Abstract
Bariatric surgery is the most effective and durable treatment option for obesity today. More importantly, beyond weight loss, bariatric procedures have many advantageous metabolic effects including reversal of obesity-related liver disease – nonalcoholic steatohepatitis (NASH). NASH is an important comorbidity of obesity given that it is a precursor to the development of liver cirrhosis that may necessitate liver transplantation in the long run. Simultaneously, we and others have observed increased serum bile acids in humans and animals that undergo bariatric surgery. Specifically, our pre-clinical studies have included experimental procedures such as ‘ileal transposition’ or bile diversion and established procedures such as Roux-en-Y gastric bypass and the adjustable gastric band. Importantly, these effects are not simply the result of weight loss since our data show that the resolution of NASH and increase in serum bile acids are not seen in rodents that lose an equivalent amount of weight via food restriction. In particular, we have studied the role of altered bile acid signaling, in the potent impact of a bariatric procedure termed ‘vertical sleeve gastrectomy’ (VSG). In this review we focus on the mechanisms of NASH resolution and weight loss after VSG surgery. We highlight the fact that bariatric surgeries can be used as ‘laboratories’ to dissect the mechanisms by which these procedures work to improve obesity and fatty liver disease. We describe key bile acid signaling elements that may provide potential therapeutic targets for ‘bariatric-mimetic technologies’ that could produce benefits similar to bariatric surgery – but without the surgery!
Keywords: Bile acid metabolism, Nonalcoholic fatty liver disease, Bariatric surgery, Obesity
Background
Obesity and its comorbidities are a public health tsunami for the modern world [1]. Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the developed world today. The increasing prevalence of fatty liver disease and its more severe form, non-alcoholic steatohepatitis (NASH), has now outpaced the rise of overall childhood obesity itself [2]. Hepatocyte steatosis is understood to be the hallmark of NAFLD [3] and in a recent population-based study the prevalence of NAFLD was found to be 46 with 12.2% of the total cohort having the more severe form – NASH [4]. NASH will soon be the foremost indication for adult liver transplantation in the United States. However, the mainstay of NAFLD and NASH treatment remains weight reduction. A recent meta-analysis of NAFLD in adults indicated that weight loss can improve both the histology and metabolic parameters found in NASH in a safe manner [5]. Vitamin E has also been recently shown to be protective against NASH, but unfortunately does not produce weight loss and is therefore an incomplete therapy [6].
Diet and exercise usually result in regained body weight over time, in part probably due to powerful central mechanisms that seek to defend body weight against voluntary weight loss attempts. But efficacy of lifestyle modification and/or approved limited efficacy medications pale in comparison to the weight loss produced by bariatric procedures such as Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG). These procedures are the most successful and effective treatment options for obese adults with long-term changes in body weight and reduction of overall mortality despite surgical risks [7, 8], and have now been studied prospectively, with support from the National Institutes of Health (NIH), in adolescents with morbid obesity and associated disorders including NASH [9–11]. While the weight loss achieved by bariatric surgery has a profound metabolic impact, we recognize that the benefits of bariatric procedures go beyond what can be explained by weight loss alone and the long-held dogma of ‘restrictive’ and/or ‘malabsorptive’ processes [12]. As a case in point, we and others have now clearly shown that the VSG procedure has no element of malabsorption and that its weight loss outcomes are driven by altered metabolic pathways [13, 14]. Bariatric surgeries have been shown to impact several critical signaling and metabolic pathways to improve type 2 diabetes and dyslipidemia, even before significant weight loss occurs [12]. In the case of NAFLD, VSG produces a significant improvement of liver enzymes and liver triglyceride levels [15].
Recent evidence suggests that in addition to their role in lipid digestion, bile acids can behave as signaling molecules. Almost in the same time frame, data have now come about from various cohorts that serum bile acid levels are increased after RYGB and VSG surgeries [16]. Patti et al. [17] reported elevated serum bile acid levels in humans after RYGB wherein the bile acid subfractions taurochenodeoxycholic, taurodeoxycholic, glycocholic, glycochenodeoxycholic, and glycodeoxycholic acids were all significantly higher in the RYGB subjects compared to overweight and morbidly obese weight-matched controls. Nakatani et al. [18] also showed that ‘restrictive’ procedures (9 of this group of 15 patients had VSG) resulted in increased total serum bile acid levels as the subjects lost weight. Further, Jansen et al. [19] reported elevated levels of the bile acid – ileal farnesoid X receptor (FXR) induced hormone FGF19/15 in a cohort of RYGB patients 3 months after surgery. We have now further shown that this bile acid phenomenon is procedure dependent, given that patients who lost weight with the gastric banding surgery (LAGB) or weight-loss-matched patients do not have an increase in serum bile acid levels similar to that seen in RYGB patients with the same excess weight lost after surgery [20]. The logical question is of course, as to whether there is a role for these elevated bile acids in signaling metabolic improvements after bariatric surgery, including in NASH resolution?
In addition to the data obtained in the clinical studies, we have observed and reported a similar increase in serum bile acids, with profound changes in serum bile acid composition, in obese mice subjected to VSG and experimental bariatric surgery rat models of ileal transposition and bile diversion [13, 21–23]. Using appropriate calorie-restricted and weight-matched controls, we observed that VSG surgery in obese mice resulted in sustained weight loss and improvement in parameters of the metabolic syndrome, including a calorie-intake and weight-independent reduction of hepatic steatosis. Serum bile acids in the murine VSG model correlated with weight lost after surgery, and changes in serum bile composition could explain suppression of hepatic genes responsible for lipogenesis [13]. Together these data provide evidence for the putative role of bile acid signaling in the mechanisms behind bariatric procedures.
Putative Bile Acid Signaling Targets
One target for bile acid signaling that is being tested clinically is FXR [24, 25]. Recently an NIH supported multicentered, double-blind placebo-controlled study using a specific FXR agonist, obeticholic acid (OCA), was stopped by the Data Safety and Monitoring Board with concurrence of its NIH sponsors, secondary to ‘preliminary, interim results showing that OCA has a significant beneficial effect on liver damage due to NASH’. However, along with these intriguing interim results it was also reported by the NIH that ‘disproportionate lipid abnormalities’ (increased total cholesterol with increased LDL and decreased HDL cholesterol) were observed in patients on OCA compared to those on placebo.
Interestingly, our group recently reported, in a paper by Ryan et al. [26], that in the absence of FXR signaling (Fxr-knockout mice subjected to VSG), the ability of this bariatric surgery to reduce body weight and improve glucose tolerance was substantially reduced, further pointing to the importance of the bile acid-signaled FXR pathway in metabolic improvements observed after VSG. In addition, we found that intestinal microbiota composition was significantly different in wild-type and Fxr-knockout mice which underwent VSG. These data point out that clearly efficacious FXR agonism may have its own perils. Small heterodimer partner (SHP) located downstream of FXR is an atypical orphan nuclear receptor which regulates important processes in the liver, including bile acid, lipid, glucose homeostasis and immune responses [27]. Cholic acid administration in the diet reduces triglyceride levels in the wild-type but not in SHP-deficient mice [28]. Therefore, SHP-knockout mice do not respond to the lipid-reducing effect of bile acids.
In a follow-up study to Ryan et al. [26], we assessed the importance of SHP activation by bile acids for the hepatic steatosis improvement observed after VSG by using genetically modified mice with hepatocyte-specific overexpression and whole-body SHP deletion [23]. Both SHP transgenic and knockout mice gained weight on a high-fat diet and VSG resulted in weight loss in both strains. Transgenic mice developed steatosis on a high-fat diet that was reduced after VSG, but knockout mice were resistant to hepatic steatosis. Further, these mice on the high-fat diet had a proinflammatory hepatic phenotype that exacerbated despite weight loss after VSG [23]. SHP-knockout mice show resistance to diet-induced obesity due to the increased basal expression of a dominant regulator of energy metabolism, PGC-1α, in brown adipocytes and increased energy expenditure [29].
Some reports suggest that SHP lowers triglyceride levels via downregulation of Srebp-1c when stimulated by cholic acid [30], while hepatic steatosis and Srebp-1c expression are increased in SHP-knockout mice compared to wild-type mice when treated with cholic acid [28]. Another study has described that SHP-deficient mice have reduced hepatic triglyceride content as SHP negatively modulates hepatic lipid export, uptake, and synthesis [31]. Boulias et al. [32] showed that sustained expression of SHP leads to the depletion of the hepatic bile acid pool and a concomitant accumulation of liver triglycerides. In our study, SHP-deficient mice gained less weight than their wild-type counterparts; however, they were still obese. SHP-knockout mice being lighter, with less body fat and higher serum bile acid levels, did not show either improvement or impairment of hepatic triglyceride accumulation after VSG [23].
SHP also appears to take part in the systemic inflammatory response as SHP-deficient mice are more susceptible to endotoxin-induced sepsis [33]. Flow cytometry analysis performed on the livers of wild-type and SHP-knockout high-fat-diet-fed mice showed that the ratios of T-lymphocyte CD4−CD69+, CD8−CD69+ and NK-CD69+ immune cells were higher in the SHP-deficient compared to the wild-type mice, pointing to the SHP-knockout mice proinflammatory phenotype. Under the excess stress conditions, which are VSG and chronic high-fat diet administration, it is possible that the proinflammatory phenotype could be exacerbated in SHP-knockout mice. Indeed, hepatic inflammation developed in SHP-knockout mice despite weight loss after VSG in our study [23]. Therefore, SHP plays a role in control of hepatic inflammation overall, and the lack of SHP signaling prevents VSG, despite producing weight loss, from resulting in averting hepatic inflammation.
Another enterohepatic bile acid signaling pathway comprised of fibroblast growth factor 15 (FGF15) and FGF receptor 4 (FGFR4) (FGF15-β-Klotho-FGFR4 axis) through which bile acids are able to regulate metabolic pathways is well studied [34]. The studies performed by our group suggest that FGF15 expression is increased, and cholic acid absorption and serum levels are elevated after VSG [13, 26]. Therefore, this bile acid-FGF15-FGFR4 pathway may also play a key mechanistic role, although it requires further investigation in a murine VSG surgery model. Stimulated in a postprandial state by the intestinal FXR, FGF15 may synergize with SHP and downregulate bile acid synthesis as described in a study by Inagaki et al. [35].
Bariatric Surgery Alters Bile Acid Enterohepatic Circulation
Clinical and rodent studies have now clearly shown that not only are serum bile acid levels increased after VSG and RYGB, but a strong inverse correlation exists between total serum bile acids and blood glucose levels, bile acids and thyroid-stimulating hormone, and between bile acid levels and increased lipid oxidation [17, 36]. On the other hand, levels of several intestinal hormones and growth factors positively correlated with bile acid levels after RYGB, i.e. adiponectin, glucagon-like peptide-1 and FGF [17, 37]. It may be speculated that these changes observed in bile acid enterohepatic circulation in patients that have undergone RYGB are because of significant rerouting of the upper gastrointestinal tract, resulting in changes to the location where bile and chyme mix. However, the same cannot be applied to that of VSG surgery wherein only a section of the stomach is resected. Clearly, there is more to the benefits of RYGB and VSG than just malabsorption and gastric restriction.
Digging deeper into clinical studies of obese populations, one finds data supporting decreased levels of taurine-conjugated bile acids; however, their postprandial levels are significantly increased after RYGB indicating that a taurine-conjugated subset of bile acids may contribute to the metabolic improvements seen after bariatric procedures [38]. In our murine VSG studies, we have also reported that serum levels of total bile acids were increased with a particular elevation in cholic acid and tauroursodeoxycholic acid [13]. Haeusler et al. [39] recently reported elevated liver and plasma triglyceride levels in FoxO1-deficient mice that were associated with a deficiency of 12α-hydroxylated cholic acid and its synthetic enzyme Cyp8b1. This triglyceride imbalance was ameliorated when mice received FXR agonist cholic acid in their diet. On the other hand, it has been shown that oral administration of tauroursodeoxycholic acid markedly improved hepatic steatosis in ob/ob mice by reducing expression of de novo lipogenesis genes [40].
Bile acids have the ability to suppress hepatic lipogenesis through activation of the FXR pathway and we have identified that the bile acid-responsive nuclear receptor FXR is a metabolic target responsible for positive outcomes after murine VSG [26]. A clinical study reported that in NAFLD patients levels of Fasn and Srebp-1c were increased, while those of FXR were depleted [41]. In another mouse study it was shown that cholic acid could decrease liver triglycerides via activation of the FXR-SHP pathway [30]. In the VSG mouse study performed by our group we found that lipogenic genes, i.e. Fasn and Cpt1a, were downregulated in the VSG group compared to their weight and caloric intake-matched controls [13].
However, it is not clearly understood how these changes in serum bile acid levels are originated despite the lack of biliary obstruction or intestinal manipulation performed during VSG surgery. It has been reported that ghrelin-O-acyltransferase knockout mice exhibited elevated serum bile acid levels. Ghrelin-O-acyltransferase is the activating enzyme for the orexigenic hormone ghrelin, produced primarily in the gastric fundus which is removed as part of the VSG surgery [42]. In our mouse study we detected that active ghrelin levels were lower in VSG compared to sham mice [13]. Further, bile acid re-absorption in the terminal ileum occurs in an active manner against its concentration gradient predominantly through the apical sodium bile salt transporter (ASBT) [43]. In addition, a greater ASBT-stained area, increased villi length and total surface area were observed after VSG [23]. This intestinal adaptive response is similar to that seen in other experimental bariatric cohorts that have been shown to have higher serum bile acid levels [21, 22, 44]. All of these facts may potentially explain the elevated serum bile acid levels.
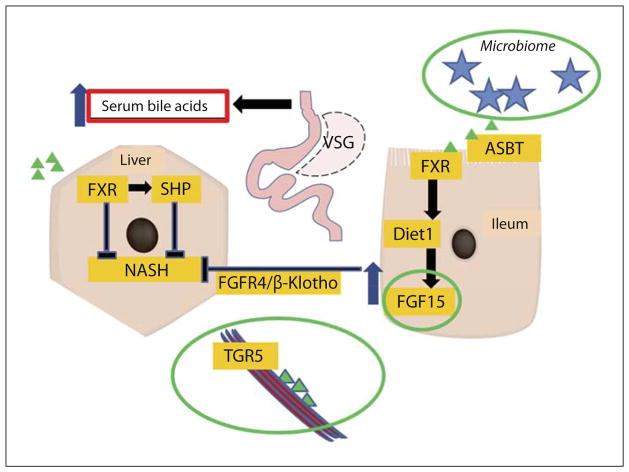
Another potential explanation for the elevation in serum bile acids could be increased synthesis in the liver. Two key rate-limiting enzymes for total bile acids are Cyp7a1 and Cyp8b1 [45, 46]. Rather than being upregulated, however, expressions of both of these genes are reduced after VSG. Higher bile acids lead to suppression of hepatocyte bile acid production, but more importantly, higher bile acid levels also suppress the liver cell bile uptake mechanisms through the FXR activation [47]. Ntcp is the major bile acid transporter into hepatocytes from serum [48], while Oatp4 and other Oatp family members play important roles in this process as well [49]. In our mouse studies, hepatic expression levels of Ntcp and Oatp4 were downregulated in the VSG mice [13, 23]. Similar suppression of bile acid uptake has been used to explain elevated serum bile acids by Vos et al. [50] in a rat cholestasis model. On the contrary, obese patients with NAFLD were reported to have increased expression of Cyp7a1 and Ntcp, indicating failure to activate SHP upon FXR stimulation [51]. All of these data point to a feedback loop, which may result in a higher steady level of serum bile acids after VSG (fig. 1).
Fig. 1.
Putative bile acid driven enterohepatic signaling after bariatric surgery. Bile acid concentration is increased in the intestinal lumen. Bile acids are actively reabsorbed in the terminal ileum due to the increased activity of ASBT and dramatically enlarged reabsorption area. Bile acids affect transcription factor FXR in the enterocyte and in combination with Diet1 may increase FGF15/19 secretion from the enterocyte. FGF15/19 is known to suppress bile acid synthesis and hepatic lipogenesis through FGFR4/β-Klotho signaling. Reabsorbed bile acids are transported to the liver by the portal vein blood flow, where their hepatocyte uptake is regulated by the import genes (Ntcp and Oatps). Bile acids’ interaction with FXR affects SHP transcription, which in turn may also inhibit bile and lipid synthesis in the liver. There are more potential mechanisms at play in this postbariatric surgery construct including (green circles; color in online version only) changes in the microbiome, and the role of FGF15/19 enterohepatic and TGR5 muscle signaling that may all together or individually impact bile acid synthesis, energy balance and negative feedback regulation of lipid metabolism genes in the liver.
Conclusions
In summary, bariatric surgery in general and VSG in particular are successful treatments for obesity and improve a number of components of the metabolic syndrome, including NAFLD. In rodent bariatric surgery models, we observed ileal villus proliferation and increased ASBT protein expression, with resultant increased bile acid reabsorption through the ileum. These models also produce improvement in various comorbidities of obesity to a degree that is considerably greater compared to when it is achieved by weight loss via food restriction alone. These existing data specifically suggest a putative role for bile acid signaling in the hepatic improvements seen after bariatric surgery.
This not only highlights VSG as an important therapy for NAFLD/NASH but also offers alternative treatment targets and strategies based on the novel implication of the bile acid composition changes in the hepatoprotective effects of VSG surgery. FXR is ‘a’ target for the beneficial weight loss-dependent and -independent effects of VSG; similarly, its downstream target SHP and indirect enterohepatic signal FGF15/19 also merit future investigation. Clarity of this enterohepatic cycle of bile acid signaling is extremely necessary to understand the metabolic changes observed after bariatric surgery. This will hopefully lead to development of less invasive ‘bariatric-mimetic’ technologies that will provide similar metabolic benefits to a much wider population of patients suffering from obesity, NASH and other related comorbidities.
Footnotes
Disclosure Statement
RJS receives research support from Ablaris, Johnson and Johnson, Novo Nordisk, and Pfizer, is a paid speaker for Johnson and Johnson, Merck, Novo Nordisk, and Pfizer, serves as a consultant for Angiogen, Eli Lilly, Johnson and Johnson, Novartis, Novo Nor-disk, Takeda and Zafgen, and has equity in Zafgen. RK receives research support from Johnson and Johnson. The other co-authors have no disclosures.
References
- 1.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162:496–500e1. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Pichiri I, Sorgato C, Zenari L, Bonora E. Prevalence of non-alcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J Hepatol. 2010;53:713–718. doi: 10.1016/j.jhep.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 4.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 6.Sanyal AJ, Chalasani N, Kowdley KV, Mc-Cullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 9.Stefater MA, Wilson-Perez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev. 2012;33:595–622. doi: 10.1210/er.2011-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inge T, Xanthakos S. Obesity at the extremes: the eyes only see what the mind is prepared to comprehend. J Pediatr. 2010;157:3–4. doi: 10.1016/j.jpeds.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 11.Inge TH, Zeller MH, Jenkins TM, et al. Peri-operative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr. 2014;168:47–53. doi: 10.1001/jamapediatrics.2013.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 2014;22:390–400. doi: 10.1002/oby.20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karcz WK, Krawczykowski D, Kuesters S, Marjanovic G, Kulemann B, Grobe H, et al. Influence of sleeve gastrectomy on NASH and type 2 diabetes mellitus. J Obes. 2011;2011:765473. doi: 10.1155/2011/765473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs C, Claudel T, Trauner M. Bile acid-mediated control of liver triglycerides. Semin Liver Dis. 2013;33:330–342. doi: 10.1055/s-0033-1358520. [DOI] [PubMed] [Google Scholar]
- 17.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–1407. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Jansen PL, van Werven J, Aarts E, Berends F, Janssen I, Stoker J, Schaap FG. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis. 2011;29:48–51. doi: 10.1159/000324128. [DOI] [PubMed] [Google Scholar]
- 20.Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708–E712. doi: 10.1210/jc.2012-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohli R, Kirby M, Setchell KD, Jha P, Klustaitis K, Woollett LA, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastroin-test Liver Physiol. 2010;299:G652–G660. doi: 10.1152/ajpgi.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohli R, Setchell KD, Kirby M, Myronovych A, Ryan KK, Ibrahim SH, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154:2341–2351. doi: 10.1210/en.2012-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myronovych A, Salazar-Gonzalez RM, Ryan KK, Miles L, Zhang W, Jha P, et al. The role of small heterodimer partner in nonalcoholic fatty liver disease improvement after vertical sleeve gastrectomy in mice. Obesity (Silver Spring) 2014;22:2301–2311. doi: 10.1002/oby.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov Today. 2012;17:988–997. doi: 10.1016/j.drudis.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771–784. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Hagedorn CH, Wang L. Role of nuclear receptor SHP in metabolism and cancer. Biochim Biophys Acta. 2011;1812:893–908. doi: 10.1016/j.bbadis.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, et al. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002;2:721–731. doi: 10.1016/s1534-5807(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Liu J, Saha P, Huang J, Chan L, Spiegelman B, et al. The orphan nuclear receptor SHP regulates PGC-1alpha expression and energy production in brown adipocytes. Cell Metab. 2005;2:227–238. doi: 10.1016/j.cmet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Han Y, Kim CS, Lee YK, Moore DD. Resistance of SHP-null mice to bile acid-induced liver damage. J Biol Chem. 2003;278:44475–44481. doi: 10.1074/jbc.M305258200. [DOI] [PubMed] [Google Scholar]
- 32.Boulias K, Katrakili N, Bamberg K, Underhill P, Greenfield A, Talianidis I. Regulation of hepatic metabolic pathways by the orphan nuclear receptor SHP. EMBO J. 2005;24:2624–2633. doi: 10.1038/sj.emboj.7600728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuk JM, Shin DM, Lee HM, Kim JJ, Kim SW, Jin HS, et al. The orphan nuclear receptor SHP acts as a negative regulator in inflammatory signaling triggered by Toll-like receptors. Nat Immunol. 2011;12:742–751. doi: 10.1038/ni.2064. [DOI] [PubMed] [Google Scholar]
- 34.Kir S, Kliewer SA, Mangelsdorf DJ. Roles of FGF19 in liver metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:139–144. doi: 10.1101/sqb.2011.76.010710. [DOI] [PubMed] [Google Scholar]
- 35.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Kakela P, Paakkonen M, et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg. 2012;22:1473–1480. doi: 10.1007/s11695-012-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted post-prandial bile acid excursion associated with obesity. Int J Obes (Lond) 2013;37:1553–1559. doi: 10.1038/ijo.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haeusler RA, Pratt-Hyatt M, Welch CL, Klaassen CD, Accili D. Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab. 2012;15:65–74. doi: 10.1016/j.cmet.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang JS, Kim JT, Jeon J, Park HS, Kang GH, Park KS, et al. Changes in hepatic gene expression upon oral administration of taurine-conjugated ursodeoxycholic acid in ob/ob mice. PloS One. 2010;5:e13858. doi: 10.1371/journal.pone.0013858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int. 2010;4:741–748. doi: 10.1007/s12072-010-9202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang K, Schmahl J, Lee JM, Garcia K, Patil K, Chen A, et al. Mouse ghrelin-O-acyltransferase (GOAT) plays a critical role in bile acid reabsorption. FASEB J. 2012;26:259–271. doi: 10.1096/fj.11-191460. [DOI] [PubMed] [Google Scholar]
- 43.Mottino AD, Hoffman T, Dawson PA, Luquita MG, Monti JA, Sanchez Pozzi EJ, et al. Increased expression of ileal apical sodium-dependent bile acid transporter in postpartum rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G41–G50. doi: 10.1152/ajpgi.00309.2001. [DOI] [PubMed] [Google Scholar]
- 44.Habegger KM, Al-Massadi O, Heppner KM, Myronovych A, Holland J, Berger J, et al. Duodenal nutrient exclusion improves metabolic syndrome and stimulates villus hyperplasia. Gut. 2014;63:1238–1246. doi: 10.1136/gutjnl-2013-304583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishibashi S, Schwarz M, Frykman PK, Herz J, Russell DW. Disruption of cholesterol 7α-hydroxylase gene in mice. I. Postnatal lethality reversed by bile acid and vitamin supplementation. J Biol Chem. 1996;271:18017–18023. doi: 10.1074/jbc.271.30.18017. [DOI] [PubMed] [Google Scholar]
- 46.Pandak WM, Bohdan P, Franklund C, Mallonee DH, Eggertsen G, Bjorkhem I, et al. Expression of sterol 12α-hydroxylase alters bile acid pool composition in primary rat hepatocytes and in vivo. Gastroenterology. 2001;120:1801–1809. doi: 10.1053/gast.2001.24833. [DOI] [PubMed] [Google Scholar]
- 47.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 48.Eloranta JJ, Jung D, Kullak-Ublick GA. The human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator-1α, and suppressed by bile acids via a small heterodimer partner-dependent mechanism. Mol Endocrinol. 2006;20:65–79. doi: 10.1210/me.2005-0159. [DOI] [PubMed] [Google Scholar]
- 49.Rost D, Herrmann T, Sauer P, Schmidts HL, Stieger B, Meier PJ, et al. Regulation of rat organic anion transporters in bile salt-induced cholestatic hepatitis: effect of ursodeoxycholate. Hepatology. 2003;38:187–195. doi: 10.1053/jhep.2003.50256. [DOI] [PubMed] [Google Scholar]
- 50.Vos TA, Ros JE, Havinga R, Moshage H, Kuipers F, Jansen PL, et al. Regulation of hepatic transport systems involved in bile secretion during liver regeneration in rats. Hepatology. 1999;29:1833–1839. doi: 10.1002/hep.510290638. [DOI] [PubMed] [Google Scholar]
- 51.Bechmann LP, Kocabayoglu P, Sowa JP, Sydor S, Best J, Schlattjan M, et al. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology. 2013;57:1394–1406. doi: 10.1002/hep.26225. [DOI] [PubMed] [Google Scholar]