Abstract
Psychostimulant reinforcement is mediated by stimulation of both dopamine (DA) D1-like and D2-like receptors, suggesting that pharmacotherapy agents with a dual DA receptor mechanism may be useful for managing psychostimulant abuse. (−)-Stepholidine (L-SPD) is a Chinese herbal extract that functions as a D1-like receptor agonist and D2-like receptor antagonist. L-SPD has been shown to attenuate the reinforcing effects of heroin; however, its effects on the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) have not been examined. The current study determined the effects of L-SPD on reinstatement of MDPV-seeking behavior in the drug intravenous self-administration (IVSA) and conditioned place preference (CPP) paradigms. To determine whether the effects of L-SPD were specific to psychostimulant reinforcement, we also examined its effects on sucrose-seeking behavior. Using a locomotor activity assay, we tested the locomotor effects of L-SPD, as well as its effects on MDPV-induced hyperactivity. The results of a battery of in vitro binding and functional assays confirmed that L-SPD functioned as a D1-like receptor agonist and D2-like receptor antagonist. In behavioral experiments, L-SPD dose-dependently attenuated cue plus MDPV-primed reinstatement of MDPV-seeking behavior in the IVSA model. The highest dose of L-SPD also attenuated MDPV-primed reinstatement of MDPV CPP, as well as cue-induced reinstatement of sucrose-seeking. L-SPD had no significant locomotor effects, and did not modulate the robust hyperactivity induced by MDPV. The current findings show for the first time a robust reinstatement effect with MDPV, which can be reduced by L-SPD. These results establish a role for DA receptors in drug-seeking behavior for MDPV.
Keywords: conditioned place preference, dopamine receptor, MDPV, reinstatement, self-administration, Stepholidine
Introduction
3,4-Methylenedioxypyrovalerone (MDPV) is a popular constituent of the synthetic cathinone class of novel psychoactive substances that emerged on the illicit drug scene in the late 2000’s 1. Despite being controlled in Schedule I of the U.S. Controlled Substances Act in 2011, MDPV is still available on the “dark web”, and has remained attractive to drug users due to its acute euphoric effects and ability to increase energy and sexual arousal 2. However, there have been reports of tachycardia, hypertension and paranoia following repeated use, which in some cases were fatal 3 4.
Rodent studies indicate that the psychomotor and rewarding effects of MDPV mirror that of other amphetamine-derived psychostimulants (e.g. cocaine and methamphetamine) 5. Most notably, MDPV produces a robust conditioned place preference (CPP) 6–8, and maintains high rates of drug intravenous self-administration (IVSA) behavior 9–13. Similar to cocaine, the high abuse liability of MDPV stems from its pharmacological action as a potent dopamine (DAT) and norepinephrine (NET) transport inhibitor that increases extracellular dopamine (DA) and norepinephrine (NE) levels in brain reward regions 14 15.
A large body of research demonstrates a central role for the DA system in the rewarding and reinforcing properties of psychostimulants 16. Two opposing classes of DA receptors primarily mediate these effects: low-affinity D1-like (D1 and D5) receptors, and high-affinity D2-like (D2, D3 and D4) receptors 17. Pharmacological manipulation of D1- or D2-like receptors can, in some cases, differentially affect cocaine-induced reinforcement. For example, both D1-like and D2-like receptor agonists decrease cocaine self-administration on the descending limb of the dose-response curve for cocaine 18. However, D1-like and D2-like agonists produce opposite effects on cocaine-primed reinstatement of cocaine-seeking behavior; namely, D1-like agonists inhibit whereas D2-like receptor agonists enhance reinstatement, respectively 19. In addition, selective D1/D5, D2 and D3 receptor antagonists inhibit the propensity for relapse to cocaine-seeking behavior 20–22. Taken together, these results suggest that pharmacotherapy agents that differentially modulate multiple DA receptor subtypes may be useful for managing the reinforcing effects of psychostimulants.
(−)-Stepholidine (L-SPD; Chemical name: (S)-3,9-Dimethoxy-6,8,13,13a-tetrahydro-5H-isoquinolino[3,2-a]isoquinoline-2,10-diol) is a tetrahydroberberine alkaloid extracted from the Chinese herb Stephania intermedia that was originally used in traditional medicine to treat a myriad of ailments 23. The pharmacological mechanism of L-SPD is generally considered to be that of a D1-like receptor agonist and D2-like receptor antagonist 23–27. A number of preclinical studies in rodents suggest that L-SPD may attenuate drug-induced reinforcement. Most notably, L-SPD reduced morphine-induced place preference 28, inhibited heroin self-administration 29, and blocked cue-induced 29 as well as drug-primed 30 reinstatement of heroin-seeking behavior.
In the current study, we first conducted an in vitro binding and functional screen to evaluate the activity of L-SPD at the D1-like and D2-like receptors. In contrast to cocaine, no study to date has examined the propensity for relapse to MDPV-seeking behavior that is a hallmark of psychostimulant addiction. Therefore, we determined if animals repeatedly exposed to MDPV reinstate their drug-seeking behavior in response to MDPV-associated cues and/or an MDPV prime following a period of extinction. We employed two established behavioral assays of drug-induced reinforcement, the IVSA and CPP models. We examined whether L-SPD modulates reinstatement of MDPV-seeking behavior, given its dual DA receptor action and inhibitory effects on heroin-induced reinforcement. To determine whether the effects of L-SPD were specific to psychostimulant-induced reinforcement, we assessed whether L-SPD inhibits cue-induced reinstatement of sucrose-seeking. Lastly, we conducted a locomotor activity assay to determine if L-SPD affects MDPV-induced hyperactivity, and whether L-SPD itself has any motoric effects.
Results and Discussion
The current study was conducted to determine whether the Chinese herbal extract L-SPD, which displays a unique pharmacological profile as a D1-like receptor agonist and D2-like receptor antagonist, can attenuate reinstatement of MDPV-seeking behavior that is a core feature of psychostimulant addiction.
We first confirmed the receptor pharmacology of L-SPD using a series of in vitro binding and functional screens. Results from binding assays showed that L-SPD binds to D1, D2, D3 and D5 receptors with Ki values ranging from 5–20 nM. The affinity of L-SPD at the D4R was very low with a Ki value higher than 1 μM (Table 1). Therefore, L-SPD in vivo likely binds to the D1R, D2R, D3R, and D5R, but not the D4R.
Table 1.
Ki values (nM) of L-SPD to the human D1, D4 and D5 receptors stably expressed in HEK293 cells and human D2 and D3 receptors stably expressed in CHO cells. Competitive inhibition by L-SPD of ~1nM [3H]SCH23390 binding to D1 and D5 receptors and of ~1nM [3H]methylspiperone binding to D2, D3 and D4 receptors was conducted, and its Ki values were determined. Each value represents mean ± S.E.M. of three experiments.
| D1-like receptors | D2-like receptors | ||||
|---|---|---|---|---|---|
| D1R | D5R | D2R | D3R | D4R | |
| L-SPD | 5.6 ± 0.7 | 18.4 ± 2.5 | 15.0 ± 1.4 | 7.8 ± 0.6 | >1000 |
For functional assays, D1 and D5 receptors were coupled to Gs proteins and stimulation of adenylyl cyclase was used as the functional end point. For these experiments, DA was used as the reference full agonist. As shown in Table 2 and Fig. 1, L-SPD functioned as a full agonist at the D1R, and as a low-efficacy partial agonist at the D5R (t(4) = 14.97, p < 0.001). For the D5R, it should be noted that L-SPD still activated the receptor, as evidenced by the fold change in stimulated cAMP level compared with the basal level (Table 2).
Table 2.
Potencies (EC50) and efficacies (Emax) of dopamine (DA) and L-SPD in stimulating D1 or D5 receptors to enhance cAMP formation in HEK cells expressing the human D1 or D5 receptor. Each value represents mean ± S.E.M. (n = 3–4).
| D1R | D5R | |||||
|---|---|---|---|---|---|---|
| Log[EC50(M)] | EC50 (μM) | Emax (% of 10−5 M DA response) [fold of basal] | Log[EC50(M)] | EC50 (μM) | Emax (% of 10−5 M DA response) [fold of basal] | |
| DA | −7.01 ± 0.20 | 0.098 ± 0.046 | 108.1 ± 9.7 [17.4] | −6.69 ± 0.12 | 0.20 ± 0.06 | 108.4 ± 5.3 [25.5] |
| L-SPD | −7.68 ± 0.12 | 0.021 ± 0.006 | 108.8 ± 4.0 [17.5] | −7.20 ± 0.28 | 0.063 ± 0.043 | 23.6 ± 2.0*** [6.5] |
p < 0.001 compared to DA using t-tests.
Figure 1. L-SPD acts as a full and partial agonist at the D1 and D5 receptor, respectively, in comparison to dopamine (DA).
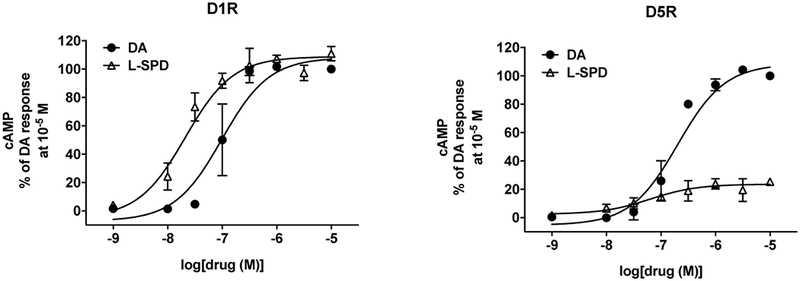
DA and L-SPD stimulated cAMP formation in HEK cells expressing D1 or D5 receptors in a dose-dependent manner. cAMP levels were examined as previously described, and data were normalized to DA at 10−5 M. Potencies and efficacies were determined by curve fitting and are shown in Table 2. Data represent mean ± S.E.M. (n = 3–4).
[35S]GTPγS binding was used as a functional measure of D2 and D3 receptor activation. Due to the low affinity of L-SPD for the D4R, we did not perform D4R-mediated [35S]GTPγS binding. L-SPD at 10 μM had no effect on [35S]GTPγS binding in membranes of cells expressing D2 or D3 receptors (Fig. 2a), indicating that it is not an agonist at these two receptor subtypes. We next examined the potency of L-SPD as an antagonist at the D2 and D3 receptors. For antagonist effects of L-SPD on DA-induced increase of [35S]GTPγS binding, membranes were pretreated with 1 μM of L-SPD followed by 10−9 M to 10−4 M of DA 10 min later. Data were analyzed and EC50 and Emax values were determined. At the D2R, L-SPD at 1 μM shifted the dose-response curve of DA to the right and increased the EC50 value of DA by 21-fold (t(7) = 7.002, p < 0.001 (Log[EC50]) and t(7) = 2.53, p < 0.05 (EC50)). In addition, L-SPD significantly reduced the Emax value of DA (Table 3 and Fig. 2b) (t(7) = 7.24, p < 0.001). At the D3R, L-SPD at 1 μM shifted the dose-response curve of DA to the right and increased the EC50 value of DA by 50-fold (t(6) = 4.22, p < 0.01 (Log[EC50]) and t(6) = 2.68, p < 0.05 (EC50)), without changing the Emax value of DA (Table 3 and Fig. 2b).
Figure 2. L-SPD acts as an antagonist at the D2 and D3 receptor.
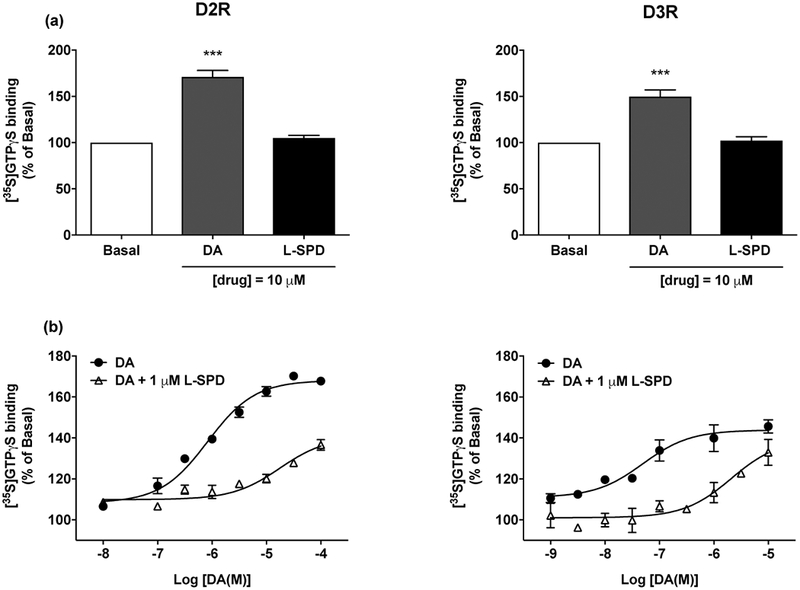
Dopamine (DA), but not L-SPD, stimulated [35S]GTPγS binding in CHO cells expressing D2 or D3 receptors at doses of 10 μM (a). L-SPD at 1 μM shifted the dose-response curve of DA to the right and increased the EC50 value of DA by 21-fold at the D2R, and 50-fold at the D3R (b). Potencies and efficacies were determined by curve fitting and are shown in Table 3. Data represent mean ± S.E.M. (n = 4–5). ***p < 0.001 relative to Basal using one-way ANOVA with Bonferroni post-hoc tests.
Table 3.
Effects of the presence of 1 μM of L-SPD on potency (EC50) and efficacy (Emax) of dopamine (DA) in stimulating D2 or D3 receptors to enhance [35S]GTPγS binding in CHO cells expressing the human D2 or D3 receptor. Basal [35S]GTPγS binding values were ~2000–3000 dpm. Each value represents mean ± S.E.M. (n = 3–5).
| D2R | D3R | |||||
|---|---|---|---|---|---|---|
| Log[EC50(M)] | EC50 (μM) [Fold of DA] | Emax(% of basal) | Log[EC50(M)] | EC50 (μM) [Fold of DA] | Emax(% of basal) | |
| DA | −6.08 ± 0.08 | 0.83 ± 0.14 [1] | 168.1 ± 1.6 | −7.30 ± 0.24 | 0.042 ± 0.019 [1] | 142.5 ± 2.9 |
| DA + L-SPD 1 μM | −4.75 ± 0.19*** | 17.6 ± 7.9* [21] | 140.6 ± 3.8*** | −5.68 ± 0.30** | 2.08 ± 1.04* [50] | 139.9 ± 9.3 |
p < 0.001
p < 0.01
p < 0.05, relative to DA by t-tests.
In accordance with previous reports 23, 24, 31, L-SPD produced antagonist effects at the D2 and D3 receptors. In contrast, L-SPD was a full agonist at the D1R and a low-efficacy partial agonist at the D5R. Our findings are in line with the widely accepted view that L-SPD has a dual action as a partial or full agonist at D1-like receptors and an antagonist at D2-like receptors 23–27. It should be noted that L-SPD has been reported to have antagonist effects on D1-like receptors (both D1R and D5R) 23, 31. These discrepancies may be due to the ability of L-SPD, as a partial agonist at D1-like receptors 23, to display a large range of intrinsic activities at the same receptor depending on the conditions (e.g., receptor level/reserve) and model (different transfected cells) used 32. Interestingly, L-SPD may function as a more potent D1R agonist in a DA-depleted state (e.g., after unilateral 6-hydroxydopamine (6-OHDA) lesions in rats), in which the D1R reserve/expression level is significantly elevated 27.
In the first behavioral experiment (Experiment 1), we determined whether rats repeatedly exposed to MDPV would reinstate their drug-seeking behavior in response to MDPV associated cues or a priming injection of MDPV in the IVSA paradigm. Results indicated that responses on both the active (main effect of IVSA phase: F(3,39) = 25.26, p < 0.001; see Fig. 3a) and inactive (main effect of IVSA phase: F(3,39) = 8.53, p < 0.001; see Fig. 3b) levers differed significantly between the self-administration phase, extinction training and the reinstatement tests (see also Fig. S1). Post-hoc tests indicated that extinction training significantly decreased active lever presses (p < 0.001), but significantly increased inactive lever presses (p < 0.01), when compared to the acquisition phase. There was a significant increase in active lever responses during both the cue-induced (p < 0.001), and MDPV-primed reinstatement tests (p < 0.05), relative to the extinction phase. However, inactive lever presses during the reinstatement tests did not differ significantly from extinction training (both p > 0.05). Therefore, similar to cocaine 33, we demonstrate for the first time that rats reinstate their drug-seeking behavior following a period of extinction when exposed to MDPV-associated cues or a priming injection of MDPV. However, they show noticeably less robust reinstatement in response to an MDPV prime.
Figure 3. MDPV-associated cues or an MDPV prime significantly reinstate MDPV-seeking behavior in the IVSA model.
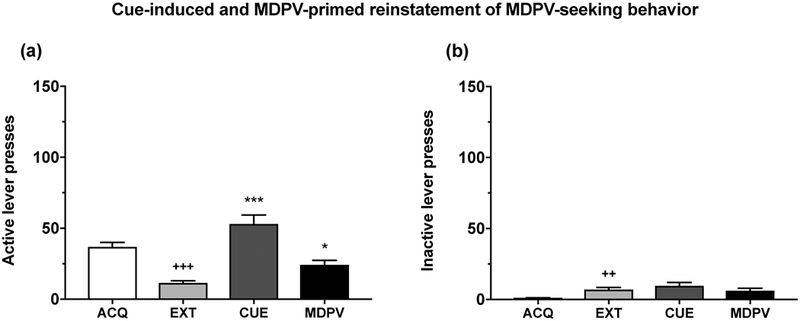
Data for acquisition (ACQ) and extinction (EXT) training was based on average performance across the last 3 days of each phase. EXT significantly decreased active lever presses (a) (p < 0.001), but significantly increased inactive lever presses (b) (p < 0.01), when compared to ACQ. There was a significant increase in active lever responses during both the cue-induced (CUE) (p < 0.001), and MDPV-primed reinstatement tests (MDPV) (p < 0.05), relative to EXT. Data are shown as mean + S.E.M. (n = 14). +++p < 0.001, ++p < 0.01 relative to ACQ; ***p < 0.001, *p < 0.05 relative to EXT.
In Experiment 2, we examined the propensity for reinstatement of MDPV-seeking behavior using both MDPV-associated stimuli and a priming injection of MDPV in the IVSA model. We also determined whether L-SPD could modulate cue plus MDPV-primed reinstatement given its dual DA receptor action. Firstly, to confirm that extinction training effectively reduced MDPV self-administration behavior, we used a paired-samples t-test to compare performance between the acquisition and extinction phases. Results showed that extinction training significantly reduced active lever presses relative to the acquisition phase (ACQ vs. EXT: t(11) = 4.37, p < 0.01; see Fig. 4a). During the reinstatement tests, there was an overall effect of L-SPD treatment on responses on the active lever (main effect of L-SPD dose: F(3,30) = 4.04, p < 0.05; see Fig. 4a), but not the inactive lever (main effect of L-SPD dose: F(3,30) = 1.30, p > 0.05; see Fig. 4b). Post-hoc tests showed that only the highest dose of L-SPD (i.e. 10 mg/kg) significantly reduced active lever presses relative to vehicle (p < 0.01). Although the 1 and 3 mg/kg doses of L-SPD showed trends of a decrease, they did not reach statistical significance. The inhibitory effects of L-SPD were not due to a nonspecific suppression of operant behavior, since there were no differences in inactive lever responses during reinstatement testing. Our results are the first to demonstrate that rats with a history of MDPV exposure will robustly reinstate their drug-seeking behavior in response to MDPV-associated cues and an MDPV prime, and that systemic injection of L-SPD dose-dependently attenuates reinstatement of MDPV-seeking behavior.
Figure 4. L-SPD dose-dependently attenuates cue plus MDPV-primed reinstatement of MDPV-seeking behavior in the IVSA model.
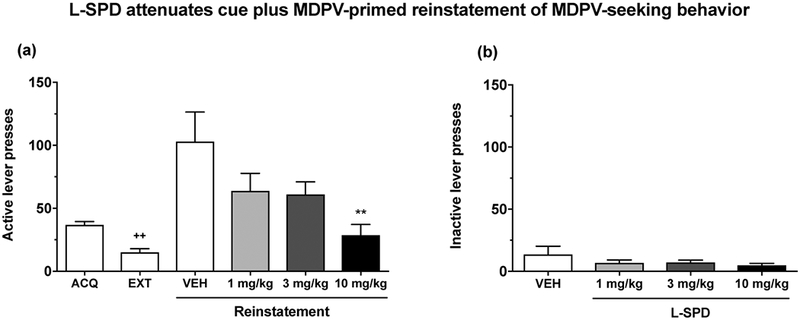
Data for acquisition (ACQ) and extinction (EXT) training was based on average performance across the last 3 days of each phase. EXT significantly reduced active lever responses in comparison to ACQ (p < 0.01) (a). L-SPD at 10 mg/kg (p < 0.01), but not 1 or 3 mg/kg (both p > 0.05), significantly reduced active lever presses during reinstatement testing (a). However, L-SPD did not significantly affect responses on the inactive lever (b). Data are shown as mean + S.E.M. (n = 11). ++p < 0.01 relative to ACQ; **p < 0.01 relative to vehicle (VEH).
To determine whether the inhibitory effects of L-SPD (10 mg/kg) were specific to psychostimulant-induced reinforcement, we examined its effects on cue-induced reinstatement of sucrose-seeking in Experiment 3 (see Fig. 5). Regardless of L-SPD treatment, active (main effect of experimental phase: F(1,13) = 15.6, p < 0.01; see Fig. 5a) but not inactive lever presses (main effect of experimental phase: F(1,13) = 0.44, p > 0.05; see Fig. 5b) were significantly higher during cue-induced reinstatement relative to extinction. Overall, L-SPD treatment decreased active (main effect of L-SPD treatment: F(1,13) = 16.71, p < 0.01) but not inactive lever presses (main effect of L-SPD treatment: F(1,13) = 1.17, p > 0.05), although this differed significantly between the extinction and reinstatement phases (interaction effect on active lever presses: F(1,13) = 14.85, p < 0.01). Post-hoc tests indicated that only vehicle-treated rats showed a significant increase in active lever presses during reinstatement compared to extinction training (p < 0.001). During the cue-induced reinstatement test, rats given L-SPD had significantly fewer active lever presses relative to vehicle-treated rats (p < 0.001). The current results indicate that the inhibitory effects of L-SPD extend to natural rewards, and are not specific to psychostimulant-induced reinforcement.
Figure 5. L-SPD inhibits cue-induced reinstatement of sucrose-seeking.
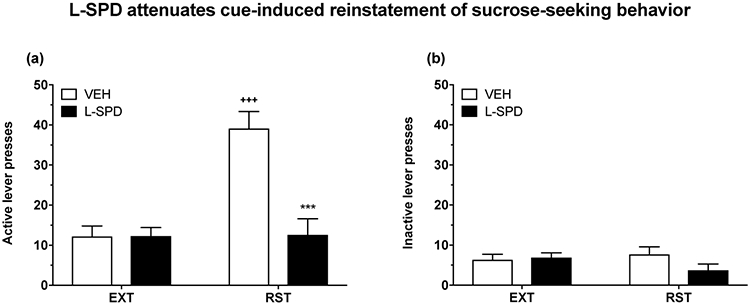
Data for extinction (EXT) training was based on average performance across the last 3 sessions. Vehicle-treated rats showed a significant increase in active lever presses during cue-induced reinstatement (RST) relative to EXT (p < 0.001; within-group comparison) (a). L-SPD (10 mg/kg) significantly reduced active lever presses during RST when compared to vehicle (p < 0.001; between-group comparison) (a). There were no significant differences within or between treatment groups in terms of inactive lever presses across EXT and RST (b). Data are shown as mean + S.E.M. (n = 7–8 per group). +++p < 0.001 relative to EXT (within-group comparison); ***p < 0.001 relative to vehicle (VEH) (between-group comparison).
In Experiment 4, we employed the CPP paradigm to determine the effects of L-SPD (10 mg/kg) on drug prime-induced reinstatement of MDPV place preference (see Fig. 6). Time in the MDPV-paired compartment differed depending on the particular experimental phase (PRE, POST, EXT or RST) of the CPP paradigm regardless of L-SPD treatment (main effect of experimental phase: F(3,66) = 15.39, p < 0.001). L-SPD treatment had a significant overall effect on time in the MDPV-paired side (main effect of L-SPD treatment: F(1,22) = 8.81, p < 0.01), however, this showed a very strong tendency to differ depending on the experimental phase (interaction effect: F(3,66) = 2.73, p = 0.051). Post-hoc tests indicated that both vehicle and L-SPD rats acquired a CPP (PRE vs. POST: both p < 0.01), which was abolished as a result of extinction training (PRE vs. EXT: both p > 0.05). In response to an MDPV prime, rats administered L-SPD showed significantly reduced reinstatement of the previously extinguished CPP compared to vehicle-treated animals (p < 0.001). These results show for the first time that L-SPD attenuates reinstatement of MDPV-seeking behavior in the CPP paradigm, which is another well-established behavioral measure of drug-induced reinforcement.
Figure 6. L-SPD inhibits MDPV-primed reinstatement of MDPV CPP.
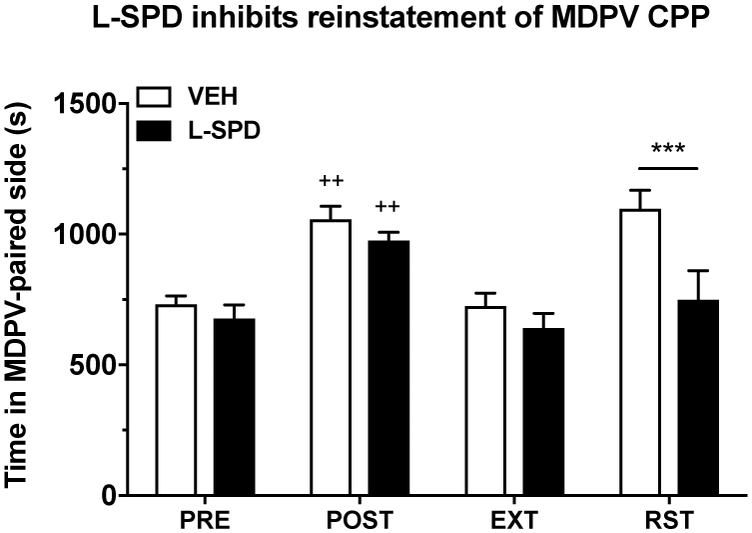
Note that L-SPD (10 mg/kg) was only administered 30 min prior to the reinstatement test. Rats in both treatment groups successfully acquired a CPP (PRE vs. POST; both p < 0.01) with MDPV (2 mg/kg), which was abolished following extinction training (PRE vs. EXT; both p > 0.05). Rats given L-SPD (10 mg/kg) spent significantly less time in the MDPV-paired side during the reinstatement test relative to vehicle-treated rats (p < 0.001). Data are presented as mean + S.E.M. (n = 12 per group). ++p < 0.01 relative to pre-conditioning (within-group comparison); ***p < 0.001 relative to vehicle (between-group comparison). VEH, vehicle; PRE, preconditioning; POST, post-conditioning; EXT, extinction; RST, reinstatement.
From the current results, we cannot determine whether the inhibitory effects of L-SPD on MDPV-seeking behavior were due to a combined D1-like receptor agonist and D2-like receptor antagonist action, or whether either mechanism alone was sufficient to attenuate MDPV reinforcement. The D1-like receptor agonist potency of L-SPD is enhanced under conditions where endogenous DA levels are depleted (e.g., unilateral 6-OHDA-lesioned rats) 27, as may be the case during MDPV withdrawal. Selective D1-like agonists have been shown to produce a downward shift in the dose-response function for cocaine self-administration 18, 34, as well as inhibit cue-induced and cocaine-primed reinstatement of cocaine-seeking behavior 35. On the other hand, pharmacological stimulation of D1-like receptors can, in some cases, be intrinsically rewarding. Systemic delivery of D1-like agonists have been shown to independently maintain self-administration behavior 36 and reinstate drug-seeking behavior 35. Therefore, it is possible that L-SPD by itself may produce rewarding or reinforcing effects. However, Wang and colleagues 28 showed that L-SPD alone failed to produce a place preference or place aversion in the CPP paradigm. Levo-tetrahydropalmatine (L-THP), a closely related analog of L-SPD, also exhibited no significant rewarding properties itself when tested in the brain stimulation reward paradigm 37.
In addition to the D1-like receptors, the D2-like receptors, in particular the D3R, may underlie the effects of L-SPD given that the D3R is involved in cocaine-induced reward and reinforcement 38. A number of previous studies have shown that selective D3R antagonists or partial agonists attenuate cocaine self-administration, inhibit cocaine-induced CPP, and reduce relapse to cocaine-seeking behavior 38. Interestingly, a recent study found that the D3R antagonist NGB2904 reduced cocaine-seeking behavior in the IVSA and CPP paradigms only when co-administered with the D1R partial agonist SKF77434 39, suggesting that D1 and D3 receptors might interact to regulate the motivational properties of cocaine, and perhaps also MDPV. It would be interesting in the future to use D1-like or D2-like receptor knockout mice to tease apart the relative contribution of these receptors in L-SPD’s effects on MDPV reinstatement.
In addition to its D1-like receptor agonist and D2-like receptor antagonist actions, L-SPD may also function as a partial agonist at serotonin 5-HT1A receptors (5-HT1aR’s) 40. A large body of research identifies an important role for 5-HT1aR’s in the rewarding and reinforcing effects of psychostimulants 41. This is supported by evidence that pharmacological stimulation of 5-HT1AR’s regulates a number of psychostimulant-induced behaviors including hyperlocomotion, drug-seeking and self-administration 41. Previously, stimulation of 5-HT1aR’s has been shown to reduce cocaine self-administration possibly by increasing the reinforcing strength of cocaine 42–44, while the 5-HT1AR antagonist WAY-100635 inhibited cocaine-primed reinstatement of cocaine-seeking behavior 45. Therefore, we cannot rule out the possibility that partial agonism of 5-HT1AR’s may have mediated some of the observed effects of L-SPD on MDPV-seeking behavior in the current study.
To examine the effects of L-SPD (10 mg/kg) on the robust hyperactivity induced by MDPV (2 mg/kg), and to determine whether L-SPD by itself had intrinsic locomotor effects, we employed a locomotor activity test in Experiment 5. Data on acute ambulatory activity (see Fig. 7a) and stereotypy (see Fig. 7b) were recorded as counts and summed over 90 min. The behavior of one rat in the vehicle + saline group was greater than 2 SD’s from the mean, and therefore, was excluded from the analysis. Pretreatment with L-SPD itself had no significant effects on ambulatory activity (main effect of L-SPD pretreatment: F(1,27) = 0.33, p > 0.05) or stereotypy (main effect of L-SPD pretreatment: F(1,27) = 0.22, p > 0.05). MDPV acutely increased both behaviors (main effect of MDPV treatment on ambulatory activity: F(1,27) = 53.67, p < 0.001; stereotypy: F(1,27) = 52.06, p < 0.001) regardless of L-SPD pretreatment (interaction effect on ambulatory activity: F(1,27) = 0.60, p > 0.05; stereotypy: F(1,27) = 1.20, p > 0.05).
Figure 7. MDPV-induced hyperactivity is not significantly affected by L-SPD.
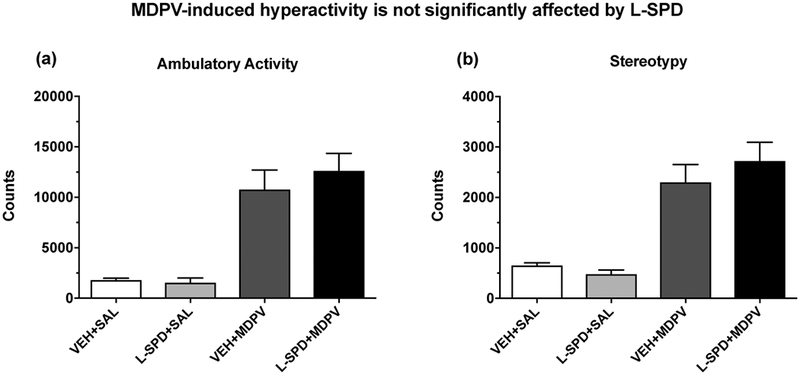
Data were measured as counts and summed over 90 min. L-SPD pretreatment (10 mg/kg) by itself had no effects on either ambulatory activity (a) or stereotypy (b) (both p > 0.05). MDPV (2 mg/kg) treatment significantly increased ambulation (a) and stereotypy (b) (both p < 0.001), neither of which were affected by pretreatment with L-SPD (10 mg/kg) (both p > 0.05). Data are shown as mean + S.E.M. (n = 7–8 per group). VEH, vehicle; SAL, saline.
Consistent with previous studies 28, 29, the behaviorally effective dose of L-SPD (i.e. 10 mg/kg) had no locomotor effects when tested alone. However, somewhat surprisingly, L-SPD failed to modulate the robust increase in ambulatory activity and stereotypy induced by MDPV. Previous reports suggest that the locomotor-activating effects of MDPV are DA-mediated since both a D1/D5 (SCH23390) and D2 receptor (haloperidol) antagonist attenuate the hyperactivity induced by MDPV 15, 46. However, in both these studies, MDPV was administered at 1 mg/kg in comparison to the 2 mg/kg dose employed in the current study. In addition, the DA antagonists were either selective for the D1/D5 receptors or the D2 receptor, in contrast to the dual DA receptor mechanism of L-SPD. It is also interesting that L-SPD has been shown to dose-dependently inhibit amphetamine-induced hyperactivity 25. In this study, the authors administered d-amphetamine at 1.5 mg/kg, which would equate to a much lower dose of MDPV based on the increased DAT potency of MDPV 14. Therefore, methodological differences may explain the lack of an effect of L-SPD on MDPV-induced hyperactivity in the current study.
In conclusion, we are the first to show that rats with a history of MDPV exposure will reinstate their drug-seeking behavior in response to MDPV-associated cues, and to a lesser extent an MDPV prime, following a period of extinction. We also demonstrate for the first time that pretreatment with the dual D1-like receptor agonist and D2-like receptor antagonist L-SPD inhibits the motivation to reinstate MDPV-seeking behavior. The results of the current study highlight an important role for DA receptors in drug-seeking behavior for the synthetic cathinone MDPV.
Methods
Cell Culture, Binding and Functional Assays
Materials
[3H]Methylspiperone (85.5 Ci/mmol), [3H]SCH23390 (73.1 Ci/mmol), [3H]cAMP (25 Ci/mmol) and [35S]GTPγS (1250 Ci/mmol) were purchased from PerkinElmer (Boston, MA). Tetracycline, hygromycin, blasticidin, DA, (+)-butaclamol, fluphenazine, phenylmethylsulfonyl fluoride (PMSF), GDP, GTPγS, cAMP, isobutylmethylxanthine and ascorbic acid were obtained from Sigma-Aldrich (St. Louis, MO). G418 was obtained from Gemini Bio-Products (West Sacramento, CA), while cell culture reagents were obtained from Invitrogen (Carlsbad, CA). (−)-L-SPD was generously provided by Dr. David Y.W. Lee from the Bio-Organic and Natural Products Laboratory at McLean Hospital (Harvard Medical School, Belmont, MA).
Cell culture
For cell culture experiments, human embryonic kidney 293 cells (HEK293 cells) stably transfected with the human D1, D4 or D5 receptor (HEK-D1R, HEK-D4R and HEK-D5R cells), and Chinese hamster ovary cells (CHO cells) stably expressing the human D2 or D3 receptor (CHO-D2R and CHO-D3R cells), were used 47. Cells were grown in 100-mm culture dishes in Minimum Essential Medium supplemented with 10% fetal bovine serum, 100 units/mL penicillin and 100 μg/mL streptomycin in a 5% CO2 incubator at 37°C. Specifically, HEK-D1R cells, which have the CMV promoter controlled by a Tet-on mechanism, were grown in the presence of 50 μg/mL hygromycin and 1 μg/mL blasticidin to maintain TetR and receptor selection, respectively. HEK-D1R cells were induced to express D1R with 1 μg/mL tetracycline for 12 to 24 h before binding or functional assays. To maintain stable receptor expression, CHO-D2R, CHO-D3R or HEK-D4R cells were grown in the presence of 0.2 mg/mL G418, and HEK-D5R in the presence of 1 μg/mL blasticidin.
Receptor binding
DA receptor binding was performed according to published procedures 48. Briefly, for D1 and D5 receptors, [3H]SCH23390 (~2 nM) was used as the radiolabeled ligand and fluphenazine (10 μM) was used to define nonspecific binding. For D2, D3 and D4 receptors, [3H]methylspiperone (~1 nM) was used as the radiolabeled ligand and (+)-butaclamol (4 μM) was used to define nonspecific binding. Binding was carried out in 50 mM Tris-HCl buffer containing 120 mM NaCl, 5 mM KCl, 2 mM CaCh and 1 mM MgCh at room temperature for 1 h in a volume of 250 μL with 10–200 μg membrane protein depending on receptor expression level. Incubations were terminated by filtration through Whatman GF/B filters and radioactivity on filters was measured. Competitive inhibition of [3H]SCH23390 binding to the D1 and D5 receptors or [3H]methylspiperone binding to the D2, D3 and D4 receptors by L-SPD, was performed with various concentrations (10–11 M to 10–5 M) of L-SPD. Binding data were analyzed with GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA) and Ki values determined.
cAMP assay
The cAMP assay was performed as described previously 49. Briefly, HEK293 cells stably expressing the D1 or D5 receptors were harvested, re-suspended and counted. Cells (0.6–1 × 106) were added to assay tubes containing isobutylmethylxanthine (final concentration 2.5 mM) in the Opti-MEM reduced serum medium as well as 10−11 M to 10−5 M of L-SPD or DA, and incubated at 37°C for 10 min. The reaction was terminated by boiling for 5 min. [3H]cAMP (~3 pmol/~ 30,000 dpm in 0.02 M citrate phosphate buffer, pH 5.0) was added to all tubes. cAMP binding protein was added at an amount that gave 10,000 to 20,000 dpm [3H]cAMP binding in the absence of cold cAMP. The mixture was incubated for 2 h at 4°C. Bound and free [3H]cAMP were separated by adsorption of free [3H]cAMP by charcoal suspension. The amounts of cAMP were calculated based on the standard curve and converted to pmol/106 cells/10 min.
[35S]GTPyS binding assay
[35S]GTPγS binding was used as a functional measure of D2 and D3 receptor activation. Determination of [35S]GTPγS binding to G proteins was carried out using a modification of our published procedure 50. Briefly, membranes of CHO cells stably expressing the D2 or D3 receptors were prepared. Membranes (10 μg protein) were incubated in reaction buffer containing [35S]GTPγS (80–100 pM) and 10 μM GDP with or without 10 μM DA or L-SPD for 60 min at 30°C. Nonspecific binding was determined in the presence of 10 μM GTPγS. Bound and free [35S]GTPγS was separated by filtration.
Drugs and Drug Preparation
For all experiments, (−)-L-SPD was dissolved in 0.1 mmol/L H2SO4, then diluted and pH neutralized with 0.1 mmol/L NaOH (pH 5.0). Water was used as the vehicle control. (±)-MDPV was synthesized according to previously published methods 51, 52 by colleagues at Fox Chase Chemical Diversity Center (Doylestown, PA, USA) and dissolved in physiological saline (0.9%). For behavioral tests, L-SPD and MDPV were administered by intraperitoneal (i.p.) injection at a volume of 1 mL/kg. Equivalent injections of vehicle or saline were used for the control conditions. For self-administration sessions, MDPV was dissolved in filtered physiological saline (0.9%) and given by intravenous (i.v.) infusion.
Animals
Male Sprague-Dawley rats (250–275 g) obtained from Harlan Laboratories (Indianapolis, IN) were used. Rats were pair-housed in a humidity- and temperature-controlled vivarium on a 12 h light/dark cycle (lights on at 07:00h), except for MDPV and sucrose self-administration experiments where rats were single-housed in a separate vivarium maintained on a reverse light cycle (lights off at 07:00h). Rats were provided with food and water ad libitum, except during behavioral testing. Experimental procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and Temple University’s Guidelines for the Care of Animals.
MDPV Intravenous Self-Administration (IVSA) Procedure
Surgical procedures were performed as previously described 10. Briefly, rats were administered an analgesic (meloxicam, 5 mg/mL, s.c.) before being anaesthetized (2% isoflurane in oxygen, 2 L/min) and implanted with an i.v. catheter into the right jugular vein. A backmount mesh secured to a 22-gauge stainless steel vascular port (PlasticsOne, Roanoke, VA, USA) was mounted on the mid-scapular region of the rats’ dorsal surface. Rats were given injectable analgesia (meloxicam 2.0 mg/kg, s.c.) post-operatively for 2 days, and allowed 7 days to recover before starting self-administration training.
Self-administration sessions were conducted during the dark cycle in standard selfadministration chambers (Med Associates, St. Albans, VT, USA) equipped with two retractable levers designated as active (right lever) or inactive (left lever). Rats were trained to self-administer 0.056 mg/kg/infusion of MDPV 9,10 in daily 2 h sessions for 14 days on a fixed-ratio 1 (FR1) schedule of reinforcement with a 20-sec inter-trial interval. Responses on the active lever produced a 50 μL infusion of MDPV delivered over 3-sec via an infusion pump connected to a tether system attached to the catheter port of each rat. Drug infusions were paired with the presentation of a tone (~4.5 kHz, 78 dB tone), illumination of a cue light (50 mm above the active lever) and deactivation of the house-light. Responses on the inactive lever were recorded during the sessions but had no scheduled consequences. Before each session, catheters were flushed with 0.1 mL of 100 IU/mL heparinized saline. After each session, catheters were flushed with 0.2 mL of antibiotic (enrofloxacin, 2 mg/mL) in heparinized saline. At the conclusion of the acquisition phase, rats were considered to have acquired reliable self-administration behavior based on the criterion of ≥ 10 infusions per session for 10 consecutive sessions 53.
Extinction training commenced immediately after the acquisition period. Rats were placed into the self-administration chambers for 2 h during which responses on the active and inactive levers were recorded, but had no scheduled consequences. Extinction training lasted 13–14 days, or until rats met the criterion of ≤ 20 active lever presses 53.
Following extinction, rats underwent a series of cue and/or MDPV-primed reinstatement tests (2 h duration). For the cue-induced reinstatement tests, depression of the active lever triggered the infusion pump and the same tone and light stimulus complex used during self-administration training. The MDPV-primed reinstatement tests were run under extinction conditions where active and inactive lever responses had no scheduled consequences. However, an experimenter injection of MDPV (0.5 mg/kg; i.p.) was given immediately before rats were placed into the chambers. The cue plus MDPV-primed reinstatement tests employed both the MDPV-associated stimuli and a priming injection of MDPV. No infusions of MDPV were delivered during all reinstatement tests. Each reinstatement test was separated by at least 2 extinction sessions, or until rats regained the extinction criterion.
Sucrose Self-Administration Procedure
Sucrose self-administration sessions were performed during the dark cycle in the same chambers used for MDPV IVSA. Rats were trained to self-administer sucrose pellets in daily 2 h sessions for 10 days on a FR1 reinforcement schedule with a 20-sec inter-trial interval. Responses on the active lever resulted in delivery of a single 45-mg sucrose pellet (Bio-Serv, Flemington, NJ, USA) paired with the same tone and light stimulus complex used during MDPV IVSA. Responses on the inactive lever were recorded but had no scheduled consequences.
Extinction training commenced immediately after the self-administration sessions and followed the same procedure used for MDPV IVSA. Extinction training was conducted over 9 days, or until rats met the criterion of ≤ 20 active lever responses.
Following extinction, a cue-induced reinstatement test (2 h duration) was conducted where responses on the active lever triggered the same tone and light stimulus complex used during self-administration sessions.
Conditioned Place Preference (CPP) Procedure
A 30-min preference test was conducted prior to conditioning to determine baseline preference. During this test, rats were allowed to freely explore both sides of the two-compartment CPP apparatus (each compartment, 45 cm × 20 cm × 20 cm) in a drug-free state. Each compartment was environmentally distinguishable based on the walls (black or white with vertical black stripes) and floor (textured or smooth). The time spent in each compartment was recorded and manually scored after the test by a naïve experimenter. The compartment in which rats spentless time was considered their ‘less-preferred compartment’, and designated as the MDPV-paired side.
Following the initial preference test, a 4-day ‘biased’ conditioning phase commenced in which two 30-min sessions were run each day during the light cycle. In the first conditioning session, rats were administered MDPV (2 mg/kg) and immediately placed in their initially less-preferred compartment. In the second session conducted 4 h later, rats were given a saline injection (1 mL/kg) and placed in their initially preferred compartment. To determine whether MDPV had produced a significant place preference, a ‘post-conditioning’ preference test was conducted the day after the last conditioning session that followed the same procedure used in the initial preference test. A significantly greater amount of time spent in the MDPV-paired compartment during the post-conditioning test relative to the initial preference test indicated the acquisition of CPP.
Following the post-conditioning test, rats underwent an extinction phase that followed the same procedure employed during conditioning, with the exception that saline was paired with both compartments. On the day after the last extinction session, a ‘post-extinction’ preference test was performed that was identical to the initial preference test. CPP was considered to be extinguished if there was no significant difference between the time spent in the MDPV-paired compartment during the post-extinction test relative to the initial preference test.
An MDPV-primed reinstatement test was conducted the day after the post-extinction test. Rats were given MDPV (0.5 mg/kg; i.p.) immediately prior to being placed into the CPP apparatus, and then allowed to explore both compartments for 30 min.
Locomotor Activity Test
A locomotor activity test was performed during the light cycle according to previously published procedures 6. Briefly, rats were placed into activity chambers that consisted of transparent plastic boxes (45 cm × 20 cm × 20 cm) mounted inside metal frames equipped with 16 infrared light emitters and detectors. Two forms of locomotor activity were automatically measured using computer software (Digiscan DMicro System; Accuscan Inc., Columbus, OH): 1) ambulatory activity produced by horizontal movements, and 2) stereotypy resulting from recurring or focused movements.
Experimental Procedures
Experiment 1: Cue or MDPV-primed reinstatement of MDPV-seeking behavior in the IVSA model
To determine whether rats exposed to MDPV would reinstate their drug-seeking behavior in response to only MDPV-associated cues, or only a priming injection of MDPV, rats (n = 14) were tested in a cue-induced reinstatement test followed by an MDPV-primed reinstatement test in Experiment 1.
Experiment 2: The effects of L-SPD on reinstatement of MDPV-seeking behavior in the IVSA model
In Experiment 2, we examined the propensity for reinstatement of MDPV-seeking behavior using both MDPV-associated cues and an MDPV prime. We also determined the dose-related effects of L-SPD on cue plus MDPV-primed reinstatement of MDPV-seeking behavior. Rats (n = 11) were administered vehicle, and three doses of L-SPD (1, 3 and 10 mg/kg) 30, in a randomized counterbalanced order 30 min prior to the reinstatement tests. We decided to test the effects of L-SPD against cue plus MDPV-primed reinstatement as this produces greater drug-seeking behavior than either stimulus alone, which provided us with the optimal conditions to observe behavioral effects with L-SPD.
Experiment 3: The effects of L-SPD on reinstatement of sucrose-seeking
In Experiment 3, we examined the effects of L-SPD on cue-induced reinstatement of sucrose-seeking to assess whether its inhibitory effects were specific to psychostimulant-induced reinforcement. Rats were matched on active lever responses across the last 3 days of self-administration training, and then allocated to receive either vehicle (1 mL/kg) or the highest dose of L-SPD (i.e. 10 mg/kg) (n = 7–8 per group). Drug treatment was administered 30 min prior to the reinstatement test.
Experiment 4: The effects of L-SPD on reinstatement of MDPV-induced CPP
To determine the effects of L-SPD in the CPP paradigm, the effective dose from Experiment 2 (i.e. 10 mg/kg) was tested against MDPV-primed reinstatement of MDPV place preference. Following the post-extinction test, treatment groups (n = 12 per group) were allocated to receive either vehicle (1 mL/kg) or L-SPD matched on their initial preference test times. Vehicle or L-SPD was administered 30 min prior to the start of the reinstatement test.
Experiment 5: The effects of L-SPD on MDPV-induced hyperactivity
In Experiment 5, we conducted a locomotor activity test to examine the effects of L-SPD on MDPV-induced hyperactivity, and whether L-SPD itself had any locomotor effects. Rats (n = 8 per group) were randomly allocated to 4 treatment conditions: 1) vehicle + saline, 2) L-SPD + saline, 3) vehicle + MDPV and 4) L-SPD + MDPV. Basal locomotor activity was recorded for 30 min prior to vehicle or L-SPD administration. Thirty minutes later, saline or MDPV was given and locomotor activity measured for 90 min. MDPV was administered at 2 mg/kg to match the dose used in Experiment 4. L-SPD was given at the highest dose of 10 mg/kg.
Data Analysis
A one-way repeated measures ANOVA was used to analyze the data from Experiment 1 (cue-induced and MDPV-primed reinstatement tests) and Experiment 2 (dose-related effects of L-SPD on cue plus MDPV-primed reinstatement). The effects of L-SPD treatment on reinstatement of sucrose-seeking (Experiment 3) or MDPV place preference (Experiment 4) were determined using a two-way mixed model ANOVA with the between-subject factor of ‘L-SPD treatment’ and the within-subject factor of ‘experimental phase’. The effects of L-SPD on MDPV-induced hyperactivity (Experiment 5) were analyzed with the two-way between-subjects ANOVA procedure. Where appropriate, the overall ANOVA’s were followed by Bonferroni post-hoc tests to enable specific comparisons between treatment groups. GraphPad Prism 6.0 was used for all statistical analyses with significance considered at p < 0.05.
Supplementary Material
Funding
Research was funded by National Institute on Drug Abuse (NIDA) grants R01DA039139 to S.M.R., R01AT006899 to L-Y.L-C., P30DA013429 and T32DA007237 to E.M.U.
Abbreviations
- CHO
Chinese hamster ovary
- CPP
conditioned place preference
- DA
dopamine
- DAT
dopamine transporter; fixed-ratio 1 (FR1)
- HEK
human embryonic kidney
- i.p.
intraperitoneal
- i.v.
intravenous
- IVSA
intravenous self-adminsitration
- L-SPD
(−)-Stepholidine
- L-THP
Levo-tetrahydropalmatine
- MDMA
3,4-methylenedioxymethamphetamine
- MDPV
3,4-methylenedioxypyrovalerone
- NE
norepinephrine
- NET
norepinephrine transporter
- NIDA
National Institute on Drug Abuse
- PMSF
phenylmethylsulfonyl fluoride
- s.c.
subcutaneous
- 5-HT
5-hydroxytryptamine (serotonin)
- 6-OHDA
6-hydroxydopamine
Footnotes
Supporting Information:
MDPV and sucrose self-administration performance across the acquisition and extinction phases in Experiments 1, 2 and 3
Author Contributions
C.H., S.M.R. and L-Y.L-C. were responsible for the study concept and design. P.H. performed all the in vitro binding and functional assays. A.B.R. and G.R.S. carried out the synthesis of MDPV, while L-SPD was generously provided by D.Y.W.L. C.H. performed all the in vivo behavioral experiments and drafted the manuscript. L.R., S.U.N. and Y.C. assisted with the behavioral experiments. S.M.R., L-Y.L-C. and P.H. provided critical revisions of the manuscript. All authors critically reviewed the content and approved the final version for publication.
The authors declare no competing financial interests.
References
- [1].Baumann MH, Bukhari MO, Lehner KR, Anizan S, Rice KC, Concheiro M, and Huestis MA (2017) Neuropharmacology of 3,4-methylenedioxypyrovalerone (MDPV), its metabolites, and related analogs. Curr. Top. Behav. Neurosci 32, 93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Marinetti LJ, and Antonides HM (2013) Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results. J. Anal. Toxicol 37, 135–146. [DOI] [PubMed] [Google Scholar]
- [3].Valsalan R, Varghese B, Soman D, Buckmaster J, Yew S, and Cooper D (2017) Multi-organ dysfunction due to bath salts: are we aware of this entity? Intern. Med. J 47, 109–111. [DOI] [PubMed] [Google Scholar]
- [4].Young AC, Schwarz ES, Velez LI, and Gardner M (2013) Two cases of disseminated intravascular coagulation due to “bath salts” resulting in fatalities, with laboratory confirmation. Am. J. Emerg. Med 31, 445e443–445. [DOI] [PubMed] [Google Scholar]
- [5].Glennon RA, and Young R (2016) Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP). Brain Res. Bull 126, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gregg RA, Hicks C, Nayak SU, Tallarida CS, Nucero P, Smith GR, Reitz AB, and Rawls SM (2016) Synthetic cathinone MDPV downregulates glutamate transporter subtype I (GLT-1) and produces rewarding and locomotor-activating effects that are reduced by a GLT-1 activator. Neuropharmacology 108, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].King HE, Wetzell B, Rice KC, and Riley AL (2015) An assessment of MDPV-induced place preference in adult Sprague-Dawley rats. Drug Alcohol Depend. 146, 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hicks C, Gregg RA, Nayak SU, Cannella LA, Schena GJ, Tallarida CS, Reitz AB, Smith GR, and Rawls SM (2017) Glutamate carboxypeptidase II (GCPII) inhibitor 2-PMPA reduces rewarding effects of the synthetic cathinone MDPV in rats: a role for N-acetylaspartylglutamate (NAAG). Psychopharmacology 234, 1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, and Olive MF (2014) Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict. Biol 19, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Simmons SJ, Gregg RA, Tran FH, Mo L, Weltin E, Barker DJ, Gentile TA, Watterson LR, Rawls SM, and Muschamp JW (2018) Comparing rewarding and reinforcing properties between ‘bath salt’ 3,4-methylenedioxypyrovalerone (MDPV) and cocaine using ultrasonic vocalizations in rats. Addict. Biol 23, 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gannon BM, Galindo KI, Rice KC, and Collins GT (2017) Individual differences in the relative reinforcing effects of 3,4-methylenedioxypyrovalerone (MDPV) under fixed and progressive ratio schedules of reinforcement in rats. J. Pharmacol. Exp. Ther 361, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aarde SM, Huang PK, Creehan KM, Dickerson TJ, and Taffe MA (2013) The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 71, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, and Taffe MA (2015) In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology 232, 3045–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, and Sitte HH (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38, 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, and Baumann MH (2014) Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 87, 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nutt DJ, Lingford-Hughes A, Erritzoe D, and Stokes PR (2015) The dopamine theory of addiction: 40 years of highs and lows. Nature Rev. Neurosci 16, 305–312. [DOI] [PubMed] [Google Scholar]
- [17].Sibley DR, and Monsma FJ (1992) Molecular biology of dopamine receptors. Trends Pharmacol. Sci 13, 61–69. [DOI] [PubMed] [Google Scholar]
- [18].Caine SB, Negus SS, Mello NK, and Bergman J (1999) Effects of dopamine D1-like and D2-like agonists in rats that self-administer cocaine. J. Pharm. Exp. Ther 291, 353–360. [PubMed] [Google Scholar]
- [19].Self DW, Barnhart WJ, Lehman DA, and Nestler EJ (1996) Opposite modulation of cocaine-seeking behavior by D1-and D2-like dopamine receptor agonists. Science 271, 1586–1589. [DOI] [PubMed] [Google Scholar]
- [20].Froger-Colléaux C, and Castagné V (2016) Effects of baclofen and raclopride on reinstatement of cocaine self-administration in the rat. Eur. J. Pharmacol 777, 147–155. [DOI] [PubMed] [Google Scholar]
- [21].Alleweireldt AT, Kirschner KF, Blake CB, and Neisewander JL (2003) D1-receptor drugs and cocaine-seeking behavior: investigation of receptor mediation and behavioral disruption in rats. Psychopharmacology 168, 109–117. [DOI] [PubMed] [Google Scholar]
- [22].Galaj E, Ananthan S, Saliba M, and Ranaldi R (2014) The effects of the novel DA D3 receptor antagonist SR 21502 on cocaine reward, cocaine seeking and cocaine-induced locomotor activity in rats. Psychopharmacology 231, 501–510. [DOI] [PubMed] [Google Scholar]
- [23].Yang K, Jin G, and Wu J (2007) The neuropharmacology of (−)-stepholidine and its potential applications. Curr. Neuropharmacol 5, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jin G-Z, Zhu Z-T, and Fu Y (2002) (−)-Stepholidine: a potential novel antipsychotic drug with dual D1 receptor agonist and D2 receptor antagonist actions. Trends Pharmacol. Sci 23, 4–7. [DOI] [PubMed] [Google Scholar]
- [25].Natesan S, Reckless GE, Barlow KB, Odontiadis J, Nobrega JN, Baker GB, George SR, Mamo D, and Kapur S (2008) The antipsychotic potential of l-stepholidine - a naturally occurring dopamine receptor D1 agonist and D2 antagonist. Psychopharmacology 199, 275–289. [DOI] [PubMed] [Google Scholar]
- [26].Dong Z-J, Guo X, Chen L-J, Han Y-F, and Jin G-Z (1997) Dual actions of (−)-stepholidine on the dopamine receptor-mediated adenylate cyclase activity in rat corpus striatum. Life Sci. 61, 465–472. [DOI] [PubMed] [Google Scholar]
- [27].Zou L-L, Liu J, and Jin G-Z (1997) Involvement of receptor reserve in D1 agonistic action of (−)-stepholidine in lesioned rats. Biochem. Pharmacol 54, 233–240. [DOI] [PubMed] [Google Scholar]
- [28].Wang W, Zhou Y, Sun J, Pan L, Kang L, Dai Z, Yu R, Jin G, and Ma L (2007) The effect of L-stepholidine, a novel extract of Chinese herb, on the acquisition, expression, maintenance, and re-acquisition of morphine conditioned place preference in rats. Neuropharmacology 52, 355–361. [DOI] [PubMed] [Google Scholar]
- [29].Yue K, Ma B, Chen L, Tian X, Ru Q, Gan Y, Wang D, Jin G, and Li C (2014) L-Stepholidine, a naturally occurring dopamine D1 receptor agonist and D2 receptor antagonist, attenuates heroin self-administration and cue-induced reinstatement in rats. Neuroreport 25, 7–11. [DOI] [PubMed] [Google Scholar]
- [30].Ma B, Yue K, Chen L, Tian X, Ru Q, Gan Y, Wang D, Jin G, and Li C (2014) L-Stepholidine, a natural dopamine receptor D1 agonist and D2 antagonist, inhibits heroin-induced reinstatement. Neurosci. Lett 559, 67–71. [DOI] [PubMed] [Google Scholar]
- [31].Meade JA, Free RB, Miller NR, Chun LS, Doyle TB, Moritz AE, Conroy JL, Watts VJ, and Sibley DR (2015) (−)-Stepholidine is a potent pan-dopamine receptor antagonist of both G protein- and β-arrestin-mediated signaling. Psychopharmacology 232, 917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hoyer D, and Boddeke HW (1993) Partial agonists, full agonists, antagonists: dilemmas of definition. Trends Pharmacol. Sci 14, 270–275. [DOI] [PubMed] [Google Scholar]
- [33].Venniro M, Caprioli D, and Shaham Y (2016) Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog. Brain Res 224, 25–52. [DOI] [PubMed] [Google Scholar]
- [34].Platt DM, Rowlett JK, and Spealman RD (2001) Modulation of cocaine and food self-administration by low- and high-efficacy D1 agonists in squirrel monkeys. Psychopharmacology 157, 208–216. [DOI] [PubMed] [Google Scholar]
- [35].Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, and Neisewander JL (2002) Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology 159, 284–293. [DOI] [PubMed] [Google Scholar]
- [36].Self DW, and Stein L (1992) The D1 agonists SKF 82958 and SKF 77434 are self administered by rats. Brain Res. 582, 349–352. [DOI] [PubMed] [Google Scholar]
- [37].Xi Z-X, Yang Z, Li S-J, Li X, Dillon C, Peng X-Q, Spiller K, and Gardner EL (2007) Levo-tetrahydropalmatine inhibits cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropharmacology 53, 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Newman AH, Blaylock BL, Nader MA, Bergman J, Sibley DR, and Skolnick P (2012) Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem. Pharmacol 84, 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Galaj E, Harding W, and Ranaldi R (2016) Dopamine D1 and D3 receptor interactions in cocaine reward and seeking in rats. Psychopharmacology 233, 3881–3890. [DOI] [PubMed] [Google Scholar]
- [40].Gao M, Chu HY, Jin GZ, Zhang ZJ, Wu J, and Zhen XC (2011) l-Stepholidine-induced excitation of dopamine neurons in rat ventral tegmental area is associated with its 5-HT1A receptor partial agonistic activity. Synapse 65, 379–387. [DOI] [PubMed] [Google Scholar]
- [41].Müller CP, Carey RJ, Huston JP, and De Souza Silva MA (2007) Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog. Neurobiol 81, 133–178. [DOI] [PubMed] [Google Scholar]
- [42].Czoty P, McCabe C, and Nader M (2005) Effects of the 5-HT1A agonist (±)-8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on cocaine choice in cynomolgus monkeys. Behav. Pharmacol 16, 187–191. [DOI] [PubMed] [Google Scholar]
- [43].Homberg JR, Arends B, Wardeh G, Raasø HS, Schoffelmeer AN, and De Vries TJ (2004) Individual differences in the effects of serotonergic anxiolytic drugs on the motivation to self-administer cocaine. Neuroscience 128, 121–130. [DOI] [PubMed] [Google Scholar]
- [44].Gold LH, and Balster RL (1992) Effects of buspirone and gepirone on iv cocaine self-administration in rhesus monkeys. Psychopharmacology 108, 289–294. [DOI] [PubMed] [Google Scholar]
- [45].Burmeister JJ, Lungren EM, Kirschner KF, and Neisewander JL (2004) Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology 29, 660–668. [DOI] [PubMed] [Google Scholar]
- [46].Novellas J, López-Arnau R, Pubill D, Camarasa J, and Escubedo E (2015) Concentrations of MDPV in rat striatum correlate with the psychostimulant effect. J. Psychopharm 29, 1209–1218. [DOI] [PubMed] [Google Scholar]
- [47].Xu W, Wang X, Tocker AM, Huang P, Reith ME, Liu-Chen L-Y, Smith AB III, and Kortagere S (2017) Functional characterization of a novel series of biased signaling dopamine D3 receptor agonists. ACS Chem. Neurosci 8, 486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Xu W, Wang Y, Ma Z, Chiu Y-T, Huang P, Rasakham K, Unterwald E, Lee DY-W, and Liu-Chen L-Y (2013) L-isocorypalmine reduces behavioral sensitization and rewarding effects of cocaine in mice by acting on dopamine receptors. Drug Alcohol Depend. 133, 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Huang P, Kehner GB, Cowan A, and Liu-Chen L-Y (2001) Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J. Pharmacol. Exp. Ther 297, 688–695. [PubMed] [Google Scholar]
- [50].Zhu J, Luo L-Y, Li J-G, Chen C, and Liu-Chen L-Y (1997) Activation of the cloned human kappa opioid receptor by agonists enhances [35S] GTPγS binding to membranes: determination of potencies and efficacies of ligands. J. Pharmacol. Exp. Ther 282, 676–684. [PubMed] [Google Scholar]
- [51].Abiedalla YFH, Abdel-Hay K, DeRuiter J, and Clark CR (2012) Synthesis and GC-MS analysis of a series of homologs and regioisomers of 3,4-methylenedioxypyrovalerone (MDPV). Forensic Sci. Int 223, 189–197. [DOI] [PubMed] [Google Scholar]
- [52].Kolanos R, Partilla J, Baumann M, Hutsell B, Banks M, Negus S, and Glennon R (2015) Stereoselective actions of methylenedioxypyrovalerone (MDPV) to inhibit dopamine and norepinephrine transporters and facilitate intracranial self-stimulation in rats. ACS Chem. Neurosci 6, 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Reichel CM, Moussawi K, Do PH, Kalivas PW, and See RE (2011) Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J. Pharm. Exp. Ther 337, 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


