Abstract
The objective of this study was to determine the impact of manganese (Mn2+) and heme on the biofilm formation characteristics of six B. cereus food isolates and two reference strains (ATCC 10987 and ATCC 14579). The data obtained from the crystal violet assay revealed that addition of a combination of Mn2+ and heme to BHI growth medium induced B. cereus biofilm formation. However, the induction of biofilm formation was strictly strain-dependent. In all of the induced strains, the impact of Mn2+ was greater than that of heme. The impact of these two molecules on the phenotypic characteristics related to biofilm formation, such as cell density, sporulation and swarming ability, was determined in a selected food isolate (GIHE 72–5). Addition of Mn2+ and heme to BHI significantly (p < 0.05) increased the number of cells, which was correlated with the results of crystal violet assays as well as scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) analyses. In addition, induced biofilms showed higher numbers of spores and greater resistance to benzalkonium chloride. The swarming ability of B. cereus planktonic cells was increased in the presence of Mn2+ and heme in BHI. The expression levels of a number of selected genes, which are involved in mobility and extracellular polymeric substances (EPS) formation in B. cereus, were positively correlated with biofilm formation in the presence of Mn2+ and heme in BHI. These results further confirming the role of these molecules in swarming mobility and making matrix components related to B. cereus biofilm formation. These data indicate that signaling molecules present in the food environment might substantially trigger B. cereus biofilm formation, which could pose a threat to the food industry.
Introduction
Bacterial biofilms are surface-attached multicellular communities enclosed by extracellular polymeric substances (EPS) [1]. Biofilm formation is significant in the food industry and in the environment as biofilms are very difficult to remove from attached surfaces and are resistant to disinfectants compared to their free-floating planktonic counterparts [2]. Bacterial biofilm formation can be influenced by a number of environmental factors, including nutrient composition, metabolites and co-factors present on attachment surfaces, and signaling molecules produced by bacteria [3–5]. Certain molecules induce a signaling pathway that leads to the synthesis of several EPS, and this plays a major role in promoting other biofilm-related phenotypes, including virulence and resistance to disinfectants [5–8].
B. cereus is a soil-dwelling, gram-positive, aerobic or facultative anaerobic bacterium. In addition to its presence in soil, it is ubiquitously found in other environments including raw and processed foods, milk, and water [9]. B. cereus is significant as it causes two types of food poisoning (emetic and diarrheal syndromes) and several local and systematic infections, including nosocomial infections, skin lesions and meningitis [10]. In addition, B. cereus is responsible for food spoilage, leading to huge economic losses in the food processing industry [11]. This bacterium is well known to attach to several biotic and abiotic surfaces and to form biofilms [12]. The phenotype of B. cereus biofilm formation is closely associated with high cell density, spore formation and resistance to disinfectants, making it a matter of serious public health concern and an issue with regard to food safety [3].
All organisms require manganese (Mn2+) and iron for their physiological needs and survival. The ability to sense Mn2+ and iron is particularly important for bacterial pathogenesis and colonization of specific environments [13]. Iron is the most abundant metal in the environment, but free ferrous iron (Fe2+) has extremely poor bioavailability. Heme, which constitutes an important source of iron, contains a single Fe2+ atom encircled by a tetrapyrrole ring [14]. Pathogenic bacteria use several strategies to acquire iron, including import of Fe2+ by ATP- or GTP-dependent inner membrane transporters and TonB-ExbB-ExbD-dependent transport of ferric siderophores, transferrins, and heme or heme-bound proteins through specific outer membrane receptors [15]. The impact of Mn2+ on biofilm formation by Bacillus subtilis is well established [7,16]. Recently, it was reported that a combination of glycerol and Mn2+ can also trigger B. cereus biofilm formation [7,8]. Comparative genomic studies of iron transport mechanisms have revealed that iron intake significantly affects B. cereus biofilm formation [5]. However, the effects of Mn2+ and heme on biofilm formation-related phenotypes, including cell density, sporulation, motility and gene expression, are not well understood.
In B. subtilis, biofilm formation is dependent on the level of phosphorylation of Spo0A (Spo0A~P), which is mainly controlled by a network of histidine kinases [17,18]. Low levels of Spo0A~P induce a network for expression of EPS, leading to biofilm formation [17]. Exposure of B. subtilis to a combination of Mn2+ and glycerol in LB decreases the activation of histidine kinase HinD, which reduces Spo0A~P levels and subsequently activates the gene expression cascade responsible for EPS production [8]. For instance, Spo0A~P triggers the upregulation of SinI, an anti-repressor, which in turn activates the central biofilm master repressor SinR [19]. Homologs of spo0A, sinI and sinR of B. subtilis are also present in B. cereus and are known to play an important role in biofilm formation [20]. In B. subtilis, SinR directly regulates the activities of tapA-sipW-tasA and eps operons which are responsible for biofilm matrix formation [21]. The tapA-sipW-tasA operon of B. subtilis is closely related to the sipW-tasA loci that is required for biofilm formation in B. cereus [22]. However, in a recent study, the homologous of eps operon of B. subtilis has shown not to be important for biofilm matrix formation in B. cereus [6]. In addition, the regulator gene abrB along with sp0A suppresses the expression of a cascade of genes involved in motility and sporulation of B. subtilis [23]. However, the role of spo0A and abrB in B. cereus biofilm formation-related characteristics is presently unclear.
Because B. cereus forms biofilms in particular under nutrient-rich conditions such as those that exist in the food processing industry, it is important to identify the molecules that might induce biofilm formation. Therefore, we performed this study to understand the impact of Mn2+ and heme on biofilm formation and other biofilm-related phenotypes in several B. cereus food isolates.
Materials and methods
Strains and culture conditions
A total of six B. cereus strains isolated from Korean soybean paste, including two reference strains, were used in this study (Table 1). Stock cultures were stored at -80°C in brain heart infusion (BHI) (Becton Dickinson, Sparks, USA) containing 15% glycerol (Daejung, Busan, Korea). The stock cultures were routinely grown on BHI agar plates and incubated at 30°C for 24 h to prepare working cultures. Overnight (18 h) broth cultures were inoculated from single colonies at 30°C. The OD at 600 nm of the culture was measured to maintain an approximate cell concentration of 7 logCFU/ml of each strain in buffered peptone water (BPW). These cultures were used in all further experiments.
Table 1. List of B. cereus strains used in this study.
| B. cereus strain* | Type of strain | Reference |
|---|---|---|
| ATCC 14579 | Reference | [37] |
| ATCC 10987 | Reference | [37] |
| GIHE 72–2 | Soybean paste | This study |
| GIHE 72–4 | Soybean paste | This study |
| GIHE 72–5 | Soybean paste | This study |
| GIHE 72–6 | Soybean paste | This study |
| GIHE 72–7 | Soybean paste | This study |
| GIHE 72–8 | Soybean paste | This study |
*ATCC, American Type Culture Collection; GIHE, Gangwon Institute of Health and Environment.
Biofilm formation
Static biofilms were formed on stainless steel (SS) (AISI type 304L) (18 x 18 mm) coupons placed vertically in the wells of a 12-well microtiter plate (SPL LifeSciences, Gyeonggi, South Korea) as described previously by Hayrapetyan et al. [24]. The SS coupons were pretreated to remove dirt and other organic compounds using the method described by Castelijn et al. [25]. Biofilms were grown in BHI because this medium promotes higher biofilm formation than other media for several B. cereus food isolates [24]. Manganese sulfate (Mn2+)) (Sigma, St. Louis, USA) and heme (Sigma, St. Louis, USA) at final concentrations of 0.005% and 10 μg/ml, respectively were added to BHI broth where indicated [26]. In addition, BHI was supplemented with either glucose (Merck, Darmstadt, Germany) or glycerol to a final concentration of 2% [6]. Each well of the microtiter plate containing an SS coupon was filled with 3 ml of BHI supplemented with Mn2+, heme, glucose and glycerol alone or in combination. The BHI broth was then inoculated with 1.0% volume of an overnight culture. The plates were incubated at 30°C for 48 h under static conditions.
Biofilm quantification
The crystal violet assay described previously by Castelijn et al. [25] was used to measure biofilm formation. Briefly, after incubation, the SS coupons were carefully washed three times by dipping into phosphate-buffered saline (PBS) (Life Technologies, Grand Island, USA) using sterile forceps [24]. The attached biofilms were stained with 0.1% crystal violet (Difco, Detroit, USA) for 30 minutes. Crystal violet that did not bind to biofilms was discarded. The coupons were washed again three times with PBS and incubated in 70% ethanol for 30 min to release the biofilms bound by the crystal violet. Solubilized crystal violet was quantified by measuring the absorbance at a wavelength of 595 nm (Molecular Devices, Berkshire, UK). crystal violet assays were performed in three independent experiments.
The number of cells in biofilms on SS coupons incubated with isolate GIHE 72–5 was determined using a previously published protocol [25]. In brief, the coupons were dipped into sterile PBS three times. The coupons were then transferred to a tube containing 10 ml PBS and 0.5 g sterile glass beads (<106 μm, Sigma, St. Louis, USA). The coupons with attached cells were vortexed at maximum speed for 1 min, the resulting suspension was transferred to a new 96-well plate, and appropriate serial dilutions were made in PBS [24]. One hundred microliters of the serially diluted samples were spread on BHI agar plates and incubated at 30°C for 24 h. Subsequently, the number of colonies was counted and expressed as logCFU/cm2. Three independent sets of cell enumeration experiments were performed.
The number of spores in the biofilms was measured using a modification of published protocols [24]. GIHE 72–5 biofilms were grown on plastic SS coupons in BHI supplemented with Mn2+, heme and glycerol alone or in combination in 12-well plates at 30°C for 48 h. After incubation, the medium was removed, the coupons were washed with PBS, and the attached cells were separated as described in previous section. The suspension was heated at 80°C for 10 min to inactivate vegetative cells. One hundred microliters of the spore suspension was spread on BHI agar plates followed by incubation at 30°C for 24 h. Subsequently, the number of colonies was enumerated in logCFU/cm2. At least three independent experiments were performed to determine the number of spores in the biofilm matrix.
Scanning electron microscopy
B. cereus GIHE 72–5 biofilms formed on SS coupons were prepared for SEM imaging as described previously [25]. Briefly, biofilms on SS coupons were fixed in 3% glycerol for 1 h. The coupons were rinsed three times with PBS, treated with osmium tetroxide (TCI, Tokyo, Japan) for 1 h, washed twice with distilled water and dehydrated using an acetone series (10%, 30%, 50%, 70%, 90% and 100%) with a 15-min incubation at each acetone concentration. The samples were then critical-point dried using carbon dioxide. For image analysis, the specimens were sputter-coated with iridium, and images were acquired using an FESEM electron microscope (Model 5430, Hitachi, Tokyo, Japan). SEM image analysis was performed in two independent experiments.
Confocal laser scanning microscopy
The live/dead status of GIHE 72–5 cells in the biofilms was investigated using confocal laser scanning microscopy (CLSM). Biofilms were formed on plastic coupons (18 x 18 mm) (Rinzl, Electron Microscopy Sciences, PA, USA) placed vertically in the wells of a 12-well microtiter plate containing BHI supplemented with Mn2+, heme and glycerol alone or in combination as described in the previous section. After incubation, the coupons were stained with 4 ml of LIVE/DEAD Viability kit reagent (Invitrogen, MA, USA) in saline (0.9% NaCl) containing a mixture of SYTO-9 and propidium iodide (1.5 μl/ml of each) and incubated for 15 minutes in the dark [27]. CLSM was then performed using a Zeiss LSM710 microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) equipped with excitation lasers at 488 nm and 514 nm and an EC Plan-Neofluar 40x /1.30 oil lens. Five microscopic fields from each coupon were randomly recorded for image acquisition. Two CLSM image analysis experiments were performed.
Benzalkonium chloride resistance
Biofilms of selected B. cereus food isolate GIHE 72–5 formed on SS were treated with disinfectant benzalkonium chloride (BAC) at 200 ppm according to published method of Poimenidou et al. [28]. Briefly, biofilms on SS slides in BHI were incubated at 30°C for 48 h. After incubation, SS was washed twice by dipping twice into PBS to remove loosely attached cells. Subsequently, SS was transferred to a 50-ml tube filled with 10 ml of BAC. Following 5 min exposure to BAC, biofilm cells were separated by vortexing vigorously with beads for 1 min with maximum speed on a vortex. Then 1 ml of biofilm suspension was transferred to a new tube filled with 9 ml of D/E Neutralizing Broth (Becton, Dickinson and Company, NJ, USA). Neutralized samples were then used for cell enumeration as described previously.
Swarming motility test
The swarming mobility of GIHE 72–5 planktonic cells was measured according to Yan el al. [8]. Briefly, an overnight culture was grown in BHI supplemented with heme, Mn2+, and glycerol alone or in combination at 30°C for 18 h. One milliliter of this culture was washed twice with PBS and resuspended in 100 μl of PBS. Five microliters of the resuspension was spotted on BHI soft agar (0.5% agar), and the plate was dried in a laminar hood for 10 min. The swarming plates were incubated at 37°C for 12 h. Subsequently, the plates were dried for one hour in a laminar flow hood and incubated for 12 h at room temperature prior to measurement of the swarming zone. Swarming motility was tested in at least three independent experiments.
RNA preparation and quantitative RT-PCR
Total RNA was extracted from B. cereus GIHE 72–5 biofilms formed on SS in BHI supplemented with heme, Mn2+ and glycerol at 30°C for 48 h using an RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Total RNA was also extracted from planktonic overnight cultures grown in BHI supplemented with heme, Mn2+ and glycerol alone or in combination at 30°C for 18 h. RNA quantity was measured using a NanoDrop spectrophotometer. cDNA samples were prepared using a SuperScriptH III Reverse transcriptase kit (Invitrogen, MA, USA) according to the manufacturer’s instructions. Real-time PCR measurements were performed using an ABI System (Applied Biosystems Inc, CA, USA) as described previously [29]. The primers’ sequences used in this study were selected from previous study and are shown in Table 2. The 16S rRNA sequence was used as an internal standard. RT-PCR was performed in three independent experiments with three to five replicates in each experiment.
Table 2. List of primers used in real-time PCR.
| Gene | Primer | Sequence (5’ to 3’) | Source |
|---|---|---|---|
| 16SrRNA | 16SrRNA_F | GGAGGAAGGTGGGGATGACG | [38] |
| 16SrRNA_R | ATGGTGTGACGGGCGGTGTG | ||
| abrB | abrB_F | TCGTGTAGTAATTCCGATTGA | [38] |
| abrB_R | TGAAGCTCGTTTAAGATTTGC | ||
| spoOA | spoOA_F | GAAGATCTACTCCACAAAAAGACAACGGTG | [6] |
| spoOA_R | CGACGCGTGCCGTTCCTTCATCATTTAATA | ||
| sinI | sinI_F | CATGCCATGGAGGAACATTTGCATTCTTTAGC | [6] |
| sinI_R | CGACGCGTCTAATTTTTCTTTCGTGTCTGC | ||
| sinR | sinR_F | GAAGTAGAGTATCAACTGGA | [8] |
| sinR_R | GCTGGTGTTGCTAAATCTTAC | ||
| tasA | tasA_F | AGCAGCTTTAGTTGGTGGAG | [22] |
| tasA_R | R GTAACTTATCGCCTTGGAATTG | ||
| sipW | sipW_F | AGATAATTAGCAACGCGATCTC | [22] |
| sipW_R | AGAAATAGCGGAATAACCAAGC |
Statistical analysis
The data shown are average values obtained in at least three independent experiments with standard deviations. For assessment of significant differences, one-way analysis of variance (ANOVA) with Tukey's post hoc test was performed using SPSS (IBM SPSS Statistics, version 22, USA). Statistical significance was considered when the p value was less than 0.05.
Results
Impact of Mn2+ and heme on biomass formation by B. cereus food isolates
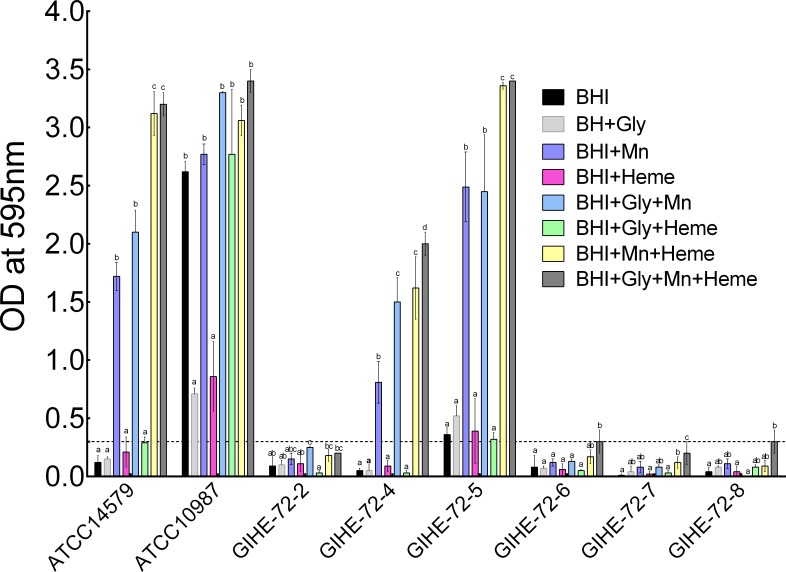
The impact of Mn2+ and heme on the biofilm formation capacity of six B. cereus food isolates and two reference strains (ATCC 14579 and ATCC 10987) was investigated. Biofilms were grown on SS coupons with or without Mn2+ and/or heme in BHI supplemented with glucose or glycerol. The results are shown in Fig 1. In the crystal violet assay, addition of Mn2+ to BHI medium (BHI+Mn) dramatically promoted biofilm formation by three of the strains under almost all conditions tested; the responding strains included one of the reference strains (ATCC 14579) and two of the food isolates (GIHE 72–4 and GIHE 72–5). After the addition of Mn2+ and heme to BHI (BHI+Mn+Heme), more biofilms were grown by these strains than after the addition of Mn2+ alone (BHI+Mn). In Mn2+- and heme-containing BHI supplemented with glycerol (BHI+Gly+Mn+Heme), a large amount of biofilm was produced only by the GIHE 72–4 isolate. Although higher crystal violet values were obtained for the GIHE 72–6, GIHE 72–7 and GIHE 72–8 isolates grown in BHI supplemented with Mn2+, heme and glycerol (BHI+Gly+Mn+Heme), the values were lower than the threshold values for biofilm formation (OD ≤ 0.3 at 595 nm). Growth of B. cereus in Mn2+- and heme-containing BHI supplemented with glycerol promotes biofilm formation on SS coupons. However, under conditions of supplementation of BHI with excess glucose in the presence of Mn2+ and/or heme (BHI+Glc+Mn+Heme), substantial biofilm formation on SS coupons did not occur (S1 Fig). Moreover, addition of heme alone to BHI or supplementation of BHI with heme and glycerol (BHI+Gly+Heme) had no impact on biofilm formation in any of the B. cereus strains tested.
Fig 1. Impact of Mn2+ and heme on biofilm formation by B. cereus food isolates.
Biofilms were grown on SS coupons with or without Mn2+ and/or heme in BHI supplemented with glycerol at 30°C for 48 h. Established biofilms were quantified using the crystal violet assay. The threshold of biofilm formation (solid line) is equal to the background absorbance value plus three times the standard deviation (OD = 0.3). Each data point represents the average value obtained in three biological experiments for each strain. The error bars indicate the standard deviation. To compare effects on biofilm formation in each strain, one-way ANOVA and Tukey's post hoc test (p < 0.05) were performed. Groups marked with different letters in each strain display significant differences.
Because the results of the crystal violet assays showed that growth of the B. cereus food isolate GIHE 72–5 in BHI medium containing added Mn2+ and heme dramatically induced robust biofilm formation (Fig 1), this strain was selected for further experiments in which the impact of Mn2+ and heme on B. cereus biofilm formation was studied.
Impact of Mn2+ and heme on the number of cells and spores in biofilms of B. cereus
The impact of Mn2+ and heme on the number of cells within the biofilm matrix was determined for the selected B. cereus GIHE 72–5 isolate using cell enumeration. For these experiments, biofilms were grown with or without Mn2+ and/or heme in BHI supplemented with glycerol on SS coupons at 30°C for 48 h. The results are shown in Fig 2. The largest number of cells (p < 0.05) was found in biofilms grown in Mn2+- and heme-containing BHI supplemented with glycerol (BHI+Gly+Mn+Heme), as shown in Fig 2A. Significantly greater (p < 0.05) numbers of cells were present in biofilms grown in BHI supplemented with Mn2+ (BHI+Mn) than in biofilms grown in BHI alone. However, there was no significant (p > 0.05) difference in the number of cells in biofilms grown in BHI containing Mn2+ and heme (BHI+Mn+Heme) and those grown in BHI supplemented only with Mn2+ (BHI+Mn). The correlation between total biomass formation measured in the crystal violet assay and the number of cells in biofilms obtained with or without Mn2+ and/or heme supplementation of BHI was calculated. In scatter plots (Fig 2B), the number of cells and the OD values obtained from the crystal violet assay showed a good correlation (R2 = 0.91). Notably, the impact of Mn2+ and heme on the planktonically grown cells was also determined for the selected B. cereus GIHE 72–5 isolate using cell enumeration. However, no correlation was found between planktonically grown cells and biofilm formation by addition of Mn(II) and Heme (S2 Fig). Sporulation of GIHE 72–5 in biofilms grown on SS coupons at 30°C for 48 h was determined in the presence and absence of Mn2+ and/or heme supplementation of BHI. A higher degree of sporulation was seen in biofilms grown in BHI supplemented with either Mn2+ or heme (BHI+Mn2+ or BHI+Heme) than in BHI alone (Fig 2C). However, under almost all conditions, higher sporulation in biofilms was found after the addition of Mn2+ than after the addition of heme to BHI. The maximum number of spores occurred in biofilms grown in BHI supplemented with both Mn2+ and heme (BHI+Mn+Heme). Notably, in biofilms grown in Mn2+- and heme-supplemented BHI (BHI+Mn+Heme), the spore-forming efficacy (the number of spores formed compared to the number of cells) was as high as 43%, whereas in BHI alone it was only 2%. The linear coefficient value (R2) for the correlation between the number of spores formed and OD values obtained from the crystal violet assay was 0.84, indicating good correlation between spore formation and biofilm formation (Fig 2D).
Fig 2. Number of cells and spores in B. cereus biofilms.
B. cereus GIHE 72–5 was grown in BHI supplemented with glycerol with or without Mn2+ and heme at 30°C for 48 h. Number of cells in logCFU/cm2 (A); scatter plot showing the relationship between the number of cells and the results of crystal violet assays (B); number of spores in logCFU/cm2 (C); scatter plot showing the relationship between spore formation and the results of crystal violet assays (D). While, squares (C) show the percentage of spore formation compared to the number of cells in biofilms. Each data point represents the average value obtained in three biological experiments, and the standard deviation. To compare the number of cells and spores obtained among the conditions used, one-way ANOVA and Tukey's post hoc test (p < 0.05) were performed. Groups marked with different letters in each growth condition display significant differences.
SEM analysis of B. cereus biofilms grown in BHI with added Mn2+ and heme
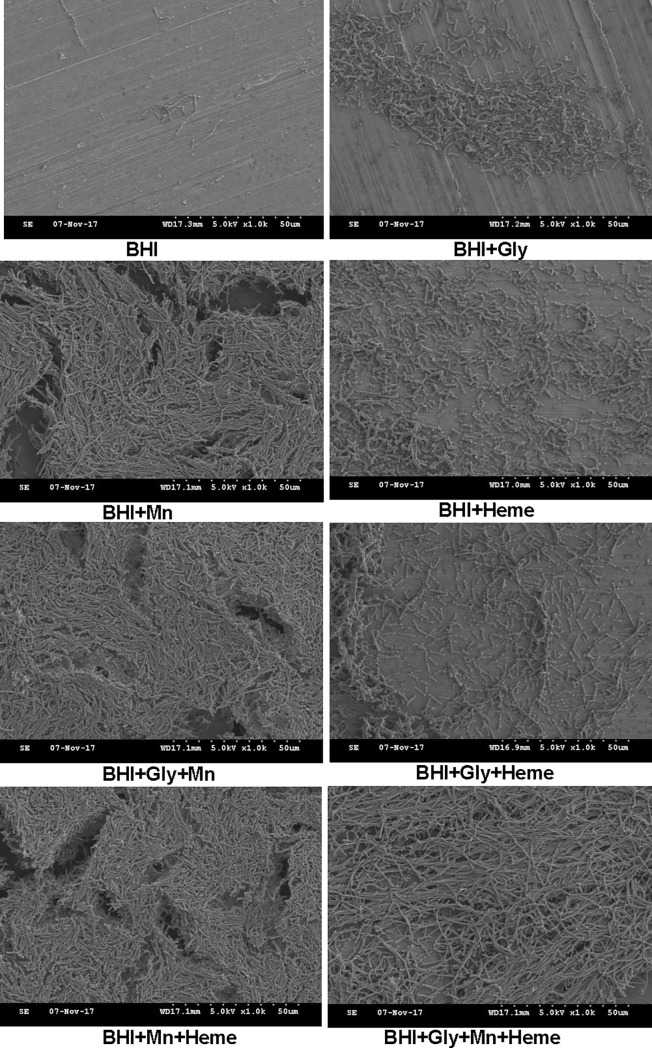
The biofilm morphology, cell heterogeneity and structure of the selected B. cereus food isolate GIHE 72–5 were visualized by SEM analysis. Biofilms grown on SS coupons in Mn2+-supplemented BHI displayed a complex structure with greater numbers of cell clusters compared to other conditions (Fig 3). Interestingly, in biofilms produced in BHI containing added Mn2+, heme and glycerol (BHI+Gly+Mn+Heme), a complex structure with relatively longer cell clusters was observed. SEM analysis revealed consistent results; in particular, biofilms grown in Mn2+-supplemented BHI, which yielded higher values in the crystal violet and cell enumeration assays, also contained higher numbers of cells within the biofilms.
Fig 3. Representative scanning electron microscope (SEM) images of biofilms formed by B. cereus GIHE 72–5.
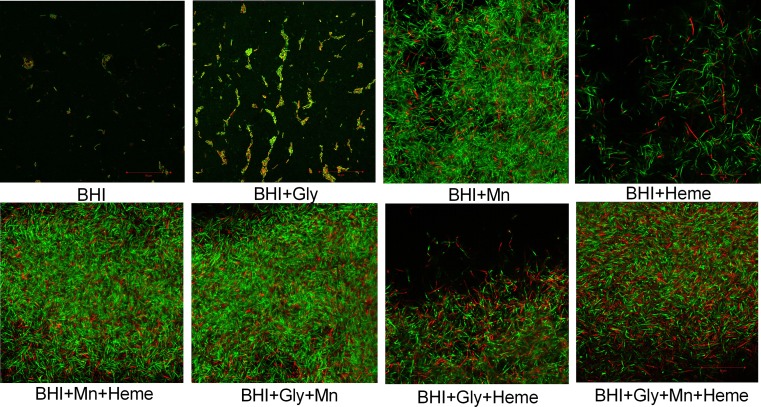
Viability of cells within B. cereus biofilms grown in BHI with added Mn2+ and heme
The viability of B. cereus GIHE 72–5 cells within biofilms grown on plastic coupons in BHI with or without Mn2+ and/or heme supplementation was assessed using LIVE/DEAD staining. In biofilms grown in Mn2+-supplemented BHI with or without the addition of glycerol, most cells were stained green, indicating that the cells were viable (Fig 4). In contrast, a high number of the cells in biofilms grown in heme-supplemented BHI were stained red, indicating that the membranes of these cells were compromised during biofilm formation. Moreover, in biofilms grown on plastic coupons in BHI with added Mn2+, a greater number of cell clusters was found compared to other conditions (Fig 4). CLSM analysis of biofilms grown on plastic coupons showed consistent results; in particular, biofilms grown in Mn2+-supplemented BHI showed higher values in the crystal violet assay and higher numbers of cells in cell enumeration experiments (S3 Fig).
Fig 4. Representative confocal laser scanning microscopy (CLSM) images of biofilm formation by B. cereus GIHE 72–5.
B. cereus GIHE 72–5 biofilms were grown on plastic coupons with or without Mn2+ andr heme in BHI supplemented with glycerol at 30°C for 48 h. Subsequently, the biofilms were stained using a LIVE/DEAD BacLight bacterial viability staining kit. The scale bar represents 50 μm.
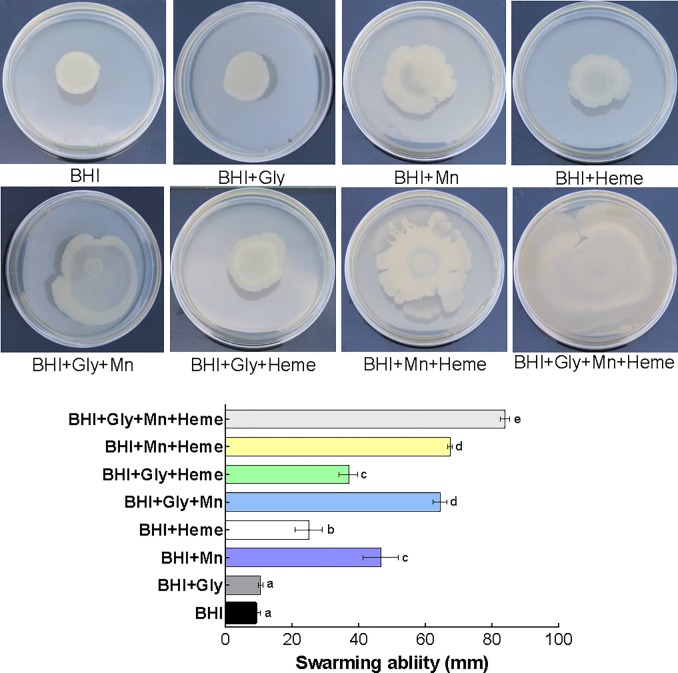
Swarming ability of B. cereus planktonic cells grown in BHI containing Mn2+ and heme
B. cereus GIHE 72–5 planktonic cells were tested for their swarming ability after growth in Mn2+- and/or heme-supplemented BHI to determine whether this property might be related to growth conditions and/or to subsequent biofilm formation. Swarming zones were measured on BHI soft agar (0.5%). Planktonic cells grown in BHI with added Mn2+ showed higher swarming ability under almost all conditions (Fig 5). The largest swarming zone was observed after growth of the cells in BHI supplemented with Mn2+, heme and additional glycerol (BHI+Gly+Mn+Heme). Importantly, the results of crystal violet assays as well as those of cell enumeration assays indicated that high amounts of biofilms were formed on SS coupons under these growth conditions.
Fig 5. Swarming motility of B. cereus food isolate GIHE 72–5 planktonic cells.
Planktonic cells were grown in BHI with or without Mn2+ and/or heme in BHI supplemented with glycerol at 30°C overnight (18) h. The overnight cultures were washed in PBS, and their swarming mobility on BHI soft agar (0.5%) plates was examined. The data represent the average swarming mobility obtained in three biological experiments; the standard deviation is shown. To compare the swarming mobility under different conditions, one-way ANOVA and Tukey's post hoc test (p < 0.05) were performed. Groups marked with different letters display significant differences.
Impact of Mn2+ and heme in BHI on the resistance of B. cereus biofilm cells to benzalkonium chloride (BAC)
The resistance of biofilms grown in Mn2+- and/or heme-supplemented BHI to BAC of the selected B. cereus GIHE 72–5 isolate was determined. The results showed that the biofilms grown on SS coupons in Mn2+-supplemented BHI, in almost all cases, displayed higher resistance to exposure to BAC (200 μg/ml) for 5 min (Fig 6). For example, in biofilms grown in BHI supplemented with Mn2+, heme, and glycerol, the number of cells within the biofilm matrix was reduced by only 0.88 logCFU/cm2 after BAC treatment, whereas the number of cells was reduced by 1.6 logCFU/cm2 when biofilms grown in BHI alone were exposed to the same treatment.
Fig 6. Impact of Mn2+ and heme on the resistance of B. cereus biofilm cells to benzalkonium chloride.
Biofilms of the B. cereus food isolate GIHE 72–5 were grown on SS coupons with or without Mn2+ and/or heme in BHI supplemented with glycerol at 30°C for 48 h. After maturation, biofilms were washed with PBS and subsequently treated with BAC (200 μg/ml) for 5 min, and the number of surviving cells was determined (A). The average number of surviving cells in logCFU/cm2 (A) is shown as a scatter plot of the Log reduction versus the crystal violet assay results (OD values) (B). Each data point represents the average value obtained in three biological experiments; the standard deviation for each condition is shown. To compare the number of surviving cells obtained under different conditions, one-way ANOVA and Tukey's post hoc test (p < 0.05) were performed. Groups marked with different letters display significant differences.
Impact of Mn2+ and heme on the expression of spoOA and abrB during the planktonic and biofilm growth phases of B. cereus
The effects of Mn2+ and heme supplementation of BHI on the expression of selected genes, spoOA, abrB, sinI, sinR, sipW and tasA during planktonic and biofilm growth conditions of GIHE 72–5 were tested. In general, spoOA, sinI, sipW and tasA were upregulated in BHI supplemented with Mn2+ under almost all conditions tested in the biofilm growth phases (Fig 7) compared to growth in BHI alone. Whereas, abrB and sinR were downregulated under this conditions (Fig 7B and 7D). Meanwhile, all of the tested genes were upregulated except sinR, which was downregulated, during planktonic growth conditions (S3 Fig). Maximal expression of spo0A, sinI and tasA were observed in biofilm cells grown in BHI supplemented with glycerol, Mn2+ and/or heme (BHI+Gly+Mn) or (BHI+Gly+Mn+Heme). In contract, sinR was minimally downregulated under these conditions. Importantly, there was no significant (p > 0.05) difference among the tested biofilm growth conditions for the downregulation of abrB (Fig 7B). In the planktonic growth phase, although, sp0A, sinI, sipW and tasA were upregulated but there were no significant (p > 0.05) differences during addition of Gly, Mn, and Heme in BHI (S4 Fig). Like spo0A, sinI, sipW and tasA, abrB was also upregulated but significantly (p < 0.05) higher after the addition of Mn2+ to BHI under almost all conditions tested in the planktonic growth phase (Fig 7B).
Fig 7. Impact of Mn2+ and heme on the expression of a number of selected genes in B. cereus in the biofilm growth phases.
The graphs show the expression of spoOA (A), abrB (B), sinI (C), sinR (D), sipW (E) and tasA (F) in the biofilm phase of GIHE 72–5. Biofilms were grown on SS coupons in Mn2+ and/or heme in BHI supplemented with glycerol at 30°C for 48 h. The fold change in expression relative to the expression in biofilm cells grown in BHI is shown. Each data point represents the average value obtained in at least two biological experiments; the error bars indicate standard deviation. To compare fold changes in expression under different conditions, one-way ANOVA and Tukey's post hoc test were performed. Groups marked with different letters in each growth condition display significant differences (p < 0.05).
Discussion
In this study, we demonstrated that Mn2+ supplementation dramatically induces B. cereus biofilm formation during growth in BHI medium. The combination of glycerol and Mn2+ has been reported previously to induce B. cereus biofilm formation in LB growth medium [7,8]. Genome-wide transcriptome analysis shows that addition of glycerol and Mn2+ to LB results in a metabolic shift that leads to increased fermentation and higher production of small fermentation products (i.e., acetone, lactate, and ethanol) compared to LB alone, and this in turn results in robust biofilm formation [8]. Moreover, glycerol and Mn2+ may induce a network of gene expression leading to purine biosynthesis, GTP homeostasis and nucleotide signaling that transforms low-biofilm-forming LB into a robust biofilm-inducing medium [8]. It is well established that Mn2+ promotes biofilm formation by B. subtilis in several growth media [7,16].
Here, we established that the promotion of B. cereus biofilm formation by Mn2+ is strictly dependent on the strain tested. Moreover, we showed that the role of glycerol in Mn2+-dependent induction of B. cereus biofilm formation is very minor (Fig 1). The addition of glucose and Mn2+ to BHI (BHI+Glc+Mn) resulted in less biofilm formation than the addition of Mn2+ alone. These results indicate that Mn2+-related induction of B. cereus biofilm formation through the KinD system is independent of the presence of excess glycerol or glucose in BHI medium. These results are consistent with the results of a previous study by Gao el al. [6] in which it was shown that addition of excess glucose and glycerol to BHI does not promote biofilm formation in B. cereus. Moreover, although the addition of glucose to tryptic soy broth (TSB) promotes biofilm formation, the addition of glycerol to TSB reduces biofilm formation. However, these data are inconsistent with the results of previous studies by Shemesh et al. [7] and Yan et al. [8], who reported that a combination of glycerol and Mn2+ in LB medium only promotes biofilm formation in B. cereus. These conflicting results might be related to the different compositions of BHI and LB media; the former contains glucose (2 g/L), whereas LB lacks glucose. The idea that the composition of LB might play a major role in biofilm formation is also suggested by the study of Mhatre et al. [16], which showed that Mn2+ related induction not only depends on the addition of glycerol but that addition of glucose to LB can also induce higher biofilm formation.
Our data show that supplementation of BHI medium with a combination of Mn2+ and heme can transform BHI into a medium that supports robust biofilm formation by several B. cereus strains. However, the addition of heme alone to BHI had no effect on biofilm formation by B. cereus. The study of Hayrapetyan et al. [5] demonstrated that B. cereus can utilize heme (hemin) as an iron source but that this ability is highly strain-specific. Furthermore, the combination of Mn2+ and heme in BHI might also play an important role in Mn2+ and heme intake and cellular metabolism utilization proficiency. Previously, it has been shown that the ratio of Mn2+ to heme plays an important role in spore resistance in B. subtilis [30].
We performed cell enumeration to determine the impact of the addition of Mn2+ and heme to BHI on the number of cells within biofilm complexes. Our results revealed a good correlation between the results of crystal violet assays and the number of cells present, in agreement with our recent study of B. cereus biofilms [31]. In addition, the results of crystal violet assays and enumeration of the number of cells were consistent with the results obtained by SEM image analysis. Moreover, the viability of GIHE 72–5 cells within biofilm matrix on plastic coupons tested using LIVE/DEAD staining showed results consistent with the crystal violet assays, cell enumeration experiments and SEM images. The fact that there were no significant (p > 0.05) in the percentage of dead cells under addition of Mn2+ or heme, in particular heme, indicated that heme did not result in cell lysis due to heme-related toxicity (S1 Table).
Here, we showed that addition of Mn2+ to BHI significantly increased sporulation efficacy in B. cereus compared to BHI alone. Previous studies have shown that addition of Mn2+ to the culture medium induces sporulation in Bacillus biofilms [16]. In the planktonic growth phase of Bacillus, a similar induction of sporulation in several growth media after the addition of Mn2+ has been reported [32–34]. However, Mn2+-dependent sporulation in Bacillus requires the presence of an additional carbon source such as glucose or glycerol [32]. Transcriptome analysis in B. subtilis shows that Mn2+-dependent induction is related to the expression of spore core proteins (i.e., gerPB, gerPD and gerPE), which are downregulated in the absence of Mn2+ [16]. Our results revealed that a combination of Mn2+ and/or heme in BHI was favorable for high sporulation in B. cereus biofilms on SS coupons, although heme alone had no effect. Similar results have been reported for B. subtilis planktonic cells, in which iron has been shown to induce sporulation in several growth media [34]. Moreover, sporulation efficacy depends on the form in which iron is supplied; soluble iron salts are more active than insoluble iron in inducing sporulation [34]. To date, there have been no studies of the effects of supplementation of BHI with a combination of Mn2+ and heme on sporulation efficiency in Bacillus. However, it was previously demonstrated that although Mn2+ is not essential for the resistance properties of B. subtilis spores, the ratio of Mn2+ and iron is important in determining spore resistance to disinfectants (ionizing radiation) [30].
The data on bacterial motility showed that addition of Mn2+ to BHI was positively correlated with swarming ability and a robust biofilm formation phenotype. This result is consistent with the results of previous studies in which it was shown that the swarming ability of B. cereus plays a major role in the initial attachment of the bacteria to a suitable surface and in subsequent biofilm formation [20,35,36]. A deletion mutation of clpYQ in B. cereus AR 156 showed reduced swarming zone size and low biofilm formation compared to wild type in LB medium supplemented with Mn2+ and glycerol (LBGM), suggesting a role of clpYQ in swarming ability and biofilm formation [35]. Interestingly, here we found that addition of heme to BHI also increased swarming ability; however, heme addition had no major impact on biofilm formation (Figs 1 and 4). These data indicate that B. cereus biofilm formation is not only dependent on swarming ability but is a complex process that involves several pathways [5,8].
The role of Mn2+ and heme in the motility of B. cereus grown in BHI and in subsequent robust biofilm formation was further established based on the results obtained regarding the expression of a number of selected genes. After addition of Mn2+ to BHI, in almost all conditions, spo0A, sinI, sipW and tasA were expressed at several-fold higher levels under biofilm growth phase conditions compared to its expression during growth in BHI alone (Fig 7C). These results indicate that spo0A-sinI-sinR and sipW-tasA regulatory pathways play an important role in the Mn2+-regulated induction of B. cereus biofilm formation. The higher expression of the spo0A and sinI as well as lower expression of sinR genes during B. cereus biofilm formation is consistent with the results of previous studies [6,20]. Recently, Xu et al. [20] reported that the spo0A-sinI-sinR expression pathway plays the key regulatory role during biofilm formation by B. cereus. In addition, deletion of sp0A and sinI genes were shown to result in defects in swarming ability [23] and pellicle formation in B. cereus [6]. Moreover, the higher expression of sipW and tasA during addition of Gly, Mn and heme indicate that these compounds promote B. cereus biofilm formation through sipW-tasA genetic pathway. The expression of TasA protein that forms the amyloid-like fibers in response to Gly, Mn and heme in BHI was also clearly visible in swarming experiment photos (Fig 5). These results further revealed that the robust biofilm formation in response to Gly, heme and Mn of B. cereus might be mediated through the sipW-tasA regulatory pathway. In contrast, here we found that abrB gene expression was downregulated under almost all conditions during the biofilm growth phase, whereas in the planktonic growth phase the addition of Mn2+ to BHI resulted in several-fold higher expression of the gene compared to BHI alone. These data indicate that abrB, a central regulatory gene that plays a key role in biofilm formation in B. subtilis by regulating cell mobility and differentiation [23], plays a major role in the response of B. cereus to the addition of Mn2+ to BHI during planktonic growth conditions. At the early stage of biofilm formation, planktonic cells require a suitable surface for attachment; abrB gene expression is upregulated at this stage, leading to increased expression of a network of motility-related genes such as lytA and lytF. However, in mature biofilms, abrB expression is downregulated, which turns on the expression of matrix genes (eps, tapA, bslA) and turns off the expression of motility-related genes [23]. The downregulation of abrB gene expression after the addition of Mn2+ to BHI observed in our study suggests that motility plays a key role in inducing B. cereus biofilm formation at the early stage. Our results showed a clear correlation between BAC resistance and robust biofilm formation in B. cereus grown in BHI containing Mn2+ and heme. The data suggest that robust biofilm formation by B. cereus grown in BHI in the presence of Mn2+ and heme may be due to high biomass formation (indicated by the results of the crystal violet assay), the formation of dense cell clusters, and sporulation under these growth conditions.
In conclusion, in this study we showed that addition of Mn2+ and heme to BHI induced robust biofilm formation by B. cereus and that this occurred in a strictly strain-dependent manner. Biofilm formation phenotypes such as cell density, sporulation, biofilm architecture and resistance to disinfectant were largely affected by addition of Mn2+ and heme to BHI. The pattern of expression of a number of selected genes suggest the involvement of Mn2+ and heme in the motility and biofilm formation of B. cereus through sp0A-sinI-sinR and sipW-tasA regulatory pathways. The results of this study indicate that B. cereus biofilm formation can be altered in the presence of signaling molecules such as Mn2+ and heme. Because these molecules are widely available in the food-processing environment, this could have a significant detrimental effect on the food industry.
Supporting information
Biofilms were grown on SS coupons with or without Mn2+ and/or heme in BHI supplemented with glucose at 30°C for 48 h. Established biofilms were quantified using the crystal violet assay. The threshold of biofilm formation (solid line), is equal to the background absorbance value plus three times the standard deviation (OD = 0.3). Each data point represents the average value obtained in three biological experiments with each strain; the error bars indicate standard deviation. To compare effects on biofilm formation in each strain, one-way ANOVA and Tukey's post hoc test (p < 0.05) were performed. Groups marked with different letters in each strain display significant differences.
(TIF)
B. cereus GIHE 72–5 planktonic cells were grown in BHI supplemented with glycerol with or without Mn2+ and/or heme at 30°C for 48 h. Number of cells in logCFU/ml (A); scatter plot showing the relationship between the number of cells and the results of crystal violet assays for biofilm formation (B). Each data point represents the average value obtained in three biological experiments, and the standard deviation. To compare the number of cells and spores obtained among the conditions used, one-way ANOVA and Tukey's post hoc test (p < 0.05) were performed. Groups marked with different letters in each growth condition display significant differences.
(TIF)
Biofilms were grown with or without Mn2+ and/or heme in BHI supplemented with glycerol at 30°C for 48 h. Established biofilms were quantified using the crystal violet assay (A) and cell enumeration (B). The threshold of biofilm formation (solid line) is equal to the background absorbance value plus three times the standard deviation (OD = 0.3). Each data point represents the average value obtained in two biological experiments for each strain; error bars indicate the standard deviations. To compare effects on biofilm formation in each strain, one-way ANOVA and Tukey's post hoc test (p < 0.05) were performed. Groups marked with different letters in each strain display significant differences.
(TIF)
The graph shows the expression of spoOA (A), abrB (B), sinI (C), sinR (D), sipW (E) and tasA (F) in the planktonic growth phase of GIHE 72–5. Planktonic cells were grown in Mn2+ and/or heme in BHI supplemented with glycerol at 30°C for 48 h. The fold change in expression relative to the expression in planktonic cells grown in BHI is shown. Each data point represents the average value obtained in at least two biological experiments; the error bars indicate standard deviation. To compare fold changes in expression under different conditions, one-way ANOVA and Tukey's post hoc test were performed. Groups marked with different letters in each growth condition display significant differences (p < 0.05).
(TIF)
* the percentage of live and dead cells were determined according to previously published protocols [1]. Groups marked with different letters in each column show significant differences (one-way ANOVA and Tukey's post hoc test, p < 0.05).
(DOCX)
Acknowledgments
The authors would like to thank Mr. Park-Yong Ik of Kangwon National University, Central Laboratory, for training and technical support with SEM analysis and Ms. Lee Hyun Ah for CLSM analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant from the Brain Korea (BK) 21 Plus project (grant no. 22A20153713433) funded by the Korean Government. It was also supported by a grant from Kangwon National University in 2015. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as Complex Differentiated Communities. Annu Rev Microbiol. 2002;56: 187–209. 10.1146/annurev.micro.56.012302.160705 [DOI] [PubMed] [Google Scholar]
- 2.Halstead FD, Rauf M, Moiemen NS, Bamford A, Wearn CM, Fraise AP, et al. The antibacterial activity of acetic acid against biofilm-producing pathogens of relevance to burns patients. PLOS One. 2015;10 10.1371/journal.pone.0136190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain MS, Oh DH. Substratum attachment location and biofilm formation by Bacillus cereus strains isolated from different sources: Effect on total biomass production and sporulation in different growth conditions. Food Control. 2017;77: 270–280. 10.1016/j.foodcont.2017.02.014 [DOI] [Google Scholar]
- 4.Lebeer S, Verhoeven TLA, Vélez MP, Vanderleyden J, De Keersmaecker SCJ. Impact of environmental and genetic factors on biofilm formation by the probiotic strain Lactobacillus rhamnosus GG. Appl Environ Microbiol. 2007;73: 6768–6775. 10.1128/AEM.01393-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayrapetyan H, Siezen R, Abee T, Groot MN. Comparative genomics of iron-transporting systems in Bacillus cereus strains and impact of iron sources on growth and biofilm formation. Front Microbiol. 2016;7 10.3389/fmicb.2016.00842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao T, Foulston L, Chai Y, Wang Q, Losick R. Alternative modes of biofilm formation by plant-associated Bacillus cereus. Microbiologyopen. 2015;4: 452–464. 10.1002/mbo3.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shemesh M, Chaia Y. A combination of glycerol and manganese promotes biofilm formation in Bacillus subtilis via histidine kinase KinD signaling. J Bacteriol. 2013;195: 2747–2754. 10.1128/JB.00028-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan F, Yu Y, Gozzi K, Chen Y, Guo JH, Chai Y. Genome-wide investigation of biofilm formation in Bacillus cereus. Appl Environ Microbiol. 2017;83 10.1128/AEM.00561-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenfors Arnesen LP, Fagerlund A, Granum PE. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiology Reviews. 2008. pp. 579–606. 10.1111/j.1574-6976.2008.00112.x [DOI] [PubMed] [Google Scholar]
- 10.Bottone EJ. Bacillus cereus, a volatile human pathogen. Clinical Microbiology Reviews. 2010. pp. 382–398. 10.1128/CMR.00073-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopal N, Hill C, Ross PR, Beresford TP, Fenelon MA, Cotter PD. The prevalence and control of Bacillus and related spore-forming bacteria in the dairy industry. Frontiers in Microbiology. 2015. 10.3389/fmicb.2015.01418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majed R, Faille C, Kallassy M, Gohar M. Bacillus cereus Biofilms-same, only different. Frontiers in Microbiology. 2016. 10.3389/fmicb.2016.01054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auwaerter PG. Borrelia: Molecular Biology, Host Interaction and Pathogenesis [Internet]. Clinical Infectious Diseases. 2011. pp. 965–965. 10.1093/cid/cir528 [DOI] [PubMed] [Google Scholar]
- 14.Ems T, Huecker MR. Biochemistry, Iron Absorption [Internet]. StatPearls. 2017. Available: http://www.ncbi.nlm.nih.gov/pubmed/28846259 [PubMed] [Google Scholar]
- 15.Hood MI, Skaar EP. Nutritional immunity: Transition metals at the pathogen-host interface. Nature Reviews Microbiology. 2012. pp. 525–537. 10.1038/nrmicro2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mhatre E, Troszok A, Gallegos-Monterrosa R, Lindstädt S, Hölscher T, Kuipers OP, et al. The impact of manganese on biofilm development of Bacillus subtilis. Microbiol (United Kingdom). 2016;162: 1468–1478. 10.1099/mic.0.000320 [DOI] [PubMed] [Google Scholar]
- 17.McLoon AL, Kolodkin-Gal I, Rubinstein SM, Kolter R, Losick R. Spatial regulation of histidine kinases governing biofilm formation in Bacillus subtilis. J Bacteriol. 2011;193: 679–685. 10.1128/JB.01186-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chai Y, Norman T, Kolter R, Losick R. Evidence that metabolism and chromosome copy number control mutually exclusive cell fates in Bacillus subtilis. EMBO J. 2011;30: 1402–1413. 10.1038/emboj.2011.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman JA, Rodrigues C, Lewis RJ. Molecular basis of the activity of SinR Protein, the master regulator of biofilm formation in Bacillus subtilis. J Biol Chem. 2013;288: 10766–10778. 10.1074/jbc.M113.455592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu S, Yang N, Zheng S, Yan F, Jiang C, Yu Y, et al. The spo0A-sinI-sinR regulatory circuit plays an essential role in biofilm formation, nematicidal activities, and plant protection in Bacillus cereus AR156. Mol Plant-Microbe Interact. 2017;X: MPMI-02-17-0042-R 10.1094/MPMI-02-17-0042-R [DOI] [PubMed] [Google Scholar]
- 21.Chu F, Kearns DB, Branda SS, Kolter R, Losick R. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol Microbiol. 2006;59: 1216–1228. 10.1111/j.1365-2958.2005.05019.x [DOI] [PubMed] [Google Scholar]
- 22.Caro-Astorga J, Pérez-García A, de Vicente A, Romero D. A genomic region involved in the formation of adhesin fibers in Bacillus cereus biofilms. Front Microbiol. 2014;5 10.3389/fmicb.2014.00745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma W, Peng D, Walker SL, Cao B, Gao C-H, Huang Q, et al. Bacillus subtilis biofilm development in the presence of soil clay minerals and iron oxides. npj Biofilms Microbiomes. Springer US; 2017;3: 4 10.1038/s41522-017-0013-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayrapetyan H, Muller L, Tempelaars M, Abee T, Nierop Groot M. Comparative analysis of biofilm formation by Bacillus cereus reference strains and undomesticated food isolates and the effect of free iron. Int J Food Microbiol. 2015;200: 72–79. 10.1016/j.ijfoodmicro.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 25.Castelijn GA, van der Veen S, Zwietering MH, Moezelaar R, Abee T. Diversity in biofilm formation and production of curli fimbriae and cellulose of Salmonella Typhimurium strains of different origin in high and low nutrient medium. Biofouling. 2012;28: 51–63. 10.1080/08927014.2011.648927 [DOI] [PubMed] [Google Scholar]
- 26.Van der Veen S, Abee T. Mixed species biofilms of Listeria monocytogenes and Lactobacillus plantarum show enhanced resistance to benzalkonium chloride and peracetic acid. Int J Food Microbiol. 2011;144: 421–431. 10.1016/j.ijfoodmicro.2010.10.029 [DOI] [PubMed] [Google Scholar]
- 27.Bojsen R, Regenberg B, Gresham D, Folkesson A. A common mechanism involving the TORC1 pathway can lead to amphotericin B-persistence in biofilm and planktonic Saccharomyces cerevisiae populations. Sci Rep. Nature Publishing Group; 2016;6: 21874 10.1038/srep21874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poimenidou S V., Chrysadakou M, Tzakoniati A, Bikouli VC, Nychas GJ, Skandamis PN. Variability of Listeria monocytogenes strains in biofilm formation on stainless steel and polystyrene materials and resistance to peracetic acid and quaternary ammonium compounds. Int J Food Microbiol. 2016;237: 164–171. 10.1016/j.ijfoodmicro.2016.08.029 [DOI] [PubMed] [Google Scholar]
- 29.Forghani F, Wei S, Oh D-H. A Rapid Multiplex Real-Time PCR High-Resolution Melt Curve Assay for the Simultaneous Detection of Bacillus cereus, Listeria monocytogenes, and Staphylococcus aureus in Food. J Food Prot. 2016;79: 810–815. 10.4315/0362-028X.JFP-15-428 [DOI] [PubMed] [Google Scholar]
- 30.Granger AC, Gaidamakova EK, Matrosova VY, Daly MJ, Setlow P. Effects of Mn and Fe levels on Bacillus subtilis spore resistance and effects of Mn2+, other divalent cations, orthophosphate, and dipicolinic acid on protein resistance to ionizing radiation. Appl Environ Microbiol. 2011;77: 32–40. 10.1128/AEM.01965-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wani A.Hprdmamma M. Shelf-life extension of pear (Pyrus communis L.) by gamma-irradiation. J Food Sci Technol. 2007;44: 138–142. Available: http://www.scopus.com/inward/record.url?eid=2-s2.0-33947630145&partnerID=40&md5=b2543c4d02bbbb473c41c0cbb6e276b9 [Google Scholar]
- 32.Vasantha N, Freese E. The role of manganese in growth and sporulation of Bacillus subtilis. J Gen Microbiol. 1979;112: 329–336. 10.1099/00221287-112-2-329 [DOI] [PubMed] [Google Scholar]
- 33.Ryu JH, Kim HK, Beuchat LR. Spore formation by Bacillus cereus in broth as affected by temperature, nutrient availability, and manganese. J Food Prot. 2005;68: 1734–1738. 10.4315/0362-028X-68.8.1734 [DOI] [PubMed] [Google Scholar]
- 34.Charney J, Fisher WP, Hegarty CP. Managanese as an essential element for sporulation in the genus Bacillus. J Bacteriol. 1951;62: 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan F, Yu Y, Gozzi K, Chen Y, Guo JH, Chai Y. Genome-wide investigation of biofilm formation in Bacillus cereus. Appl Environ Microbiol. 2017;83 10.1128/AEM.00561-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houry A, Briandet R, Aymerlch S, Gohar M. Involvement of motility and flagella in Bacillus cereus biofilm formation. Microbiology. 2010;156: 1009–1018. 10.1099/mic.0.034827-0 [DOI] [PubMed] [Google Scholar]
- 37.Wijman JGE, De Leeuw PPLA, Moezelaar R, Zwietering MH, Abee T. Air-liquid interface biofilms of Bacillus cereus: Formation, sporulation, and dispersion. Appl Environ Microbiol. 2007;73: 1481–1488. 10.1128/AEM.01781-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lücking G, Dommel MK, Scherer S, Fouot A, Ehling-Schulz M. Cereulide synthesis in emetic Bacillus cereus is controlled by the transition state regulator AbrB, but not by the virulence regulator PlcR. Microbiology. 2009;155: 922–931. 10.1099/mic.0.024125-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Biofilms were grown on SS coupons with or without Mn2+ and/or heme in BHI supplemented with glucose at 30°C for 48 h. Established biofilms were quantified using the crystal violet assay. The threshold of biofilm formation (solid line), is equal to the background absorbance value plus three times the standard deviation (OD = 0.3). Each data point represents the average value obtained in three biological experiments with each strain; the error bars indicate standard deviation. To compare effects on biofilm formation in each strain, one-way ANOVA and Tukey's post hoc test (p < 0.05) were performed. Groups marked with different letters in each strain display significant differences.
(TIF)
B. cereus GIHE 72–5 planktonic cells were grown in BHI supplemented with glycerol with or without Mn2+ and/or heme at 30°C for 48 h. Number of cells in logCFU/ml (A); scatter plot showing the relationship between the number of cells and the results of crystal violet assays for biofilm formation (B). Each data point represents the average value obtained in three biological experiments, and the standard deviation. To compare the number of cells and spores obtained among the conditions used, one-way ANOVA and Tukey's post hoc test (p < 0.05) were performed. Groups marked with different letters in each growth condition display significant differences.
(TIF)
Biofilms were grown with or without Mn2+ and/or heme in BHI supplemented with glycerol at 30°C for 48 h. Established biofilms were quantified using the crystal violet assay (A) and cell enumeration (B). The threshold of biofilm formation (solid line) is equal to the background absorbance value plus three times the standard deviation (OD = 0.3). Each data point represents the average value obtained in two biological experiments for each strain; error bars indicate the standard deviations. To compare effects on biofilm formation in each strain, one-way ANOVA and Tukey's post hoc test (p < 0.05) were performed. Groups marked with different letters in each strain display significant differences.
(TIF)
The graph shows the expression of spoOA (A), abrB (B), sinI (C), sinR (D), sipW (E) and tasA (F) in the planktonic growth phase of GIHE 72–5. Planktonic cells were grown in Mn2+ and/or heme in BHI supplemented with glycerol at 30°C for 48 h. The fold change in expression relative to the expression in planktonic cells grown in BHI is shown. Each data point represents the average value obtained in at least two biological experiments; the error bars indicate standard deviation. To compare fold changes in expression under different conditions, one-way ANOVA and Tukey's post hoc test were performed. Groups marked with different letters in each growth condition display significant differences (p < 0.05).
(TIF)
* the percentage of live and dead cells were determined according to previously published protocols [1]. Groups marked with different letters in each column show significant differences (one-way ANOVA and Tukey's post hoc test, p < 0.05).
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.