Abstract
Rationale
Compulsive cocaine use is a key feature of cocaine addiction and understanding the factors that promote the development of such a behavior will provide important insights into the mechanism of cocaine addiction and is essential for the clinical management of the disorder.
Objectives
This study aimed to determine how the preexisting compulsive reward-seeking behavior is related to the development of compulsive cocaine-seeking behavior in male and female rats and the potential impact of the reward value and estrous cycle on such behaviors.
Methods
Adult male and female Wistar rats were first trained to self-administer sucrose pellets under a chained schedule and then the intensity-response effects of footshock punishment on sucrose SA reinforced by different values of sucrose were measured. Subsequently, the same rats went on to self-administer intravenous cocaine and the punishment intensity-response effects on cocaine SA reinforced by different doses of cocaine were similarly determined. For the female rats, the measurements were made during different phases of the estrous cycle.
Results
The rats showed a wide range of levels of the compulsive behaviors despite the similar training history. Surprisingly, the compulsive sucrose-seeking behavior did not predict the compulsive cocaine-seeking behavior in either sex. Increasing cocaine dose significantly increased the compulsive cocaine-seeking behavior in the female but not male rats. Estrous cycle did not have impact on the compulsive behaviors.
Conclusion
Preexisting differences in compulsive sucrose-seeking behavior does not predict compulsive cocaine-seeking behavior. Compulsive cocaine-seeking behavior is influenced by cocaine dose but not estrous cycle in the female rats.
Keywords: cocaine, self-administration, compulsive behavior, motivation, estrous cycle
Introduction
Recreational cocaine use gradually becomes out of control and ultimately leads to cocaine addiction in some individuals (Gawin 1991; Wagner and Anthony 2002). Understanding the factors facilitating such a transition provides important clues on the underlying mechanism and has important clinical implications. A core feature of cocaine addiction is compulsive cocaine use, defined as the continued use despite the negative consequences (American Psychiatric Association 2013). Significant progress has been made in understanding the factors contributing to the development of such a behavior by the preclinical studies on male animals. For example, the duration of cocaine use appears to be an important factor. After a prolonged period (> 70 but not < 40 days) of cocaine self-administration (SA), a subpopulation of rats showed addiction-like behaviors including the continued cocaine SA in the face of electric footshock punishment (Deroche-Gamonet et al. 2004). Besides the duration, the amount of cocaine allowed to consume in daily sessions is also important. The rats self-administering cocaine in a daily extended (6 hr) but not limited (1 hr) session for about two weeks are more likely to continue cocaine SA in the face of footshock punishment (Pelloux et al. 2007; Xue et al. 2012). In addition, the pattern that cocaine is self-administered appears to be important too. Using an intermittent daily access procedure to generate spiking concentrations in the brain, Kawa et al (Kawa et al. 2016) demonstrate that more rats develop compulsive cocaine SA. Note that the development of the compulsive behavior is not due to impairment of fear conditioning or pain perception (Pelloux et al. 2007). Neither can the stronger habit conditioning under the extended daily access condition explain the compulsive behavior (Jonkman et al. 2012). One important conclusion from these studies is that extensive cocaine exposure is required but not sufficient to promote the compulsive behavior.
Besides the impact of cocaine exposure, sex is another important factor involved in the risk of cocaine addiction. Women show faster escalation of cocaine intake, a shorter time from initial use to seek treatment, and higher total consumption by the time when they seek treatment (Becker and Hu 2008; becLozano et al. 2008; Greenfield et al. 2010; Griffin et al. 1989; Haas and Peters 2000). The faster transition in females has also been reported for several other drugs including alcohol, nicotine, and opioids (Randall et al. 1999; Westermeyer and Boedicker 2000). Understanding the factors that facilitate the development of cocaine addiction in females may provide important information into the mechanism of cocaine addiction and shed lights on the sex-specific mechanisms involved.
Compulsive cocaine use reflects the decreased regulation by the negative consequences of cocaine use. Such a decrease may result from the deficit in the brain punishment function that mediates the negative consequence-induced behavioral inhibition. The deficit could result from chronic cocaine use-induced impairment of or preexisting deficit in the punishment function. A recent study demonstrates that cocaine SA recovers more rapidly in the female rats selectively bred for high intake of saccharin after histamine is co-administered with cocaine (Holtz et al. 2013). Because histamine can act as a punisher (Negus 2005; Woolverton et al. 2012), these data suggest that individuals with such a trait may have a higher risk to develop compulsive cocaine SA. The current study aimed to examine whether the preexisting differences in the punishment function can predict compulsive cocaine-seeking behavior. To characterize the preexisting punishment function, the intensity-response effects of footshock punishment on sucrose SA were determined in the cocaine-naïve rats. To determine whether the preexisting differences in the function can predict the later development of compulsive cocaine-seeking behavior, the same rats were trained to self-administer intravenous cocaine and subsequently, the punishment intensity-response effects on cocaine SA were determined. In addition, the impact of reward or drug value on the compulsive sucrose- and cocaine-seeking was also determined. Given that the estrous cycle has impact on some of behavioral effects of cocaine (Becker and Koob 2016; Carroll and Anker 2010), the compulsive behaviors were also determined in the different estrous phases to determine whether the compulsive behaviors vary with the estrous cycle.
Materials and Methods
Subjects and Drugs
The outbred Wistar rats (Male: 300–390 g, n = 41; Female: 240–320 g, n = 32, Charles River) were housed individually in plastic home cages in a temperature- and humidity-controlled colony room on a 12-h reverse light-dark cycle (lights off at 08:00). Part of the data from the male rats were previously published (Datta et al. 2018) and used here for comparison. Experiments were conducted during the dark phase (between 09:00 and 18:00). Cocaine hydrochloride (the National Institute on Drug Abuse, Bethesda, MD) was dissolved in physiological saline to prepare the solutions with concentrations of 2.5, and 5 mg/ml (salt), respectively. All procedures followed the Guidelines for the Care and Use of Laboratory Animals (National Research Council 2011) and were approved by University of Tennessee Health Science Center Animal Care and Use Committee.
Sucrose SA
The rats were placed on a restricted diet to reach ~85% of free feeding body weights and thereafter, fed daily with ~20 g and ~15 g regular rat chow for male and female rats, respectively. They were trained to self-administer sucrose pellets (45 mg, Research Diet, New Brunswick, NJ) under the chained schedule (Olmstead et al. 2000) in a standard operant conditioning chamber equipped with two metal levers (Med Associates Inc., St. Albans, VT). The chained schedule requires sequential responses on the two levers, designated as the seeking and taking levers, respectively, to earn sucrose pellets. Only one lever is available at one time and pressing the seeking lever leads to lever retraction and access to the taking lever after a delay. Pressing the taking lever results in delivery of one sucrose pellet and lever retraction followed by next trial after a timeout period. Thus, the fixed-ratio 1 (FR1) schedule is effective for both levers (chained FR1FR1). Under such a schedule, reward-seeking and reward-taking behaviors can be investigated separately (Pelloux et al. 2007; Vanderschuren and Everitt 2004; Xue et al. 2012). In this study, 2-second delay between extensions of the seeking- and taking levers and 5-second timeout after each reinforcement were used in daily 30-minute sessions. The session ended either when 30 minutes had passed or when 100 (male) or 60 (female) pellets had been earned, whichever occurred first. Training continued until the rats reached the criteria: the rates of reinforcement varied < 10% for three consecutive sessions.
Sucrose-seeking behavior in the face of footshock punishment
To determine the preexisting differences in the punishment function, the intensity-response effects of footshock punishment on the sucrose-seeking behavior were measured before the rats were exposed to cocaine SA. The tests were conducted after the training criteria were reached and a minimum of 10 training sessions. The footshock was delivered to the grid floor of the chamber immediately after the seeking but not the taking responses. The four different levels of current intensity including 0.2, 0.3, 0.4, and 0.5 mA with a duration of 0.5 second were tested in a session consisting of four 15-min blocks. The first response on the seeking lever marked the onset of the session and the current intensity was increased in each subsequent block beginning from 0.2 mA. To determine whether an increase in reward value alters the punished sucrose-seeking behavior, the intensity-response effects of punishment were determined for one and three sucrose pellets in different test sessions in the same rats. To minimize the potential impact of satiation on performance, the maximum number of reinforcements was set for each block: 25 or eight reinforcements for one or three pellets, respectively. The chained schedule was used to avoid simultaneous delivery of sucrose and footshock that facilitates counterconditioning between reward and footshock (Dickinson and Pearce 1976; Pearce and Dickinson 1975). The counterconditioning may turn foot-shock into part of the stimuli predicting reward and consequently, reduce the effect of punishment. A recent study showed that the effect of counterconditioning can be minimized by temporally separating reward and punishment (Pelloux et al. 2007). In addition, the mechanisms underlying the reward-seeking and reward-taking behaviors are not entirely overlapping (Roberts et al. 2013), the goal of the current study was to determine how the seeking behavior is regulated. Thus, the seeking but not taking response was punished. Note that the during the punishment test, the pellets were delivered after each response on the taking-lever. This is in contrast to the schedule used by others (Pelloux et al. 2007; Vanderschuren and Everitt 2004).
The effects of punishment were determined for the female rats during the proestrus/estrus and diestrus using a mixed-subject design with estrous phase and value as the between- and within-subject factors, respectively. The vaginal smears were collected half hour before the test sessions during the dark phase of the light cycle and assessed by the vaginal cytology to determine the phase (Marcondes et al. 2002). The proestrus and estrus were combined because most females did not show sequential transition of the phase in the dark phase as reported before (Becker et al. 2005; Perry et al. 2015). The rats went through two normal estrous cycles (4–5 days per cycle) before testing. The order of the two tests with different values of sucrose in each phase was counterbalanced among the rats. Sucrose SA training continued between the tests to ensure the stable SA behavior before next test.
Surgery
After the sucrose tests, a subpopulation of the rats (Male: n = 30; Female; n = 25) randomly selected were catheterized under the anesthesia with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). The catheter was made of a ~12-cm polyurethane tubing (MRE-037; Braintree Scientific, Inc. MA, USA) connected to the rat vascular access button (VAB95BS; Instech, PA, USA). The button was placed subcutaneously in the mid-scapular region and the tubing was tunneled under the skin and inserted into the right external jugular vein with a length of ~3.5 cm. Buprenorphine (0.03 mg/kg, subcutaneously) was given for the post-surgical analgesia. Catheter patency was evaluated by injecting 0.1 ml Brevital (1%) through the catheters as necessary and loss of muscle tone within five seconds indicates patency of the catheter.
Cocaine SA
After 5- or 6-day recovery from surgery, the rats began to self-administer 0.125 mg/infusion of cocaine in daily 2-hour sessions under the same chained schedule as described for sucrose SA except that the timeout period was 20 seconds and a 10-second compound stimulus consisting of the two flashing cue lights above the levers and tone was presented after the onset of cocaine infusions. The session ended when two hours had passed or 80 infusions had been self-administered, whichever occurred first. The training was conducted 6–7 days per week until they reached the criterion: the number of cocaine infusions varied by < 20% for three consecutive sessions. Following the motivational and punishment tests for the dose of 0.125 mg (see below), the rats began to self-administer 0.25 mg/infusion of cocaine and were trained for another 10 sessions followed by the motivational and punishment tests for the dose of 0.25 mg. A volume of 0.05 ml of different concentrations of cocaine (2.5 mg/ml or 5 mg/ml) was infused over a period of 0.55 second to deliver 0.125 or 0.25 mg, respectively.
Motivation for cocaine
The cocaine-seeking behavior is regulated by the value of cocaine and aversive effects of punishment (Grove and Schuster 1974; Johanson 1977). The motivational value of the same dose of cocaine may be different for different individuals. Thus, individual differences in cocaine-seeking behavior in the face of punishment could be due to the differences in the motivation for cocaine. If so, individual differences in the level of motivation for cocaine should correlate with the punished cocaine-seeking behavior. To this end, the breakpoint (BP) defined as the last ratio finished under the progressive-ratio (PR) schedule of reinforcement (Roberts et al. 1989a) was determined in a 4-hour test session. The tests were conducted after the training criteria were reached and a minimum of 10 training sessions. The PR schedule was only effective for the seeking lever while the schedule for the taking lever remained the same as during cocaine SA. The PR session ended if the rats had failed to respond within a half hour or 4-hour had passed, whichever occurred first. In addition, we reasoned that increasing cocaine dose may enhance the punished cocaine-seeking behavior via increasing the motivation for cocaine. Thus, the BPs for two doses of cocaine were determined in the same rats. The order of the two tests was counterbalanced among the rats and the SA training continued between the tests to ensure the stable SA behavior before next test. The BPs were determined during the proestrus/estrus and diestrus using the mixed-subject design with estrous phase and value as the between- and within-subject factors, respectively
Cocaine-seeking behavior in the face of footshock punishment
The intensity-response effects of punishment on cocaine-seeking behavior were similarly determined as described for sucrose except that no maximum number of cocaine infusions was set and each block lasted half hour. Because the rats regulated the number of cocaine infusions in daily 2-hr sessions, there was less concern over the satiation effect from the early blocks on the performance of the later blocks. To determine whether an increase in cocaine dose affects the punished cocaine-seeking behavior, the intensity-response effects of punishment were sequentially determined in the same rats. Again, the mixed-subject design was used to determine the impact of the estrous cycle on the punished cocaine-seeking behavior.
Statistics
The rates of reinforcement were calculated as the number of reinforcements divided by the session durations. Because the durations of the SA and punishment sessions were different for cocaine and sucrose, the rates were scaled to half hour to facilitate comparisons. The rates from the SA training sessions before the punishment tests were used as the baseline controls. After normalizing the rates from each block of the punishment session to the baseline controls, the intensity-response curves were constructed and the area under the curves (AUCs) were calculated for each rat with the trapezoid method, a measurement of the effects of punishment. Note that the AUCs should be equal to 0.5 if the punishment has no effects. Because the rates of punished SA could be higher than the control at lower levels of the punishment intensity, it is possible that the AUCs are greater than 0.5. The mean of the AUCs were compared with the parametric (paired/unpaired t test) or nonparametric (paired Wilcoxon signed-rank/unpaired Mann-Whitney) test, respectively, depending on whether the data passed the normality test. Either the D’Agostino & Pearson omnibus or Shapiro-Wilk normality test was used for this purpose (the latter test was used if the sample size was too small for the former test).
A two-way analysis of variance (ANOVAs) was used to analyze the interaction between punishment intensity and reward value or estrous phase. The Pearson or Spearman correlation coefficients between punished sucrose- and cocaine-seeking behaviors were calculated. All the analyses were conducted with the GraphPad Prism version 7.03 (GraphPad Software, La Jolla California). The significance level was set at 0.05.
Results
Relationship between the Punished Sucrose- and Cocaine-seeking Behaviors
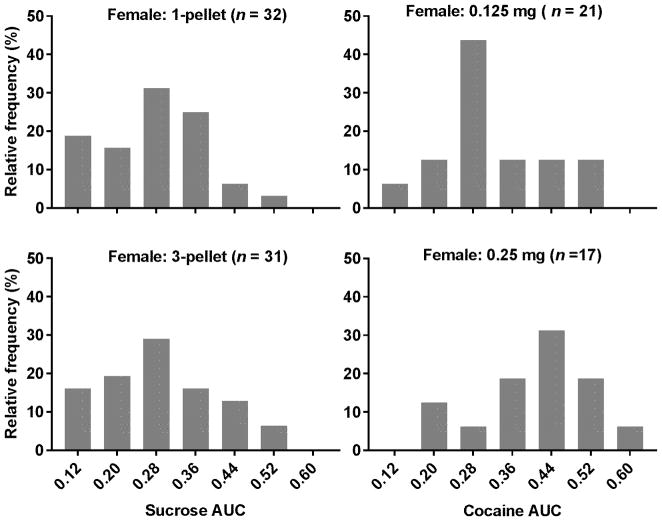
For the sucrose SA training, it took an average of 8 ± 0.80 (Mean ± SEM) sessions for the female rats to reach the training criteria. The average of SA rates for the first three sessions that met the training criteria was 171 ± 4.3 pellets/30 min. Individual rats showed different levels of punished sucrose-seeking behavior indicated by the differences in the AUCs as shown in the left column of Figure (Fig) 1. The AUCs ranged from 0.12 to 0.51 for one pellet and 0.11 to 0.54 for three pellets, respectively. The D’Agostino & Pearson omnibus test revealed no significant deviation from the normal distribution (1-pellet: p = 0.72; 3-pellet: p = 0.48). Similar results were also observed in the male rats (Datta et al. 2018).
Fig. 1.
Individual differences in compulsive sucrose- and cocaine-seeking behaviors. Upper Left Panel: Distribution of the AUCs for one sucrose pellet; Lower Left Panel: Distribution of the AUCs for three sucrose pellet; Upper Right Panel: Distribution of the AUCs for 0.125 mg/infusion of cocaine; Lower Right Panel: Distribution of the AUCs for 0.25 mg/infusion. The distributions did not significantly deviate from the normal distribution.
For the cocaine SA training, it took an average of 8 ± 1.1 sessions for the female rats to reach the training criteria. The average of SA rates for the first three sessions that met the training criteria was 10 ± 0.6 infusions/30 min. As shown in the right column of Fig. 1, the AUCs ranged from 0.14 to 0.54 and 0.20 to 0.62 for 0.125 and 0.25 mg/infusion, respectively. The distributions of the AUCs did not significantly deviate from the normal distribution (0.125 mg: n = 0.65; 0.25 mg: p = 0.98). The similar results were also observed in the male rats (Datta et al. 2018).
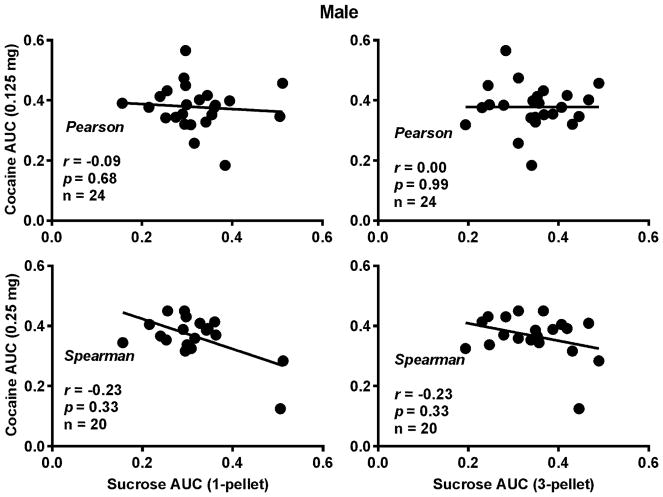
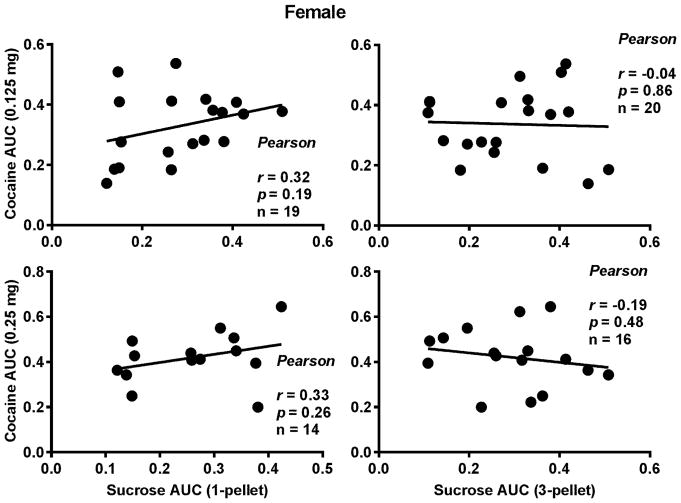
To determine whether the punished sucrose-seeking behavior can predict the punished cocaine-seeking behavior, the correlation coefficients between the AUCs of sucrose and cocaine were calculated. Note that there were two sucrose values and two doses of cocaine. Thus, the coefficients for the four possible combinations were calculated. As shown in Fig. 2 and 3, no significant correlations were found between the two in either sex.
Fig. 2.
Correlations between the compulsive sucrose- and cocaine-seeking behaviors in the male rats. Upper Left Panel: Correlation between one pellet and 0.125 mg/infusion of cocaine; Lower Left Panel: Correlation between one sucrose pellet and 0.25 mg/infusion of cocaine; Upper Right Panel: Correlation between three sucrose pellets and 0.125 mg/infusion of cocaine; Lower Right Panel: Correlation between three pellets and 0.25 mg/infusion of cocaine. No significant correlation was found.
Fig. 3.
Correlations between the compulsive sucrose- and cocaine-seeking behaviors in the female rats. Upper Left Panel: Correlation between one pellet and 0.125 mg/infusion of cocaine; Lower Left Panel: Correlation between one sucrose pellet and 0.25 mg/infusion of cocaine; Upper Right Panel: Correlation between three sucrose pellets and 0.125 mg/infusion of cocaine; Lower Right Panel: Correlation between three pellets and 0.25 mg/infusion of cocaine. No significant correlation was found.
Impact of Sucrose Value or Cocaine Dose on the Punished Sucrose- and Cocaine-seeking Behaviors
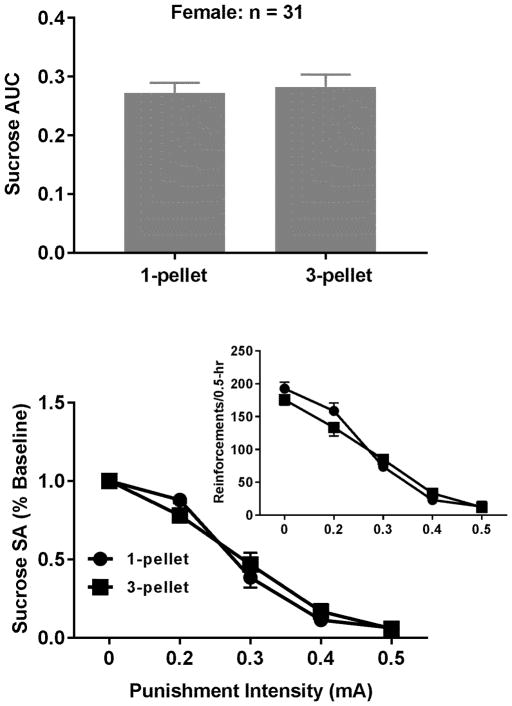
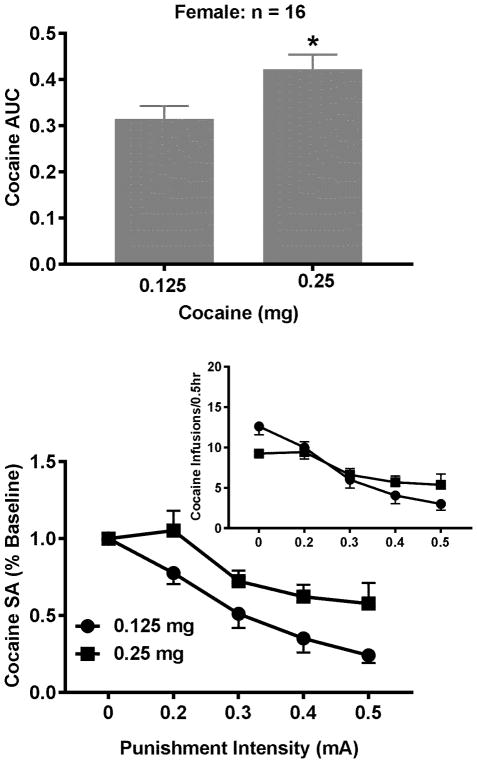
To determine the impact of sucrose value on the punished sucrose-seeking behavior, a paired t test revealed no significant differences in the AUCs between one and three pellets (Fig. 4, t = 0.35, p = 0.73). Similarly, the sucrose value had no impact on the punished sucrose-seeking behavior in the male rats (Datta et al. 2018). In contrast, the paired t test revealed significant differences in the AUCs between 0.125 and 0.25 mg of cocaine in the female rats (Fig. 5, t = 3.33, p < 0.01). Such an effect is sex-specific because the effect was not observed in the male rats (Datta et al. 2018). To determine whether the dose-induced increase in the punished cocaine-seeking behavior is due to the increased motivation for cocaine, the Wilcoxon test was used to determine the differences in the BPs between the two doses. Surprisingly, no significant increase was found although there was an increasing trend (p = 0.06). Given such a result, we cannot rule out the possibility that the elevated motivation for cocaine is involved in the increased compulsive behavior. To further address issue, we analyzed the correlational relationship between individual differences in the BPs and AUCs and the Spearman’s correlation between the two was not significant for either dose (data not shown). Similar results were also observed in the male rats (Datta et al. 2018).
Fig. 4.
Effects of sucrose value on the compulsive sucrose-seeking behavior. Upper Panel: The AUCs did not significantly differ between one and three sucrose pellets. The data are represented as means ± SEM. Lower Panel: The punishment intensity-response effects on sucrose SA. The two-way repeated measures ANOVA analysis on the normalized data revealed that there was no significant interaction between intensity and value but there was a significant main effect of intensity but not value. Inset: The intensity-response effects without normalization.
Fig. 5.
Effects of cocaine dose on the compulsive cocaine-seeking behavior. Upper Panel: The AUCs significantly increased by 0.25 mg/infusion of cocaine. Lower Panel: The punishment intensity-response effects on cocaine SA. The two-way repeated measures ANOVA analysis revealed that there was no significant interaction between intensity and dose but there were significant main effects of intensity and dose. Inset: The intensity-response effects without normalization. * P < 0.01 in comparison to the dose of 0.125 mg.
To further determine whether the punishment function varies with sucrose value or cocaine dose, the interaction between the two was analyzed. No significant interactions between sucrose value and punishment intensity were found in either males (Datta et al. 2018) or females (F4, 124 = 0.99, p = 0.42) although the main effect of intensity was significant for both males (Datta et al. 2018) and females (Fig. 4, F4, 124 = 150.0, p < 0.0001). Similarly, no significant interactions between cocaine dose and punishment intensity were found in either sex (female: F4, 60 = 0.99, p = 0.09). Although the main effect of intensity was observed in both males (Datta et al. 2018) and females (Fig. 5, F4, 60 = 29.91, p < 0.0001), the main effect of dose was only observed in the females (F1, 15 = 11.44, p < 0.01) but not males (Datta et al. 2018).
Impact of Estrous Cycle on the Punished Sucrose- and Cocaine-seeking Behaviors
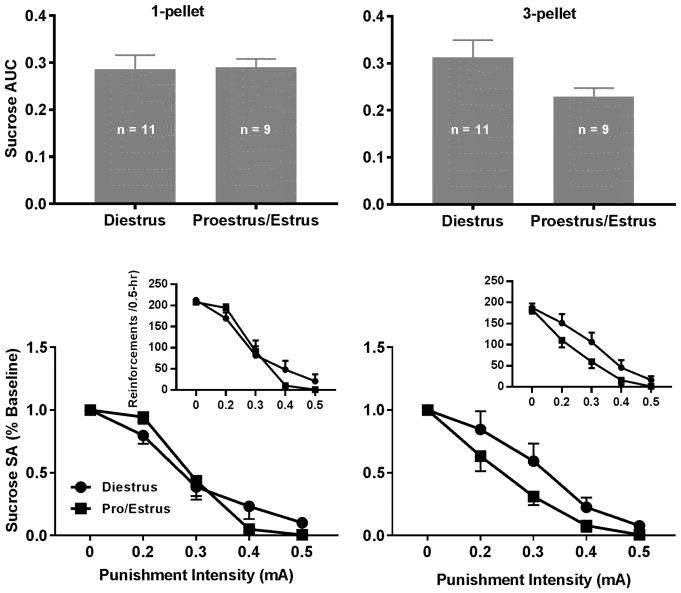
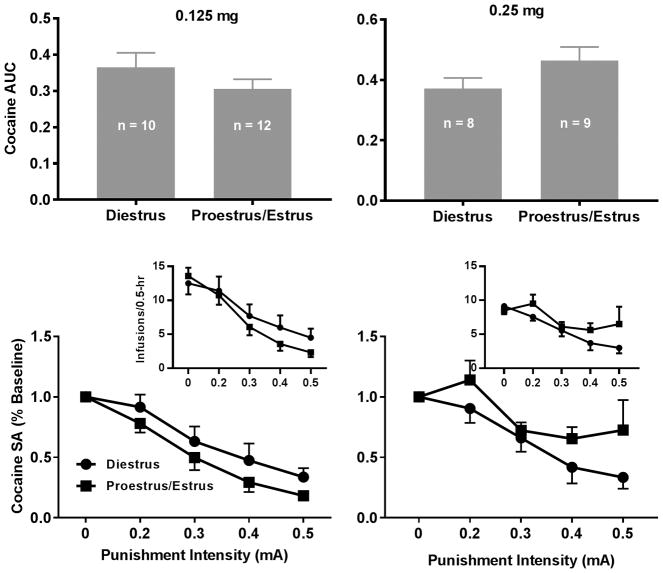
The unpaired t test revealed no significant differences in the AUCs between the phases for either sucrose value although the AUCs with three pellets appeared to be lower in the proestrus/estrus compared to diestrus (Fig. 6, 1-pellet: t = 0.92, p = 0.11; 3-pellet: t = 1.87, p = 0.08). The two-way mixed ANOVA revealed no significant main effects of phase (1-pellet: F1, 18 = 0.05, p = 0.83; 3-pellet: F1, 17 = 3.33, p = 0.09) although there were significant main effects of intensity (1-pellet: F4, 72 = 110.1, p < 0.0001; 3-pellet: F4, 68 = 48.71, p < 0.0001). No significant interaction between phase and intensity was found (1-pellet: F4, 72 = 2.45, p = 0.05; 3-pellet: F4, 68 = 0.95, p = 0.44). For the effects of the estrous cycle on the punished cocaine-seeking behavior, the unpaired t test did not reveal significant differences in the AUCs between the phases (Fig. 7, 0.125 mg: t = 1.27, p = 0.22; 0.25 mg: t = 1.61, p = 0.13). The two-way mixed ANOVA analysis did not reveal significant main effects of phase (0.125 mg: F1, 20 = 1.79, p = 0.20; 0.25 mg: F1, 13 = 2.18, p = 0.16) although there were significant main effects of intensity (0.125 mg: F4, 80 = 49.83, p < 0.0001; 0.25 mg: F4, 52 = 10.99, p < 0.0001). No significant interaction between phase and intensity was found (0.125 mg: F4, 80 = 0.64, p = 0.63; 0.25 mg: F4, 52 = 1.14, p = 0.35).
Fig. 6.
Effects of the estrous cycle on compulsive sucrose-seeking behavior. Upper Left Panel: The AUCs did not significantly differ between the two phases for one sucrose pellet. Lower Left Panel: The punishment intensity-response effects on sucrose SA. The two-way repeated measures ANOVA analysis revealed that there was no significant interaction between intensity and phase but there was a significant main effect of intensity but not phase. Upper Right Panel: The AUCs did not significantly differ between the two phases for three sucrose pellets although there was a decreasing trend. Lower Right Panel: The punishment intensity-response effects on sucrose SA. The two-way repeated measures ANOVA analysis revealed that there was no significant interaction between intensity and phase but there was a significant main effect of intensity but not phase. Insets: The intensity-response effects without normalization.
Fig. 7.
Effects of the estrous cycle on compulsive cocaine-seeking behavior. Upper Left Panel: The AUCs did not significantly differ between the two phases for 0.125 mg/infusion of cocaine. Lower Left Panel: The punishment intensity-response effects on cocaine SA. The two-way repeated measures ANOVA analysis revealed that there was no significant interaction between intensity and phase but there was a significant main effect of intensity but not phase. Upper Right Panel: The AUCs did not significantly differ between the two phases for 0.25 mg/infusion of cocaine. Lower Right Panel: The punishment intensity-response effects on cocaine SA. The two-way repeated measures ANOVA analysis revealed that there was no significant interaction between intensity and phase but there was a significant main effect of intensity but not phase. Insets: The intensity-response effects without normalization.
Discussion
Our results demonstrate that the AUCs of some rats are close to the theoretic maximum value of 0.5 after a short period of cocaine SA (0.125 mg for ~2-week) indicating that these rats are insensitive to punishment and therefore, highly compulsive. Importantly, the preexisting differences in the punishment function cannot predict the levels of the compulsive cocaine-seeking behavior in either sex. The impact of cocaine dose on such behavior appears to be sex-specific. Neither compulsive cocaine- nor sucrose-seeking behavior is influenced by the estrous cycle.
Identifying behavioral traits that predict the development of compulsive cocaine use will provide important information on the mechanisms of cocaine addiction. The previous studies demonstrate that impulsivity measured as the increased premature responses in the 5-Choice serial reaction time (5-CSRT) task reinforced by food pellets predicts the development of compulsive cocaine-seeking behavior (Belin et al. 2008; Dalley et al. 2007). These studies suggest that the deficit in the inhibitory control plays a role in the vulnerability to cocaine addiction. Because the inhibitory control is also involved in the punishment-induced inhibition of reward-seeking behaviors, we reasoned that the preexisting deficit in the punishment function may predict compulsive cocaine-seeking behavior. To this end, we first measured the effects of punishment on sucrose SA in cocaine-naïve rats as an indicator of the baseline punishment function and then on cocaine SA in the same rats after a period of cocaine SA. To our surprise, the compulsive sucrose-seeking behavior did not predict the compulsive cocaine-seeking behavior. One interpretation is that the effects of punishment on reward-seeking behavior depend on the nature or type of the reward (sucrose vs cocaine). Alternatively, repeated cocaine uses may alter the punishment function that is independent of the basal punishment function. These results suggest that the inhibitory processes triggered by the punishment and impulsive responses during the 5-CSRT task may be different. The inhibitory control is triggered by the loss of reward under the 5-CSRT task in contrast to punishment under which the presentation of the noxious stimuli triggers the process. Thus, the different neural circuits are likely involved in the inhibitory processes under the two conditions. Such a difference could explain the different results between the previous and our studies. Future studies are needed to address why the inhibitory process involved in loss of reward but not footshock punishment predicts the development of compulsive cocaine-seeking behavior.
The previous studies demonstrate that the addiction-like behaviors only occur after extensive experiences with cocaine SA (Belin et al. 2016; Piazza and Deroche-Gamonet 2014). The results from the current study, however, demonstrate that high levels of compulsive cocaine-seeking behavior occurred after a limited period of cocaine SA in both sexes. Recently, the intermittent access procedure used to generate the spiking levels of cocaine in the brain was shown to promote the compulsive cocaine SA despite the fact that the rats took much less cocaine compared with the extended access condition (Kawa et al. 2016). Together, these data suggest that the extensive cocaine experiences may facilitate but are not required for the development of compulsive cocaine SA. It is likely that the genetic factors play a role in the quick transition to cocaine addiction. Human studies demonstrate that the decreased function of dopamine DRD2/3 receptor is associated with cocaine and other drug addictions (Comings and Blum 2000; Comings et al. 1999). The TaqI A1 allele of the DRD2 genes is associated with the decreased expression of the receptor (Pohjalainen et al. 1998). The preclinical study demonstrates that the decreased level of Drd2/3 receptors in the nucleus accumbens is associated with impulsivity (Dalley et al. 2007) and the rats with high impulsivity develop the compulsive cocaine-seeking behavior more rapidly than the rats sampled from a population not selected for such a trait (Belin et al. 2008; Deroche-Gamonet et al. 2004). Thus, individuals with the decreased DRD2/3 receptor function in the nucleus accumbens may make transition to addiction more quickly. The mechanisms underlying the compulsive behavior after limited and extensive cocaine experience likely differ. Identifying the mechanisms involved in the rapid transition will provide new insights into cocaine addiction and have the potential to identify new molecular targets for medicine development.
Cocaine SA under the punishment condition is regulated by the motivation for cocaine and aversive effects of punishment (Grove and Schuster 1974; Johanson 1977). Thus, the compulsive cocaine-seeking behavior could result from either elevated motivation or impaired punishment function or a combination of the two. The previous studies demonstrate that compulsive cocaine SA can occur in the presence or absence of the elevated BPs (Deroche-Gamonet et al. 2004; Pelloux et al. 2007) suggesting that the two processes may not be necessarily related. The current study showed that increasing cocaine dose promoted the compulsive cocaine-seeking behavior in the female rats. This effect, however, cannot be attributed to the increase in the motivation induced by the higher dose of cocaine because the BPs were not significantly higher at the higher dose. It is not entirely surprising that we did not observe the increase in the BPs because the dose supporting the maximum BP varies among individuals and the doses larger or smaller than the optimum dose produce lower BPs (Roberts et al. 1989b). Indeed, the failure to increase the BPs at the higher dose of cocaine is due to the fact that the BPs were increased in some and decreased in others in the current study. To further address this issue, the correlational relationship between the motivation and compulsive behavior was analyzed and found not significant at either dose of cocaine. Together, these results support the idea that that the motivation may not be the driving force for the dose-related increase in the compulsive cocaine-seeking behavior observed in the current study. We should, however, point out that there are some caveats in using the PR schedule to measure the motivational levels. For example, it is known that the BP is influenced by the step size of the PR schedule and the duration of the interval defining the last ratio (Bradshaw and Killeen 2012; Hursh and Silberberg 2008). Thus, other procedures such as those of the behavioral economics should be used to validate our conclusion. Alternatively, the dose-related increase in the compulsive cocaine-seeking behavior may result from the differential impact of the two doses on the punishment function. Our results showed no significant interaction between cocaine dose and punishment intensity suggesting that the two doses do not differentially change the sensitivity or responsiveness of the punishment function. Thus, it is possible that the higher dose may have a greater impact on the magnitude of the punishment function. A decrease in the response magnitude is expected to promote the compulsive cocaine-seeking behavior.
There are sex differences in the cocaine-related behaviors (Becker et al. 2012). Sex hormones appear to be responsible for the majority of the differences (Becker and Koob 2016; Carroll and Anker 2010). The effects of sex hormones may be organizational due to their impact on the brain development or activational due to the acute effects on the brain (Sanchis-Segura and Becker 2016). Because sex hormones fluctuate during the estrous cycle (Staley and Scharfman 2005), the behavior regulated by the activational effects of sex hormones also varies with the cycle. Indeed, the menstrual cycle alters the intensity of the subjective effects of cocaine (Evans et al. 2002; Sofuoglu et al. 1999). Preclinical studies demonstrate that the motivation for cocaine varies with the estrous cycle (Hecht et al. 1999; Roberts et al. 1989a) and sex hormones play a significant role (Lynch and Taylor 2005; Perry et al. 2013). To the best of our knowledge, we are not aware of any studies on the impact of estrous cycle on the compulsive cocaine-seeking behavior. We found that the compulsive sucrose- and cocaine-seeking behaviors did not vary with the estrous cycle suggesting that the sex hormones may not play a significant role in the regulation of cocaine-related compulsive behaviors.
Note that punishment was delivered immediately after the cocaine-seeking responses in the current study. Because the negative consequences associated with cocaine use in humans do not typically occur immediately, it has been suggested that the animal model should simulate this uncertainty. With the uncertainty animals may, however, underestimate or discount the probability of punishment. Indeed, there is evidence that the effect of punishment is decreased under the uncertain punishment condition (Simon et al. 2009). If animals believe that no negative consequences will occur, we cannot conclude that their cocaine-seeking behavior is compulsive. The core feature of cocaine addiction is the continued cocaine use despite being fully aware of the negative consequences. Regardless of whether the negative consequences occur immediately or not, addicted patients are fully aware of the fact that it is just a matter of time that the dire consequences will occur. Such knowledge, however, has minimal impact on their drug use. Thus, the model used in the current study captures this compulsive aspect of cocaine-seeking behavior and thus, may play an important role in identifying the mechanism of cocaine addiction.
Acknowledgments
The project was supported by Grant Number DA034776 (WLS) from the National Institute on Drug Abuse and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDA or NIH. All procedures followed the National Institute of Health Guidelines for the Care and Use of Laboratory Animals. There is no conflict of interest in relation to this article.
Footnotes
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Publishing, Incorporated; Washington, DC: 2013. [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex Differences in Drug Abuse. Frontiers in neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev. 2016;68:242–63. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biology of sex differences. 2012;3:14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- becLozano OM, Domingo-Salvany A, Martinez-Alonso M, Brugal MT, Alonso J, de la Fuente L Investigators I. Health-related quality of life in young cocaine users and associated factors. Quality of Life Research. 2008;17:977–985. doi: 10.1007/s11136-008-9376-8. [DOI] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Everitt BJ, Dalley JW. In search of predictive endophenotypes in addiction: insights from preclinical research. Genes Brain Behav. 2016;15:74–88. doi: 10.1111/gbb.12265. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw CM, Killeen PR. A theory of behaviour on progressive ratio schedules, with applications in behavioural pharmacology. Psychopharmacology (Berl) 2012;222:549–64. doi: 10.1007/s00213-012-2771-4. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–41. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Comings DE, Gonzalez N, Wu S, Saucier G, Johnson P, Verde R, MacMurray JP. Homozygosity at the dopamine DRD3 receptor gene in cocaine dependence. Mol Psychiatry. 1999;4:484–487. doi: 10.1038/sj.mp.4000542. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta U, Martini M, Sun W. Different functional domains measured by cocaine self-administration under the progressive-ratio and punishment schedules in male Wistar rats. Psychopharmacology (Berl) 2018;235:897–907. doi: 10.1007/s00213-017-4808-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Pearce JM. Preference and response suppression under different correlations between shock and a positive reinforcer in rats. Learn Motiv. 1976;7:66–85. [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–6. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33:339–55. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989;46:122–6. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Grove RN, Schuster CR. Suppression of cocaine self-administration by extinction and punishment. Pharmacol Biochem Behav. 1974;2:199–208. doi: 10.1016/0091-3057(74)90053-7. [DOI] [PubMed] [Google Scholar]
- Haas AL, Peters RH. Development of substance abuse problems among drug-involved offenders - Evidence for the telescoping effect. J Subst Abuse. 2000;12:241–253. doi: 10.1016/s0899-3289(00)00053-5. [DOI] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol. 1999;35:136–45. [PubMed] [Google Scholar]
- Holtz NA, Anker JJ, Regier PS, Claxton A, Carroll ME. Cocaine self-administration punished by i.v. histamine in rat models of high and low drug abuse vulnerability: effects of saccharin preference, impulsivity, and sex. Physiol Behav. 2013;122:32–8. doi: 10.1016/j.physbeh.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–98. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Johanson CE. The effects of electric shock on responding maintained by cocaine injections in a choice procedure in the rhesus monkey. Psychopharmacology (Berl) 1977;53:277–82. doi: 10.1007/BF00492364. [DOI] [PubMed] [Google Scholar]
- Jonkman S, Pelloux Y, Everitt BJ. Drug intake is sufficient, but conditioning is not necessary for the emergence of compulsive cocaine seeking after extended self-administration. Neuropsychopharmacology. 2012;37:1612–9. doi: 10.1038/npp.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, Robinson TE. Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology (Berl) 2016;233:3587–602. doi: 10.1007/s00213-016-4393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Persistent changes in motivation to self-administer cocaine following modulation of cyclic AMP-dependent protein kinase A (PKA) activity in the nucleus accumbens. Eur J Neurosci. 2005;22:1214–20. doi: 10.1111/j.1460-9568.2005.04305.x. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–14. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. The National Academies; Washington, DC: 2011. [Google Scholar]
- Negus SS. Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 2005;181:244–52. doi: 10.1007/s00213-005-2266-7. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Parkinson JA, Miles FJ, Everitt BJ, Dickinson A. Cocaine-seeking by rats: regulation, reinforcement and activation. Psychopharmacology (Berl) 2000;152:123–31. doi: 10.1007/s002130000498. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Dickinson A. Pavlovian counterconditioning: changing the suppressive properties of shock by association with food. J Exp Psychol Anim Behav Process. 1975;1:170–7. doi: 10.1037//0097-7403.1.2.170. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology (Berl) 2007;194:127–37. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. Impact of pubertal and adult estradiol treatments on cocaine self-administration. Horm Behav. 2013;64:573–8. doi: 10.1016/j.yhbeh.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Jagannathan L, Becker JB. The Roles of Dopamine and alpha1-Adrenergic Receptors in Cocaine Preferences in Female and Male Rats. Neuropsychopharmacology. 2015;40:2696–704. doi: 10.1038/npp.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V. A general theory of transition to addiction it was and a general theory of transition to addiction it is: Reply to the commentaries of Ahmed, Badiani, George & Koob, Kalivas & Gipson, and Tiffany. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, Hietala J. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–60. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60:252–60. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989a;98:408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Gabriele A, Zimmer BA. Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: methodological and interpretational considerations. Neurosci Biobehav Rev. 2013;37:2026–36. doi: 10.1016/j.neubiorev.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989b;97:535–8. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Becker JB. Why we should consider sex (and study sex differences) in addiction research. Addict Biol. 2016;21:995–1006. doi: 10.1111/adb.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34:2208–17. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–83. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Staley K, Scharfman H. A woman’s prerogative. Nat Neurosci. 2005;8:697–699. doi: 10.1038/nn0605-697. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–9. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence: Developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse. 2000;26:523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Freeman KB, Myerson J, Green L. Suppression of cocaine self-administration in monkeys: effects of delayed punishment. Psychopharmacology (Berl) 2012;220:509–17. doi: 10.1007/s00213-011-2501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Steketee JD, Sun W. Inactivation of the central nucleus of the amygdala reduces the effect of punishment on cocaine self-administration in rats. Eur J Neurosci. 2012;35:775–83. doi: 10.1111/j.1460-9568.2012.08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]