Abstract
Purpose
Lung cancer is the deadliest known cancer in the world, with the highest number of mutations in proto-oncogenes and tumor suppressor genes. Therefore, this study was conducted to determine the status of hotspot regions in DDR2 and KRAS genes for the first time, as well as in TP53 gene, in lung cancer patients within the Iranian population.
Experimental design
The mutations in exon 2 of KRAS, exon 18 of DDR2, and exons 5–6 of TP53 genes were screened in lung cancer samples, including non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) using PCR and sequencing techniques.
Results
Analysis of the KRAS gene showed only a G12C variation in one large cell carcinoma (LCC) patient, whereas variants were not found in adenocarcinoma (ADC) and squamous cell carcinoma (SCC) cases. The Q808H variation in the DDR2 gene was detected in one SCC sample, while no variant was seen in the ADC and LCC subtypes. Variations in the TP53 gene were seen in all NSCLC subtypes, including six ADC (13.63%), seven SCC (15.9%) and two LCC (4.54%). Forty-eight variants were found in the TP53 gene. Of these, 15 variants were found in coding regions V147A, V157F, Q167Q, D186G, H193R, T211T, F212L and P222P, 33 variants in intronic regions rs1625895 (HGVS: c.672+62A>G), rs766856111 (HGVS: c.672+6G>A) and two new variants (c.560-12A>G and c.672+86T>C).
Conclusions
In conclusion, KRAS, DDR2, and TP53 variants were detected in 2%, 2.17% and 79.54% of all cases, respectively. The frequency of DDR2 mutation is nearly close to other studies, while KRAS and TP53 mutation frequencies are lower and higher than other populations, respectively. Three new putative pathogenic variants, for the first time, have been detected in Iranian patients with lung cancer, including Q808H in DDR2, F212L, and D186G in coding regions of TP53. In addition, we observed five novel benign variants, including Q167Q, P222P and T211T in coding sequence, and c.560-12A>G and c.672+86T>C, in intronic region of TP53. Mutations of KRAS and DDR2 were found in LCC and SCC subtypes, respectively, whereas mutations of TP53 were seen in SCC and ADC subtypes with higher frequencies and LCC subtype with lower frequency. Therefore, Iranian lung cancer patients can benefit from mutational analysis before starting the conventional treatment. A better understanding of the biology of these genes and their mutations will be critical for developing future targeted therapies.
Introduction
Lung cancer is the leading cause of cancer-related death in both men and women worldwide. Non-small cell lung cancer (NSCLC), with an incidence of 80% to 85%, is the most common type of lung cancer [1]. Lung cancer is often diagnosed when a person is in advanced stages of the disease and the prognosis is poor [2].
Many efforts have been made to treat patients with lung cancer. Surgery, chemotherapy, radiotherapy, and targeted therapies are conventional lung cancer treatments [3]. Targeted therapies with tyrosine kinase inhibitors (TKIs) comprise epidermal growth factor receptor (EGFR) inhibitors, such as erlotinib or gefitinib, and anaplastic lymphoma kinase (ALK) inhibitors, such as crizotinib [4, 5]. Considering the high mortality and morbidity rates of lung cancer and the emergence of drug resistance to chemoradiotherapy regimens and TKIs, determining targetable genetic changes is of paramount importance [6].
Research has shown that the genetic variation in lung cancer is higher than that of other cancers [7]. The DDR2 gene, which is located on the long arm of chromosome 1 (1q23.3) is a tyrosine kinase receptor that plays a critical role in cellular connectivity, survival, migration and cell proliferation [8]. In tumor cells, driver mutations in kinase domain activation loops, autoinhibitory juxtamembrane regions, and ligand binding domains, can interrupt kinase function and initiate pro‑migratory and pro‑invasive cascades [9]. A substitution of serine to arginine at position 768 (S768R) of exon 18 has been reported as the most common mutation in the DDR2 gene [8, 10]. In one study, Hammerman et al. found that DDR2 mutations account for nearly 4% of squamous cell carcinoma (SCC) subtype [8]. Further evaluations in Korea, China, and France populations revealed that the frequencies of DDR2 mutations were 2%, 4.6%, and 4% in SCC, respectively [10–12]. However, Kenmotsu et al. and Yashima et al. did not find any mutations in DDR2 gene of Japanese SCC patients [13, 14]. In addition, despite the broader range of mutated genes in SCC, there is no effective targeted treatment for this subtype [15–17]. Some studies have shown that the targeting of DDR2 by FDA-approved kinase inhibitors including dasatinib, imatinib, nilotinib, and ponatinib can suppress the proliferation of this gene in mutated cancer cell lines [18, 19]. Dramatic response to dasatinib has been reported in SCC patients harboring S768R mutations in exon 18 of DDR2, and thus this region has been introduced as a valuable molecular target of TKIs in these patients [10, 20].
KRAS proto-oncogene (12p12.1) is a GTPase that is located on the downstream pathway of the tyrosine kinase receptors and involved in cell growth, differentiation, and apoptosis. Investigations of KRAS status in NSCLC patients revealed a wide spectrum of mutations in different countries: 8.4% in China, 21% in Japan, 27% in Greece and Italy, 29% in France, and 43.3% in Spain [21–26]. The most prevalent mutated region of KRAS in lung cancer is codon 12 (exon 2) with 75% frequency, whereas mutations in other regions of KRAS are less frequent including codon 13 (exon 2) and codon 63 (exon 3) [27, 28]. A previous meta-analysis demonstrated that the presence of KRAS mutations was a negative prognostic factor for the overall survival of patients with lung cancer, but a more recent study showed that only the presence of KRAS mutations in exon 2 had a predictive value in adenocarcinoma (ADC) patients [27, 29]. Targeted therapies with TKIs have been effective in ADC, but the presence of KRAS mutations induces resistance to treatment with EGFR-independent mechanisms [6]. The RAS/MAPK pathway, with the key component of KRAS, is one of the major signaling networks linking to EGFR signaling. Hence, mutations in downstream effectors of EGFR signaling could lead to resistance to EGFR inhibitors [30]. Moreover, the response to TKIs varies among lung cancer patients with KRAS mutations and may be affected by such factors as coexistence of mutations in tumor suppressor genes (TP53 or PTEN) [31, 32]. Simultaneous analysis of KRAS and TP53 mutations has an important role in determining the prognosis and appropriate treatment strategies for lung cancer patients [33]. The gene TP53, 17p13.1, encodes a tumor suppressor protein that plays a role in regulating the cell cycle. In genomic damage, TP53 plays an anti-cancer role by preventing and suppressing abnormal cell growth by cell cycle arrest, DNA repair, control of metabolism, and apoptosis. Mutations within the TP53 gene itself or mutations of downstream mediators of TP53 lead to inactivation of its function [34, 35]. Prevalence of TP53 gene mutation accounts for nearly 39% of ADC, 51% of SCC, 68% of large cell carcinoma (LCC), and 80% of small cell lung cancer (SCLC) [34, 36]. In addition, a frequent variation has been found in TP53 mutations in lung cancer patients with different ethnicities [37].
Previous studies have shown that TP53 gene in exons 5 to 8 has a considerably higher mutation rate and exons 5–6 have been identified as the mutational hotspot regions [38, 39]. A more recent and comprehensive study by Baugh et al determined a list of the 50 most common mutations in the TP53 gene are associated with disruption of protein structures and highly deleterious VIPUR scores (> 0.5)[40]. They demonstrated that R175H (exon 5) mutation had the highest frequency, whereas R248Q (exon 7) and R273H (exon 8) mutations were located in the next positions.
No studies were found on the status of the KRAS and DDR2 genes in the Iranian population [41]. To date, we have only found two studies on TP53 mutations in SCC, however, other subtypes (ADC, LCC, SCLC) have not yet been evaluated in Iranian patients [42, 43]. The above-mentioned lines of evidence and geographical variation in the prevalence of gene mutations indicate that studying the status of KRAS, DDR2, and TP53 may have important implications for diagnosis, prognosis, cancer recurrence prevention, and designing clinical trials and targeted therapies for Iranian population with lung cancer. Therefore, we conducted this study to explore the status of KRAS, DDR2, and TP53 genes in hotspot regions on a panel of lung cancer samples including three major NSCLC subtypes (ADC, SCC, and LCC) and SCLC in the Iranian population. Moreover, we examined the potential correlations among mutational status of KRAS, DDR, and TP53 genes with clinicopathological parameters in this study.
Materials and methods
Patient characteristics
Fifty-five formalin-fixed paraffin-embedded (FFPE) samples of lung cancer, including NSCLC and SCLC, were collected from several referral hospitals in Tehran, Iran. All samples were investigated by an expert pathologist and had a histologic diagnosis of primary lung carcinoma, containing at least 50% tumor cells [44, 45]. We selected cases with sufficient material for molecular analyses. The specimens were obtained before any systematic treatment. The clinicopathological parameters of the patients, including tumor types, histological grade (in SCC and ADC) and inflammation (in SCC) were obtained by reviewing their medical records. This research was approved by the Iran University of Medical Sciences (IUMS) Research Ethics Committee. Patients’ data were kept fully anonymous.
Mutational analysis
DNA extraction
After removing the surrounding paraffin, the tissues were cut into seven micrometer thick sections, xylene (Merck Co., Germany) was added and the samples were incubated at 60°C for deparaffinization. The samples were then hydrated with a decreased serial dilution of ethanol and incubated at 60°C at each step. A lysis solution and proteinase K were added to the tissue samples, which were then incubated at 60°C overnight. DNA extraction was performed using the FavorPrep™ Tissue Genomic DNA Extraction Mini Kit (Cat number: FATGK001, Favorgen, Taiwan) following the manufacturer’s recommendations. Extracted DNA was quantified on the NanoDrop 8000 (Thermo Scientific).
PCR
PCR was carried out using a super PCR Master Mix 2X (Cat number: YT1553, Yekta Tajhiz Azma Co., Iran) according to the manufacturer’s protocol. The PCR program, which was repeated for each gene over 35 cycles, was as follows; 94°C for one minute, annealing phase at 57.5°C for KRAS, 55.5°C for DDR2 and 52°C for TP53 for one minute, and extension phase at 72°C for three minutes. The PCR products were electrophoresed on a 1% agarose gel.
Most of the tumor samples collected were of the SCC type and S768R substitutions are commonly reported in SCC patients. As such, we decided to design the primers for exon 18 of DDR2 to be 5’-GGGTATAGCTGCAGATTATGAA-3´ for forward and 5´-CATTCATCCCCAACAGTTCTTA-3´ for reverse. Primers were designed by an online website (http://simgene.com/Primer3). We also used the previously described primer pairs (5’-TTTCTTTGCTGCCGTCTTC-3´ as forward and 5´-TTGCACATCTCATGGGGTTA-3´ as reverse) for exons 5–6 of TP53 and 5’-AAAGGTACTGGTGGAGTATTTGATAGTG-3´ as forward and 5´-TCATGAAAATGGTCAGAGAAACCT-3´ as reverse primers for exon 2 (codon 12) of KRAS (23, 24). To confirm the quality of these primers, we examined the number of nucleotides, Tm temperature, GC ratio, the possibility of forming secondary structures and their proper attachment to the desired gene with the help of online tools, such as the NCBI Primer BLAST (www.ncbi.nlm.nih.gov/tools/primer-blast/) and the Beacon Designer program (http://www.premierbiosoft.com/). All the primers are listed in S1 Table. We also included appropriate negative control at each PCR process, as mentioned in the Sanger sequencing guidelines [46].
Sequencing
After confirming the band for each gene, the PCR products were purified and screened for mutations using the Sanger sequencing analysis (DNA Analyzer ABI PRISM® 3700).
Data analysis
Statistical analyses were performed using SPSS software version 20 (SPSS, Chicago, IL, USA). The associations of TP53 status with clinicopathological parameters were assessed using Pearson’s χ 2 or the Fisher's exact test, where appropriate. A p- value of < 0.05 was considered statistically significant.
All sequences were analyzed by mutation surveyor V3.30 (Softgenetics, Pennsylvania, US). Quality scores for all the sequences were more than 20, with less than 5% noise. We only confirmed the variations that did not have noise in the region of interest (ROI) of the sequence. We excluded the sequences without these criteria. We also evaluated the quality of the sequences and variations by Sequence Scanner v1.0. For each variant, Phred scores were more than 30 (between 48 to 62). The variants were described in HGVS nomenclature (GRCh38) by an online tool (https://mutalyzer.nl). We also used the following in-silico tools, as described in the ACMG guidelines [47, 48], for interpretation of sequence variants: MutationTaster (http://www.mutationtaster.org/), CADD (http://cadd.gs.washington.edu/), varsome (https://varsome.com/) and CGI (https://www.cancergenomeinterpreter.org). For checking the previously reported variants, we reviewed the following online databases: The UniProt database (http://www.uniprot.org; UniProtKB ID Q8IYM9), the NCBI dbSNP database (https://www.ncbi.nlm.nih.gov/SNP/), the Catalogue of Somatic Mutations in Cancer (COSMIC; http://cancer.sanger.ac.uk/cosmic) and 1000 Genomes (http://www.1000genomes.org/).
Results and discussion
Study population
Fifty-five tumor samples were used for the KRAS gene mutation analysis. Due to a lack of genomic DNA, TP53 and DDR2 were examined in 44 and 46 samples, respectively. The patient characteristics are summarized in S2 Table. The median age of the patients was 65.7 years (range, 37–83 years). They were 46 male (83.6%) and 9 female patients (16.36%) (male to female ratio = 5.1). Thirteen patients (23.63%) had ADC, 34 (61.81%) SCC, four (7.27%) LCC, one (1.81%) mixed LCC/SCC, two (3.63%) NSCLC without mentioned subtype and one (1.81%) SCLC. SCC was the major histologic type. The histologic grade of patients was as follows: three (23.07%): poor, four (30.76%): moderate, and two (15.38%): well differentiated in ADC, and 10 (26.41%): poor, six (17.64%): moderate, and 13 (38.23%): well differentiated in SCC. A total of 12 (35.29%) patients with SCC had inflammation.
KRAS Mutations analysis
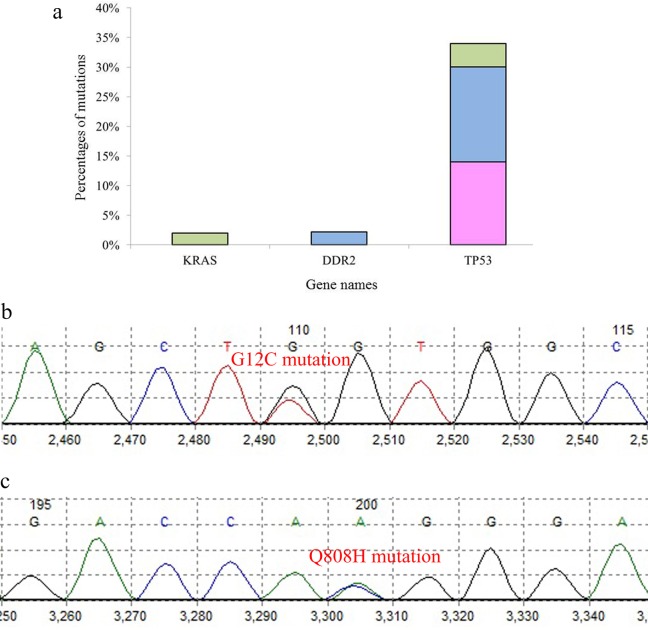
G12C substitution, with G>T transversion (GGT>TGT), was observed in a 67-year-old man with LCC (2%), but there was no other mutation in patients with ADC and SCC (Figs 1 and 2). An rs1625895 variant was another finding in the TP53 gene of this patient (Tables 1–4).
Fig 1. The PCR products on the gel agarose electrophoresis.
Fig 2. Analysis of the lung cancer samples for KRAS, DDR2 and TP53 gene mutations.
(a) percentage of KRAS, DDR2 and TP53 mutations in different subtypes of lung cancer; (green: SCLC, blue: SCC, pink: ADC). (b) G12C mutation in KRAS and (c) Q808H mutation in DDR2.
Table 1. Frequency and type of coding variants in KRAS, DDR2, and TP53 genes in lung tumor samples.
| Pt. ID | Gender | Age | Tumor type | KRAS | DDR2 | TP53 | Phred Score (ROI) |
|---|---|---|---|---|---|---|---|
| 8 | M | 67 | SCC | No. | No. | D186G | 52 |
| H193R | 51 | ||||||
| P222P | 50 | ||||||
| 13 | M | 69 | SCC | No. | No. | Q167Q | 48 |
| 15 | M | 67 | SCC | No. | Q808H | NA | 51 |
| 25 | M | 58 | ADC | No. | No. | V157F | 54 |
| 26 | M | 67 | ADC | No. | No. | D186G | 55 |
| H193R | 52 | ||||||
| P222P | 51 | ||||||
| 35 | M | 45 | SCC | No. | No. | F212L | 62 |
| 36 | M | 64 | SCC | No. | No. | F212L | 50 |
| 41 | M | 72 | SCC | No. | No. | V147A | 50 |
| 49 | F | 68 | ADC | NA | No. | T211T | 62 |
| P222P | 62 | ||||||
| 67 | M | 62 | LCC | No. | No. | D186G | 53 |
| H193R | 53 | ||||||
| 70 | M | 67 | LCC | G12C | No. | No. | 57 |
M = male, F = female, ROI = region of interest
NA = Not available
Table 4. The predictions of variants effect based on in silico tools.
| Gene | Variant | Cadd phred | Raw Score | Oncogenic classification* | Mutation taster | SIFTcat | PolyPhenCat | DANN score | ClinVar |
|---|---|---|---|---|---|---|---|---|---|
| KRAS | G12C | 31 | 6.5 | NSCLC; OV; LUAD; THCA; COREAD; | Disease causing | Deleterious | Possibly damaging | 0.9987 | Pathogenic |
| DDR2 | Q808H | 24.7 | 4.7 | TIER 2 | Disease causing | Deleterious | Probably damaging | 0.9953 | NM |
| TP53 | D186G | 22.9 | 3.3 | Passenger | Disease causing | Tolerated | Probably damaging | 0.9943 | NM |
| H193R | 23.5 | 3.9 | known in any cancer type | Disease causing | Deleterious | Probably damaging | 0.9876 | Likely pathogenic | |
| P222P | 21.2 | 2.7 | Not protein affecting | Disease causing | NM | NM | 0.5001 | Likely benign | |
| Q167Q | 8.331 | 0.6 | Not protein affecting | Disease causing | NM | NM | 0.5293 | NM | |
| V157F | 24.2 | 4.4 | Hepatocellular carcinoma | Disease causing | Deleterious | Probably damaging | 0.9909 | Pathogenic/Likely pathogenic | |
| F212L | 10.92 | 1.0 | TIER 1 | Polymorphism | Tolerated | Benign | 0.7743 | NM | |
| V147A | 25.9 | 5.3 | TIER 1 | Disease causing | Deleterious | Probably damaging | 0.9917 | NM | |
| T211T | 3.703 | 0.1 | Not protein affecting | Disease causing | NM | NM | 0.487 | NM |
According to the oncodriveMUT method (tier 1 and 2 represent higher and lower level of stringency of the driver prediction, respectively).
NSCLC: Non-small Cell Lung Cancer, OV: Ovary Cancer, LUAD: Lung Adenocarcinoma, THCA: Thyroid Carcinoma, COREAD: Colorectal Adenocarcinoma, NM: Not Mention.
Table 2. The data of observed variations based on HGVS38 in coding sequence.
| Transcript ID | RefSeq | Gene | Gene role | Variant | HGVS38 (Chromosomal variant) | HGVS38 (transcripts variant) | MAF | db SNP ID/ COSMIC ID |
|---|---|---|---|---|---|---|---|---|
| ENST00000311936.7 | NM_004985 | KRAS | OG | G12C | NC_000012.12:g.25245351C>T | NM_004985.4:c.34G>A | 1.976e-05 | rs121913530 |
| ENST00000367922.7 | NM_001014796 | DDR2 | OG | Q808H | NC_000001.11:g.162778720A>C | NM_001014796.1:c.2424A>C | 0.0002393 | rs765660823 |
| ENST00000269305.8 | NM_000546 | TP53 | TSG | D186G | NC_000017.11:g.7675055T>C | NM_000546.5:c.557A>G | NM. | COSM46287 |
| H193R | NC_000017.11:g.7674953T>C | NM_000546.5:c.578A>G | NM. | rs786201838 | ||||
| P222P | NC_000017.11:g.7674865C>T | NM_000546.5:c.666G>A | 6.748e-05 | rs72661118 | ||||
| Q167Q | NC_000017.11:g.7675111C>T | NM_000546.5:c.501G>A | NM. | COSM44299 | ||||
| V157F | NC_000017.11:g.7675143C>A | NM_000546.5:c.469G>T | 0.00006/7 | rs121912654 | ||||
| F212L | NC_000017.11:g.7674897A>G | NM_000546.5:c.634T>C | NM. | COSM45477 | ||||
| V147A | NC_000017.11:g.7675172A>G | NM_000546.5:c.440T>C | NM. | COSM45819 | ||||
| T211T | NC_000017.11:g.7674898A>G | NM_000546.5:c.633T>C | NM. | COSM46211 |
OG: Oncogene, TSG: Tumor Suppressor Gene, MAF: Minor Allele Frequency, NM: Not Mention.
Table 3. The data of observed variations based on HGVS38 in non-coding sequence.
| gene | HGVS38 (Chromosomal variant) | HGVS38 (transcripts variant) | db SNP ID/ COSMIC ID | Mutation taster |
|---|---|---|---|---|
| TP53 | NC_000017.11:g.7674983T>C | NM_000546.5:c.560-12A>G | Novel | Polymorphism |
| NC_000017.11:g.7674773A>G | NM_000546.5:c.672+86T>C | Novel | Polymorphism | |
| NC_000017.11:g.7674853C>T | NM_000546.5:c.672+6G>A | rs766856111 | Polymorphism | |
| NC_000017.11:g.7674797T>C | NM_000546.5:c.672+62A>G | rs1625895 | Polymorphism |
DDR2 mutations analysis
Q808H substitution with A>C transversion (CAA>CAC) was observed in the SCC tumor specimen of a 67-year-old male (2.17%; Figs 1 and 2), but no mutation was seen in the ADC and LCC subtypes (Tables 1–4).
TP53 mutations analysis
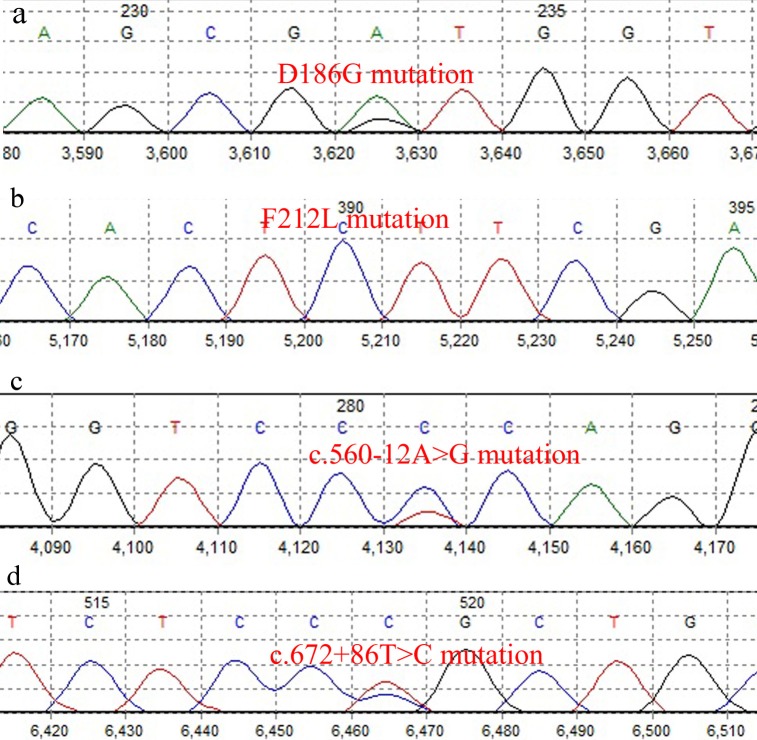
We found 48 variants in 35 of the 44 (79.54%) patients, in which 15 variants (31.25%) were in coding regions and 33 variants (68.75%) were intronic. V147A, V157F, Q167Q, D186G, H193R, T211T, F212L and P222P were the coding variants which were detected in nine patients (20.45%), including six (13.63%) in ADC, seven (15.9%) in SCC and two (4.54%) in LCC (Figs 1 and 3). The most frequently mutated sites were codons 186 (n = 3) and 193 (n = 3) with A>G transition, codon 222 (n = 3) with G>A transition and codon 212 (n = 2) with T>C transition. G>A transition in codon 167, G>T transversion in codon 157, T>C transition in codon 147 and T>C transition in codon 211 were other mutated base sites in TP53. Among all patients, the A>G transition was the most frequent (n = 6, 40%) base change in the coding region. Previously identified as polymorphism, rs1625895 (HGVS: c.672+62A>G) was a frequent intronic variant in 31 of the 44 patients (70.45%). rs766856111 (HGVS: c.672+6G>A) was another intronic variant in the SCC tumor sample of a 61-year-old male with intensive inflammation. We also found two new variants in two SCC male patients. c.560-12A>G was found in a 72-year-old patient and c.672+86T>C in a 37-year-old patient (Tables 1–4).
Fig 3. Analysis of TP53 gene mutations in lung cancer samples.
(a) D186G mutation in coding sequence (b) F212L mutation in coding sequence (c) c.560-12A>G mutation in intronic region (d) c.672+86T>C mutation in intronic region.
The exploration of TP53 status and clinicopathologic factors revealed a relative positive correlation between the presence of mutation in TP53 with age (P = 0.08)(Table 5). The correlations between TP53 status and other clinicopathological parameters are summarized in Table 5.
Table 5. Correlations between TP53 mutational status and clinicopathological parameters.
| Characteristics | Total number (%) | TP53 mutant |
TP53 wild-type |
P-value | ||
|---|---|---|---|---|---|---|
| Age, year | ≤ 65 years | 18(41) | 12(67) | 6(33) | 0.08 | |
| > 65 years | 26(59) | 23(89) | 3(11) | |||
| Gender | Male | 37(84) | 29(78) | 8(22) | 0.55 | |
| Female | 7(16) | 6(86) | 1(14) | |||
| Tumor type | NSCLC | ADC | 13(30) | 10(77) | 3(23) | 0.13 |
| SCC | 24(55) | 18(75) | 6(25) | |||
| LCC | 3(7) | 3(100) | 0(0) | |||
| LCC/SCC | 1(2) | 1(100) | 0(0) | |||
| NM | 2(4) | 2(100) | 0(0) | |||
| SCLC | SCLC | 1(2) | 1(100) | 0(0) | ||
| Histological Grade | ADC | Well | 2(22) | 1(50) | 1(50) | 0.56 |
| Moderate | 4(45) | 3(75) | 1(25) | |||
| Poor | 3(33) | 2(67) | 1(33) | |||
| SCC | Well | 9(43) | 6(67) | 3(33) | 0.49 | |
| Moderate | 5(24) | 5(100) | 0(0) | |||
| Poor | 7(33) | 5(71) | 2(29) | |||
| Inflammation (SCC) | Yes | 0(0) | 6(67) | 3(33) | 0.54 | |
| No | 0(0) | 4(80) | 1(20) | |||
NSCLC = non-small cell lung cancer, SCLC = small cell lung cancer, ADC = adenocarcinoma, SCC = squamous cell carcinoma, LCC = large cell carcinoma.
NM = not mention.
As previously established, lung cancer is the second most common and the most lethal type of cancer [49]. In Iran, lung cancer is the second cause of cancer-related death, after stomach malignancies [50]. Surgery, radiotherapy and chemotherapy are some of the commonly used treatments for lung cancer and are often used in the early stages of lung cancer. For some cases of lung cancer, targeted therapies or immunotherapy can also be used. Due to the toxicity of some medications, and the lack of response in some patients to common treatments, researchers face serious challenges in the treatment of lung cancer [51, 52]. A review of the genetic variations of lung cancer can be effective in detecting the disease as quickly as possible and choosing an effective treatment.
For the first time, this study was designed to investigate the status of KRAS, DDR2, and TP53 in hotspot regions in a panel of lung cancer, including NSCLC and SCLC, in Iranian population. In addition, we examined the association between mutational status of these genes and clinicopathological parameters.
Almost 15% to 25% of patients with NSCLC have KRAS mutations [27]. These mutations occur more frequently in ADC (approximately 30%) and less frequently in the SCC subtype (approximately 5%). More than 97% of KRAS-mutant cases affect exon 2 (G12, G13), which disrupts common targeted therapies in lung cancer [53, 54]. Therefore, targeted therapies have been provided based on the KRAS mutations (S3 Table). In this study, 23.63% of patients were diagnosed with ADC and 61.81% with SCC, but the G12C variant was seen in LCC, which contained only 7.27% of the tumor samples. There is evidence that the frequency of KRAS mutations in ADC varies among different ethnic groups, with a lower frequency observed among Asians compared to Caucasians [55]. Mutation frequency of KRAS in Chinese, Japanese, and Korean populations with ADC was 5.7% (range: 0.0%– 18.2%), 11.3% (range: 6.6%– 14.2%), and 9% (range: 7.3%– 9.5%), respectively, whereas this amount was 28.1% in Europe [55]. It can be concluded that the frequency of KRAS mutations in Iran, as a Western Asian country, may vary from 0 to 1.8 per 10 patients with ADC (0/10 to 1.8/10). These findings may indicate a different distribution of KRAS mutations in patients with lung cancer in the Iranian population.
We also examined the status of DDR2, which has been reported as a variable factor in lung cancer, most commonly in SCC, with a frequency of 3.8%. The mutations in this gene do not correlate with the gender or age of patients [10]. DDR2 mutations have been observed in conjunction with the KRAS (G12C) mutation [56].
Our results showed Q808H substitution (rs765660823) with A>C transversion (CAA>CAC) in SCC samples, which was previously reported by Exome Aggregation Consortium. This variant is in the tyrosine kinase domain (563–849) of DDR2, which may result in hyper-activation of this oncogene [57]. We investigated the COSMIC to find this variant in different cancers. However, there were no journal citations for this particular variant. Thus, we searched the Greater Middle East (GME) Variome Project (http://igm.ucsd.edu/gme/) website but still did not find any reported data on this variant in the countries of the Greater Middle East. Analysis using in silico tools, such as MutationTaster and CADD, revealed disease-causing and pathogenic effects for this variant, which is categorized as tier II, with potential clinical significance in CGI. No study was conducted about this variant, and to the best of our knowledge, our study was the first to report this variant in a cancer study.
As a tumor suppressor gene, TP53 is reported as the most mutated gene in lung cancer and its mutations are observed in 50% of NSCLC and 65% of SCC cases, which is higher than in ADC [58]. In the current study, all variations, including benign, pathogenic or intermediate, in coding and intronic sequences of hotspot regions of TP53 have been reported, using Oncogenic classification (https://www.cancergenomeinterpreter.org) that is a reliable database [59, 60] (Table 4). In our study, TP53 variants were observed in 79.54% of the samples, including 31.25% in conding and 68.75% in intronic regions, and had the highest frequency of variations among the three genes. Coding variants V147A, V157F, Q167Q, D186G, H193R, T211T, F212L and P222P were detected in nine patients (20.45%). Among these variants, V147A, V157F and H193R were already documented in lung cancers [61–63]. D186G and F212L were reported in some malignant tumors, including ADC of large intestine and maxillary sinus SCC, but we did not find any reported data about these variants in lung cancer [64, 65](S4 Table).
Chromatogram study of patient 35 showed homozygous mutations in F212L. Considering that the age of the patient was less than the mean age of patients with lung cancer, it may be possible that lung cancer in this patient was familial. Unfortunately, the patient died at the time of the study and samples of blood or other tissues were not available. The patient's family was not able to be located for supplemental studies. rs1625895 (HGVS: c.672+62A>G) was a frequent intronic polymorphism in our study, seen in 31 of the 44 patients (70.45%). A significant association between TP53 intron 6 variant (rs1625895) with increased risk of lung cancer has been reported [66].
The association of TP53 status and clinicopathological parameters revealed a marginal trend between the presence of TP53 mutation and older age. However, no data exist in the literature on the association of TP53 status and clinicopathological characteristics in the Iranian population with lung cancer [42, 43].
Many researchers have claimed that mutations in TP53 are prognostic, or predictive, to treatment response, while others have failed to demonstrate this association [36, 67]. Since most chemo-therapeutics induce DNA damage and consequently activate the p53 protein, mutations in the TP53 gene can negatively affect responses to this treatment [68]. In addition, cancer stem cells (CSCs) within the tumors are one of the reasons for resistance to treatment, relapse, and metastasis of the tumors. It is suggested that the level of expression of these genes be evaluated with important indicators of the CSC population including CD44, CD133, and ALDH1 in subsequent studies [69, 70]. Moreover, conducting large population-based studies is highly recommended.
Application of the next generation sequencing (NGS) will help increase sensitivity and quality of data in finding mutation(s), but selecting a method is determined by several factors including sample type (fresh, frozen, or FFPE), quality and quantity of DNA, or RNA [71]. The PCR-based enrichment is the most preferred methodology for FFPE samples, which can efficiently amplify targeted regions of interest for sequencing analysis from low amounts of FFPE DNA; thus, the direct DNA sequencing methods, such as Sanger sequencing, are still accepted as the gold standard for mutations diagnosis [72]. In addition, to improve the sensitivity of molecular analysis, a pathologist can be asked to evaluate the tissue samples using a microscope to select a suitable area with high tumor cells proportion. Thus, in the present survey, we selected lung tumor samples containing at least 50% tumor cells [44, 45].
Conclusions
In conclusion, KRAS, DDR2, and TP53 variants were detected in 2%, 2.17% and 79.54% of all cases, respectively. The frequency of DDR2 mutation is nearly close to other studies, while KRAS and TP53 mutation frequencies are lower and higher than other populations, respectively. Three new putative pathogenic variants, for the first time, have been detected in Iranian patients with lung cancer, including Q808H in DDR2, F212L, and D186G in coding regions of TP53. In addition, we observed five novel benign variants, including Q167Q, P222P and T211T in coding sequence, and c.560-12A>G and c.672+86T>C, in intronic region of TP53. Mutations of KRAS and DDR2 were found in LCC and SCC subtypes, respectively, whereas mutations of TP53 were seen in SCC and ADC subtypes with higher frequencies and LCC subtype with lower frequency. Therefore, Iranian lung cancer patients can benefit from mutational analysis before starting the conventional treatment. A better understanding of the biology of these genes and their mutations will be critical for developing future targeted therapies.
Supporting information
(DOC)
(DOC)
* https://www.cancergenomeinterpreter.org.
(DOC)
LIP: Liposarcoma, HNC: Head and neck cancer, BRCA: Breast Adenocarcinoma, BCL: B cell Lymphoma, FGCT: Female Germ Cell Tumor, MGCT: Male Germ Cell Tumor, AML: Acute Myeloid Leukemia, MDPS: Myelodysplastic Proliferative Syndrome, OV: Ovary Cancer, THYM: Thymic.
* https://www.cancergenomeinterpreter.org
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by a grant from Iran University of Medical Sciences (Grant #95-02-30-27795). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization, Cancer, Fact sheet no297 (http://www.who.int/mediacentre/factsheets/fs297/en/). 2017.
- 2.Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest Journal. 2003;123(1_suppl):21S–49S. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Li R, Tiselius E, Roudi R, Teghararian O, Suo C, et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. The Cochrane database of systematic reviews. 2017;12:Cd011300 Epub 2017/12/17. 10.1002/14651858.CD011300.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roviello G, Zanotti L, Cappelletti MR, Gobbi A, Dester M, Paganini G, et al. Are EGFR tyrosine kinase inhibitors effective in elderly patients with EGFR-mutated non-small cell lung cancer? Clinical and experimental medicine. 2017. Epub 2017/04/10. 10.1007/s10238-017-0460-7 . [DOI] [PubMed] [Google Scholar]
- 5.Yashima H, Shimizu K, Araki T, Aomori T, Ohtaki Y, Nagashima T, et al. Assessment of DDR2, BRAF, EGFR and KRAS mutations as therapeutic targets in non-adenocarcinoma lung cancer patients. Molecular and clinical oncology. 2014;2(5):714–8. 10.3892/mco.2014.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim SM, Syn NL, Cho BC, Soo RA. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer treatment reviews. 2018;65:1–10. Epub 2018/02/27. 10.1016/j.ctrv.2018.02.006 . [DOI] [PubMed] [Google Scholar]
- 7.Heist RS, Sequist LV, Engelman JA. Genetic changes in squamous cell lung cancer: a review. Journal of Thoracic Oncology. 2012;7(5):924–33. 10.1097/JTO.0b013e31824cc334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, Zhou W, et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer discovery. 2011;1(1):78–89. 10.1158/2159-8274.CD-11-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer and Metastasis Reviews. 2012;31(1–2):295–321. 10.1007/s10555-012-9346-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricordel C, Lespagnol A, Llamas-Gutierrez F, de Tayrac M, Kerjouan M, Fievet A, et al. Mutational Landscape of DDR2 Gene in Lung Squamous Cell Carcinoma Using Next-generation Sequencing. Clinical lung cancer. 2018;19(2):163–9.e4. Epub 2017/11/14. 10.1016/j.cllc.2017.10.006 . [DOI] [PubMed] [Google Scholar]
- 11.Miao L, Wang Y, Zhu S, Shi M, Li Y, Ding J, et al. Identification of novel driver mutations of the discoidin domain receptor 2 (DDR2) gene in squamous cell lung cancer of Chinese patients. BMC cancer. 2014;14:369 Epub 2014/06/03. 10.1186/1471-2407-14-369 ; PubMed Central PMCID: PMCPmc4039546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MS, Jung EA, An SB, Kim YJ, Oh DY, Song JY, et al. Prevalence of Mutations in Discoidin Domain-Containing Receptor Tyrosine Kinase 2 (DDR2) in Squamous Cell Lung Cancers in Korean Patients. Cancer research and treatment: official journal of Korean Cancer Association. 2017;49(4):1065–76. Epub 2017/02/07. 10.4143/crt.2016.347 ; PubMed Central PMCID: PMCPmc5654160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenmotsu H, Serizawa M, Koh Y, Isaka M, Takahashi T, Taira T, et al. Prospective genetic profiling of squamous cell lung cancer and adenosquamous carcinoma in Japanese patients by multitarget assays. BMC cancer. 2014;14:786 Epub 2014/10/29. 10.1186/1471-2407-14-786 ; PubMed Central PMCID: PMCPmc4221703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yashima H, Shimizu K, Araki T, Aomori T, Ohtaki Y, Nagashima T, et al. Assessment of DDR2, BRAF, EGFR and KRAS mutations as therapeutic targets in non-adenocarcinoma lung cancer patients. Mol Clin Oncol. 2014;2(5):714–8. Epub 2014/07/24. 10.3892/mco.2014.302 ; PubMed Central PMCID: PMCPmc4106743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. Jama. 2014;311(19):1998–2006. 10.1001/jama.2014.3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA: a cancer journal for clinicians. 2016;66(4):271–89. [DOI] [PubMed] [Google Scholar]
- 17.Poghosyan H, Sheldon LK, Leveille SG, Cooley ME. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer. 2013;81(1):11–26. 10.1016/j.lungcan.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 18.Richters A, Nguyen HD, Phan T, Simard JR, Grutter C, Engel J, et al. Identification of type II and III DDR2 inhibitors. Journal of medicinal chemistry. 2014;57(10):4252–62. Epub 2014/04/24. 10.1021/jm500167q . [DOI] [PubMed] [Google Scholar]
- 19.Terai H, Tan L, Beauchamp EM, Hatcher JM, Liu Q, Meyerson M, et al. Characterization of DDR2 Inhibitors for the Treatment of DDR2 Mutated Nonsmall Cell Lung Cancer. ACS chemical biology. 2015;10(12):2687–96. Epub 2015/09/22. 10.1021/acschembio.5b00655 ; PubMed Central PMCID: PMCPMC4685943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitini V, Arrigo C, Di Mirto C, Mondello P, Altavilla G. Response to dasatinib in a patient with SQCC of the lung harboring a discoid-receptor-2 and synchronous chronic myelogenous leukemia. Lung Cancer. 2013;82(1):171–2. Epub 2013/08/13. 10.1016/j.lungcan.2013.07.004 . [DOI] [PubMed] [Google Scholar]
- 21.Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet (London, England). 2016;387(10026):1415–26. Epub 2016/01/19. 10.1016/s0140-6736(16)00004-0 . [DOI] [PubMed] [Google Scholar]
- 22.Gobbini E, Galetta D, Tiseo M, Graziano P, Rossi A, Bria E, et al. Molecular profiling in Italian patients with advanced non-small-cell lung cancer: An observational prospective study. Lung Cancer. 2017;111:30–7. Epub 2017/08/26. 10.1016/j.lungcan.2017.06.009 . [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto S, Tsuchihara K, Yoh K, Zenke Y, Kohno T, Ishii G, et al. A new nationwide genomic screening system in Japan for the development of targeted therapies against advanced non-small lung cancers with rare driver mutations. American Society of Clinical Oncology; 2014. [Google Scholar]
- 24.Li S, Li L, Zhu Y, Huang C, Qin Y, Liu H, et al. Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. British journal of cancer. 2014;110(11):2812 10.1038/bjc.2014.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatziandreou I, Tsioli P, Sakellariou S, Mourkioti I, Giannopoulou I, Levidou G, et al. Comprehensive Molecular Analysis of NSCLC; Clinicopathological Associations. PloS one. 2015;10(7):e0133859 Epub 2015/07/25. 10.1371/journal.pone.0133859 ; PubMed Central PMCID: PMCPMC4514742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin Martorell P, Huerta M, Compan Quilis A, Abellan R, Seda E, Blesa S, et al. Coexistence of EGFR, KRAS, BRAF, and PIK3CA Mutations and ALK Rearrangement in a Comprehensive Cohort of 326 Consecutive Spanish Nonsquamous NSCLC Patients. Clinical lung cancer. 2017;18(6):e395–e402. Epub 2017/05/30. 10.1016/j.cllc.2017.04.006 . [DOI] [PubMed] [Google Scholar]
- 27.Yang IS, Kim S. Isoform specific gene expression analysis of KRAS in the prognosis of lung adenocarcinoma patients. BMC bioinformatics. 2018;19(Suppl 1):40 Epub 2018/03/06. 10.1186/s12859-018-2011-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalela R, Curull V, Enriquez C, Pijuan L, Bellosillo B, Gea J. Lung adenocarcinoma: from molecular basis to genome-guided therapy and immunotherapy. Journal of thoracic disease. 2017;9(7):2142–58. Epub 2017/08/26. 10.21037/jtd.2017.06.20 ; PubMed Central PMCID: PMCPMC5542927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mascaux C, Iannino N, Martin B, Paesmans M, Berghmans T, Dusart M, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. British journal of cancer. 2005;92(1):131–9. 10.1038/sj.bjc.6602258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(25):5900–9. Epub 2005/07/27. 10.1200/jco.2005.02.857 . [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483(7391):613–7. Epub 2012/03/20. 10.1038/nature10937 ; PubMed Central PMCID: PMCPMC3385933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dry JR, Pavey S, Pratilas CA, Harbron C, Runswick S, Hodgson D, et al. Transcriptional pathway signatures predict MEK addiction and response to selumetinib (AZD6244). Cancer research. 2010;70(6):2264–73. Epub 2010/03/11. 10.1158/0008-5472.CAN-09-1577 ; PubMed Central PMCID: PMCPMC3166660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang CL, Taki T, Adachi M, Konishi T, Higashiyama M, Kinoshita M, et al. Mutations of p53 and K-ras genes as prognostic factors for non-small cell lung cancer. International journal of oncology. 1998;12(3):553–63. Epub 1998/04/18. . [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Le Teuff G, Lacas B, Tsao MS, Graziano S, Pignon JP, et al. Prognostic and Predictive Effect of TP53 Mutations in Patients with Non-Small Cell Lung Cancer from Adjuvant Cisplatin-Based Therapy Randomized Trials: A LACE-Bio Pooled Analysis. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2016;11(6):850–61. Epub 2016/02/24. 10.1016/j.jtho.2016.02.002 . [DOI] [PubMed] [Google Scholar]
- 35.Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer cell. 2014;25(3):304–17. Epub 2014/03/22. 10.1016/j.ccr.2014.01.021 ; PubMed Central PMCID: PMCPMC3970583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Lin X, Wang C, Yan K, Zhao L, An W, et al. Association between smoking and p53 mutation in lung cancer: a meta-analysis. Clinical Oncology. 2014;26(1):18–24. 10.1016/j.clon.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Lin XJ, Wang CP, Yan KK, Zhao LY, An WX, et al. Association between smoking and p53 mutation in lung cancer: a meta-analysis. Clinical oncology (Royal College of Radiologists (Great Britain)). 2014;26(1):18–24. Epub 2013/10/16. 10.1016/j.clon.2013.09.003 . [DOI] [PubMed] [Google Scholar]
- 38.Deben C, Deschoolmeester V, Lardon F, Rolfo C, Pauwels P. TP53 and MDM2 genetic alterations in non-small cell lung cancer: evaluating their prognostic and predictive value. Critical reviews in oncology/hematology. 2016;99:63–73. 10.1016/j.critrevonc.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 39.Soussi T, Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nature reviews Cancer. 2001;1(3):233–40. Epub 2002/03/21. 10.1038/35106009 . [DOI] [PubMed] [Google Scholar]
- 40.Baugh EH, Ke H, Levine AJ, Bonneau RA, Chan CS. Why are there hotspot mutations in the TP53 gene in human cancers? Cell death and differentiation. 2018;25(1):154–60. Epub 2017/11/04. 10.1038/cdd.2017.180 ; PubMed Central PMCID: PMCPMC5729536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fathi Z, Syn NL, Zhou J-G, Roudi R. Molecular epidemiology of lung cancer in Iran: implications for drug development and cancer prevention. Journal of Human Genetics. 2018. 10.1038/s10038-018-0450-y [DOI] [PubMed] [Google Scholar]
- 42.Jafari H. Genotyping of human papillomavirus and TP53 mutaions at exons 5 to 7 in lung cancer patients from Iran. BioImpacts: BI. 2013;3(3):135 10.5681/bi.2013.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammadi A, Vaziri Gohar A, Shakibaie MR. Mutations in tumor suppressor TP53 gene in formalin-fixed, paraffin embedded tissues of squamous cell carcinoma (SCC) of lung cancer. Am J Bioch Biotechnol. 2008;4(1):1–6. [Google Scholar]
- 44.Lee HJ, Xu X, Kim H, Jin Y, Sun P, Kim JE, et al. Comparison of Direct Sequencing, PNA Clamping-Real Time Polymerase Chain Reaction, and Pyrosequencing Methods for the Detection of EGFR Mutations in Non-small Cell Lung Carcinoma and the Correlation with Clinical Responses to EGFR Tyrosine Kinase Inhibitor Treatment. Korean journal of pathology. 2013;47(1):52–60. Epub 2013/03/14. 10.4132/KoreanJPathol.2013.47.1.52 ; PubMed Central PMCID: PMCPMC3589609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HJ, Lee KY, Kim YC, Kim KS, Lee SY, Jang TW, et al. Detection and comparison of peptide nucleic acid-mediated real-time polymerase chain reaction clamping and direct gene sequencing for epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Lung Cancer. 2012;75(3):321–5. Epub 2011/09/21. 10.1016/j.lungcan.2011.08.005 . [DOI] [PubMed] [Google Scholar]
- 46.Ellard S, Charlton R, Yau S, Gokhale D, Taylor G, Wallace A, et al. Practice guidelines for Sanger sequencing analysis and interpretation. 2016. [Google Scholar]
- 47.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in medicine. 2015;17(5):405–23. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. The Journal of Molecular Diagnostics. 2017;19(1):4–23. 10.1016/j.jmoldx.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. Epub 2016/01/09. 10.3322/caac.21332 . [DOI] [PubMed] [Google Scholar]
- 50.Karami-Matin B, Najafi F, Rezaei S, Khosravi A, Soofi M. Estimating the Economic Burden of Premature Mortality Caused by Cancer in Iran: 2006–2010. Asian Pacific journal of cancer prevention: APJCP. 2016;17(4):2131–6. Epub 2016/05/26. . [DOI] [PubMed] [Google Scholar]
- 51.Zarogoulidis K, Zarogoulidis P, Darwiche K, Boutsikou E, Machairiotis N, Tsakiridis K, et al. Treatment of non-small cell lung cancer (NSCLC). Journal of thoracic disease. 2013;5 Suppl 4:S389–96. Epub 2013/10/09. 10.3978/j.issn.2072-1439.2013.07.10 ; PubMed Central PMCID: PMCPMC3791496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine. 2016;375(19):1823–33. Epub 2016/10/11. 10.1056/NEJMoa1606774 . [DOI] [PubMed] [Google Scholar]
- 53.Nadal E, Chen G, Prensner JR, Shiratsuchi H, Sam C, Zhao L, et al. KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014;9(10):1513–22. Epub 2014/08/30. 10.1097/jto.0000000000000305 . [DOI] [PubMed] [Google Scholar]
- 54.Timar J. The clinical relevance of KRAS gene mutation in non-small-cell lung cancer. Current opinion in oncology. 2014;26(2):138–44. Epub 2014/01/28. 10.1097/CCO.0000000000000051 . [DOI] [PubMed] [Google Scholar]
- 55.Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Annals of oncology: official journal of the European Society for Medical Oncology. 2013;24(9):2371–6. Epub 2013/06/01. 10.1093/annonc/mdt205 ; PubMed Central PMCID: PMCPMC3755331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicoś M, Powrózek T, Krawczyk P, Jarosz B, Pająk B, Sawicki M, et al. Sensitive methods for detection of the S768R substitution in exon 18 of the DDR2 gene in patients with central nervous system metastases of non-small cell lung cancer. Medical Oncology. 2014;31(10):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terashima M, Togashi Y, Sato K, Mizuuchi H, Sakai K, Suda K, et al. Functional Analyses of Mutations in Receptor Tyrosine Kinase Genes in Non-Small Cell Lung Cancer: Double-Edged Sword of DDR2. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22(14):3663–71. Epub 2016/01/31. 10.1158/1078-0432.ccr-15-2093 . [DOI] [PubMed] [Google Scholar]
- 58.Warth A, Endris V, Stenzinger A, Penzel R, Harms A, Duell T, et al. Genetic changes of non-small cell lung cancer under neoadjuvant therapy. Oncotarget. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mardis ER. New additions to the cancer precision medicine toolkit. Genome medicine. 2018;10(1):28 Epub 2018/04/15. 10.1186/s13073-018-0540-7 ; PubMed Central PMCID: PMCPmc5899382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsang H, Addepalli K, Davis SR. Resources for Interpreting Variants in Precision Genomic Oncology Applications. Frontiers in oncology. 2017;7:214 10.3389/fonc.2017.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Anta JM, Jassem E, Rosell R, Martinez-Roca M, Jassem J, Martinez-Lopez E, et al. TP53 mutational pattern in Spanish and Polish non-small cell lung cancer patients: null mutations are associated with poor prognosis. Oncogene. 1997;15(24):2951–8. Epub 1998/01/07. 10.1038/sj.onc.1201475 . [DOI] [PubMed] [Google Scholar]
- 62.Andriani F, Conte D, Mastrangelo T, Leon M, Ratcliffe C, Roz L, et al. Detecting lung cancer in plasma with the use of multiple genetic markers. International journal of cancer. 2004;108(1):91–6. Epub 2003/11/18. 10.1002/ijc.11510 . [DOI] [PubMed] [Google Scholar]
- 63.Liu D, Huang CL, Kameyama K, Hayashi E, Yamauchi A, Sumitomo S, et al. Topoisomerase IIalpha gene expression is regulated by the p53 tumor suppressor gene in nonsmall cell lung carcinoma patients. Cancer. 2002;94(8):2239–47. Epub 2002/05/10. 10.1002/cncr.10450 . [DOI] [PubMed] [Google Scholar]
- 64.Bandoh N, Hayashi T, Kishibe K, Takahara M, Imada M, Nonaka S, et al. Prognostic value of p53 mutations, bax, and spontaneous apoptosis in maxillary sinus squamous cell carcinoma. Cancer. 2002;94(7):1968–80. [DOI] [PubMed] [Google Scholar]
- 65.Ashktorab H, Mokarram P, Azimi H, Olumi H, Varma S, Nickerson ML, et al. Targeted exome sequencing reveals distinct pathogenic variants in Iranians with colorectal cancer. Oncotarget. 2017;8(5):7852–66. Epub 2016/12/22. 10.18632/oncotarget.13977 ; PubMed Central PMCID: PMCPmc5341754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang W, Spitz MR, Yang H, Lu C, Stewart DJ, Wu X. Genetic variants in cell cycle control pathway confer susceptibility to lung cancer. Clinical cancer research. 2007;13(19):5974–81. 10.1158/1078-0432.CCR-07-0113 [DOI] [PubMed] [Google Scholar]
- 67.Mitsudomi T, Hamajima N, Ogawa M, Takahashi T. Prognostic significance of p53 alterations in patients with non-small cell lung cancer: a meta-analysis. Clinical Cancer Research. 2000;6(10):4055–63. [PubMed] [Google Scholar]
- 68.Viktorsson K, De Petris L, Lewensohn R. The role of p53 in treatment responses of lung cancer. Biochemical and biophysical research communications. 2005;331(3):868–80. 10.1016/j.bbrc.2005.03.192 [DOI] [PubMed] [Google Scholar]
- 69.Roudi R, Korourian A, Shariftabrizi A, Madjd Z. Differential Expression of Cancer Stem Cell Markers ALDH1 and CD133 in Various Lung Cancer Subtypes. Cancer investigation. 2015;33(7):294–302. Epub 2015/06/06. 10.3109/07357907.2015.1034869 . [DOI] [PubMed] [Google Scholar]
- 70.Roudi R, Madjd Z, Korourian A, Mehrazma M, Molanae S, Sabet MN, et al. Clinical significance of putative cancer stem cell marker CD44 in different histological subtypes of lung cancer. Cancer biomarkers: section A of Disease markers. 2014;14(6):457–67. Epub 2014/10/23. 10.3233/CBM-140424 . [DOI] [PubMed] [Google Scholar]
- 71.Luthra R, Chen H, Roy-Chowdhuri S, Singh RR. Next-Generation Sequencing in Clinical Molecular Diagnostics of Cancer: Advantages and Challenges. Cancers. 2015;7(4):2023–36. Epub 2015/10/17. 10.3390/cancers7040874 ; PubMed Central PMCID: PMCPMC4695874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nicos M, Powrozek T, Krawczyk P, Jarosz B, Pajak B, Sawicki M, et al. Sensitive methods for detection of the S768R substitution in exon 18 of the DDR2 gene in patients with central nervous system metastases of non-small cell lung cancer. Medical oncology (Northwood, London, England). 2014;31(10):176 Epub 2014/09/01. 10.1007/s12032-014-0176-4 ; PubMed Central PMCID: PMCPMC4180903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
* https://www.cancergenomeinterpreter.org.
(DOC)
LIP: Liposarcoma, HNC: Head and neck cancer, BRCA: Breast Adenocarcinoma, BCL: B cell Lymphoma, FGCT: Female Germ Cell Tumor, MGCT: Male Germ Cell Tumor, AML: Acute Myeloid Leukemia, MDPS: Myelodysplastic Proliferative Syndrome, OV: Ovary Cancer, THYM: Thymic.
* https://www.cancergenomeinterpreter.org
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.