 Many in vivo studies have revealed that the cytotoxic potential of gold nanoparticles results in controversial conclusions.
Many in vivo studies have revealed that the cytotoxic potential of gold nanoparticles results in controversial conclusions.
Abstract
Background: Many in vivo studies have revealed that the cytotoxic potential of gold nanoparticles results in controversial conclusions. The aim of this study is to establish a systematic method for determining the biological effects of gold nanoparticles in rats. Methods: In the present investigation AuNPs were prepared using Helianthus tuberosus extract as a reducing agent. The synthesized AuNPs were characterized using various techniques, such as Bio-TEM, SEM-EDS, X-ray diffraction and FT-IR. Cytotoxicity of the synthesized AuNPs was assessed using the rat as an animal model. Subchronic oral administration of AuNPs (5 and 10 mg kg–1) and its effect on major organs (liver, kidney, lungs, and spleen) and its accumulation were analyzed using haematoxylin & eosin staining and ICP-MS respectively. The extent of apoptosis in the liver cells was determined using western blotting. Results: The results of the current study revealed that the synthesized AuNPs at a mild concentration of 5 mg kg–1 have been found to cause a hypoglycemic state and an increase in the HDL cholesterol level in normal rats. Nevertheless, histopathological results revealed that AuNPs could cause inflammation in the lungs at increasing concentrations. Conclusion: The biologically synthesized AuNPs were evaluated in this study showed a hypoglycemic effect at a concentration of 5 mg kg–1 AuNPs. A systemic study on the accumulation of AuNPs revealed that the lung is the major target organ and further suggests that enduring administration could lead to organ damage as majorly observed in lung tissue. This study highlights the necessity of complete in vivo toxicity analysis, prior to introducing nanoparticles in any application field. Further, this study warrants the application of the synthesized AuNPs in drug delivery related to lung disorders.
Background
Green synthesis of metallic nanoparticles has shown promising potential applications in multiple segments of day-to-day life, and such advances apply from materials science to consumer products.1 The extensive application of nanoparticles in many industries increases the quantity of nanomaterials in the environment and leads to increased public exposure.2 The accumulation of nanoparticles differs significantly from traditional materials and may cause toxicity to the living systems. Consequently, the detailed mechanism of cell-specific cytotoxicity of nanoparticles will be useful for assessing the risk of nanoparticles.3–5 Among the metallic nanoparticles, gold nanoparticles (AuNPs) in particular are known for their multiple biological applications in the fields of medicine and science and technology. Due to their unique physicochemical properties, AuNPs constitute promising candidates in the fields of biomedicine6 and drug delivery7 and as anticancer agents.8,9
As an alternative to chemical reductants, biological extracts have shown the potential to reduce the metal ions into nanoparticles.10–13 The biosynthesis of AuNPs offers many advantages including biocompatibility and large-scale production.14 Several studies reported AuNP synthesis using biological materials.15,16 A wide range of AuNPs synthesized based on organics, lipids and proteins as well as synthetic methods are shown for their promising results for various purposes. Nanoparticles have a higher surface to volume ratio, and they have the potential to modulate the pharmacokinetic and pharmacodynamic profile of a drug, therefore, they are likely to have an impact on the distribution of drugs in various organs.17,18 Among the biological materials, the synthesis of AuNPs using plant materials has been actively pursued in the last few years, due to their mass production. Thus, the present study focused on the biosynthesis of AuNPs from Helianthus tuberosus (H. tuberosus), a perennial herb. Several studies reported the pharmacological applications of H. tuberosus.19,20 However, this is the first report on the synthesis of gold nanoparticles from H. tuberosus and their in vivo toxicity studies in rats.
Apart from the great excitement about the potential applications of AuNPs, the toxicity of AuNPs must be investigated before using them for any in vivo applications.21 Rats are a suitable in vivo animal model for rapid screening of AuNP toxicity because of their user friendliness and a high degree of homology to the human genome. Lasagna-Reeves et al.22 reported the bioaccumulation and toxicity of AuNPs in mice. The liver and spleen are the major organs where AuNPs accumulate upon continuous administration, followed by lungs, kidney and heart. However, little is known about the biodistribution, accumulation and toxicity of AuNPs after repeated administration. Hence, the objectives of the present study were (i) synthesis and characterization of AuNPs from H. tuberosus, (ii) analysis of bioaccumulation of Au in the organs of the rat by ICP-MS, (iii) assessment of AuNP induced apoptosis in the liver and (iv) histopathological examination of organs following the subchronic oral administration for 21 days.
Results and discussion
Characterization of AuNPs
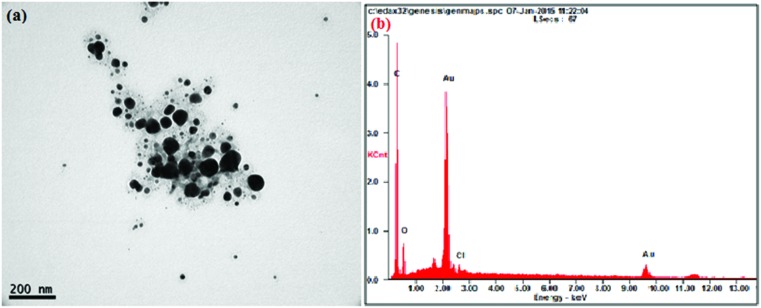
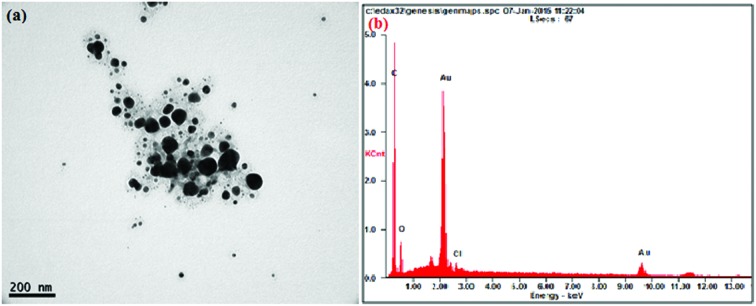
The AuNPs synthesized by sunroot extract mediated synthesis were analyzed for their physical properties such as morphology and size using TEM. The results evidently show that the AuNPs possess a typical spherical shape with an average size of 10 μm (Fig. 1a). To further confirm the presence of Au, the samples were analyzed by SEM-EDS and the results are shown in Fig. 1b. The results showed strong gold signals (2 keV), along with weak oxygen and carbon peaks, which might have originated from the plant extract. EDS quantitative analysis showed the presence of gold (100%) without any impurity.
Fig. 1. (a) A representative TEM image of H. tuberosus extract mediated gold nanoparticles. The AuNPs were probe-sonicated and diluted prior to the analysis. (b) SEM-EDX pattern of gold nanoparticles, the visible peak confirms the presence of Au and oxygen in the sample.
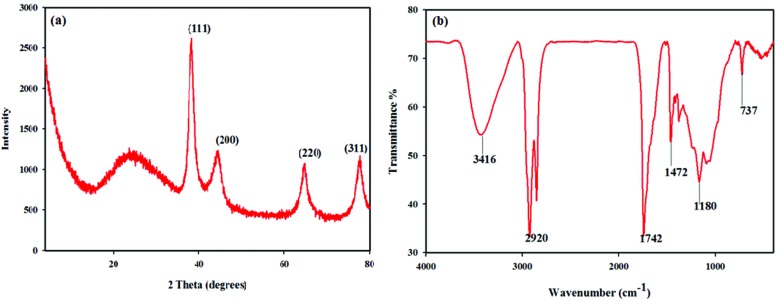
The XRD profile of the biosynthesized AuNPs is shown in Fig. 2a. Four diffraction peaks at 38.26° (111), 44.48° (200), 66.29° (220), and 79.28° (311) were corresponding to the reflections of the face centered cubic structure of gold. The results are in agreement with the previous studies reported for the cubic nature of the biologically synthesized AuNPs.16 FTIR analysis of the synthesized AuNPs was performed to identify the biomolecules responsible for the reduction of the Au+ ions. The FTIR spectrum (Fig. 2b) of AuNPs showed that a strong peak at 3416 cm–1 was assigned to the –NH stretching of primary amines and amides. The peaks observed around 2920, 1742, 1472 and 1180 cm–1 could be assigned to the C–H stretching vibration of aldehyde groups and the O–H, N–H and C–O stretching of alcohols, phenols and carboxylic anions. The small peak observed at 737 cm–1 was associated with the stretching vibrations of O–H respectively.15
Fig. 2. (a) X-ray diffraction pattern of synthesized gold nanoparticles from H. tuberosus extract. (b) Fourier transform infrared (FT-IR) spectrum of AuNPs.
Animal observation, body and organ weight
Throughout the study period all the animals were observed carefully for any abnormal behavior and there were no death and morbidity of the animals recorded. There were no significant differences in food and water consumption between the groups observed (data not shown). In the AuNP treated group there was no significant weight change observed. There was a reduction in size of the lungs in the 10 mg kg–1 AuNP group. No significant changes were observed in the organ weight among the various groups, however spleen was found to have a higher weight in the AuNP administered group (Fig. 3b). Previous research on silver nanoparticles has shown that the administration of nanoparticles does not influence the body as well as the organ weight significantly, despite the size and the dosage for a period of 30 days.23
Fig. 3. (a) Oral glucose tolerance test (OGTT); following the administration of AuNPs, rats were fed with glucose (2 g kg–1) and the glucose levels in each group were monitored until 2 h. Each value represents the mean ± SD of six animals. *P < 0.05 versus control. (b) The relative organ weights of male Sprague Dawley rats, each value represents the average of six animals’ weights ±SD.
Hypoglycemic effect of AuNPs
The hypoglycemic effect of AuNPs over the high glucose dose was assessed using OGTT. The blood glucose level of each group was measured following an overnight fasting. After the oral glucose administration, the blood glucose level of each group was measured up to 2 h with a frequency interval of 30 min and the results are shown in Fig. 3(a). The animals which received AuNPs orally are shown to have a hypoglycemic effect when compared to normal control. Furthermore, AuNPs at a dose of 5 mg kg–1 have shown to significantly (p < 0.05) reduce the blood glucose level after 30 min of administration of glucose, compared to the 10 mg kg–1 AuNP administered animals. Previous research on the hypoglycemic effect of AuNPs in diabetic mice24 and rats25 have shown promising results, and our finding is also in line with the previous reports and found to significantly reduce the glucose level after 30 min in the case of the 5 mg kg–1 AuNP administered animals, however the 10 mg kg–1 treated group did not show significant variation from the control group.
Serum lipid and antioxidant level
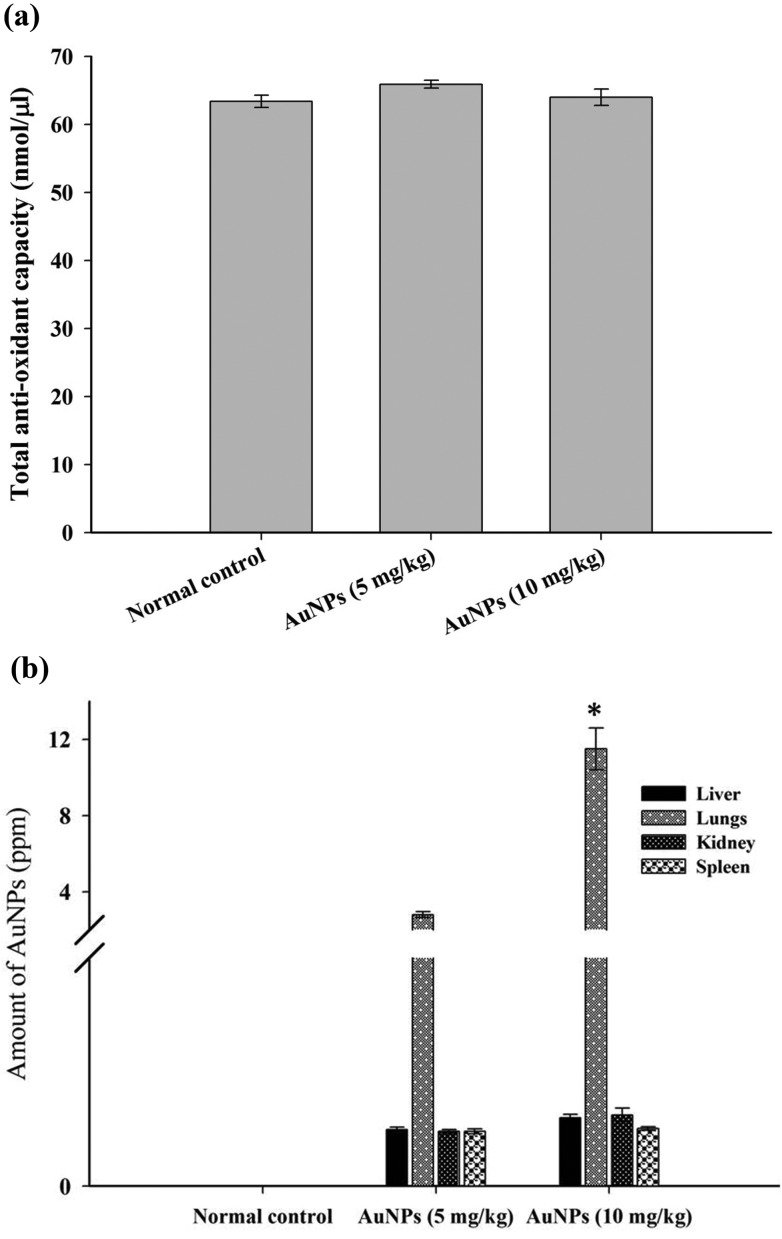
Owing to the continuous metabolic process, it is necessary for an organism to maintain a homeostatic balance of harmful oxidant production via various anti-oxidant mechanisms.26 The anti-oxidant molecules are key members in preventing disorders, therefore we estimated the total antioxidant capacity (TAC) level of serum, and the results (Fig. 4a) have revealed that the AuNP treated animals displayed a slightly higher level of TAC than the normal control; despite the higher level of TAC in both treated groups, the elevation of the total antioxidant capacity was not found to be statistically significant compared to the control group. These results suggest that AuNPs do not significantly alter the TAC level when it is administered orally in normal animals. Several research studies have shown that the ability of AuNPs either elevates or decreases the anti-oxidant level depending on the physical and chemical properties of nanoparticles.27–29 The lipid profile is also one of the critical factors to evaluate when administering with nanoparticles due to their ability to control the lipid level in diabetic rats.25,30 In the present study normal animals were orally administered with different concentrations of AuNPs, after the treatment period, and serum lipid levels such as TC and LDL and HDL cholesterol were analyzed. The results disclosed that H. tuberosus extract mediated AuNPs were found to have a mild hypolipidemic effect on normal animals, the change observed in the total cholesterol level was not significant, whereas the HDL-C level was significantly increased in the 5 mg kg–1 treated animals (Table 1).
Fig. 4. (a) Total antioxidant capacity (TAC) of AuNP administered rats: the rats were sacrificed following the administration period (21 days) and blood serum was analyzed for TAC of the 5 and 10 mg kg–1 AuNP administered rats and compared with normal control. (b) Bioaccumulation of AuNPs in various organs of rats: ICP-MS data reveals that the accumulation of orally administered AuNPs is found to have a greater rate in the lungs compared to other vital organs (liver, kidney and spleen) in a dose dependant manner. *P < 0.05 versus the AuNP (5 mg kg–1) group.
Table 1. Serum cholesterol level of AuNP administered Sprague Dawley rats for 21 days.
| Groups | Cholesterol level (mg dL–1) |
||
| Total cholesterol | HDL-C | LDL-C | |
| Normal control | 89 ± 3 | 74.3 ± 3 | 10 ± 0.56 |
| AuNPs (5 mg kg–1) | 84 ± 4 | 85.6 ± 3.2* | 9.5 ± 0.5 |
| AuNPs (10 mg kg–1) | 83 ± 6 | 72.2 ± 3.8 | 10.4 ± 1.1 |
ICP-MS revealed that the AuNP deposition is more prominent in the lungs
The AuNPs were deposited in various organs, and the distribution pattern is shown in Fig. 4(b). ICP-MS reveals the amount of gold in various organs (g–1, wet weight). The accumulated gold element concentration (ppm) in the liver, lung, kidney and spleen, was found to be 0.13, 2.8, 0.12 and 0.10 in the 5 mg kg–1 and 0.15, 11.5, 0.16 and 0.13 in the 10 mg kg–1 treated groups respectively. The amount of gold element deposition in the lungs of the 10 mg kg–1 AuNP treated group showed a significant difference (P < 0.05) compared to the 5 mg kg–1 administered group.
Lanone and Boczkowski31 have shown that AuNPs deposit with the highest rate in the lungs; our results are also in line with their findings. Furthermore, the deposition and toxicity of AuNPs in various organs depend on the size of the nanoparticles.32,33 Several research studies on in vivo analysis of nanoparticles revealed that they tend to settle and induce toxicity in various organs.34 Schleh et al.35 have studied the effect of the surface charge and size of AuNPs on their deposition in various secondary organs following the oral administration and their results suggest that 2.8 nm AuNPs with a negative charge tend to settle more in the lungs.
Phytosynthesised AuNPs induce apoptosis via the mitochondrial mediated pathway in the liver
Apoptosis is the important mechanism known for maintaining the homeostasis between cell division and cell death. The apoptosis can be induced by the presence of xenobiotics in organisms and the relationship between the xenobiotics induced apoptosis and liver injury has been elucidated.36,37 The apoptotic pathway is majorly regarded under two broad categories: (1) extrinsic and (2) intrinsic pathways. Extrinsic apoptosis is initiated by extracellular substances which bind to the death receptor (a member of the tumor necrosis factor superfamily). Binding of ligands to the death receptor protein leads to dimerization and activation of caspase-8, which in turn leads to cell death without the involvement of mitochondrial factors.
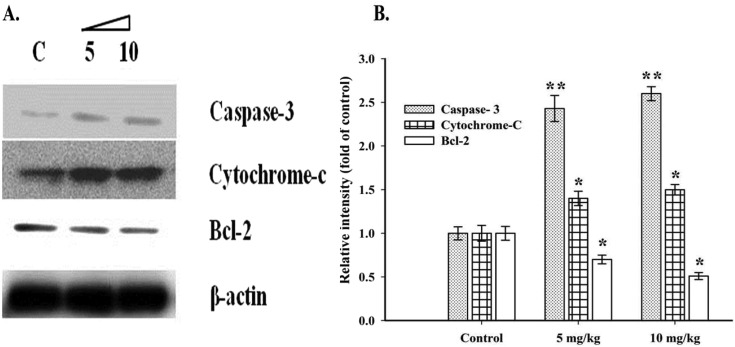
The mitochondrial (intrinsic) apoptotic pathway persuaded by the presence of various factors which can induce cellular stress, followed by the release of cytochrome C from mitochondria and ultimately lead to cell death via various executioner caspases such as caspase 3, 6 and 7.38 The western blotting results (Fig. 5) showed that there was a significant increase (P < 0.05) in the proapoptotic protein caspase-3 (2.4 and 2.6 fold) and cytochrome-c (1.4 and 1.5 fold) with a decreased expression of the anti-apoptotic protein Bcl-2 (0.7 and 0.5 fold) with respect to loading control (β-actin) in a dose dependant fashion in the liver of the 5 and the 10 mg kg–1 AuNP administered rats respectively. Liu et al.39 have also reported that AuNPs could induce apoptosis in cancerous lung cells (A549) via down regulation of Bcl-2 proteins. Meanwhile, increasing the expression of the Bax (pro-apoptotic) protein leads to mitochondria mediated cell death. The upregulation of Bax leads to the formation of a heterodimer with the Bcl-2 protein, which further cascades the permeabilization and release of proapoptotic proteins (e.g. cytochrome-c) from mitochondria that execute apoptosis. Our results suggest that AuNPs induce cell death in the rat liver via mitochondria mediated (intrinsic) apoptosis.
Fig. 5. Western blot analysis of AuNP induced apoptosis in the liver cells. A. Western blot image of pro- and anti-apoptotic proteins (cas-3, cytochrome-c and Bcl-2) with β-actin as a loading control. B. A bar diagram representing the relative intensity of cas-3, cytochrome-c and Bcl-2 with respect to the control group. Each group was tested in triplicate and the representative data are shown. *P < 0.05 and **P < 0.01 with the control group.
Histopatholgy reveals the toxicity of AuNPs in several organs
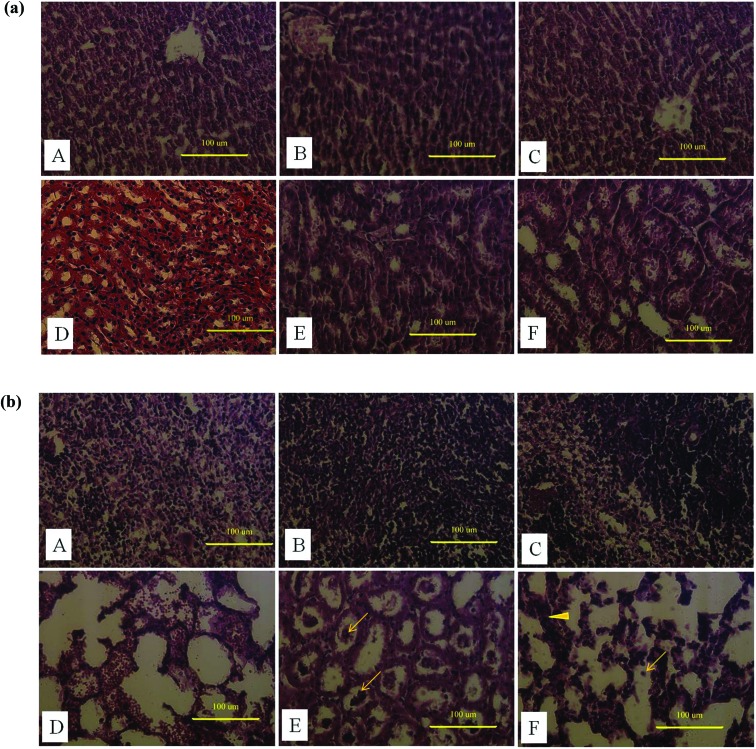
The histopathology of the liver and kidney of the AuNP treated and non-treated animals is shown in Fig. 6(a). The animals that received 5 and 10 mg kg–1 AuNPs showed a more significant change in histology than the control animals. The sinusoidal Kupffer cells in the liver became prominent, owing to AuNP exposure. The increase in the population of Kupffer cells indicates that nanoparticles activated the phagocytic activity of the cell which in turn helps in clearing nanoparticles by the intracellular breakdown of the engulfed particle with the help of lysosomes. Furthermore, the research on the size dependant toxicity of AuNPs shows histological alteration in the liver, particularly necrosis, in 10 nm sized AuNP treated rats.40
Fig. 6. (a) Administration of synthesized AuNP induced damage in Sprague Dawley rats. Histological sections of the liver (A–C) and the kidney (D–F); A & D are the histological sections of a control rat administered only with saline. B, C & E, F represent the histological alternations induced by 5 and 10 mg kg–1 AuNPs in the liver and the kidney respectively. (b) Administration of synthesized AuNP induced damage in Sprague Dawley rats. Histological sections of the spleen (A–C) and the lungs (D–F). A & D are the histological sections of a control rat administered only with saline. B, C & E, F represent the histological alternations induced by 5 and 10 mg kg–1 AuNPs in the spleen and the lungs respectively (arrow indicates the pulmonary edema and arrow head shows the thickened alveolar walls in lung tissue).
The histopathological micrograph of a kidney section clearly indicates the abnormality in the AuNP received animals compared to normal control. The enlarged capsules in renal corpuscles were distinctly evident due to the toxicity of the nanoparticles in the kidney, similar reports were also discussed by Doudi and Setorki.41 Studies on other copper nanoparticles revealed that the toxicity is related to accumulation in extracellular spaces and its subsequent solubility in intracellular spaces.42
The liver and spleen play a vital role in metabolizing any xenobiotics mixed with blood by various routes, therefore their vulnerability causes less damage to the body.43 The present study revealed that the lung is the primary organ which is severely affected by AuNPs, indicating that the nanoparticles through blood circulation have reached the lung and subsequently caused the damage. The histological evaluation of AuNP induced lung inflammation at 21 days after oral administration demonstrated severe congestion of the alveolar septae accompanying acute pulmonary edema. Furthermore, the spleen histology reveals mild hyperplasia (Fig. 6(b)), probably involved in clearing the accumulated nanoparticles.
Materials and methods
Plant material and synthesis of AuNPs
The dried tuber of H. tuberosus was purchased from a local market, Iksan, South Korea. The extraction of the plant material was carried out according to Aravinthan et al.12 The solvent free aqueous extract was used as a reducing agent. Gold(iii) chloride hydrate (HAuCl4) was purchased from Sigma-Aldrich (St Louis, MO). All other chemicals used were of analytical grade. The tuber extract (4 mL) was added to 96 mL of 1 mM HAuCl4 solution and shaken for 2 h. The production of AuNPs was visually indicated by a color change from yellow to dark red (wine color).
Characterization of AuNPs
UV-vis spectra were recorded for the preliminary assessment of AuNP formation by using a UV-1800 UV–VIS spectrophotometer (Shimadzu, Japan) in the range of 300–800 nm. The size and morphological appearance of the particles were examined using transmission electron microscopy (TEM) using an FEI Tecnai TF 20 high resolution TEM instrument operated at an accelerating voltage of 200 kV. The crystalline nature of the gold nanoparticles was determined based on X-ray powder diffraction (XRD, XPERT-Pro diffractometer using Cu Kα radiation). The scanning was performed in the region of 2θ = 30 to 80° at 0.041° min–1 with a time constant of 2 s. The presence of elemental gold was determined by using scanning electron microscopy with energy dispersive X-ray analysis (SEM–EDS; JEOL-64000, Japan). The Fourier transform infrared spectrum (FTIR) of the AuNPs was obtained on a Perkin-Elmer FTIR spectrophotometer (USA) in the diffuse reflectance mode at a resolution of 4 cm–1 in KBr pellets.10,11
Animals and treatment
The Sprague Dawley (6 weeks old) rats were purchased from Koatech, South Korea. The rats were housed on a 12 h light–dark cycle with a temperature of 23 ± 1 °C and relative humidity of 60–70%. Animals were given free access to food and water ad libitum, and acclimatized for two weeks before the commencement of experiments. All experimental procedures involving animals were approved by the Chonbuk National University Animal Ethics Committee in accordance with the detailed guidelines of the Korean Council on Animal Care. The animals were divided into 3 equal groups, each containing 6 rats. Group I (control group) receives only saline, group II receives a low dose of AuNPs (5 mg kg–1) and group III receives a mild dose of AuNPs (10 mg kg–1). The AuNPs in saline were probe-sonicated everyday before the administration to animals, to ensure homogeneous distribution. The AuNP dispersions were administered with a rigid oral gavage needle. The administration was performed once a day for 3 consecutive weeks (21 days). The rats were sacrificed the next day, following the period of administration of AuNPs and fasting overnight before euthanization. The rats were sacrificed by an intraperitonial injection of Zoletil 50 (50 mg kg–1) followed by cervical dislocation, and the organs were dissected, weighed and stored either in 10% buffered formalin or –80 °C until further analysis.
Toxicity assessment
Acute toxicity of biologically synthesized AuNPs was carried out in adult rats with four different concentrations (5, 10, 20 and 40 mg mL–1) before the commencement of the actual experiments, the animals were administered with AuNPs orally for three days and observed for any morbidity or lethality for 72 h. Furthermore, an OGTT (explained elsewhere) was performed to assess whether the sunroot (H. tuberosus) AuNPs exert an anti-hypoglycemic effect on normal healthy rats. Based on the preliminary experimental results (data not shown) optimal concentrations of 5 and 10 mg mL–1 were used in the experiments.
Histopathological examinations
Organs (lungs, spleen, kidney and liver) were carefully removed, and sliced into small fragments, stored in 10% buffered formalin until analysis. Organ fragments were embedded in paraffin, sectioned into 6 μm and stained using hemotoxylin and eosin following a standard procedure.44 Stained sections from control and the AuNP treated rats were observed under a light microscope (Olympus, BX 50) at 200× magnification.
Biochemical assay
Hypoglycemic effect of AuNPs
In order to assess the efficacy of AuNPs as a hypoglycemic agent, the overnight fasted rats were administered 2 g kg–1 glucose orally and the glycemic conditions of the treated and untreated rats were measured thereafter at intervals of 30 min until 2 h.45 The blood was obtained from the tail vein, and the glucose level was measured using a glucometer (Accu-Chek, Roche Diagnostic, Germany).
Serum lipids and antioxidant levels
Serum lipids (TC, HDL and LDL) and antioxidant levels were estimated using the HDL and LDL/VLDL quantification kit (Biovision, Milpitas, CA, USA) and the total anti-oxidant kit (Biovision, CA, USA) respectively, following the manufacture's instruction.
Accumulation of gold nanoparticles in various organs
The dissected organs (lungs, kidney, liver and spleen) from control as well as the AuNP treated groups were analyzed for the accumulation of gold element using inductively coupled plasma mass spectrometry (ICP-MS) as a proposition to assess the bio-distribution of AuNPs following the oral administration according to Barathmanikanth et al.24 Briefly, 10% (w/v) wet tissue was dissolved in 75% HNO3 and heated overnight at 50 °C with occasional venting, the digested product was diluted to 200 fold with deionized water and kept at 50 °C overnight and filtered using a 0.45 μm filter before analysis in ICP-MS (Leemans Labs, USA). The samples were analyzed in triplicate and the values represent the average ± SD of the triplicate.
Western blot analysis
Rat livers were homogenized in protein extraction solution (Pro-prep™, Intron-Biotechnology) following the manufacturer's instruction, the protein content was quantified using the Bicinchoninic acid method (BCA kit, Thermo Scientific) and the equal amount of proteins were resolved in 15% SDS gel and transferred to a PVDF membrane (GE Healthcare Life Sciences, Germany). The membrane was incubated with 5% skimmed milk in Tris-buffered saline (pH 7.4) and probed with antibodies for caspase-3 (D175), Cyt-C and Bcl-2 (50E3) from Cell Signaling, and β-actin (C4, SC-47778, Santa Cruz) was used as a loading control. Primary antibodies were incubated with their respective membranes overnight at 4 °C followed by the addition of HRP-conjugated secondary antibodies for 1 h at room temperature, and then visualized using an enhanced chemiluminescence detection unit (Fluorchem®, Alpha Innotech) and quantified using an image J software (version: 1.45).
Statistical analysis
All statistics were calculated using SigmaPlot 12.0 (SYSTAT Software, Inc., IL, USA). Data were analyzed with one-way analysis of variance (ANOVA). The values from the control group and those of the remaining groups were compared using the Bonferroni t test. For all the experiments, statistical significance was considered at *p < 0.05 and **P < 0.01.
Authors contribution
AA participated in experimental design and played a role in carrying out the experiments (animal study) in this study with particular efforts in histology, western blotting, other biochemical analysis and the preparation of the manuscript. MG participated in experimental design, synthesis, characterization of gold nanoparticles, the ICP-MS study and the preparation of the manuscript. JHK monitored and participated in the studies as well as in the preparation of the manuscript. All the authors read, contributed feedback to, and approved the final manuscript.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A1A2055893).
References
- Cardoso E., Rezin G. T., Zanoni E. T., Notoyaa F. S., Leffa D. D., Damiani A. P., Daumann F., Rodriguez J. O., Benavides R., Silva L., Andrade V. M., Paulaa M. S. Mutat. Res. 2014;766–767:25–30. doi: 10.1016/j.mrfmmm.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Chuang S. M., Lee Y. H., Liang R. Y., Roam G. D., Zeng Z. M., Tu H. F., Wang S. K., Chueh P. J. Biochim. Biophys. Acta. 2013;1830:4960–4973. doi: 10.1016/j.bbagen.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Dhawan A., Sharma V. Anal. Bioanal. Chem. 2010;398:589–605. doi: 10.1007/s00216-010-3996-x. [DOI] [PubMed] [Google Scholar]
- Faraji A. H., Wipf P. Bioorg. Med. Chem. 2009;17:2950–2962. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- Limbach L. K., Wick P., Manser P., Grass R. N., Bruinink A., Stark W. J. Environ. Sci. Technol. 2007;41:4158–4163. doi: 10.1021/es062629t. [DOI] [PubMed] [Google Scholar]
- Boisselier E., Astruc D. Chem. Soc. Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- Pissuwan D., Niidome T., Cortie M. B. J. Controlled Release. 2011;149:65–71. doi: 10.1016/j.jconrel.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Kong T., Zeng J., Wang X., Yang X., Yang J., McQuarrie S., McEwan A., Roa W., Chen J., Xing J. Z. Small. 2008;4(9):1537–1543. doi: 10.1002/smll.200700794. [DOI] [PubMed] [Google Scholar]
- Malugin A., Ghandehari H. J. Appl. Toxicol. 2010;30:212–217. doi: 10.1002/jat.1486. [DOI] [PubMed] [Google Scholar]
- Lee K. J., Park S. H., Govarthanan M., Hwang P. H., Seo Y. S., Cho M., Lee W. H., Lee J. Y., Kamala-kannan S., Oh B. T. Mater. Lett. 2013;105:128–131. [Google Scholar]
- Govarthanan M., Selvankumar T., Manoharan K., Rathika R., Shanthi K., Lee K. J., Cho M., Kamala-kannan S., Oh B. T. Int. J. Nanomed. 2014;9:1593–1599. doi: 10.2147/IJN.S58932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravinthan A., Govarthanan M., Selvam K., Praburaman L., Selvankumar T., Balamurugan R., Kamala-Kannan S., Kim J. H. Int. J. Nanomed. 2015;10:1977–1983. doi: 10.2147/IJN.S79106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praburaman L., Jang J. S., Govarthanan M., Sengottaiyan A., Manoharan K., Cho K. M., Cho M., Kamala-Kannan S., Oh B. T. Artif. Cells, Nanomed., Biotechnol. 2016;44(6):1400–1405. doi: 10.3109/21691401.2015.1029630. [DOI] [PubMed] [Google Scholar]
- Hwang S. J., Jun S. H., Park Y., Cha S. H., Yoon M., Cho S., Lee H. J., Park Y. Nanomedicine. 2015;11(7):1677–1688. doi: 10.1016/j.nano.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Suman T. Y., Radhika Rajasree S. R., Ramkumar R., Rajthilak C., Perumal P. Spectrochim. Acta, Part A. 2014;118:11–16. doi: 10.1016/j.saa.2013.08.066. [DOI] [PubMed] [Google Scholar]
- Gopinath K., Venkatesh K. S., Ilangovan R., Sankaranarayanan K., Arumugam A. Ind. Crops Prod. 2013;50:737–742. [Google Scholar]
- Prabhakar U., Maeda H., Jain R. K., Sevick-Muraca M., Zamboni W., Farokhzad O. C., Barry S. T., Gabizon A., Grodzinski P., Blakey D. C. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicki A., Witzigmann D., Balasubramanian V., Huwyler J. J. Controlled Release. 2015;200:138–157. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Talipova M. Chem. Nat. Compd. 2001;37(3):213–215. [Google Scholar]
- Pan L., Sinden M. R., Kennedy A. H., Chai H., Watson L. E., Graham T. L. Phytochem. Lett. 2009;2(1):15–18. [Google Scholar]
- Heiden T. C., Dengler E., Kao W. J., Heideman W., Peterson R. E. Toxicol. Appl. Pharmacol. 2007;225:70–79. doi: 10.1016/j.taap.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves C., Gonzalez-Romero D., Barria M. A., Olmedo I., Clos A., Sadagopa Ramanujam V. M., Urayama A., Vergara L., Kogan M. J., Soto C. Biochem. Biophys. Res. Commun. 2010;393(4):649–655. doi: 10.1016/j.bbrc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Kim Y. S., Song K. S., Ryu H. R., Sung J. H., Park J. D., Park H. M., Song N. W., Shin B. S., Marshak D., Ahn K., Lee J. E., Yu I. J. Part. Fibre Toxicol. 2013;10:36. doi: 10.1186/1743-8977-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BarathManiKanth S., Kalishwaralal K., Sriram M., Pandian S. R. K., Youn H.-S., Eom S. H., Gurunathan S. J. Nanobiotechnol. 2010;8:16. doi: 10.1186/1477-3155-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daisy P., Saipriya K. Int. J. Nanomed. 2012;7:1189–1202. doi: 10.2147/IJN.S26650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erel O. Clin. Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kowluru R. A., Kennedy A. Expert Opin. Invest. Drugs. 2001;10:1665–1676. doi: 10.1517/13543784.10.9.1665. [DOI] [PubMed] [Google Scholar]
- Gerber A., Bundschuh M., Klingelhofer D., Groneberg D. A. J. Occup. Med. Toxicol. 2013;8:32. doi: 10.1186/1745-6673-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. S., Vishakante G. D., Siddaramaiah H. Adv. Colloid Interface Sci. 2013;199–200:44–58. doi: 10.1016/j.cis.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Karthick V., Ganesh Kumar V., Stalin Dhas T., Singaravelu G., Sadiq A. M., Govindaraju K. Colloids Surf., B. 2014;122:505–511. doi: 10.1016/j.colsurfb.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Lanone S., Boczkowski J. Curr. Mol. Med. 2006;6(6):651–663. doi: 10.2174/156652406778195026. [DOI] [PubMed] [Google Scholar]
- De Jong W. H., Hagens W. I., Krystek P., Burger M. C., Sips A. J., Geertsma R. E. Biomaterials. 2008;29(12):1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Cho W. S., Cho M. J., Jeong J. Toxicol. Appl. Pharmacol. 2009;236(1):16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Baati T., Njim L., Neffati F., Kerkeni A., Bouttemi M., Gref R., Najjar M. F., Zakhama A., Couvreur P., Serre C., Horcajada P. Chem. Sci. 2013;4:1597–1607. [Google Scholar]
- Schleh C., Semmler-Behnke M., Lipka J., Wenk A., Hirn S., Schaffler M., Schmid G., Simon U., Kreyling W. G. Nanotoxicology. 2012;6(1):36–46. doi: 10.3109/17435390.2011.552811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara T., Hayashi N., Mita E., Kanto T., Tatsumi T., Sasaki Y., Kasahara A., Hori M. Hepatology. 1998;27:1643–1651. doi: 10.1002/hep.510270625. [DOI] [PubMed] [Google Scholar]
- Horn T. L., O'Brien T. D., Schook L. B., Rutherford M. S. Toxicol. Sci. 2000;54:262–273. doi: 10.1093/toxsci/54.1.262. [DOI] [PubMed] [Google Scholar]
- Mcllwain D. R., Berger T., Mak T. W. Cold Spring Harbor Perspect. Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Gu X., Zhang K., Ding Y., Wei X., Zhang X., Zhao Y. J. Nanopart. Res. 2013;15:1745. [Google Scholar]
- Abdelhalim M. A. K., Jarar B. M. J. Nanobiotechnol. 2012;10:5. [Google Scholar]
- Doudi M., Setorki M. Nanomed. J. 2014;1(4):248–257. [Google Scholar]
- Studer A. M., Limbach L. K., Van Duc L., Krumeich F., Athanassiou E. K., Gerber L. C. Toxicol. Lett. 2010;197(3):169–174. doi: 10.1016/j.toxlet.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Ajdari M., Ghahnavieh M. Z. Int. Nano Lett. 2014;4:113. [Google Scholar]
- Bancroft J. D. and Stevens A., Theory and Practice of Histological Techniques, Churchill-Livingstone, London, UK, 4th edn, 1999. [Google Scholar]
- Damge C., Maincent P., Ubrich N. J. Controlled Release. 2007;117:163–170. doi: 10.1016/j.jconrel.2006.10.023. [DOI] [PubMed] [Google Scholar]