 Propyl paraben is a widely used preservative in pharmaceuticals, cosmetics, and foods preventing microbial and fungal contamination.
Propyl paraben is a widely used preservative in pharmaceuticals, cosmetics, and foods preventing microbial and fungal contamination.
Abstract
Propyl paraben is a widely used preservative in pharmaceuticals, cosmetics, and foods preventing microbial and fungal contamination. This study was designed to investigate antiandrogenic profiles of propyl paraben following oral doses at 10, 250, and 750 mg kg–1 day to immature male rats using the Hershberger Bioassay. Rats were divided into six groups including solvent control, negative control (0.4 mg kg–1 day testosterone propionate = TP), positive control (3 mg kg–1 day flutamide = FLU) and treatment groups (10, 250, and 750 mg kg–1 day testosterone propionate + Propyl paraben). Propyl paraben (PP) significantly decreased all accessory sex organ weights at each dose of 250 and 750 mg kg–1 day compared to control groups. Thus, we found that propyl paraben had antiandrogenic activity within the supported results of increasing LH levels and histopathologic results such atrophy, hyalinization, and anastomosis on androgenic tissues.
1. Introduction
Parabens are esters of p-hydroxybenzoic acid typically identified by their alkyl chains. Because of their strong activity against yeasts, molds, and bacteria, chemical stability, and low costs of production, parabens are commonly used in cosmetics, pharmaceuticals, and foods for more than 50 years.1,2 For combination uses in products, methyl and propyl paraben have been the most commonly used parabens for decades.3 Humans can be exposed to parabens via absorption through dermal contact or by ingestion of related products4 as well as inhalation of air.4–6 Monitoring and data use indicate that the general population may be exposed via dermal contact with products containing this compound, ingestion of some foods, inhalation, contact with house dust, and administration of pharmaceutical products.7 Cosmetic exposure is believed to be the predominant paraben exposure route for most adults in developed nations, as indicated from estimates of dermal total paraben internal exposure.8 In most cosmetics such as toothpastes, sunscreens, body lotions, facial lotions and cleansers, mascara, assorted lipsticks, and hand soaps, as well as hair products including shampoos, conditioners, and sprays, parabens are used at very low levels ranging from 0.01 to 0.3% and they have been found in 99% of leave-on products and in 77% of rinse-off cosmetics.9 Usage of the parabens in the food industry includes cakes, pastries, pie-crusts, icings, toppings and fillings, soft drinks, creams and pastes, jams, jellies and preserves, olives, and syrups.10
Parabens are rapidly metabolized into p-hydroxybenzoic acid or their conjugates and excreted in urine.11 The average daily total personal paraben exposure is estimated to be 76 mg, including cosmetics at 50 mg, pharmaceuticals at 25 mg and food at 1 mg.12
Besides being known to have weak estrogenic activity,13 parabens also have been demonstrated to effect male reproductive systems. A spermicidal activity of propyl paraben was evaluated and spermicidal potency of it was found to be 3 mg ml–1.14 Research critically related to antiandrogenic effects of propyl paraben, including male rats exposed to 0, 0.01, 0.1, and 1% for 4 weeks, showed a decrease in cauda epididymal sperm reserves and concentrations.15 Furthermore, an androgenic receptor gene assay indicated that propyl paraben has antiandrogenic activity with results of acceptable daily intake (ADI) as 10 mg kg–1 day.16
Since there is no ban for use of propyl paraben, this study was undertaken to support published evidence about antiandrogenic properties of propyl paraben on the male reproductive system. Our study investigated antiandrogenic consequences of propyl paraben on castrated 6-week old immature male rats using the Hershberger Bioassay which provides an effective screening method.
2. Materials and methods
2.1. Chemicals
Testosterone propionate (TP, 97%) was obtained from Hangzhou Dayang Chem. Co., Ltd. Flutamide (FLU, 98%) and propyl paraben (PP, 98%) were obtained from Sigma-Aldrich, USA. FSH and LH Elisa kits were purchased from Eastbiopharm Company. All other chemicals were of analytical grade and obtained from Sigma-Aldrich, USA. Since many androgen ligands tend to be hydrophobic, all test chemicals were dissolved in corn oil.
2.2. Animals and housing
Thirty six prepubertal male Wistar albino rats (Rattus norvecigus), 6-weeks old, weighing 170–210 g were purchased from the Experimental Animals Production Center, Hacettepe University in Ankara, Turkey. The experimental protocol and usage of rats was approved by Hacettepe University Research Ethics Committee, Turkey (permit number: 2015/86-13). All rats were housed in standard polypropylene cages with stainless steel covers in an air-conditioned room (12 h light/dark cycle with at 22 ± 2 °C and relative humidity of 50 ± 5%). During the experimental period rats were provided with pellet food and drinking water was available ad libitum in glass bottles. After acclimatization, at the age of 6-weeks, rats were castrated under anesthesia and both testes and epididymitis were removed. Rats were observed daily and any animal with evidence of disease was removed.
2.3. Dose level selection and experimental design
To investigate antiandrogenic activity, the dose levels used in this study were determined according to previous studies that investigated the endocrine-disrupting potency of propyl paraben in rats in reports of the US EPA; CDR, (Chemical Data Reporting17), and based on previous toxicokinetic and experimental findings.15,18 The highest dose level should first take into consideration the LD50 and/or acute toxicity information in order to avoid death, severe suffering or distress in the animals. The objective in the case of the Hershberger Bioassay is to select doses that ensure animal survival and that are without significant toxicity or distress to the animals after ten consecutive days of chemical administration up to a limit dose of 1000 mg kg–1 day.
The daily doses of TP (0.4 mg kg–1 day) and FLU (3 mg kg–1 day) were taken from the Hershberger Bioassay OCSPP Guideline 890.1400. The dosage levels employed daily were based on daily measured body weights. For oral gavage, a stomach tube was used. For subcutaneous injection, doses were administered to the dorsal-scapular region via sterile needles. The total dosing volume of 0.5 ml per kg bw and 5 ml per kg bw were adjusted for subcutaneous and oral administration relatively.19
2.4. Hershberger bioassay
Hershberger bioassay was performed in accordance with the OCSPP Guideline 890.1400.19 The bioassay serves as an in vivo screening method for androgen agonists or antagonists and other 5α-reductase inhibitors. The Hershberger bioassay includes changes of five androgen-dependent tissue weights in immature castrated male rats. The five androgen-dependent accessory reproductive tissues included in this assay include: the ventral prostate (VP), seminal vesicles (SV) (plus fluids and coagulating glands), levator plus-bulbocavernosus muscles (LABC), Cowper's gland (COW), and glans penis (GP). These tissues respond to antiandrogens with a difference in absolute tissue weight.
Prior to the assay, all animals were checked for any clinical signs of illness. At their 6-week age, rats were castrated and given 8 days to recover. After the recovery period, according to body weights, animals were randomly divided into six groups (n = 6) including a solvent control group (5 mg kg–1 day corn oil), negative control group (0.4 mg kg–1 day TP), positive control group (3 mg kg–1 day FLU plus 0.4 mg kg–1 day TP), and three propyl paraben dose groups (10, 250, and 750 mg kg–1 day plus 0.4 mg kg–1 day TP). While TP was administered by subcutaneous injection, FLU and propyl paraben were administered by oral gavage for 10 days. Food and water consumption were measured, body weights were recorded daily, and the dose administered each day was adjusted for body weight. After 10 days of treatment, the rats were killed within 24 h after the last administration. The ventral prostate, paired seminal vesicles, levator plus bulbocavernosus muscles, Cowper's glands, glans penis, liver, and paired kidneys were carefully removed and weighed.
2.5. Blood sampling and serum androgen measurement
Blood samples were collected from the animals by cardiac puncture for serum preparation, per each group and after the completion of the treatments, at the same day and a time interval from 8 to 9 in the morning. Serum was separated after centrifugation at 3000g for 25 minutes to pipette into silicon microcentrifuge tubes and stored at –80 °C until hormone analysis. Follicle stimuli hormone (FSH) and luteinizing hormone (LH) were measured by using commercially available ELISA kits for rats according to the manufacturer's instructions. All samples were measured in duplicate with the same assay; the intra- and inter-assay coefficients of variations were less than 9.1%.
2.6. Histopathological analysis
After weighing all tissue samples, they were fixed in Bouin's and 10% formal solutions for 8 h. After fixation, the tissues were embedded and sectioned at 4 μm thicknesses and stained with Harris hematoxylin and eosin (H&E) using standard methodologies. Pathologic abnormalities and potential treatment-related effects and abnormalities/lesions were noted. All slides were examined using an Olympus BX51 system light microscope. Photographs were captured using Bs200prop software and all histopathologic changes recorded for each animal.
2.7. Statistical analysis
All data are presented as the mean ± S.D. The results were statistically analyzed for characteristics such as homogeneity of variance with appropriate data transformations. Groups with homogeneous variances were analyzed using ANOVA following Tukey's range test to determine differences among groups followed by pair-wise comparisons with the controls. P < 0.05 was considered evidence for statistical significance.
3. Results
3.1. Clinical signs and food-water consumption changes
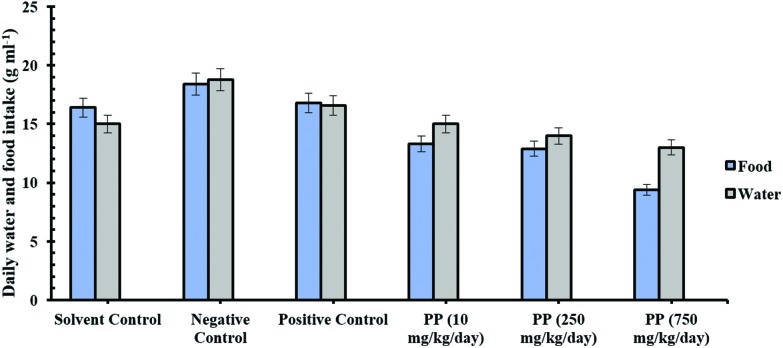
During the experiment, no unexpected deaths were observed in any of the rats. The daily food and water consumption changes in the experimental groups are presented in Fig. 1. Our results showed no significant effects from water and food consumption between the groups. However, food consumption at the 10, 250, and 750 mg kg–1 day propyl paraben-treated groups showed a tendency for decreased uptake compared to the solvent control group, but no statistically significant effects (Fig. 1).
Fig. 1. Effects of orally administered propyl paraben and flutamide on daily food and water consumption of testosterone propionate provided (0.4 mg kg–1 day–1 subcutaneously) to castrated rats.
3.2. Measurement of body and organ weights
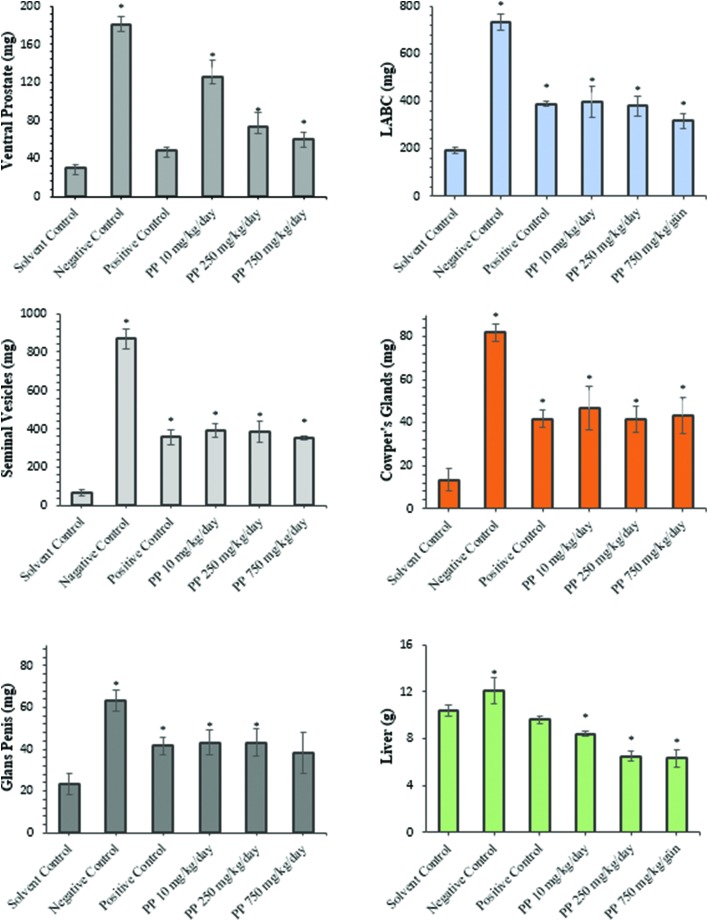
The effects of propyl paraben on the weight of the androgenic tissues, liver, and kidney tissues were compared to the other groups. Organ and body weights and overall means are summarized with the average weight of the target tissues in castrated rats and shown in Table 1. Our results showed that body weight gain decreased significantly in all propyl paraben treatment groups. Otherwise, body weight increased extremely in the negative control group (0.4 mg kg–1 day TP). Seminal vesicle, LABC, Cowper's gland, and glans penis weight significantly decreased in the testosterone propionate group, but increased in the vehicle control group. However, statistics indicated that weights of LABC muscles, seminal vesicles, Cowper's gland, and ventral prostate were significantly increased in rats treated only with testosterone propionate. Additionally, organ weights in rats given propyl paraben (10, 250, and 750 mg kg–1 day) plus testosterone propionate and flutamide plus testosterone propionate were significantly lower than those in the testosterone propionate (negative) group and significant decreases were observed with the 250 and 750 mg kg–1 day PP and FLU (positive) groups. Weight changes of the glans penis in castrated rats given propyl paraben showed the weakest response among other organs to control groups. Flutamide significantly decreased all relative accessory organ weights in castrated rats (Table 1). The weights of livers decreased in castrated rats given 10, 250, and 750 mg kg–1 day of PP and positive control group (FLU 3 mg kg–1 day) compared with the vehicle control group (Fig. 2). A significant increase was observed in the negative control (0.4 mg kg–1 day TP) group while flutamide presented a weight decrease in castrated rats compared to the vehicle group. In the current study we found that there was no significant statistical difference in kidney weights among the groups.
Table 1. Effects of orally administered propyl paraben and flutamide on body and organ weights of testosterone propionate supplemented-castrated rats. Castrated rats were concomitantly treated with TP and PP or flutamide for ten days.
| Treatment | N | Dose (mg kg–1 day) | Body weight (g) |
Androgen-sensitive tissue weights (mg) |
Optional tissue weights (g) |
||||||
| Initial | Terminal | Ventral prostate | Seminal vesicles | LABC | Cowper's gland | Glans penis | Liver | Kidneys | |||
| TP | 6 | 0.4 | 210 ± 10.3 | 274 ± 2.6* | 181 ± 7.5* | 868.3 ± 53.1* | 733.3 ± 32.6* | 81.7 ± 4.1* | 63.3 ± 5.2* | 12.04 ± 1.1* | 1.66 ± 0.8 |
| TP + PP | 6 | 10 | 183.6 ± 6.8 | 223.3 ± 9.3* | 126 ± 17.5* | 391.7 ± 37.1* | 396.7 ± 64.7* | 46.7 ± 20.6* | 43.3 ± 12.1* | 8.44 ± 0.2* | 1.28 ± 0.3 |
| TP + PP | 6 | 250 | 169.6 ± 9.9* | 204 ± 9.9* | 73.3 ± 15.1* | 383.3 ± 55.7* | 380 ± 40.8* | 41.7 ± 16.1* | 43.3 ± 16.3* | 6.59 ± 0.4* | 1.36 ± 0.06 |
| TP + PP | 6 | 750 | 176 ± 12.4* | 187.1 ± 9.7* | 60 ± 7.5* | 353.3 ± 8.2* | 316.7 ± 32.6* | 43.3 ± 8.2* | 38.3 ± 9.8 | 6.39 ± 0.7* | 1.27 ± 0.1 |
| TP + FLU | 6 | 3 | 179.6 ± 13.1* | 226.8 ± 10* | 48.3 ± 4.1 | 356.7 ± 39.3* | 388.3 ± 9.8* | 41.7 ± 4.1* | 45.7 ± 4.1* | 9.63 ± 0.4 | 1.70 ± 0.1 |
| Vehicle only | 6 | — | 201 ± 15.3 | 246.3 ± 10.5 | 30 ± 4.1 | 66.7 ± 16.3 | 191.7 ± 11.7 | 13.3 ± 5.2 | 23.3 ± 5.2 | 10.42 ± 0.5 | 1.76 ± 0.1 |
Fig. 2. Effects of orally administered propyl paraben and flutamide on organ weights of testosterone propionate provided (TP, 0.4 mg kg–1 day subcutaneously) to castrated rats. Data are expressed as mean ± S.D. (six animals per group). * p < 0.05 different from solvent control group.
3.3. Serum measurement
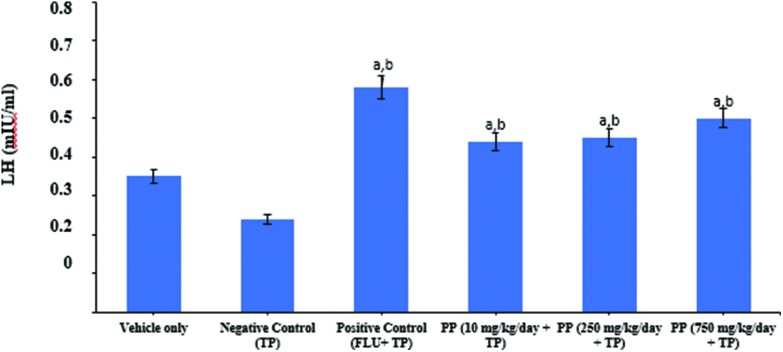
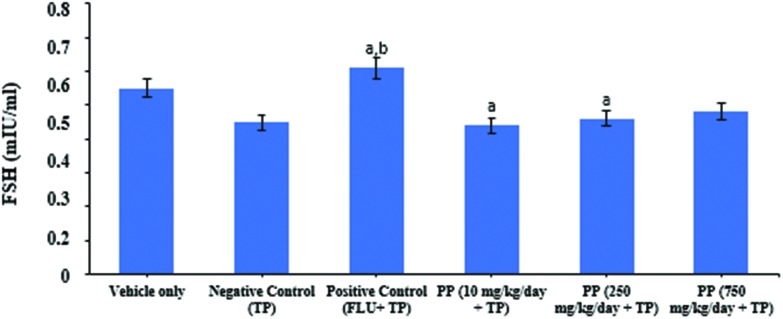
Both serum LH and FSH levels were significantly decreased with testosterone propionate treatment in castrated male rats compared to the vehicle control group. But, serum LH levels were significantly increased in given flutamide+ testosterone propionate groups compared to the vehicle and negative control group. LH serum concentrations at doses of 10, 250, and 750 mg kg–1 day PP were significantly higher than controls (Fig. 3). FSH serum concentrations at doses of 10 and 250 mg kg–1 day PP groups were significantly lower than vehicle and positive control groups (Fig. 4). However, at 750 mg kg–1 day PP treated animals, the levels of FSH were similar to control values.
Fig. 3. Effects of flutamide and propyl paraben on plasma LH levels in castrated male rats treated with testosterone propionate. Treatment was initiated 8 days after castration, 24 h after the last dosing; plasma LH levels were measured using an ELISA kit. Data are expressed as mean ± S.D. (six animals per group). aDifferent from vehicle control group, bdifferent from negative control group, p < 0.05.
Fig. 4. Effects of flutamide and propyl paraben on plasma FSH levels in castrated male rats treated with testosterone propionate. Treatment was initiated 8 days after castration, 24 h after the last dosing; plasma FSH levels were measured using an ELISA kit. Data are expressed as mean ± S.D. (six animals per group). aDifferent from vehicle control group, bdifferent from negative control group, p < 0.05.
3.4. Histopathological analysis
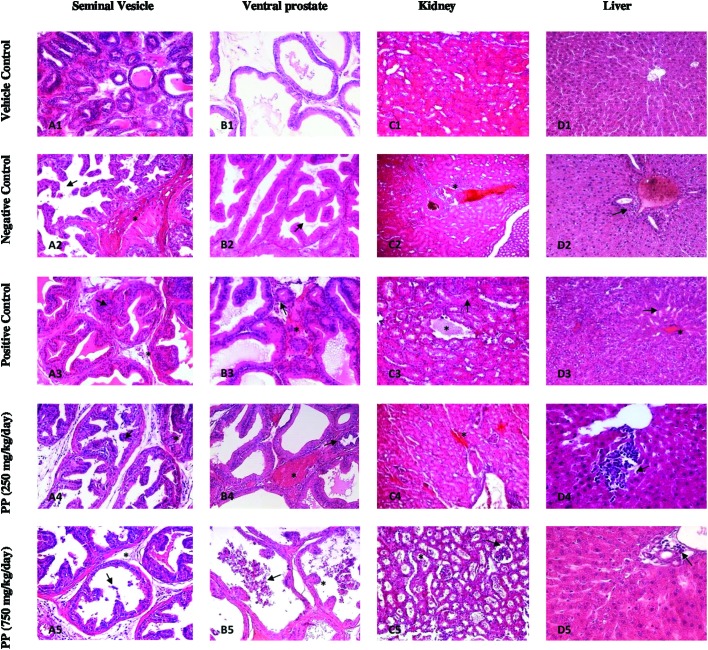
In the vehicle control group (Fig. 5A1, B1, C1, and D1), seminal vesicle, ventral prostate, liver, and kidney tissue cells showed normal morphology and complete arrangement. In the TP-negative control group (TP = 0.4 mg kg–1 day), there was a disruption of the histoarchitecture of all organs (Fig. 5). In seminal vesicles, hyperplasic vesicles and fibromuscular tissue are shown (Fig. 5A2). In the positive control group, flutamide given with TP, hyalinization, and atrophic vesicles (Fig. 5A3), and in the 250 mg kg–1 day group, PP given with TP, hyalinization and anastomosis (Fig. 5A4), and in the 750 mg kg–1 day group, PP given with TP, hyalinization and a mass of cells in vesicle lumen were observed (Fig. 5A5). In the ventral prostate, the negative control group given TP only showed hyperplasic nodules (Fig. 5B2), but in the positive control group, flutamide given with TP, and 250 mg kg–1 day, and PP given with TP showed tubular atrophy and minimal congestion (Fig. 5B3, B4), while 750 mg kg–1 day PP given with TP, caused an increase of the alveolar volume, polyp structures, and a mass of cells in prostate lumen (Fig. 5B5). FLU treatment also showed apoptosis on the ventral prostate and seminal vesicle within the observable hyalinization. In kidney tissue, the negative control group given TP-only, showed cellular degeneration (Fig. 5C3), while in the positive control group flutamide given with TP, showed tubular degeneration and oedema (Fig. 5C3). But 250 mg kg–1 day PP given with TP, showed congestion (Fig. 5C4), and 750 mg kg–1 day PP given with TP, showed enlargement in the glomerular capsule and tubular degeneration (Fig. 5C5). In liver tissue, the negative control group given TP-only showed congestion and Kupffer cell proliferation around the portal vein (Fig. 5D2). Administration of FLU (3 mg kg–1 day) enhanced sinusoidal expansion and congestion in liver tissue (Fig. 5D3). At 250 mg kg–1 day propyl paraben, mononuclear inflammatory cells occurred in liver tissue (Fig. 5D4). At 750 mg kg–1 day propyl paraben, alterations in liver tissue occurred such as Kupffer cell proliferation among the portal veins (Fig. 5D5).
Fig. 5. (A1) Photomicrograph of the seminal vesicle of castrated rat H&E 20×. (A2) Negative group given TP-only, showing marked fibromuscular stroma (*) and hyperplasic vesicles (arrow) H&E 20×. (A3) Positive group flutamide given with TP, showing hyalinization (*) and atrophic vesicles (arrow) H&E 20×. (A4) 250 mg kg–1 day PP given with TP, showing hyalinization (*) and anastomosis (arrow) H&E 20×. (A5) 750 mg kg–1 day PP given with TP, showing hyalinization (*) and cells in vesicle lumen (arrow) H&E 20×. (B1) Photomicrograph of the ventral prostate of castrated rat H&E 20×. (B2) Negative control group given TP-only, showing hyperplasic nodules (*) H&E 20×. (B3) Positive control group flutamide given with TP, showing tubular atrophy (arrow), minimal congestion (*) H&E 20×. (B4) 250 mg kg–1 day PP given with TP, showing minimal congestion (*) and tubular atrophy (arrow) H&E 20×. (B5) 750 mg kg–1 day PP given with TP, showing increased volume of the alveolar lumen, in detriment of epithelial cells height (atrophy of structural elements), polyp structures (*), and mass of cells in the tubule lumen (arrow) H&E 40×. Increased volume of the alveolar lumen, associated with emptiness (arrow) of the great majority of alveoli content. (C1) Photomicrograph of the kidney of castrated rat H&E 20×. (C2) Negative group given TP-only, showing cellular degeneration (*) H&E 20×. (C3) Positive group flutamide given with TP, showing tubular degeneration (arrow) and oedema (*) H&E 20×. (C4) 250 mg kg–1 day PP given with TP, showing congestion H&E 20×. (C5) 750 mg kg–1 day PP given with TP, showing enlargement in the glomerular capsule (arrow) and tubular degeneration (*) H&E 40×. (D1) Photomicrograph of the liver of castrated rat H&E 20×. (D2) Negative control group given TP-only, showing congestion and Kupffer cell proliferation around the portal vein (arrow) H&E 20×. (D3) Positive control group flutamide given with TP, showing minimal congestion (*) and sinusoidal expansion (arrow) H&E 20×. (D4) 250 mg kg–1 day PP given with TP, showing mononuclear inflammatory cells (arrow) H&E 40×. (D5) 750 mg kg–1 day PP given with TP, showing Kupffer cells proliferation around the portal vein (arrow) H&E 40×.
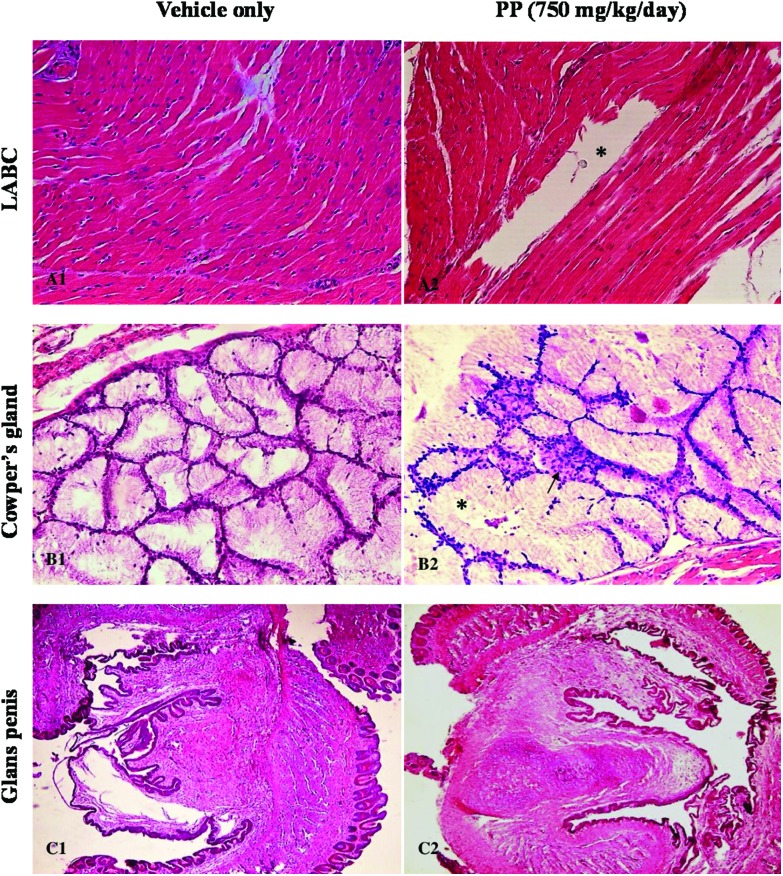
In the vehicle control group (Fig. 6A1, B1, C1), LABC, Cowper's gland and glans penis tissue cells showed normal morphology and complete arrangement. Since 250 and 750 mg kg–1 day PP doses showed similar endpoints on LABC, Cowper's gland, and glans penis, we only mentioned the results from the 750 mg kg–1 day PP group. As shown in Fig. 6(A2), massive ruptures of striated muscle fibers were observed in LABC tissue at the 750 mg kg–1 day propyl paraben dose group. In Cowper's glands, enlargement of nodules and hypertrophy on basal cells were shown (Fig. 6B2). There were no histopathological changes observed on glans penis (Fig. 6B3).
Fig. 6. (A1) Photomicrograph of the LABC of castrated rat H&E 20×. (A2) 750 mg kg–1 day PP given with TP, showing massive rupture of striated muscle fibers (*) H&E 20×. (B1) Photomicrograph of the Cowper's gland of castrated rat H&E 20×. (B2) 750 mg kg–1 day PP given with TP, showing hypertrophy on basal cells (arrow) and enlargement of nodules (*) H&E 20×. (C1) Photomicrograph of the glans penis of castrated rat H&E 20×. (C2) 750 mg kg–1 day PP given with TP H&E 20×.
4. Discussion
Reproductive and developmental disorders can be considered as a significant source of health detriment. Some known endocrine disruptors can bind to the androgen receptor and inhibit the conversion of testosterone to dihydrotestosterone.20 The present study focused on the effects of propyl paraben exposures on androgenic organs, liver, and kidney tissues of male rats. Such substances present a risk for adverse health effects regarding the male reproductive function. Oishi reported that oral dosing with 0, 10, 100, and 1000 mg kg–1 day propyl paraben (PND 21) for 4 weeks reduced spermatogenesis and testosterone hormone levels.15 In that study, Oishi indicated the lowest-observed adverse effect level (LOAEL) as 10 mg per kg bw day for propyl paraben.15 The dosage level at which antiandrogenic activities were observed was lower than the ADI (10 mg kg–1 day) value.16 To prove the androgenic property of propyl paraben, an androgen transcriptional activity assay was employed to assess the antiandrogenic activity of propyl paraben at a concentration of 10 μM. At this concentration, propyl paraben inhibited testosterone (T)-induced transcriptional activity by 33% (p < 0.05).21,22 However, in a GLP-compliant juvenile toxicity study, at 3, 10, 100, or 1000 mg kg–1 propyl paraben was employed and no evidence was found of an effect of propyl paraben on the weight of the male reproductive organs, epididymal sperm parameters, serum hormone levels, or histopathologic endpoints. In addition, no effects on the developing male reproductive organs could be observed when male rats were treated from the neonatal period and the dose of 1000 mg kg–1 day was mentioned as the no-observed adverse effect level (NOAEL).23 Due to insufficient human data on antiandrogenic profiles of propyl paraben, the relation between exposure and effects cannot be estimated based on an animal-to-human exposure comparison. However, one neonates study indicated a kinetic model in human neonates for propyl paraben exposure and the dose of 2 mg kg–1 day for propyl paraben was mentioned as a permitted daily exposure in adults.24
Because some studies on male reproductive system provided conflicting results, we wanted to enlighten this area by using the in vivo Hershberger bioassay screening method. This is the first study to examine whether propyl paraben interferes with AR-mediated mechanisms using the Hershberger bioassay and supported by detailed histopathological aspects. Hershberger bioassay is a short term and quantitative method for assessing androgenic or antiandrogenic properties of xenobiotics by measuring the organ weights of seminal vesicles, ventral prostate, LABC, glans penis and Cowper's gland.25 The advantages of this assay are that it is a specific procedure to assess the xenobiotics with AR-mediated mechanisms in vivo.26 Therefore, the Hershberger bioassay was used to assess the disruptive effects of propyl paraben on the castrated 6-week old immature male rats. The reason why immature male rats were chosen is that they are more sensitive to the negative feedback of testosterone when it is compared with adult males having a higher threshold for the gonadal mechanisms.27,28 Also, androgen-dependent tissues respond with rapid and vigorous growth to stimulation by androgens, particularly in castrate-peripubertal male rats. Therefore, the assay version using the castrated peripubertal rat and the five target tissues in this assay are appropriate for in vivo screening of androgen agonists and antagonists and 5α-reductase inhibitors. Also, castration enhances the precision of the assay to detect weak androgens and antiandrogens by eliminating compensatory endocrine feed-back mechanisms present in the intact animal that can attenuate the effects of administered androgens and antiandrogens and by eliminating the large inter-individual variability in serum testosterone levels. Hence, castration reduces the numbers of animals required to screen for these endocrine activities.19
The recovery time also varied and ranged from no recovery to 11 days.9 In this study, the recovery period was 8 days. There are also many different protocols for a Hershberger bioassay. But, generally oral administration was used as an exposure route for test chemicals according to the EDSTAC protocol12 and further findings indicated that subcutaneous (s.c.) injection is highly sensitive to the androgenic effects (testosterone propionate).29 Regarding s.c. TP treatment, the method prolongs hormone effectiveness because of the short half-life of steroids.30
In the current study, all propyl paraben treatment groups indicated a decrease in body weight. Otherwise, body weight was extremely increased in the negative control group. All organ weights of rats given TP alone were higher than in rats given the vehicle alone, flutamide, and all propyl paraben dose groups. FLU is known to inhibit the development of sex organ weights.31 In our experiment, oral FLU administration resulted in a decrease of all sex accessory tissue weights. A similar effect was observed for propyl paraben at 250 mg kg–1 day and 750 mg kg–1 day treatment groups and in these groups we observed a significant decrease of accessory sex organ weights. This data clearly indicates that 250 and 750 mg kg–1 day propyl paraben doses have significant antiandrogenic effects on sex accessory organs of castrated immature rats. Srinivasan et al. reported that weight loss of measured tissues (sex accessory organs) may be attributed to the degeneration of basement membrane and interstitial tissues and an absence of cell proliferation.31 Among optional organs, liver weights in positive and all paraben dose groups significantly decreased compared to solvent and negative control groups. It is known that FLU inhibits secretion of androgens, which leads to a change of androgen-dependent serum hormone levels.32 The LH levels of male rats in all exposed propyl paraben groups and positive control group (FLU), were increased compared to vehicle and negative control levels. Contrary to this, 10 and 250 mg kg–1 day PP doses, except for 750 mg kg–1 day PP dose and positive control, FSH levels were decreased compared to vehicle control. Interference with cellular processes has been shown in rodents exposed to endocrine disruptors, which indicates that Sertoli cells did not respond to the FSH trigger for spermatogenesis or inhibin production.33,34 In our study, the mentioned increasing of plasma LH and decreasing of plasma FSH levels may occur due to inhibition of negative feedback regulation by testosterone35 and also a castration operation may affect these obtained endpoints.
In histopathologic examinations, we found remarkable histopathologic results in all groups. The differences in fibromuscular volume fractions in the TP-treated group was interpreted as an indication that fibrous and fibromuscular tissues respond to testosterone treatment slightly more than with other groups.32,33 The histopathologic endpoints of the FLU-treated group showed slight histological changes, such as atrophy and hyalinization on seminal vesicles and ventral prostates. Since flutamide has the ability to suppress T antigen-driven carcinogenesis and results in a significant decrease in the incidence of prostate cancer, similar histological results were reported in other studies.36
At the 750 mg kg–1 day–1 dose of propyl paraben, LABC showed massive ruptures of striated muscular fibbers in muscle samples. Similar results of some antiandrogens were also mentioned as damages in LABC tissues.37 No histopathological alterations were found on glans penis. In our study, enlargement of Cowper's gland nodules and the hypertrophy on basal cells at 750 mg kg–1 day propyl paraben may indicate potential basal cell carcinoma but further immunohistochemical studies on Cowper glands are needed to clarify the endpoints.38
In addition to marked sinusoidal expansion, mononuclear inflammatory cells and congestions in liver tissues (respectively FLU and 250 mg kg–1 day propyl paraben treatment), proliferation of Kupffer cells from the obtained liver results allow a conclusion that propyl paraben and TP can be toxic to liver cells. This toxicity is related to an increas in proliferation of Kupffer cells at the 750 mg kg–1 day PP. It is known that Kupffer cell activation occurs in response to liver injury.39 Furthermore, propyl paraben may also present toxicity with increased production of superoxide anions and lead to an impairment of antioxidant mechanisms.40
With a dose of 250 mg kg–1 day PP, glomerular and tubular deformations and marked cells in the tubule lumen were observed. A similar study also claimed the related anti-androgenic potential of propyl paraben assessing inhibition of testosterone-induced transcriptional activity in a kidney cell line.22 Overall, our study did not confirm results reported in Gazin's study that included contrary results about propyl paraben. Indeed, Gazin's results indicated that propyl paraben had no antiandrogenic effects on non-castrated juvenile male rats and showed the effects of propyl paraben from the accessory male organs only on ventral prostate and seminal vesicles.18 In terms of our detailed investigation, we castrated 6-week old male rats to avoid the oscillation of the androgenic hormone system and prepared an effective experimental design instead. For more specific endpoints, we measured the weights of the rest of the accessory male organs such as LABC, Cowper's gland, and glans penis; this supported our results within the histopathological aspects including kidney and liver tissues. This assay has the advantage that it can assess all accessory male reproductive tracts and evaluate the histopathological effects of propyl paraben. Hence, we present that this study provides needed aspects about the antiandrogenic properties of propyl paraben on male reproductive health.
The European Commission is required to reduce the concentration of propyl paraben in related products, including propyl paraben (up to 0.14% in formulations), and to avoid using propyl paraben in products which are designed for application on the nappy area of children under the age of three.41 We wanted to embark on a discussion about this opinion within the mentioned results on propyl paraben. Further supportive data using in vitro assays are needed to determine the antagonistic effects of propyl paraben.
5. Conclusions
In this study, we found that propyl paraben significantly decreased all the organ weights at each dose of 250 and 750 mg kg–1 day. Also, we observed that most obvious histological changes (atrophy and hyalinization) occurred in all propyl paraben dose groups. Inspected tissue weight and histoarchitecture suggested that propyl paraben has an important role as an endocrine disruptor. Within the supported results of increasing LH levels and histopathologic results (such as atrophy and hyalinization on androgenic tissues), we strongly believe that this study gives detailed information about the antiandrogenic profiles of propyl paraben.
Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank the authors for their commitment to this project. Development of this study was financially supported by the Hacettepe University Scientific Research Projects Coordination Unit (Project no: FHD-2017-13477).
References
- Soni M. G., Carabin I. G., Burdock G. A. Food Chem. Toxicol. 2005;43:985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Guo Y., Kannan K. A. Environ. Sci. Technol. 2013;47:14442–14449. doi: 10.1021/es4042034. [DOI] [PubMed] [Google Scholar]
- Melo L. P., Queiroz M. E. J. Sep. Sci. 2010;33:1849–1855. doi: 10.1002/jssc.201000024. [DOI] [PubMed] [Google Scholar]
- Genuis S. J., Birkholz D., Curtis L., Sandau C. ISRN Toxicol. 2013:507897. doi: 10.1155/2013/507897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canosa P., Rodriguez I., Rubi E., Cela R. Anal. Chem. 2007;79:1675–1681. doi: 10.1021/ac061896e. [DOI] [PubMed] [Google Scholar]
- Wang L., Liao C., Liu F., Wu Q., Guo Y., Moon H. B., Nakata H., Kannan K. Environ. Sci. Technol. 2012;46:11584–11593. doi: 10.1021/es303516u. [DOI] [PubMed] [Google Scholar]
- N.O.E.S., National Occupational Exposure Survey conducted from 1981–1983. Estimated numbers of employees potentially exposed to specific agents by 2-digit standard industrial classification (SIC), Jan 16, 2007: http://www.cdc.gov/noes/.
- Boberg J., Taxvig C., Christiansen S., Hass U. Reprod. Toxicol. 2010;30:301–312. doi: 10.1016/j.reprotox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Andersen F. A. Int. J. Toxic. 2008;27(Suppl. 4):1–82. [Google Scholar]
- Aubert N., Ameller T., Legrand J. J. Food Chem.Toxicol. 2012;50:445–454. doi: 10.1016/j.fct.2011.12.045. [DOI] [PubMed] [Google Scholar]
- Frederiksen H., Jørgensen N., Andersson A. M. J. Exposure Sci. Environ. Epidemiol. 2011;21:262–271. doi: 10.1038/jes.2010.6. [DOI] [PubMed] [Google Scholar]
- Soni M. G., Carabin I. G., Burdock G. A. Food Chem. Toxicol. 2005;43(7):985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Routledge E. J., Parker J., Odum J., Ashby J., Sumpter J. P. Toxicol. Appl. Pharmacol. 1998;153:12–19. doi: 10.1006/taap.1998.8544. [DOI] [PubMed] [Google Scholar]
- Bao-Liang S., Hai-Ying L., Dunren P. Contraception. 1989;39:331–335. [Google Scholar]
- Oishi S. Food Chem. Toxicol. 2002;40:1807–1813. doi: 10.1016/s0278-6915(02)00204-1. [DOI] [PubMed] [Google Scholar]
- Satoh K., Nonaka R., Ohyama K., Nagai F. J. Health Sci. 2005;51:557–568. [Google Scholar]
- U.S. E.P.A, Chemical Data Reporting (CDR). Non-confidential 2012 Chemical Data Reporting information on chemical production and use in the United States. Oct 27, 2016: http://www.epa.gov/chemical-data-reporting.
- Gazin V., Marsden E., Marguerite F. Toxicol. Sci. 2013;136:392–401. doi: 10.1093/toxsci/kft211. [DOI] [PubMed] [Google Scholar]
- U.S.E.P.A .
- Breiner M., Romalo G., Schweikert H. U. Wien. Klin. Wochenschr. 1986;64:732–737. doi: 10.1007/BF01734339. [DOI] [PubMed] [Google Scholar]
- Chen J., Ahn K. C., Gee N. A. Toxicol. Appl. Pharmacol. 2007;221:278–284. doi: 10.1016/j.taap.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulla H., Yakkundi S., McElnay J., Lutsar I., Metsvaht T., Varendi H., Nellis G., Nunn A., Duncan J., Pandya H., Turner M. Pharm. Res. 2015;32:1084–1093. doi: 10.1007/s11095-014-1520-2. [DOI] [PubMed] [Google Scholar]
- Hershberger L., Shipley E., Meyer R. Proc. Soc. Exp. Biol. Med. 1953;83:175–180. doi: 10.3181/00379727-83-20301. [DOI] [PubMed] [Google Scholar]
- Gray L. E. Reprod. Toxicol. 1997;11:719–750. doi: 10.1016/s0890-6238(97)00025-7. [DOI] [PubMed] [Google Scholar]
- Owens W., Zeiger E., Walker M., Ashby J., Onyon L., Gray Jr. L. E. Environ. Health Perspect. 2006;114(8):1259–1265. doi: 10.1289/ehp.8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez J. M., Nazian S. J., Mahesh V. B. Biol. Reprod. 1980;22:486–487. doi: 10.1095/biolreprod22.3.486. [DOI] [PubMed] [Google Scholar]
- O'Connor J. C., Frame S. R., Davis J. C., Cook L. G. Toxicol. Sci. 1999;51:44–53. doi: 10.1093/toxsci/51.1.44. [DOI] [PubMed] [Google Scholar]
- Howell T. J., MacDougall D. E., Jones P. J. H. J. Lipid Res. 1998;39:892–900. [PubMed] [Google Scholar]
- Hoberman A. M., Schreur D. K., Leazer T., Daston G. P., Carthew P., Re T., Loretz L., Mann P. Birth Defects Res., Part B. 2008;83(2):123–133. doi: 10.1002/bdrb.20153. [DOI] [PubMed] [Google Scholar]
- Tinwell H., Santini C. Toxicol. Sci. 2007;100:54–65. doi: 10.1093/toxsci/kfm208. [DOI] [PubMed] [Google Scholar]
- Srinivasan N., Micheal M., Govindarajulu P. J. Androl. 1986;7:55–58. doi: 10.1002/j.1939-4640.1986.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Yamada T., KunimatsuT T., Sako H. Toxicol. Sci. 2000;53:289–296. doi: 10.1093/toxsci/53.2.289. [DOI] [PubMed] [Google Scholar]
- David R. M. Toxicol. Pathol. 2006;34:209–219. doi: 10.1080/01926230600642625. [DOI] [PubMed] [Google Scholar]
- Treinen K. A., Dodson W. C., Heindel J. J. Toxicol. Appl. Pharmacol. 1990;106:334–340. doi: 10.1016/0041-008x(90)90252-p. [DOI] [PubMed] [Google Scholar]
- Viguier-Martinez M. C., Hochereau de Reviers M. T., Barenton B., Perreau C. Acta Endocrinol. 1983;104:246–252. doi: 10.1530/acta.0.1040246. [DOI] [PubMed] [Google Scholar]
- Raghow S., Kuliyev E., Steakley M., Greenberg N., Steiner M. S. Cancer Res. 2000;60:4093–4097. [PubMed] [Google Scholar]
- Cristina R. T., Hangau F., Brezovan D., Dumitrescu E., Muselin F., Chiurciu V., Stancu A. C., Pentea M. C., Motoc A. G. M. Rom. J. Morphol. Embryol. 2014;55:1143–1148. [PubMed] [Google Scholar]
- Dermer G. B. Cancer. 1978;41:1857–1862. doi: 10.1002/1097-0142(197805)41:5<1857::aid-cncr2820410529>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Han S. Y., Huh C. S., Ahn Y. T. Arch. Pharmacal Res. 2005;28:325–329. doi: 10.1007/BF02977800. [DOI] [PubMed] [Google Scholar]
- Szeląg S., Zabłocka A. Toxicol. In Vitro. 2016;31:30–34. doi: 10.1016/j.tiv.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Scientific Committee on Consumer Safety (SCCS), Clarification on Opinion SCCS/1348/10 in the light of the Danish clause of safeguard banning the use of parabens in cosmetic products intended for children under three years, of age, 2011, SCCS/1446/11