Abstract
Context
Growth Hormone (GH) is prescribed for an increasing range of indications, but there has been concern that it might raise cancer risk. Published data are limited.
Objective
To examine cancer risks in relation to GH treatment.
Design
Cohort study.
Setting
Population-based.
Patients
The cohort comprised 23,984 patients treated with recombinant GH (r-hGH) in 8 European countries since this treatment was first used in 1984. Cancer expectations were from country-specific national population statistics.
Main Outcome Measures
Cancer incidence and cancer mortality.
Results
Incidence and mortality risks in the cohort were raised for several cancer sites, largely consequent on second primary malignancies in patients given r-hGH after cancer treatment. There was no clear raised risk in patients with growth failure without other major disease. Only for bone (standardised incidence ratio 2.8 (95% confidence interval 1.1-7.5) and bladder (16.3 (5.2-50.4)) cancers was incidence significantly raised in GH-treated patients without previous cancer. Cancer risk was unrelated to duration or cumulative dose of r-hGH treatment, but for patients treated after previous cancer, risk of cancer mortality increased significantly with increasing daily r-hGH dose (p trend<0.001). Hodgkin lymphoma incidence increased significantly with longer follow-up (p trend=0.001 for patients overall and 0.002 for patients without previous cancer).
Conclusions
Our results do not generally support a carcinogenic effect of r-hGH, but the unexplained trend in cancer mortality risk in relation to GH dose in patients with previous cancer, and the indication of possible effects on bone cancer, bladder cancer and Hodgkin lymphoma risks, need further investigation.
Key terms: Cancer, incidence, mortality, risk, growth hormone
Introduction
GH has been prescribed since 1957 to treat GH deficiency and short stature due to other causes. The hormone used was initially extracted from human pituitaries (p-hGH), but after an outbreak of Creutzfeldt-Jakob disease consequent on prion infection from these pituitaries, this was discontinued in 1985 and all subsequent treatment has been with recombinant hormone (r-hGH).
GH raises serum concentrations of IGF-1 (insulin-like growth factor-1), which is mitogenic and antiapoptotic in vitro, and adult levels of which have been associated in most studies with risks of subsequent breast, colorectal and prostate cancers, and in some studies with other cancers(1, 2). Furthermore, cohort studies of patients with endogenously raised GH concentrations, acromegaly, have found raised risks of several cancers, most consistently colorectal(3, 4). Potential effects on leukaemia(5, 6) and other malignancy(1) risks have been suggested, and second primary malignancy risk has been shown raised in patients receiving GH after childhood cancer(7, 8). While these data give suspicion that there might be carcinogenic effects, however, no risks have been consistently shown or established. Cohort studies of r-hGH treatment have either comprised at most a few hundred patients(7, 9) or been conducted by pharmaceutical companies(10–14) with too short follow-up to cover the likely lag period of carcinogenesis, and there has been an absence of dose- and duration-response data. We therefore assembled a large cross-European cohort, the SAGhE (Safety and Appropriateness of Growth Hormone Treatments in Europe) study, with follow-up and analysis independent of pharmaceutical companies, to examine whether or not treatment with r-hGH affects cancer incidence and mortality risks in patients who have taken this treatment.
Materials and Methods
In each of eight European countries (Table 1) we assembled cohorts of patients treated with r-hGH at paediatric ages since such treatment was first used in that country (1984-6, depending on the country), and never treated with p-hGH. Data on demographic and GH-related variables were extracted from existing databases and case-notes. Subjects were followed for mortality and cancer incidence via national population-based registries in Belgium, the Netherlands, Sweden, and UK and by a range of methods in the other four countries. Details are given in(15).
Table 1.
The SAGhE cohort: descriptive variables
| Characteristic | Mortality cohorta |
Cancer incidence cohorta |
|||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sex | Male | 13268 | 55.3 | 11002 | 54.2 |
| Female | 10716 | 44.7 | 9312 | 45.8 | |
| Country | Belgium | 1363 | 5.7 | 1327 | 6.5 |
| France | 10202 | 42.5 | 8614 | 42.4 | |
| Germany | 1779 | 7.4 | 558 | 2.7 | |
| Italy | 1361 | 5.7 | 736 | 3.6 | |
| Netherlands | 1746 | 7.3 | 1685 | 8.3 | |
| Sweden | 2955 | 12.3 | 2822 | 13.9 | |
| Switzerland | 743 | 3.1 | 737 | 3.6 | |
| UK | 3835 | 16.0 | 3835 | 18.9 | |
| Age started GH treatment (years) | 0-4 | 2008 | 8.4 | 1801 | 8.9 |
| 5-9 | 7665 | 32.0 | 6535 | 32.2 | |
| 10-14 | 12136 | 50.6 | 10181 | 50.1 | |
| 15-19 | 2175 | 9.1 | 1797 | 8.8 | |
| Year started GH treatment | <1990 | 5239 | 21.8 | 4685 | 23.1 |
| 1990-94 | 10394 | 43.3 | 9264 | 45.6 | |
| 1995-99 | 5796 | 24.2 | 4598 | 22.6 | |
| ≥2000 | 2555 | 10.7 | 1766 | 8.7 | |
| Diagnosis leading to GH treatment | CNS tumour | 2221 | 9.3 | 1357 | 6.7 |
| Non CNS solid tumour | 151 | 0.6 | 100 | 0.5 | |
| Hematological malignancy | 730 | 3.0 | 428 | 2.1 | |
| Chronic renal failure and renal diseases diseases | 288 | 1.2 | 155 | 0.8 | |
| Turner syndrome | 3503 | 14.6 | 3189 | 15.7 | |
| Other syndromes and chronic diseases | 1446 | 6.0 | 1264 | 6.2 | |
| Multiple pituitary hormone deficiency organic GHD | 2497 | 10.4 | 2261 | 11.1 | |
| Skeletal dysplasias | 358 | 1.5 | 337 | 1.7 | |
| Isolated growth failureb | 12468 | 52.0 | 11062 | 54.5 | |
| Non-classifiable | 322 | 1.3 | 161 | 0.8 | |
| Total | 23984 | 100.0 | 20314c | 100.0 | |
Subjects included in follow-up for mortality, and for cancer incidence, excluding “high risk” initial diagnoses (see Methods).
Including isolated growth hormone deficiency, idiopathic short stature, and prenatal growth failure (small for gestational age).
10,406 of these subjects were from Belgium, the Netherlands, Sweden, Switzerland and the UK, and are included in the person-years based analyses of cancer incidence risk presented in Tables 2-5, and Supplemental Tables 1 and 2; 9,908 are from France, Germany and Italy and are presented in the Supplement for the reasons specified in the Methods.
In each country appropriate ethics committee agreement was obtained. For all patients, either we obtained written informed consent, or an ethics committee decided that consent was not required.
In Belgium, France, the Netherlands, Sweden and the UK the cohorts were national and population-based, or virtually so, while in Switzerland, Germany and Italy they were mainly clinic-based and sub-national. Vital status follow-up was highly complete except for uncertainty on this in France and Italy(15). Cancer incidence follow-up based on cancer registration was highly complete in Belgium, the Netherlands, Sweden, Switzerland and the UK, but less complete in France, Germany and Italy, where there was no national cancer registration. We therefore restricted cancer incidence risk calculations to the former five countries(15), and present numbers of cancers from the latter three in the Supplemental data.
Because certain rare conditions (e.g. neurofibromatosis) that lead to GH therapy are themselves strong predisposing factors for cancer, we followed previous practice(11, 12, 16) in excluding individuals with such conditions (listed in Supplemental data) from analysis.
Statistical Analysis
We calculated person-years at risk of cancer incidence and mortality, and used these with national population rates to calculate standardised mortality ratios (SMRs), standardised incidence ratios (SIRs), absolute excess rates (AERs) and trends in risk(17) by standard methods, as detailed in the Supplemental data. The analyses investigated risks of all primary malignancies except non-melanoma skin cancer, for which cancer registration tends to be highly incomplete. All p values are 2-sided.
Results
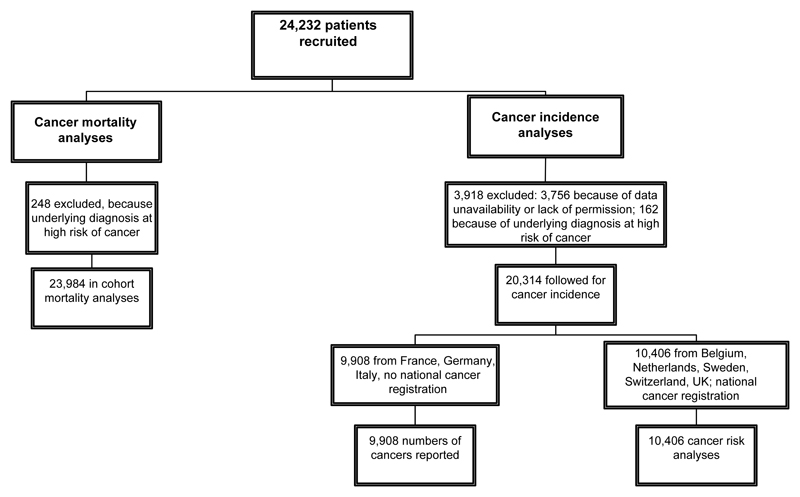
After exclusions for high-risk diagnoses, data unavailability and lack of permission (see Supplemental data and Fig. 1), the cohort for mortality risk analyses comprised 23,984 patients and for cancer incidence 10,406 patients. (For a further 9,908 from France, Germany and Italy, incident cancers are reported in the Supplemental data but risks not analysed (see Methods)). Half the cohort were first treated at ages 10-14, and about half received GH for isolated growth failure (Table 1).
Figure 1.
Numbers of patients recruited, excluded, and analysed, SAGhE cohort
Follow-up for mortality totalled 396,344 person-years, an average of 16.5 years per patient, and for cancer incidence 154,371 person-years, averaging 14.8 years per patient. The mean age at the end of follow-up was 27.1 years for the cancer mortality analyses and 25.8 years for the incidence analyses. There were 251 cancer deaths in the cohort, and 137 incident cancers in the countries for which incidence risk was analysed.
Cancer risks in the cohort overall
Cancer mortality in the cohort overall was over 13-fold raised and cancer incidence risk doubled (Table 2). AERs were 5.9 (95% confidence interval (CI) 5.1-6.7) for cancer mortality and 4.8 (3.4-6.4) for cancer incidence. There was significantly raised risk of both cancer mortality and incidence for cancers of the bone, kidney, CNS and thyroid, and significantly raised risks based on >1 case for mortality from tongue, mouth and pharynx cancer, soft tissue cancer, non-Hodgkin lymphoma and leukaemia, and incidence of melanoma and ovarian and bladder cancers. With the exception of bone and bladder cancers, these raised risks were essentially a consequence of risks in patients whose original diagnosis leading to GH treatment was cancer. In patients treated after cancer, there was additionally a significantly raised risk based on >1 case for colorectal cancer incidence. Risk estimates for the major adult cancers, e.g. breast, lung and prostate, had wide confidence intervals, based on few person-years of follow-up.
Table 2.
Cancer mortality and incidence risks, SAGhE cohort, by site and initial diagnosis leading to GH treatment
| All initial diagnoses |
|||||
|---|---|---|---|---|---|
| Outcome |
Cancer mortality |
Cancer incidencec |
|||
| ICD 10, C code | Cancer sitea | No. of cases | SMRb (95% CI) | No. of cases | SIR (95% CI) |
| 01-14 | Tongue, mouth, pharynx | 3 | 6.8 (2.2- 21.2)d | 1 | 1.4 (0.0-7.5) |
| 18-21 | Colon and rectum | 2 | 3.6 (0.9-14.6) | 4 | 2.3 (0.9-6.2) |
| 25 | Pancreas | 1 | 7.7 (0.2-42.6) | 0 | 0.0 (0.0-28.0) |
| 33-34 | Lung | 1 | 1.9 (0.1-10.9) | 1 | 2.6 (0.1-14.5) |
| 40-41 | Bone | 12 | 6.9 (3.9-12.1)f | 9 | 5.2 (2.7-10.1)f |
| 43 | Melanoma | 1 | 1.4 (0.0-8.0) | 12 | 2.1 (1.2-3.8)d |
| 47-49 | Soft tissue | 5 | 5.9 (2.5-14.2)e | 4 | 2.7 (1.0-7.2) |
| 50 | Breast | 1 | 1.9 (0.1-10.4) | 2 | 0.9 (0.2-3.4) |
| 51 | Vulva | 0 | 0.0 (0.0-1749.0) | 1 | 5.0 (0.1-27.8) |
| 53 | Cervix | 0 | 0.0 (0.0-18.8) | 17 | 0.9 (0.6-1.5) |
| 54-55 | Corpus uteri | 1 | 24.3 (0.6-135.4) | 0 | 0.0 (0.0-35.4) |
| 56 | Ovary | 1 | 4.2 (0.1-23.4) | 4 | 3.0 (1.1-7.9)d |
| 61 | Prostate | 1 | 50.7 (1.3-282.3)d | 0 | 0.0 (0.0-174.7) |
| 62 | Testis | 0 | 0.0 (0.0-13.0) | 7 | 1.2 (0.6-2.4) |
| 64-66 | Kidney | 2 | 13.8 (3.5-437.5)d | 3 | 6.8 (2.2-21.2)d |
| 67-68 | Bladder | 1 | 11.7 (0.3-65.0) | 3 | 14.0 (4.5-43.4)e |
| 70-72 | CNS | 156 | 45.8 (39.2-53.6)f | 29 | 6.5 (4.5-9.4)f |
| 73 | Thyroid | 1 | 54.6 (1.4-304.2)d | 12 | 6.0 (3.4-10.5)f |
| 81 | Hodgkin lymphoma | 0 | 0.0 (0.0-6.4) | 8 | 1.8 (0.9-3.6) |
| 82-85,96 | Non-Hodgkin lymphoma | 4 | 3.4 (1.3-9.0)d | 3 | 1.3 (0.4-4.2) |
| 91-95 | Leukaemia | 27 | 6.4 (4.4-9.4)f | 7 | 2.2 (1.0-4.6) |
| 00-43, 45, 47-85, 89-97 | All sites except non-melanoma skin cancer | 251 | 13.7 (12.1-15.5)f | 138 | 2.2 (1.9-2.6)f |
| Initial diagnosis cancer |
Initial diagnosis non- cancer |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ICD 10, C code | Cancer site | Cancer mortality |
Cancer incidence |
Cancer mortality |
Cancer incidence |
||||
| n. | SMR (95% CI) | n. | SIR (95% CI) | n. | SMR (95% CI) | n. | SIR (95% CI) | ||
| 01-14 | Tongue, mouth, pharynx | 2 | 24.2 (6.1-96.8)e | 1 | 7.9 (0.2-43.8) | 1 | 2.8 (0.1-15.7) | 0 | 0.0 (0.0-6.0) |
| 18-21 | Colon and rectum | 1 | 14.7 (0.4-81.7) | 2 | 7.4 (1.9-29.7)d | 1 | 2.1 (0.1-11.6) | 2 | 1.4 (0.3-5.6) |
| 25 | Pancreas | 1 | 61.6 (1.6-343.4)d | 0 | 0.0 (0.0-182.7) | 0 | 0.0 (0.0-32.2) | 0 | 0.0 (0.0-33.1) |
| 33-34 | Lung | 0 | 0.0 (0.0-62.2) | 0 | 0.0 (0.0-57.0) | 1 | 2.2 (0.1-12.3) | 1 | 3.1 (0.4-22.2) |
| 40-41 | Bone | 8 | 35.5 (17.7-70.9)f | 5 | 17.2 (7.2-41.4)f | 4 | 2.6 (1.0-7.0) | 4 | 2.8 (1.1-7.5)d |
| 43 | Melanoma | 0 | 0.0 (0.0-41.4) | 5 | 5.8 (2.4-13.9)e | 1 | 1.7 (0.0-9.2) | 7 | 1.5 (0.7-3.1) |
| 47-49 | Soft tissue | 5 | 47.2 (19.7-113.4)f | 2 | 8.5 (2.1-33.9)d | 0 | 0.0 (0.0-5.0) | 2 | 1.6 (0.4-6.4) |
| 50 | Breast | 1 | 16.9 (0.4-94.1) | 1 | 3.0 (0.1-16.6) | 0 | 0.0 (0.0-7.7) | 1 | 0.5 (0.0-2.8) |
| 51 | Vulva | 0 | 0.0 (0.0-9281.9) | 0 | 0.0 (0.0-141.6) | 0 | 0.0 (0.0-2155.1) | 1 | 5.7 (0.2-31.9) |
| 53 | Cervix | 0 | 0.0 (0.0-128.6) | 2 | 0.8 (0.2-3.2) | 0 | 0.0 (0.0-22.0) | 15 | 0.9 (0.6-1.5) |
| 54-55 | Corpus uteri | 1 | 259.7 (6.6-1446.7)e | 0 | 0.0 (0.0-240.9) | 0 | 0.0 (0.0-98.9) | 0 | 0.0 (0.0-41.4) |
| 56 | Ovary | 1 | 39.7 (1.0-221.2)d | 3 | 14.8 (4.8-45.9)e | 0 | 0.0 (0.0-17.4) | 1 | 0.9 (0.0-4.9) |
| 61 | Prostate | 0 | 0.0 (0.0-1622.2) | 0 | 0.0 (0.0-850.4) | 1 | 57.3 (1.5-319.1)d | 0 | 0.0 (0.0-219.8) |
| 62 | Testis | 0 | 0.0 (0.0-99.8) | 3 | 2.7 (0.9-8.5) | 0 | 0.0 (0.0-15.0) | 4 | 0.8 (0.3-2.2) |
| 64-66 | Kidney | 2 | 138.1 (34.5-552.0)f | 3 | 44.1 (14.2-136.8)f | 0 | 0.0 (0.0-28.4) | 0 | 0.0 (0.0-10.0) |
| 67-68 | Bladder | 0 | 0.0 (0.0-397.0) | 0 | 0.0 (0.0-123.2) | 1 | 13.1 (0.3-72.9) | 3 | 16.3 (5.2-50.4)e |
| 70-72 | CNS | 153 | 373.4 (318.6-437.5)f | 23 | 34.7 (23.1-52.2)f | 3 | 1.0 (0.3-3.1) | 6 | 1.6 (0.7-3.5) |
| 73 | Thyroid | 1 | 496.6 (12.6-2767.0)e | 10 | 32.2 (17.3-59.8)f | 0 | 0.0 (0.0-226.3) | 2 | 1.2 (0.3-4.7) |
| 81 | Hodgkin lymphoma | 0 | 0.0 (0.0-50.7) | 1 | 1.3 (0.0-7.2) | 0 | 0.0 (0.0-7.4) | 7 | 1.9 (0.9-4.0) |
| 82-85,96 | Non-Hodgkin lymphoma | 4 | 26.8 (10.1-71.4)f | 1 | 2.6 (0.1-14.4) | 0 | 0.0 (0.0-3.6) | 2 | 1.1 (0.3-4.3) |
| 91-95 | Leukaemia | 23 | 45.5 (30.2-68.5)f | 4 | 7.7 (2.9-20.4)e | 4 | 1.1 (0.4-2.9) | 3 | 1.1 (0.4-3.5) |
| 00-43, 45, 47-85, 89-97 | All sites except non-melanoma skin cancer | 230 | 101.9 (89.6-116.0)f | 72 | 7.6 (6.1-9.6)f | 21 | 1.3 (0.9-2.0) | 66 | 1.2 (1.0-1.6) |
ICD = International Classification of Diseases; SMR = Standardised mortality ratio; SIR = Standardised incidence ratio; CI = Confidence interval
The sites selected are those for which any cancer deaths or incident cases occurred.
Using Swiss rates as expecteds for Germany, and Belgian rates as expecteds for both France and the Netherlands, for cancer sites for which sufficient detail was not available from home-country national rates.
Excluding France, Germany and Italy.
p<0.05
p<0.01
p<0.001
ICD = International Classification of Diseases; SMR = Standardised mortality ratio; SIR = Standardised incidence ratio; CI = Confidence interval
p<0.05
p<0.01
p<0.001
Risks by underlying diagnosis
In patients whose initial diagnosis was “isolated growth failure” (i.e. growth failure without other major disease: isolated growth hormone deficiency, idiopathic short stature, and prenatal growth failure), overall cancer risk was not raised and there were no significantly raised site-specific risks, based on small numbers of cases (Table 3). For patients whose initial diagnosis was not isolated growth failure or cancer, there were significantly raised risks of cancer incidence (SIR 1.4; 95% CI 1.1-1.9) and mortality (SMR 2.2; 95% CI 1.3-3.7) overall, and of bone (SIR 4.1; 95% CI 1.3-12.6) and bladder SIR 27.8 (7.0-111.3) cancer incidence, reflecting cases after several different initial diagnoses, with no obvious common factor, although based on small numbers for each cancer site.
Table 3.
Cancer mortality and incidence risks, SAGhE cohort, for patients in whom a non-cancer diagnosis led to GH treatment
| Initial diagnosis isolated growth failure | Initial diagnosis non-cancer, non-isolated growth failure | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome (cancer site) |
Cancer mortality |
Cancer incidence |
Cancer mortality |
Cancer incidence |
||||
| n | SMR (95% CI) | n | SIR (95% CI) | n | SMR (95% CI) | n | SIR (95% CI) | |
| Colon and rectum | 0 | 0.0 (0.0-12.1) | 0 | 0.0 (0.0-5.2) | 1 | 5.7 (0.1-31.8) | 2 | 2.7 (0.7-10.9) |
| Bone | 3 | 3.1 (1.0-9.6) | 1 | 1.4 (0.0-8.0) | 1 | 1.8 (0.1-10.1) | 3 | 4.1 (1.3-12.6)a |
| Melanoma | 1 | 2.6 (0.1-14.5) | 3 | 1.5 (0.5-4.5) | 0 | 0.0 (0.0-16.7) | 4 | 1.5 (0.6-4.0) |
| Soft tissue | 0 | 0.0 (0.0-8.2) | 0 | 0.0 (0.0-6.0) | 0 | 0.0 (0.0-12.7) | 2 | 3.1 (0.8-12.6) |
| Cervix | 0 | 0.0 (0.0-64.4) | 7 | 1.1 (0.5-2.4) | 0 | 0.0 (0.0-33.4) | 8 | 0.8 (0.4-1.6) |
| Testis | 0 | 0.0 (0.0-19.7) | 3 | 1.0 (0.3-3.0) | 0 | 0.0 (0.0-62.3) | 1 | 0.5 (0.0-3.0) |
| Bladder | 0 | 0.0 (0.0-83.4) | 1 | 8.9 (0.2-49.4) | 1 | 31.0 (0.8-172.9) | 2 | 27.8 (7.0-111.3)b |
| CNS | 0 | 0.0 (0.0-2.0) | 3 | 1.6 (0.5-4.8) | 3 | 2.6 (0.8-8.0) | 3 | 1.6 (0.5-5.1) |
| Thyroid | 0 | 0.0 (0.0-371.7) | 0 | 0.0 (0.0-5.5) | 0 | 0.0 (0.0-578.6) | 2 | 2.0 (0.5-7.8) |
| Hodgkin lymphoma | 0 | 0.0 (0.0-11.5) | 3 | 1.7 (0.6-5.4) | 0 | 0.0 (0.0-20.3) | 4 | 2.0 (0.8-5.4) |
| NHL | 0 | 0.0 (0.0-5.5) | 0 | 0.0 (0.0-3.9) | 0 | 0.0 (0.0-10.5) | 2 | 2.2 (0.6-8.8) |
| Leukaemia | 2 | 0.8 (0.2-3.4) | 1 | 0.8 (0.0-4.2) | 2 | 1.5 (0.4-6.1) | 2 | 1.5 (0.4-5.9) |
| All sites except non-melanoma skin cancer | 8 | 0.8 (0.4-1.6) | 23 | 1.0 (0.6-1.4) | 13 | 2.2 (1.3-3.7)a | 42 | 1.4 (1.1-1.9)a |
SMR = Standardised mortality ratio; SIR = Standardised incidence ratio; CI = Confidence interval
p<0.05
p<0.01
Risks by demographic characteristics and GH treatment variables
Cancer risks in the cohort were similar in males and females (Table 4). Risks varied over twofold between countries, paralleling approximately the proportions of subjects in these countries who had cancer as their initial diagnosis(15). Cancer risks did not relate to age at starting r-hGH treatment, but for cancer mortality not incidence risks decreased with duration since starting treatment, and for cancer mortality especially risks decreased with longer duration of treatment. The effect of duration of treatment disappeared for cancer incidence (p=0.72) and greatly diminished for cancer mortality (p=0.04) when we censored from analysis the person-time during treatment plus the first 2 years after ending treatment (not in Table), suggesting that it had been an artefact of cessations of treatment because of cancer occurrence. Risk of cancer incidence but not mortality decreased with increasing mean GH dose, and both incidence and mortality risks tended to diminish with cumulative dose.
Table 4.
Cancera mortality and incidence risks, SAGhE cohort, by demographic and GH treatment variables, and initial diagnosis leading to GH treatment
| Demographic or treatment variable | All initial diagnoses, total |
||||
|---|---|---|---|---|---|
| Cancer mortality |
Cancer incidencec |
||||
| n | SMRb (95% CI) | n | SMR (95% CI) | ||
| Sex | Male | 138 | 12.4 (10.5-14.6)g | 52 | 2.2 (1.7-2.9)g |
| Female | 113 | 15.8 (13.1-19.0)g | 86 | 2.2 (1.8-2.7)g | |
| Country | Belgium | 23 | 22.1 (14.7-33.3)g | 7 | 1.9 (0.9-4.0) |
| France | 88 | 9.9 (8.1-12.2)g | - | ||
| Germany | 10 | 11.5 (6.2-21.4)g | - | ||
| Italy | 1 | 1.7 (0.0-9.4) | - | ||
| Netherlands | 27 | 23.6 (16.2-34.5)g | 22 | 2.5 (1.6-3.8)g | |
| Sweden | 30 | 12.9 (9.0-18.5)g | 50 | 1.5 (1.2-2.0)f | |
| Switzerland | 6 | 19.7 (8.8-43.7)g | 2 | 2.5 (0.6-10.1) | |
| UK | 66 | 20.7 (16.3-26.4)g | 57 | 3.4 (2.6-4.4)g | |
| Age started treatment (years) | 0-4 | 9 | 7.1 (3.7-13.6)g | 7 | 1.7 (0.8-3.5) |
| 5-9 | 70 | 13.9 (11.0-17.6)g | 46 | 2.5 (1.8-3.3)g | |
| 10-14 | 149 | 15.4 (13.1-18.0)g | 71 | 2.1 (1.7-2.6)g | |
| 15-19 | 23 | 10.0 (6.6-15.0)g | 14 | 2.3 (1.4-3.9)f | |
| p trend | 0.55 | 1.00 | |||
| Time since started treatment (years) | 0-4 | 103 | 24.4 (20.1-29.6)g | 25 | 3.5 (2.4-5.2)g |
| 5-9 | 78 | 17.2 (13.8-21.5)g | 21 | 1.8 (1.2-2.8)e | |
| 10-14 | 37 | 8.2 (6.0-11.4)g | 47 | 2.3 (1.8-3.1)g | |
| 15-19 | 25 | 6.7 (4.5-9.8)g | 30 | 1.6 (1.1-2.3)e | |
| ≥20 | 8 | 6.1 (3.1-12.3)g | 15 | 2.7 (1.7-4.6)f | |
| p trend | <0.001 | 0.13 | |||
| Duration of treatment (years)d | <3 | 118 | 21.1 (17.6-25.3)g | 40 | 2.8 (2.1-3.9)g |
| 3-6 | 80 | 12.4 (10.0-15.5)g | 52 | 2.7 (2.1-3.5)g | |
| ≥7 | 35 | 7.5 (5.4-10.4)g | 33 | 1.9 (1.3-2.6)f | |
| p trend | <0.001 | 0.07 | |||
| Mean GH dose (µg/kg/day)d | <20 | 37 | 9.6 (7.0-13.3)g | 18 | 4.0 (2.6-6.4)g |
| 20-9 | 94 | 19.5 (15.9-23.8)g | 40 | 3.3 (2.4-4.4)g | |
| 30-9 | 52 | 16.8 (12.8-22.0)g | 41 | 2.1 (1.6-2.9)g | |
| ≥40 | 7 | 3.8 (1.8-8.0)f | 11 | 1.1 (0.6-2.0) | |
| p trend | 0.39 | <0.001 | |||
| Cumulative GH dose (mg/kg)d | <25 | 91 | 14.9 (12.1-18.3)g | 38 | 3.4 (2.5-4.7)g |
| 25-49 | 73 | 16.9 (13.4-21.2)g | 30 | 2.1 (1.5-3.0)g | |
| 50-99 | 36 | 11.1 (8.0-15.3)g | 40 | 2.3 (1.7-3.2)g | |
| ≥100 | 3 | 2.7 (0.9-8.3) | 11 | 1.6 (0.9-2.9) | |
| p trend | 0.003 | 0.02 | |||
| Total | 251 | 13.7 (12.1-15.5)g | 138 | 2.2 (1.9-2.6)g | |
| Initial diagnosis cancer |
Initial diagnosis non-cancer |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer mortality |
Cancer incidence c |
Cancer mortality |
Cancer incidence c |
|||||||
| n | SMRb (95% CI) | n | SIR (95% CI) | n | SMRb (95% CI) | n | SIR (95%CI) | |||
| Sex | Male | 131 | 90.2 (76.0-107.0)g | 30 | 7.2 (5.0-10.3)g | 7 | 0.7 (0.3-1.5) | 22 | 1.1 (0.7-1.7) | |
| Female | 99 | 123.1 (101.1-149.9)g | 42 | 8.0 (5.9-10.8)g | 14 | 2.2 (1.3-3.7)e | 44 | 1.3 (1.0-1.7) | ||
| Country | Belgium | 20 | 113.9 (73.5-176.6)g | 3 | 5.0 (1.6-15.4)e | 3 | 3.5 (1.1-10.8)e | 4 | 1.3 (0.5-3.5) | |
| France | 79 | 96.6 (77.5-120.5)g | - | 9 | 1.1 (0.6-2.2) | - | ||||
| Germany | 9 | 114.9 (59.8-220.9)g | - | 1 | 1.3 (0.0-7.0) | - | ||||
| Italy | 0 | 0.0 (0.0-252.6) | - | 1 | 1.7 (0.0-9.6) | - | ||||
| Netherlands | 26 | 138.2 (94.1-203.0)g | 14 | 9.9 (5.8-16.7)g | 1 | 1.0 (0.0-5.8) | 8 | 1.1 (0.5-2.2) | ||
| Sweden | 27 | 120.6 (82.7-175.9)g | 21 | 5.6 (3.6-8.6)g | 3 | 1.4 (0.5-4.4) | 29 | 1.0 (0.7-1.4) | ||
| Switzerland | 5 | 168.4 (70.1-404.5)g | 2 | 31.0 (7.7-123.9)f | 1 | 3.6 (0.1-20.2) | 0 | 0.0 (0.0-5.1) | ||
| UK | 64 | 87.8 (68.7-112.1)g | 32 | 8.9 (6.3-12.6)g | 2 | 0.8 (0.2-3.3) | 25 | 1.9 (1.3-2.8)f | ||
| Age started treatment (years) | 0-4 | 8 | 127.4 (63.7-254.7)g | 4 | 16.1 (6.1-43.0)g | 1 | 0.8 (0.0-4.6) | 3 | 0.8 (0.2-2.4) | |
| 5-9 | 65 | 100.1 (78.5-127.7)g | 18 | 7.1 (4.5-11.3)g | 5 | 1.1 (0.5-2.7) | 28 | 1.7 (1.2-2.5)f | ||
| 10-14 | 137 | 108.7 (91.9-128.5)g | 42 | 7.3 (5.4-9.9)g | 12 | 1.4 (0.8-2.5) | 29 | 1.0 (0.7-1.5) | ||
| 15-19 | 20 | 70.3 (45.4-109.0)g | 8 | 8.6 (4.3-17.2)g | 3 | 1.5 (0.5-4.6) | 6 | 1.2 (0.5-2.6) | ||
| p trend | 0.30 | 0.71 | 0.53 | 0.44 | ||||||
| Time since started treatment (years) | 0-4 | 100 | 184.8 (151.9-224.8)g | 20 | 16.0 (10.3-24.9)g | 3 | 0.8 (0.3-2.5) | 5 | 0.9 (0.4-2.1) | |
| 5-9 | 71 | 124.4 (98.6-157.0)g | 12 | 5.9 (3.4-10.5)g | 7 | 1.8 (0.8-3.7) | 9 | 0.9 (0.5-1.8) | ||
| 10-14 | 33 | 60.7 (43.2-85.4)g | 24 | 8.2 (5.5-12.2)g | 4 | 1.0 (0.4-2.7) | 23 | 1.3 (0.9-2.0) | ||
| 15-19 | 19 | 45.9 (29.3-71.9)g | 11 | 4.5 (2.5-8.2)g | 6 | 1.8 (0.8-4.0) | 19 | 1.2 (0.7-1.8) | ||
| ≥20 | 7 | 37.4 (17.8-78.4)g | 5 | 6.1 (2.5-14.7)f | 1 | 0.9 (0.0-5.0) | 10 | 2.2 (1.2-4.0)e | ||
| p trend | <0.001 | 0.005 | 0.65 | 0.11 | ||||||
| Duration of treatment (years) | <3 | 110 | 174.9 (145.1-210.8)g | 25 | 10.4 (7.1-15.5)g | 8 | 1.6 (0.8-3.2) | 15 | 1.3 (0.8-2.1) | |
| 3-5 | 74 | 87.0 (69.3-109.3)g | 29 | 8.6 (6.0-12.4)g | 6 | 1.1 (0.5-2.4) | 23 | 1.4 (1.0-2.2)e | ||
| ≥6 | 31 | 50.2 (35.3-71.4)g | 12 | 4.0 (2.2-7.0)g | 4 | 1.0 (0.4-2.6) | 21 | 1.4 (0.9-2.2) | ||
| p trend | <0.001 | 0.006 | 0.76 | 0.77 | ||||||
| Mean GH dose (µg/kg/day) | <20 | 35 | 64.1 (46.0-89.3)g | 12 | 6.5 (3.7-11.4)g | 2 | 0.6 (0.2-2.4) | 6 | 2.3 (1.0-5.2) | |
| 20-9 | 89 | 102.1 (82.9-125.6)g | 26 | 7.6 (5.2-11.2)g | 5 | 1.3 (0.5-3.0) | 14 | 1.6 (0.9-2.7) | ||
| 30-9 | 50 | 178.9 (135.6-236.1)g | 19 | 10.2 (6.5-16.0)g | 2 | 0.7 (0.2-2.8) | 22 | 1.3 (0.8-1.9) | ||
| ≥40 | 5 | 101.5 (42.3-243.9)g | 3 | 5.0 (1.6-15.5)e | 2 | 1.1 (0.3-4.5) | 8 | 0.9 (0.4-1.7) | ||
| p trend | <0.001 | 0.59 | 0.74 | 0.05 | ||||||
| Cumulative GH dose (mg/kg) | <25 | 87 | 108.8 (88.2-134.2)g | 25 | 9.9 (6.7-14.6)g | 4 | 0.8 (0.3-2.0) | 13 | 1.5 (0.9-2.6) | |
| 25-49 | 70 | 108.1 (85.5-136.7)g | 18 | 6.6 (4.1-10.4)g | 3 | 0.8 (0.3-2.5) | 12 | 1.0 (0.6-1.8) | ||
| 50-99 | 30 | 79.5 (55.6-113.6)g | 18 | 6.8 (4.3-10.9)g | 6 | 2.1 (0.9-4.6) | 22 | 1.5 (1.0-2.3) | ||
| ≥100 | 2 | 34.2 (8.6-136.9)f | 5 | 9.6 (4.0-23.1)g | 1 | 0.9 (0.0-5.3) | 6 | 1.0 (0.4-2.1) | ||
| p trend | 0.05 | 0.43 | 0.24 | 0.63 | ||||||
| Total | 230 | 101.9 (89.6-116.0)g | 72 | 7.6 (6.1-9.6)g | 21 | 1.3 (0.9-2.0) | 66 | 1.2 (1.0-1.6) | ||
SMR = Standardised mortality ratio; SIR = Standardised incidence ratio; CI = Confidence interval
All malignancies except non-melanoma skin cancer.
Using Swiss rates as expecteds for Germany, and Belgian rates as expecteds for both France and the Netherlands, for cancer sites for which sufficient detail was not available from home-country national rates.
Excluding France, Germany and Italy.
Unknown, all initial diagnoses: duration of treatment mortality 18, incidence 13; mean GH dose mortality 61, incidence 28; cumulative GH dose mortality 48, incidence 19.
p<0.05
p<0.01
p<0.001
SMR = Standardised mortality ratio; SIR = Standardised incidence ratio; CI = Confidence interval
All malignancies except non-melanoma skin cancer.
Using Swiss rates as expecteds for Germany, and Belgian rates as expecteds for both France and the Netherlands, for cancer sites for which sufficient detail was not available from home-country national rates.
Excluding France, Germany and Italy.
p<0.05
p<0.01
p<0.001
Examining these risks within the patients whose initial diagnosis was cancer (Table 4), the only indications of different patterns from above were that incidence as well as mortality decreased with duration since starting treatment and duration of treatment, cumulative GH dose did not significantly affect mortality or incidence, and mortality but not incidence increased highly significantly (p<0.001) with increasing mean GH dose. For patients whose initial diagnosis was not cancer, neither cancer mortality nor incidence was significantly related to any of the treatment variables. The diminutions in risk seen with mean GH dose and cumulative dose for cohort members overall were at least in part due to confounding by initial diagnosis: patients with initial non-cancer diagnoses tended to have received greater mean and cumulative GH doses than did cancer patients (e.g. 33% of non-cancer patients but only 17% of cancer patients received doses of ≥30µg/Kg/day). Analyses separately for patients with isolated growth failure, and for those with Turner syndrome (Supplemental Table 1), showed significant rising incidence risks with time since first treatment and with duration of treatment (both p=0.02) for isolated growth failure patients, but otherwise no significant risks for incidence or mortality. Mean daily doses of GH were 26.0µg/Kg/day for the patients with isolated growth hormone deficiency, 33.8 for those with idiopathic short stature, and 49.5 for those born small for gestational age.
The rising risk of cancer mortality in cancer patients in relation to daily GH dose was similar for each of the three cancer sites with sufficient deaths for such subanalysis (Supplemental Table 2), and also separately in patients who were and were not known to have been treated with any radiotherapy, with craniospinal radiotherapy, and with chemotherapy (each based on limited data on these treatments), and in subgroups by time since starting GH treatment. Strong significant dose-response trends were seen in every sub-group, except where there were small numbers (not in Table).
Examining site-specific cancer risks by duration since first GH treatment (Table 5), CNS tumour mortality decreased significantly (p<0.001) and Hodgkin lymphoma incidence increased significantly (p=0.001), with longer follow-up. The decreasing CNS tumour trend derived from patients whose underlying diagnosis was cancer (trend p<0.001) and the Hodgkin lymphoma trend from patients whose initial diagnosis was not cancer (a wide range of non-cancer diagnoses) (trend p=0.002).
Table 5.
Cancer mortality and incidence risks at selected cancer sites, SAGhE cohort, by duration since first treatment and initial diagnosis leading to GH treatment
| All initial diagnoses, total | |||||
|---|---|---|---|---|---|
| Cancer mortality |
Cancer incidenceb |
||||
| Cancer site (outcome) | Duration since first treatment (years) | n | SMRa (95% CI) | n | SIR (95% CI) |
| Colorectal cancer | 0-9 | 1 | 10.2 (0.3-56.7) | 2 | 4.2 (1.1-16.9)c |
| 10-19 | 1 | 2.7 (0.1-15.1) | 2 | 2.0 (0.5-7.9) | |
| 20-29 | 0 | 0.0 (0.0-45.2) | 0 | 0.0 (0.0-16.4) | |
| p trend | 0.25 | 0.25 | |||
| Bone cancer | 0-9 | 9 | 8.5 (4.4-16.3)e | 5 | 4.6 (1.9-11.0)c |
| 10-19 | 3 | 4.6 (1.5-14.2)c | 4 | 6.8 (2.6-18.1)d | |
| 20-29 | 0 | 0.0 (0.0-114.7) | 0 | 0.0 (0.0-86.2) | |
| p trend | 0.30 | 0.76 | |||
| Melanoma | 0-9 | 0 | 0.0 (0.0-25.2) | 2 | 1.6 (0.4, 6.2) |
| 10-19 | 1 | 2.1 (0.1-11.9) | 7 | 1.9 (0.9, 4.0) | |
| 20-29 | 0 | 0.0 (0.0-45.4) | 3 | 4.5 (1.4, 13.8)c | |
| p trend | 0.86 | 0.25 | |||
| CNS | 0-9 | 120 | 62.9 (52.6-75.2)e | 15 | 6.2 (3.8-10.3)e |
| 10-19 | 32 | 24.2 (17.1-34.3)e | 13 | 7.2 (4.2-12.4)e | |
| 20-29 | 4 | 22.9 (8.6-61.0)e | 1 | 4.7 (0.1-26.2) | |
| p trend | <0.001 | 0.90 | |||
| Thyroid | 0-9 | 0 | 0.0 (0.0-1284.3) | 6 | 10.9 (4.9-24.2)e |
| 10-19 | 1 | 78.1 (2.0-435.4)c | 5 | 4.1 (1.7-9.8)c | |
| 20-29 | 0 | 0.0 (0.0-1393.9) | 1 | 4.4 (0.1-24.6) | |
| p trend | 0.98 | 0.14 | |||
| Hodgkin lymphoma | 0-9 | 0 | 0.0 (0.0-17.0) | 0 | 0.0 (0.0-1.8) |
| 10-19 | 0 | 0.0 (0.0-11.3) | 6 | 2.7 (1.2-6.0)c | |
| 20-29 | 0 | 0.0 (0.0-119.2) | 2 | 9.6 (2.4-38.2)c | |
| p trend | - | 0.001 | |||
| Leukaemia | 0-9 | 20 | 7.7 (5.0-11.9)e | 4 | 2.0 (0.8-5.4) |
| 10-19 | 7 | 4.7 (2.3-9.9)d | 3 | 2.7 (0.9-8.4) | |
| 20-29 | 0 | 0.0 (0.0-32.6) | 0 | 0.0 (0.0-30.5) | |
| p trend | 0.16 | 0.98 | |||
| Initial diagnosis cancer |
Initial diagnosis non-cancer |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cancer mortality |
Cancer incidenceb |
Cancer mortality |
Cancer incidenceb |
||||||
| Cancer site (outcome) | Duration since first treatment (years) | n | SMRa (95% CI) | n | SIR (95% CI) | n | SMRa (95% CI) | n | SIR (95% CI) |
| Colorectal cancer | 0-9 | 1 | 74.4 (1.9-414.4)c | 2 | 24.4 (6.1-97.6)d | 0 | 0.0 (0.0-43.5) | 0 | 0.0 (0.0-9.4) |
| 10-19 | 0 | 0.0 (0.0-85.4) | 0 | 0.0 (0.0-24.7) | 1 | 3.1 (0.1-17.1) | 2 | 2.3 (0.6-9.3) | |
| 20-29 | 0 | 0.0 (0.0-319.9) | 0 | 0.0 (0.0-96.7) | 0 | 0.0 (0.0-52.6) | 0 | 0.0 (0.0-19.7) | |
| p trend | 0.11 | 0.06 | 0.97 | 0.75 | |||||
| Bone cancer | 0-9 | 7 | 47.8 (22.8-100.3)e | 3 | 14.8 (4.8-45.9)d | 2 | 2.2 (0.5-8.7) | 2 | 2.3 (0.6-9.0) |
| 10-19 | 1 | 13.3 (0.3-74.2) | 2 | 24.7 (6.2-98.8)d | 2 | 3.5 (0.9-13.8) | 2 | 3.9 (1.0-15.8) | |
| 20-29 | 0 | 0.0 (0.0-915.9) | 0 | 0.0 (0.0-561.5) | 0 | 0.0 (0.0-131.2) | 0 | 0.0 (0.0-101.8) | |
| p trend | 0.19 | 0.82 | 0.76 | 0.74 | |||||
| Melanoma | 0-9 | 0 | 0.0 (0.0-178.8) | 1 | 4.3 (0.1-23.9) | 0 | 0.0 (0.0-29.4) | 1 | 0.9 (0.0-5.3) |
| 10-19 | 0 | 0.0 (0.0-65.6) | 2 | 3.8 (0.9-15.1) | 1 | 2.4 (0.1-13.6) | 5 | 1.6 (0.7-3.9) | |
| 20-29 | 0 | 0.0 (0.0-299.2) | 2 | 18.9 (4.7-75.6)c | 0 | 0.0 (0.0-53.5) | 1 | 1.8 (0.0-9.8) | |
| p trend | - | 0.22 | 0.86 | 0.64 | |||||
| CNS | 0-9 | 118 | 503.2 (420.1-602.7)e | 11 | 29.3 (16.2-52.9)e | 2 | 1.2 (0.3-4.8) | 4 | 2.0 (0.7-5.2) |
| 10-19 | 31 | 207.3 (145.8-294.8)e | 11 | 43.4 (24.0-78.3)e | 1 | 0.9 (0.0-4.8) | 2 | 1.3 (0.3-5.1) | |
| 20-29 | 4 | 155.1 (58.2-413.3)e | 1 | 29.8 (0.8-165.9) | 0 | 0.0 (0.0-24.8) | 0 | 0.0 (0.0-20.6) | |
| p trend | <0.001 | 0.43 | 0.64 | 0.47 | |||||
| Thyroid | 0-9 | 0 | 0.0 (0.0-10035.5) | 5 | 51.3 (21.4-123.3)e | 0 | 0.0 (0.0-1472.7) | 1 | 2.2 (0.1-12.3) |
| 10-19 | 1 | 772.6 (19.6-4304.7)d | 4 | 22.5 (8.4-59.9)e | 0 | 0.0 (0.0-320.7) | 1 | 1.0 (0.0-5.3) | |
| 20-29 | 0 | 0.0 (0.0-10488.7) | 1 | 28.4 (0.7-158.1) | 0 | 0.0 (0.0-1607.5) | 0 | 0.0 (0.0-19.3) | |
| p trend | 0.99 | 0.29 | - | 0.41 | |||||
| Hodgkin lymphoma | 0-9 | 0 | 0.0 (0.0-123.2) | 0 | 0.0 (0.0-9.3) | 0 | 0.0 (0.0-19.8) | 0 | 0.0 (0.0-2.3) |
| 10-19 | 0 | 0.0 (0.0-95.4) | 1 | 2.9 (0.4-20.7) | 0 | 0.0 (0.0-12.8) | 5 | 2.6 (1.1-6.3)c | |
| 20-29 | 0 | 0.0 (0.0-887.9) | 0 | 0.0 (0.0-116.5) | 0 | 0.0 (0.0-137.7) | 2 | 11.3 (2.8-45.1)c | |
| p trend | - | 0.40 | - | 0.002 | |||||
| Leukaemia | 0-9 | 18 | 55.7 (35.1-88.4)e | 3 | 8.8 (2.9-27.4)c | 2 | 0.9 (0.2-3.5) | 1 | 0.6 (0.0-3.4) |
| 10-19 | 5 | 29.9 (12.5-71.9)e | 1 | 6.2 (0.2-34.3) | 2 | 1.5 (0.4-6.1) | 2 | 2.1 (0.5-8.4) | |
| 20-29 | 0 | 0.0 (0.0-239.3) | 0 | 0.0 (0.0-186.3) | 0 | 0.0 (0.0-37.7) | 0 | 0.0 (0.0-36.5) | |
| p trend | 0.10 | 0.63 | 0.74 | 0.47 | |||||
SMR = Standardised mortality ratio; SIR = Standardised incidence ratio; CI = Confidence interval
Using Swiss rates as expecteds for Germany, and Belgian rates as expecteds for both France and the Netherlands, for cancer sites for which sufficient detail was not available from home-country national rates.
Excluding France, Germany and Italy.
p<0.05
p<0.01
p<0.001
SMR = Standardised mortality ratio; SIR = Standardised incidence ratio; CI = Confidence interval
Using Swiss rates as expecteds for Germany, and Belgian rates as expecteds for both France and the Netherlands, for cancer sites for which sufficient detail was not available from home-country national rates.
Excluding France, Germany and Italy
p<0.05
p<0.01
p<0.001
There was no indication that risk related to cumulative GH dose (Supplemental Table 3), except that CNS tumour mortality diminished with increasing dose in the cohort overall and bone cancer mortality diminished with increasing dose in patients with an initial diagnosis of cancer.
Discussion
GH therapy is widely used and a range of biological data suggest that hormone levels in the GH-IGF1 axis may affect cancer risks(1, 2). It is therefore important clinically to determine whether cancer risks are raised by GH treatment. Information on this has been very limited, however. Generally, the larger studies have had short follow-up(10–13, 16) and the studies with long follow-up have been small. With the exception of two cohorts of patients treated with p-hGH(6, 18, 19), the only studies with mean follow-up of >6 years have been a cohort examining solely leukaemia as an outcome(5), and cohorts of a few hundred GH-treated cancer patients(7, 20).
This paucity of large-scale long-term follow-up is important because with few exceptions (e.g. certain cancers after immunosuppression, several causes of leukaemia(21)), most known causes of cancer act after a lag period of many years and hence short-term follow-up would give little information regarding risks after likely lag periods. Furthermore, most cancer types occur almost entirely in adulthood, so information on short-term cancer risks after childhood treatment (i.e. when the patient is still young) would be virtually uninformative about risks of these malignancies. There have been almost no published data by duration of follow-up(12), however, and none beyond 10 years. In our cohort, for patients with an initial diagnosis of cancer there was no indication of rising risk of cancer incidence or mortality with longer follow-up. For patients with initial non-cancer diagnoses, however, cancer incidence was significantly raised beyond 20 years of follow-up and there was a highly significant increase in incidence with longer follow-up for Hodgkin lymphoma incidence. For patients with isolated growth failure, separately, there were inconsistent findings based on modest numbers: significant trends of incidence with duration of treatment and time since first treatment, but not for mean dose (p=0.52), not clearly for cumulative dose (p=0.08), and not for cancer mortality. For Turner syndrome separately, there were no consistent or significant relations.
Potentially, the cancer risks in GH-treated patients could reflect the underlying condition leading to GH treatment, and the non-GH treatments (e.g. radiotherapy) given for this condition, as well as the effect of GH itself. This is clearest for patients receiving GH because of malignancy or chromosomal instability syndromes, but applies to some extent to virtually every underlying diagnosis, e.g. hypopituitarism(9, 22) or Turner syndrome(23). The underlying diagnoses are numerous and heterogeneous, and we do not hold information on the non-GH treatments, so we cannot give explanation of the results in relation to specific confounders. In principle, this might be overcome by comparing GH-treated patients with others with the same condition who had not received GH. This has been done in some studies for patients with underlying cancer(7, 24, 25). We did not have comparison data for untreated patients, however. Furthermore this would not entirely solve the problem since selective factors leading to GH treatment may themselves cause differences in cancer risk between treated and untreated groups. Our analyses using general population rates to generate expectations need to be interpreted cautiously in this light.
High completeness of follow-up to a fixed end-date is critical if cohort study results are to be valid, especially for safety assessment because deficient follow-up can artefactually produce an apparent lack of raised risk. Previous large r-hGH cohorts with one exception(16) have censored follow-up at last clinic visit not at a fixed end-date(10, 12, 13, 26). Since frequency of medical contact depends on health status, this could be seriously biased. The large post-marketing surveillance studies(10–12, 14, 16) have also depended on active reporting of cancers to the pharmaceutical company by physicians, the completeness of which is unknown, especially after patients leave paediatric endocrine care. Our follow-up for mortality and cancer incidence, like that in the p-hGH cohorts(18, 19), was to a fixed end-date, and had high completeness through routine national data systems(15).
If GH affects cancer risk, one might expect dose- and duration-response relationships for risk. No data have been published on this, however: only statements of no relation for leukaemia in one cohort(5) and for overall cancer risk in another(19). Our results did not suggest an increase in cancer mortality or incidence risks with increasing cumulative GH dose: apparent decreases in risk with higher doses appeared to be largely or entirely an artefact of confounding by initial diagnosis, and apparent increases with shorter duration an artefact of stopping GH treatment because of cancer occurrence (see Results).
However there was a significant increase in cancer mortality with increasing mean daily r-hGH dose for patients with previous cancer. Interpretation is uncertain. Favouring a causal explanation, the association was highly significant so very unlikely to be due to chance; the results did not appear to be due to potentially confounding treatments such as craniospinal radiotherapy, as far as data were available to assess this; and the lack of similar associations for cancer incidence or for patients with initial non-cancer diagnoses could be plausible if GH affects cancer survival rather than cancer occurrence. Against a causal explanation is the lack of relation of risk to increasing cumulative GH dose or treatment duration, and the existence of potential for confounding by underlying disease or non-GH treatment factors not captured by the relatively crude measures of these we had available. Further data are needed to resolve whether high GH doses affect cancer survival.
For the three cancer sites for which there is most published support for an association with IGF1 levels, colorectum, breast and prostate(2), the evidence from our cohort and previously(9, 10, 18, 19) is too sparse to reach a conclusion on relations to GH treatment, reflecting the rarity of these cancers at childhood and young adult ages. Concerns about leukaemia risk after GH were raised by case-reports(5) and a significantly raised risk in a cohort of p-hGH patients(6). However, others(19), and cohorts that excluded “high-risk” patients(11, 12), have found no excess, although several leukaemias occurred in the high-risk group. In our cohort there was a highly significant excess of leukaemia incidence and mortality confined to patients with prior cancer. The data overall suggest that GH treatment does not substantially increase leukaemia risk in patients without prior high risk, but leave it unclear whether risk is affected in high-risk individuals.
A cohort study of p-hGH patients found a significant excess of Hodgkin lymphoma (HL) mortality(19). The only other cohort findings have been a non-significant excess(18), or deficit(12), based on small numbers. In our cohort, 8 HL cases occurred, a non-significant excess, but there was a highly significant trend with longer follow-up (although no trend with GH dose). The previous studies finding raised HL risk have been those with longest follow-up, so it remains possible that GH treatment at young ages may affect long-term HL risk.
Our cohort showed significant raised bone cancer incidence in GH-treated patients, both those with and without an initial cancer diagnosis. Bone cancer has been one of the most common second primaries in previous childhood GH-treated cohorts(18, 24). The few risk analyses have been non-significant, based on very small numbers(12, 19). The three bone cancer deaths after isolated growth failure in our data were included in a French SAGhE publication (27), but the other bone cancer deaths, and all of the incident cases of bone cancer, were not. There was no evidence in our data that bone cancer risk was related to GH dose, but the significant bone cancer excess in both cancer and non-cancer patients, and the anatomical and age distributions of bone cancer and association with height in the general population(28), argue that the relation needs re-examination in future data.
Bladder cancer risk was greatly and significantly (P=0.002) raised in patients without previous cancer, but based on small numbers. There appear to be no previous data about this and until such data are available, little weight can be put upon it.
We found significant excesses of incidence and mortality from cancers of the soft tissue, kidney, CNS and thyroid, and of incidence of melanoma and cancer of the ovary and mortality from NHL, all restricted to patients with cancer as the reason for GH treatment. Mainly, these are cancer sites for which raised risk of second cancer after radiotherapy and/or chemotherapy is well-known(29, 30) although this does not preclude GH raising the risks further. Melanoma, however, is not a tumour usually raised after radiotherapy and chemotherapy, although it has been in at least one instance(31). Only for CNS tumours are there previous data on risks as a second malignancy after GH, with no raised risk relating to GH(25). An excess of CNS tumours has been found in patients treated with GH who did not have previous malignancy(12).
Our study had weaknesses, detailed further in(15). We did not have information on GH treatment beyond paediatric ages, so we may have underestimated treatment duration for some patients with consequent dilution of any true effect of duration on cancer risk. Aggregation of data from eight countries adds the complexity of heterogeneity in patient mix and treatments, but without such a pooling the large numbers and hence statistical power of this study could not have been achieved. We did not have information on IGF-I levels. In addition, although our follow-up was much longer than in previous large cohort studies of childhood-treated patients(10–14), it still included few person-years beyond age 35, and hence had limited power for cancers prevalent at middle ages and older (and indeed for cancers prevalent at younger ages, even though the cohort is large, numbers of cases are often not large and therefore confidence intervals tended to be wide). Interpretation of our data must therefore be cautious, and future longer follow-up of the cohort will be important. In Germany and Italy, ascertainment of GH-treated patients may have been substantially incomplete, in Italy there was incompleteness in mortality follow-up, and in France and Italy regulations and reimbursement rules gave incentives to prescribers to overstate isolated growth failure as an underlying diagnosis. These weaknesses seem unlikely to have biased the cancer analyses presented, however, since removal of France, Germany and Italy from the analyses did not alter the conclusions.
Overall, our study, with much larger numbers of GH-treated patients followed long-term than previously, does not suggest that GH treatment affects the risk of cancer incidence or mortality for the outcomes and durations of follow-up for which our analyses have substantial data. The lack of increased risk with greater cumulative dose or duration of treatment, key variables for which data have not been published previously, makes a causal relation less likely. There was also no clear raised risk in patients with isolated growth failure. These factors argue against a major risk of cancer overall within the length of follow-up currently available. Nevertheless, continued vigilance during follow-up is desirable, both because of the lack of data for longer follow-up than in our study, and because of the presence of some significant raised risks in the results. The rising cancer mortality with greater daily dose in cancer patients, however, leaves open the possibility of an effect on cancer survival. Also, the raised risks of bone and bladder cancers in patients with initial non-cancer diagnoses, and the rising risk of Hodgkin lymphoma with longer follow-up in such patients, leave possibilities of effects on site-specific cancer causation for which further data are needed.
Supplementary Material
Acknowledgements
We gratefully acknowledge the assistance in all countries of the GH patients and their families. We also thank: in Belgium, Colienne de la Barre, Christine Derycke, Siska Verlinde for collecting and verifying data, the members of the Belgian Society for Pediatric Endocrinology and Diabetology (BESPEED, previously BSGPE), the persons of the National Population Registry, the Federal and Regional Death Registries for their essential contribution sharing their data with us, and Professor Marc Maes; in France, Caroline Arnaud-Sarthou, Vean-Eng Ly, Florentia Kaguelidou for their invaluable contributions. Members of the French SAGhE study steering committee, Michel Andrejak (CHU d'Amiens), Juliette Bloch (IVS), Anne Castot (AFSSAPS), Jean-Louis Chaussain (Académie de Médecine), Dominique Costagliola (INSERM), Nathalie Hoog-Labouret (INCa), Carmen Kreft-Jaïs (AFSSAPS), Anne Périllat (DGS), Béatrice Porokhov (AFSSAPS), Catherine Rey-Quinio (AFSSAPS). We thank all the physicians involved in the follow-up of patients and in the review process at Association France Hypophyse and, in particular, those who contributed to the collection of data for the study; in Germany, the physicians and nurses caring for the GH patients and their families, and Mandy Vogel, Leipzig; in Italy, Flavia Pricci, Rome; Cristina Fazzini, Rome; Pietro Panei, Rome; Giuseppe Scirè, Rome; Gian Luigi Spadoni, Rome; Franco Cavallo, Torino; Patrizia Matarazzo, Torino; Gianluca Aimaretti, Torino; Laura Perrone, Napoli; in the Netherlands, we thank Sandra de Zeeuw and Eefje Koopman for verifying data. We thank the members of the Dutch Growth Hormone Advisory Board of the Dutch Society for Paediatrics and the registration teams of the Comprehensive Cancer Centre Netherlands and Comprehensive Cancer Centre South for the collection of data for the Netherlands Cancer Registry and the scientific staff of the Comprehensive Cancer Centre Netherlands. We also thank all paediatric endocrinologists of the Advisory Group Growth Hormone involved in GH treatment of children; in Sweden, the statistician team led by Nils-Gunnar Pehrsson, the Board of the National Growth Hormone Registry; Kerstin Albertsson-Wikland, Stefan A Aronson, Peter Bang, Jovanna Dahlgren, Maria Elfving, Jan Gustafsson, Lars Hagenäs, Anders Häger, Sten A Ivarsson, Berit Kriström, Claude Marcus, Christian Moell, Karl Olof Nilsson, Svante Norgren, Martin Ritzen, Johan Svensson, Torsten Tuvemo, Ulf Westgren, Otto Westphal, Jan Åman, and all paediatric endocrinologists and paediatricians involved in the GH treatment of children for their assistance in collecting and verifying data; in Switzerland, Rahel Kuonen, Bern; U. Eiholzer, Zurich; J. Girard, Basel; S. Gschwendt, Zug; M. Hauschild, Lausanne; M. Janner, Bern; B. Kuhlmann, Aarau; D. L‘Allemand, St. Gallen; U. Meinhardt, Zurich; P-E. Mullis, Bern; F. Phan-Hug, Lausanne; E. Schoenle, Zurich; M. Steigert; Chur; U. Zumsteg, Basel; R. Zurbruegg, Bienne; in the UK, the members of the British Society for Paediatric Endocrinology and Diabetes in the 21 historic UK Growth centres for tracking down and contacting former and current patients, in particular; Justin Davies, Southampton; Jo Blair, Liverpool; Fiona Ryan, Oxford; Liz Crowne, Bristol; Savitha Shenoy, Leicester; Carlo Acerini, Cambridge; Jeremy Kirk, Birmingham; Tim Cheetham, Newcastle; Leo Dunkel, London Barts; Tabitha Randell, Nottingham; Sabah Alvi, Leeds; Neil Wright, Sheffield; Justin Warner, Cardiff; Amalia Mayo, Aberdeen; Guftar Shaikh, Glasgow; Louise Bath, Edinburgh; the Medicines for Children Research Network and the Scottish Medicines for Children Network who supported the network of research nurses; the former Pfizer UK KIGS team for assistance in making electronic database records of treated patients available to the local investigators at each of the UK Growth Centres, and Sharon Squires, London.
Funding:
This work was supported in all countries by the European Union (HEALTH-F2-2009-223497); in addition, there was funding in Belgium from the Belgian Study Group for Paediatric Endocrinology (BSGPE); in France, the Agence Française de Sécurité Sanitaire des Produits de Santé (AFSSAPS, the French drug safety agency), Direction Générale de la Santé (DGS, French Ministry of Health), Institut National du Cancer (INCa); in Italy, from the EC European Commission (FP7-HEALTH-2007-3. 1-5); in Sweden, from the Swedish Research Council, Regional University Hospital Grants (ALF), the Swedish Cancer Society, and the Swedish Childhood Cancer Foundation; in Switzerland from the Swiss Cancer League (KLS-02586-02-2010; KLS-2948-02-2012), Pfizer AG, Novo Nordisk Pharma AG, Sandoz Pharmaceuticals AG; and in the UK, University College London, from the UK Child Growth Foundation.
Footnotes
Disclosure summary: PC is a consultant for MerckSerono. LS is a member of the NordiNet International Study Committee (Novo Nordisk) and recipient of investigator-initiated independent research awards from MerckSerono (GGI award), NovoNordisk and Pfizer, and lecture honoraria from Ferring, Novo Nordisk, Pfizer, and Merck Serono. J-CC is an investigator in clinical trials using GH sponsored by Pfizer and by Lilly and in post-marketing studies using several brands of GH, support for travel to international meetings from several GH manufacturers. SC has received lecture fees from Ipsen, Merck-Serono, Novo Nordisk and Pfizer, research grants from Merck Serono, Eli Lilly and Pfizer, support for travel to international meetings from several GH manufacturers and is member of PRISM advisory board (Ipsen). WK is a member of the Novo Nordisk Advisory Board on GH treatment, and has received lecture and consultancy honoraria from Pfizer, Ipsen and Sandoz. AT has received lecture fees from Pfizer. All other authors declare that they have no conflict of interest.
References
- 1.Swerdlow AJ. Does growth hormone therapy increase the risk of cancer? Nat Clin Pract Endocrinol Metab. 2006 Oct;2(10):530–1. doi: 10.1038/ncpendmet0295. [DOI] [PubMed] [Google Scholar]
- 2.Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. 2011 Jan;7(1):11–24. doi: 10.1038/nrendo.2010.171. [DOI] [PubMed] [Google Scholar]
- 3.Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 1998 Aug;83(8):2730–4. doi: 10.1210/jcem.83.8.5007. [DOI] [PubMed] [Google Scholar]
- 4.Baris D, Gridley G, Ron E, et al. Acromegaly and cancer risk: a cohort study in Sweden and Denmark. Cancer Causes Control. 2002 Jun;13(5):395–400. doi: 10.1023/a:1015713732717. [DOI] [PubMed] [Google Scholar]
- 5.Nishi Y, Tanaka T, Takano K, et al. Recent status in the occurrence of leukemia in growth hormone-treated patients in Japan. GH Treatment Study Committee of the Foundation for Growth Science, Japan. J Clin Endocrinol Metab. 1999 Jun;84(6):1961–5. doi: 10.1210/jcem.84.6.5716. [DOI] [PubMed] [Google Scholar]
- 6.Fradkin JE, Mills JL, Schonberger LB, et al. Risk of leukemia after treatment with pituitary growth hormone. JAMA. 1993 Dec 15;270(23):2829–32. [PubMed] [Google Scholar]
- 7.Ergun-Longmire B, Mertens AC, Mitby P, et al. Growth hormone treatment and risk of second neoplasms in the childhood cancer survivor. J Clin Endocrinol Metab. 2006 Sep;91(9):3494–8. doi: 10.1210/jc.2006-0656. [DOI] [PubMed] [Google Scholar]
- 8.Deodati A, Ferroli BB, Cianfarani S. Association between growth hormone therapy and mortality, cancer and cardiovascular risk: systematic review and meta-analysis. Growth Horm IGF Res. 2014 Aug;24(4):105–11. doi: 10.1016/j.ghir.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Svensson J, Bengtsson BA, Rosen T, Oden A, Johannsson G. Malignant disease and cardiovascular morbidity in hypopituitary adults with or without growth hormone replacement therapy. J Clin Endocrinol Metab. 2004 Jul;89(7):3306–12. doi: 10.1210/jc.2003-031601. [DOI] [PubMed] [Google Scholar]
- 10.Child CJ, Zimmermann AG, Woodmansee WW, et al. Assessment of primary cancers in GH-treated adult hypopituitary patients: an analysis from the Hypopituitary Control and Complications Study. Eur J Endocrinol. 2011 Aug;165(2):217–23. doi: 10.1530/EJE-11-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab. 2010 Jan;95(1):167–77. doi: 10.1210/jc.2009-0178. [DOI] [PubMed] [Google Scholar]
- 12.Wilton P, Mattsson AF, Darendeliler F. Growth hormone treatment in children is not associated with an increase in the incidence of cancer: experience from KIGS (Pfizer International Growth Database) J Pediatr. 2010 Aug;157(2):265–70. doi: 10.1016/j.jpeds.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 13.Gaillard RC, Mattsson AF, Akerblad AC, et al. Overall and cause-specific mortality in GH-deficient adults on GH replacement. Eur J Endocrinol. 2012 Jun;166(6):1069–77. doi: 10.1530/EJE-11-1028. [DOI] [PubMed] [Google Scholar]
- 14.Hartman ML, Xu R, Crowe BJ, et al. Prospective safety surveillance of GH-deficient adults: comparison of GH-treated vs untreated patients. J Clin Endocrinol Metab. 2013 Mar;98(3):980–8. doi: 10.1210/jc.2012-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swerdlow AJ, Cooke R, Albertsson-Wikland K, et al. Description of the SAGhE Cohort: A Large European Study of Mortality and Cancer Incidence Risks after Childhood Treatment with Recombinant Growth Hormone. Horm Res Paediatr. 2015 Jul 23; doi: 10.1159/000435856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuffli GA, Johanson A, Rundle AC, Allen DB. Lack of increased risk for extracranial, nonleukemic neoplasms in recipients of recombinant deoxyribonucleic acid growth hormone. J Clin Endocrinol Metab. 1995 Apr;80(4):1416–22. doi: 10.1210/jcem.80.4.7714117. [DOI] [PubMed] [Google Scholar]
- 17.Breslow NE, Day NE. Statistical methods in cancer research. Volume I - The analysis of case-control studies. IARC Sci Publ. 1980;(32):5–338. [PubMed] [Google Scholar]
- 18.Mills JL, Schonberger LB, Wysowski DK, et al. Long-term mortality in the United States cohort of pituitary-derived growth hormone recipients. J Pediatr. 2004 Apr;144(4):430–6. doi: 10.1016/j.jpeds.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Swerdlow AJ, Higgins CD, Adlard P, Preece MA. Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959-85: a cohort study. Lancet. 2002 Jul 27;360(9329):273–7. doi: 10.1016/s0140-6736(02)09519-3. [DOI] [PubMed] [Google Scholar]
- 20.Mackenzie S, Craven T, Gattamaneni HR, Swindell R, Shalet SM, Brabant G. Long-term safety of growth hormone replacement after CNS irradiation. J Clin Endocrinol Metab. 2011 Sep;96(9):2756–61. doi: 10.1210/jc.2011-0112. [DOI] [PubMed] [Google Scholar]
- 21.Linet MS, Devesa SS, Morgan GR. The Leukemias. Third ed. Oxford: Oxford University Press; 2006. [Google Scholar]
- 22.Stochholm K, Gravholt CH, Laursen T, et al. Mortality and GH deficiency: a nationwide study. Eur J Endocrinol. 2007 Jul;157(1):9–18. doi: 10.1530/EJE-07-0013. [DOI] [PubMed] [Google Scholar]
- 23.Schoemaker MJ, Swerdlow AJ, Higgins CD, Wright AF, Jacobs PA, Group UKCC Cancer incidence in women with Turner syndrome in Great Britain: a national cohort study. Lancet Oncol. 2008 Mar;9(3):239–46. doi: 10.1016/S1470-2045(08)70033-0. [DOI] [PubMed] [Google Scholar]
- 24.Sklar CA, Mertens AC, Mitby P, et al. Risk of disease recurrence and second neoplasms in survivors of childhood cancer treated with growth hormone: a report from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2002 Jul;87(7):3136–41. doi: 10.1210/jcem.87.7.8606. [DOI] [PubMed] [Google Scholar]
- 25.Patterson BC, Chen Y, Sklar CA, et al. Growth hormone exposure as a risk factor for the development of subsequent neoplasms of the central nervous system: a report from the childhood cancer survivor study. J Clin Endocrinol Metab. 2014 Jun;99(6):2030–7. doi: 10.1210/jc.2013-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Bunderen CC, van Nieuwpoort IC, Arwert LI, et al. Does growth hormone replacement therapy reduce mortality in adults with growth hormone deficiency? Data from the Dutch National Registry of Growth Hormone Treatment in adults. J Clin Endocrinol Metab. 2011 Oct;96(10):3151–9. doi: 10.1210/jc.2011-1215. [DOI] [PubMed] [Google Scholar]
- 27.Carel JC, Ecosse E, Landier F, et al. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab. 2012 Feb;97(2):416–25. doi: 10.1210/jc.2011-1995. [DOI] [PubMed] [Google Scholar]
- 28.Mirabello L, Pfeiffer R, Murphy G, et al. Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes Control. 2011 Jun;22(6):899–908. doi: 10.1007/s10552-011-9763-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Leeuwen FE, Travis LB. Second Cancers. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 30.Curtis RE, Freedman DM, Ron E, et al. New malignancies among cancer survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Cancer institute; 2006. NIH Publ. No 05-5302. [Google Scholar]
- 31.Swerdlow AJ, Higgins CD, Smith P, et al. Second cancer risk after chemotherapy for Hodgkin's lymphoma: a collaborative British cohort study. J Clin Oncol. 2011 Nov 1;29(31):4096–104. doi: 10.1200/JCO.2011.34.8268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.