OH-GQDs exhibit evident cytotoxicity on lung carcinoma cells via inducing cells senescence in both p53-dependent and -independent manner.
OH-GQDs exhibit evident cytotoxicity on lung carcinoma cells via inducing cells senescence in both p53-dependent and -independent manner.
Abstract
The numerous particular chemical/physical properties make graphene quantum dots (GQDs) attractive for various biomedical applications such as drug delivery, bioimaging and tumor photodynamic therapy (PDT). In the present study, the critical roles of hydroxyl-modified GQDs (OH-GQDs) on lung carcinoma A549 (wild type p53) and H1299 (p53-null) cells were investigated. Our data showed that a medium concentration (50 μg mL–1) of OH-GQDs significantly decreased the viability of A549 and H1299 cells. OH-GQDs treatment enhanced intracellular reactive oxygen species (ROS) generation. Furthermore, we found that treatment with ROS scavenger N-acetylcysteine (NAC) at least partially abolished the cytotoxic effect of OH-GQDs on A549 and H1299 cells. Hydroxylated GQDs lead to G0–G1 arrest and cells senescence. Signal pathway analysis revealed that OH-GQDs activated the expression of p21 in both a p53-dependent and -independent manner. Consistent with this, OH-GQDs could also inhibit the phosphorylation of Rb in both A549 and H1299 cells. These findings provide valuable information for the consideration of biomedical application of GQDs in the future.
Introduction
Graphene quantum dots (GQDs), as a new type of zero-dimensional (0D) carbon material, are smaller than 100 nm in size and consist of layers of single-atom thickness.1,2 Owing to their particular chemical/physical properties, GQDs have great potential in various areas such as energy, electrochemical, environmental and biomedical applications.1,3–6 Among them, biomedical application is a new field; the biological safety of GQDs has caught much attention in recent years. Due to their high water solubility and stability, GQDs have better biocompatibility when compared with other carbon-based nanomaterials.7 For instance, GQDs can serve as good vectors for drug or protein molecular delivery. Wang et al. found that, without any pre-modification, GQDs could efficiently deliver doxorubicin (DOX) into the nucleus and significantly increase its binding affinity to DNA, thereby improving its DNA cleavage activity and cytotoxicity even in drug resistant cancer cells.8 GQDs exhibit higher potency in drug delivery and cancer therapy than graphene oxide (GO) or other nanoparticles.7,8 In addition, based on the pH dependence of the binding of DOX with GQDs, GQDs can be designed as a novel pH-sensitive drug delivery system to improve drug release, particularly in cancer cells that are slightly more acidic than normal tissue and blood.9 Recently, Ge and colleagues revealed that GQDs are novel photodynamic therapy agents that can produce singlet oxygen in a multistate sensitization process.10 The excellent luminescent properties of GQDs also enable them to simultaneously act as good fluorescent probes for bioimaging.11,12 Despite the fact that these studies have proposed that GQDs appear to be bio-friendly materials,13 their biomedical applications will still be limited until their potential toxicity is carefully assessed.
Several recent studies have been performed to evaluate the in vivo and in vitro toxicity of GQDs in different biosystems. Wu's study demonstrated that GQDs exhibit much lower cytotoxicity in gastric and breast cancer cells when compared with micrometer-size GO.14 Furthermore, Zhang's group found that GQDs with sizes varying from 3 to 5 nm have no obvious toxic effect on mice. The biodistribution experiment revealed no GQD accumulation in the main organs of mice.15 On the other hand, Qin and colleagues proved that GQDs could significantly enhance the generation of reactive oxygen species (ROS) and then lead to activation of inflammatory response through activating the p38MAPK and NF-κB signaling pathways in macrophages.16 This study also revealed that GQDs result in autophagy, which has been recognized as the effector and player in genotoxicants-induced DNA damage response (DDR).17 Consistent with this report, GQDs also induce ROS generation in NIH-3T3 cells, in which GQDs increase the expression of DNA damage response proteins, including Rad51, OGG1 and p53.18 These results indicated that GQDs are potential genotoxic agents. In response to DNA damage, the cell will activate DNA damage response, a process in which the cells stop cell cycle progression to provide enough time to repair DNA lesions. If the damage is irreparable, cells will ultimately launch cell death program to prevent the genomic instability which is highly correlated with pathophysiology and disease, such as cancer.19 Therefore, the toxicity of GQDs is still a critical obstacle for their widespread application in biomedical fields.
Surface modification of nanomaterials can change their characteristics. For instance, partially hydroxylated and carboxylated GQDs exhibit a half-metallic state under almost same electric-field intensity.20 These modifications also provide a platform for conjugation with different functional molecules to facilitate greater biocompatibility. Yuan et al. compared the toxic effect of GQDs with different surface modifications (NH2, COOH and CO–N (CH3)2) and found that all of these modified GQDs had good biocompatibility even in high concentrations.21 However, Wang et al. analyzed the toxicity of nitrogen-doped graphene quantum dots (N-GQDs) in red blood cells (RBCs) and found that N-GQDs disturb the order and conformation of the lipids and lead to the formation of echinocytes.22 Herein, we studied the cytotoxicity of hydroxyl-modified GQDs (OH-GQDs) and demonstrated that 50 μg mL–1 OH-GQDs significantly inhibited the viability of human lung carcinoma cells (A549 and H1299). OH-GQDs also enhanced the ROS generation and block the cell cycle at G0–G1 phase. Finally, OH-GQDs induce cells senescence but not apoptosis (data not shown) by promoting p21 expression and inhibiting phosphorylation of Rb in both p53-dependent and -independent manners. Our study will provide valuable information for GQDs toxicity assessment.
Materials and methods
Material characterization
The hydroxyl-modified GQDs were purchased from XFNANO Materials Tech Co. (Nanjing, Jiangsu, China). Physical properties of GQDs were characterized by various techniques, including transmission electron microscopy (TEM), dynamic light scattering (DLS) and zeta potential analysis. The diameter of GQDs was observed by TEM (FEI, Tecnai G2). Hydrodynamic diameter of GQDs in PBS buffer was analyzed by a DLS instrument. Zeta potential testing was conducted using a particle analyzer (Malvern, Zetasizer Nano-ZS).
Cell culture and treatment
The human lung carcinoma cell lines A549 and H1299 were kindly provided by Dr Hongying Yang at Medical College of Soochow University, Suzhou, Jiangsu, China, and grown in RPMI 1640 medium with 10% FBS (HyClone, Hudson, NH, USA) at 37 °C with 5% CO2. Cells were treated with various concentrations of hydroxyl-modified GQDs (XFNANO Materials Tech Co., Nanjing, Jiangsu, China) for different times.
CCK-8 assay analysis of cell viability
A549 and H1299 cells were cultured in a 96-well plate at density of 5 × 103 cells per well. After 24 hours, cells were treated with 12.5, 25, 50 and 100 μg mL–1 hydroxyl-modified GQDs for 24 and 48 hours. The negative control groups were incubated with equal amount of PBS. The CCK-8 assay was performed according to the instructions. In brief, 10 μL CCK-8 solution (Beyotime, Shanghai, China) was added in each well and incubated for 1 hour at 37 °C. Absorbance was monitored at 450 nm and normalized to controls.
Cell cycle distribution analysis
A549 and H1299 cells were treated with 50 μg mL–1 OH-GQDs for 24 and 48 hours, respectively. Cells were harvested and then treated as previously described.23
Measurement of intracellular ROS generation
A549 and H1299 cells were seeded into 60 mm2 dish at a density of 1.5 × 105 mL–1. After 24 and 48 hours of 50 μg mL–1 OH-GQDs treatment, cells were incubated with 200 μL of 100 μM DCFH-DA (Sigma-Aldrich, St Louis, MO, USA) at 37 °C with 5% CO2 for 30 min. After the aspiration of the DCFH-DA, cells were harvested and the ROS probe was measured by flow cytometry. The data was presented as the average ROS intensity.
Senescence-associated β-galactosidase staining
Cells were treated with 50 μg mL–1 OH-GQDs for 24 and 48 hours and then fixed in 2% fomaldehyde/0.2% glutaraldehyde for 5 min at room temperature. The fixed cells were washed twice with PBS and then β-galactosidase staining solution containing 20 mg mL–1 X-gal (Beyotime, Shanghai, China) was added and cells were incubated for 6–10 hours at 37 °C without CO2.
Immunoblotting and immunofluorescence analysis
Whole-cell lysate preparation and western blotting were mentioned in our published paper.24 The primary antibodies used for western blotting analysis are anti-p21 (1 : 1000, Cell Signaling Technology, Danvers, MA, USA), anti-p53 (1 : 1000, Cell Signaling Technology, Danvers, MA, USA), anti-PIG3 (1 : 1000, Santa Cruz, California, CA, USA), anti-pRb, Rb (1 : 1000, Cell Signaling Technology, Danvers, MA, USA) and GAPDH (1 : 1000, Santa Cruz, California, CA, USA). Secondary antibodies were the goat, anti-rabbit and -mouse, IgG-horseradish peroxidase conjugated (Pierce, Rockford, IL).
For immunofluorescence assay, A549 and H1299 cells were seeded on slide covers in 35 mm2 dishes. Cells were treated with 50 μg mL–1 OH-GQDs for 24 and 48 hours and then fixed with 4% (v/v) paraformaldehyde. The immunofluorescent stainings were performed as previously described.25 In brief, the fixed cells were blocked in blocking buffer (2% BSA/PBS) for 30 min at room temperature. Cells were incubated with an anti-p21 (1 : 200, Cell Signaling Technology, Danvers, MA, USA) antibody and an anti-p53 (1 : 200, Cell Signaling Technology, Danvers, MA, USA) antibody for 4 hours at room temperature. The cells were then incubated with Alexa-568- and Alexa-488-conjugated secondary antibodies (BD Pharmingen, San Diego, CA, USA) for 1 h. Nuclei were stained with 100 μg mL–1 DAPI in the mounting solution. Confocal immunofluorescence microscopy was performed using an LSM 510 laser-scanning confocal microscope (Carl Zeiss, Oberkochen, Germany).
Statistical analysis
Data is presented as the mean ± SD of at least three independent experiments. The results were tested for significance using the unpaired Student's t test.
Results
Characteristics and morphology of OH-GQDs
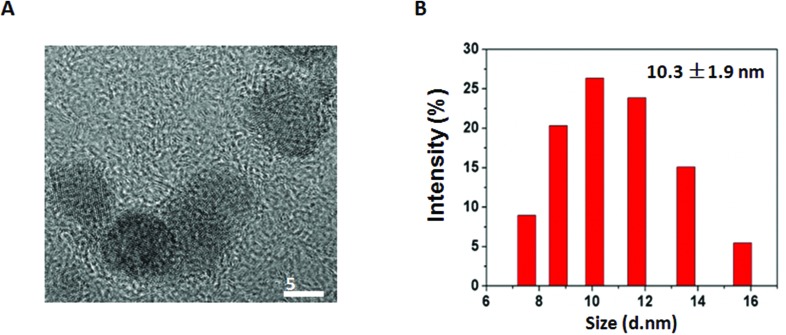
The hydroxylated GQDs were obtained commercially. GQDs were monodispersed with an average diameter of 5.6 ± 1.1 nm, which was calculated by measuring at least 100 GQDs under transmission electron microscopy (TEM). We used the dynamic light scattering (DLS) method to measure their hydrodynamic diameters. As shown in Fig. 1B, the hydrodynamic diameter of GQD is 10.3 ± 1.9 nm. The GQDs were negatively charged, as evidenced by –5.0 mV zeta potential, which was analyzed using a particle analyzer. GQDs were ultrasonicated for 5 min and vortexed for 1 min before use.
Fig. 1. Characterization of GQD. (A) Typical TEM image of GQD (scale 5 nm). (B) The hydrodynamic diameter of GQD.
The effect of hydroxylated GQDs on cellular viability of lung carcinoma cells
Wang et al. reported that GQDs could induce the expression of p53, which is a tumor suppressor and is correlated with DNA damage response.18 Here, we determined the effect of hydroxylated GQDs on proliferation and cell cycle progression using cells with different p53 gene status. Human lung carcinoma A549 (wild type p53) and H1299 (p53-null) cells were incubated with different concentrations (12.5, 25, 50 and 100 μg mL–1) of hydroxylated GQDs for 24 and 48 hours. As shown in Fig. 2A and B, a significant decrease of cell proliferation was induced by OH-GQDs in a concentration dependent manner for both cell lines. The A549 cell viability was found to be 76.8% and 65.2% when cells were exposed to 50 and 100 μg mL–1 OH-GQDs for 24 h, respectively. The cell viability further decreased to 58.9% and 31.7% when the cells were incubated with 50 and 100 μg mL–1 OH-GQDs for 48 h, respectively. The cell viability for H1299 cells was reduced to 86.9% and 64% when the cells were exposed to 50 and 100 μg mL–1 OH-GQDs for 24 h, and to 88.4% and 34.1% when the cells were exposed to 50 and 100 μg mL–1 OH-GQDs for 48 h (Fig. 2A and B). We further treated A549 and H1299 cells with 50 μg mL–1 OH-GQDs for different time periods (24, 48, 72 and 96 h), and cells proliferation speed was determined by cell counting. As shown in Fig. 2C and D, 50 μg mL–1 OH-GQDs almost totally inhibited the proliferation of A549 cells after 72 h of treatment. H1299 cells were still growing after 96 h of exposure to 50 μg mL–1 OH-GQDs; however, the proliferation speed was significantly slowed when compared with the control cells. These results indicated that hydroxylated GQDs have evident cytotoxicity towards lung cancer cells in a concentration dependent manner.
Fig. 2. Evaluation of cytotoxicity of hydroxyl-modified GQDs. (A, B) Cell viability after exposure to indicated concentration of OH-GQDs (12.5, 25, 50, 100 μg mL–1) for 24 and 48 hours, respectively. Data are expressed as mean ± standard deviation (S.D.), *p < 0.05, **p < 0.01, n = 5. (C, D) 1.6 × 105 A549 cells and 1.4 × 105 H1299 cells were plated at day 0 and cells were treated or untreated with 50 μg mL–1 OH-GQDs for indicated time. Cell numbers were counted at indicated day to generate a growth curve. The data are the mean ± S.D. from three tests, **p < 0.01 compared with control group at the same time point. (E, F) A549 and H1299 cells were exposed to 50 μg mL–1 OH-GQDs, meanwhile, cells were treated with indicated concentration of ROS scavenger NAC (1, 2 and 5 mM) for 48 hours. Cell viability was determined by CCK-8 assay. The data are the mean ± S.D. from five tests.
Hydroxylated GQDs induced the accumulation of G0–G1 cells population of cell cycle
To further ascertain the effect of OH-GQDs on cell growth, the cell cycle distribution of A549 and H1299 cells were analyzed by flow cytometry. The A549 and H1299 cells were incubated with 50 μg mL–1 OH-GQDs for 24 and 48 hours. This resulted in significantly increased accumulation of the G0–G1 population in both A549 and H1299 cells. The percentage of G0–G1 cells increased from 55.1% to 81.1% in A549 cells, and from 42.9% to 69.1% in H1299 cells after OH-GQD treatment for 48 hours. Such data suggested that a medium concentration of OH-GQDs was enough to induce robust G0–G1 cell accumulation through an unknown mechanism of cell cycle arrest, quiescence or senescence induction in both A549 and H1299 cells (Fig. 3A and B).
Fig. 3. Effect of hydroxylated GQDs on cell cycle distribution. (A, B) A549 and H1299 cells were treated with 50 μg mL–1 OH-GQDs for 24 and 48 hours, cell cycle distributions of these groups were detected by flow cytometry assay. The data are the mean ± S.D. from three tests.
OH-GQDs enhance ROS generation in A549 and H1299 cells
Several groups have revealed that GQDs could promote the generation of ROS in different types of cells. To further confirm the ROS generation in lung carcinoma cells after treatment with OH-GQDs, a DCFH-DA measurement was performed. We treated A549 and H1299 cells with 50 μg mL–1 OH-GQDs for 24 and 48 hours. As shown in Fig. 4, OH-GQD treatment significantly increased the ROS levels in A549 cells 48 hours after exposure. H1299 cells exhibited higher basal level of ROS. These results demonstrated that OH-GQDs could further enhance ROS generation.
Fig. 4. Hydroxyl-modified GQDs enhanced the generation of ROS. A549 and H1299 cells were treated with 50 μg mL–1 OH-GQDs for 24 and 48 hours. Cells were then incubated with 200 μL of 100 μM DCFH-DA for another 30 min at 37 °C. Cells were collected and detected using a 525 nm bandpass filter by flow-cytometric analysis. (A, C) Representative pictures of flow cytometry for the DCHF-DA probes. (B, D) Analysis by flow cytometry of the DCF+ incorporation rate in OH-GQDs treated or control groups of A549 and H1299 cells.
To further determine whether ROS plays an essential role in OH-GQD-induced cytotoxicity, different concentrations (1, 2 and 5 mM) of ROS scavenger N-acetylcysteine (NAC) were incubated with A549 and H1299 cells. At the same time, the cells were exposed to 50 μg mL–1 OH-GQDs. As shown in Fig. 2E and F, NAC significantly rescued the cell growth inhibited by OH-GQDs.
OH-GQDs induce senescence in lung carcinoma cells
Continuous generation of ROS can lead to permanent exit of the cells from the cell cycle, which is known as cellular senescence. We investigated the effect of OH-GQDs on senescent phenotype in both A549 and H1299 cell lines. Senescence-associated beta-galactosidase (SA-β-gal) activity was detected using SA-β-gal staining. Fig. 5 clearly shows that 50 μg mL–1 OH-GQDs treatment led to marked increase of senescence in both A549 and H1299 cells. Consistent with this result, we also found that OH-GQD-treated cells displayed an enlarged cell size, which is recognized as a marker of senescent cells (Fig. 5).
Fig. 5. Hydroxyl-modified GQDs induced cellular senescence in lung carcinoma cells. A549 and H1299 cells were treated with 50 μg mL–1 OH-GQDs for 24 and 48 hours and then stained with X-Gal. (A, C) Representative image of SA-β-Gal activity after 24 and 48 hours treatment. (B, D) Quantitative analysis of senescent cells. The data are presented as the means ± SD of three independent experiments.
Because p53–p21 pathway plays an important role in ROS induced senescence, we examined the expression levels of p53 and p21 in these cells using immunofluorescent staining and western blotting assays. We found that OH-GQDs dramatically promoted the accumulation of p21 in A549 cells 48 h after treatment, but did not affect the expression of another p53-induced gene, PIG3, which is reported to be correlated with p53-dependent apoptosis. Interestingly, OH-GQDs also induced the expression of p21 in p53-deficient H1299 cells despite the fact that the signal was weaker in comparison with the signal in p53-wt A549 cells, suggesting another upstream regulator also participates in OH-GQDs induced cell senescence (Fig. 6). We have also observed that OH-GQDs inhibited the phosphorylation of Rb protein (Fig. 6). These findings indicated that OH-GQDs induce ROS production, through which OH-GQDs activate p21 signal pathway in both p53-dependent and -independent manners. The activated p21 plays an important role in mediating cell cycle arrest and inducing senescence (Fig. 6C).
Fig. 6. Signaling pathway analysis after hydroxyl-modified GQDs treatment. (A) A549 and H1299 ells were fixed 24 and 48 hours after exposure to 50 μg mL–1 OH-GQDs and subjected to immunofluorescent analysis, the signal of p53 and p21 were detected. (B) A549 and H1299 cells were lysed 24 and 48 hours after exposure to 50 μg mL–1 OH-GQDs and subjected to western blot analysis; the expression of p53, p21, Rb, phosphorylated Rb and PIG3 were determined. (C) The work model of the effect of OH-GQDs on induction of cell cycle arrest and senescence through activation of p21, Rb signal pathways in both p53-dependent and -independent manner.
Discussion
Although the cytotoxicity of GQDs with or without modifications has been reported in a few recent studies, the conclusions are still contradictory.14,16,18,21 Moreover, the effects of hydroxylated GQDs on biosystems as a whole have not been elucidated. Here, our study demonstrated that the OH-GQDs exhibit obvious cytotoxicity on lung carcinoma A549 and H1299 cells, which have different p53 gene status (Fig. 2). A medium concentration of OH-GQDs (50 μg mL–1) almost totally inhibit the cell growth in A549 (wild type p53) cells and can also dramatically slow down H1299 (p53 null) cell growth speed (Fig. 2). p53 is one of the most studied tumor suppressors and plays an important role in genomic stability maintenance through its response to various environmental stimuli.26 Wang et al. revealed that GQDs could induce the expression of p53 in NIH-3T3 cells using flow cytometry analysis.18 In the present study, we did not find an increased expression of p53 in OH-GQD-treated A549 cells using western blotting method (Fig. 6). However, we did observe that OH-GQDs significantly promoted the accumulation of p53 in the nucleus 24 and 48 hours after exposure (Fig. 6A). As a transcription factor, nuclear translocation of p53 is necessary for its binding with DNA and achieving its activation.27 Therefore, our data indicated that OH-GQDs might contribute to p53 activation through facilitating p53 nuclear retention. Why unmodified GQDs enhance the expression of p53, whereas hydroxyl-modified GQDs regulate the cellular localization of p53 remains an interesting question.
In response to diverse stresses, activated p53 binds to DNA as a tetramer to transactivate or transrepress numerous target genes which are involved in different cellular responses, including cell cycle arrest, cellular senescence and apoptosis.28 p21 is one of the p53-activated factors and was first discovered as a mediator of cell cycle arrest by inhibiting the activity of cyclin-dependent kinase (CDK). Our results showed that OH-GQDs markedly increased the expression of p21 in A549 cells (Fig. 6). Interestingly, we also observed significantly increased expression of p21 in p53-null lung cancer H1299 cells after OH-GQDs treatment. In addition to p53, several other transcription factors can bind to p21 promoter to regulate p21 expression. For example, forkhead box A1/2 is required for p21 transcription in p53-null H1299 cells.29 Chk2 can activate p21 transcription in p53-deficient breast cancer HaCaT and SK-BR-3 cells.30 Furthermore, transcription factors including SP1,31 ATM,32 Brca133 and c-Myc34 were demonstrated to involve in p21 activation, even in p53 deficient cells. The potential signal pathways that participate in OH-GQD-induced p21 activation in p53-null H1299 cells merit further exploration. Due to the important role of p21 in cell cycle arrest, we analyzed the cell cycle distribution of A549 and H1299 cells and discovered that treatment with 50 μg mL–1 OH-GQDs resulted in robust increased accumulation of G0–G1 cell population in both of the two lung cancer cell lines, used in this study (Fig. 3). Together, our results indicated that OH-GQDs could halt cell cycle progression by activating the expression of p21 in both p53-dependent and -independent manner.
In addition to GQDs, several other nanoparticles have been shown to activate p21 signaling, most probably via induction of oxidative stress,35 such as silica nanoparticles36 and zinc oxide nanoparticles.37 Wang18 and Qin16 have shown that GQD treatment increase intracellular ROS generation. Consistent with this report, our findings showed that OH-GQDs significantly promote ROS generation in lung carcinoma cells. Furthermore, we demonstrated that treatment of cells with NAC, a ROS scavenger, could at least partially protect them against OH-GQD-mediated cytotoxicity (Fig. 2E and F). p53 has both pro-oxidant and antioxidant properties in response to various stresses through activating different groups of downstream target genes. For example, p53 has been implicated in regulation of a subset of important antioxidant factors including glutathione peroxidase 1 (GPX1) and mitochondrial superoxide dismutase 2, suggesting it has an essential role in the antioxidant defence system.38,39 Meanwhile, p53 also transactivates a series of gene-encoding proteins that promote intracellular ROS generation.40,41 In the p53-null H1299 cells, we observed a higher basal level of ROS when compared with A549 cells, indicating that p53 might play an important role in inhibiting oxidative stress in lung carcinoma. On the other hand, Polyak et al. first proposed a model in which p53-induced apoptosis is associated with the generation of cellular ROS mediated by PIGs, such as the p53-induced gene 3 (PIG3), which is a homolog of NADPH:quinone oxidoreductase and has been implicated in p53-induced apoptosis.41 In the present study, we found that OH-GQDs did not affect the expression level of PIG3. Consistent with this, we did not find an effect of OH-GQDs on cells apoptosis (data not shown). How OH-GQDs influence the p53-induced genes expression profile and ultimately determine the fate of the cells still remains unclear.
Sustained generation of ROS induces intracellular oxidative stress which will eventually lead to cellular senescence through activating p21 signal pathway.42 The retinoblastoma protein (Rb) plays an essential role in cellular senescence maintenance. The tumor suppressor Rb binds to the transcription factor E2F and blocks its interaction with target genes, which function to facilitate G1/S cell cycle transition. The cyclin/CDK mediated phosphorylation of Rb disrupts the binding of Rb to E2F,43 allowing the promotion of normal cell cycle progression. p53-dependent or -independent p21 activation can inhibit cyclin/CDK activity and phosphorylation of Rb. Our data showed that OH-GQDs induce cellular senescence and that phosphorylation of Rb was suppressed in both lung carcinoma p53 wild-type A549 and p53 deficient H1299 cells. Altogether, our investigation demonstrated that nanomaterial OH-GQDs induce cell cycle arrest and senescence, which might occur via activation of p21-Rb signal pathway in both p53-dependent and -independent manner. Recently, Ulusoy and colleagues’ work evaluated the cytotoxicity effects of CdTe/CdS/ZnS quantum dots on three-dimensional (3D) spheroid cultured human adipose-derived mesenchymal stem cells (hAD-MSCs).44 It warrants further investigation to assess the cytotoxicity of OH-GQDs using the 3D in vitro model.
Conclusions
Our study indicated that hydroxyl-modified GQDs exhibit evident cytotoxicity on lung carcinoma cells. OH-GQDs are able to regulate intracellular ROS generation and G0–G1 cell cycle arrest. In addition, hydroxylated GQD treatment leads to cellular senescence, which might be mediated by activating p21-Rb signal pathway in both p53-dependent and -independent manner.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81530085, 81472919, 81573079), and the Natural Science Foundation of Jiangsu Province of China (BK20131149), the Six Talent Peaks Project of Jiangsu Province of China (WSN095), the Suzhou Administration of Science and Technology (SYS201360) and Suzhou Key Medical Center (SZZX201506).
References
- Shen J. H., Zhu Y. H., Yang X. L., Li C. Z. Chem. Commun. 2012;48:3686–3699. doi: 10.1039/c2cc00110a. [DOI] [PubMed] [Google Scholar]
- Pan D. Y., Zhang J. C., Li Z., Wu M. H. Adv. Mater. 2010;22:734–738. doi: 10.1002/adma.200902825. [DOI] [PubMed] [Google Scholar]
- Li Y., Hu Y., Zhao Y., Shi G. Q., Deng L. E., Hou Y. B., Qu L. T. Adv. Mater. 2011;23:776–780. doi: 10.1002/adma.201003819. [DOI] [PubMed] [Google Scholar]
- Yan X., Cui X., Li B. S., Li L. S. Nano Lett. 2010;10:1869–1873. doi: 10.1021/nl101060h. [DOI] [PubMed] [Google Scholar]
- Zheng X. T., Than A., Ananthanaraya A., Kim D. H., Chen P. ACS Nano. 2013;7:6278–6286. doi: 10.1021/nn4023137. [DOI] [PubMed] [Google Scholar]
- Markovic Z. M., Ristic B. Z., Arsikin K. M., Klisic D. G., Harhaji-Trajkovic L. M., Todorovic-Markovic B. M., Kepic D. P., Kravic-Stevovic T. K., Jovanovic S. P., Milenkovic M. M., Milivojevic D. D., Bumbasirevic V. Z., Dramicanin M. D., Trajkovic V. S. Biomaterials. 2012;33:7084–7092. doi: 10.1016/j.biomaterials.2012.06.060. [DOI] [PubMed] [Google Scholar]
- Zhang L. M., Xing Y. D., He N. Y., Zhang Y., Lu Z. X., Zhang J. P., Zhang Z. J. J. Nanosci. Nanotechnol. 2012;12:2924–2928. doi: 10.1166/jnn.2012.5698. [DOI] [PubMed] [Google Scholar]
- Wang C., Wu C. Y., Zhou X. J., Han T., Xin X. Z., Wu J. Y., Zhang J. Y., Guo S. W. Sci. Rep. 2013;3:2852. doi: 10.1038/srep02852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J. C., Zhang R. B., Li J. H., Sang Y. H., Tang W., Gil P. R., Liu H. Int. J. Nanomed. 2015;10:6709–6724. doi: 10.2147/IJN.S91864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J. C., Lan M. H., Zhou B. J., Liu W. M., Guo L., Wang H., Jia Q. Y., Niu G. L., Huang X., Zhou H. Y., Meng X. M., Wang P. F., Lee C. S., Zhang W. J., Han X. D. Nat. Commun. 2014;5:4596. doi: 10.1038/ncomms5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. H., Xia J. F., Zhou C. F., Via B., Xia Y. Z., Zhang F. F., Li Y. H., Xia L. H., Tang J. Colloids Surf., B. 2013;112:192–196. doi: 10.1016/j.colsurfb.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Zhu S. J., Zhang J. H., Qiao C. Y., Tang S. J., Li Y. F., Yuan W. J., Li B., Tian L., Liu F., Hu R., Gao H. N., Wei H. T., Zhang H., Sun H. C., Yang B. Chem. Commun. 2011;47:6858–6860. doi: 10.1039/c1cc11122a. [DOI] [PubMed] [Google Scholar]
- Feng L. Z., Liu Z. A. Nanomedicine. 2011;6:317–324. doi: 10.2217/nnm.10.158. [DOI] [PubMed] [Google Scholar]
- Wu C. Y., Wang C., Han T., Zhou X. J., Guo S. W., Zhang J. Y. Adv. Healthcare Mater. 2013;2:1613–1619. doi: 10.1002/adhm.201300066. [DOI] [PubMed] [Google Scholar]
- Chong Y., Ma Y. F., Shen H., Tu X. L., Zhou X., Xu J. Y., Dai J. W., Fan S. J., Zhang Z. J. Biomaterials. 2014;35:5041–5048. doi: 10.1016/j.biomaterials.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Qin Y. R., Zhou Z. W., Pan S. T., He Z. X., Zhang X. J., Qiu J. X., Duan W., Yang T. X., Zhou S. F. Toxicology. 2015;327:62–76. doi: 10.1016/j.tox.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Zhang S. M., Shang Z. F., Zhou P. K. Toxicol. Res. 2015;4:613–622. [Google Scholar]
- Wang D., Zhu L., Chen J. F., Dai L. M. Nanoscale. 2015;7:9894–9901. doi: 10.1039/c5nr01734c. [DOI] [PubMed] [Google Scholar]
- Matt S., Hofmann T. G. Cell. Mol. Life Sci. 2016;3:2829–2850. doi: 10.1007/s00018-016-2130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Yang F., Xue Y., Xiao D., Guo Y. ChemPhysChem. 2014;15:157–164. doi: 10.1002/cphc.201300768. [DOI] [PubMed] [Google Scholar]
- Yuan X., Liu Z., Guo Z., Ji Y., Jin M., Wang X. Nanoscale Res. Lett. 2014;9:108. doi: 10.1186/1556-276X-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. T., Zhu S. J., Jiang X. E. Toxicol. Res. 2015;4:885–894. [Google Scholar]
- Yu L., Shang Z. F., Hsu F. M., Zhang Z., Tumati V., Lin Y. F., Chen B. P. C., Saha D. Oncotarget. 2015;6:3848–3860. doi: 10.18632/oncotarget.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L. H., Shang Z. F., Tan W., Liu X. D., Xu Q. Z., Song M., Wang Y., Guan H., Zhang S. M., Yu L., Zhong C. G., Zhou P. K. Oncotarget. 2015;6:7011–7022. doi: 10.18632/oncotarget.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Z. F., Huang B., Xu Q. Z., Zhang S. M., Fan R., Liu X. D., Wang Y., Zhou P. K. Cancer Res. 2010;70:3657–3666. doi: 10.1158/0008-5472.CAN-09-3362. [DOI] [PubMed] [Google Scholar]
- Jimenez G. S., Khan S. H., Stommel J. M., Wahl G. M. Oncogene. 1999;18:7656–7665. doi: 10.1038/sj.onc.1203013. [DOI] [PubMed] [Google Scholar]
- Li Q., Falsey R. R., Gaitonde S., Sotello V., Kislin K., Martinez J. D. Oncogene. 2007;26:7885–7893. doi: 10.1038/sj.onc.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieging K. T., Mello S. S., Attardi L. D. Nat. Rev. Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J. H., Jang S. M., Kim J. W., Kim C. H., Song P. I., Choi K. H. FEBS Lett. 2014;588:4065–4070. doi: 10.1016/j.febslet.2014.09.033. [DOI] [PubMed] [Google Scholar]
- Aliouat-Denis C. M., Dendouga N., Van den Wyngaert I., Goehlmann H., Steller U., van de Weyer I., Van Slycken N., Andries L., Kass S., Luyten W., Janicot M., Vialard J. E. Mol. Cancer Res. 2005;3:627–634. doi: 10.1158/1541-7786.MCR-05-0121. [DOI] [PubMed] [Google Scholar]
- Gartel A. L., Goufman E., Najmabadi F., Tyner A. L. Oncogene. 2000;19:5182–5188. doi: 10.1038/sj.onc.1203900. [DOI] [PubMed] [Google Scholar]
- Ju R., Muller M. T. Cancer Res. 2003;63:2891–2897. [PubMed] [Google Scholar]
- Somasundaram K., Zhang H., Zeng Y. X., Houvras Y., Peng Y., Wu G. S., Licht J. D., Weber B. L., El-Deiry W. S. Nature. 1997;389:187–190. doi: 10.1038/38291. [DOI] [PubMed] [Google Scholar]
- Li H., Wu X. Biochem. Biophys. Res. Commun. 2004;324:860–867. doi: 10.1016/j.bbrc.2004.09.130. [DOI] [PubMed] [Google Scholar]
- Dutto I., Tillhon M., Cazzalini O., Stivala L. A., Prosperi E. Arch. Toxicol. 2015;89:155–178. doi: 10.1007/s00204-014-1430-4. [DOI] [PubMed] [Google Scholar]
- Ye Y., Liu J., Chen M., Sun L., Lan M. Environ. Toxicol. Pharmacol. 2010;29:131–137. doi: 10.1016/j.etap.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Roy R., Singh S. K., Chauhan L. K., Das M., Tripathi A., Dwivedi P. D. Toxicol. Lett. 2014;227:29–40. doi: 10.1016/j.toxlet.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Hussain S. P., Amstad P., He P., Robles A., Lupold S., Kaneko I., Ichimiya M., Sengupta S., Mechanic L., Okamura S., Hofseth L. J., Moake M., Nagashima M., Forrester K. S., Harris C. C. Cancer Res. 2004;64:2350–2356. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- Tan M., Li S., Swaroop M., Guan K., Oberley L. W., Sun Y. J. Biol. Chem. 1999;274:12061–12066. doi: 10.1074/jbc.274.17.12061. [DOI] [PubMed] [Google Scholar]
- Kang M. Y., Kim H. B., Piao C., Lee K. H., Hyun J. W., Chang I. Y., You H. J. Cell Death Differ. 2013;20:117–129. doi: 10.1038/cdd.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Xia Y., Zweier J. L., Kinzler K. W., Vogelstein B. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- Sharpless N. E., Sherr C. J. Nat. Rev. Cancer. 2015;15:397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- Narasimha A. M., Kaulich M., Shapiro G. S., Choi Y. J., Sicinski P., Dowdy S. F. eLife. 2014;3:e02872. doi: 10.7554/eLife.02872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulusoy M., Lavrentieva A., Walter J. G., Sambale F., Green M., Stahl F., Scheper T. Toxicol. Res. 2016;5:126–135. doi: 10.1039/c5tx00236b. [DOI] [PMC free article] [PubMed] [Google Scholar]