Parkinsonism often fails to correlate with nigrostriatal pathology. By studying rare cases of parkinsonism caused by focal brain lesions, Joutsa et al. identify the claustrum as a common neuroanatomical substrate and potential therapeutic target.
Keywords: Parkinson’s disease, basal ganglia, deep brain stimulation, movement disorders, imaging
Abstract
Bradykinesia, rigidity, and tremor frequently co-occur, a clinical syndrome known as parkinsonism. Because this syndrome is commonly seen in Parkinson’s disease, symptoms are often attributed to cell loss in the substantia nigra. However, parkinsonism occurs in several other neurological disorders and often fails to correlate with nigrostriatal pathology, raising the question of which brain region(s) cause this syndrome. Here, we studied cases of new-onset parkinsonism following focal brain lesions. We identified 29 cases, only 31% of which hit the substantia nigra. Lesions were located in a variety of different cortical and subcortical locations. To determine whether these heterogeneous lesion locations were part of a common brain network, we leveraged the human brain connectome and a recently validated technique termed lesion network mapping. Lesion locations causing parkinsonism were functionally connected to a common network of regions including the midbrain, basal ganglia, cingulate cortex, and cerebellum. The most sensitive and specific connectivity was to the claustrum. This lesion connectivity pattern matched atrophy patterns seen in Parkinson’s disease, progressive supranuclear palsy, and multiple system atrophy, suggesting a shared neuroanatomical substrate for parkinsonism. Lesion connectivity also predicted medication response and matched the pattern of effective deep brain stimulation, suggesting relevance as a treatment target. Our results, based on causal brain lesions, lend insight into the localization of parkinsonism, one of the most common syndromes in neurology. Because many patients with parkinsonism fail to respond to dopaminergic medication, these results may aid the development of alternative treatments.
Introduction
The most common cause of parkinsonism is idiopathic Parkinson’s disease, characterized by loss of dopamine neurons in the substantia nigra (Dickson, 2012; Surmeier et al., 2017). However, parkinsonism can be seen in patients without a dopaminergic deficit on brain imaging (Marek et al., 2014) and in patients with brain lesions located outside the nigrostriatal tract (Alarcón et al., 2004; Handley et al., 2009). Further, parkinsonism is not specific to Parkinson’s disease but is prevalent in other neurological disorders including progressive supranuclear palsy (PSP), multiple system atrophy (MSA) and microvascular disease (Dickson, 2012), conditions that respond poorly to dopaminergic medication. These observations suggest that the clinical syndrome of parkinsonism may stem from brain regions outside the substantia nigra.
Neuroimaging studies suggest that activity in multiple different brain regions correlate with parkinsonian symptoms (Eidelberg, 2009). However, these studies are unable to determine which if any of these imaging correlates are causing clinical symptoms. Focal brain lesions allow for causal links between the lesion location and resulting symptoms (Karnath et al., 2017). However, cases of lesion-induced parkinsonism are rare and have been reported across very different brain locations, leaving localization unclear (Alarcón et al., 2004; Handley et al., 2009).
Recently, it has become possible to link lesions in different locations causing the same symptom to a common network using maps of human brain connectivity (Boes et al., 2015). This approach, termed lesion network mapping, has been used to localize hemichorea (Laganiere et al., 2016), freezing of gait (Fasano et al., 2017), and many other neuropsychiatric symptoms (Boes et al., 2015; Fischer et al., 2016; Darby et al., 2017, 2018). For example, lesions causing hemichorea occur in different brain locations, but all lesion locations are functionally connected to the posterolateral putamen, a region implicated in other causes of hemichorea (Laganiere et al., 2016). Here, we apply this approach to parkinsonism, one of the most common symptom complexes encountered in neurology. Such localization may identify treatment targets for parkinsonism refractory to dopaminergic medication.
Materials and methods
Case selection
Case reports of lesion-induced parkinsonism from PubMed using search terms ‘(parkinsonism) AND (lesion OR tumor OR tumour OR stroke OR infarct OR hemorrhage OR haemorrhage OR bleeding OR traumatic) AND (case report OR case series)’ in English in June 2017. The search identified 717 articles, and the abstract or full text was read from 215 publications that were considered possibly relevant based on the titles. The inclusion criteria were: (i) unilateral or bilateral ‘parkinsonism’ as reported by the authors; (ii) symptoms attributed by the authors to a focal brain lesion; (iii) the brain lesion was caused by a stroke, intracerebral haemorrhage, cerebral parenchymal tumour, traumatic brain injury (TBI) or hypoxia; and (iv) an image of the brain lesion was shown in the article in which lesion borders could be identified. In addition, acute neurological symptoms attributable to the lesion or temporal correlation between the brain lesion and symptoms were required to avoid incidental brain imaging findings. The exclusion criteria were: (i) age < 16 years; (ii) lesion could not be reliably localized, or showed large mass effect distorting brain structures or oedema that could be the main cause of symptoms; (iii) lesion was caused by a surgical complication; (iv) clearly abnormal brain anatomy prior to the lesion causing parkinsonism; (v) more than 1 year between the occurrence of an acute brain lesion and onset of clinical symptoms; and (vi) other movement disorders (asterixis, chorea, ballismus, dystonia, athetosis or other involuntary movements), or exacerbation of pre-existing parkinsonism. Cases in which insufficient clinical detail was presented to assess compliance with our inclusion/exclusion criteria were excluded.
Using the above criteria, we identified 28 cases of lesion-induced parkinsonism published between 1988 and 2017. We identified one additional case from a case series with sufficient detail to meet our inclusion/exclusion criteria (Case 11), resulting in 29 total cases (Table 1). Cardinal motor symptoms of parkinsonism (bradykinesia, tremor, rigidity), symptom laterality (unilateral/bilateral) and antiparkinsonian medication response were noted. Symptom onset was categorized as acute, subacute (within 1 month of the lesion, or evident immediately after other lesion-induced symptoms improved), delayed (symptoms developed over a month after the presentation), or gradual (no clear time of onset).
Table 1.
Cases secondary parkinsonism caused by brain lesions
| Publication | Patient | Lesion | Parkinsonism symptoms | Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case number | Publication | Age, sexa | Type | Locationb | Side | Brady | Rigid | Tremor | Side | Onset | Medic. resp.c | Clinical outcome (follow-up)d | |
| 1 | Straube and Sigel, 1988 | 53 F | Neoplasia | SMA | L | Yes | Yes | Yes | Bil | Gradual | Yes | NA | |
| 2 | Abe and Yanagihara, 1996 | 53 F | Haemorrhage | SNr | L | Yes | Yes | Yes | R | Subacute | Yes | Stable (1y 10 mo) | |
| 3 | Doder et al., 1999 | 24 M | TBI | Globus pallidus, striatum | L | Yes | - | Yes | Bil | Delayed (6 w) | No | Progressive (>13 y) | |
| 4 | Yoshii et al., 1998 | 50 M | Hypoxiae | Globus pallidus | Bil | Yes | Yes | - | Bil | Subacute | Yes | Resolved (4 mo) | |
| 5 | Cicarelli et al., 1999 | 39 F | Neoplasia | Pontomesencephalic | Bil | Yes | Yes | Yes | Bil | Gradual | No | Improved (>2 mo)f | |
| 6 | Moro and Albanese, 1999, Case 1 | 26 F | Neoplasia | Midbrain tegmentum | Bil | Yes | Yes | Yes | Bil | Gradual | Yes | Resolved (>1 y) | |
| 7 | Bhatt et al., 2000, Case 2 | 39 M | TBI | SNr | L | Yes | Yes | Yes | Bil | Subacute | Yes | Resolved (1 y) | |
| 8 | Bhatt et al., 2000, Case 3 | 35 M | TBI | SNr, sublentiform nucleus | Bil | Yes | - | - | R | Delayed (3 mo) | - | Progressive (6 mo) | |
| 9 | Li et al., 2000 | 72 M | Hypoxia | Basal ganglia | Bil | Yes | Yes | Yes | Bil | Subacute | No | Died (3 mo) | |
| 10 | Haussermann et al., 2001 | 56 M | Neoplasia | SMA, precentral gyrus | Bil | Yes | Yes | Yes | Bil | Gradual | No | NA | |
| 11 | Kim, 2001, Case 1 | 79 F | Stroke | Medial frontal lobe | R | Yes | Yes | Yes | L | Subacute | No | Improved (3 y) | |
| 12 | Peters et al., 2001 | 56 M | Stroke | Cerebral peduncle | L | Yes | Yes | - | R | Acute | No | Stable (3 y) | |
| 13 | Ling et al., 2002 | 46 M | Haemorrhage | Temporal lobe | R | Yes | Yes | - | Bil | Subacute | Yes | Stable (2 y) | |
| 14 | Yoshimura et al., 2002 | 63 F | Neoplasia | Mesencephalic | L | Yes | Yes | Yes | R | Gradual | Yes | Stable (8 y) | |
| 15 | Morgan and Sethi, 2003 | 65 F | Stroke | Midbrain (SNr, red nucleus) | L | Yes | - | - | R | Acute | Yes | Improved (2 mo) | |
| 16 | Orimo et al., 2004 | 71 F | Stroke | SNr | R | - | Yes | Yes | L | Delayed (2 mo) | Yes | Stable (>7 y) | |
| 17 | Peralta et al., 2004 | 78 M | Stroke | Striatum | L | Yes | Yes | Yes | Bil | Subacute | No | Stable (11 mo) | |
| 18 | Schramm et al., 2004 | 41 M | Hypoxia | Striatum | Bil | Yes | Yes | - | L | Subacute | No | Stable (1 y) | |
| 19 | Akyol et al., 2006 | 80 M | Stroke | SNr | R | Yes | Yes | Yes | Bil | Acute | Yes | Improved (1 mo) | |
| 20 | Ho et al., 2008 | 60 M | Neoplasia | Basal ganglia, medial temporal lobe, insula | L | Yes | Yes | Yes | R | Gradual | No | Died (1 y) | |
| 21 | Frosini et al., 2009 | 65 M | Neoplasia | Thalamus | Bil | Yes | Yes | Yes | Bil | Gradual | No | Progressive (>1 y) | |
| 22 | Ghaemi et al., 2009 | 16 M | Haemorrhage | Pontomesencephalic | L | Yes | Yes | - | R | Subacute | - | Improved (>6 mo)f | |
| 23 | Miao et al., 2009 | 21 F | Hypoxia | Basal ganglia | Bil | Yes | Yes | Yes | Bil | Subacute | No | Resolved (1 y) | |
| 24 | Amin et al., 2011 | 28 F | Stroke | Basal ganglia | Bil | Yes | Yes | - | Bil | Acute | - | NA | |
| 25 | Kobayashi et al., 2011 | 83 F | Stroke | Medial frontal lobe | Bil | Yes | Yes | Yes | Bil | Acute | Yes | NA | |
| 26 | Wächter et al., 2011 | 70 M | Neoplasia | Thalamus | Bil | Yes | Yes | Yes | Bil | Gradual | Yes | NA | |
| 27 | Chen et al., 2012 | 57 F | Hypoxia, haemorrhagee | SNr, basal ganglia | Bil | Yes | Yes | - | Bil | Subacute | Yes | Improved (7 mo) | |
| 28 | Robles, 2016 | 61 M | Stroke | SNr | R | Yes | Yes | Yes | L | Acute | - | Stable (5 y) | |
| 29 | Bejr-Kasem Marco et al., 2017 | 68 F | Stroke | Brainstem | Bil | Yes | Yes | Yes | Bil | Acute | Yes | NA | |
aEstimated age when the lesion occurred or when first symptoms of parkinsonism emerged in cases with gradual onset.
bMain lesion location(s) as shown in the original publication.
cMedication response: yes = more than minimal or mild clinical benefit with any antiparkinsonian dopaminergic medication according to the authors, no = no response or minimal to mild benefit.
dOutcome: clinical stability of the symptoms at the end of follow-up, as described by the authors. The follow-up time in parentheses is calculated from the time of symptom onset to the end of the follow-up.
eCarbon monoxide poisoning.
fClinical outcome after surgery.
Bil = bilateral; F = female; L = left; M = male; mo = months; NA = not available; R = right; SMA = supplementary motor area; SNr = substantia nigra; TBI = traumatic brain injury; y = years.
To ensure that results were dependent on our choice of inclusion/exclusion, we created two subgroups using more stringent criteria. Subgroup 1 included only patients with all three cardinal parkinsonism symptoms (bradykinesia, rigidity and tremor) (n = 19, 66% of the cases). Subgroup 2 included only patients with acute or subacute parkinsonism caused by a focal ischaemic stroke or haemorrhage (n = 12, 41% of the cases).
Lesions
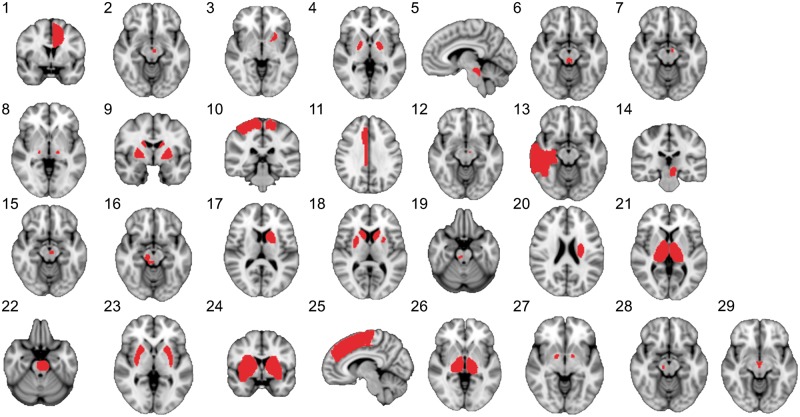
Lesion locations as displayed in the original publications were traced onto a common brain atlas as described previously (Boes et al., 2015; Fischer et al., 2016; Laganiere et al., 2016; Darby et al., 2017; Fasano et al., 2017). Briefly, lesions were drawn on the MNI152 T1 template with 2 mm isotropic voxels using FSL (Jenkinson et al., 2012) to create binary lesion masks (value 1 for voxels included to the lesions and value 0 for other voxels). It should be noted that using this approach, it was not possible to capture the entire 3D lesion volume but only representative 2D slices. This is an important limitation (see ‘Discussion’ section), but prior work suggests that 2D slices can serve as a reasonable approximation of 3D lesions for lesion network mapping (Boes et al., 2015; Darby et al., 2017). The spatial correlation coefficient between lesion networks derived from 3D versus 2D representations of the same lesion were 0.96 for small lesions and 0.89–0.91 for larger lesions (Boes et al., 2015; Darby et al., 2017). In case of multiple lesions, all lesions were combined to a single mask without trying to interpret which one(s) would be the symptomatic lesion(s). A representative slice of each lesion is illustrated in Fig. 1 and lesion mask sizes are presented in Supplementary Table 1.
Figure 1.
Lesions causing parkinsonism. The case numbers correspond to Table 1.
To evaluate whether the lesions involved the substantia nigra, lesion locations were overlaid with a 3D mask of the substantia nigra in atlas space (Ewert et al., 2018). Overlap of one or more voxels was considered indicative of nigral involvement. Nigral involvement in lesions causing parkinsonism was compared to a database of lesions causing other non-specific symptoms (n = 135) (Corbetta et al., 2015) and lesions causing other movement disorders (n = 73). Other lesion-induced movement disorders included asterixis (n = 30), hemichorea-hemiballismus (n = 29) and freezing of gait (n = 14) as detailed in prior publications by our group (Boes et al., 2015; Laganiere et al., 2016; Fasano et al., 2017). Group differences were tested using Fisher’s exact test.
Lesion network mapping
The network of brain regions functionally connected to each lesion location was identified as described previously (Boes et al., 2015; Fischer et al., 2016; Laganiere et al., 2016; Darby et al., 2017; Fasano et al., 2017). A publicly available database of resting state functional connectivity MRI (rs-fcMRI) data from 1000 neurologically healthy volunteers (Yeo et al., 2011; Holmes et al., 2015) was used to determine connectivity between each lesion location and all other brain voxels (referred to as lesion networks). The processed data and code for lesion connectivity analysis are freely available through LEAD-DBS software [www.lead-dbs.org; (Horn and Kühn, 2015)] and described in our previous publications (Darby et al., 2018; Horn et al., 2017). Lesion masks are available upon request. Voxel-based functional connectivity maps across the whole brain were created for each lesion by running the lesion as a seed region by averaging the time course of all voxels within the binary lesion mask in rs-fcMRI. Seed-based rs-fcMRI analysis provides connectivity strength estimates from the lesion to each voxel in the brain, creating a whole brain connectivity map. Lesion networks were thresholded (t = ± 7, P < 10−11) and overlaid to identify brain regions functionally connected to all or most lesion locations. The binary maps were created separately for negative and positive maps to differentiate between correlated and anti-correlated networks, as these networks are also likely to have biologically different functions. Lesion location was included in lesion network maps as part of the positively correlated network. Next, the binary maps were overlaid and the number of cases overlapping networks in each voxel was calculated. The final images were then thresholded to >90% of the cases (≥27/29, ≥18/19 or ≥11/12 for the whole sample, Subgroups 1 and 2, respectively).
The specificity of lesion networks of parkinsonism lesions was compared to the database of lesions causing other non-specific symptoms (n = 135) and lesions causing other movement disorders (n = 73). To ensure that results were not dependent on the statistical approach, two different approaches were used to compare connectivity profiles between groups: voxel-wise t-test, and Liebermeister test for binarized lesion connectivity maps. This approach ensures that our results are not driven, for example, by high connectivity values in the lesion locations. First, the strength of the connectivity in each voxel was compared between lesions causing parkinsonism and other lesions using two sample t-test with Statistical Parametric Mapping software (SPM12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) (Friston et al., 2004). Family-wise error (FWE)-corrected P-values below 0.05 at voxel-level were considered significant. Second, the lesion connectivity maps were compared using a Liebermeister test using MRIcron voxel-based lesion-symptom mapping (VLSM) (Rorden et al., 2007) after binarizing the maps using |t| ≥ 7 (P < 10−11) as threshold corresponding to the lesion network mapping overlap threshold. The correlated (t ≥ 7, P < 10−11) and anticorrelated (t ≤ − 7, P < 10−11) networks were analysed separately. The analyses were conducted across the whole brain ignoring voxels affected in less than 10% of all cases. False discovery rate (FDR) corrected P-values below 0.05 were considered significant. For both t-tests and Liebermeister tests, correction for multiple comparisons was conducted across a search volume covering the entire brain and, critically, only voxels that were both significantly different between the groups and located within ≥90% (≥27/29 cases) lesion network mapping overlap of the whole sample were considered specific for parkinsonism.
Relevance of the lesion network mapping for neurodegenerative parkinsonism
To test whether parkinsonism caused by focal brain lesions shares neuroanatomy with neurodegenerative parkinsonism, we computed functional connectivity between each lesion location causing parkinsonism and maps of brain atrophy from neurodegenerative forms of parkinsonism [Parkinson’s disease, PSP, and multiple system atrophy, parkinsonism variant (MSA-P)]. For Parkinson’s disease, we used a publicly available voxel-wise map of brain atrophy (Zeighami et al., 2015). For PSP and MSA-P, such maps were not available so we used atrophy coordinates from previously published voxel-based morphometry (VBM) studies (Supplementary material). Functional connectivity values between each lesion location and each atrophy map (Pearson’s correlation coefficients) were converted to a normal distribution using Fisher’s r to z transform. Specificity was assessed in two ways. First, we tested whether lesions causing parkinsonism were more connected to atrophy maps associated with parkinsonism than lesions causing other symptoms. Second, we tested whether lesions causing parkinsonism were more connected to atrophy maps associated with parkinsonism (Parkinson’s disease, PSP and MSA-P) than atrophy maps not associated with parkinsonism (mild cognitive impairment, Supplementary material). All group comparisons were investigated using two-sample t-tests.
Relevance of the lesion network mapping for treatment response
First, we tested whether connectivity with the lesion location was different between patients whose parkinsonism responded to dopaminergic medication compared to those who did not (Table 1 and Supplementary Fig. 1). Specifically, we computed connectivity between each lesion location and an a priori region of interest in the putamen, the site of action for dopaminergic medications (Dauer and Przedborski, 2003). The group differences were investigated using two-sample t-test. To assess specificity, we repeated this analysis on a voxel-wise basis using two-sample t-test.
Second, we tested whether connectivity with our lesion locations might relate to deep brain stimulation (DBS) response. No patients with lesion-induced parkinsonism underwent DBS. We therefore used a recently published cohort of 95 patients with idiopathic Parkinson’s disease who underwent subthalamic nucleus (STN) DBS (Horn et al., 2017). These patients were used to identify network connections predictive of DBS response (Horn et al., 2017). The connectivity of the lesions with the treatment response map was analysed similarly as with the atrophy patterns described in the previous section.
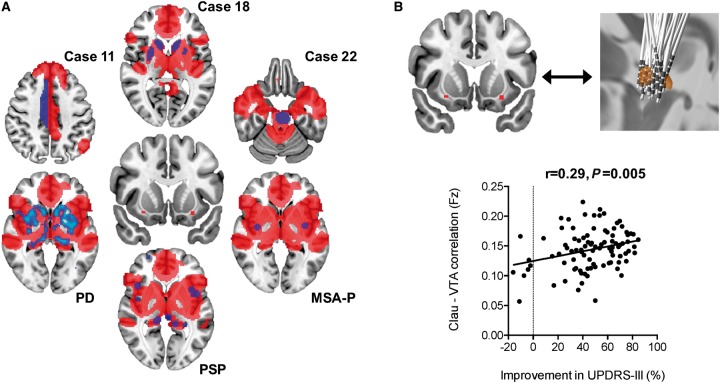
Finally, to investigate whether the lesion network hotspot in the claustrum has potential as a novel treatment target for parkinsonism, the connectivity between the significant cluster in the claustrum and a set of DBS electrodes implanted in two centres (Horn et al., 2017) was calculated based on the same normative functional connectome (Yeo et al., 2011) used in the main analysis. For details about the patients sample treated with DBS, see Horn et al. (2017). Briefly, it consisted of 95 patients (30 female, mean age = 60.2 ± 8.0 years; six akinetic-rigid, 19 mixed and 88 tremor-dominant cases), 51 of whom were operated on at Charité – University Medicine Berlin and 44 at University Hospital Würzburg. Motor improvement scores were as expected for standard DBS Parkinson cohorts (47.2 ± 22.4%) and are again reported in detail elsewhere (Horn et al., 2017). DBS electrodes were localized using Lead-DBS software version 2.1.5 (www.lead-dbs.org) (Horn and Kühn, 2015), including correction for brain shift, and normalized into standard stereotactic (MNI) space using Advanced Normalization Tools (http://stnava.github.io/ANTs/) (Avants et al., 2008). Based on localization and clinical stimulation parameters, a volume of tissue activated (VTA) was calculated using a finite element approach and E-field thresholding as described in Horn et al. (2017). The two VTAs for each hemisphere served as seeds to estimate functional connectivity to the claustrum region defined in the lesion network analysis. Connectivity strength between each pair of VTA and the claustrum region was then correlated with empirical motor improvement [measured by per cent improvement on the Unified Parkinson’s Disease Rating Scale (UPDRS-III)] in the DBS sample. The correlation between the connectivity and clinical improvement was analysed using Pearson's correlation coefficient, both with and without correction for dataset. The study was approved by the internal review board of Charité–Universitätsmedizin or Würzburg University Hospital, and carried out in accordance with the Declaration of Helsinki.
Results
Lesions and clinical symptoms
Twenty-nine lesions causing parkinsonism were identified and occurred in a variety of different brain locations (Fig. 1): 14 (48%) involved the midbrain, 12 (41%) involved the basal ganglia and six (21%) involved the cerebral cortex. Five cortical lesions (17%) did not involve any part of the midbrain or basal ganglia and thus fell outside the nigrostriatal tract (Fig. 1 and Table 1: Cases 1, 10, 11, 13 and 25). Nine (31%) of the lesions directly involved the substantia nigra, which was more than among lesions causing other movement disorders (5/73, P = 0.003) or non-specific symptoms (15/135, P = 0.02). Fifteen (52%) of the patients had unilateral lesions and 11 (38%) had unilateral parkinsonism (Table 1). Twenty-five (86%) received antiparkinsonian medication, of whom 14 (56%) responded (Table 1).
Lesion network mapping
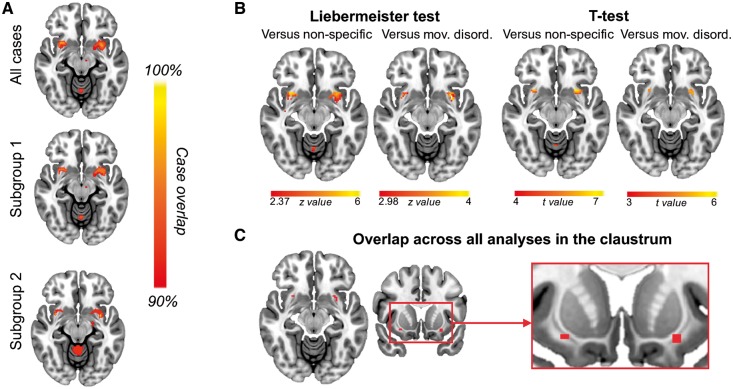
Although lesion locations were heterogeneous, they were functionally connected to a common set of brain regions. Over 90% of lesion locations were connected to the midbrain, basal ganglia, anterior cingulate cortex and cerebellum (Supplementary Fig. 2). Results were similar when limited to cases with all three cardinal motor symptoms (Subgroup 1, n = 19) or cases with acute/subacute parkinsonism from focal stroke (Subgroup 2, n = 12) (Supplementary Fig. 2). When compared to lesions causing non-specific symptoms or lesions causing other movement disorders, the claustrum was the only region that was specific to lesions causing parkinsonism (Fig. 2). Adding lesion mask size as a covariate did not change the significance of these results. Connectivity to the claustrum was also highly sensitive, with connectivity to 28 of the 29 lesion locations causing parkinsonism. The only exception involved an infiltrative lymphoma (Case 10). Lesion network mapping also identified parietal regions that were anticorrelated to lesion locations causing parkinsonism (Supplementary Fig. 3A). However, unlike positive connectivity to the claustrum, results depended somewhat on the statistical test employed (Supplementary Fig. 3B).
Figure 2.
Parkinsonism lesion network overlap and specificity. (A) Lesion network overlap of all cases of parkinsonism, cases causing all three cardinal symptoms of parkinsonism (Subgroup 1) and cases of focal stroke or haemorrhage causing acute/subacute parkinsonism (Subgroup 2). (B) Specificity when compared to lesions causing non-specific symptoms or other movement disorders using two different statistical approaches. (C) The only brain region that was sensitive and specific for parkinsonism lesions across all analyses was the claustrum. Section at z = − 11.5 mm is shown in all axial panels.
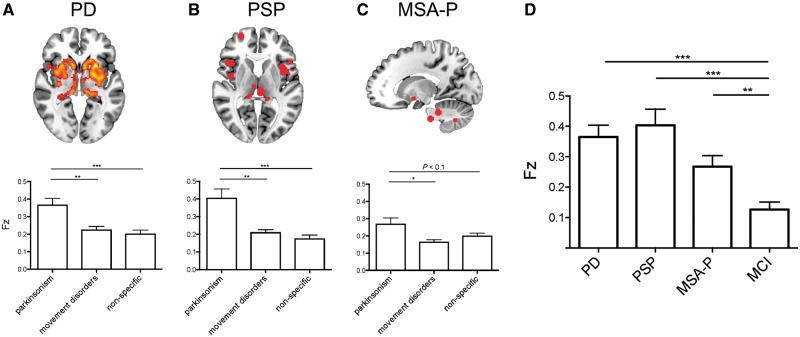
Relevance for neurodegenerative parkinsonism
Lesion locations causing parkinsonism were functionally connected to atrophy maps from patients with idiopathic Parkinson’s disease [Fz mean (95% confidence interval, CI) 0.37 (0.29–0.45), t(28) = 9.44, P < 0.001], PSP [0.40 (0.29–0.51), t(28) = 7.53, P < 0.001], and MSA-P [0.27 (0.19–0.34), t(28) = 7.29, P < 0.001]. This connectivity was specific to lesion locations causing parkinsonism compared to lesion locations causing other movement disorders or lesion locations causing non-specific symptoms (Fig. 3A–C). This connectivity was also specific to atrophy patterns of parkinsonian syndromes (Parkinson’s disease, PSP, and MSA-P) compared to the atrophy pattern of mild cognitive impairment (Fig. 3D).
Figure 3.
Relevance of lesion connectivity to neurodegenerative parkinsonism syndromes. Lesion locations causing parkinsonism are connected to areas of brain atrophy in patients with idiopathic Parkinson's disease (A), PSP (B), and MSA-P (C). (D) Lesion locations causing parkinsonism are more connected to areas of atrophy in patients with parkinsonism syndromes than patients with mild cognitive impairment (MCI). Fz = Fisher’s z. *P < 0.05, **P < 0.01, ***P < 0.001.
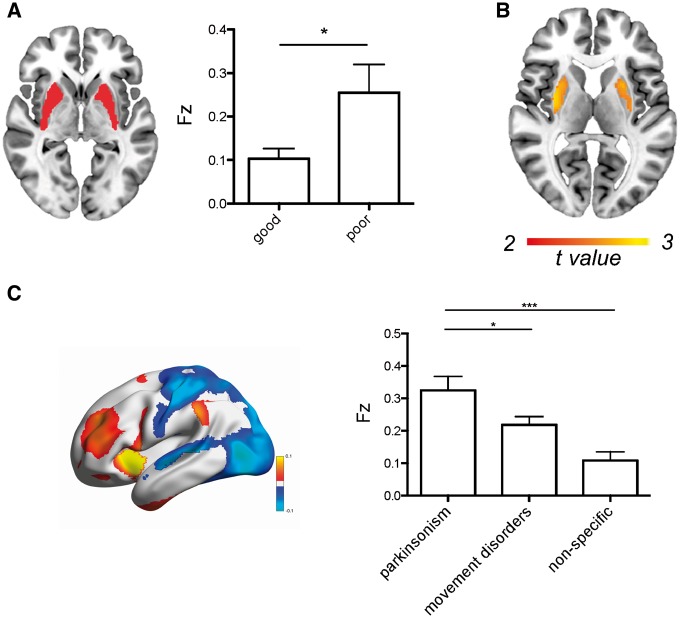
Relevance for treatment
Lesion locations causing a DOPA-responsive parkinsonism (n = 14) showed a significant difference in connectivity to the putamen compared to lesion locations causing a non-DOPA-responsive parkinsonism (n = 11) (Fig. 4A). This result was specific to the putamen in a whole brain voxel-wise analysis (Fig. 4B). The results remained significant when excluding nigral lesions, leaving eight DOPA-responsive cases versus 10 non-DOPA-responsive cases [t(16) = 2.15, P = 0.05]. Second, lesion locations causing parkinsonism were significantly connected to the same brain regions that DBS sites that relieve parkinsonism symptoms are connected to [Fz mean (95% CI) 0.32 (0.24–0.41), t(28) = 7.53, P < 0.001]. This connectivity was specific to lesions causing parkinsonism compared to lesions causing other movement disorders or non-specific symptoms (Fig. 4C).
Figure 4.
Relevance of the lesion connectivity to treatment efficacy. Connectivity between lesion locations causing parkinsonism and the putamen is associated with medication response (A), an effect that is specific to the putamen (uncorrected P < 0.01) (B). (C) Lesion locations causing parkinsonism are connected to the same brain regions as DBS electrodes that relieve parkinsonism. Fz = Fisher’s z. *P < 0.05, ***P < 0.001.
Claustrum connectivity
Because the claustrum was the single most sensitive and specific brain region for lesion-induced parkinsonism, we computed the connectivity between our claustrum region and all other brain regions. By definition, this network encompassed all lesion locations causing parkinsonism (except for Case 10). It also aligned well with parkinsonism atrophy patterns (Fig. 5A). Finally, we examined if connectivity between the claustrum region and STN electrode location was associated with clinical response in patients with Parkinson’s disease who had undergone DBS surgery. We found that connectivity between DBS locations and the claustrum was associated with improvement in UPDRS motor scores (r = 0.29, p = 0.005, Fig. 5B), which remained unchanged when correcting for dataset (r = 0.28, p = 0.007).
Figure 5.
The claustrum network links diverse causes and treatments of parkinsonism. (A) Connectivity with the claustrum (middle) defines a brain network (red) that encompasses lesion locations casing parkinsonism (purple) and areas of atrophy in parkinsonism syndromes (blue–purple). (B) Connectivity between the claustrum and STN DBS electrode location correlates with clinical improvement in patients with Parkinson’s disease. Clau = claustrum; Fz = Fisher’s z; PD = Parkinson’s disease; UPDRS-III = Unified Parkinson’s Disease Rating Scale, motor score; VTA = volume tissue activation.
Discussion
There are four important findings in the present study, each of which will be discussed in turn. First, lesions in very different brain regions and outside the nigrostriatal tract can cause clinical parkinsonism. Second, these lesion locations are part of a common intrinsically connected brain network, with connectivity to the claustrum being the most sensitive and specific marker of lesion-induced parkinsonism. Third, the current network identified based on focal brain lesions is convergent with atrophy patterns seen in neurodegenerative parkinsonism, suggesting a common neuroanatomical substrate. Finally, lesion connectivity was associated with treatment outcomes, suggesting that these findings could be relevant for development of novel therapeutic targets.
The substantia nigra has an established role in the neuropathophysiology of Parkinson’s disease and lesions to this location are known to result in clinical parkinsonism (Alarcón et al., 2004; Handley et al., 2009; Dickson, 2012). Not surprisingly, the substantia nigra was a common lesion location in our cases and significantly more common compared to lesions causing other movement disorders. However, in a majority of cases the lesion was not in the substantia nigra and some of the lesions were definitely outside the entire nigrostriatal tract, indicating that not all parkinsonism can be attributed to nigrostriatal damage. Thus, although direct damage to nigrostriatal neurons can cause motor parkinsonism, it is not required.
Despite heterogeneity in lesion location, lesions causing parkinsonism were functionally connected to a common network of brain regions. This is consistent with recent lesion network mapping studies of other lesion-induced symptoms (Boes et al., 2015; Fischer et al., 2016; Laganiere et al., 2016; Darby et al., 2017; Fasano et al., 2017). In all cases, lesion locations causing similar symptoms were connected to a common brain region. For example, lesions causing visual hallucinations were connected to extrastriate visual cortex, lesions causing central post-stroke pain were connected to the posterior insula, and lesions causing hemichorea were connected to the posterior putamen (Boes et al., 2015; Laganiere et al., 2016). In the current study, lesions causing parkinsonism were connected to several regions, but the most sensitive and specific connectivity was to the claustrum.
The claustrum, which some have considered to be a part of the basal ganglia, is located bilaterally between the putamen and insular cortex, separated from these structures by the white matter bundles of external and extreme capsules, respectively. It has widespread projections, including input directly from the substantia nigra and projections to/from the striatum and cortical motor regions (Crick and Koch, 2005; Goll et al., 2015). The role of the claustrum in the human brain is still largely unclear but is thought to include motor, perceptual and cognitive functions (Crick and Koch, 2005; Goll et al., 2015), all of which can be impaired in Parkinson’s disease (Kalia and Lang, 2015). Primate studies suggest that different parts of the claustrum have different connectivity patterns, but segmentation of the human claustrum remains uncertain.
So far, the role of the claustrum has received very little attention in Parkinson’s disease and related disorders. One of the reasons for the oversight may be that the claustrum is a relatively small structure and located very close to the striatum, making it difficult to investigate with brain imaging. In this study, we employed a very large dataset (n = 1000), which greatly improves the power to detect weak signals. Neuropathological studies have demonstrated that the claustrum is affected early in Parkinson’s disease (Surmeier et al., 2017), including substantial loss of dopamine and noradrenaline (Sitte et al., 2017). The claustrum is also atrophied in other neurodegenerative syndromes with parkinsonism, such as MSA-P and PSP (Yu et al., 2015; Mueller et al., 2017). Interestingly, ‘bright claustrum’ is a classic neuroimaging sign in Wilson’s disease, a disorder of copper metabolism, which can cause parkinsonism (Sener, 1993).
Although the findings in the claustrum were robust and specific across all analyses, one lesion location was not connected with the claustrum (Case 10). This patient had a rapidly progressive infiltrative lymphoma in the supplementary motor area. Thus, it is possible that the key regions relevant for development of parkinsonism in this patient were not evident from the published image. It is also possible that this lesion caused white matter damage disconnecting pre-supplementary motor area from the claustrum (Tanné-Gariépy et al., 2002). Interestingly, this is the second time a case of CNS lymphoma was the only lesion that failed to show similar connectivity to other lesions causing the same motor symptoms (Fasano et al., 2017), suggesting that the location of this particular lesion type on neuroimaging may be unreliable.
Beyond the claustrum, lesion locations causing parkinsonism shared connectivity to a variety of other brain regions. This is not surprising, given that parkinsonism is actually defined as a combination of individual symptoms. It is also not surprising that some of this connectivity was not specific to Parkinson’s disease. For example, the basal ganglia were connected to lesion locations causing parkinsonism but also to lesion locations causing other movement disorders, consistent with involvement across many different movement disorders (Obeso et al., 2014).
An important question is whether brain lesions that cause parkinsonism have any relevance to more common neurodegenerative forms of parkinsonism. The current results suggest that they do. Lesions causing parkinsonism were specifically connected to the brain atrophy patterns found in Parkinson’s disease, PSP, and MSA-P. This convergence across heterogeneous lesion locations and different forms of neurodegenerative parkinsonism suggests that parkinsonism, as a syndrome, shares a common neuroanatomical substrate that is independent of aetiology. This result is consistent with lesion network mapping of other symptoms in which the identified substrate aligns well with alternative causes of the same symptom (Boes et al., 2015; Fischer et al., 2016; Laganiere et al., 2016; Darby et al., 2017; Fasano et al., 2017). For example, lesion network mapping of hemichorea identified specific and sensitive connectivity to the posterolateral putamen, the exact area that becomes hyperintense on MRI when hemichorea is caused by hyperglycaemia (Laganiere et al., 2016).
Medication response in the cases with lesion-induced parkinsonism is not obvious based on the lesion location alone (Supplementary Fig. 1). However, connectivity between each lesion location and the putamen was associated with medication response. Because the putamen is where dopamine exerts its action (Dauer and Przedborski, 2003), this finding is consistent with the interpretation that lesions connected to the putamen alter its function in a way that interfere with the symptom relieving benefit of dopaminergic medication.
Brain intrinsic connectivity is also thought to underlie the efficacy of invasive and non-invasive neuromodulation treatments, including in Parkinson’s disease (Ressler and Mayberg, 2007; Fox et al., 2012, 2014; Horn et al., 2017). Recently, the connectivity pattern of effective STN DBS has been described (Horn et al., 2017). Here, we show that lesion locations causing parkinsonism were connected to the same set of regions as STN DBS sites that relieve parkinsonism (Horn et al., 2017), suggesting that lesion network localization might be useful in identifying neuroanatomical targets for neuromodulation treatments. Supporting this hypothesis, the connectivity between each patient’s DBS electrode location and our lesion-derived region of interest in the claustrum was correlated with clinical DBS response. These findings indicate that lesion network mapping of secondary parkinsonism cases may provide useful information for development of novel neuroanatomical treatment targets.
It should be noted that many forms of parkinsonism do not respond to DBS of established neuroanatomical targets such as the STN (Shih and Tarsy, 2007). These DBS targets, similar to l-DOPA, were identified by studying idiopathic Parkinson’s disease. Other parkinsonian disorders may be caused by dysfunction in different brain regions, requiring different DBS targets. Based on the results of this study, we speculate that the claustrum may represent a common node where different parkinsonian syndromes overlap. STN DBS electrodes providing the greatest clinical benefit in idiopathic Parkinson’s disease are connected to the claustrum, but this may not be the best way to access the claustrum for other causes of parkinsonism. Whether the claustrum will prove a valuable therapeutic target across parkinsonian syndromes remains unknown but a testable hypothesis.
The lesion network mapping method used in this study and most others is based on functional connectivity and resting state functional MRI (Boes et al., 2015; Fischer et al., 2016; Laganiere et al., 2016; Sutterer et al., 2016; Darby et al., 2017, 2018; Fasano et al., 2017). Although white matter contributes to the functional MRI signal and connectivity estimates (Fox et al., 2014; Wu et al., 2017; Ding et al., 2018), lesion network mapping is likely dominated by grey matter. A complimentary approach for investigating lesion networks more focused on white matter disconnection is based on magnetic resonance diffusion imaging (Thiebaut de Schotten et al., 2015; Foulon et al., 2018). How these measures relate to one another and how to best combine them to map lesion networks is an important topic for future work (Thiebaut de Schotten and Foulon, 2018).
Limitations
Several potential limitations of lesion network mapping such as using a 2D tracing to represent a 3D lesion and using a connectome that is not age-matched to the lesion patient have been addressed previously and found to have minimal impact on results (Boes et al., 2015; Darby et al., 2017). Although we excluded cases in which the anatomical location or borders of the lesion could not be reliably identified, there is some inherent error in tracing a lesion image from a published case report onto an anatomical template. That said, this error should bias us against the present findings, namely a common network for lesion locations causing parkinsonism. In addition, although a systematic search for published case reports was conducted, our sample may not be representative of all lesions causing parkinsonism. The published cases are heterogeneous making the definition of comprehensive inclusion/exclusion criteria challenging. Thus, it is likely that there are additional cases that weren’t captured by our search. We included only case reports with clearly localizable lesions but there is still uncertainty in localization of the brain lesions and whether the image shown in the case report is causing the clinical symptom. We also recognize the possibility of a publication bias, as cases where lesions do not hit the nigrostriatal tract may not be recognized to be causal to parkinsonism. The current study included heterogeneous lesion aetiologies, including TBI and tumour that may cause damage outside our lesion masks (e.g. Case 10). Although we are reassured that results are similar using a subset of lesions due to focal stroke, heterogeneity in lesion aetiology may decrease our power to detect important associations. Finally, because of the rarity of lesion-induced parkinsonism and our stringent inclusion criteria, analyses were limited to 29 cases (only 12 cases of acute stroke). Although these numbers are similar to prior lesion network mapping studies (Boes et al., 2015; Fischer et al., 2016; Laganiere et al., 2016; Darby et al., 2017, 2018; Fasano et al., 2017) replication in an independent lesion dataset would be valuable.
Conclusions
The results of the present study suggest that parkinsonism is a brain network disorder that can be caused by lesions in multiple different brain locations. Our findings highlight the claustrum as a potential key region in parkinsonism, providing a new and testable therapeutic target.
Supplementary Material
Acknowledgements
The authors wish to thank Prof. Andea Kühn, Prof. Jens Volkmann, and Prof. Maurizio Corbetta for contributing data and valuable feedback during the study.
Glossary
Abbreviations
- DBS
deep brain stimulation
- MSA-P
multiple system atrophy, parkinsonism variant
- PSP
progressive supranuclear palsy
- STN
subthalamic nucleus
Funding
J.J. was supported by the Academy of Finland (grant # 295580), the Finnish Medical Foundation, the Orion Research Foundation and the Finnish Brain Foundation. A.H. was supported by DFG KFO247, Thiemann Foundation, Berlin Institute of Health, Stiftung Charité. M.D.F. was supported by the Dystonia Medical Research Foundation, National Parkinson Foundation, Nancy Lurie Marks Foundation, Mather’s Foundation, and the NIH (K23NS083741, R01MH113929). This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program; specifically, grant number(s) S10RR023043 and S10RR023401.
Conflict of interest
J.J. reports grants from Academy of Finland, grants from Finnish Medical Foundation, grants from Orion Research Foundation, during the conduct of the study; grants from Orion, grants from Abbvie, outside the submitted work; A.H. reports grants from DFG KFO247, grants from Thiemann Foundation, grants from Berlin Institute of Health, grants from Stiftung Charité, during the conduct of the study; M.D.F. reports personal fees from Qmenta, outside the submitted work; In addition, M.D.F has a patent Lesion Network Mapping pending.
Supplementary material
Supplementary material is available at Brain online.
References
- Abe K, Yanagihara T. Hemiparkinsonism following haemorrhage in the contralateral substantia nigra. Neuroradiology 1996; 38: S67–9. [DOI] [PubMed] [Google Scholar]
- Akyol A, Akyildiz UO, Tataroglu C. Vascular Parkinsonism: a case of lacunar infarction localized to mesencephalic substantia nigra. Parkinsonism Relat Disord 2006; 12: 459–61. [DOI] [PubMed] [Google Scholar]
- Alarcón F, Zijlmans JC, Dueñas G, Cevallos N. Post-stroke movement disorders: report of 56 patients. J Neurol Neurosurg Psychiatry 2004; 75: 1568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin OS, Shwani SS, Zangana HM, Ameen NA. Progressive obtundation in a young woman with bilateral corpus striatum infarction: a case report. J Med Case Rep 2011; 5: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008; 12: 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejr-Kasem Marco H, Lorenzo-Bosquet C, Alvarez-Sabin J, Hernandez-Vara J. Parkinsonism related to Percheron artery infarct. J Neurol Sci 2017; 373: 21–2. [DOI] [PubMed] [Google Scholar]
- Bhatt M, Desai J, Mankodi A, Elias M, Wadia N. Posttraumatic akinetic-rigid syndrome resembling Parkinson's disease: a report on three patients. Mov Disord 2000; 15: 313–7. [DOI] [PubMed] [Google Scholar]
- Boes AD, Prasad S, Liu H, Liu Q, Pascual-Leone A, Caviness VS et al. . Network localization of neurological symptoms from focal brain lesions. Brain 2015; 138 (Pt 10): 3061–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NC, Lui CC, Huang SH, Huang CW, Lee CC, Chang WN, et al. Pallidoreticular lesion in carbon monoxide intoxication by gradient echo: report of a case with parkinsonism features and review of the literature. Acta Neurol Taiwan 2012; 21: 44–8. [PubMed] [Google Scholar]
- Cicarelli G, Pellecchia MT, Maiuri F, Barone P. Brain stem cystic astrocytoma presenting with “pure” parkinsonism. Mov Disord 1999; 14: 364–6. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Ramsey L, Callejas A, Baldassarre A, Hacker CD, Siegel JS et al. . Common behavioral clusters and subcortical anatomy in stroke. Neuron 2015; 85: 927–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick FC, Koch C. What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci 2005; 360: 1271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby RR, Horn A, Cushman F, Fox MD. Lesion network localization of criminal behavior. Proc Natl Acad Sci USA 2018; 115: 601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby RR, Laganiere S, Pascual-Leone A, Prasad S, Fox MD. Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain 2017; 140 (Pt 2): 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron 2003; 39: 889–909. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med 2012; 2: a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Huang Y, Bailey SK, Gao Y, Cutting LE, Rogers BP et al. . Detection of synchronous brain activity in white matter tracts at rest and under functional loading. Proc Natl Acad Sci USA 2018; 115: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doder M, Jahanshahi M, Turjanski N, Moseley IF, Lees AJ. Parkinson's syndrome after closed head injury: a single case report. J Neurol Neurosurg Psychiatry 1999; 66: 380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci 2009; 32: 548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert S, Plettig P, Li N, Chakravarty MM, Collins DL, Herrington TM et al. . Toward defining deep brain stimulation targets in MNI space: a subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage 2018; 170: 271–82. [DOI] [PubMed] [Google Scholar]
- Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol 2017; 81: 129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DB, Boes AD, Demertzi A, Evrard HC, Laureys S, Edlow BL et al. . A human brain network derived from coma-causing brainstem lesions. Neurology 2016; 87: 2427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosini D, Ceravolo R, Rossi C, Pesaresi I, Cosottini M, Bonuccelli U. Bilateral thalamic glioma presenting with parkinsonism. Mov Disord 2009; 24: 2168–9. [DOI] [PubMed] [Google Scholar]
- Foulon C, Cerliani L, Kinkingnéhun S, Levy R, Rosso C, Urbanski M et al. . Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. Gigascience 2018; 7: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci USA 2014; 111: E4367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry 2012; 72: 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2004; 2: 189–210. [Google Scholar]
- Ghaemi K, Krauss JK, Nakamura M. Hemiparkinsonism due to a pontomesencephalic cavernoma: improvement after resection. Case report. J Neurosurg Pediatr 2009; 4: 143–6. [DOI] [PubMed] [Google Scholar]
- Goll Y, Atlan G, Citri A. Attention: the claustrum. Trends Neurosci 2015; 38: 486–95. [DOI] [PubMed] [Google Scholar]
- Handley A, Medcalf P, Hellier K, Dutta D. Movement disorders after stroke. Age Ageing 2009; 38: 260–6. [DOI] [PubMed] [Google Scholar]
- Haussermann P, Wilhelm T, Keinath S, Stölzle C, Conrad B, Ceballos-Baumann A. Primary central nervous system lymphoma in the SMA presenting as rapidly progressive parkinsonism. Mov Disord 2001; 16: 962–5. [DOI] [PubMed] [Google Scholar]
- Ho BL, Lieu AS, Hsu CY. Hemiparkinsonism secondary to an infiltrative astrocytoma. Neurologist 2008; 14: 258–61. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Hollinshead MO, O’Keefe TM, Petrov VI, Fariello GR, Wald LL et al. . Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Sci Data 2015; 2: 150031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, Kühn AA. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage 2015; 107: 127–35. [DOI] [PubMed] [Google Scholar]
- Horn A, Reich M, Vorwerk J, Li N, Wenzel G, Fang Q et al. . Connectivity predicts deep brain stimulation outcome in Parkinson’s disease. Ann Neurol 2017; 82: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage 2012; 62: 782–90. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Lang AE. Parkinson’s disease. Lancet 2015; 386: 896–912. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Sperber C, Rorden C. Mapping human brain lesions and their functional consequences. Neuroimage 2017; 165: 180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS. Involuntary movements after anterior cerebral artery territory infarction. Stroke 2001; 32: 258–61. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Maki T, Kunimoto M. Clinical symptoms of bilateral anterior cerebral artery territory infarction. J Clin Neurosci 2011; 18: 218–22. [DOI] [PubMed] [Google Scholar]
- Laganiere S, Boes AD, Fox MD. Network localization of hemichorea-hemiballismus. Neurology 2016; 86: 2187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Lai PH, Chen CY, Wang JS, Lo YK. Postanoxic parkinsonism: clinical, radiologic, and pathologic correlation. Neurology 2000; 55: 591–3. [DOI] [PubMed] [Google Scholar]
- Ling MJ, Aggarwal A, Morris JG. Dopa-responsive parkinsonism secondary to right temporal lobe haemorrahage. Mov Disord 2002; 17: 402–4. [DOI] [PubMed] [Google Scholar]
- Marek K, Seibyl J, Eberly S, Oakes D, Shoulson I, Lang AE et al. . Longitudinal follow-up of SWEDD subjects in the PRECEPT Study. Neurology 2014; 82: 1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Su C, Wang W, Lin H, Li H, Lei G, et al. Delayed parkinsonism with a selective symmetric basal ganglia lesion after manual strangulation. J Clin Neurosci 2009; 16: 573–5. [DOI] [PubMed] [Google Scholar]
- Morgan JC, Sethi KD. Midbrain infarct with parkinsonism. Neurology 2003; 60: E10. [DOI] [PubMed] [Google Scholar]
- Moro E, Albanese A. Apomorphine and levodopa challenge in patients with a focal midbrain lesion. Mov Disord 1999; 14: 269–75. [DOI] [PubMed] [Google Scholar]
- Mueller K, Jech R, Bonnet C, Tintěra J, Hanuška J, Möller HE et al. . Disease-specific regions outperform whole-brain approaches in identifying progressive supranuclear palsy: a Multicentric MRI Study. Front Neurosci 2017; 11: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Stamelou M, Bhatia KP, Burn DJ. The expanding universe of disorders of the basal ganglia. Lancet 2014; 384: 523–31. [DOI] [PubMed] [Google Scholar]
- Orimo S, Amino T, Tanaka H, Mitani K, Ishiwata K, Ishii K. A case of hemiparkinsonism following ischemic lesion of the contralateral substantia nigra: a PET study. Eur Neurol 2004; 51: 175–7. [DOI] [PubMed] [Google Scholar]
- Peralta C, Werner P, Holl B, Kiechl S, Willeit J, Seppi K, et al. Parkinsonism following striatal infarcts: incidence in a prospective stroke unit cohort. J Neural Transm (Vienna) 2004; 111: 1473–83. [DOI] [PubMed] [Google Scholar]
- Peters S, Eising EG, Przuntek H, Müller T. Vascular Parkinsonism: a case report and review of the literature. J Clin Neurosci 2001; 8: 268–71. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 2007; 10: 1116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles LA. Pure hemiparkinsonism secondary to contralateral lacunar stroke in the substantia nigra. J Stroke Cerebrovasc Dis 2016; 25: e20–1. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci 2007; 19: 1081–8. [DOI] [PubMed] [Google Scholar]
- Schramm A, Grunewald S, Lorenz R, Classen J, Naumann M. Parkinsonism due to bilateral basal ganglia lesions following mastocytosis-induced hypoxia. J Neurol 2004; 251: 1270–2. [DOI] [PubMed] [Google Scholar]
- Sener RN. The claustrum on MRI: normal anatomy, and the bright claustrum as a new sign in Wilson’s disease. Pediatr Radiol 1993; 23: 594–6. [DOI] [PubMed] [Google Scholar]
- Shih LC, Tarsy D. Deep brain stimulation for the treatment of atypical parkinsonism. Mov Disord 2007; 22: 2149–55. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Pifl C, Rajput AH, Hörtnagl H, Tong J, Lloyd GK et al. . Dopamine and noradrenaline, but not serotonin, in the human claustrum are greatly reduced in patients with Parkinson’s disease: possible functional implications. Eur J Neurosci 2017; 45: 1356. [DOI] [PubMed] [Google Scholar]
- Straube A, Sigel K. Parkinsonian syndrome caused by a tumour of the left supplementary motor area. J Neurol Neurosurg Psychiatry 1988; 51: 730–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 2017; 18: 101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterer MJ, Bruss J, Boes AD, Voss MW, Bechara A, Tranel D. Canceled connections: Lesion-derived network mapping helps explain differences in performance on a complex decision-making task. Cortex 2016; 78: 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanné-Gariépy J, Boussaoud D, Rouiller EM. Projections of the claustrum to the primary motor, premotor, and prefrontal cortices in the macaque monkey. J Comp Neurol 2002; 454: 140–57. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Ratiu P, Leslie A, Howells H, Cabanis E et al. . From phineas gage and monsieur leborgne to H.M.: revisiting disconnection syndromes. Cereb Cortex 2015; 25: 4812–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Foulon C. The rise of a new associationist school for lesion-symptom mapping. Brain 2018; 141: 2–4. [DOI] [PubMed] [Google Scholar]
- Wächter T, Engeholm M, Bisdas S, Schittenhelm J, Gasser T, Krüger R. Slowly progressive Parkinson syndrome due to thalamic butterfly astrocytoma. Neurology 2011; 77: 404–5. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang Z, Bailey SK, Zhou J, Cutting LE, Gore JC et al. . Functional connectivity and activity of white matter in somatosensory pathways under tactile stimulations. Neuroimage 2017; 152: 371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M et al. . The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106: 1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii F, Kozuma R, Takahashi W, Haida M, Takagi S, Shinohara Y. Magnetic resonance imaging and 11C-N-methylspiperone/positron emission tomography studies in a patient with the interval form of carbon monoxide poisoning. J Neurol Sci 1998; 160: 87–91. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Yamamoto T, Iso-o N, Imafuku I, Momose T, Shirouzu I, et al. Hemiparkinsonism associated with a mesencephalic tumor. J Neurol Sci 2002; 197: 89–92. [DOI] [PubMed] [Google Scholar]
- Yu F, Barron DS, Tantiwongkosi B, Fox P. Patterns of gray matter atrophy in atypical parkinsonism syndromes: a VBM meta-analysis. Brain Behav 2015; 5: e00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeighami Y, Ulla M, Iturria-Medina Y, Dadar M, Zhang Y, Larcher KM et al. . Network structure of brain atrophy in de novo Parkinson’s disease. Elife 2015; 4: e08440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.