 Developmental exposure to bisphenol A (BPA) has been linked to impaired glucose homeostasis and pancreatic function in adulthood, which has been hypothesized to result from the disruption of pancreatic β-cell development at early life.
Developmental exposure to bisphenol A (BPA) has been linked to impaired glucose homeostasis and pancreatic function in adulthood, which has been hypothesized to result from the disruption of pancreatic β-cell development at early life.
Abstract
Developmental exposure to bisphenol A (BPA) has been linked to impaired glucose homeostasis and pancreatic function in adulthood, which has been hypothesized to result from the disruption of pancreatic β-cell development at early life. Here we evaluated whether maternal BPA exposure disrupts β-cell development and glucose tolerance and the role of epigenetic modifications of key regulator in this process. We found that maternal exposure to BPA (10 μg kg–1 d–1) reduced the pancreatic β-cell mass and the expression of pancreatic and duodenal homeobox 1 (Pdx1) at birth, as well as the expression of Pdx1 at gestational day (GD) 15.5. In parallel with the decreased expression of Pdx1, histones H3 and H4 deacetylation, along with demethylation of histone 3 lysine 4 (H3K4) and methylation of histone 3 lysine 9 (H3K9), were found at the promoter of Pdx1, while no significant changes in DNA methylation status were detected at this region. Moreover, these alterations were observed in adult life along with impaired glucose tolerance. We conclude that maternal exposure to BPA reduces pancreatic β-cell mass at birth by reducing PDX1+ progenitors during fetal development through altering the histone modifications of Pdx1, which can be propagated to later life and increase the susceptibility to glucose intolerance.
Introduction
The rapidly increasing prevalence of obesity and type 2 diabetes (T2D) has posed huge health and economic burden globally in recent years. Data from the World Health Organization revealed that diabetes was responsible for 1.5 million deaths and 89 million disability-adjusted life years (DALYs) in 2012.1 Although genetic predisposition, lifestyle and socioeconomic factors are the generally accepted risk factors for developing T2D, it is now recognized that early-life chemical exposure also plays a significant role in the growing epidemic of diabetes and obesity in later life.2 For example, prenatal exposure to nicotine has been proved to be a risk factor for obesity and metabolic disorders.3 Endocrine disrupting chemicals (EDCs), to which people are widely exposed, have recently drawn much attention because of their association with energy imbalance, obesity and T2D.4
Bisphenol A (BPA) is one of the highest production-volume EDCs used in manufacturing polycarbonate plastics and epoxy resins. Data from more than 80 biomonitoring studies all over the world indicates that humans are widely exposed to BPA.5 Animal studies have raised the concern that perinatal exposure to BPA may induce obesity and/or metabolic syndrome.6,7 However, the underlying mechanisms are still not clarified.8 Previous studies have suggested that pancreatic β-cells may serve as the target of BPA. Prenatal exposure to BPA has been shown to lead to alterations in Ca2+ signaling and insulin secretion in the islets of Langerhans in adult male offspring, and reduction in proliferating insulin-secreting β-cells.9 Exposure to di-2-ethylhexyl phthalate (DEHP), another kind of EDCs, through gestation and lactation reduced pancreatic β-cell mass, pancreatic insulin content and the expression of pancreatic and duodenal homeobox 1 (Pdx1) in weaning Wistar rats.10
Previous studies have also linked the development of T2D with impaired pancreatic β-cell development in early-life.3 The development of pancreatic β-cells represents a concert of a hierarchy of transcription factors,11 which is initiated by the expression of Pdx1 in endodermal cells.12Pdx1 is a critical regulator of pancreatic development and β-cell ontogeny during fetal development. Both genetic and acquired reduction in the expression of Pdx1 have verified the role of Pdx1 in pancreatic development in both humans and animal models,13,14 while overexpression of Pdx1 in pancreatic β-cells has been shown to restore cell mass and prevent the development of diabetes.15,16
The fact that maternal exposure to environmental chemicals leads to metabolic disorders even long after the exposure is removed indicates that epigenetic mechanisms may be involved in this process. A number of studies have linked EDCs with the potential role of epigenetics in the development of T2D.17 Interestingly, it is reported that point mutations of the proximal promoter drastically impaired the Pdx1 promoter activity, indicating that epigenetic modifications in this region may have a huge influence on the Pdx1 expression.18 Indeed, altered epigenetic modifications of Pdx1 have been implicated as the reason of β-cell anomaly and occurrence of T2D in intrauterine growth retarded (IUGR) rats.19 However, to our knowledge, no evidence has ever linked maternal exposure to BPA with the pancreatic β-cell development and the role of epigenetic modifications of Pdx1 in this process.
In the present study, we aim to verify the hypothesis that maternal BPA exposure disturbs epigenetic modifications of key regulators in the development of pancreatic β-cells, which may contribute to the development of T2D in adulthood.
Materials and methods
Animals and treatment
Wistar rats were purchased from Hubei Research Center of Experimental Animals (Wuhan, China) and were housed on a 12 : 12 h light–dark cycle. Rats were given ad libitum access to water and a standard rat chow. The water bottles and cages were made of BPA-free polypropylene. Two female rats were mated with 1 male rat after acclimatization for one week. The day on which a vaginal plug or a sperm positive vaginal smear was found was defined as gestational day (GD) 0.5. Pregnant rats were randomly assigned to two weight-matched treatment groups: BPA (10 μg kg–1 d–1) or the vehicle corn oil (both were from Sigma-Aldrich, Saint Louis, MO). This exposure dose was proved to make the internal exposure level similar to that detected in humans.20 BPA was first dissolved in corn oil to make a 10 μg mL–1 solution and both reagents were given at a volume of 1 mL per kg body weight via gavage throughout gestation and lactation. Neonates were weighed and distributed within each group to balance the litter size (4 males and 4 females per dam), and caged with dams until weaning. For all experiments, both male and female animals were used due to the technical challenge of simultaneously identifying fetal sexes when isolating pancreatic buds. All the procedures were reviewed and approved by the Ethics Committee of Tongji Medical College (Huazhong University of Science and Technology, Wuhan, China).
Isolation of the pancreatic islets
The islets of Langerhans from adult rats were isolated as previously described.21 Briefly, rats were first anesthetized. Then the peritoneal cavity was opened and the hepatopancreatic ampulla was located following the common bile duct and the pancreatic duct. After the duodenum was clamped off with hemostats at both up and downstream of the ampulla, the common bile duct was injected with collagenase V (1 mg ml–1, Sigma-Aldrich, Saint Louis, MO) dissolved in Hank's balanced sodium solution (HBSS) plus 1% w/v BSA until the pancreas was fully distended. The pancreas was removed to a 15 ml conical tube and put in a 37 °C water bath for 18 min. Following washing twice, the resulting tissue debris was re-suspended in a discontinuous Ficoll 400 gradient (25%, 23%, 20.5% and 11% w/v; Amresco, Solon, OH, USA) and centrifuged at 800g for 20 min. The tissue debris at the 23/20.5 and 20.5/11 interface was collected and washed twice with cold HBSS.
Immunostaining
For immunofluorescence, fetuses from 6 dams were delivered by caesarean at GD 15.5 and fixed in 4% w/v paraformaldehyde overnight, followed by being embedded in paraffin. Four-micrometer sagittal sections were cut, of which those containing the pancreas were stained with rabbit antiserum (Millipore, Temecula, CA), and visualized with a goat anti-rabbit secondary antibody conjugated to Alexa Fluo 555 (CST, CA). The PDX1+ cell fraction was calculated by dividing the PDX1+ cell number with the whole cell number in the pancreas area of the section. Given the regional variation in islet distribution and cell composition, every 30th section was analyzed, which yielded 5 to 7 sections from each pancreas. For immunohistochemistry, pancreas was excised from neonates from six randomly chosen dams. Pancreas were sectioned and immunostained with a mouse anti-insulin + proinsulin monoclonal antibody (Abcam, Cambridge, MA) at a dilution of 1 : 1250. Sections were detected by a secondary anti-mouse macromolecule ligated to horse radish peroxidase (Vector Laboratories, Burlingame, CA) and visualized in brown with 3,3-diaminobenzidine. As described above, every 30th section was analyzed and 5 to 7 sections yielded from each pancreas. All images were taken by Olympus IX71 (Olympus, Tokyo, Japan) equipped with Image-Pro Plus software (version 5.0; Media Cybernetics, Inc., Rockville, MD, USA). The pancreatic β-cell mass was calculated by multiplying the total weight of the unfixed pancreas by the β-cell fraction, which was calculated as the ratio of insulin-positive area to the total tissue area of the section.
Real-time PCR
At GD 15.5 and birth, total RNA and DNA were extracted from the pancreas using an AllPrep DNA/RNA Micro Kit (QIAGEN, Hilden, Germany). The mRNA was first reversely transcribed to cDNA using a RevertAid First Strand cDNA synthesis kit (Carlsbad, CA, USA). The resulting cDNA was subjected to real-time PCR using a FastStart Universal SYBR Green Master (Rox) (Roche, Germany) on a 7900HT Fast Real-Time PCR system. The primers for Pdx1 amplification are as follows: forward, 5′-CGGACATCTCCCCATACG-3′; reverse, 5′-AAAGGGAGATGAACGCGG-3′. Primers for insulin amplification are: forward, 5′-TCTTCTACACACCCATGTCCC-3′; reverse, 5′-GGTGCAGCACTGATCCAC-3′. The PCR conditions are as follows: initial denaturing at 95 °C for 10 min; followed by 40 cycles of denaturing at 95 °C for 15 s, annealing and extension at 60 °C for 1 min. Data was analyzed using the 2–ΔΔCt method,22 with cyclophilin used as the internal control.
DNA methylation analysis of the proximal promoter of Pdx1
DNA extracted from fetal and neonatal pancreas, as well as from the islets isolated from 8 week-old offspring were treated with bisulphite and subjected to methyl-specific PCR amplification. Pancreas from three fetuses at GD 15.5 and pancreas from two neonates were pooled together to get 1 tube of DNA. The primer pair used in this amplification is as follows: forward, 5′-GGGGGATTAGTATTGAATTTTGGTA-3′; reverse, 5′-AAACCTCCTTCTTAAAACAAAACCA-3′. The resulting PCR products were transcribed to RNA using the T7 RNA polymerase in vitro, followed by RNase A cleavage into fragments containing the CpG sites. These RNA fragments were finally analyzed by the Matrix-Assisted Laser Desorption/Ionization Time of Flight (MALDI-TOF) mass spectrometry.
Chromatin immunoprecipitation assay
Pancreas from fetuses and neonates, and the islets from 8 week-old offspring were cut into pieces and cross-linked in PBS containing 1% w/v formaldehyde (Sigma-Aldrich, Saint Louis, MO) and a protease inhibitor cocktail for 10 min on a rocking platform. Cells were lysed using SDS lysis buffer and the resulting chromatin was sheared into fragments ranging from 200 to 1000 bp. DNA shearing was conducted using a VCX 750 ultrasonic processor with a time-course of six 15 s pulses at 50% output with a 60 s ice-rest between pulses. Supernatants were pre-cleared with protein A-sepharose (Millipore, Temecula, CA). An aliquot of the supernatant was 1 : 100 diluted and used as the input control. The pre-cleared supernatants were incubated with rabbit anti-acetyl-histone H3/H4, rabbit anti-dimethyl-histone H3 (Lys 4) or mouse anti-trimethyl-histone H3 (Lys 9) (all were purchased from Millipore, Temecula, CA) overnight at 4 °C on a rocking platform. DNA–protein–antibody complexes were precipitated by protein A-sepharose and washed serially with low-salt buffer, high-salt buffer, IP wash buffer and TE buffer. Precipitated complexes were eluted from protein A with an elution buffer (1% w/v NaHCO3, 1% w/v SDS), followed by reverse cross-linking using 5 M NaOH and thorough digestion by proteinase K. The resulting DNA was purified with a QIAquick PCR purification kit (QIAGEN, Hilden, Germany) and subjected to PCR amplification. The primer set used in this study was obtained from previous research (ref. 15), which is as follows: forward, 5′-GCAGGACAGGAGAGATCAGC-3′; reverse, 5′-CCCAGATCGCTTTGACAGTT-3′.
Intraperitoneal glucose or insulin tolerance tests
Rat offspring randomly chosen at 4 weeks, 8 weeks and 20 weeks were subjected to intraperitoneal glucose tolerance test (ipGTT) or intraperitoneal insulin tolerance test (ipITT) to evaluate the glucose homeostasis and insulin sensitivity. For ipGTT, rats were fasted overnight and injected with 2 g kg–1 glucose dissolved in saline intraperitoneally. Blood glucose was measured at 0, 15, 30, 60, and 120 min later using an Accu-chek glucometer and test strips (Roche, Mannheim, Germany). For ipITT, rats were fasted for 6 h and given an i.p. injection of 0.75 IU kg–1 human insulin (Novo Nordisk, Bagsvaerd, Denmark) and the time points for blood glucose measurement was 0, 15, 30, 45, and 60 min. Rats were excluded from data analysis if they did not exhibit a rise in blood glucose greater than 20 mmol L–1 (360 mg dl–1) in the first 15 min or if they exhibited diarrhea.
Statistics
Statistical analysis was carried out with SPSS 13.0. A repeated measure analysis of variances (ANOVA) was used in the analysis of data from ipGTT and ipITT. Other statistical analyses were performed using two-tailed Student's t-test. Data were presented as mean ± SEM, p < 0.05 was considered significant in all the analyses.
Results
Maternal exposure to BPA reduces pancreatic β-cell mass at birth
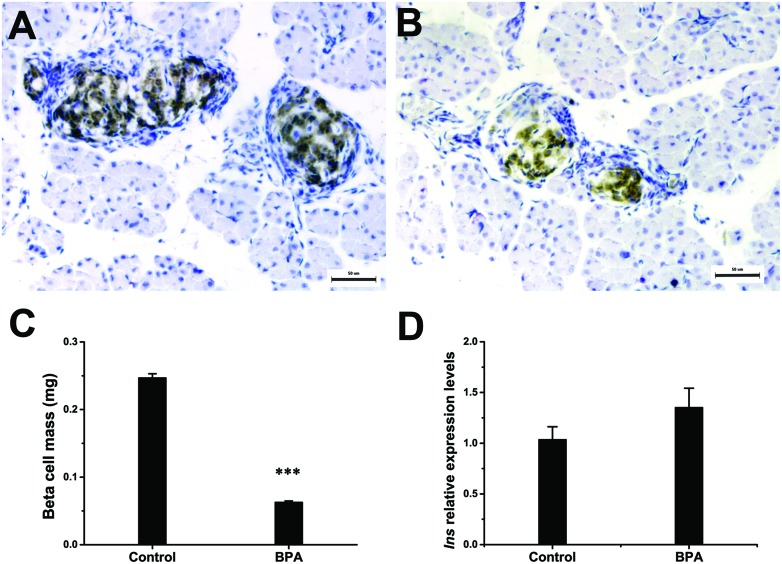
The results of birth weight, pancreas weight, and organ coefficient are shown in Table 1. The birth weight and pancreas weight were significantly reduced (p < 0.001) due to maternal exposure to BPA, while no significant variation in the pancreas organ coefficient (organ weight/body weight) was observed. Fig. 1 shows that BPA exposure significantly reduced (p < 0.001) the pancreatic β-cell mass (BCM) at birth (Fig. 1A–C). However, this reduction in cell mass did not alter the content of mRNA for insulin in isolated pancreas (Fig. 1D).
Table 1. General parameters of neonatal rats.
| Body weight (g) | Pancreas weight (g) | Organ coefficient | |
| Control (n = 61) | 4.638 ± 0.011 | 0.027 ± 6.557 × 10–5 | 0.006 ± 5.882 × 10–6 |
| BPA (n = 73) | 3.954 ± 0.010* | 0.022 ± 6.849 × 10–5* | 0.006 ± 1.127 × 10–5 |
Fig. 1. Pancreatic β-cell mass and insulin expression at birth. (A, B) Representative images of pancreatic β-cells (brown) in pancreas isolated from the control (A) and BPA (B) group at birth (n = 6). Bars = 50 μm. (C) The pancreatic β-cell mass at birth. Mean pancreatic β-cell fraction was used to calculate the absolute pancreatic β-cell mass as described in the Materials and methods section (n = 6). (D) The relative mRNA expression level of insulin at birth (n = 6). Data are represented as mean ± SEM, ***p < 0.001 by two-tailed Student's t-test.
Maternal exposure to BPA disrupts the expression and epigenetic modifications of Pdx1 at birth
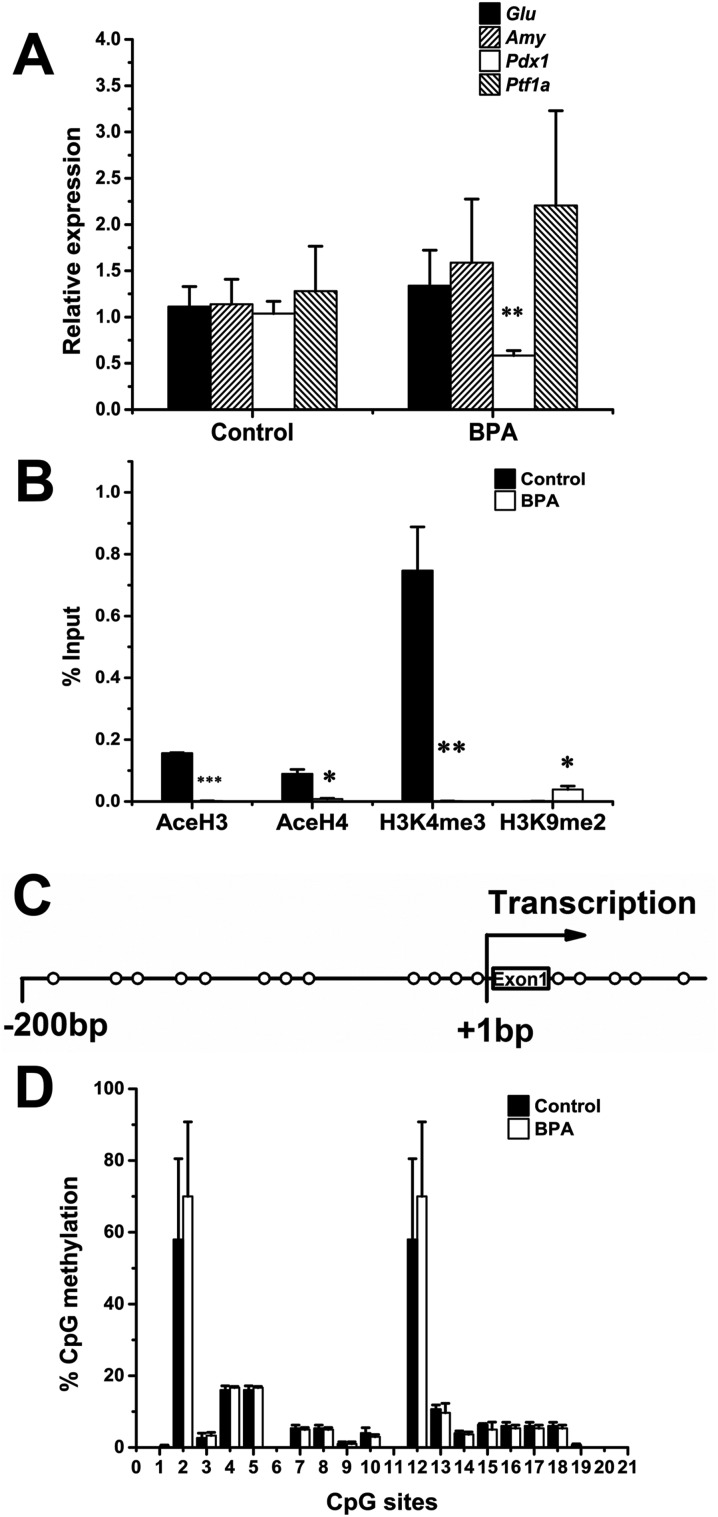
We also examined the expression of mRNA for some key regulators which are important for the pancreatic β-cell development. The expression of mRNA for Pdx1 was decreased in the BPA group, whereas no changes were observed in the expression of pancreas transcription factor 1 subunit alpha (Ptf1a), glucagon (Gcg), and amylase (Amy) (Fig. 2A).
Fig. 2. The expression and epigenetic profiling of Pdx1 at birth. (A) The expression level of mRNA for Pdx1 was determined at birth (n = 6). (B) The abundance of acetylated H3 (AceH3), acetylated H4 (AceH4), H3K4me3, and H3K9me2 at the Pdx1 promoter (n = 3). Data are represented as percent of input control, which undergo analyses exactly as the test samples except for the IP step. (C) A schematic illustration of the analyzed CpG sites at the Pdx1 proximal promoter. CpG sites are marked with open circles relative to the translational start site (+1 bp). (D) DNA methylation status of the Pdx1 promoter (n = 3). Data are represented as mean ± SEM. *p < 0.05, **p < 0.01; ***p < 0.001 by two-tailed Student's t-test.
We next investigated whether this change in the expression of Pdx1 correlates with epigenetic modifications of the proximal promoter of Pdx1. The acetylation status of core histones H3 and H4, trimethylation of lysine 4 at H3 (H3K4me3) and dimethylation of lysine 9 at H3 (H3K9me2) were determined by chromatin immunoprecipitation (ChIP). Maternal exposure to BPA significantly reduced the abundance of histones H3 (p < 0.001) and H4 (p < 0.05) acetylation and H3K4me3 (p < 0.01), whereas increased the abundance of H3K9me2 (p < 0.05), leading to a repressed chromatin structure (Fig. 2B). In addition to histone modifications, we also determined the DNA methylation status of the proximal promoter of Pdx1. The CpG sites examined in the present study are illustrated in Fig. 2C. However, no substantial changes in the DNA methylation status were observed at birth. Two sites (2 and 12) were consistently methylated across the study.
Maternal exposure to BPA disrupts the expression and epigenetic modifications of Pdx1 at GD 15.5
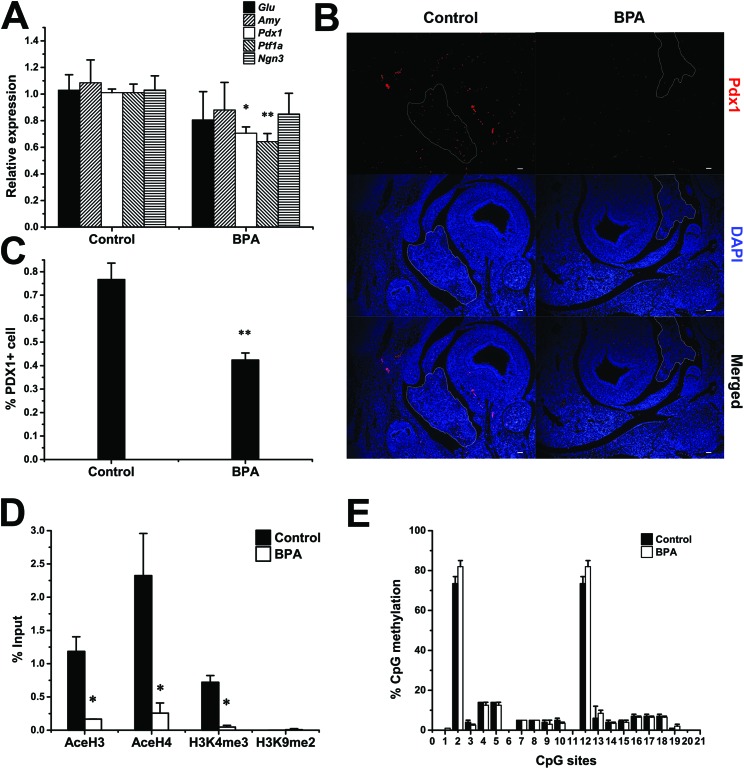
Since the development of pancreatic β-cells initiates and develops under the control of Pdx1 at fetal stages before birth, we also examined the expression and epigenetic modifications of Pdx1 at gestational day (GD) 15.5. The expression of mRNA for Pdx1 and Ptf1a were reduced by 30.9% and 63.6% respectively, indicating a potential decrease in the abundance of pancreatic progenitor cells, while the expression of mRNA for insulin (Ins), Gcg, Amy, and neurogenin 3 (Ngn3) remained unchanged (Fig. 3A). In addition to the reduction in expression of mRNA for Pdx1, morphological results also showed that BPA exposure significantly reduced the PDX1+ cell fraction (p < 0.01) at GD 15.5 compared to the control group (Fig. 3B and C).
Fig. 3. The expression and epigenetic profiling of Pdx1 at GD 15.5. (A) The expression level of mRNA for Pdx1 was determined at GD 15.5 (n = 6). (B) PDX1+ cell was stained red with the anti-PDX1 antibody and the pancreas at GD 15.5 was outlined (n = 6). Bars = 50 μm. (C) PDX1+ cell fraction at GD 15.5 (n = 6). (D) The abundance of AceH3, AceH4, H3K4me3, and H3K9me2 at the Pdx1 promoter (n = 3). Data are represented as percent of input control, which undergo analyses exactly as the test samples except for the IP step. (E) DNA methylation status at the Pdx1 promoter (n = 3). Data are represented as mean ± SEM. *p < 0.05, **p < 0.01 by two-tailed Student's t-test.
At this life stage, BPA also significantly reduced the acetylation of histones H3 and H4, as well as the H3K4me3 (p < 0.05), while H3K9me2 was not detected in the control group. Still, we did not observe any changes in the DNA methylation status at the promoter of Pdx1.
Altered epigenetic modifications of Pdx1 propagates to 8 weeks of age
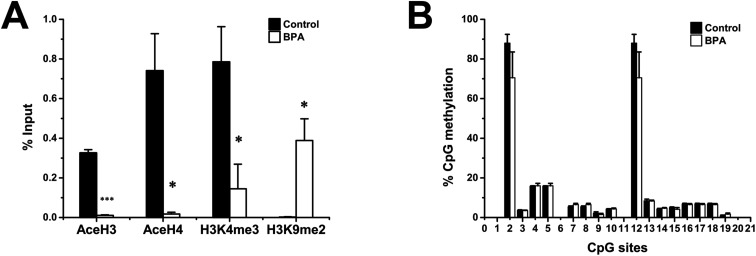
To verify the continuity and progressiveness of the epigenetic modifications, we also investigated the epigenetic modifications of Pdx1 at 8 weeks of age. The difference in histone code between two groups strengthened at this life stage. Acetylated histones H3 and H4 were barely detected by 8 weeks in the BPA exposed group whereas H3K9me2 was significantly increased compared to the control (Fig. 4A). Surprisingly, DNA methylation status still did not show any changes between the two groups (Fig. 4B).
Fig. 4. Epigenetic profiling of Pdx1 at 8 weeks of age. (A) The abundance of AceH3, AceH4, H3K4me3, and H3K9me2 at the Pdx1 promoter. Data are represented as percent of input control, which went through the analyses exactly as the test samples except for the IP step (n = 3). (B) DNA methylation status at the Pdx1 promoter (n = 3). Data are represented as mean ± SEM. *p < 0.05, ***p < 0.001 by two-tailed Student's t-test.
Maternal exposure to BPA induces glucose intolerance and insulin resistance in adulthood
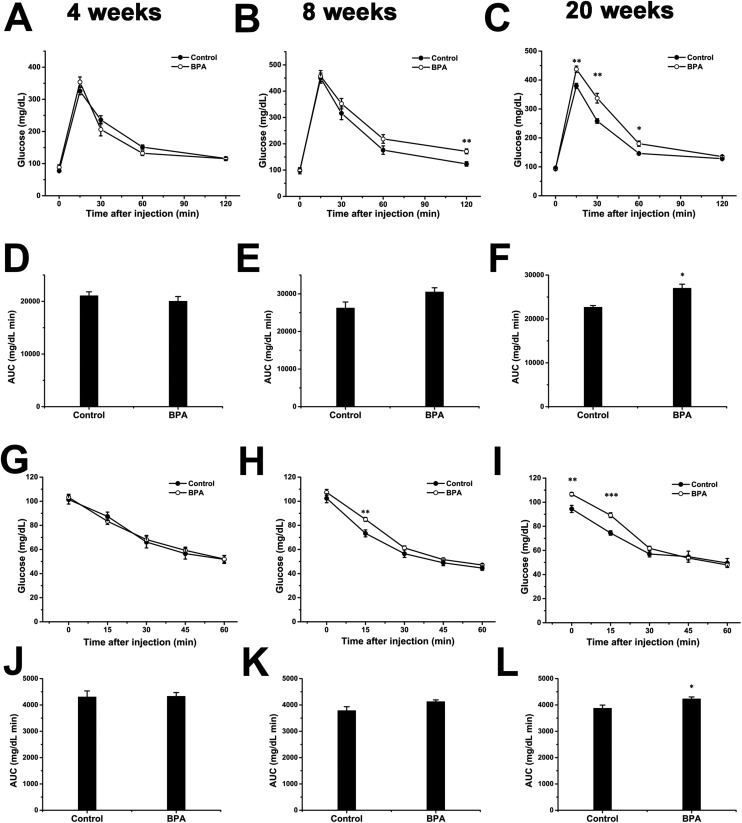
We also tried to verify if the disrupted pancreatic β-cell development at birth correlates with the glucose homeostasis at adulthood. There was no significant difference in both ipGTT and ipITT at 4 weeks (Fig. 5A and G). At 8 weeks, the results of ipGTT and ipITT showed slight differences after the injection (Fig. 5B and H), with the area under curve (AUC) of ipGTT showed a marginal difference (p = 0.088; Fig. 5E). At 20 weeks, repeated measures ANOVA showed significant differences in interactions between treatment and time (p = 0.001 and 0.026 for ipGTT and ipITT respectively). The BPA group exhibited higher blood glucose levels at 15, 30 and 60 min after glucose injection (Fig. 5C). There were significant differences in blood glucose at 0 and 15 min after the injection during the ipITT (Fig. 5I). The AUC also showed significant differences at this stage (Fig. 5F and L).
Fig. 5. Glucose homeostasis and insulin sensitivity in adult rats. ipGTT was performed at 4 weeks (A), 8 weeks (B), and 20 weeks (C), n = 10–19 and 8–17 for control and BPA respectively. Panels D–F show the ipGTT AUC. The ipITT was performed in the same groups at 4 weeks (G), 8 weeks (H) and 20 weeks (I), n = 11–16 and 12–17 for control and BPA respectively. Panels J–L show the ipITT AUC. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 by two-tailed Student's t-test.
Separated analyses of data for males and females revealed no differences for both sexes at 4 weeks of age (ESI Fig. S1A–H†). At 8 weeks, BPA exposed males first exhibited insulin resistance (ESI Fig. S2C†), which was confirmed by the increased AUC in BPA rats (ESI Fig. S2D†), whereas females showed no difference between the control and BPA group (ESI Fig. S2E–H†). At 20 weeks, BPA exposed males showed both glucose intolerance and insulin resistance compared to control males (ESI Fig. S3A–D†). Glucose intolerance was also detected in BPA exposed females at this age (ESI Fig. S3E and F†).
Discussion
Previous studies have demonstrated that in utero exposure to low-dose BPA disrupts glucose homeostasis and induces diabetes.6,9,17 However, the underlying molecular mechanisms remain unclear. In this study, we found that perinatal exposure to BPA impaired the fetal development of pancreatic β-cells, characterized by decreased PDX1+ cell fraction at GD 15.5, and reduced the pancreatic β-cell mass at birth. Furthermore, alterations in the histone modification of the Pdx1 promoter towards an inactivated chromatin were observed in parallel with reduction in the PDX1+ cell fraction at GD 15.5. These alterations propagated to 8 weeks of age, when glucose intolerance and insulin resistance were observed. To our knowledge, this is the first study to report the correlation between pancreatic β-cell development and the histone modification of the Pdx1 gene in a BPA exposure model. We believe this will help understand the underlying mechanisms by which early life exposure to BPA may impact on the development of diabetes in adulthood.
This research further verifies that the perinatal period is the critical time window for BPA exposure. Rodent models and human studies have shown that BPA can be transferred across the placenta,23,24 and through the milk,25 which makes the developing organ systems particularly susceptible to the potential adverse effects of BPA, including brain and behavior,26,27 reproductive system,28 and metabolism.29 The perinatal period being one of the most crucial time windows is due to not only directly exposure but also the effects of BPA on maternal behavior.30
Both in vivo and in vitro studies have suggested the pancreatic β-cells as the potential target, by which BPA may exert its effects on blood glucose homeostasis and diabetes.31,32 However, to our knowledge, no evidence has ever linked BPA exposure with the development of endocrine pancreas in vivo, of which impairment has been proposed to greatly increase the susceptibility to the development of T2D.33 In our present study, we found that perinatal exposure to BPA significantly reduced the pancreatic β-cell mass at birth, which, at least in part, account for the association between prenatal exposure to BPA and the onset of T2D. The involvement of endocrine pancreas in the development of T2D has been verified by various previous studies involving developmental malnutrition and/or developmental exposure to environmental chemicals. Both maternal low-protein and low-energy diet during pregnancy significantly decreased the rat fetal β-cell mass.34 Similar results have been demonstrated in vitamin A deficiency and exposure to DEHP, nicotine, and dexamethasone models during development.10,35–37 Furthermore, studies indicate that even at a 37% increase in pancreatic β-cell proliferation in IUGR animals at 3 months of age, the pancreatic β-cell mass remained lower than that of the normally nourished ones, indicating a persistent reduction in the β-cell mass in adulthood.38 This may in turn strengthen the reduction in the β-cell mass, because adult pancreatic β-cells are maintained by self-duplication of existing cells rather than stem-cell differentiation.39 When this persistent reduction in the pancreatic β-cell mass encounters aging, and glucose intolerance was triggered.40 Intriguingly, the reduced beta cell mass by BPA was not mirrored by a reduction of insulin mRNA, suggesting a higher insulin content per beta cell.34
Pdx1 is a key regulator in determining cell lineage in pancreatic development and maintaining β-cell function in adulthood. Increasing evidence suggests that the decreased expression level of Pdx1 in development may be responsible for the reduction in the pancreatic β-cell mass induced by developmental deficiency in nutrition.19,34 Restoration of expression of Pdx1 by neonatal administration of exendin-4 counteracted the progressive reduction in the pancreatic β-cell mass after intrauterine growth retardation.16 Consistent with the reduction in the pancreatic β-cell mass, our data suggest that the expression of Pdx1 was significantly reduced by BPA exposure at birth. We also found that the expression levels of Pdx1 and Ptf1a were significantly reduced compared to that of the control group at GD 15.5. Genetic tracing experiments have demonstrated that both endocrine and exocrine cells of the pancreas derive from a pool of progenitor cells that express the transcription factors Pdx1 41 and Ptf1a.42 These results indicate that reduction in the PDX1+ progenitor cells at an early developmental stage may be responsible for the impairment of pancreatic β-cell development in our experiment. Conditional progenitor cell ablation revealed that the pancreas size was limited by the size of the progenitor cell pool that was set aside in early development and was not subject to growth compensation.43 The same study also suggested that early ablation of the PDX1+ progenitor cells decreased the insulin+ cell area at E18.5 while the amylase+ (exocrine) and DBA+ (duct) cell area showed small non-significant changes, and adult mice derived from the early ablation of pancreatic progenitor cells exhibited glucose intolerance at 11 weeks.43 These results indicate that BPA induced reduction in PDX1+ progenitor cells may have profound and permanent effects on pancreatic β-cell development and glucose homeostasis in adulthood. Indeed, in addition to the reduced pancreatic β-cell mass at birth, we also observed slight changes in glucose metabolism and insulin response in BPA exposed offspring at as early as 8 weeks, and this propagated to insulin resistance at 20 weeks of age. Although we believe that perinatal exposure to BPA may possibly decrease the pancreatic β-cell mass by reducing the PDX1+ progenitor cell pool at the early stage, studies are still needed to elucidate the exact events mediating the effects. It is worth noting that previous studies indicate that females are more resistant to the toxicity of BPA on glucose homeostasis.9 However, identifying the fetal sexes when isolating the pancreatic buds at GD 15.5 turned out to be technically challenging for us. In this case, subsequent experiments were conducted on rats irrespective of their sex. However, we still tried to analyze the GTT and ITT data separately based on sex (see the ESI†), and found that the glucose intolerance in females emerged later than that in males, which is consistent with previous studies.6
Epigenetic modifications provide a mechanism, by which the activity of gene expression can be reprogrammed and propagated to the later life stage. The dynamic epigenome is sensitive to environmental signals, which makes it an interface between the dynamic environment and the static genome.44 Moreover, pancreas development and T2D have both been implicated to be associated with epigenetic modifications.45,46 In mammals, epigenetic modifications are mainly mediated by DNA methylation and chromatin modifications. In vitro studies suggest that the proximal promoter region of Pdx1 contains a highly conserved CpG island and is heavily acetylated at histone H3 and H4.18,47 We first observed a decrease in the acetylated H3 and H4, and H3K4me3 at GD 15.5 in BPA exposed fetuses, followed by an increase in H3K9me2 at birth. These results indicate that BPA significantly altered the chromatin modifications of the Pdx1 promoter towards an inactive direction, through which the expression level of Pdx1 was significantly decreased. Moreover, these epigenetic modifications were progressively propagated to 8 weeks of age, indicating permanent impairments in the expression level of Pdx1.
We also detected the DNA methylation status of the proximal promoter region in parallel with histone modifications in the same region. Surprisingly, we did not detect any significant changes at all the 19 CpG sites at different time points. Emerging evidence supports the view that DNA methylation and histone modifications may be mutually reinforcing and interdependent, and histone modifications are readily reversible whereas DNA methylation is stable.48 Given that methylation of H3K9 precedes DNA methylation,49 it is possible that the histone modifications of the Pdx1 promoter in the BPA exposed group may represent an early stage of DNA methylation in this study. However, to our knowledge, no previous studies have ever identified the DNA methylation status of the promoter of Pdx1 in primary islet tissue when exposed to BPA during fetal development. Future studies are required to fully verify the long-term effects of BPA on DNA methylation of the Pdx1 promoter and the molecular interactions in this process.
Conclusions
In conclusion, our study demonstrates that perinatal exposure to BPA induces altered histone modifications towards an inactive status in the proximal promoter of Pdx1. This leads to the reduction in PDX1+ progenitor cells during development, which in turn impairs the development of the pancreatic β-cells. And these finally disrupt glucose homeostasis at adulthood. We believe that our research provides new insights into the mechanisms by which perinatal exposure to BPA may impair pancreatic β-cell development and induce T2D.
Conflict of interest
There are no conflicts of interest to declare.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 21177046, 21437002, and 81372959); the National Program on Key Basic Research Project of China (973 Program) (grant number 2012CB722401); the R&D Special Fund for Public Welfare Industry (Environment) (grant number 201309048); the National Basic Research Development Program of China (grant number 2008CB418206); the Fundamental Research Funds for the Central Universities, HUST (grant numbers 2012QN240, 2012TS072); and the Doctoral Fund of Ministry of Education of China (grant number 20120142120017).
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tx00047a
References
- WHO, Global status report on noncommunicable diseases 2014, http://www.who.int/nmh/publications/ncd-status-report-2014/en/. [DOI] [PubMed]
- Inadera H. Environ. Health Prev. Med. 2013;18:185–197. doi: 10.1007/s12199-013-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M., Rao D., Aagaard K., Davidson T. L., Levin E. D., Slotkin T. A., Srinivasan S., Wallinga D., White M. F., Walker V. R., Thayer K. A., Holloway A. C. Environ. Health Perspect. 2013;121:170. doi: 10.1289/ehp.1205404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P., Quesada I., Nadal A. Nat. Rev. Endocrinol. 2011;7:346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N., Chahoud I., Heindel J. J., Padmanabhan V., Paumgartten F. J., Schoenfelder G. Environ. Health Perspect. 2010:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Lin Y., Li Y., Ying C., Chen J., Song L., Zhou Z., Lv Z., Xia W., Chen X., Xu S. Endocrinology. 2011;152:3049–3061. doi: 10.1210/en.2011-0045. [DOI] [PubMed] [Google Scholar]
- Susiarjo M., Xin F., Bansal A., Stefaniak M., Li C., Simmons R. A., Bartolomei M. S. Endocrinology. 2015;156:2049–2058. doi: 10.1210/en.2014-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezg R., El-Fazaa S., Gharbi N., Mornagui B. Environ. Int. 2014;64:83–90. doi: 10.1016/j.envint.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P., Vieira E., Soriano S., Menes L., Burks D., Quesada I., Nadal A. Environ. Health Perspect. 2010;118:1243–1250. doi: 10.1289/ehp.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Wei J., Li Y., Chen J., Zhou Z., Song L., Wei Z., Lv Z., Chen X., Xia W., Xu S. Endocrinol. Metab. 2011;301:E527–E538. doi: 10.1152/ajpendo.00233.2011. [DOI] [PubMed] [Google Scholar]
- Oliver-Krasinski J. M., Stoffers D. A. Genes Dev. 2008;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon C., Docherty K. Diabetologia. 2001;44:1203–1214. doi: 10.1007/s001250100628. [DOI] [PubMed] [Google Scholar]
- Jonsson J., Carlsson L., Edlund T., Edlund H. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Stoffers D. A., Zinkin N. T., Stanojevic V., Clarke W. L., Habener J. F. Nat. Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- Kushner J. A., Ye J., Schubert M., Burks D. J., Dow M. A., Flint C. L., Dutta S., Wright C. V. E., Montminy M. R., White M. F. J. Clin. Invest. 2002;109:1193–1201. doi: 10.1172/JCI14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers D. A., Desai B. M., DeLeon D. D., Simmons R. A. Diabetes. 2003;52:734–740. doi: 10.2337/diabetes.52.3.734. [DOI] [PubMed] [Google Scholar]
- Ma Y., Xia W., Wang D., Wan Y., Xu B., Chen X., Li Y., Xu S. Diabetologia. 2013;56:2059–2067. doi: 10.1007/s00125-013-2944-7. [DOI] [PubMed] [Google Scholar]
- Sharma S., Leonard J., Lee S., Chapman H. D., Leiter E. H., Montminy M. R. J. Biol. Chem. 1996;271:2294–2299. doi: 10.1074/jbc.271.4.2294. [DOI] [PubMed] [Google Scholar]
- Park J. H., Stoffers D. A., Nicholls R. D., Simmons R. A. J. Clin. Invest. 2008;118:2316–2324. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins G. S., Ye S.-H., Birch L., Ho S.-M., Kannan K. Reprod. Toxicol. 2011;31:1–9. doi: 10.1016/j.reprotox.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. D., Dula S. B., Corbin K. L., Wu R., Nunemaker C. S. Biol. Proced. Online. 2009;11:3–31. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Balakrishnan B., Henare K., Thorstensen E. B., Ponnampalam A. P., Mitchell M. D. Am. J. Obstet. Gynecol. 2010;202:393–395. doi: 10.1016/j.ajog.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Iwano H., Yanagisawa R., Koike N., Inoue H., Yokota H. Environ. Health Perspect. 2010;118:1196–1203. doi: 10.1289/ehp.0901575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy B. F., English K. R., Jagals P., Sly P. D. J. Exposure Sci. Environ. Epidemiol. 2015;25:544–556. doi: 10.1038/jes.2015.49. [DOI] [PubMed] [Google Scholar]
- Chang H., Wang M., Xia W., Chen T., Huo W., Mao Z., Zhu Y., Li Y., Xu S. Toxicol. Res. 2016;5:828–835. doi: 10.1039/c5tx00449g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.-H., Yang Y., Ge M.-M., Xu L., Tang Y., Hu F., Xu Y., Wang H.-L. Toxicol. Res. 2015;4:686–694. [Google Scholar]
- Peretz J., Vrooman L., Ricke W. A., Hunt P. A., Ehrlich S., Hauser R., Padmanabhan V., Taylor H. S., Swan S. H., VandeVoort C. A., Flaws J. A. Environ. Health Perspect. 2014;122:775–786. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer K. A., Heindel J. J., Bucher J. R., Gallo M. A. Environ. Health Perspect. 2012;120:779. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanese M. C., Suvorov A., Vandenberg L. N. Toxicol. Res. 2015;4:592–612. [Google Scholar]
- Alonso-Magdalena P., Morimoto S., Ripoll C., Fuentes E. and Nadal A., The Estrogenic Effect of Bisphenol A DisruptsThe Estrogenic Effect of Bisphenol A Disrupts Pancreatic β-Cell Function In Vivo and Induces Insulin ResistancePancreatic β-Cell Function In Vivo and Induces Insulin Resistance, Environ. Health Perspect, 2005, 114, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A., Alonso-Magdalena P., Soriano S., Quesada I., Ropero A. B. Mol. Cell. Endocrinol. 2009;304:63–68. doi: 10.1016/j.mce.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Hales C. N., Barker D. J. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Dumortier O., Blondeau B., Duvillie B., Reusens B., Breant B., Remacle C. Diabetologia. 2007;50:2495–2503. doi: 10.1007/s00125-007-0811-0. [DOI] [PubMed] [Google Scholar]
- Matthews K. A., Rhoten W. B., Driscoll H. K., Chertow B. S. J. Nutr. 2004;134:1958–1963. doi: 10.1093/jn/134.8.1958. [DOI] [PubMed] [Google Scholar]
- Somm E., Schwitzgebel V. M., Vauthay D. M., Camm E. J., Chen C. Y., Giacobino J. P., Sizonenko S. V., Aubert M. L., Huppi P. S. Endocrinology. 2008;149:6289–6299. doi: 10.1210/en.2008-0361. [DOI] [PubMed] [Google Scholar]
- Dumortier O., Theys N., Ahn M. T., Remacle C., Reusens B. PLoS One. 2011;6:e25576. doi: 10.1371/journal.pone.0025576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofano A., Czernichow P., Breant B. Diabetologia. 1998;41:1114–1120. doi: 10.1007/s001250051038. [DOI] [PubMed] [Google Scholar]
- Dor Y., Brown J., Martinez O. I., Melton D. A. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Garofano A., Czernichow P., Breant B. Diabetologia. 1999;42:711–718. doi: 10.1007/s001250051219. [DOI] [PubMed] [Google Scholar]
- Gu G., Dubauskaite J., Melton D. A. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Cooper B., Gannon M., Ray M., MacDonald R. J., Wright C. V. Nat. Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Stanger B. Z., Tanaka A. J., Melton D. A. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- Feil R., Fraga M. F. Nat. Rev. Genet. 2012;13:97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- Avrahami D., Kaestner K. H. Semin. Cell Dev. Biol. 2012;23:693–700. doi: 10.1016/j.semcdb.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert E. R., Liu D. Epigenetics. 2012;7:841–852. doi: 10.4161/epi.21238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrish K., Van Velkinburgh J. C., Stein R. Mol. Endocrinol. 2004;18:533–548. doi: 10.1210/me.2003-0371. [DOI] [PubMed] [Google Scholar]
- Cedar H., Bergman Y. Nat. Rev. Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- Bachman K. E., Park B. H., Rhee I., Rajagopalan H., Herman J. G., Baylin S. B., Kinzler K. W., Vogelstein B. Cancer Cell. 2003;3:89–95. doi: 10.1016/s1535-6108(02)00234-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.