A cross-sectional study was conducted in a sample of 571 workers to explore the toxic effect and early sensitive biomarker of the health effects of low-dose benzene exposure (LDBE), as well as the correlation between DNA methylation and the toxic effect of LDBE.
A cross-sectional study was conducted in a sample of 571 workers to explore the toxic effect and early sensitive biomarker of the health effects of low-dose benzene exposure (LDBE), as well as the correlation between DNA methylation and the toxic effect of LDBE.
Abstract
Alterations in DNA methylation patterns play an essential role in disease process and are associated with cancer risk. To explore the toxic effect and early sensitive biomarker of the health effects of low-dose benzene exposure (LDBE), and investigate the correlation between DNA methylation and the toxic effect of LDBE, a cross-sectional study was conducted in a sample of 571 workers; 312 workers who were exposed to a 1.82 ± 1.16 mg m–3 air benzene concentration were assigned to the LDBE group, while 259 non-known benzene exposure (NBE) workers were assigned to the control group, with an air benzene concentration of 0.06 ± 0.01 mg m–3. Routine blood indexes, alanine transaminase (ALT), oxidative stress parameters and signal transducer and activator of transcription 3 (STAT3) methylation were detected. Compared with the NBE population, the STAT3 methylation level (P = 0.001), Platelets (PLTs) (P = 0.002) and 8-isoprostane-PGFs (8-iso-PGF2a) (P = 0.001) manifested a significant reduction, while ALT (P = 0.002) and 8-hydroxy-2 deoxyguanosine (8-OHdG) (P = 0.002) showed a significant rise in the LDBE population. In addition, a significant correlation was observed between STAT3 methylation and oxidative stress, namely 8-OhdG and 8-iso-PGF2a. Furthermore, a multivariate analysis showed that the STAT3 methylation (structure loadings = 0.909) was the most strongly correlated with the other set of variables, especially with white blood cells (WBCs) (structure loadings = 0.675). Taken together, STAT3 methylation may be the underlying mechanism involved in the early toxic effect of LDBE, therefore, STAT3 methylation can be a novel sensitive biomarker for the toxic effect of low-dose benzene exposure.
1. Introduction
Benzene, a kind of important industrial solvent, diluent and substrate for the synthesis of various chemicals, widely exists in the working and living environment. Benzene is confirmed as a human carcinogen by the international agency for research on cancer (IARC).1 Long-term exposure to high concentrations of benzene leads to a toxic effect on the hematopoietic system, including a decrease in white blood cells, bone marrow suppression, and acute and chronic leukemia.2 With the improvement of the working environment and the progress of technology, the high content of benzene is gradually being replaced by a low concentration of benzene. However, some studies have shown that exposure to low-dose benzene still induced a toxic effect,3–6 but evidence from human studies is much more limited and the mechanism of the toxic effect is controversial and indefinite. Therefore, it is necessary to explore the toxic effect and mechanisms of low-dose benzene exposure, and to offer an early sensitive biomarker of the health effects.
Nowadays it is clearly acknowledged that cytogenetic damage is accompanied by equally important epigenetic modifications during the deterioration of a tumor.7 In particular, DNA methylation is one of the earliest epigenetic modifications which is closely associated with the occurrence and development of many human diseases and it appears earlier than the obvious malignant phenotype.8 Abundant evidence shows that DNA methylation can be used as a biomarker for health surveillance and early diagnosis of diseases.9–14 In our previous study, a signal-net analysis of differentially methylated genes showed that hypomethylated signal transducer and activator of transcription 3 (STAT3) was the key gene involved in chronic benzene poisoning.15 Intriguingly, silencing genes through hypermethylation or activating genes through hypomethylation play an important role in causing disease.16 We infer that hypomethylated STAT3, which continues to be activated, has been implicated as a key participant in the toxicity of benzene. In previous studies, the association between STAT3 methylation and cancer risk was investigated, and evidence for STAT3 methylation as a diagnostic and prognostic biomarker is increasing.17–20 A leukemia study found that STAT3 activation may result from the expression of oncogenic protein tyrosine kinases or from autocrine stimulation by hematopoietic growth factors.21
Based on the above background, we postulated that STAT3 hypomethylation was an early event and a potential sensitive biomarker for assessing the toxic effect of low-dose benzene exposure (LDBE).
2. Materials and methods
2.1. Subjects
In a spray painting unit, a cross-sectional study was conducted in a sample of 571 workers.
Among them, 312 workers were engaged in the LDBE group, while 259 workers with non-known benzene exposure (NBE) were recruited into the control group. The inclusion criteria of the exposure and control groups were as follows:
Exposure group: (A) the occupational history of exposure to benzene is for more than 2 years (including 2 years), (B) no history of chronic benzene poisoning; NBE group: (A) without known benzene exposure and whose blood routine examination is normal, (B) age, sex and other conditions are similar to the exposure group. In addition, comprehensive exclusion criteria were also made for both the exposure and control groups: all subjects with recent or long-term non-occupational benzene exposure, a long history of drinking or smoking, recent infection, physical injury, blood system diseases, diseases of the nervous system, splenic dysfunction caused by radiation or ionizing radiation, or taking cytotoxic drugs such as cyclophosphamide. Demographic, life-style and occupational related information of all subjects including age, gender, smoking, drinking, medication, and family history of health status were collected through questionnaires. Written informed consent was obtained from each subject, and this study was approved by the Ethical Committee of Capital Medical University.
2.2. The exposure assessment
Personal exposure to airborne benzene was determined using passive samplers worn by the study participants near the breathing zone during their work shifts for 5–6 h, namely Radiello® passive samplers, equipped with a 35- to 50-mesh charcoal cartridge (Sigma-Aldrich, USA). The content of airborne benzene was determined using gas chromatography/mass spectrometry analysis and the detailed steps are described elsewhere.22
2.3. Sample collection and storage
During the workers’ occupational health examination, two copies of venous blood samples (2 ml) were collected from each participant; one sample was taken in vacuum negative pressure tubes containing Ethylene Diamine Tetraacetic Acid (EDTA), which was used to analyze the blood routine and gene methylation, and the other sample was taken in vacuum negative pressure tubes not containing EDTA. Serum was then collected through centrifugation at 5000 rpm for 5 min, which was used to analyze the ALT and oxidative stress indicators. All collected blood samples were processed within 8 hours and stored in freezers (–80 °C) before measurement.
2.4. Routine blood indexes and ALT
A hospital automated blood analyzer was used to detect the routine blood indexes including white blood cells (WBCs), neutrophil (NEUT), hemoglobin (HGB), red blood cells (RBCs) and platelets (PLTs). A hospital biochemical analyzer was used to detect alanine transaminase (ALT), which is the most sensitive index reflecting liver damage.
2.5. Oxidative stress
The oxidative stress level of the serum was measured using an oxidative stress kit (Nanjing Jiancheng Bioengineering Institute, China) with an enzyme-linked immune sorbent assay (ELISA), composed of 8-hydroxy-2 deoxyguanosine (8-OHdG), malondialdehyde (MDA) and 8-isoprostane-PGFs (8-iso-PGF2a). The oxidative stress indexes were detected in accordance with the protocols from the 8-OHdG kit, MDA kit and 8-iso-PGF2a kit (Abcam, Baltimore, MD 21230 USA).
2.6. STAT3 methylation
The methylation-specific PCR (MSP) method was used to evaluate the STAT3 methylation status. Genomic DNA was extracted in accordance with the protocols from the EZNA-DNA kit (Omega). Then, 1 μg of the purified DNA was subjected to bisulfite modification which was performed using a CpGenome DNA Modification Kit (Chemicon International, USA) according to the manufacturer's instructions. The sequences of primers were as follows:
For methylated DNA: MF-STAT3(5′-TATCGTTTTTTGTATTCGTTTGTAC-3′) and MR-STAT3(5′-CCTACTTTAAACTTCAATTTCTACGTA-3′), a 192 bp fragment (–1249 to –1440 relative to the transcription start site), was chosen as the M primer. For unmethylated DNA: UF-STAT3 (5′-TTGTTTTTTGTATTTGTTTGTATGG-3′) and UR-STAT3 (5′-CCTACTTTAAACTTCAATTTCTACATAT-3′), a 190 bp fragment (–1251 to –1440 relative to the transcription start site), was chosen as the U primer.
We adopted two methods to judge the methylation status: real time PCR quantitatively measuring the level of methylation expression, and Agarose Gel electrophoresis (AGE) qualitatively judging methylation or not.
2.7. Statistical analysis
Descriptive statistics and univariate analysis were performed using the Statistical Package for the Social Sciences (SPSS) software version 17.0. Normality distributions of all variables were checked using the Kolmogorov–Smirnov tests. Independent-sample t tests or two independent-sample nonparametric tests were used to compare the differences between the two groups, and the results were presented as mean ± SD values. Moreover, correlation analyses were performed to estimate the relationship between STAT3 methylation and oxidative stress indexes using Pearson or Spearman's rank correlation method. The P value in 2 sides <0.05 was considered to be statistically significant.
After the traditional correlation analysis, Canonical Correlation Analysis (CCA), which is an important method of the multivariate statistical analysis, was carried out using SAS version 8.2. Thinking about the principal components, we can use a few comprehensive variables to reflect the linear relationship between two sets of variables. The CCA was conducted using the parameters of methylation and oxidative stress (x) as predictors of the routine blood and ALT related effective indexes (y) to further examine the relationships between the two sets after the data standardization. CCA is a generalization of multiple correlations for analyzing the relationship between two sets of variables through examining the correlation between linear combinations of a set of x variables and linear combinations of a set of y variables. These linear combinations are called canonical variables. The identified variables with a statistically significant impact on the canonical variables were judged using the canonical loadings. Generally, an absolute value which was greater than 0.3 was used for identifying significant loadings.
3. Results
3.1. Characteristics of study sample
Our analysis was restricted to 571 individuals who had completed the questionnaire and laboratory results. In our present study, 312 individuals were assigned to the LDBE group (157 males and 155 females) and 259 individuals were assigned to the NBE group (130 males and 129 females). The average age in the LDBE group was 36.91 ± 9.06 years, while the NBE group had an average age of 37.66 ± 10.24 years. The age and gender difference between the two groups was not significant (P > 0.05). In addition, smoking, alcohol consumption and life styles for the two groups were prebalanced and matched. The air benzene concentrations in the LDBE and NBE control groups were 1.82 ± 1.16 mg m–3 and 0.06 ± 0.01 mg m–3, respectively.
3.2. The change in the routine blood indexes and ALT induced by LDBE
We analyzed the differences in some biological response markers between the LDBE and NBE groups. Primarily, we detected the routine blood indexes and ALT. Because there is a gender difference in RBC and HGB levels, we analyzed them separately. Compared with the NBE group, PLTs manifested a significant reduction (P = 0.002) and ALT showed a remarkable rise (P = 0.002) in the LDBE group, whereas for the other indicators, no significant differences were found between the two groups (Table 1).
Table 1. Analysis of routine blood indexes and ALT for LDBE and NBE groups.
| NBE group | LDBE group | |
| X[combining macron] ± S | X[combining macron] ± S | |
| WBCs (109 L–1) | 6.28 ± 1.43 | 6.19 ± 1.56 |
| NEUT (109 L–1) | 3.72 ± 2.79 | 3.63 ± 1.31 |
| PLTs (109 L–1) | 227.21 ± 52.48 | 213.76 ± 46.95* |
| RBCs (1012 L–1) | ||
| Male | 4.96 ± 0.29 | 4.91 ± 0.34 |
| Female | 4.38 ± 0.32 | 4.33 ± 0.34 |
| HGB (g L–1) | ||
| Male | 153.53 ± 9.30 | 153.17 ± 9.22 |
| Female | 130.37 ± 10.77 | 133.13 ± 9.98 |
| ALT (U L–1) | 22.22 ± 17.82 | 24.30 ± 14.61* |
3.3. LDBE impact on oxidative stress
We detected MDA, 8-OHdG and 8-iso-PGF2a which could reflect the oxidative stress state caused by benzene exposure. Table 2 describes the differences in the oxidative stress indicators between the two groups. Significant differences between the two groups were found for 8-OhdG (P = 0.001) and 8-iso-PGF2a (P = 0.001). Moreover, as shown in Table 2, there were significant differences in 8-OhdG (P = 0.002) and 8-iso-PGF2a (P = 0.001) for men, which indicated that men were more susceptible to oxidative stress injury. However, MDA only had a rising trend; there were no statistically significant differences when considering the differences between males and females.
Table 2. Analysis of oxidative stress indicators for LDBE and NBE groups.
| NBE group | LDBE group | |
| X[combining macron] ± S | X[combining macron] ± S | |
| 8-OHdG (ng L–1) | 212.32 ± 42.69 | 227.49 ± 49.27* |
| Male | 212.49 ± 43.06 | 229.89 ± 48.77* |
| Female | 212.21 ± 42.57 | 213.79 ± 50.41 |
| MDA (mmol L–1) | 6.28 ± 1.18 | 6.44 ± 1.08 |
| Male | 6.22 ± 1.25 | 6.44 ± 1.06 |
| Female | 6.33 ± 1.11 | 6.46 ± 1.17 |
| 8-iso-PGF2α (ng mL–1) | 24.26 ± 4.84 | 22.59 ± 4.72* |
| Male | 25.06 ± 4.88 | 22.58 ± 4.64* |
| Female | 23.71 ± 4.75 | 22.65 ± 5.18 |
3.4. STAT3 methylation expression decreased significantly due to LDBE
Furthermore, we adopted two methods to measure the STAT3 methylation status, including a quantitative method and a qualitative method. Compared with the control group, the level of STAT3 methylation decreased significantly in the LDBE group (P = 0.001) (Fig. 1). In addition, we chose the quantitative values of 10–1, 100, 101, 103, 105 and 107 to perform the qualitative detection using agarose gel electrophoresis. As shown in Fig. 2, when the quantitative level of methylation was higher than 100, the stripe was observed clearly, and the intensity of the stripes ascended sharply with the increase in the quantitative values. Therefore, the methylation quantity which was higher than 1 was identified as hypermethylation, thus the lower one was called hypomethylation. This was consistent with the cell experiment in our previous study. Accordingly, the frequencies of hypomethylation and hypermethylation in the two groups are summarized in Table 3. According to the concept and formula of OR, we assessed that the risk of STAT3 hypomethylation for the LDBE group was 3.7 times higher than for the control group, with a 95% confidence interval (2.37,5.82).
Fig. 1. Relative expression of STAT3 methylation for the two groups. LDBE: low-dose benzene exposure; NBE: non-benzene exposure; * compared with the NBE group, P < 0.01.
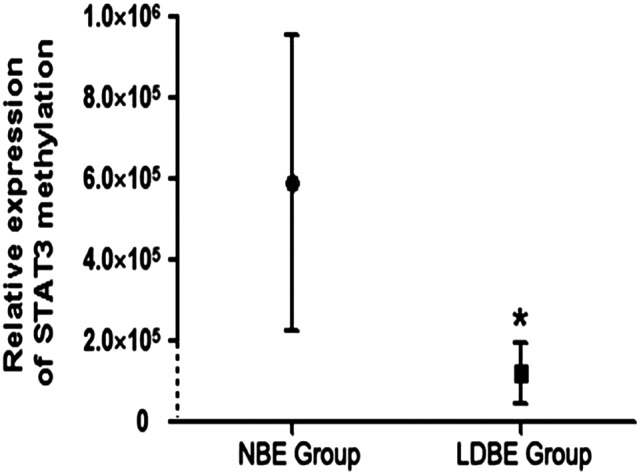
Fig. 2. Methylation status of the STAT3 gene using agarose gel electrophoresis. Numbers 1–6 respectively represent the expression quantities of 10–1, 100, 101, 103, 105 and 107 using real time-MSP.

Table 3. Chi-square test of STAT3 methylation for LDBE and NBE groups.
| Hypo-methylation | Hyper-methylation | χ 2 | P* | |
| LDBE group | 265 (84.94%) | 47 (15.06%) | 32.81 | P < 0.01* |
| NBE group | 164 (63.32%) | 95 (36.68%) |
3.5. The relationship between STAT3 methylation and oxidative stress indicators
Next, we evaluated the association between STAT3 methylation and the oxidative stress indicators, and the correlation coefficients between STAT3 methylation and the oxidative stress indicators including 8-OhdG and 8-iso-PGF2a are recorded in Table 4. There was a significant correlation between STAT3 methylation and the levels of 8-OhdG (P = 0.001) and 8-iso-PGF2a (P = 0.005). In addition, 8-OhdG was significantly correlated with 8-iso-PGF2a (P = 0.001).
Table 4. Correlation coefficients of STAT3 with 8-OHdG and 8-iso-PGF2α.
| Correlation coefficient | STAT3 methylation | 8-OHdG | 8-iso-PGF2α |
| STAT3 methylation | 1 | 0.175* | 0.145* |
| 8-OHdG | 1 | 0.174* | |
| 8-Iso-PGF2α | 1 |
3.6. Multivariate analyses using canonical correlation analysis
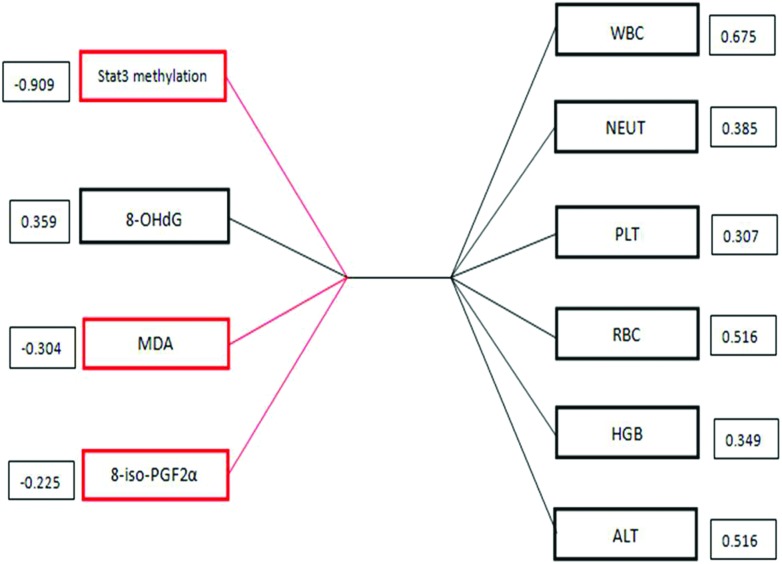
Since the present study observed that STAT3 methylation was significantly correlated with oxidative stress variables, they were seen as a set of variables. CCA was used to assess the intrinsic correlations between the two sets of variables replaced by a few comprehensive variables. In addition, the CCA was more efficient in estimating the largest contribution to the outcome in the two sets of variables. The results showed that there were four pairs of canonical variables, with canonical correlations of 0.57 (F = 1.72, P = 0.017), 0.35 (F = 1.19, P = 0.276), 0.07 (F = 0.41, P = 0.917) and 0.006 (F = 0.09, P = 0.968), for each successive pair using the CCA. Only the first canonical set was statistically significant, indicating that STAT3 methylation and oxidative stress variables were significantly correlated with the routine blood indexes and ALT variables. The canonical structures of the first pair of canonical variants are shown in Fig. 3. STAT3 methylation and the oxidative stress indicators tended to be associated with the routine blood indexes and ALT. Furthermore, STAT3 methylation (structure loadings = 0.909) was the parameter most correlated with the other set of variables, especially with WBCs (structure loadings = 0.675).
Fig. 3. Canonical correlation of STAT3 methylation and oxidative stress variables, and the routine blood indexes and ALT variables in the first canonical set. The absolute value of canonical loadings which were significant loadings was greater than 0.3. A positive relationship is expressed by a black box, while a negative relationship is shown in a red box.
4. Discussion
Our cross-sectional study is the first attempt to investigate an early sensitive epigenetic marker in a large number of LDBE workers. The study identified that STAT3 hypomethylation is a sensitive biomarker for early toxic effects on individuals exposed to low-dose benzene for the first time. The emergence and development of molecular markers opens the “black box” of disease development. Alterations of DNA methylation and oxidative stress are observed during the toxicity of benzene, and our study has confirmed that STAT3 hypomethylation accompanies oxidative stress, thus relationships between STAT3 methylation and oxidative stress provide a scientific basis for clarifying benzene toxicity.
From our knowledge, biomarkers are the biological responses of the body to a certain chemical substance, which can be used to measure chemical exposure and toxic effects.23,24 With the development of molecular biology technology, the molecular markers of disease may provide a new possibility for its early diagnosis and personalized therapy. Molecular markers should be capable of being sensitive and effective in reflecting the biological changes that occur in the body, and can accurately evaluate the status and potential hazards of living organisms, and provide an early warning for toxic damage.
Studies have indicated that exposure to low-dose benzene (<1 ppm) still causes hemototoxicity including a decrease in the number of white blood cells and platelets.3 However, some researchers believed that the routine blood indexes are not sensitive biomarkers for individuals exposed to a low concentration of benzene.6 In our study, although the numbers of HGB, RBCs and WBCs in the LDBE group were lower than those in the control group, they were not statistically significant (P > 0.05). The number of PLTs in the LDBE group was significantly decreased (P < 0.05), but the value did not exceed the normal reference range. These results were consistent with the previous studies that the changes in routine blood indexes are not sensitive indicators for workers with LDBE. Therefore, a further study on the biological markers in the LDBE group is needed.
ALT is recommended as the most sensitive indicator of liver function damage by the World Health Organization, and its normal value is 0–40 U L–1. Our study found that ALT in the LDBE group significantly increased compared with the NBE group, but not beyond the clinical normal reference value range, and the LDBE group had no liver injury symptoms. The results suggested that LDBE had a certain effect on liver function, but did not result in significant qualitative changes.
The development of benzene toxicity in humans is a complex and multistep process. It involves many molecular and cellular alterations which in turn contribute to benzene toxicity. Oxidative stress plays an important role in the process of benzene poisoning, which has been recognized by many domestic and foreign scholars.25,26 In our study, MDA, 8-OHdG and 8-iso-PGF2a were used to evaluate the oxidative stress state of the body caused by the changes in oxygen free radicals in lipids and nucleic acids. 8-Iso-PGF2a is a new discovery in bioactive compounds, which is the end product of lipid peroxidation.27 MDA is the final product of lipid peroxidation metabolism and a rising level suggests that lipid oxidation is enhanced,28 which can indirectly reflect damage of the body's cells by free radical attack. As a sensitive biomarker, 8-OHdG is a representative product of DNA base oxidation modification, which has a strong mutagenic ability.29 The results of this study showed that the level of 8-OHdG in the LDBE group was significantly increased, indicating that low-dose benzene may lead to oxidative damage. The experimental results also showed that males in the LDBE group present a greater degree of oxidative stress change, which is consistent with a number of studies that have indicated there was a gender difference in oxidative stress injury caused by various chemical substances.30–32 Studies show that the content of 8-iso-PGF2a is rising with human exposure to hazardous substances.33 To our regret, the level of 8-iso-PGF2a in the LDBE group was significantly lower than that of the NBE group. However, some studies have shown that there was a significant correlation between the expression of NADPH oxidase and 8-iso-PGF2a in the patients with atherosclerosis risk, suggesting the increase or decrease of 8-iso-PGF2a was affected by the activation or inactivation of NADPH oxidase.34–38 The reduction of 8-iso-PGFs in the LDBE group could be influenced by the inactivation of NADPH oxidase due to the entry of benzene into the body.35 Based on the above results, we came to the conclusion that 8-OHdG was more sensitive than 8-iso-PGF2 and MDA in our study.
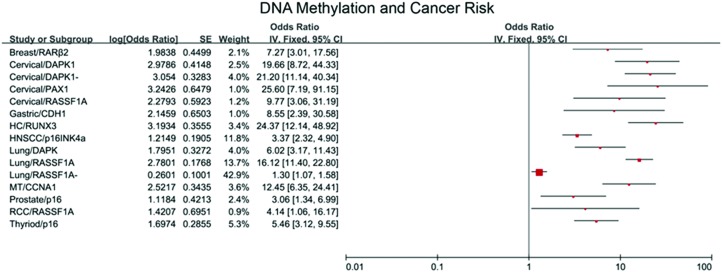
Data on the association of DNA methylation with cancer risk is rapidly emerging. DNA methylation can serve as a useful biomarker for cancers including breast cancer, gastric cancer and so on. We summarize some literature of the meta analysis, which is listed in Fig. 4.39–53 In our previous study, the signal-net analysis of differentially methylated genes demonstrated that hypomethylated STAT3 was the key gene involved in chronic benzene poisoning.15 Exhilaratingly, in our present study, compared with the control group, the level of STAT3 methylation decreased significantly in the LDBE group which is consistent with that observed in chronic benzene poisoning patients, which implied that STAT3 hypomethylation occurred early and existed in all stages of benzene toxication. Hypomethylated STAT3, which was activated, played a pivotal role in the toxic effect of LDBE. We assessed that the risk of STAT3 hypomethylation for the LDBE group was 3.7 times higher than for the control group, with a 95% confidence interval (2.37, 5.82), which suggested that STAT3 hypomethylation was a novel sensitive biomarker for the toxic effect of LDBE.
Fig. 4. Forest plot of cancer risk associated with DNA methylation. 15 independent studies on meta analysis were used in the present forest plot. The strength of the association between DNA methylation and cancer risk was assessed using the combined odds ratio (OR) and 95% confidence interval (CI).
The previous studies suggested that oxidative stress could regulate the expression level of the methylated genes, which have shown that excessive ROS can directly attack a DNA base to form a variety of modified bases.54,55 In our previous study, the association between STAT3 hypomethylation and oxidative stress was confirmed in vitro. In order to demonstrate the relationship between oxidative stress and DNA methylation in vivo, we made the correlation between the two in statistics. Our present study indicates there are significant relationships between STAT3 methylation and oxidative stress including 8-OhdG and 8-iso-PGF2a, which provides a scientific basis for impeding benzene toxicity.
Since the present study observed that STAT3 methylation was significantly correlated with oxidative stress variables, they were seen as a set of variables. The merit of the CCA analysis is that it not only explores all of the information from two sets of variables, but also overcomes the shortcoming of the small sample size of the study; therefore, the CCA is more efficient in assessing the correlation between the level of STAT3 methylation and oxidative stress, and the routine blood indexes and ALT than multiple linear regressions. Meanwhile, the CCA helps to solve the problem of multicollinearity induced by the change in risk factors to find the greatest contribution of variables. In the multivariate analysis using the CCA, we found STAT3 methylation and the oxidative stress indicators tended to be associated with the routine blood indexes and ALT. Furthermore, STAT3 methylation (structure loadings = 0.909) was the indicator most correlated with the other set of variables, especially with WBCs (structure loadings = 0.675). The current and previous studies strongly supported our initial hypothesis that STAT3 methylation was a novel sensitive biomarker in white blood cells for assessing the toxic effect of LDBE. However, this study was a cross-sectional study, lacking information on the time sequence of events, so the strength of association between STAT3 hypomethylation and disease risk was unclear in this study.
5. Conclusion
These findings suggest the important role of STAT3 hypomethylation in the initiation and development of the toxicity of benzene, and it has the potential to collude with oxidative damage. STAT3 hypomethylation is an event which appears early in the process of benzene intoxication. Accordingly, hypomethylated STAT3 provided an important basis for early hazard screening and evaluation of health effects in individuals exposed to low-dose benzene.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work has been supported by grants from the National Natural Science Foundation of China (81172639, 81472957), the Beijing Natural Science Foundation (7142020), the Importation and Development of High-Caliber Talents Project of Beijing Municipal Institutions (CIT&TCD201404187) and the Scientific Research Common Program of Beijing Municipal Commission of Education (KM201410025008).
References
- Wingo B. Occup. Health Saf. 2015;84:28. [PubMed] [Google Scholar]
- Stenehjem J. S., Kjaerheim K., Bratveit M., Samuelsen S. O., Barone-Adesi F., Rothman N., Lan Q., Grimsrud T. K. Br. J. Cancer. 2015;113:1641. doi: 10.1038/bjc.2015.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini S., Maffei F., Bermejo J. L., Ravegnini G., L'Insalata D., Cantelli-Forti G., Violante F. S., Hrelia P. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2012;743:99–104. doi: 10.1016/j.mrgentox.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Koh D. H., Jeon H. K., Lee S. G., Ryu H. W. Occup. Environ. Med. 2015;72:421–427. doi: 10.1136/oemed-2014-102227. [DOI] [PubMed] [Google Scholar]
- Snyder R. Int. J. Environ. Res. Public Health. 2012;9:2875–2893. doi: 10.3390/ijerph9082875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R., Hubbard A. E., McHale C. M., Zhang L. P., Rappaport S. M., Lan Q., Rothman N., Vermeulen R., Guyton K. Z., Jinot J., Sonawane B. R., Smith M. T. PLoS One. 2014:9. doi: 10.1371/journal.pone.0091828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whayne T. F. Mol. Biol. Rep. 2015;42:765–776. doi: 10.1007/s11033-014-3727-z. [DOI] [PubMed] [Google Scholar]
- Niu W. B., Gui S. L., Lin Y. L., Fu X. L., Ma J. G., Li W. P. Med. Sci. Monit. 2014;20:2584–2589. doi: 10.12659/MSM.893083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amissah F., Duverna R., Aguilar B. J., Poku R. A., Kiros G. E., Lamango N. S. Am. J. Cancer Res. 2014;4:116–134. [PMC free article] [PubMed] [Google Scholar]
- Creech A. L., Taylor J. E., Maier V. K., Wu X., Feeney C. M., Udeshi N. D., Peach S. E., Boehm J. S., Lee J. T., Carr S. A., Jaffe J. D. Methods. 2015;72:57–64. doi: 10.1016/j.ymeth.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallol A., Al-Maghrabi J., Buhmeida A., Gari M. A., Chaudhary A. G., Schulten H. J., Abuzenadah A. M., Al-Ahwal M. S., Sibiany A., Al-Qahtani M. H. Cancer Epidemiol., Biomarkers Prev. 2012;21:2069–2075. doi: 10.1158/1055-9965.EPI-12-0755. [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Xie P. G., Ma J. G. Med. Sci. Monit. 2014;20:1572–1577. doi: 10.12659/MSM.892130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Akutsu T., Sakurada K. J. Forensic Sci. 2015 doi: 10.1111/1556-4029.12941. [DOI] [Google Scholar]
- Zhang J. C., Gao B., Yu Z. T., Liu X. B., Lu J., Xie F., Luo H. J., Li H. P. Tumor Biol. 2014;35:2795–2802. doi: 10.1007/s13277-013-1372-0. [DOI] [PubMed] [Google Scholar]
- Yang J., Bai W. L., Niu P. Y., Tian L., Gao A. Exp. Mol. Pathol. 2014;96:346–353. doi: 10.1016/j.yexmp.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Doolittle-Hall J. M., Cunningham Glasspoole D. L., Seaman W. T., Webster-Cyriaque J. Cancers. 2015;7:2217–2235. doi: 10.3390/cancers7040887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescenzo R., Abate F., Lasorsa E., Tabbo’ F., Gaudiano M., Chiesa N., Di Giacomo F., Spaccarotella E., Barbarossa L., Ercole E., Todaro M., Boi M., Acquaviva A., Ficarra E., Novero D., Rinaldi A., Tousseyn T., Rosenwald A., Kenner L., Cerroni L., Tzankov A., Ponzoni M., Paulli M., Weisenburger D., Chan W. C., Iqbal J., Piris M. A., Zamo’ A., Ciardullo C., Rossi D., Gaidano G., Pileri S., Tiacci E., Falini B., Shultz L. D., Mevellec L., Vialard J. E., Piva R., Bertoni F., Rabadan R., Inghirami G., Grp E. T.-C. L. S. Cancer Cell. 2015;27:744–744. doi: 10.1016/j.ccell.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Lu Y., Li J., Liu Y., Liu J., Wang W. APMIS. 2015;123(10):837–846. doi: 10.1111/apm.12427. [DOI] [PubMed] [Google Scholar]
- Yeh J. E., Kreimer S., Walker S. R., Emori M. M., Krystal H., Richardson A., Ivanov A. R., Frank D. A. Genes Cancer. 2015;6:153–168. doi: 10.18632/genesandcancer.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Zhao Q., Wang Z., Liu X. Y. Cancer Chemother. Pharmacol. 2015;75:917–922. doi: 10.1007/s00280-015-2710-2. [DOI] [PubMed] [Google Scholar]
- Ghoshal Gupta S., Baumann H., Wetzler M. Leuk. Res. 2008;32:1005–1014. doi: 10.1016/j.leukres.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fustinoni S., Rossella F., Campo L., Mercadante R., Bertazzi P. A. Sci. Total Environ. 2010;408:2840–2849. doi: 10.1016/j.scitotenv.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Freitas E. C., Printes L. B., Fernandes M. N., Rocha O. Ecotoxicol. Environ. Saf. 2014;101:70–76. doi: 10.1016/j.ecoenv.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Jordaan M. S., Reinecke S. A., Reinecke A. J. Aquat. Toxicol. 2013;144:133–140. doi: 10.1016/j.aquatox.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Li J. N., Lu S. Y., Liu G. H., Zhou Y. X., Lv Y. S., She J. W., Fan R. F. Sci. Total Environ. 2015;524:74–80. doi: 10.1016/j.scitotenv.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Murugesan K., Baumann S., Wissenbach D. K., Kliemt S., Kalkhof S., Otto W., Mogel I., Kohajda T., von Bergen M., Tomm J. M. Proteomics. 2013;13:3211–3221. doi: 10.1002/pmic.201300126. [DOI] [PubMed] [Google Scholar]
- Ryu Y., Reid M. J., Thomas K. V. J. Chromatogr., A. 2015;1409:146–151. doi: 10.1016/j.chroma.2015.07.060. [DOI] [PubMed] [Google Scholar]
- Karki K., Pande D., Negi R., Khanna S., Khanna R. S., Khanna H. D. J. Environ. Pathol., Toxicol. Oncol. 2015;34:1–10. doi: 10.1615/jenvironpatholtoxicoloncol.2015010089. [DOI] [PubMed] [Google Scholar]
- Chang F. K., Mao I. F., Chen M. L., Cheng S. F. Ann. Occup. Hyg. 2011;55:519–525. doi: 10.1093/annhyg/mer010. [DOI] [PubMed] [Google Scholar]
- Chang K. A., Lin I. C., Sheen J. M., Chen Y. C., Chen C. C., Tain Y. L., Hsieh C. S., Huang L. T. Pediatr. Neonatol. 2013;54:95–101. doi: 10.1016/j.pedneo.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Guo H., Huang K., Zhang X., Zhang W. Z., Guan L., Kuang D., Deng Q. F., Deng H. X., Zhang X. M., He M. A., Christiani D., Wu T. C. Environ. Mol. Mutagen. 2014;55:472–481. doi: 10.1002/em.21866. [DOI] [PubMed] [Google Scholar]
- Zhang X. H., Zhang X., Wang X. C., Jin L. F., Yang Z. P., Jiang C. X., Chen Q., Ren X. B., Cao J. Z., Wang Q., Zhu Y. M. BMC Public Health. 2011:11. doi: 10.1186/1471-2458-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannhoff A., Bolck B., Kubler A. C., Bloch W., Reuther T. Toxicol. in Vitro. 2013;27:915–921. doi: 10.1016/j.tiv.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Cangemi R., Pignatelli P., Carnevale R., Nigro C., Proietti M., Angelico F., Lauro D., Basili S., Violi F. Diabetes. 2012;61:1626–1632. doi: 10.2337/db11-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincz L. F., Scorgie F. E., Robertson R., Enno A. Leuk. Res. 2007;31:759–763. doi: 10.1016/j.leukres.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Liu Y. N., Davidson B. P., Yue Q., Belcik T., Xie A., Inaba Y., McCarty O. J. T., Tormoen G. W., Zhao Y., Ruggeri Z. M., Kaufmann B. A., Lindner J. R. Circ. Cardiovasc. Imaging. 2013;6:74–82. doi: 10.1161/CIRCIMAGING.112.975193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo L., Martino F., Carnevale R., Pignatelli P., Catasca E., Perri L., Calabrese C. M., Palumbo M. M., Baratta F., Del Ben M., Angelico F., Violi F. J. Pediatr. 2012;161:1004–1009. doi: 10.1016/j.jpeds.2012.05.042. [DOI] [PubMed] [Google Scholar]
- Violi F., Pignatelli P., Pignata C., Plebani A., Rossi P., Sanguigni V., Carnevale R., Soresina A., Finocchi A., Cirillo E., Catasca E., Angelico F., Loffredo L. Arterioscler., Thromb., Vasc. Biol. 2013;33:406–412. doi: 10.1161/ATVBAHA.112.300438. [DOI] [PubMed] [Google Scholar]
- Agodi A., Barchitta M., Quattrocchi A., Maugeri A., Vinciguerra M. FASEB J. 2015:29. [Google Scholar]
- Fang C., Jian Z. Y., Shen X. F., Wei X. M., Yu G. Z., Zeng X. T. PLoS One. 2015:10. doi: 10.1371/journal.pone.0140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W., Han Z., Zhu R., Liu P., Liu S. J. BUON. 2015;20:1074–1080. [PubMed] [Google Scholar]
- Huang Y. Z., Wu W., Wu K., Xu X. N., Tang W. R. Asian Pac. J. Cancer Prev. 2014;15:10325–10328. doi: 10.7314/apjcp.2014.15.23.10325. [DOI] [PubMed] [Google Scholar]
- Li J. Y., Huang T., Zhang C., Jiang D. J., Hong Q. X., Ji H. H., Ye M., Duan S. W. Asian Pac. J. Cancer Prev. 2015;16:5749–5754. doi: 10.7314/apjcp.2015.16.14.5749. [DOI] [PubMed] [Google Scholar]
- Nikolaidis C., Nena E., Panagopoulou M., Balgkouranidou I., Karaglani M., Chatzaki E., Agorastos T., Constantinidis T. C. Cancer Epidemiol. 2015;39:682–686. doi: 10.1016/j.canep.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Shi H., Chen X., Lu C., Gu C., Jiang H., Meng R., Niu X., Huang Y., Lu M. PLoS One. 2015;10:e0122302. doi: 10.1371/journal.pone.0122302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Fang N., Guo L., Wu Z., Zhou Q. Zhongguo Fei Ai Za Zhi. 2015;18:443–450. doi: 10.3779/j.issn.1009-3419.2015.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Yang S. F., Liu F. F., Zhang J. H. Asian Pac. J. Cancer Prev. 2015;16:7111–7115. doi: 10.7314/apjcp.2015.16.16.7111. [DOI] [PubMed] [Google Scholar]
- Xiong J. Q., Li Y., Huang K. C., Lu M. X., Shi H., Ma L. F., Luo A. Y., Yang S. H., Lu Z. Y., Zhang J., Yang L. L., Wang S. X. PLoS One. 2014:9. doi: 10.1371/journal.pone.0107272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Miao S., Zhang L. N., Sun H. B., Xu Z. N., Han C. S. BioMed Res. Int. 2015;2015:134027. doi: 10.1155/2015/134027. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yu G. S., Lai C. Y., Xu Y., Bu C. F., Su Z. X. Asian Pac. J. Cancer Prev. 2015;16:4665–4669. doi: 10.7314/apjcp.2015.16.11.4665. [DOI] [PubMed] [Google Scholar]
- Zeng W., Zhu J., Shan L., Han Z., Aerxiding P., Quhai A., Zeng F., Wang Z., Li H. Drug Des., Dev. Ther. 2015;9:2149–2157. doi: 10.2147/DDDT.S75429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yu X. L., Zheng G. F., Zhao F. Cancer Biomarkers. 2015;15:609–617. doi: 10.3233/CBM-150501. [DOI] [PubMed] [Google Scholar]
- Zhang X., He H., Guo W., Wang Y. Cancer Invest. 2015;33:121–125. doi: 10.3109/07357907.2014.1003934. [DOI] [PubMed] [Google Scholar]
- Afanas'ev I. Aging Dis. 2014;5:52–62. doi: 10.14336/AD.2014.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S., Sardesai N. P., Liu H., Rusling J. F. Toxicol. Res. 2013;2:375–378. doi: 10.1039/C3TX50022E. [DOI] [PMC free article] [PubMed] [Google Scholar]