset forms also occur, which are commonly undiagnosed (Wassif et al., 2016). Neurodegeneration is a prominent phenotype in nearly all LSDs, emphasizing the importance of lysosomal degradation in maintaining neuronal health (Fraldi et al., 2016).
Parkinson’s disease is the second most common neurodegenerative disorder of ageing, affecting ∼1% of the population over 65 years old. Parkinson’s disease symptoms and signs can include tremor, bradykinesia, muscular rigidity, speech difficulties and can occasionally present with cognitive disturbances, but clinical features can be variable. The histopathological hallmark of Parkinson’s disease is the intracellular accumulation of α-synuclein in the form of Lewy body inclusions, and death of dopaminergic neurons in the substantia nigra as well as other circumscribed regions of the nervous system (Braak and Del Tredici, 2017). Accumulation of α-synuclein is a common feature observed in many LSDs (Shachar et al., 2011).
Most individuals with early onset Parkinson’s disease, which correspond to ∼10% of all cases, show Mendelian inheritance. Familial cases of Parkinson’s disease can be due to variants in several genes, including LRRK2, PARK7, PINK1, PRKN, ATP13A2 (which encodes for a component of the lysosomal acidification machinery) and SNCA (which encodes for α-synuclein protein). However, most Parkinson’s disease cases are idiopathic without a single clear genetic link. Early studies describing anecdotal Parkinson’s disease cases bearing variants in other lysosomal genes in addition to ATP13A2, including variants in the GBA1 gene were reported (Goker-Alpan et al., 2004). The GBA1 gene encodes for the lysosomal β-glucocerebrosidase (GlcCerase), an enzyme responsible for degrading the lipid glucosylceramide into ceramide and glucose. Originally it was believed that the GBA1–Parkinson’s disease association was not causative. However, a multi-centre analysis of idiopathic Parkinson’s disease patients demonstrated that loss-of-function mutations in one allele of GBA1 is a significant risk factor for developing disease (Sidransky et al., 2009).
While heterozygote GBA1 mutations increase risk for developing Parkinson’s disease, homozygous variants cause Gaucher disease with an incidence of 1:50 000 births in the general population and rising up to 1:800 in Ashkenazi Jews (Stirnemann et al., 2017). Clinically, Gaucher disease is a heterogeneous syndrome and is divided into three subtypes: type 1, non-neuropathic; and types 2 and 3, the neuropathic forms. These distinctions are not absolute, and currently Gaucher disease is largely recognized as continuum spectrum of phenotypes (Sidransky, 2012). Gaucher disease-causing GBA1 mutations result in an accumulation of glucosylceramide within lysosomes. The mechanisms that lead to cell death in Gaucher disease are still under investigation; however, disruptions in autophagic-lysosomal pathway (Sun and Grabowski, 2010; Schöndorf et al., 2014), mitophagy (Osellame et al., 2013), necroptosis (Vitner et al., 2014) and enhanced inflammation through activation of complement C5a receptor 1 (Pandey et al., 2017) are thought to play a role.
Genetics implicates multiple lysosomal variants in Parkinson's disease aetiology
Newly emerging data have now indicated that the lysosomal connection in Parkinson’s disease likely extends far beyond GBA1. Using an unbiased approach, a recent genome wide association study (GWAS) encompassing the largest cohort to date (26 035 idiopathic Parkinson’s disease cases and 403 190 controls) showed that the majority of variants identified were components of the autophagic-lysosomal pathway (Chang et al., 2017). Of the loci identified, novel genes included CTSB (cathepsin B), ATP6V0A1 (ATPase H+ transporting V0 subunit A1), and GALC (galactocerebrosidase) of the lysosomal pathway, and KAT8 (lysine acetyltransferase), which has been shown to alter autophagic flux (Füllgrabe et al., 2013). An independent study recently published in Brain also demonstrated an excessive burden of rare, likely damaging LSD gene variants in association with Parkinson’s disease risk. In this study, a cohort of 1156 patients with Parkinson’s disease and 1679 control subjects was analysed for variants within 54 lysosomal genes known to cause paediatric LSDs, and novel variants within CTSD (cathepsin D), SLC17A5 (sialin), and ASAH1 (acid ceramidase) were identified. Most (56%) of the Parkinson’s disease patients presented with at least one putative damaging variant in a LSD gene, and 21% carried multiple alleles (Robak et al., 2017). Consistent with a focus on lysosomal-related proteins, previous studies have shown an association of Parkinson’s disease with variants in NPC1, a lysosomal cholesterol transporter that regulates vesicular trafficking and causes Niemann-Pick type C disease (Josephs et al., 2004; Kluenemann et al., 2013), and α-N-acetylglucosaminidase (NAGLU), the gene responsible for Sanfilippo syndrome (Winder-Rhodes et al., 2012). Independent of direct mutations in lysosomal components, variants in vesicular trafficking machinery have been associated with Parkinson’s disease including Rab GTPases (MacLeod et al., 2013; Lesage et al., 2015), retromer components such as VPS35 (Zimprich et al., 2011), LIMP2, which is essential for the targeting of GlcCerase to lysosomes (Do et al., 2011), and LRRK2, which can phosphorylate and deregulate Rab GTPases to influence vesicular trafficking (Satake et al., 2009; Steger et al., 2017). These mutations likely lead to cellular dysfunction by affecting multiple pathways, possibly by disrupting protein secretion or targeting of essential proteins in the synapse such as dopamine transporter (DAT), which may lead to aberrant dopamine metabolism and oxidation (Oaks et al., 2013). However, disruptions in the early secretory pathway strongly affects the maturation and targeting of lysosomal hydrolases, which may particularly influence protein accumulation and aggregation. Since efficient cellular clearance involves the concerted action of proper enzyme targeting, vesicular trafficking or secretion, and cargo delivery processes, a combination of damaging variants in one or both of these pathways are likely to amplify the perturbation, culminating in protein aggregation. Indeed, recent mechanistic studies have demonstrated the interaction of different Parkinson’s disease-linked variants including Rab7L1/LRRK2, which contribute to endosomal-lysosomal dysfunction (MacLeod et al., 2013). Together, these studies suggest that not a single variant, but a combination of many common variants converge in the same pathway ultimately leading to dysfunction, consistent with the notion of idiopathic Parkinson’s disease as a complex genetic disorder that converges on lysosomal dysfunction (Fig. 1). The identification of non-Mendelian variants that are not individually causative, but rather predispose one to disease, have been notoriously difficult to identify due to difficulties in achieving adequate statistical power. These recent large-scale studies bring us one step closer to identifying critical pathogenic pathways to prioritize for therapeutic development centred on the lysosomal system.
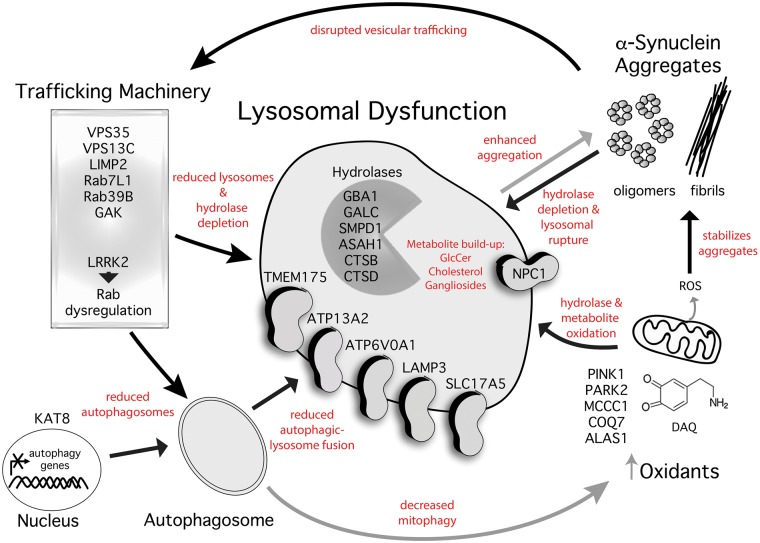
Figure 1.
Parkinson’s disease genes reveal an association of multiple variants that converge on deficiencies in cellular clearance. The emergence of several genes associated with Parkinson’s disease have highlighted essential cellular pathways key to pathogenesis. Genetic variants associated with idiopathic or familial Parkinson’s disease are listed above organized by their normal cellular pathway and function. Lysosomes are critical for the degradation of α-synuclein, and disruptive lysosomal gene variants are expected to enhance the formation of pathogenic α-synuclein oligomers and fibrils directly and indirectly through the interaction of lysosomal membrane lipids. Once formed, these aggregates actively disrupt trafficking and lysosomal clearance pathways, which permits their persistence in neurons and ultimately results in cellular self-destruction. Other Parkinson’s disease genes, such as LRRK2, can influence trafficking and autophagic-lysosomal function through phosphorylating Rab proteins. Parkinson’s disease genes that disrupt mitochondrial function can lead to reactive oxygen species (ROS) or dopamine-o-quinone (DAQ) that can damage lysosomal machinery, leading to α-synuclein aggregate formation. Black arrows indicate an inhibitory process and grey arrows indicate enhancement of potential pathogenic processes described in red text. GlcCer = glucosylceramide.
Lysosomal dysfunction can influence α-synuclein aggregation
Mechanistic studies have begun to illuminate the relationship between lysosomal dysfunction and α-synuclein aggregation. Physiological α-synuclein can be degraded through the lysosomal system (Cuervo et al., 2004) and mutations that perturb lysosomal function are expected to affect α-synuclein levels. As α-synuclein is an abundant protein in physiological conditions, even a subtle elevation in protein concentration may drive the protein towards pathological aggregation since the polymerization process is highly concentration dependent (Giasson et al., 1999). Generalized lysosomal dysfunction may be expected to induce widespread protein aggregation; however, several factors may explain the relative selectivity of α-synuclein aggregation. The amino acid sequence of α-synuclein contains a region in the middle of the protein with an abundance of hydrophobic residues that renders the protein susceptible to polymerization (Giasson et al., 2001). Multiple studies have shown that sphingolipid metabolites that accumulate in LSDs, such as glucosylceramide, psychosine, glucosylsphingosine, and gangliosides can specifically interact and induce α-synuclein aggregation (Mazzulli et al., 2011; Smith et al., 2014; Suzuki et al., 2015; Taguchi et al., 2017). This process is thought to initiate through a conversion of physiological α-synuclein conformers into an alternate, assembly-state oligomer that can subsequently seed the formation of amyloidogenic fibrils (Zunke et al., 2017). Cholesterol may also be an important player in Parkinson’s disease pathogenesis. In addition to the genetic association with NPC1 with Parkinson’s disease, accumulated oxidized metabolites of cholesterol have been identified in synucleinopathy brain and can directly induce α-synuclein fibrilization (Bosco et al., 2006). A recent study has shown that fibroblasts derived from Parkinson’s disease patients bearing the N370S-GBA1 variant accumulate lysosomal cholesterol and present multilamellar bodies, analogous to what occurs in the lysosomal cholesterol storage disorder Niemann-Pick type C (García-Sanz et al., 2017). Related to Gaucher disease pathogenesis, increased intracellular cholesterol can modify GlcCerase processing by inducing its degradation through endoplasmic reticulum (ER)-associated degradation (ERAD) in proteasomes, an activity that is associated with disease severity (Ron and Horowitz, 2008). This, in turn, may lower lysosomal GlcCerase activity and alter glucosylceramide and α-synuclein levels. However, whether this occurs in the context of Parkinson’s disease requires further investigation. Together, this indicates that lysosomal storage of certain toxic metabolites can influence the structural state of α-synuclein, converting it into a pathogenic conformation capable of inducing neurodegeneration. This may explain the selectivity and relationship between certain lysosomal mutations and synucleinopathies.
Genetic variants may modify the development of Gaucher and Parkinson's disease
Important information pertaining to the genetic variants that modify the severity of Gaucher disease has also been obtained through the analysis of mouse models. The in vivo use of a GlcCerase inhibitor in many pure inbred strains has led to the identification of candidate modifier genes of Gaucher disease severity. These findings may also relate to modifiers of idiopathic Parkinson’s disease through the study of long-lived Gaucher disease mouse models where age-related Parkinsonism can be studied (Klein et al., 2016). This work has identified NMDA receptor signalling as an important modifier of disease onset, indicating a possible novel therapeutic pathway in Gaucher disease and Parkinson’s disease. Importantly, this may provide clues not only into the heterogeneity that underlies Gaucher disease, but may also identify novel genetic variants that predispose one to the development of neurological disease.
A bidirectional relationship between α-synuclein and lysosomes
A critical pathogenic factor that could combine with predisposing genetic variants involves the accumulation of α-synuclein. In a remarkable connection to the genetic studies, α-synuclein aggregates have been shown to inhibit autophagic-lysosomal pathways either through direct disruption of lysosomal components (Cuervo et al., 2004; Martinez-Vicente et al., 2008; Yap et al., 2011; Freeman et al., 2013), or through inhibiting trafficking events (Cooper et al., 2006; Mazzulli et al., 2011, 2016a; Chung et al., 2013). Previous work demonstrated that the build-up of α-synuclein blocks the trafficking of newly synthesized GlcCerase to the lysosome and thus amplifies glucosylceramide accumulation, creating a bidirectional pathogenic loop (Mazzulli et al., 2011, 2016a). In addition to GlcCerase, recent studies have found that the enzyme deficient in Fabry’s disease, alpha-galactosidase A, is also deficient in idiopathic Parkinson’s disease brain and correlates with pathological α-synuclein accumulation (Nelson et al., 2018). Loss of NPC1 function, which affects vesicular trafficking and lysosomal function, can also impede the clearance of α-synuclein (Ko et al., 2001; Liao et al., 2007; Eriksson et al., 2017). Since build-up of α-synuclein has been documented in many lysosomal disorders (Shachar et al., 2011), LSD patients may develop α-synuclein deposits by mechanisms that are similar to idiopathic Parkinson’s disease. Although the precise function of α-synuclein is not completely understood, it plays a role in synaptic vesicle recycling and transmission at presynaptic terminals (Bendor et al., 2013). Specifically, α-synuclein can interact with membrane lipids, such as acidic phospholipids, cholesterol, gangliosides, glucosylceramide and others that accumulate in many LSDs and may regulate its function (Perrin et al., 2000; Galvagnion, 2017). Previous work showed that α-synuclein may work as a chaperone that aids the function of synaptic SNARE proteins, regulating the activity of synaptic-vesicle fusion machinery at nerve terminals (Burré et al., 2010; Garcia-Reitböck et al., 2010). Additional studies suggested that multiple copies of α-synuclein coalesce on synaptic vesicles, possibly forming multimeric structures (Burré et al., 2014). It is possible that pathology is initiated at the synapse, since elevated local concentrations will potentiate its aggregation propensity. Autophagic delivery of substrates, such as α-synuclein, from distal axons into lysosomes at the cell body for degradation require intact retrograde axonal transport machinery. Therefore, subtle perturbations in axonal trafficking or lysosomal function, which may occur through heterozygote mutations in LSD genes, may influence the transport of α-synuclein from the presynaptic terminal into the cell body for degradation (Maday and Holzbaur, 2016). Consistent with this, impairments in retrograde axonal transport have been documented in Krabbes disease mouse models with GALC deficiency that also accumulate pathological α-synuclein, as well as in Niemann-Pick type C mice (Ohara et al., 2004; Smith et al., 2014; Teixeira et al., 2014). Aggregation of α-synuclein in neurites or cell bodies may contribute to neurodegeneration by impeding axonal transport (Volpicelli-Daley et al., 2014), or through reducing its putative physiological chaperoning activity at synapses with consequent presynaptic failure (Burgoyne and Morgan, 2011). The relationship between α-synuclein accumulation, lysosomal hydrolase dysfunction, and build-up of lysosomal membrane lipids indicates that any synucleinopathy including Parkinson’s disease exhibits similar but more mild features compared to paediatric LSDs, such as neuronopathic Gaucher disease or other storage diseases characterized by a multiple hydrolase deficiency.
Convergence of mitochondrial and lysosomal dysfunction in Parkinson's disease
In addition to lysosomes and trafficking dysfunction, mitochondria dysfunction has been linked to Parkinson’s disease both genetically and pathologically. Interestingly, mutations that cause early-onset familial Parkinson’s disease have been shown to impede mitophagy, the process of mitochondrial degradation by autophagic-lysosomal system (Pickrell and Youle, 2015). Mechanistic evidence that may explain the convergence of mitochondrial and lysosomal dysfunction in Parkinson’s disease has recently emerged. Using midbrain induced pluripotent stem cell (iPSC) neurons and mouse models, a recent study showed that GlcCerase was susceptible to modification and inactivation by oxidized dopamine, which can induce α-synuclein oligomerization directly or by inhibiting lysosomal function (Burbulla et al., 2017; Mor et al., 2017). Using live-cell imaging techniques, direct contacts between lysosomes and mitochondria have been documented, providing the possibility for aberrant contacts of damaged mitochondria that oxidize lysosomal enzymes in the disease state (Wong et al., 2018). This may also lead to oxidation of accumulating lysosomal metabolites, such as cholesterol that occurs in NPC1, to potentiate α-synuclein aggregation (Bosco et al., 2006). Oxidation and mitochondrial dysfunction are well-established pathological features of Parkinson’s disease and many LSDs (Takamura et al., 2008; Vázquez et al., 2012; Won et al., 2016), and have been implicated in disease initiation. It is possible that interaction of oxidant stress, α-synuclein, and subtle genetic perturbations that affect the lysosomal system combine to produce prominent deficits in cellular clearance (Fig. 1).
Genetic and pathological similarities of Parkinson's disease and lysosome storage disorders
An additional interesting connection between idiopathic Parkinson’s disease-associated genetic variants and mutations that are causative for LSDs is the significant degree of overlap in the cellular pathways involved. Similar to newly discovered factors thought to influence the onset of idiopathic Parkinson’s disease, LSDs can be caused by mutations in different components of the lysosomal system ranging from hydrolases, trafficking machinery, metabolite transporters, or co-activator proteins (Futerman and van Meer, 2004). Mutations that disrupt a key trafficking enzyme located at the Golgi, N-acetylglucosamine-1-phosphotransferase, result in near-complete depletion of multiple hydrolases in the lysosomal compartment. These mutations cause a paediatric LSD with severe neurodegeneration called mucolipidosis type II or inclusion (I)-cell disease, and the multiple hydrolase deficiency results in prominent storage of protein, lipid, and oligosaccharides within lysosomes (Futerman and van Meer, 2004; Klein and Futerman, 2013). Although less severe, the biochemical phenotype of I-cell disease have features that resemble long-lived midbrain cultures of Parkinson’s disease patients, which exhibit a deficiency in multiple lysosomal hydrolases and substrate accumulation as a result of α-synuclein-induced disruptions in ER-to-Golgi trafficking (Cooper et al., 2006; Mazzulli et al., 2016a). As ageing is a critical risk factor for synucleinopathies, it is possible that subtle and chronic perturbations in trafficking machinery eventually reach a threshold leading to severe lysosomal dysfunction, substrate accumulation, and neuronal cell death. The study of post-mortem Parkinson’s disease brain has shown a deficiency of GlcCerase and other lysosomal components (Chu et al., 2009; Alvarez-Erviti et al., 2010; Murphy et al., 2014). Accumulation of lipids, ubiquitin, and the autophagic marker LC3 has been demonstrated in Parkinson’s disease brain (Gai et al., 2000; Dehay et al., 2010), suggestive of autophagic accumulation and general dysfunction in cellular clearance. Studies in transgenic mouse models demonstrate that α-synuclein overexpression induces an LSD-like pathology, as demonstrated by the presence of enlarged lysosomes containing electron-dense inclusion bodies and accumulation of double-membrane vacuoles reminiscent of autophagosomes (Rockenstein et al., 2005).
Collectively, these data suggest that idiopathic Parkinson’s disease and other synucleinopathies exhibit remarkably similar features of LSDs at both the genetic and biochemical level, indicating an overlap in the pathogenesis of these disorders. An important distinction between Mendelian LSDs and genetically complex neurodegenerative disorders such as Parkinson’s disease is the age-at-onset and rate of disease progression. LSDs can result in a severe neurological phenotype that occurs very early in life, likely due to the severity and penetrance of the mutations involved. For example, severe neuronopathic forms of Gaucher disease are often caused by highly destabilizing mutations (such as L444P) that result in near-complete depletion of GlcCerase with little residual activity. Death can ensue rapidly, within 1 year after birth, which can preclude the diagnosis of Parkinson’s disease. As a chronic disease of ageing, lysosomal dysfunction in idiopathic Parkinson’s disease likely occurs by mild heterozygote mutations that combine with age-related complications such as oxidant stress. This suggests that LSDs represent a continuum of diseases that span from severe early onset to late-stage neurodegeneration.
Developing lysosomal therapies for Parkinson's disease
The convergence of pathogenic pathways that culminate in lysosomal dysfunction indicate the importance of cellular degradation in Parkinson’s disease aetiology, and have identified many targets that may be translated into Parkinson’s disease therapies. Several strategies for treating LSDs have been investigated or are under development, and these may provide novel opportunities for treating Parkinson’s disease. These include: (i) gene therapy or enzyme replacement; (ii) substrate reduction therapies, aimed at decreasing storage material; (iii) chaperones to rescue misfolded or unstable enzymes, or direct allosteric activators; (iv) cell therapies to replace injured cells; (v) inhibition of pathways that cause cell death; and (vi) stimulation of bypass pathways to compensate for loss of lysosomal proteins (Klein and Futerman, 2013). Among these approaches, small molecule chaperones or activators from studies done in Parkinson’s disease iPSC models or mouse models have shown promise. Experimental approaches using iPSC-derived neurons from SNCA triplication patients and transgenic mice treated with GlcCerase chaperones or activators, have shown reduced levels of α-synuclein aggregates, opening a new therapeutic strategy for the disease (Mazzulli et al., 2016b; Migdalska-Richards et al., 2016). Brain-penetrant molecules that reduce the synthesis of glucosylceramide can also reduce α-synuclein in iPSC neurons (Zunke et al., 2017; Kim et al., 2018) and improve cognitive symptoms in synucleinopathy mouse models (Sardi et al., 2017). These studies and other work have laid the foundation for clinical trials that will evaluate the efficacy of glucosylceramide reducing agents in patients that carry a GBA1 mutation. Therapies aimed at lowering cholesterol levels with beta-cyclodextrins, which are currently used for treating Niemann-Pick type C patients, reduced accumulation of α-synuclein in a mouse model of Parkinson’s disease (Bar-On et al., 2006). Strategies to increase cellular clearance by overexpressing the transcription factor EB (TFEB), which controls lysosomal biogenesis and autophagy, have therapeutic potential since studies have shown that it can rescue midbrain dopamine neurons in a rat model of Parkinson’s disease (Decressac et al., 2013). Interestingly, beta-cyclodextrins activate TFEB nuclear translocation (Kilpatrick et al., 2015), reinforcing the therapeutic potential of these agents for Parkinson’s disease.
Concluding remarks
The identification of an overrepresentation of autophagic-lysosomal variants in Parkinson’s disease brings us closer to deciphering the aetiology of this complex disorder. Prior to the discovery of SNCA mutations as a cause for familial Parkinson’s disease more than 20 years ago, Parkinson’s disease was classified as strictly idiopathic and considered to be the archetypal non-genetic disease. Recent advancements have clearly negated this with the discovery of several Mendelian forms of Parkinson’s disease, and the identification of risk factors that predispose one to idiopathic Parkinson’s disease. Newly emerging genetic evidence is sure to direct the development of future mechanistic studies on the aetiology of idiopathic Parkinson’s disease, and personalized therapies centred on correcting specific perturbations in lysosomal components.
Funding
A.D.K. is funded by Fondo Nacional de Desarrollo Científico y Tecnológico grant No 1180337 and by the European Union’s Horizon 2020 research and innovation programme (RISE) under the Marie Skłodowska-Curie grant agreement No 734825. J.R.M. is supported by the National Institute of Neurological Disorders and Stroke grant R01NS092823.
Conflict of interest
J.R.M. is a scientific founder of Lysosomal Therapeutics, Inc. (LTI).
Glossary
Abbreviations
- GlcCerase
β-glucocerebrosidase
- LSD
lysosomal storage disorder
References
- Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA et al. . Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol 2010; 67: 1464–72. [DOI] [PubMed] [Google Scholar]
- Bar-On P, Rockenstein E, Adame A, Ho G, Hashimoto M, Masliah E. Effects of the cholesterol-lowering compound methyl-beta-cyclodextrin in models of alpha-synucleinopathy. J Neurochem 2006; 98: 1032–45. [DOI] [PubMed] [Google Scholar]
- Bendor JT, Logan TP, Edwards RH. The function of α-synuclein. Neuron 2013; 79: 1044–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco DA, Fowler DM, Zhang Q, Nieva J, Powers ET, Wentworth P et al. . Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat Chem Biol 2006; 2: 249–53. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J Parkinsons Dis 2017; 7: S73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S et al. . Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 2017; 357: 1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Chaperoning the SNAREs: a role in preventing neurodegeneration? Nat Cell Biol 2011; 13: 8–9. [DOI] [PubMed] [Google Scholar]
- Burré J, Sharma M, Südhof TC. α-synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci USA 2014; 111: E4274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010; 329: 1663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D, Nalls MA, Hallgrímsdóttir IB, Hunkapiller J, van der Brug M, Cai F et al. . A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet 2017; 49: 1511–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis 2009; 35: 385–98. [DOI] [PubMed] [Google Scholar]
- Chung CY, Khurana V, Auluck PK, Tardiff DF, Mazzulli JR, Soldner F et al. . Identification and rescue of α-synuclein toxicity in Parkinson patient-derived neurons. Science 2013; 342: 983–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B et al. . Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 2006; 313: 324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 2004; 305: 1292–5. [DOI] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc Natl Acad Sci USA 2013; 110: E1817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay B, Bové J, Rodríguez-Muela N, Perier C, Recasens A, Boya P et al. . Pathogenic lysosomal depletion in Parkinson’s disease. J Neurosci 2010; 30: 12535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U et al. . Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet 2011; 7: e1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson I, Nath S, Bornefall P, Giraldo AMV, Öllinger K. Impact of high cholesterol in a Parkinson’s disease model: prevention of lysosomal leakage versus stimulation of α-synuclein aggregation. Eur J Cell Biol 2017; 96: 99–109. [DOI] [PubMed] [Google Scholar]
- Fraldi A, Klein AD, Medina DL, Settembre C. Brain disorders due to lysomal dysfunction. Annu Rev Neurosci 2016; 39: 277–95. [DOI] [PubMed] [Google Scholar]
- Freeman D, Cedillos R, Choyke S, Lukic Z, McGuire K, Marvin S et al. . Alpha-synuclein induces lysosomal rupture and cathepsin dependent reactive oxygen species following endocytosis. PLoS One 2013; 8: e62143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füllgrabe J, Lynch-Day MA, Heldring N, Li W, Struijk RB, Ma Q et al. . The histone H4 lysine 16 acetyltransferase hMOF regulates the outcome of autophagy. Nature 2013; 500: 468–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol 2004; 5: 554–65. [DOI] [PubMed] [Google Scholar]
- Gai WP, Yuan HX, Li XQ, Power JT, Blumbergs PC, Jensen PH. In situ and in vitro study of colocalization and segregation of alpha-synuclein, ubiquitin, and lipids in Lewy bodies. Exp Neurol 2000; 166: 324–33. [DOI] [PubMed] [Google Scholar]
- Galvagnion C. The role of lipids interacting with α-synuclein in the pathogenesis of Parkinson’s disease. J Parkinsons Dis 2017; 7: 433–50. [DOI] [PubMed] [Google Scholar]
- Garcia-Reitböck P, Anichtchik O, Bellucci A, Iovino M, Ballini C, Fineberg E et al. . SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain 2010; 133: 2032–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sanz P, Orgaz L, Bueno-Gil G, Espadas I, Rodríguez-Traver E, Kulisevsky J et al. . N370S -GBA1 mutation causes lysosomal cholesterol accumulation in Parkinson’s disease. Mov Disord 2017; 32: 1409–22. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem 2001; 276: 2380–6. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem 1999; 274: 7619–22. [DOI] [PubMed] [Google Scholar]
- Goker-Alpan O, Schiffmann R, LaMarca ME, Nussbaum RL, McInerney-Leo A, Sidransky E. Parkinsonism among Gaucher disease carriers. J Med Genet 2004; 41: 937–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Matsumoto JY, Lindor NM. Heterozygous Niemann-Pick disease type C presenting with tremor. Neurology 2004; 63: 2189–90. [DOI] [PubMed] [Google Scholar]
- Kilpatrick K, Zeng Y, Hancock T, Segatori L. Genetic and chemical activation of TFEB mediates clearance of aggregated α-synuclein. PLoS One 2015; 10: e0120819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Yun SP, Lee S, Umanah GE, Bandaru VVR, Yin X et al. . GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers. Proc Natl Acad Sci USA 2018; 115: 798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AD, Ferreira NS, Ben-Dor S, Duan J, Hardy J, Cox TM et al. . Identification of modifier genes in a mouse model of Gaucher disease. Cell Rep 2016; 16: 2546–53. [DOI] [PubMed] [Google Scholar]
- Klein AD, Futerman AH. Lysosomal storage disorders: old diseases, present and future challenges. Pediatr Endocrinol Rev 2013; 11(Suppl 1): 59–63. [PubMed] [Google Scholar]
- Kluenemann HH, Nutt JG, Davis MY, Bird TD. Parkinsonism syndrome in heterozygotes for Niemann–Pick C1. J Neurol Sci 2013; 335: 219–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko DC, Gordon MD, Jin JY, Scott MP. Dynamic movements of organelles containing Niemann-Pick C1 protein: NPC1 involvement in late endocytic events. Mol Biol Cell 2001; 12: 601–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage S, Bras J, Cormier-Dequaire F, Condroyer C, Nicolas A, Darwent L et al. . Loss-of-function mutations in RAB39B are associated with typical early-onset Parkinson disease. Neurol Genet 2015; 1: e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G, Yao Y, Liu J, Yu Z, Cheung S, Xie A et al. . Cholesterol accumulation is associated with lysosomal dysfunction and autophagic stress in Npc1 -/- mouse brain. Am J Pathol 2007; 171: 962–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD et al. . RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron 2013; 77: 425–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Holzbaur ELF. Compartment-specific regulation of autophagy in primary neurons. J Neurosci 2016; 36: 5933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV et al. . Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest 2008; 118: 777–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA et al. . Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 2011; 146: 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D. α-synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci USA 2016a; 113: 1931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Zunke F, Tsunemi T, Toker NJ, Jeon S, Burbulla LF et al. . Activation of β-glucocerebrosidase reduces pathological α-synuclein and restores lysosomal function in Parkinson’s patient midbrain neurons. J Neurosci 2016b; 36: 7693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migdalska-Richards A, Daly L, Bezard E, Schapira AH. Ambroxol effects in glucocerebrosidase and α-synuclein transgenic mice. Ann Neurol 2016; 80: 766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor DE, Tsika E, Mazzulli JR, Gould NS, Kim H, Daniels MJ et al. . Dopamine induces soluble α-synuclein oligomers and nigrostriatal degeneration. Nat Neurosci 2017; 20: 1560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E et al. . Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain 2014; 137: 834–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MP, Boutin M, Tse TE, Lu H, Haley ED, Ouyang X et al. . The lysosomal enzyme alpha-Galactosidase A is deficient in Parkinson’s disease brain in association with the pathologic accumulation of alpha-synuclein. Neurobiol Dis 2018; 110: 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks AW, Marsh-Armstrong N, Jones JM, Credle JJ, Sidhu A. Synucleins antagonize endoplasmic reticulum function to modulate dopamine transporter trafficking. PLoS One 2013; 8: e70872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara S, Ukita Y, Ninomiya H, Ohno K. Axonal dystrophy of dorsal root ganglion sensory neurons in a mouse model of Niemann? Pick disease type C. Exp Neurol 2004; 187: 289–98. [DOI] [PubMed] [Google Scholar]
- Osellame LD, Rahim AA, Hargreaves IP, Gegg ME, Richard-Londt A, Brandner S et al. . Mitochondria and quality control defects in a mouse model of Gaucher disease–links to Parkinson’s disease. Cell Metab 2013; 17: 941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey MK, Burrow TA, Rani R, Martin LJ, Witte D, Setchell KD et al. . Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature 2017; 543: 108–12. [DOI] [PubMed] [Google Scholar]
- Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human alpha-synuclein and Parkinson’s disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J Biol Chem 2000; 275: 34393–8. [DOI] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015; 85: 257–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robak LA, Jansen IE, van Rooij J, Uitterlinden AG, Kraaij R, Jankovic J et al. . Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain 2017; 140: 3191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Schwach G, Ingolic E, Adame A, Crews L, Mante M et al. . Lysosomal pathology associated with alpha-synuclein accumulation in transgenic models using an eGFP fusion protein. J Neurosci Res 2005; 80: 247–59. [DOI] [PubMed] [Google Scholar]
- Ron I, Horowitz M. Intracellular cholesterol modifies the ERAD of glucocerebrosidase in Gaucher disease patients. Mol Genet Metab 2008; 93: 426–36. [DOI] [PubMed] [Google Scholar]
- Sardi SP, Viel C, Clarke J, Treleaven CM, Richards AM, Park H et al. . Glucosylceramide synthase inhibition alleviates aberrations in synucleinopathy models. Proc Natl Acad Sci USA 2017; 114: 2699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M et al. . Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet 2009; 41: 1303–7. [DOI] [PubMed] [Google Scholar]
- Schöndorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, Schmid B et al. . iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun 2014; 5: 4028. [DOI] [PubMed] [Google Scholar]
- Shachar T, Lo Bianco C, Recchia A, Wiessner C, Raas-Rothschild A, Futerman AH. Lysosomal storage disorders and Parkinson’s disease: Gaucher disease and beyond. Mov Disord 2011; 26: 1593–604. [DOI] [PubMed] [Google Scholar]
- Sidransky E. Gaucher disease: insights from a rare Mendelian disorder. Discov Med 2012; 14: 273–81. [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER et al. . Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 2009; 361: 1651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Santos MB, Marshall MS, Cantuti-Castelvetri L, Lopez-Rosas A, Li G et al. . Neuronal inclusions of α-synuclein contribute to the pathogenesis of Krabbe disease. J Pathol 2014; 232: 509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger M, Diez F, Dhekne HS, Lis P, Nirujogi RS, Karayel O et al. . Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife 2017; 6: e3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnemann J, Belmatoug N, Camou F, Serratrice C, Froissart R, Caillaud C et al. . A review of Gaucher disease pathophysiology, clinical presentation and treatments. Int J Mol Sci 2017; 18: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Grabowski GA. Impaired autophagosomes and lysosomes in neuronopathic Gaucher disease. Autophagy 2010; 6: 648–9. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Fujikake N, Takeuchi T, Kohyama-Koganeya A, Nakajima K, Hirabayashi Y et al. . Glucocerebrosidase deficiency accelerates the accumulation of proteinase K-resistant α-synuclein and aggravates neurodegeneration in a Drosophila model of Parkinson’s disease. Hum Mol Genet 2015; 24: 6675–86. [DOI] [PubMed] [Google Scholar]
- Taguchi YV, Liu J, Ruan J, Pacheco J, Zhang X, Abbasi J et al. . Glucosylsphingosine promotes α-synuclein pathology in mutant GBA-associated Parkinson’s disease. J Neurosci 2017; 37: 9617–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura A, Higaki K, Kajimaki K, Otsuka S, Ninomiya H, Matsuda J et al. . Enhanced autophagy and mitochondrial aberrations in murine GM1-gangliosidosis. Biochem Biophys Res Commun 2008; 367: 616–22. [DOI] [PubMed] [Google Scholar]
- Teixeira CA, Miranda CO, Sousa VF, Santos TE, Malheiro AR, Solomon M et al. . Early axonal loss accompanied by impaired endocytosis, abnormal axonal transport, and decreased microtubule stability occur in the model of Krabbe’s disease. Neurobiol Dis 2014; 66: 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez MC, Balboa E, Alvarez AR, Zanlungo S. Oxidative stress: a pathogenic mechanism for niemann-pick type C disease. Oxid Med Cell Longev 2012; 2012: 205713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitner EB, Salomon R, Farfel-Becker T, Meshcheriakova A, Ali M, Klein AD et al. . RIPK3 as a potential therapeutic target for Gaucher’s disease. Nat Med 2014; 20: 204–8. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Gamble KL, Schultheiss CE, Riddle DM, West AB, Lee VM. Formation of α-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol Biol Cell 2014; 25: 4010–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassif CA, Cross JL, Iben J, Sanchez-Pulido L, Cougnoux A, Platt FM et al. . High incidence of unrecognized visceral/neurological late-onset Niemann-Pick disease, type C1, predicted by analysis of massively parallel sequencing data sets. Genet Med 2016; 18: 41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder-Rhodes SE, Garcia-Reitböck P, Ban M, Evans JR, Jacques TS, Kemppinen A et al. . Genetic and pathological links between Parkinson’s disease and the lysosomal disorder Sanfilippo syndrome. Mov Disord 2012; 27: 312–15. [DOI] [PubMed] [Google Scholar]
- Won JS, Singh AK, Singh I. Biochemical, cell biological, pathological, and therapeutic aspects of Krabbe’s disease. J Neurosci Res 2016; 94: 990–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC, Ysselstein D, Krainc D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 2018; 554: 382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TL, Gruschus JM, Velayati A, Westbroek W, Goldin E, Moaven N et al. . Alpha-synuclein interacts with Glucocerebrosidase providing a molecular link between Parkinson and Gaucher diseases. J Biol Chem 2011; 286: 28080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Benet-Pagès A, Struhal W, Graf E, Eck SH, Offman MN et al. . A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet 2011; 89: 168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunke F, Moise AC, Belur NR, Gelyana E, Stojkovska I, Dzaferbegovic H et al. . Reversible conformational conversion of α-synuclein into toxic assemblies by glucosylceramide. Neuron 2017; 97: 92–107.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]