 The generation of M1dG adducts can constitute an additional molecular mechanism for formaldehyde-induced nasal carcinogenesis.
The generation of M1dG adducts can constitute an additional molecular mechanism for formaldehyde-induced nasal carcinogenesis.
Abstract
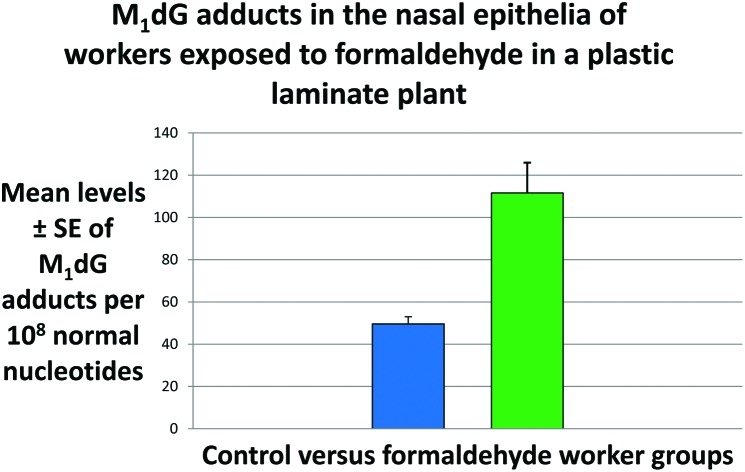
Formaldehyde is a ubiquitous volatile organic compound widely used for various industrial purposes. Formaldehyde was reclassified by the International Agency for Research on Cancer as a human carcinogen, based on sufficient evidence for a casual role for nasopharyngeal cancer. However, the mechanisms by which this compound causes nasopharyngeal cancer are not completely understood. Therefore, we have examined the formaldehyde-induced toxicity in the nasal epithelia of the workers of a plastic laminate plant in Bra, Cuneo, Piedmont region, North-Western Italy, hence in the target site for formaldehyde-related nasal carcinogenesis. We have conducted a cross-sectional study aimed at comparing the frequency of 3-(2-deoxy-β-d-erythro-pentafuranosyl)pyrimido[1,2-α]purin-10(3H)-one deoxyguanosine (M1dG) adducts, a biomarker of oxidative stress and lipid peroxidation, in 50 male exposed workers and 45 male controls using 32P-DNA post-labeling. The personal levels of formaldehyde exposure were analysed by gas-chromatography mass-spectrometry. The smoking status was estimated by measuring the concentrations of urinary cotinine by gas-chromatography mass-spectrometry. The air monitoring results showed that the exposure levels of formaldehyde were significantly greater for the plastic laminate plant workers, 211.4 ± 14.8 standard error (SE) μg m–3, than controls, 35.2 ± 3.4 (SE) μg m–3, P < 0.001. The levels of urinary cotinine were 1064 ± 118 ng ml–1 and 14.18 ± 2.5 ng ml–1 in smokers and non-smokers, respectively, P < 0.001. The M1dG adduct frequency per 108 normal nucleotides was significantly higher among the workers of the plastic laminate plant exposed to formaldehyde, 111.6 ± 14.3 (SE), compared to controls, 49.6 ± 3.4 (SE), P < 0.001. This significant association persisted also when personal dosimeters were used to measure the extent of indoor levels of formaldehyde exposure. No influences of smoking and age were observed across the study population. However, after categorization for occupational exposure, a significant effect was found in the controls, P = 0.018, where the levels of DNA damage were significantly correlated with the levels of urinary cotinine, regression coefficient (β) = 0.494 ± 0.000 (SE), P < 0.002. Our findings indicated that M1dG adducts constitute a potential mechanism of formaldehyde-induced toxicity. Persistent DNA damage contributes to the general decline of the physiological mechanisms designed to maintain cellular homeostasis.
Introduction
Formaldehyde (FA) is a ubiquitous volatile organic compound widely used for various industrial purposes as an adhesive and a bonding agent in the manufacture of particle-board, plywood, furniture and other wood products.1 FA is utilized as a fixation product in pathology laboratories, an antimicrobial agent in cosmetics and is a by-product of cigarette smoke and automotive exhausts. FA is also released from products used in building materials such as particle board and carpet. FA exposure by inhalation has been associated with a number of toxic effects, such as hepatotoxicity, neurotoxicity, reproductive toxicity, and respiratory toxicity.1 In 1980, FA was reported to be a carcinogen of the nasal epithelia of rats exposed by inhalation.2 In 2006, FA was reclassified by the International Agency for Research on Cancer as a human carcinogen (Group 1), based on sufficient evidence for a casual role for FA-induced nasopharyngeal cancer.1 In 2012, FA was classified as a human leukemogen based on epidemiological studies indicating an increased risk of leukemia.3 FA exposure in occupational settings has been considered a number of times by the American Conference Governmental Industrial Hygienists (ACGIH), starting from 1946 until the current Ceiling limit value of 370 μg m–3 has been adopted. A lower guideline value of 0.100 μg m–3 has been estimated to prevent carcinogenic effects by the World Health Organization.4
The molecular mechanisms by which FA causes nasopharyngeal cancer are not completely understood.5 Several studies showed that genotoxicity and cytotoxicity contribute together to the carcinogenic mode of action of FA in the nasal epithelia.1,6 FA related DNA–protein cross-links, chromosome mutations, sister-chromatid exchanges and micronuclei are considered relevant lesions,7 but increased production of reactive oxygen species (ROS) and subsequent DNA damage and peroxidation of lipids play a relevant role in FA-induced toxicity.8 FA causes genetic damage to the nasal tissues of both experimental animals and humans exposed by inhalation.1 In particular, FA causes the production of malondialdehyde (MDA), or more correctly β-hydroxyacrolein, a reactive lipid peroxidation by-product.8 MDA is an aldehyde capable of interacting with DNA to form 3-(2-deoxy-β-d-erythro-pentafuranosyl)pyrimido[1,2-α]purin-10(3H)-one deoxyguanosine (M1dG) adducts.9,10 Exocyclic M1dG adducts, if not repaired, may block cell replication and cause base pair and frameshift mutations in repeated sequences.11,12 M1dG adducts have been associated with the loss of DNA methylation in the Long Interspersed Nuclear Element-1 repeated sequences, and in the promoter region of the inflammatory cytokine interleukin-6 gene.13,14 DNA mutations, as well as altered patterns of DNA methylation are important hallmarks in carcinogenesis, and clinical studies indeed showed that enhanced levels of M1dG adducts may be linked to cancer development and tumor progression.15–19 Exocyclic M1dG adducts reflect also the extent of various carcinogen exposures,20–22 including FA in pathology wards.23 In particular, we have found that working in the reduction rooms and being exposed to FA at levels greater than 66 μg m–3 was associated with increased generation of M1dG adducts.23
In the present study, we have examined the mechanisms of FA-induced toxicity in the nasal epithelia of plastic laminate plant workers, hence in the target site for FA-induced nasal carcinogenesis. Our approach consisted of conducting a cross-sectional study aimed to compare the frequency of M1dG adducts, a biomarker of oxidative stress and lipid peroxidation,21–25 in the nasal epithelia of FA workers with respect to controls. DNA damage measurement analyzing exfoliated nasal epithelial cells is considered a promising approach for the detection of genotoxic compounds in the occupational setting,16,20,21,26–28 because these cells may be obtained easily by nasal brushing, a minimal invasive method.16 M1dG adducts have been examined in the nasal epithelial cells using the 32P-DNA post-labeling technique,25,29–32 whereas the indoor levels of FA have been measured by using passive personal air samplers using the gas-chromatography mass-spectrometry (GC-MS) technique.23,33,34 The tobacco smoking status has also been estimated by measuring the levels of urinary cotinine, an indicator of tobacco smoke exposure,35 using the GC-MS assay.33,36,37
Experimental
Study population
The plastic laminate plant, where the study was conducted, is located in a wide area in the suburbs of Bra, a town counting 30 000 inhabitants, in the Province of Cuneo, Piedmont region, North-Western Italy. The decorative laminate sheet produced in this plant is made of melamine and phenolic resins reacting with FA during the thermosetting process. The resins are laminated onto layers of kraft paper topped with a decorative sheet. Extractor hoods were regularly active in the plastic laminate plant, whereas personal protective equipment were provided and available as well (e.g. mask), but they were not regularly used by all the workers. The underlying basic population consisted of workers of a plastic laminate plant, whereas controls were subjects without occupational history in industries entailing exposure to known or suspected carcinogens. The final database included 95 male volunteers, 44 ± 10 years, and 40% current smokers. There were 50 FA workers, 41.0 ± 10 years, 44% current smokers, and 45 office personnel, as a control group, 47 ± 9.0 years, 36% current smokers; the latter living in residential areas with no proximity to major air-pollution sources. The participation rates were ∼95% in each study group.
Study procedures were performed in accordance with the guidelines of the General Hospital Institutional Committee that reviewed and approved the protocol. FA workers and the other subjects were contacted by the local health services. Signed, informed consent to participate in the study was obtained from all the volunteers before nasal brushing collection. As there were not enough women employed in the plant, most of the workers who had given their written informed consent were males, and therefore women were excluded from the study. A standardized life style questionnaire aimed to collect standard demographic and life-style data, including age, tobacco cigarette smoking and occupational status was filled by each participant before biological sampling collection. In detail, the description of smoking status for all the participants has been established a priori. Never and former smokers from at least one month were classified as non-smokers, whereas subjects who smoked at least one cigarette per day were classified as current smokers. An aliquot of fresh urine was collected for cotinine quantification to account for the role of tobacco smoking.
Personal dosimeter
Indoor FA samples were collected for a whole working shift (8 hours) using passive personal air samplers working with the radial symmetry (Radiello®), clipped near the breathing zone of the subject, as previously described.23,33,34 The personal air-samplers were equipped with a specific sorbent tube containing a 35–50 florisil mesh coated with 2,4-dinitrophenylhydrazine (DNPH). DNPH reacts with FA yielding 2,4-dinitrophenylhydrazone which was subsequently quantified by the GC-MS technique. Cartridges were stored at –80 °C until analysis. In brief, every sample was eluted with toluene (3 ml) and shaken for 15 min at room temperature. Then, an aliquot was injected into a GC-MS system, e.g. a capillary gas chromatograph (Agilent Technology 6890) interfaced to a single quadrupole mass spectrometer (Agilent Technology 5973 MSD). A Gerstel CIS4 PTV injection system utilized an initial temperature of 65 °C followed by heating at 5 °C s–1, with a final temperature of 320 °C, held for 10 min. The injection volume was 2 μl in splitless mode. The capillary column used was a HP-5MS of 30 m × 0.25 mm × 0.25 μm film thickness. The initial column temperature was 70 °C, and increased at 20 °C min–1 up to 220 °C, and then increased at 30 °C min–1 up to 300 °C. The carrier gas was ultrapure He (1.0 ml min–1). The transfer-line temperature was set at 280 °C. MS operated in electron impact and Selected Ion Monitoring mode. The monitored m/z values for FA were 63, 79, 180 and 210, while the ones for the internal standard (isovaleraldehyde-DNPH) were 177, 206, 223 and 166. The calibration curve was built by fortifying 3 ml of toluene so as to obtain a concentration ranging from 0.10 μg ml–1 to 10 μg ml–1. The fortified toluene was analyzed for the samples. The detection limit was calculated as the sample concentration providing a signal-to-noise ratio of 3. The quantification limit was considered to be twice the detection limit value. The detection limit and quantification limit were 0.05 and 0.10 μg ml–1, respectively. CV values were <5%.
Urinary cotinine
Aliquots of fresh urine were collected for cotinine quantification in the early morning and approximately at the same time from all the participants of the study, and stored at –80 °C prior to analysis, performed within 20 working days, as previously described.33,36 Urinary creatinine (crea) was determined by the kinetic Jaffé procedure38 so as to normalize the excretion rate of urinary cotinine. Ten ml of urine was transferred into a glass tube and 4 g NaCl, 500 μl 5 M NaOH and 10 μl of cotinine-d3 (internal standard) were added. Then, for two times, 2 ml CHCl3 were added to the sample to perform liquid–liquid extraction which was carried out in a shaking wheel for 15 min. The sample was then centrifuged for 10 min at 1000g and the resulting organic phase was collected in a new glass tube and evaporated to dryness in a rotary evaporator at room temperature. The dry residue was reconstituted in 200 μl of CHCl3 and transferred into a conical vial for GC-MS determination.36 The GC-MS analysis was performed using an Agilent Technologies 6890 GC, interfaced to a 5973 MSD Inert Agilent mass spectrometer. A Gerstel CIS4 PTV injection system utilized an initial temperature of 50 °C followed by heating at 10 °C s–1; with a final temperature of 300 °C, held for 10 min. The injection volume was 1 μl in the split mode. The capillary column used was a HP-5MS of 30 m × 0.25 mm × 0.25 μm film thickness. The initial column temperature was 50 °C, increased at 15 °C min–1 up to 300 °C. The carrier gas was ultrapure Helium (1.0 ml min–1). The transfer-line temperature was set at 280 °C. The MS operated in electron impact and SIM mode. The monitored m/z values for cotinine were: 98, 118, 176; while the ones for the internal standard were 101, 121, 179. The cotinine calibration curve was built by fortifying a blank urine pool of non-smoking subjects, to obtain a concentration range from 0.02 μg ml–1 to 2 μg ml–1. The fortified urine was extracted for the samples. LOD and LOQ were, respectively, 0.01 μg ml–1 and 0.02 μg ml–1. CV calculated to test repeatability were below 5% for cotinine and the internal standard.
Nasal brushing
Nasal epithelial samples were collected at the end of the working shift in the middle of the week from the lower turbinate in each nostril with a PAP test cytobrush during work attendance, after having gently washed the nasal cavity of volunteers with saline solution and cleaning it with a cotton swab.16,39 Since the surface of the nasal cavity is generally covered in a thin blanket of clear mucus, which serves to trap any particulates, brushing cells were incubated with 10% acetylcysteine for 30 min (100 cycles per min shaking frequency) at room temperature to break down the mucus gel structure. Nasal cell pellets were maintained at –80 °C until DNA extraction.
32P-DNA post-labeling assay
DNA was extracted using a method that requires RNase and proteinase treatments and the extraction with organic solvents.40,41 M1dG in the brushing samples of FA workers and controls were measured using a modified version of the 32P-DNA post-labeling assay22 that was developed in our laboratory for the specific detection of M1dG adducts in the genomic DNA.29 This 32P-post-labeling version is highly sensitive for the analysis of oxidative DNA adducts caused from various environmental carcinogens, including ROS-generating chemicals.32,42–44 The M1dG adducts were then expressed as relative adduct labelling (RAL) = pixels in adducted nucleotides/pixels in normal nucleotides. The M1dG levels were corrected across experiments based in the recovery of the reference standard that was prepared as previously reported.23 The presence of M1dG adducts in the reference standard was confirmed by matrix-assisted laser desorption/ionization time-of-flight-mass-spectrometry (MALDI-TOF-MS).23 Higher specificity of the 32P-labeling technique is obtained using appropriated reference standards,40,41,45 thus, co-chromatography was utilized to confirm the identity of adduct spots detected in the study population, as previously described.20
Statistical analysis
The levels of M1dG adducts were expressed as adducted nucleotides per 108 normal nucleotides. M1dG and FA data were log transformed to stabilize the variance and normalize the distribution. The study population was a priori grouped according to the personal FA exposure measurements in three categories: (a) <25 μg m–3, (b) 25–66 μg m–3, and (c) >66 μg m–3. The mean concentrations of the FA and M1dG adducts across variable levels were compared by analysis of covariance, introducing into each model terms for age (continuous), cigarette tobacco smoking (non-smokers, current smokers), exposure status, and indoor levels of FA, as appropriate. Subsequently, a multiple regression model adjusted for confounding factors was used to evaluate the correlation between the levels of M1dG adducts with the concentrations of urinary cotinine. All statistical tests were two-sided and p < 0.05 was considered to be statistically significant. The data were analyzed using SPSS 13.0 (IBM SPSS Statistics, New York, NY).
Results
Personal formaldehyde exposure
The exposure levels for 8 hour time-weighted average of FA are regulated by the Occupational Safety and Health Administration Permissible Exposure Limit (923 μg m–3) and the National Institute for Occupational Safety and Health Recommended Exposure Limit (20 μg m–3), whereas the ACGIH Threshold Limit Value (TLV)-Time Weighted Average currently has not yet been established. Given the great difference existing between these two limits, and since we have measured the exposure for 8 hours of work, we preferred to refer to the most used limit at the international level, the TLV Ceiling (TLV-C) limit value of 370 μg m–3, which refers to the limit that must not be exceeded in 15 minutes. The TLV-C is currently used as the reference level and it represents the most common and comprehensive limit internationally adopted to quantify the health protection against FA occupational exposure.
The GC-MS results showed that the indoor levels of FA were significantly increased, up to 6-fold, in the workers of the plastic laminate plant compared to the controls, P < 0.001 (Table 1). Specifically, the levels of FA experienced by the FA workers and the controls were 211.4 ± 14.8 standard error (SE) μg m–3 and 35.2 ± 3.4 (SE) μg m–3, respectively, P < 0.001. The FA concentrations ranged from 49 to 444 μg m–3 for the FA workers, and from 16 to 110 μg m–3 for the controls. There were three subjects that showed personal exposure levels greater than 370 μg m–3, the reference level that should not be exceeded according to the ACGIH. When the smoking habits and age were considered, the concentrations of FA were not found to be associated with smoking, whereas an effect of age was found with older people having significantly lower levels of FA exposure, p < 0.001. A significant trend was observed, the P-value for the trend = 0.001 (Table 1).
Table 1. Mean levels of formaldehyde (μg m–3) measured in the personal dosimeters of the workers of a plastic laminate plant and the controls.
| Indoor formaldehyde exposure levels | |||
| N | Mean levels ± SE | P-value a | |
| Age | |||
| <42 years b | 31 | 171.9 ± 24 | |
| 42–49 years | 35 | 130.5 ± 19 | <0.001 |
| >49 years | 29 | 77.8 ± 16 | <0.001 |
| P-value for trend | 0.001 | ||
| Tobacco smoking | |||
| Non-smokers b | 57 | 120.1 ± 16 | |
| Smokers | 38 | 139.7 ± 18 | 0.676 |
| Jobs | |||
| Controls b | 45 | 35.2 ± 3.4 | |
| Plastic laminate plant workers | 50 | 211.4 ± 14.8 | <0.001 |
a P-values after adjusting for confounding factors.
bReference level.
Urinary cotinine
Tobacco smoke is a known source of air pollutants, including FA and other carcinogen agents.1,46 To estimate the smoking status of the study population, the urinary cotinine was measured by the GC-MS assay,33,37 since this nicotine metabolite is considered to be a reliable indicator of tobacco smoking.35 The GC-MS analysis showed that the levels of urinary cotinine were 1064 ± 118 ng ml–1 and 14.18 ± 2.5 ng ml–1 in smokers and non-smokers, respectively, P < 0.001. The concentrations of urinary cotinine ranged from 1.0 to 3306.1 ng ml–1 for the FA workers, and from 0.80 to 1644.4 ng ml–1 for the controls. After categorization for occupational exposure, the urinary cotinine levels were 1551.9 ± 186 ng ml–1 and 16.6 ± 6.8 ng ml–1 in smokers and non-smoker workers, respectively, P < 0.001, and 595.1 ± 110 ng ml–1 and 17.4 ± 2.2 ng ml–1 in smokers and non-smoker controls, respectively, P < 0.001.
M1dG adducts
To search for the exocyclic M1dG adducts caused by occupational exposure to FA, we analyzed the amount of exocyclic DNA adducts in the nasal epithelia of the plastic laminate plant workers using the 32P-post-labeling technique.22,25,32 A characteristic pattern of the M1dG adduct spot was detected in the chromatographic plates of the study population. The intensity of M1dG adduct spots was generally stronger in the chromatograms of the FA workers compared to controls. The presence of M1dG adducts in the DNA extracted and purified from the nasal brushing samples of the FA exposed workers was confirmed by co-chromatography.
When we examined the levels of exocyclic M1dG adducts in the nasal epithelia of the FA workers, our findings showed that the adduct frequency was significantly higher, up to 2-fold, among the workers exposed to FA (Table 2). Next, the relationship between the levels of personal FA exposures and the generation of M1dG adducts has been evaluated by subgrouping the study population according to a previous paper.23 After stratification, we found that the levels of M1dG adducts were significantly higher in the groups of subjects exposed to more than 66 μg m–3. In brief, the levels of M1dG adducts were 111.6 ± 14.3 (SE) in the plastic laminate plant workers, and 49.6 ± 3.4 (SE) in the group of controls, P < 0.001, after adjusting for confounding factors. The amount of M1dG adducts was 82.0 ± 12 (SE) in the current smokers, and 82.4 ± 11 (SE) in the non-smokers, P = 0.637. Next, when stratifying for personal FA exposures, the levels of M1dG adducts in subjects who were exposed to FA levels higher than 66 μg m–3 were significantly higher compared to those exposed to values of FA lower than 25 μg m–3, P = 0.001, after correction for confounding factors. A significant trend was present, the P-value for the trend = 0.002, with the highest levels of M1dG adducts in the subjects exposed to levels of FA higher than 66 μg m–3, and with intermediate amounts in those subjects who were exposed to 25–66 μg m–3 FA values (Table 2).
Table 2. Mean levels of 3-(2-deoxy-β-d-erythro-pentafuranosyl)pyrimido[1,2-α]purin-10(3H)-one deoxyguanosine (M1dG) adducts per 108 normal nucleotides in the nasal epithelia of workers occupationally exposed to formaldehyde compared to the controls, and according to study variables.
| Nasal epithelia M1dG adducts | |||
| N | Mean levels ± SE | P-values a | |
| Age | |||
| <42 years b | 31 | 90 ± 15 | |
| 42–49 years | 35 | 87 ± 16 | 0.841 |
| >49 years | 29 | 68 ± 10 | 0.492 |
| P-value for trend | 0.348 | ||
| Tobacco smoking | |||
| Non-smokers b | 57 | 82.4 ± 11 | |
| Smokers | 38 | 82.0 ± 12 | 0.637 |
| Jobs | |||
| Controls b | 45 | 49.6 ± 3.4 | |
| Plastic laminate plant workers | 50 | 111.6 ± 14.3 | <0.001 |
| Personal formaldehyde exposure | |||
| <25 b μg m–3 | 23 | 47.6 ± 4.4 | |
| 25–66 μg m–3 | 19 | 59.2 ± 11.6 | 0.703 |
| >66 μg m–3 | 53 | 105.5 ± 13.4 | 0.001 |
| P-value for trend | 0.002 | ||
a P-values after adjusting for confounding factors.
bReference level.
No influence of smoking and age on DNA damage was observed in the study population. However, when we stratified by occupational exposure, a significant effect of smoking on M1dG adducts was found in the group of the controls, P = 0.018, but not in the plant workers, P = 0.661. Furthermore, when we examined the association between the production of the exocyclic DNA adducts and the concentrations of cotinine in the urine of the controls, the multivariate regression analysis showed that the levels of DNA damage were significantly correlated with the levels of urinary cotinine, regression coefficient (β) = 0.494 ± 0.000 (SE), P < 0.002, after correction for confounding factors.
Discussion
Since the molecular mechanisms by which FA causes nasopharyngeal cancer are not completely understood,5 we have conducted a mechanistic study to evaluate the association between M1dG adducts, a biomarker of oxidative stress and lipid peroxidation,21–25 with personal air FA exposure in the nasal epithelia of plastic laminate plant workers using 32P-post-labelling and GC-MS techniques. Our aim was to study the mechanisms of FA-induced toxicity in the portal-of-entry of air FA as well as in the target site for FA-induced nasal carcinogenesis.
The personal air monitoring results indicated that the indoor levels of FA exposure experienced from the plastic laminate plant workers were significantly higher compared to controls, but with three workers showing FA exposure levels that exceeded the ACGIH regulatory limit of 370 μg m–3. Good environmental work conditions and health status suggested that these high values might essentially be caused from improper work behavior by some workers, rather than by improper working conditions. On the other hand, the controls showed indoor levels of FA exposure equal to 40 μg m–3, and for 10% of the control subjects, FA exposure levels exceeded 70 μg m–3, indicating that some subjects were exposed to other non-reported domestic or environmental exposures. No effect of smoking was found, whereas a significant effect of age was observed, with older people who experienced lower levels of indoor pollution. Our measurements from passive personal air samplers are well representative of individual exposures and they provided evidence of the workers’ exposure to FA through ambient air with respect to the controls. Recently, Svecova et al.47 measured the concentrations of volatile organic compounds in a study aimed to evaluate the effects of air pollution in the Czech Republic. In that study, the personal levels of volatile organic compounds were associated with the atmosphere concentrations of air pollutants as well as various life-style factors including cooking, home-heating and the time spent outdoors.
Nasal epithelia was adopted since this tissue constitutes the portal-of-entry of air FA, where this chemical induces toxic effects due to its high reactivity.48 Nasal epithelia is commonly represented by 75% of columnar surface epithelium, 14% of other epithelial cells, 11% of neutrophils, 0.07% of eosinophils and 0.2% lymphocytes. Apart from the potential value of the nasal epithelial cells for the prediction of cancer risk of the respiratory tract,16,27 the use of nasal epithelia may allow mechanistic studies of various carcinogens, including those contained in tobacco smoke,16,39 industrial air pollution,20–22,28 and hair-dyeing.49 Additionally, the patterns of Phase I enzymes that have an important role in the production of ROS during the metabolism of a number of carcinogens50 are qualitatively different in the respiratory tract than in other tissues.51
As previously mentioned, the levels of M1dG adducts were measured in the nasal epithelia to investigate the molecular mechanisms in the target site for FA-induced nasal carcinogenesis, since the formation of DNA damage in this region reflects the genotoxic action of FA as well as the actions of mediators released from the nasal epithelial cells following the inhalation of this compound. The main outcome of the present study showed that the production of exocyclic DNA adducts was detected in the nasal epithelia of FA workers; indeed, the levels of M1dG adducts of the FA professionally exposed workers were significantly higher compared to the controls, although the mean FA value experienced from the plastic laminate plant workers was lower than the ACGIH reference level limit. This significant association persisted also when personal air samplers were used to measure the extent of indoor levels of FA exposure. Interestingly, the analysis of the dose–response relationship between DNA damage and the mean FA levels showed a significant increment of M1dG adducts only in the subjects that were exposed to indoor levels of FA higher than 66 μg m–3, but not in those exposed to lower FA concentrations. Our findings are in line with previous studies of our group reporting increased levels of oxidative stress and protein adducts in FA workers using a biomarker approach.33,34 In those studies, the relationships between the concentration of FA in the atmosphere and the levels of urinary of 15-F2t isoprostane, a biomarker of oxidative stress,34 and the formation of N-methylenvaline, a covalent molecular adduct of FA with primary amino groups of the hemoglobin,33 were examined in different FA exposed groups, including technicians of pathology wards and workers of the plastic laminate plants. The workers professionally exposed to FA in the above occupational settings had significantly increased levels of biomarkers of oxidative stress or hemoglobin alkylation with respect to the controls.
Plastic laminate plant workers are generally exposed to FA emitted by pressed-wood products employed in home construction, and furnishings containing urea-FA resins and phenolformaldehyde resin. Therefore, high levels of exocyclic DNA adducts may be caused by the altered cellular redox state and ROS production, including singlet oxygen, superoxide anions, hydrogen peroxide and hydroxyl radicals. Indeed, FA exposure is associated with increased ROS and the cytochrome P450 1A1 enzyme levels as well as the glutathione and the glutathione S-transferase theta 1 decreased amounts in experimental animals.52 FA induces oxidative stress by different mechanisms, as by the inhibition of scavenger systems and the activation of oxidases. FA is a substrate for the action of the cytochrome P450 2E2 isozyme and is oxidized by peroxidase, aldehyde oxidase and xanthine oxidase with free radical production.53 A pathway independent of MDA is also involved in the M1dG generation. Indeed, it was a proposed mechanism in which M1dG adducts are formed directly upon ROS exposure through base propenals.54 Impaired antioxidant enzyme activities in the metabolic detoxification of oxidative by-products were also associated with in vivo FA treatments.53,55 FA exposure also leads to inflammation and to a consequent excess of ROS.31,56 ROS, as hydrogen peroxide and hypochlorite acid, are indeed generated from the oxidative burst of activated alveolar macrophages and neutrophils.31
The genomic mechanisms of instability and the cellular tolerance pathways associated with FA exposure seem to be a key point in the mutation arising, even if it is not fully characterized.57 Many studies have shown that FA is genotoxic and mutagenic in vitro in various kinds of mammalian cells including cultured human blood and human nasal epithelial cells. FA induces DNA–protein crosslinks, sister chromatid exchanges, micronuclei, chromosome aberrations and, to a lesser extent, gene mutations.58 We have analyzed chromosomal aberrations in peripheral blood lymphocytes from workers in pathology wards who have been exposed to FA, compared with a group of unexposed subjects, as well as for the glutathione S-transferase Mu 1 and the glutathione S-transferase theta 1 metabolic gene polymorphisms. The exposed subjects showed a significant increase in the frequency of chromosomal aberrations per cell and in the percentage of cells with aberrations compared to control subjects. These findings demonstrate that air FA induces chromosomal aberrations even consequently to low levels of daily exposure, indicating an increased risk of genetic damage for workers exposed to FA.59 Additionally, M1dG adducts are a form of DNA damage that is quite persistent, with a relatively long half-life of 12.5 days.60 If unrepaired, M1dG adducts may result in base pair substitutions, e.g. M1dG → A–T > C, and frameshift mutations.61
The role of smoking habit was investigated in the present study, but a significant influence of tobacco smoking on M1dG adducts has been only found in the group of controls, where the generation of DNA damage was linearly correlated to the levels of urinary cotinine, a major proximate metabolite of nicotine, that is oxidized in the liver by the cytochrome P450 2A6 enzyme and distributed in various body fluids.35 Indeed, although the smoker workers had an increase of cotinine like the controls, the levels of DNA damage in their nasal epithelia were not correlated to the amounts of urinary cotinine. This finding is in agreement with previous studies reporting higher M1dG levels in smokers,22,23,62 but other studies did not report significant differences by smoking status.18,63 The association with tobacco smoking in the group of the controls appears as expected because smokers inhale a broad range of chemical compounds derived from tobacco and pyrolysis products, including free radicals involved in oxidative damage and peroxidation of lipids, which induce exocyclic DNA adducts. The generation of M1dG adducts is indeed promoted by a secondary reaction, that is induced by any reactive species capable of inducing oxidative stress, such as those contained in the tobacco smoke. Whereas it is possible that the effects of smoking were not discerned in the occupational exposed workers because they were less compared to the FA exposure, we have to point out that we have only analyzed a single DNA adduct, whereas cigarette smoking may induce many different types of DNA adducts.
The strengths of our study were that the indoor levels of FA were measured using passive personal air dosimeters by GC-MS,34 whereas the levels of exocyclic DNA adducts were detected using the 32P-post-labeling assay, a technique known to be sensitive for the detection of various kinds of DNA damage, including M1dG adducts.22,25,32 A good repeatability of 32P-post-labeling measurements was also reported for this assay.44,64 Nevertheless, this method is unable to determine the precise DNA adduct structure, unless if the technique is combined with appropriate internal standards,40,41 or coupled with the MALDI-TOF-MS techniques,23 as in the present study. The exact assessment of the smoking habit is important to investigate the influence of tobacco smoke exposure on biomarkers of carcinogenesis, thus, a next strength was that the smoking status was also estimated by measuring the concentrations of urinary cotinine by GC-MS.37
Conclusions
To evaluate the potential mechanisms underlying the pathogenesis of nasopharyngeal cancer, we have examined the scientific literature regarding potential interactions between FA and carcinogenic effects.1 Cytotoxicity related cellular proliferation is considered a possible mechanism for carcinogenicity of FA, whereas the typical genotoxic pattern in multiple in vitro models and in exposed humans and laboratory animals showed various types of DNA damage, such as DNA–protein cross-links, DNA cross-links, nucleotide base adducts and mutations, and micronuclei.1 Herein, the observation that exocyclic M1dG adducts were produced in the nasal epithelia of the FA workers reflects an additional potential mechanism of FA-induced toxicity, as well as FA-related carcinogenesis. Nevertheless, it is not possible to conclude that this damage was solely due to FA, since there are other chemicals to which workers were exposed. Excess ROS generation exerts detrimental effects in the nasal epithelial cells through attack on DNA and inner membrane lipids causing increased DNA oxidation and peroxidation of lipids. If this damage is unrepaired, persistent M1dG lesions60 may lead to specific cellular responses, such as mutations61 and inhibition of DNA transcription at adduct sites.65 Persistent DNA damage may ultimately contribute to the general decline of the physiological mechanisms designed to maintain cellular homeostasis, including cell death, senescence, uncontrolled proliferation and genome instability.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding sources
This work was partially supported by the Tuscany and the Piedmont regions, the INAIL Piedmont, and the Associazione Italiana per la Ricerca sul Cancro.
References
- IARC IARC Monogr. Eval. Carcinog. Risks Hum. 2006;88:1–478. [PMC free article] [PubMed] [Google Scholar]
- Swenberg J. A., Kerns W. D., Mitchell R. I., Gralla E. J., Pavkov K. L. Cancer Res. 1980;40:3398–3402. [PubMed] [Google Scholar]
- IARC IARC Monogr. Eval. Carcinog. Risks Hum. 2012;100:1–538. [PMC free article] [PubMed] [Google Scholar]
- O. World Health, Air quality guidelines for Europe, WHO regional publications, European series, 2000, vol. V–X, pp. 1–273. [PubMed] [Google Scholar]
- N. T. Program, in Review of the Formaldehyde Assessment in the National Toxicology Program 12th Report on Carcinogens, Washington (DC), 2014, 10.17226/18948. [DOI] [PubMed]
- Costa S., Garcia-Leston J., Coelho M., Coelho P., Costa C., Silva S., Porto B., Laffon B., Teixeira J. P. J. Toxicol. Environ. Health, Part A. 2013;76:217–229. doi: 10.1080/15287394.2013.757212. [DOI] [PubMed] [Google Scholar]
- Baan R., Grosse Y., Straif K., Secretan B., El Ghissassi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., Cogliano V., W. H. O. I. A. f. R. o. C. M. W. Group Lancet Oncol. 2009;10:1143–1144. [Google Scholar]
- Duong A., Steinmaus C., McHale C. M., Vaughan C. P., Zhang L. Mutat. Res. 2011;728:118–138. doi: 10.1016/j.mrrev.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plastaras J. P., Riggins J. N., Otteneder M., Marnett L. J. Chem. Res. Toxicol. 2000;13:1235–1242. doi: 10.1021/tx0001631. [DOI] [PubMed] [Google Scholar]
- Jeong Y. C., Swenberg J. A. Free Radicals Biol. Med. 2005;39:1021–1029. doi: 10.1016/j.freeradbiomed.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Marnett L. J. Toxicology. 2002;181–182:219–222. doi: 10.1016/s0300-483x(02)00448-1. [DOI] [PubMed] [Google Scholar]
- Zhou X., Taghizadeh K., Dedon P. C. J. Biol. Chem. 2005;280:25377–25382. doi: 10.1074/jbc.M503079200. [DOI] [PubMed] [Google Scholar]
- Peluso M., Bollati V., Munnia A., Srivatanakul P., Jedpiyawongse A., Sangrajrang S., Piro S., Ceppi M., Bertazzi P. A., Boffetta P., Baccarelli A. A., Int. J. Epidemiol., 2012, 41 , 1753 –1760 , ; discussion 1761–1753 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso M. E., Munnia A., Bollati V., Srivatanakul P., Jedpiyawongse A., Sangrajrang S., Ceppi M., Giese R. W., Boffetta P., Baccarelli A. A. Toxicol. Sci. 2014;137:47–54. doi: 10.1093/toxsci/kft241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Dhingra K., Hittelman W. N., Liehr J. G., de Andrade M., Li D. Cancer Epidemiol. Biomarkers Prev. 1996;5:705–710. [PubMed] [Google Scholar]
- Munnia A., Bonassi S., Verna A., Quaglia R., Pelucco D., Ceppi M., Neri M., Buratti M., Taioli E., Garte S., Peluso M. Free Radicals Biol. Med. 2006;41:1499–1505. doi: 10.1016/j.freeradbiomed.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Munnia A., Amasio M. E., Peluso M. Free Radicals Biol. Med. 2004;37:850–858. doi: 10.1016/j.freeradbiomed.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Leuratti C., Watson M. A., Deag E. J., Welch A., Singh R., Gottschalg E., Marnett L. J., Atkin W., Day N. E., Shuker D. E., Bingham S. A. Cancer Epidemiol. Biomarkers Prev. 2002;11:267–273. [PubMed] [Google Scholar]
- Peluso M., Munnia A., Risso G. G., Catarzi S., Piro S., Ceppi M., Giese R. W., Brancato B. Free Radical Res. 2011;45:477–482. doi: 10.3109/10715762.2010.549485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso M. E., Munnia A., Giese R. W., Chellini E., Ceppi M., Capacci F. Mutagenesis. 2015;30:519–525. doi: 10.1093/mutage/gev014. [DOI] [PubMed] [Google Scholar]
- Peluso M., Munnia A., Ceppi M., Giese R. W., Catelan D., Rusconi F., Godschalk R. W., Biggeri A. Mutagenesis. 2013;28:315–321. doi: 10.1093/mutage/get005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso M., Srivatanakul P., Munnia A., Jedpiyawongse A., Ceppi M., Sangrajrang S., Piro S., Boffetta P. Environ. Health Perspect. 2010;118:55–59. doi: 10.1289/ehp.0900907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono R., Romanazzi V., Munnia A., Piro S., Allione A., Ricceri F., Guarrera S., Pignata C., Matullo G., Wang P., Giese R. W., Peluso M. Chem. Res. Toxicol. 2010;23:1342–1348. doi: 10.1021/tx100083x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen M., Autrup H., Moller P., Hertel O., Jensen S. S., Vinzents P., Knudsen L. E., Loft S. Mutat. Res. 2003;544:255–271. doi: 10.1016/j.mrrev.2003.06.010. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Venitt S. Int. J. Cancer. 2013;131:2733–2753. doi: 10.1002/ijc.27827. [DOI] [PubMed] [Google Scholar]
- Knasmueller S., Holland N., Wultsch G., Jandl B., Burgaz S., Misik M., Nersesyan A. Mutagenesis. 2011;26:231–238. doi: 10.1093/mutage/geq079. [DOI] [PubMed] [Google Scholar]
- Peluso M. E. M., Munnia A. Toxicol. Res. 2014;3:42–49. [Google Scholar]
- Demircigil G. C., Coskun E., Vidinli N., Erbay Y., Yilmaz M., Cimrin A., Schins R. P., Borm P. J., Burgaz S. Mutagenesis. 2010;25:163–169. doi: 10.1093/mutage/gep057. [DOI] [PubMed] [Google Scholar]
- van Helden Y. G., Keijer J., Heil S. G., Pico C., Palou A., Oliver P., Munnia A., Briede J. J., Peluso M., Franssen-van Hal N. L., van Schooten F. J., Godschalk R. W. Carcinogenesis. 2009;30:2070–2076. doi: 10.1093/carcin/bgp186. [DOI] [PubMed] [Google Scholar]
- Vanhees K., van Schooten F. J., van Waalwijk van Doorn-Khosrovani S. B., van Helden S., Munnia A., Peluso M., Briede J. J., Haenen G. R., Godschalk R. W. Free Radicals Biol. Med. 2013;57:154–161. doi: 10.1016/j.freeradbiomed.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Gungor N., Knaapen A. M., Munnia A., Peluso M., Haenen G. R., Chiu R. K., Godschalk R. W., van Schooten F. J. Mutagenesis. 2010;25:149–154. doi: 10.1093/mutage/gep053. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Arlt V. M. Nat. Protoc. 2007;2:2772–2781. doi: 10.1038/nprot.2007.394. [DOI] [PubMed] [Google Scholar]
- Bono R., Romanazzi V., Pirro V., Degan R., Pignata C., Suppo E., Pazzi M., Vincenti M. Sci. Total Environ. 2012;414:701–707. doi: 10.1016/j.scitotenv.2011.10.047. [DOI] [PubMed] [Google Scholar]
- Romanazzi V., Pirro V., Bellisario V., Mengozzi G., Peluso M., Pazzi M., Bugiani M., Verlato G., Bono R. Sci. Total Environ. 2013;442:20–25. doi: 10.1016/j.scitotenv.2012.10.057. [DOI] [PubMed] [Google Scholar]
- Goniewicz M. L., Eisner M. D., Lazcano-Ponce E., Zielinska-Danch W., Koszowski B., Sobczak A., Havel C., Jacob P., Benowitz N. L. Nicotine Tob. Res. 2011;13:202–208. doi: 10.1093/ntr/ntq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono R., Vincenti M., Schiliro T., Traversi D., Pignata C., Scursatone E., Dotti G., Gilli G. J. Exposure Anal. Environ. Epidemiol. 2005;15:66–73. doi: 10.1038/sj.jea.7500344. [DOI] [PubMed] [Google Scholar]
- Bono R., Bellisario V., Romanazzi V., Pirro V., Piccioni P., Pazzi M., Bugiani M., Vincenti M. Int. J. Hyg. Environ. Health. 2014;217:287–293. doi: 10.1016/j.ijheh.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Bartels H., Cikes M. Clin. Chim. Acta. 1969;26:1–10. doi: 10.1016/0009-8981(69)90278-2. [DOI] [PubMed] [Google Scholar]
- Peluso M., Amasio E., Bonassi S., Munnia A., Altrupa F., Parodi S. Carcinogenesis. 1997;18:339–344. doi: 10.1093/carcin/18.2.339. [DOI] [PubMed] [Google Scholar]
- Peluso M., Castegnaro M., Malaveille C., Talaska G., Vineis P., Kadlubar F., Bartsch H. Carcinogenesis. 1990;11:1307–1311. doi: 10.1093/carcin/11.8.1307. [DOI] [PubMed] [Google Scholar]
- Peluso M., Castegnaro M., Malaveille C., Friesen M., Garren L., Hautefeuille A., Vineis P., Kadlubar F., Bartsch H. Carcinogenesis. 1991;12:713–717. doi: 10.1093/carcin/12.4.713. [DOI] [PubMed] [Google Scholar]
- Munnia A., Saletta F., Allione A., Piro S., Confortini M., Matullo G., Peluso M. Mutagenesis. 2007;22:381–385. doi: 10.1093/mutage/gem030. [DOI] [PubMed] [Google Scholar]
- Peluso M., Merlo F., Munnia A., Bolognesi C., Puntoni R., Parodi S. Cancer Epidemiol. Biomarkers Prev. 1996;5:361–369. [PubMed] [Google Scholar]
- Peluso M., Hainaut P., Airoldi L., Autrup H., Dunning A., Garte S., Gormally E., Malaveille C., Matullo G., Munnia A., Riboli E., Vineis P., E. investigators Mutat. Res. 2005;574:92–104. doi: 10.1016/j.mrfmmm.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Castegnaro M. Mutagenesis. 1999;14:301–315. doi: 10.1093/mutage/14.3.301. [DOI] [PubMed] [Google Scholar]
- IARC IARC Monogr. Eval. Carcinog. Risks Hum. 1986;38:35–394. [PubMed] [Google Scholar]
- Svecova V., Topinka J., Solansky I., Sram R. J. J. Exposure Sci. Environ. Epidemiol. 2012;22:455–460. doi: 10.1038/jes.2012.30. [DOI] [PubMed] [Google Scholar]
- Nielsen G. D., Wolkoff P. Arch. Toxicol. 2010;84:423–446. doi: 10.1007/s00204-010-0549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni T. B., Hudari F., Munnia A., Peluso M., Godschalk R. W., Zanoni M. V., den Hartog G. J., Bast A., Barros S. B., Maria-Engler S. S., Hageman G. J., de Oliveira D. P. Toxicol. Lett. 2015;239:194–204. doi: 10.1016/j.toxlet.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Cancer. 1981;47:2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Peluso M., Ceppi M., Munnia A., Puntoni R., Parodi S. Am. J. Epidemiol. 2001;153:546–558. doi: 10.1093/aje/153.6.546. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu X., McHale C., Li R., Zhang L., Wu Y., Ye X., Yang X., Ding S. PLoS One. 2013;8:e74974. doi: 10.1371/journal.pone.0074974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel A., Coskun O., Armutcu F., Kanter M., Ozen O. A. J. Chem. Neuroanat. 2005;29:173–178. doi: 10.1016/j.jchemneu.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Dedon P. C., Plastaras J. P., Rouzer C. A., Marnett L. J. Proc. Natl. Acad. Sci. U. S. A. 1998;95:11113–11116. doi: 10.1073/pnas.95.19.11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N. J., Namasivayam A. Drug Alcohol Depend. 2003;71:87–91. doi: 10.1016/s0376-8716(03)00066-8. [DOI] [PubMed] [Google Scholar]
- Gungor N., Pennings J. L., Knaapen A. M., Chiu R. K., Peluso M., Godschalk R. W., Van Schooten F. J. Respir. Res. 2010;11:24. doi: 10.1186/1465-9921-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A., Owen N., Juarez E., McCullough A. K. DNA Repair. 2015;28:73–82. doi: 10.1016/j.dnarep.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller J., Neuss S., Mueller J. U., Kuhner S., Holzmann K., Hogel J., Klingmann C., Bruckner T., Triebig G., Speit G. Mutagenesis. 2011;26:555–561. doi: 10.1093/mutage/ger016. [DOI] [PubMed] [Google Scholar]
- Santovito A., Schiliro T., Castellano S., Cervella P., Bigatti M. P., Gilli G., Bono R., DelPero M. Arch. Toxicol. 2011;85:1295–1302. doi: 10.1007/s00204-011-0668-3. [DOI] [PubMed] [Google Scholar]
- Marnett L. J. Mutat. Res. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- VanderVeen L. A., Hashim M. F., Shyr Y., Marnett L. J. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14247–14252. doi: 10.1073/pnas.2332176100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen S. Y., Hsu T., Santella R. M. Carcinogenesis. 2002;23:207–211. doi: 10.1093/carcin/23.1.207. [DOI] [PubMed] [Google Scholar]
- Everett S. M., Singh R., Leuratti C., White K. L., Neville P., Greenwood D., Marnett L. J., Schorah C. J., Forman D., Shuker D., Axon A. T. Cancer Epidemiol. Biomarkers Prev. 2001;10:369–376. [PubMed] [Google Scholar]
- Ibanez R., Munnia A., Agudo A., Berenguer A., Amiano P., Tormo M. J., Barricarte A., Quiros J. R., Sanchez M. J., Gonzalez C. A., Peluso M. Biomarkers. 2005;10:1–9. doi: 10.1080/13547500500050580. [DOI] [PubMed] [Google Scholar]
- Cline S. D., Lodeiro M. F., Marnett L. J., Cameron C. E., Arnold J. J. Nucleic Acids Res. 2010;38:7546–7557. doi: 10.1093/nar/gkq656. [DOI] [PMC free article] [PubMed] [Google Scholar]


