 NiCl2 intake induced the pulmonary oxidative stress and inflammatory responses via dietary pathway, which subsequently contributed to histopathological lesions and dysfunction.
NiCl2 intake induced the pulmonary oxidative stress and inflammatory responses via dietary pathway, which subsequently contributed to histopathological lesions and dysfunction.
Abstract
The respiratory system is the primary target of nickel or nickel compound toxicity after inhalation exposure. There are no reports on the effects of nickel or nickel compounds on the lung via dietary administration at present. This study aimed to investigate pulmonary toxicity induced by dietary NiCl2 in broiler chickens by using histopathology, qRT-PCR, and ELISA. In comparison with the control group, NiCl2 intake induced oxidative damage to DNA (upregulation of 8-OHdG) and lipid peroxidation (upregulation of MDA), which was associated with the upregulation of NO and the downregulation of the expression levels and activities of pulmonary CuZn-SOD, Mn-SOD, CAT, GSH-Px, GR and GST mRNA. Also, the T-AOC activity, GSH content, ability to inhibit the generation of hydroxyl radicals, and ratio of GSH/GSSG were decreased in the groups treated with NiCl2. Concurrently, the mRNA expression levels of iNOS, TNF-α, COX-2, IL-1β, IL-6, IL-8, IL-18 and IFN-γ were increased via the activation of NF-κB, and the mRNA expression levels of anti-inflammatory mediators including IL-2, IL-4 and IL-13 were decreased in the groups treated with NiCl2. The above-mentioned results were the first to demonstrate that NiCl2 intake induced pulmonary oxidative stress and inflammatory responses via the dietary pathway, which subsequently contributed to histopathological lesions and dysfunction.
Introduction
As one of the most abundant transition metals in the Earth's crust, Ni occurs in soil, water, and air.1,2 Owing to its unique catalytic, magnetic, and optical properties and biological effects, Ni is widely used as an important material in many processes of modern industry.3 The high consumption of Ni-containing products inevitably leads to environmental pollution, which increases the possible health risks to humans and animals.4 Ni is also considered to be essential for many animal species, microbes, and plants.5,6 However, excess exposure to Ni may be acutely toxic to humans and animals.7 Ni chloride, nitrate, sulphate, hydroxide, acetate, carbonate, and oxide are the most commercially important Ni compounds in nature.8 Exposure to Ni commonly occurs by ingestion of contaminated water and food.2,9 Workers in Ni-producing and processing industries are exposed by inhalation and, to a lesser extent, dermal contact.10 People may also be exposed via contact with stainless steel, jewelry, and coins. Ni is toxic at high doses to both humans and animals.11 A previous study has reported that Ni and Ni compounds are immunotoxic, neurotoxic, genotoxic, toxic to the reproductive and pulmonary systems, nephrotoxic, hepatotoxic and carcinogenic.12 Also, our studies have proved that dietary NiCl2 in excess of 300 mg kg–1 can cause immunotoxicity, oxidative damage, inflammatory response, apoptosis and cell cycle arrest in the kidney, spleen, small intestine, cecal tonsil and bursa of Fabricius in broiler chickens.13–30
It has been reported that oral NiCl2 in excess of 2 mg kg–1 can decrease body and liver weight and cause hepatic toxicity in mice.31 Ling and Leach reported that dietary NiCl2 concentrations of 300 mg kg–1 and above resulted in a significant reduction in growth rate. Mortality and anemia were observed in chicks receiving 1100 mg kg–1 nickel.32 Weber and Reid found a significant reduction in growth at concentrations of 700 mg kg–1 and above of NiSO4 and nickel acetate.33 Chicks that were fed diets contaminated with Ni at a concentration ranging from 250 to 300 mg kg–1 in the diet exhibited growth depression and a reduction in feed intake.34 Bersényi et al.35 reported that supplementation of 500 mg kg–1 NiCl2 reduced weight gain (by 10%), feed intake (by 4%) and FCE (by 5%) in growing broiler cockerels. Numerous studies have demonstrated that Ni can induce oxidative stress, inflammatory responses and apoptosis in vivo and in vitro.36–40 In in vivo studies, it has been reported that NiSO4 can induce oxidative stress-mediated apoptosis in the liver in Carassius auratus.41 Sun et al. and Amudha et al. have also reported that NiCl2 and NiSO4 induce oxidative stress in the testes in Spodoptera litura36 and in the kidney in rats.42 In in vitro studies, Ma et al.43 have demonstrated that nickel nanowires (Ni NWs) induce apoptosis via the production of ROS in HeLa cells. Ni and Ni compounds can cause oxidative stress in human lung epithelial A549 cells44 and human bronchial epithelial cells (BEAS-2B).45 Also, these studies all demonstrate that oxidative stress induced by Ni is accompanied by an increase in the generation of ROS, a decrease in GSH and inhibition of the activities of antioxidant enzymes.41,46,47 Lung inflammation has been observed after inhalation of NiO, NiSO4 or Ni3S2 in F344/N rats and B6C3F1 mice.48 NiCl2 can induce an inflammatory response by increasing the protein expression levels of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β in RAW264 cells, bone marrow-derived macrophages and bone marrow dendritic cells.49,50 Nickel oxide nanoparticles (NiONPs) can also increase the protein expression levels of MIP-1α, MCP-1, IL-1α and IL-1β in the lungs of rats.51 The above-mentioned studies suggest that oxidative stress and inflammatory response are the two main mechanisms involved in Ni-induced toxic effects on organs, tissues and cells.
The lung is the target organ for the acute toxicology of Ni,12 and the respiratory system is the primary target of Ni toxicity after inhalation exposure. Based on the above-mentioned references, there are no reports on the effects of Ni or Ni compounds on the lung via dietary administration at present. Therefore, this study was conducted with the objective of assessing pulmonary toxicity via oxidative stress and inflammatory responses after NiCl2 intake in broiler chickens by using histopathology, qRT-PCR, and ELISA.
Results
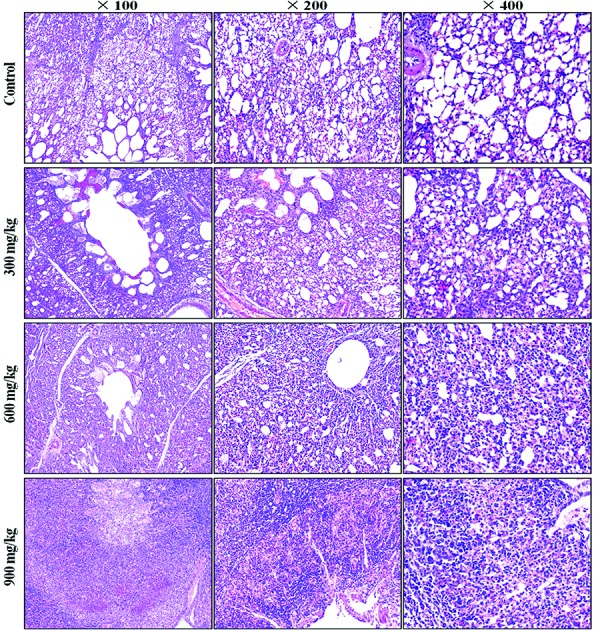
Histopathological changes in the lung
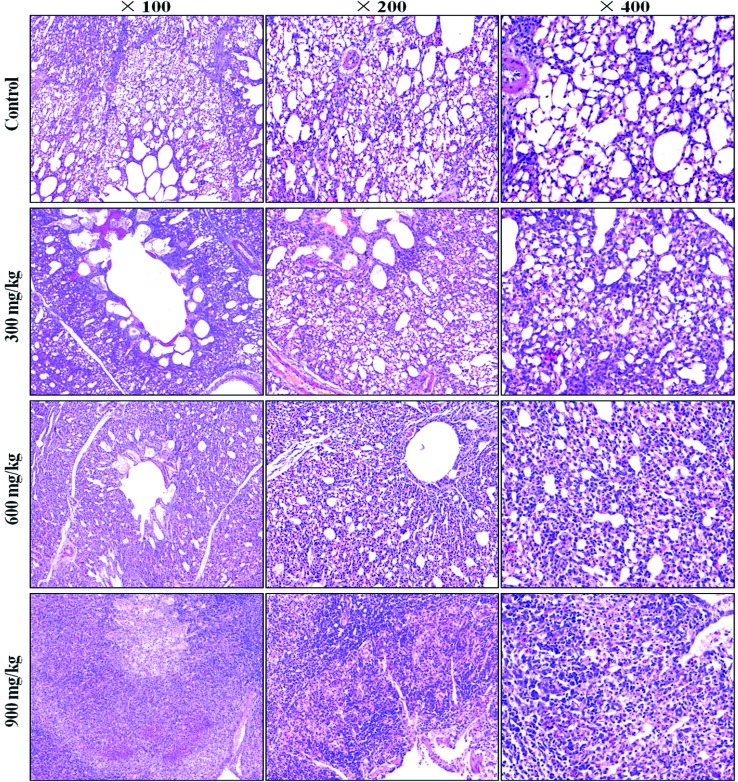
A histopathological study showed that NiCl2 caused dose- and time-dependent lesions in the lungs of the groups treated with NiCl2 when compared with the control group. The alveolar epithelial cells were swollen and exfoliated. The alveolar walls were thickened with the infiltration of inflammatory cells and congestion. The results are shown in Fig. 1.
Fig. 1. Histopathological changes in the lung at 42 days of age. Control group: no changes are observed; 300 mg kg–1 group: the alveolar epithelial cells are swollen; 600 mg kg–1 group: the alveolar epithelial cells are swollen. The alveolar walls are thickened with the infiltration of inflammatory cells and congestion; 900 mg kg–1 group: the normal alveolar architecture has disappeared. Many exfoliated and necrotic alveolar epithelial cells, alveolar macrophages and inflammatory cells are observed (H&E).
Changes in 8-OHdG, MDA and NO contents in the lung
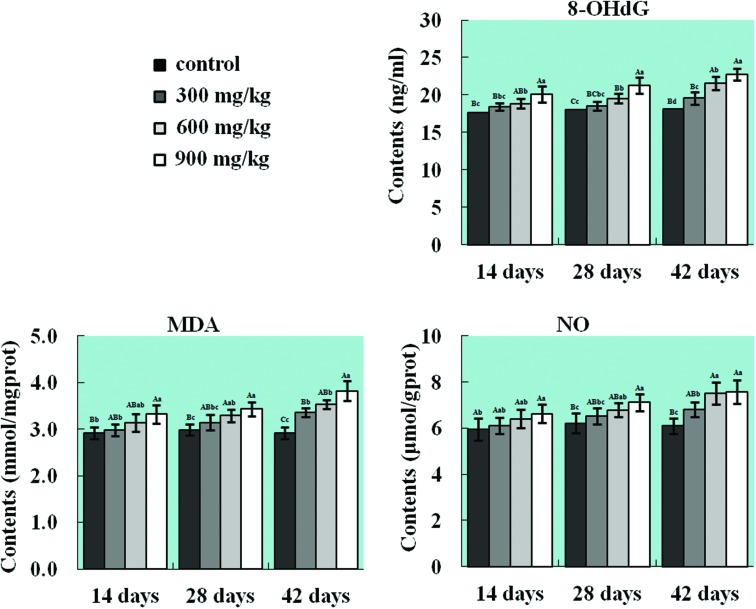
As shown in Fig. 2, NiCl2 induced the generation of NO free radicals, oxidative damage to DNA and LPO in the lung. The 8-OHdG contents were significantly higher (p < 0.05 or p < 0.01) in the 600 mg kg–1 and 900 mg kg–1 groups from 14 to 42 days of age and in the 300 mg kg–1 group at 42 days of age than those in the control group. The MDA and NO contents were significantly increased (p < 0.05 or p < 0.01) in the 900 mg kg–1 group at 14 days of age, in the 600 mg kg–1 and 900 mg kg–1 groups at 28 days of age, and in all three NiCl2-treated groups at 42 days of age in comparison with those in the control group.
Fig. 2. Changes in 8-OHdG, MDA and NO contents in the lung. The contents of NO (free radical), 8-OHdG (marker of oxidative damage to DNA), and MDA (LPO marker) are significantly increased in the NiCl2-treated groups. The letters A, B, C, and D represent a significant difference (p < 0.01) among the four groups; the letters a, b, c, and d represent a difference (p < 0.05) among the four groups.
Changes in mRNA expression levels of antioxidant enzymes in the lung
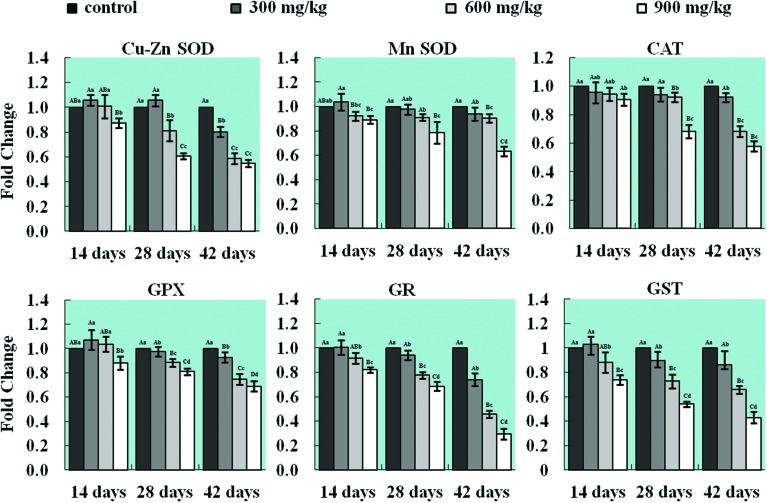
The results of qRT-PCR showed that NiCl2 decreased the mRNA expression levels of antioxidant enzymes in the lung when compared with those in the control group. The mRNA expression levels of CuZn-SOD were significantly lower (p < 0.05 or p < 0.01) in the 900 mg kg–1 group at 14 days of age, in the 600 mg kg–1 and 900 mg kg–1 groups at 28 days of age and in all three NiCl2-treated groups at 42 days of age than those in the control group. The mRNA expression levels of Mn-SOD were significantly decreased (p < 0.05 or p < 0.01) in the 900 mg kg–1 group from 14 to 42 days of age and in the 600 mg kg–1 group at 42 days of age. The mRNA expression levels of CAT and GSH-Px were significantly lower (p < 0.05 or p < 0.01) in the 900 mg kg–1 group at 14 days of age, in the 600 mg kg–1 and 900 mg kg–1 groups at 28 days of age, and in all three NiCl2-treated groups at 42 days of age than those in the control group. The mRNA expression levels of GR and GST were significantly decreased (p < 0.05 or p < 0.01) in the 600 and 900 mg kg–1 groups at 14 days of age and in all three NiCl2-treated groups from 28 to 42 days of age when compared with those in the control group. The results are shown in Fig. 3.
Fig. 3. Changes in mRNA expression levels of CuZn-SOD, Mn-SOD, CAT, GPx, GR, and GST in the lung. The mRNA expression levels of the antioxidant enzymes CuZn-SOD, Mn-SOD, CAT, GPx, GR, and GST are decreased in the lung. The letters A, B, C, and D represent a significant difference (p < 0.01) among the four groups; the letters a, b, c, and d represent a difference (p < 0.05) among the four groups.
Changes in activities of antioxidant enzymes in the lung
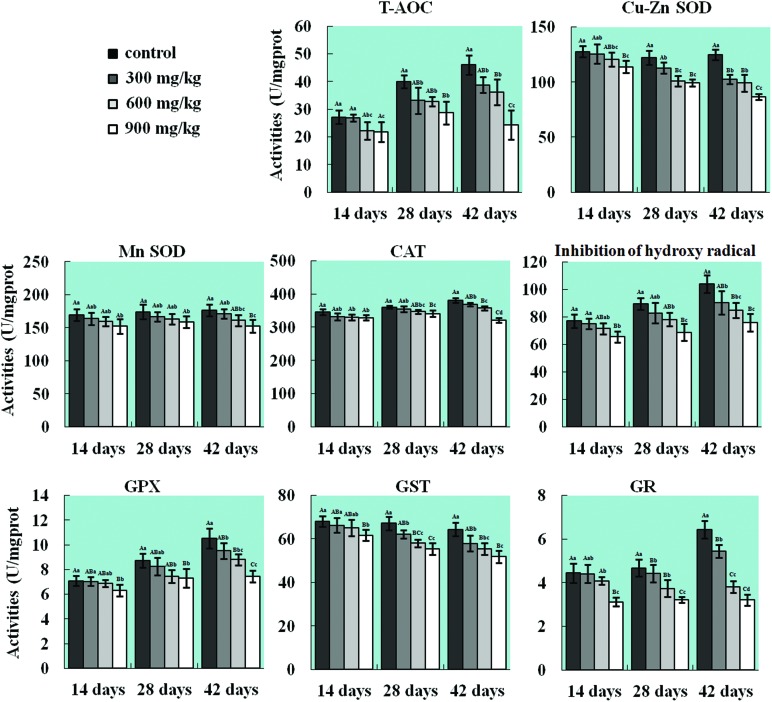
The T-AOC activities in the three NiCl2-treated groups were lower (p < 0.05 or p < 0.01) than that in the control group from 14 to 42 days of age except in the 300 mg kg–1 group at 14 days of age. The activities of CuZn-SOD and GST were significantly reduced (p < 0.05 or p < 0.01) in the 900 mg kg–1 group at 14 days of age and in all three NiCl2-treated groups at 28 and 42 days of age, and the Mn-SOD activity was significantly decreased (p < 0.05 or p < 0.01) in the 900 mg kg–1 group from 14 to 42 days of age and in the 600 mg kg–1 group at 42 days of age when compared with the control group. The CAT activity was lower (p < 0.05 or p < 0.01) in the 900 mg kg–1 group at 14 days of age, in the 600 mg kg–1 and 900 mg kg–1 groups at 28 days of age, and in all three NiCl2-treated groups at 42 days of age than that in the control group. The ability to inhibit the production of hydroxyl radical and the GSH-Px activity were significantly decreased (p < 0.05 or p < 0.01) in the 900 mg kg–1 group from 14 to 42 days of age and in the 600 mg kg–1 group from 28 to 42 days of age. Also, the GR activity was reduced (p < 0.05 or p < 0.01) in the 600 mg kg–1 and 900 mg kg–1 groups from 28 to 42 days of age and in all three NiCl2-treated groups at 42 days of age. The results are shown in Fig. 4.
Fig. 4. Changes in activities of T-AOC, CuZn-SOD, Mn-SOD, CAT, GPx, GR, GST, and ability to inhibit hydroxyl radicals in the lung. The activities of T-AOC, CuZn-SOD, Mn-SOD, CAT, GPx, GR, and GST, and the ability to inhibit hydroxyl radicals are decreased in the lung. The letters A, B, C, and D represent a significant difference (p < 0.01) among the four groups; the letters a, b, c, and d represent a difference (p < 0.05) among the four groups.
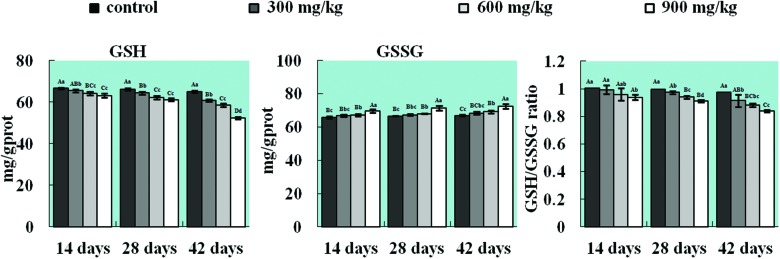
Changes in contents of GSH and GSSG and the ratio of GSH/GSSG in the lung
The GSH content was dramatically decreased (p < 0.05 or p < 0.01) in the 300, 600 and 900 mg kg–1 groups in comparison with that in the control group from 14 to 42 days of age, whereas the GSSG content was increased (p < 0.05 or p < 0.01) in the 600 and 900 mg kg–1 groups from 14 to 42 days of age. Concurrently, the ratio of GSH to GSSG was sharply reduced (p < 0.05 or p < 0.01) in the 900 mg kg–1 group from 14 to 42 days of age and in all three NiCl2-treated groups at 28 and 42 days of age. The results are shown in Fig. 5.
Fig. 5. Changes in contents of GSH and GSSG and the ratio of GSH/GSSG in the lung. The content of the non-enzymatic antioxidant molecule GSH and the ratio of GSH to GSSG are significantly decreased and the GSSG content is increased in the lung. The letters A, B, C, and D represent a significant difference (p < 0.01) among the four groups; the letters a, b, c, and d represent a difference (p < 0.05) among the four groups.
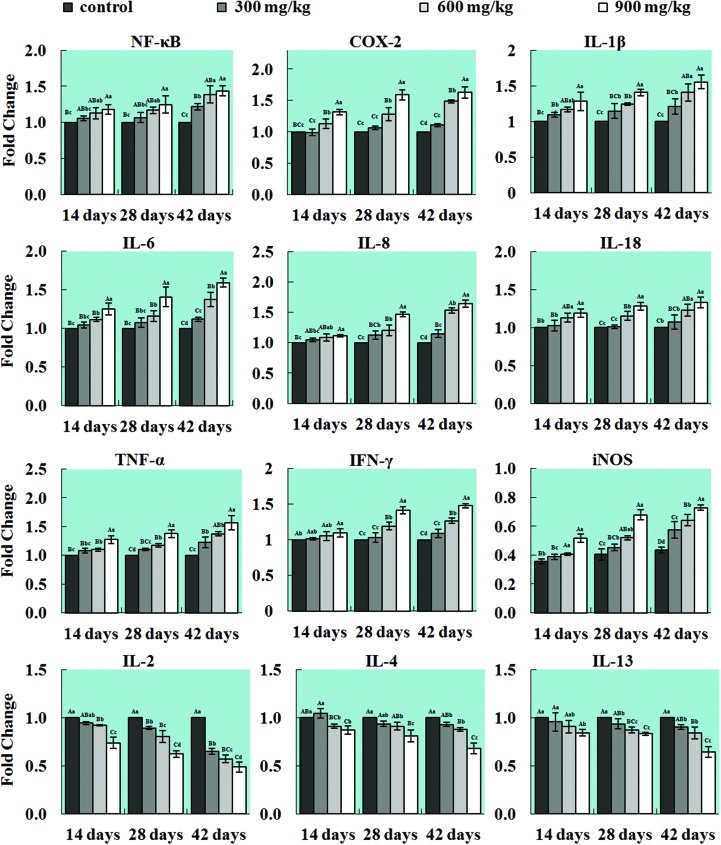
Changes in mRNA expression levels of inflammatory mediators in the lung
The mRNA expression levels of NF-κB, TNF-α, IFN-γ, iNOS, COX-2, IL-1β, IL-6, IL-8 and IL-18 were significantly higher (p < 0.05 or p < 0.01) in the three NiCl2-treated groups than those in the control groups. However, the mRNA expression levels of IL-2, IL-4 and IL-13 were decreased (p < 0.05 or p < 0.01) when compared with those in the control group. The results are shown in Fig. 6.
Fig. 6. Changes in the mRNA expression levels of inflammatory mediators in the lung. The mRNA expression levels of the pulmonary pro-inflammatory mediators NF-κB, TNF-α, IFN-γ, COX-2, iNOS, IL-1β, IL-6, IL-8 and IL-18 are increased, and the mRNA expression levels of the anti-inflammatory mediators IL-2, IL-4, and IL-13 are decreased in the lung. The letters A, B, C, and D represent a significant difference (p < 0.01) among the four groups; the letters a, b, c, and d represent a difference (p < 0.05) among the four groups.
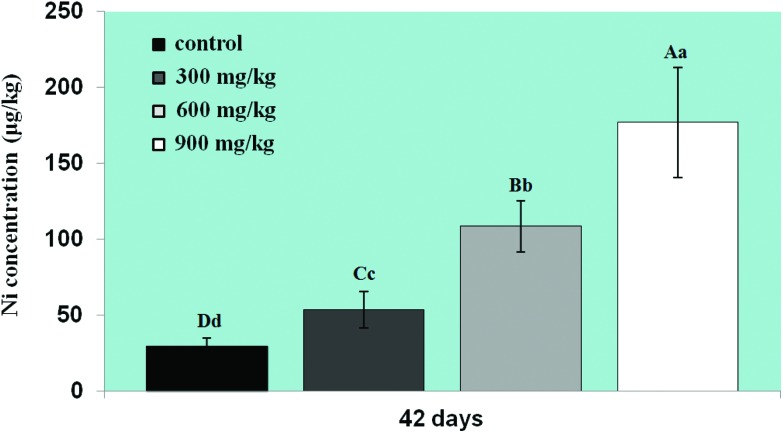
Changes in Ni residues in the lung
The Ni content was increased in the three NiCl2-treated groups at 42 days of age in comparison with that in the control group (p < 0.05 or p < 0.01), as shown in Fig. 7.
Fig. 7. Changes in Ni content in the lung at 42 days. The pulmonary Ni content has significantly increased. Data are presented as the mean ± standard deviation (n = 5). The letters A, B, C, and D represent a significant difference (p < 0.01) among the four groups; the letters a, b, c, and d represent a difference (p < 0.05) among the four groups.
Discussion
In summary, we found that NiCl2 can cause oxidative stress and inflammatory responses in this study.
It has been suggested that one possible molecular mechanism involved in the toxicity of Ni is the disruption of a delicate oxidant/antioxidant balance, which can lead to lesions via oxidative damage.12 We investigated the mechanism of NiCl2-induced oxidative stress in this study. The result showed that NiCl2 intake induced oxidative damage to DNA (upregulation of 8-OHdG) and lipid peroxidation (upregulation of MDA). 8-OHdG is an indicator of oxidant-induced damage to DNA and oxidant status.52 MDA is the most abundant aldehyde that is formed during LPO.53 Kalaivani et al.54 have also reported that the intratracheal instillation of Ni3S2, NiO, and NiSO4 into Wistar rats significantly increased the levels of 8-OHdG in the lungs. An increase in the concentration of NO free radicals and a decrease in the mRNA expression levels and activities of pulmonary antioxidant enzymes such as CuZn-SOD, Mn-SOD, CAT, GPx, GR and GST were observed in the broiler chickens treated with NiCl2. Also, the T-AOC activity, GSH content, ability to inhibit the generation of hydroxyl radicals and ratio of GSH/GSSG were decreased in the groups treated with NiCl2. An increase in the pulmonary NO content has also been observed in rats treated with NiSO4.55 Decreases in GSH levels and SOD activities and an increase in LPO have been found in the kidneys of rats fed diets contaminated with NiCl2.56 An increase in the lipid peroxidation level, depletion of GSH and decreases in the activities of antioxidant enzymes (SOD, CAT, and GPx) have also been observed in the livers of mice treated with NiCl2.57 Pari et al.58 have also demonstrated that NiSO4 can induce oxidative stress via decreasing the activities of enzymatic antioxidants such as SOD, CAT, GPx, and GST and non-enzymatic antioxidants such as GSH. The findings in this study are consistent with our previous studies on the kidney, spleen, small intestine, cecal tonsil and bursa of Fabricius of broiler chickens.17,22,29,59,60 Based on the above references, this in vivo study was the first to observe NiCl2-induced pulmonary oxidative damage via the dietary pathway. These results demonstrate that NiCl2 induces pulmonary oxidative stress via increasing the generation of free radicals and reducing antioxidant status. In this study, the decrease in the mRNA expression levels of these antioxidant enzymes shows that the inhibition of transcription is a possible way to decrease the activities of antioxidant enzymes.
There are no reports at present on the mechanism of inflammatory responses induced by dietary NiCl2 in the lung via the expression of mRNA. We also investigated the possible mechanism of inflammatory responses induced by dietary NiCl2 in this study. The results showed that dietary intake of NiCl2 increased the mRNA expression levels of the transcription factor NF-κB, TNF-α, IFN-γ, COX-2, iNOS, IL-1β, IL-6, IL-8 and IL-18. The transcription factor NF-κB is a critical intracellular mediator of the inflammatory cascade.61 The expressions of pro-inflammatory cytokines and other pro-inflammatory mediators that participate in acute inflammatory responses such as TNF-α, IFN-γ, COX-2, iNOS, IL-1β, IL-6, IL-8 and IL-18, which play an important role in initiating the body's inflammatory response to infections, injuries, or stress, are widely regulated by the transcription factor NF-κB.61,62 Our results indicated that dietary intake of NiCl2 amplifies the expression of pro-inflammatory mediators by activating the NF-κB signaling pathway, which is in agreement with the results obtained by Liu et al.63 that NiSO4 induced inflammation in livers by upregulating the levels of expression of TNF-α, IL-6, COX-2, and PGE2 via activation of the nuclear translocation of NF-κB.37 Freitas et al.64 have also reported that Ni(NO3)2 can activate the NF-κB pathway in THP-1 monocytic cells. Nickel hydroxide NPs increase the mRNA levels of TNF-α and IL-6 in the lung, spleen and heart of mice.65 Ni3S2 significantly increases mRNA expression levels of IL-8 protein in human airway epithelial cells.66 Furthermore, NiCl2 reduced the mRNA expression levels of IL-2, IL-4 and IL-13 in this study, which are the main anti-inflammatory mediators and can inhibit the production of pro-inflammatory mediators.67,68 Saito et al.69 have suggested that NiCl2 decreases the mRNA expression levels of IL-2, IL-4 and IL-13 in T cells. Also, our previous studies have proved that NiCl2 decreases the expression of IL-2 in the thymus, intestinal mucosa, cecal tonsil and spleen.13,14,20 Moreover, we have found that NiCl2 causes renal inflammatory responses by activation of the NF-κB pathway and reduction in the expression of anti-inflammatory mediators.25 The upregulation of the production of pro-inflammatory mediators and downregulation of that of anti-inflammatory mediators stimulate the lung to inflammatory responses, which impair the pulmonary function and tissue structure.
Oxidative stress induces the generation of free radicals, LPO and oxidative damage to DNA, which lead to injury to the lung tissue and cells. At the same time, the upregulation and downregulation of pro-inflammatory and anti-inflammatory mediators can induce an inflammatory response with the infiltration of inflammatory cells and congestion. Thus, we observed histopathological changes induced by NiCl2, e.g., swollen and exfoliated alveolar epithelial cells, the infiltration of inflammatory cells and congestion, which were promoted by oxidative stress and the inflammatory response. Also, the pulmonary injuries were strongly consistent with the accumulation of Ni in the lung.
Experimental procedures
Experimental design
Two hundred and eighty one-day-old healthy broiler chickens were divided into four groups (n = 70). There were seven replicates in each group and ten broilers in each replicate. The broilers were housed in cages with electrical heaters and were provided with water as well as the below-mentioned diets ad libitum for 42 days. The growth cycle of commercial broilers is about 42 days, and then they will be put to use for consumption. In this period, they grow rapidly and a lot of diet will be consumed, and broilers will easily be affected by a diet containing metal pollutants (such as Ni). The aim of our study was to evaluate the effect of dietary NiCl2 on broilers in the period of growth.
A corn-soybean meal formulated by the National Research Council 70 was the control diet, and NiCl2 (NiCl2·6H2O, Cheng Du Kelong Chemical Co., Ltd, Chengdu, China) was mixed into this basal diet to produce experimental diets containing 300, 600 and 900 mg kg–1 NiCl2, respectively. According to the ref. 32–35 doses of 300, 600 and 900 mg kg–1 NiCl2 were chosen to observe dose-dependent changes in this study.
The animal protocols and all procedures of the experiment were performed in compliance with the laws and guidelines of Sichuan Agricultural University Animal Care and Use Committee (Approval No.: 2012-024). The Ethics Committee for Animal Experiments (Institute of Animal Diseases and Environmental Hazards of Sichuan Province, Chengdu, China) approved our experimental protocol.
Histopathological examination of the lung
Five chickens in each group were humanely killed at 14, 28 and 42 days of age. The lungs were removed, fixed in 4% paraformaldehyde, dehydrated in ethanol and embedded in paraffin. Serial slices with a thickness of 5 μm were prepared, stained with haematoxylin and eosin (H&E), and examined by light microscopy.
Detection of parameters of oxidative damage in the lung
At 14, 28 and 42 days of age, after five broilers in each group had been humanely killed, the lungs were immediately removed, chilled to 0 °C in 0.85% NaCl and then dried, weighed and homogenized in 9 vol. ice-cold 0.85% NaCl in a chilled homogenizer and centrifuged at 3500g at 4 °C for 10 min, and the supernatant was finally collected. After determining the amount of total protein in the supernatant by the Bradford method,71 the activities of T-AOC, CuZn-SOD, Mn-SOD, CAT, GST, GSH-Px and GR, ability to inhibit the generation of hydroxyl radicals, contents of NO, MDA, and GSH and ratio of GSH to GSSG in the supernatant were detected by biochemical methods following the instructions of the reagent kits (T-AOC, A015; CuZn-SOD, A001-2; Mn-SOD, A001-2; CAT, A007; GST, A004; GSH-Px, A005; GR, A062; ability to inhibit the generation of hydroxyl radicals, A018; NO, A012; MDA, A003-1; GSH/GSSG, A061; total protein, A045-2, purchased from Nanjing Jiancheng Bioengineering Institute of China, Nanjing, China). Their absorbances were measured at 520, 550, 550, 240, 412, 412, 340, 550, 412, 532, 240 and 590 nm, respectively, using a microtiter plate reader (Thermo, Varioskan Flash, USA).
Determination of the 8-hydroxy-2′-deoxyguanosine (8-OHdG) content in the lung by ELISA
At 14, 28 and 42 days of age, five broilers in each group were humanely sacrificed, and the lungs were immediately stored at –70 °C until analysis was performed. Pulmonary 8-OHdG levels were measured using ELISA (DRE-C6207, RB, USA), as described by Gaca et al.72
Determination of mRNA expression of antioxidant enzymes and inflammatory mediators in the lung by qRT-PCR
The lungs from five broilers in each group were taken at 14, 28, and 42 days of age and stored in liquid nitrogen. They were then homogenized in liquid nitrogen using a mortar and pestle. The total RNA was isolated using RNAiso Plus (9108/9109, Takara, Japan). Subsequently, the RNA was transcribed into cDNA using a PrimeScript™ RT reagent kit (RR047A, Takara, Japan) according to the manufacturer's protocol. The cDNA product was used as a template for qRT-PCR analysis. Sequences for target genes were obtained from the NCBI database. Oligonucleotide primers were designed using Primer 5 software and synthesized by Takara (Dalian, China; Tables 1 and 2).
Table 1. List of primers of the antioxidant enzymes in qRT-PCR analysis.
| Gene symbol | Accession number | Primer | Primer sequence (5′–3′) | Product size | T m (°C) |
| CuZn-SOD | NM205064 | Forward | CGCAGGTGCTCACTTTAATCC | 119 bp | 57 |
| Reverse | CTATTTCTACTTCTGCCACTCCTCC | ||||
| Mn-SOD | NM204211 | Forward | CACTCTTCCTGACCTGCCTTACG | 146 bp | 57 |
| Reverse | TTGCCAGCGCCTCTTTGTATT | ||||
| CAT | NM001031215 | Forward | CTGTTGCTGGAGAATCTGGGTC | 160 bp | 61 |
| Reverse | TGGCTATGGATGAAGGATGGAA | ||||
| GST | L15387 | Forward | CAGGGAAATACAATCTCTACGGGA | 128 bp | 59 |
| Reverse | TTCTTTCACATCGGCTGCTTG | ||||
| GSH-Px | NM001277853 | Forward | TTGTAAACATCAGGGGCAAA | 140 bp | 61 |
| Reverse | TGGGCCAAGATCTTTCTGTAA | ||||
| GR | GQ853055 | Forward | CTGTGGCAAAGCCCTCCTGA | 135 bp | 61 |
| Reverse | ATGGGTGGGTGGCTGAAGAC | ||||
| β-Actin | L08165 | Forward | TGCTGTGTTCCCATCTATCG | 178 bp | 62 |
| Reverse | TTGGTGACAATACCGTGTTCA |
Table 2. List of primers of the inflammatory mediators in qRT-PCR analysis.
| Gene symbol | Accession number | Primer | Primer sequence (5′–3′) | Product size | T m (°C) |
| NF-κB | NM205134 | Forward | CTGAAACTACTGATTGCTGCTGGA | 179 bp | 62 |
| Reverse | GCTATGTGAAGAGGCGTTGTGC | ||||
| IFN-γ | Y07922 | Forward | AGCTGACGGTGGACCTATTATT | 157 bp | 58 |
| Reverse | GGCTTTGCGCTGGATTC | ||||
| TNF-α | NM204267 | Forward | CCCCTACCCTGTCCCACAA | 100 bp | 58 |
| Reverse | TGAGTACTGCGGAGGGTTCAT | ||||
| COX-2 | NM001167718 | Forward | CTTAAATTGAGACTTCGCAAGGATG | 165 bp | 62 |
| Reverse | TGGGACCAAGCCAAACACCT | ||||
| iNOS | NM204961 | Forward | CCGTGTTCCACCAGGAGATG | 168 bp | 56 |
| Reverse | GCAGGAGTAATGACGCCAAGAG | ||||
| IL-1β | Y15006 | Forward | CAGCCTCAGCGAAGAGACCTT | 106 bp | 60 |
| Reverse | CACTGTGGTGTGCTCAGAATCC | ||||
| IL-2 | AF000631 | Forward | TCTGGGACCACTGTATGCTCT | 138 bp | 60 |
| Reverse | ACACCAGTGGGAAACAGTATCA | ||||
| IL-4 | AJ621249 | Forward | ACCCAGGGCATCCAGAAG | 176 bp | 59 |
| Reverse | CAGTGCCGGCAAGAAGTT | ||||
| IL-6 | AJ309540 | Forward | AATCCCTCCTCGCCAATCTG | 106 bp | 60 |
| Reverse | GCCCTCACGGTCTTCTCCATA | ||||
| IL-8 | HM179639 | Forward | CTGGCCCTCCTCCTGGTT | 105 bp | 60 |
| Reverse | GCAGCTCATTCCCCATCTTTAC | ||||
| IL-13 | AJ621250 | Forward | AGTGCTGGACAACATGACCGA | 128 bp | 60 |
| Reverse | GCAAGAAGTTCCGCAGGTAGAT | ||||
| IL-18 | AJ277865 | Forward | TAGCCAGTTGCTTGTGGTTCG | 170 bp | 60 |
| Reverse | TCTTATCTTCTACCTGGACGCTGA | ||||
| β-Actin | L08165 | Forward | TGCTGTGTTCCCATCTATCG | 178 bp | 62 |
| Reverse | TTGGTGACAATACCGTGTTCA |
For qRT-PCR reactions, 25 μL mixtures were made up, containing 12.5 μL SYBR® Premix Ex Taq™ system (DRR820A, Takara, Japan), 1 μL forward and 1 μL reverse primer, 8.5 μL RNase-free water (RT12102, Tiangen, China) and 1 μL cDNA. A Bio-Rad C1000 thermal cycler (Bio-Rad, USA) was used to perform qRT-PCR reactions. The PCR procedure consisted of 95 °C for 3 min followed by 44 cycles of 95 °C for 10 s, Tm of a specific primer pair for 30 s, and then 95 °C for 10 s and 72 °C for 10 s. Melting curve analysis showed only one peak for each PCR product.
Data from the real-time PCR reactions were analyzed using the 2–ΔΔCT method.73
Determination of pulmonary Ni content by GFAAS
After five broilers in each group were humanely killed at 42 days of age, the lungs were immediately removed, weighed, dried, and collected for the determination of the Ni content.
The Ni content in the lungs was measured by GFAAS according to a reference method.25
Statistical analysis
The significance of differences among the four groups was analyzed by variance analysis, and the results were presented as the mean ± standard deviation (M ± SD). The variation was measured by a one-way analysis of variance (ANOVA) test using SPSS 16.0 for Windows. Statistical significance was considered to occur at p < 0.05.
Conclusions
Our data are the first to show that NiCl2 intake induces pulmonary oxidative stress and inflammatory responses via the dietary pathway, which contributes to histopathological lesions and dysfunction.
Conflict of interest
There are no conflicts of interest to declare.
Acknowledgments
The study was supported by the Program for Changjiang Scholars and Innovative Research Team in University (IRT 0848) and the Shuangzhi project of Sichuan Agricultural University (03570327; 03571198).
Appendices
Appendix
Appendix
Abbreviations appearing in the text
| Abbreviation | Name | Abbreviation | Name |
| NiCl2 | Nickel chloride | NF-κB | Nuclear factor-κB |
| Ni | Nickel | IL-1β | Interleukin-1β |
| qRT-PCR | Quantitative real-time polymerase chain reaction | IL-6 | Interleukin-6 |
| DNA | Deoxyribonucleic acid | IL-18 | Interleukin-18 |
| mRNA | Messenger ribonucleic acid | IL-2 | Interleukin-2 |
| 8-OHdG | 8-Hydroxy-2′-deoxyguanosine | IL-4 | Interleukin-4 |
| MDA | Malondialdehyde | IL-13 | Interleukin-13 |
| NO | Nitric oxide | NiSO4 | Nickel sulfate |
| CuZn-SOD | Copper–zinc superoxide dismutase | Ni NWs | Nickel nanowires |
| Mn-SOD | Manganese superoxide dismutase | ROS | Reactive oxygen species |
| CAT | Catalase | NiNPs | Nickel nanoparticles |
| GSH-Px | Glutathione peroxidase | LPO | Low pulse occupancy |
| GR | Glutathione reductase | NiONPs | Nickel oxide nanoparticles |
| GST | Glutathione-S-transferase | MIP-1α | Macrophage inflammatory protein-1α |
| T-AOC | Total antioxidant capacity | MCP-1 | Monocyte chemotactic protein-1 |
| GSH | Reduced glutathione | IL-1α | Interleukin-1α |
| GSSG | Oxidized glutathione | Ni3S2 | Nickel subsulfide |
| ˙OH | Hydroxyl free radical | Ni(NO3)2 | Nickel nitrate |
| iNOS | Inducible nitric oxide synthase | H&E | Haematoxylin and eosin |
| TNF-α | Tumor necrosis factor-α | PBS | Phosphate buffered solution |
| COX-2 | Cyclooxygenase-2 | PI | Propidium iodide staining solution |
| IFN-γ | Interferon-γ | GFAAS | Graphite furnace atomic absorption spectrometry |
References
- Huang Y., Davidson G., Li J., Yan Y., Chen F., Costa M., Chen L. C., Huang C. Environ. Health Perspect. 2002;110:835. doi: 10.1289/ehp.02110s5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cempel M., Nikel G. Pol. J. Environ. Stud. 2006;15:375–382. [Google Scholar]
- Lu H., Shi X., Costa M., Huang C. Mol. Cell. Biochem. 2005;279:45–67. doi: 10.1007/s11010-005-8215-2. [DOI] [PubMed] [Google Scholar]
- Kasprzak K. S., Sunderman Jr. F. W., Salnikow K. Mutat. Res. 2003;533:67–97. doi: 10.1016/j.mrfmmm.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Phipps T., Tank S. L., Wirtz J., Brewer L., Coyner A., Ortego L. S., Fairbrother A. Environ. Rev. 2002;10:209–261. [Google Scholar]
- Muyssen B. T., Brix K., DeForest D., Janssen C. Environ. Rev. 2004;12:113–131. [Google Scholar]
- Poonkothai M V. B. S. Int. J. Environ. Sci. 2012;1:285–288. [Google Scholar]
- Grandjean P. IARC Sci. Publ. 1984:469–485. [PubMed] [Google Scholar]
- Haber L., Erdreicht L., Diamond G., Maier A., Ratney R., Zhao Q., Dourson M. Regul. Toxicol. Pharmacol. 2000;31:210–230. doi: 10.1006/rtph.2000.1377. [DOI] [PubMed] [Google Scholar]
- Oller A. R., Costa M., Oberdorster G. Toxicol. Appl. Pharmacol. 1997;143:152–166. doi: 10.1006/taap.1996.8075. [DOI] [PubMed] [Google Scholar]
- Wataha J. C., Lockwood P. E., Schedle A., Noda M., Bouillaguet S. J. Oral Rehabil. 2002;29:133–139. doi: 10.1046/j.1365-2842.2002.00845.x. [DOI] [PubMed] [Google Scholar]
- Das K. K., Das S. N., Dhundasi S. A. Indian J. Med. Res. 2008;128:412–425. [PubMed] [Google Scholar]
- Tang K., Guo H., Deng J., Cui H., Peng X., Fang J., Zuo Z., Wang X., Wu B., Li J., Yin S. Biol. Trace Elem. Res. 2015;164:242–252. doi: 10.1007/s12011-014-0219-x. [DOI] [PubMed] [Google Scholar]
- Wu B., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Huang J. Environ. Toxicol. 2015;30:1309–1321. doi: 10.1002/tox.22001. [DOI] [PubMed] [Google Scholar]
- Wu B., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Huang J. Environ. Toxicol. 2015;30:1309–1321. doi: 10.1002/tox.22001. [DOI] [PubMed] [Google Scholar]
- Wu B., Cui H., Peng X., Fang J., Zuo Z., Deng J., Huang J. Int. J. Environ. Res. Public Health. 2014;11:8175–8192. doi: 10.3390/ijerph110808175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Cui H., Peng X., Fang J., Zuo Z., Deng J., Huang J. Food Chem. Toxicol. 2014;63:18–29. doi: 10.1016/j.fct.2013.10.033. [DOI] [PubMed] [Google Scholar]
- Wu B., Cui H., Peng X., Fang J., Zuo Z., Deng J., Huang J. Int. J. Environ. Res. Public Health. 2014;11:657–670. doi: 10.3390/ijerph110100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K., Li J., Yin S., Guo H., Deng J., Cui H. Health. 2014;6:2875. [Google Scholar]
- Huang J., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Wu B. Biol. Trace Elem. Res. 2014;159:183–191. doi: 10.1007/s12011-014-0003-y. [DOI] [PubMed] [Google Scholar]
- Huang J., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Wu B. Biol. Trace Elem. Res. 2014;158:353–358. doi: 10.1007/s12011-014-9938-2. [DOI] [PubMed] [Google Scholar]
- Guo H., Wu B., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Deng J., Yin S., Li J., Tang K. Biol. Trace Elem. Res. 2014;162:288–295. doi: 10.1007/s12011-014-0132-3. [DOI] [PubMed] [Google Scholar]
- Wu B., Cui H., Peng X., Fang J., Zuo Z., Huang J., Luo Q., Deng Y., Wang H., Liu J. Biol. Trace Elem. Res. 2013;151:234–239. doi: 10.1007/s12011-012-9554-y. [DOI] [PubMed] [Google Scholar]
- Wu B., Cui H., Peng X., Fang J., Zuo Z., Deng J., Huang J. Int. J. Environ. Res. Public Health. 2013;10:2109–2119. doi: 10.3390/ijerph10062109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Deng H., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Wu B., Chen K. Oncotarget. 2015;6:28607–28620. doi: 10.18632/oncotarget.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Wu B., Chen K., Deng J. Int. J. Mol. Sci. 2015;16:22989–23011. doi: 10.3390/ijms160922989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Wu B., Chen K., Deng J. Oncotarget. 2015;6:35964–35977. doi: 10.18632/oncotarget.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Wu B., Guo H. Oncotarget. 2016;7:125–139. doi: 10.18632/oncotarget.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Guo H., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Huang J. Chem.-Biol. Interact. 2016;243:91–106. doi: 10.1016/j.cbi.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Guo H., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Wu B., Chen K., Deng J. Oncotarget. 2016;7:17508–17519. doi: 10.18632/oncotarget.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathwan K. H., Al-Karkhi I. H. T., Jaffar Al-Mulla E. A. Res. Chem. Intermed. 2012;39:2537–2542. [Google Scholar]
- Ling J., Leach R. Poult. Sci. 1979;58:591–596. doi: 10.3382/ps.0580591. [DOI] [PubMed] [Google Scholar]
- Weber C. W., Reid B. L. J. Nutr. 1968;95:612–616. doi: 10.1093/jn/95.4.612. [DOI] [PubMed] [Google Scholar]
- Szilagyi M., Szentmihalyi S. and Anke M., in Changes in some of the biochemical parameters in Ni and Mo deficient animals [goat, sheep, pig, chicken, rat], Proceeding, Hungary, 1981, vol. 1, pp. 257–260. [Google Scholar]
- Bersényi A., Fekete S. G., Szilágyi M., Berta E., Zöldág L., Glávits R. Acta Vet. Hung. 2004;52:185–197. doi: 10.1556/AVet.52.2004.2.7. [DOI] [PubMed] [Google Scholar]
- Sun H., Wu W., Guo J., Xiao R., Jiang F., Zheng L., Zhang G. Chemosphere. 2016;148:178–187. doi: 10.1016/j.chemosphere.2015.10.068. [DOI] [PubMed] [Google Scholar]
- Capasso L., Camatini M., Gualtieri M. Toxicol. Lett. 2014;226:28–34. doi: 10.1016/j.toxlet.2014.01.040. [DOI] [PubMed] [Google Scholar]
- Alarifi S., Ali D., Alakhtani S., Al Suhaibani E. S., Al-Qahtani A. A. Biol. Trace Elem. Res. 2014;157:84–93. doi: 10.1007/s12011-013-9871-9. [DOI] [PubMed] [Google Scholar]
- Kubrak O. I., Husak V. V., Rovenko B. M., Poigner H., Kriews M., Abele D., Lushchak V. I. Chemosphere. 2013;90:971–976. doi: 10.1016/j.chemosphere.2012.06.044. [DOI] [PubMed] [Google Scholar]
- Siddiqui M. A., Ahamed M., Ahmad J., Majeed Khan M. A., Musarrat J., Al-Khedhairy A. A., Alrokayan S. A. Food Chem. Toxicol. 2012;50:641–647. doi: 10.1016/j.fct.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Zheng G. H., Liu C. M., Sun J. M., Feng Z. J., Cheng C. Aquat. Toxicol. 2014;147:105–111. doi: 10.1016/j.aquatox.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Amudha K., Pari L. Chem.-Biol. Interact. 2011;193:57–64. doi: 10.1016/j.cbi.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Ma C., Song M., Zhang Y., Yan M., Zhang M., Bi H. Toxicol. Rep. 2014;1:114–121. doi: 10.1016/j.toxrep.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahamed M. Toxicol. In Vitro. 2011;25:930–936. doi: 10.1016/j.tiv.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Pan J., Chang Q., Wang X., Son Y., Zhang Z., Chen G., Luo J., Bi Y., Chen F., Shi X. Chem. Res. Toxicol. 2010;23:568–577. doi: 10.1021/tx9003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., He M., Zhong M., Li L., Lu Y., Zhang Y., Zhang L., Yu Z., Zhou Z. Neurosci. Lett. 2015;590:52–57. doi: 10.1016/j.neulet.2015.01.065. [DOI] [PubMed] [Google Scholar]
- Gupta A. D., Patil A. M., Ambekar J. G., Das S. N., Dhundasi S. A., Das K. K. J. Basic Clin. Physiol. Pharmacol. 2006;17:87–100. doi: 10.1515/jbcpp.2006.17.2.87. [DOI] [PubMed] [Google Scholar]
- Dunnick J. K., Elwell M. R., Benson J. M., Hobbs C. H., Hahn F. F., Haly P. J., Cheng Y. S., Eidson A. F. Fundam. Appl. Toxicol. 1989;12:584–594. doi: 10.1016/0272-0590(89)90031-6. [DOI] [PubMed] [Google Scholar]
- Li X., Zhong F. Inflammation. 2014;37:457–466. doi: 10.1007/s10753-013-9759-z. [DOI] [PubMed] [Google Scholar]
- Taira M., Sasaki M., Kimura S., Araki Y. J. Mater. Sci. Mater. Med. 2008;19:2173–2178. doi: 10.1007/s10856-007-3322-0. [DOI] [PubMed] [Google Scholar]
- Morimoto Y., Ogami A., Todoroki M., Yamamoto M., Murakami M., Hirohashi M., Oyabu T., Myojo T., Nishi K.-I., Kadoya C. Nanotoxicology. 2010;4:161–176. doi: 10.3109/17435390903518479. [DOI] [PubMed] [Google Scholar]
- Abder-Rahman H. A., Nusair S. J. UOEH. 2007;29:247–258. doi: 10.7888/juoeh.29.247. [DOI] [PubMed] [Google Scholar]
- Jiang W.-D., Feng L., Liu Y., Jiang J., Hu K., Li S.-H., Zhou X.-Q. Food Chem. 2010;120:692–697. [Google Scholar]
- Kalaivani P., Saranya S., Poornima P., Prabhakaran R., Dallemer F., Vijaya Padma V., Natarajan K. Eur. J. Med. Chem. 2014;82:584–599. doi: 10.1016/j.ejmech.2014.05.075. [DOI] [PubMed] [Google Scholar]
- Hattiwale S. H., Saha S., Yendigeri S. M., Jargar J. G., Dhundasi S. A., Das K. K. Biometals. 2013;26:329–336. doi: 10.1007/s10534-013-9617-3. [DOI] [PubMed] [Google Scholar]
- Tyagi R., Rana P., Gupta M., Khan A. R., Bhatnagar D., Bhalla P. J., Chaturvedi S., Tripathi R. P., Khushu S. J. Appl. Toxicol. 2013;33:134–141. doi: 10.1002/jat.1730. [DOI] [PubMed] [Google Scholar]
- Liu C. M., Zheng G. H., Ming Q. L., Chao C., Sun J. M. J. Agric. Food. Chem. 2013;61:1146–1154. doi: 10.1021/jf304562b. [DOI] [PubMed] [Google Scholar]
- Pari L., Amudha K. Eur. J. Pharmacol. 2011;650:364–370. doi: 10.1016/j.ejphar.2010.09.068. [DOI] [PubMed] [Google Scholar]
- Huang J., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wu B. Int. J. Environ. Res. Public Health. 2013;10:7310–7326. doi: 10.3390/ijerph10127310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Guo H., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Tang K., Li J. Biol. Trace Elem. Res. 2016;171:214–223. doi: 10.1007/s12011-015-0528-8. [DOI] [PubMed] [Google Scholar]
- Sanz A. B., Sanchez-Nino M. D., Ramos A. M., Moreno J. A., Santamaria B., Ruiz-Ortega M., Egido J., Ortiz A. J. Am. Soc. Nephrol. 2010;21:1254–1262. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Karin M. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Liu C. M., Ma J. Q., Liu S. S., Feng Z. J., Wang A. M. Chem.-Biol. Interact. 2016;243:29–34. doi: 10.1016/j.cbi.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Freitas M., Fernandes E. Metallomics. 2011;3:1238–1243. doi: 10.1039/c1mt00050k. [DOI] [PubMed] [Google Scholar]
- Kang G. S., Gillespie P. A., Gunnison A., Moreira A. L., Tchou-Wong K. M., Chen L. C. Environ. Health Perspect. 2011;119:176–181. doi: 10.1289/ehp.1002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchowsky A., Soucy N. V., O'Hara K. A., Hwa J., Noreault T. L., Andrew A. S. J. Biol. Chem. 2002;277:24225–24231. doi: 10.1074/jbc.M202941200. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- Coppo R., Amore A. Pediatr. Nephrol. 2004;19:256–265. doi: 10.1007/s00467-003-1357-0. [DOI] [PubMed] [Google Scholar]
- Saito R., Hirakawa S., Ohara H., Yasuda M., Yamazaki T., Nishii S., Aiba S. Toxicol. Appl. Pharmacol. 2011;254:245–255. doi: 10.1016/j.taap.2011.04.017. [DOI] [PubMed] [Google Scholar]
- NRC, Nutrient Requirements of Poultry, National Academy Press, Washington, DC, 9th revised edn, 1994. [Google Scholar]
- Bradford M. M. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Gaca M. D., Pickering J. A., Arthur M. J., Benyon R. C. J. Hepatol. 1999;30:850–858. doi: 10.1016/s0168-8278(99)80139-1. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]