Abstract
Mutations in PARK7/DJ-1 gene are associated with familial autosomal recessive Parkinson disease. Recently, lysosomes and chaperone mediated autophagy (CMA) has been reported to participate in the degradation of DJ-1/PARK7 protein. Lamp-2A isoform is considered as the lysosomal receptor for the uptake of proteins being degraded by the CMA pathway. We have used several cell lines with disrupted LAMP2 gene expression and their respective control cells to test the possible role of lysosomal degradation and in particular CMA in DJ-1 /PARK7 degradation. Interruption of LAMP-2 expression did not result in an increase of the steady-state protein levels of DJ-1 /PARK7, as it would have been expected. Furthermore, no change in DJ-1 /PARK7 protein levels were observed upon inhibition of lysosomal function with NH4Cl or NH4Cl plus leupeptin, or after activation of CMA by serum starvation for 24h. Accordingly, we have not found any evidence that DJ-1 /PARK7 protein levels are regulated via lysosomal degradation or the CMA pathway.
Introduction
PARK7 /DJ-1 gene mutations are linked to autosomal recessive and early-onset clinical manifestations of Parkinson's disease. Pathogenic mutations identified in PARK7 /DJ-1 gene include CNVs (exonic deletions and truncations), and numerous missense mutations [1] [2].
DJ-1 is a dimeric protein with a flavodoxin-like structure [3–5] [6,7] and ubiquitously expressed [8]. DJ-1 wild type protein is a rather stable protein since DJ-1 protein levels remain essentially unchanged after 24h of incubation of cells with cycloheximide [9] [10]. In addition, pulse-chase experiments did not reveal a clear decay in wild type DJ-1 protein levels within 24 hrs [11]. Surprisingly, Wang et al [12] recently reported that DJ-1 protein levels increase after treatment of SN4741 cells with NH4Cl or NH4Cl and leupeptin for 18h. Furthermore, they show that >80% of DJ-1 is degraded after prolonged (24 hrs) serum starvation, such treatment is known to activate the pathway of chaperone mediated autophagy (CMA). In CMA, proteins with a conserved aminoacid motif selectively bind to the C-terminus of the integral lysosomal membrane protein Lamp-2A, one of the three isoforms generated from LAMP2 gene transcripts by alternative splicing. After binding to Lamp-2A, proteins are translocated to the lysosomal matrix for degradation [13]. The DJ-1 degradation observed under serum starvation conditions was prevented by addition of leupeptin and NH4Cl, and not by addition of·3-methyl adenine (3-MA) to the culture media of the cells. Those basic results, and other experiments, lead the authors [12] to conclude that DJ-1 protein is degraded by CMA pathway through binding to Lamp-2A [13]. Those results prompt us to replicate and validate the implication of the CMA pathway in degradation of wild type DJ-1. Using different cell lines which lack LAMP2 gene expression (and their respective controls) and after stimulation of CMA, we found no experimental evidence that CMA pathway is involved in the turnover of the DJ-1 wild type protein.
Materials and methods
Ethical statement
Mice for isolation of mouse embryonic fibroblasts were handle following the ethical standards set by the National Animal Care Committee of Germany. Protocols were approved by the Ministerium für Energiewende, landwirtschaft und ländliche Räume, Schleswig-Holstein (V312-72241.121–3) to work by Dr. Paul Saftig group at the animal facilities of Institute of Biochemistry, Christian-Albrechts-Universität zu Kiel, Kiel, Germany. Human cell lines used in this study were described previously with identified source and approval for the B-cell line derived from a Danon patient [14].
Cell lines
Human B-lymphoblastoid cell lines (B-LCL) were grown in RPMI medium (Gibco BRL) supplemented with 10% fetal bovine serum (FBS), penicillin/streptomycin and 2 mM glutamine, as described [14]. HeLa, HEK, SN4741 cells (provided by Dr. José Luis Zugaza, Faculty of Science and Technology, University of the Basque Country, UPV/EHU, Bilbao, Spain) and the different control and Lamp-2 deficient cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL) supplemented with 10% foetal bovine serum (Sigma-Aldrich) and 100 μg/mL gentamycin. Mouse embryonic fibroblasts (MEF) were obtained from wild type (Wt) mice and MEF Lamp-2-/y from Lamp-2 deficient mice [15] [16]. N2a cells transfected with shRNA scrambled sequence (shRNA scrmbl) and shRNA for Lamp-2 have been previously described [17] and were cultured in the presence of the selecting antibiotic G418 at 400 μg/mL. All cells were grown at 37°C (except SN4741 that were cultured at 33°C) and humidified 5% CO2. Both MEFs and N2a cell lines were provided by Drs. Judith Blanz and Paul Saftig from Institute of Biochemistry, Christian-Albrechts-Universität zu Kiel, Kiel, Germany.
Antibodies
Anti-Lamp-2A-specific antibody (Abcam ab18528) was used at 1/1000 and rabbit anti-Lamp-2A [17] was also used at 1/1000. Anti-LC-3 (Sigma) was used at 1/1000, anti-DJ-1 (Abcam ab18257) was used at 1/2000 and anti-IKappaBα rabbit polyclonal antibody from Santa Cruz was used at 1/500 dilution. As control for total protein loading, antibodies against α-tubulin (1/10,000, Sigma, clone DM1a) were used.
Studies of protein degradation
Exponentially growing cells were treated with 25 μg/ml of cycloheximide (CHX) for the times indicated. Cell viability by trypan blue exclusion was ≥95%. Cells were collected and processed for analysis by Western and immunoblot with anti-DJ-1 specific antibodies. To study the basal activity of the autophagic pathway, cells in complete medium (with serum) were untreated (controls) or treated with 20 mM NH4Cl or 20 mM NH4Cl in combination with 50 μM leupeptin (leup) for 24 h and processed for immunoblot analysis with anti-DJ-1, anti-LC3 or anti-IKappaBα antibodies. To study the effect of serum starvation on protein levels, exponentially growing cells were washed three times with HBSS with calcium and magnesium (Sigma, 55037) and incubated in serum free medium (serum starvation) for the times indicated. Cells were then cultured in the absence or in the presence of 20 mM NH4Cl, 20 mM NH4Cl and 50 μM Leup or 10 mM 3-MA. Afterwards cells were washed three times with cold PBS and lysed in SDS-Laemmli loading buffer without DTT and processed for Western and immunoblot analysis (see below).
Immunoblot analysis
After lysis, the samples were boiled for 5 min and loaded onto 10–14% SDS-PAGE (as required) and transfer to PVDF. Membranes were incubated with the corresponding primary antibodies (as indicated) and developed with anti-rabbit or anti-mouse peroxidase-labeled antibodies (1/5,000, Bio-Rad). Blots were imaged with a chemiluminiscent detector (MFChemiBIS 3.2, DNR Bio-Imaging Systems) and analyzed by quantitative densitometry using Totallab TL100 software. Protein levels were normalized respect to tubulin (protein loading control) and are expressed as mean ± s. e. m. from three different experiments.
RNA expression analysis
Total RNA from cells untreated or treated, as described above, was extracted by the using TRIzol reagent (Sigma). Isolated RNA was treated with DNase I (amplification grade from Invitrogen) to eliminate any remaining genomic DNA and inactivated. The RNA integrity was assessed by RNA chips using the Agilent 2100 Byoanalyzer. Afterwards, 1μg of total RNA was used for cDNA synthesis with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems) using random primers. Quantitative real time PCR (qRT-PCR) was performed in a 7900 HT Fast Real-Time PCR System with Fast SYBR Green Master Mix (Applied Biosystems) using the suggested standard protocol. For the target mRNAs (mouse and human DJ-1 mRNA) the following oligonucleotides were used: forward human DJ-1, 5’- CCATATGATGTGGTGGTTCTAC-3’; reverse human DJ-1, 5’-ACTTCCACAACCTATTTCATGAG-3’; forward mouse DJ-1, 5’- ATCTGAGTCGCCTATGGTGAAG-3’; reverse mouse DJ-1, 5’- ACCTACTTCGTGAGCCAACAG -3’. The oligonucleotides used for normalization were: human β-actin: forward, 5’- AGCCTCGCCTTTGCCGA-3’; reverse, 5’-CTGGTGCCTGGGGCG-3’ and mouse β-actin: forward, 5’-GACAGGATGCAGAAGGAGATTACTG-3’; reverse; 5’-GCTGATCCACATCTGCTGGAA-3’. Similar amplification efficiencies were obtained for target and reference mRNAs (100 ± 5%). Relative expression levels were analyzed by the ΔΔCT, as described [18]. Results are expressed as fold changes, taking the wild type cell (control) as a reference value of 1 (S2 Fig). The values presented are average from two independent experiments run in triplicates (technical replicas) with the corresponding range (minimum and maximum) indicated.
Results
DJ-1 steady-state protein levels in control and LAMP2 interrupted cell lines
If Lamp-2A is implicated in the degradation of DJ-1 by the CMA pathway as reported [12], it would be expected that the interruption of LAMP2 gene expression would produce an increase in DJ-1 protein levels. To test the above prediction, DJ-1 protein levels were measured in mouse embryonic fibroblasts (MEF) derived from control and LAMP2 KO mice [16], as well as in control (scrambled shRNA) and LAMP2 –deficient (shRNA for LAMP2) N2a cells obtained by stable transfection of scrambled and LAMP-2 shRNA, respectively [17]. Lack of Lamp-2(A) expression in these cell lines has been validated by using Lamp-2 and Lamp-2A specific antibodies [17], Disruption of Lamp-2 expression does not affect Lamp-1 protein expression, another abundant integral membrane lysosomal protein (see S1 Fig). We also used B- lymphoblastoid cell lines (B-LCL) derived from a healthy control and Danon disease’s patient [14], an X-linked human disease caused by mutations in LAMP2 gene.[19]. The data presented in Fig 1 show that no significant changes in DJ-1 protein levels were observed between controls and Lamp-2-deficient MEFs, N2a and B-LCL cells. Furthermore, we also found no significant changes in DJ-1 mRNA levels, as determined by qRT-PCR (see S2 Fig). These results reveal that DJ-1 steady state protein and mRNA levels are not significantly affected by lack of expression of Lamp-2.
Fig 1. Steady-state protein expression levels of DJ-1 in control and Lamp-2-deficient cell lines.
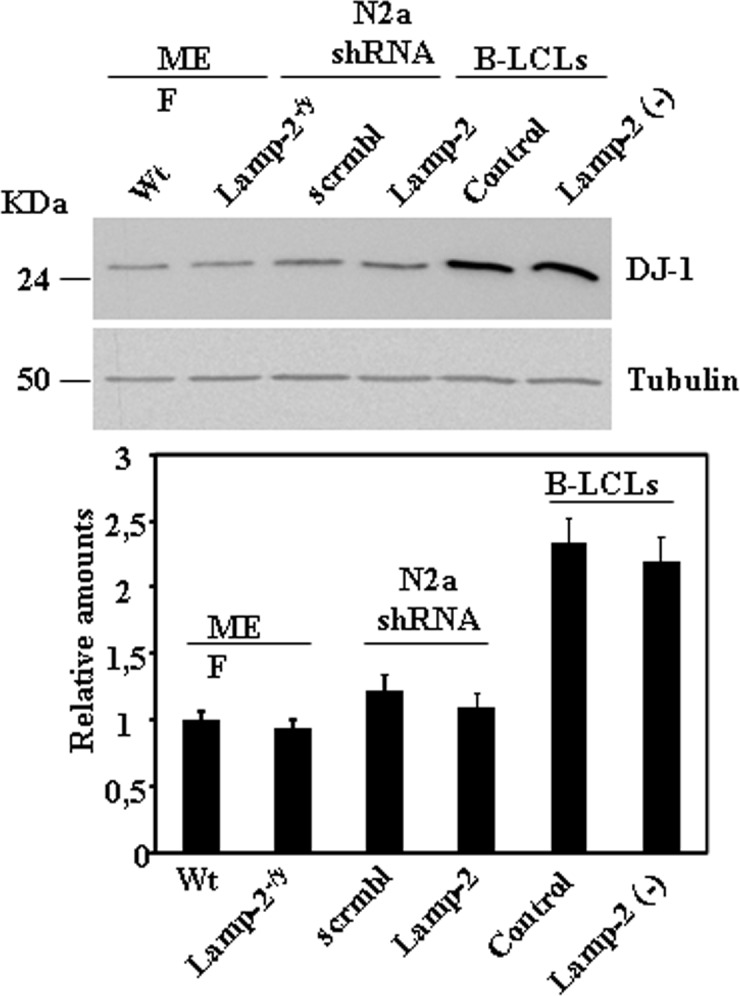
Total lysates from exponentially growing cells under basal culture conditions, control and Lamp-2-deficient MEF, N2a and B-LCLs cells were prepared and the levels of DJ-1 protein analysed by Western and immunoblot. Anti-tubulin antibodies were used as total protein loading control. Below is shown the graph of quantification of the corresponding immunoblots. Data are mean ± s. e. m. from at least three experiments. No significant difference in DJ-1 protein levels was found between controls and their corresponding Lamp-2 deficient cell lines.
Kinetic studies of DJ-1 stability after protein synthesis inhibition
The results shown above led us to test the stability of DJ-1 in the cell lines described above after protein synthesis inhibition with cycloheximide (CHX). In agreement with our previous results with N2a cells [10], treatment of these different cell lines with CHX up to 24h did not significantly change DJ-1 protein levels (Fig 2), indicating that the half-life of the DJ-1 protein is longer than 24 h. Similar results were obtained (S3 Fig) with other cell lines: HeLa, HEK293 and SN4741, the cell line used by Wang et al [12]. Under the same experimental conditions IKappaBα, used as a positive control, was degraded (S4 Fig).
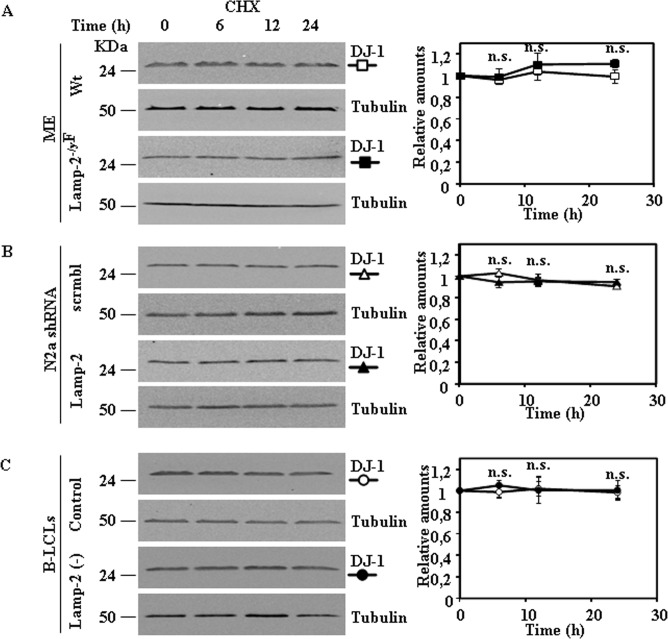
Fig 2. Stability of DJ-1 protein in control and Lamp-2-deficient cell lines after inhibition of protein synthesis.
Exponentially growing cells from control and Lamp-2-deficient cells were treated with cycloheximide (CHX) for the times indicated. Total cell lysates were prepared and DJ-1 protein levels were analysed by Western and immunoblot with specific antibodies. Anti-tubulin antibodies were used as total protein loading control. Panels show the results obtained for MEF (A), N2a (B) and B-LCLs (C), Graphs on the right show the quantification of the levels of DJ-1 protein respect to untreated cells as controls (time 0 h). Values are expressed as mean ± s.e.m. from three different experiments. n.s. not significant difference.
Effect of lysosomal inhibition on DJ-1 protein levels
Wang et al [12] reported that inhibition of lysosomal degradation by NH4Cl/leupeptin treatment (18hrs) leads to increased DJ-1 protein levels. This observation led the authors to conclude that DJ-1 is degraded in lysosomes. We aimed to reproduce those results in the same cell line (SN4741) used in their study, as well as, in other cell lines such as MEF obtained from wild type or Lamp-2-deficient mice, N2a cells either stably transfected with scrambled shRNA or LAMP-2-specific shRNA and in B-LCLs from a control or Danon disease’s patient. As shown in Fig 3, our data showed that after 24h of treatment with NH4Cl or NH4Cl/leupeptin, there is no significant change in DJ-1 protein levels in any of the cell lines studied, including SN4741 cells. In contrast and as expected, NH4Cl and NH4Cl/leupeptin treatments increased the levels of LC3-II, as expected due to autophagic inhibition (Fig 3).
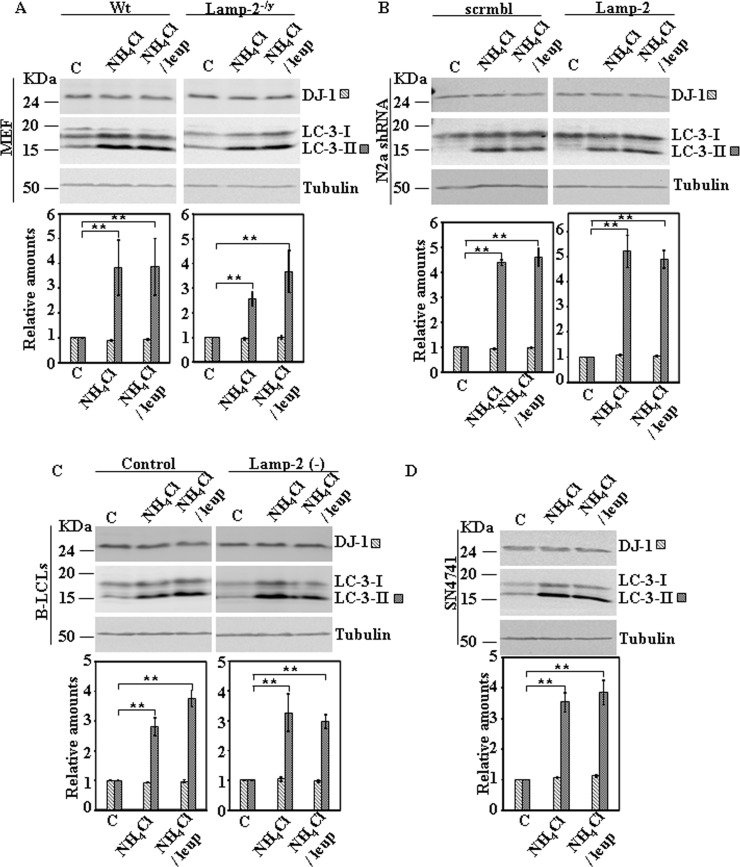
Fig 3. Protein expression levels of DJ-1 in control and Lamp-2-deficient cell lines in the presence of inhibitors of lysosomal function.
Exponentially growing cells from control and Lamp-2-deficient cells were incubated in complete medium r suplemented with 20mM NH4Cl or 20 mM NH4Cl in combination with 50 μM leupeptin (leup) for 24 h. Total cell lysates were analysed by Western and immunoblot with the corresponding specific antibodies: DJ-1 and LC-3. Anti-tubulin antibodies were used as total protein loading control. Panels show the results for MEF (A), N2a (B), B-LCLs (C) and SN4741 (D). Graphs below each panel show the quantification of the levels of the proteins analysed respect to the levels in cells kept in complete growth medium, controls. Values are expressed as mean ± s.e.m. from three different experiments. Significant differences between groups ** at p<0.01 by Student t-test are indicated.
Effect of CMA activation on DJ-1 protein levels
Next we studied the effect on DJ-1 protein levels of activation of CMA pathway by serum starvation. Serum starvation for 24h did not produce any change in DJ-1 protein levels in MEF obtained from wild type or Lamp-2-deficient mice (Fig 4). Similar results were obtained in N2a cells either stably transfected with scrambled shRNA or LAMP-2-specific shRNA (Fig 5). Also no significant changes in DJ-1 protein levels were found in B-LCLs from a control or Danon disease’s patient (Fig 6) [14], when starved for 8h. In the case of B-LCLs we could not apply 24h of serum starvation, since >60% cell died, as reported previously [14]. Finally, upon CMA activation we also did not observe any change of DJ-1 protein levels in SN4741 cells (Fig 7). The addition of either NH4Cl, NH4Cl/leupeptin or 3-MA during the starvation period did not modified DJ-1 protein levels in any of the cell lines studied (see Figs 4–7). As expected, those co-treatments during starvation resulted in the accumulation of LC3-II (NH4Cl, NH4Cl/leupeptin) and its inhibition in the presence of 3-MA (see also Figs 4–7). In contrast, under the same experimental conditions IKappaBα, used as a control, was degraded (serum starvation) and its degradation significantly prevented by co-treatment with NH4Cl, NH4Cl/leupeptin (S5 Fig).
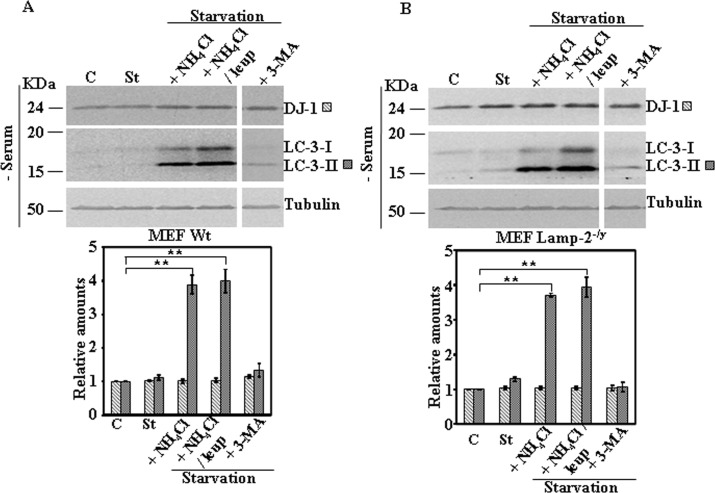
Fig 4. Protein expression levels of DJ-1 following activation of autophagy by serum starvation in control and Lamp-2-deficient MEF cells.
Exponentially growing control and Lamp-2-deficient MEF cells were kept in complete medium (C) or starved of serum for 24 h in the absence (St) or in the presence of NH4Cl, NH4Cl and leupeptin (leup), or 3-methyl adenine (3-MA). Panels A and B show the effect of serum starvation in MEF wild type (MEF Wt) and Lamp-2-deficient MEF (Lamp-2-/y) cells, respectively. Total cell lysates were analysed by Western and immunoblot with the corresponding specific antibodies, as indicated. Anti-tubulin antibodies were used as total protein loading control. Graphs below each panel show the quantification of the levels of the different proteins analysed respect to the levels in cells kept in complete growth medium, controls. Values are expressed as mean ± s.e.m. from three different experiments. Significant differences between groups ** at p<0.01 by Student t-test are indicated.
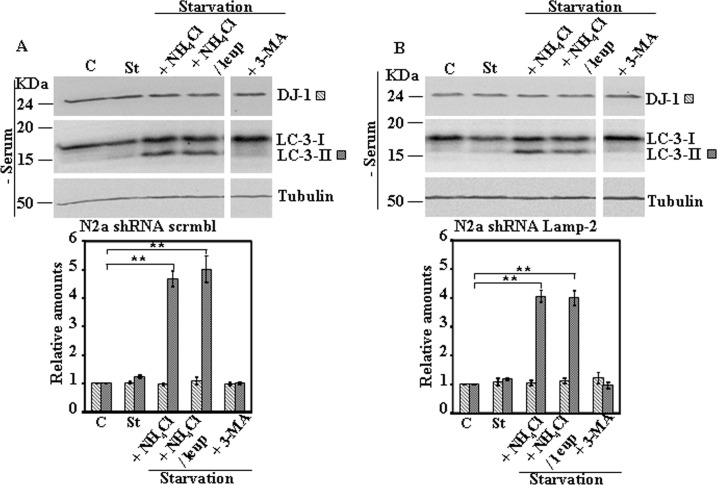
Fig 5. Protein expression levels of DJ-1 following activation of CMA by serum starvation in control and Lamp-2-deficient N2a cells.
Exponentially growing control and Lamp-2-deficient N2a cells were kept in complete medium (C) or starved of serum for 24 h in the absence (St) or in the presence of NH4Cl, NH4Cl and leupeptin (leup), or 3-methyl adenine (3-MA). Panels A and B show the effect of serum starvation in N2a shRNA scrmbl cells and in Lamp-2-deficient N2a shRNA Lamp-2 cells, respectively. Total cell lysates were analysed by Western and immunoblot with the corresponding specific antibodies. Anti-tubulin antibodies were used as total protein loading control. Graphs below each panel show the quantification of the levels of the different proteins analysed respect to the levels in cells kept in complete growth medium, controls. Values are expressed as mean ± s.e.m. from three different experiments. Significant differences between groups ** at p<0.01 by Student t-test are indicated.
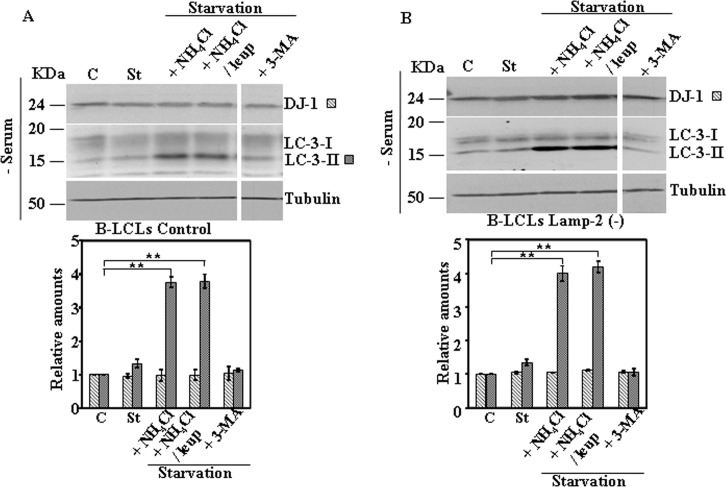
Fig 6. Protein expression levels of DJ-1 following activation of CMA by serum starvation in control and Lamp-2-deficient B-LCLs.
Exponentially growing control and Lamp-2-deficient B-LCL were kept in complete medium (C) or starved of serum (8h) in the absence (St) or in the presence of NH4Cl, NH4Cl and leupeptin (Leup), or 3-methyl adenine (3-MA). Panels A and B show the effect of serum starvatrion in B-LCLs control and Lamp-2 (-). Lamp-2-deficient B-LCL, respectively. Total cell lysates were analysed by Western and immunoblot with the corresponding specific antibodies, as indicated. Anti-tubulin antibodies were used as total protein loading control. Graphs below each panel show the quantification of the levels of the different proteins analysed respect to the levels in cells kept in complete growth medium, controls. Values are expressed as mean ± s.e.m. from three different experiments. Significant differences between groups ** at p<0.01 by Student t-test are indicated.
Fig 7. Protein expression levels of DJ-1 following activation of CMA by serum starvation in SN4741 cells.
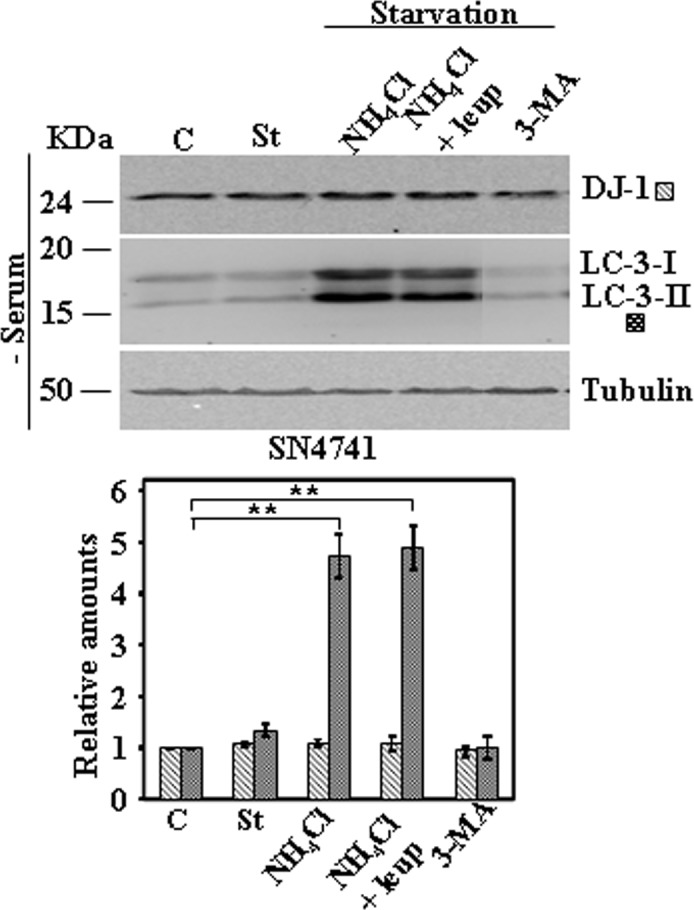
Exponentially growing SN4741 cells were kept in complete medium (C) or starved of serum for 24 h in the absence (St) or in the presence of NH4Cl, NH4Cl and leupeptin (Leup), or 3-methyl adenine (3-MA). Panels show the effect of serum deprivation in SN4741. Total cell lysates were analysed by Western and immunoblot with the corresponding specific antibodies, as indicated. Anti-tubulin antibodies were used as total protein loading control. Graphs below each panel show the quantification of the levels of the different proteins analysed respect to the levels in cells kept in complete growth medium, controls. Values are expressed as mean ± s.e.m. from three different experiments. Significant differences between groups ** at p<0.01 by Student t-test are indicated.
Discussion
The results presented here revealed that DJ-1 protein levels did not change in response to LAMP2 gene expression silencing (Fig 1) in different cell types (MEF, N2a and B-LCL). Also we showed that there was no difference in DJ-1 mRNA abundance between control and LAMP2 silenced cell lines (S2 Fig). As a consequence, the increased protein levels of DJ-1 reported by Wang et al [12] in SN4741 cells upon silencing LAMP2 gene expression can not be generalized to other cell types.
DJ-1 protein is a stable protein in the cell, here we have extended previous results to several cell lines, showing that treatment with CHX for 24h did not change the amount of DJ-1 protein in MEF from wild type or LAMP-2 knock-out mice, control N2a cells and interrupted with an shRNA for LAMP-2 or in B-LCL from control or from a patient with Danon disease. Similarly, no changes (S3 Fig) were observed in HeLa, HEK293 and in SN4741, the cell line used by Wang et al [12].
Treatment of cells with NH4Cl or NH4Cl/leupeptin resulted in no change in DJ-1 protein levels either in control cells or in LAMP2 interrupted cells mentioned above (Fig 3) and also no change was observed in SN4741 (Fig 3D). Furthermore, the results presented here show no change in DJ-1 protein levels by serum starvation for 24h, either in control or LAMP-2 interrupted cells (Figs 4–6) and in SN4741(Fig 7).
We have tried to find possible explanations for the discrepancy between our results and those of Wang et al [12]. We found differences in the buffer composition and procedure for cell lysis and protein extraction. In our hands, protein extraction using their buffer composition and procedure gave similar results to those presented here. Another difference is in the anti-DJ-1 antibody, they use Abcam, ab76008. We bought that antibody and in our hands gave similar results to Abcam ab18257 used in the present work, and we also obtained the same results using our in-house produced polyclonal anti-DJ-1 antibody [10]. As Wang al [12] only use SN4741, it could be that they are using a SN4741 cell line where DJ-1 protein for unknown reasons is more unstable.
Our results are in agreement with previous reported results on the stability of DJ-1 from typical biochemical studies [9] [11] [10]. Furthermore, quantitative proteomic studies using SILAC pulse-chase experiments and MS analysis also show that DJ-1 protein has a very long half-life (t1/2). For example; DJ-1 has a t1/2 = 187 h in mouse NIH3T3 cells [20]. The t1/2 values in HeLa and C2C12 cells are 59.2 h and 88h, respectively [21]. In vivo studies by deuterium labelling and MS also show that the t1/2 of DJ-1 in heart from mice varies from 199 to 299 h depending of the mouse strain under study [22]. Finally, recent proteomic studies by MS of the degradation of "young" (newly synthesized) and "old" (steady state) proteins in NIH3T3 report that DJ-1 degradation follows an exponential decay, exponential 1-state t1/2 = 150.26 h and steady-state 2-state model with a t1/2 = 217.1 h, similar exponential decay is observed in human RPE-1 cells with a t1/2 >300 h [23]. In all cases DJ-1 protein has a very long half-life, several days. These facts, together with the ubiquitous expression of DJ-1, are in agreement with a proposal to use DJ-1 peptides as reference for quantification of proteomic studies, being even better that commonly used peptides from house keeping proteins [24]. With a half-life of several days, even for the newly synthesized DJ-1 protein [23], the study of the pathway responsible for the degradation of DJ-1 is not easy to approach experimentally by applying treatments that can stimulate or inhibit its degradation. In particular, it could be that longer serum fasting times (> 24h) may be required to observe a significant change in DJ-1 protein levels. Unfortunately those experiments are not possible, because the cells (MEFs, N2a and B-LCL) used in this work do not survive longer fasts (36-48h). Using HeLa cells, that are more resistant, no change in DJ-1 protein levels were observed even after 48 h of serum starvation.
In conclusion, DJ-1 protein has a long half-life in the cell and we have not found any change in DJ-1 protein levels by inactivation of LAMP2 gene expression, inhibition of lysosomal function or activation of the CMA pathway by serum starvation. Accordingly, we have been unable to reproduce in other cell lines, or even in SN4741 cells, the observations reported by Wang et al [12] on the role of CMA in DJ-1 protein turn-over in their SN4741 cells.
Supporting information
Total lysates from exponentially growing control and Lamp2-deficient MEF (A, B) and N2a (C, D) cells were prepared and the expression levels of Lamp-2A (A, C) and Lamp-1 (B, D) membrane lysosomal proteins were analysed by Western and immunoblot with the corresponding specific antibodies, as indicated. Anti-tubulin antibodies were used as protein loading control. Below each panel is shown the graph of quantification of the corresponding immunoblots. Data are mean ± s. e. m. from at least three different experiments.
(TIF)
Exponentially growing control and Lamp-2-deficient MEF, N2a, B-LCLs cells were cultured in complete medium and total RNA was isolated and analyzed by qRT-PCR, as described under "Materials and methods". Graph shows the relative fold change using β-actin mRNA levels, as reference. Data are average from two experiments assayed by triplicate (technical replica), upper and lower values are represented by horizontal lines.
(TIF)
Exponentially growing HeLa (A), HEK (B) and SN4741 (C) cells were treated with cycloheximide (CHX) for the times indicated. Total cell lysates were prepared and DJ-1 protein levels were analyzed by Western and immunoblot with specific antibodies. Anti-tubulin antibodies were used as total protein loading control. Right graph shows the quantification of the levels of DJ-1 protein. Values are expressed as mean ± s.e.m. from three different experiments.
(TIF)
Exponentially growing cells from control and Lamp-2-deficient cells were treated with cycloheximide (CHX) for the times indicated. Total cell lysates were prepared and IKappaBα (Iκbα) protein levels were analysed by Western and immunoblot with specific antibodies. Anti-tubulin antibodies were used as total protein loading control. Panels show the results obtained with MEF, N2a, and B-LCLs and SN4741 cell lines, Graphs on the right side show the quantification of the levels of IKappaBα protein respect to their corresponding untreated cells as controls (time 0 h). Values are expressed as mean ± s.e.m. from three different experiments, no significant differences in degradation was found.
(TIF)
Exponentially growing control and Lamp-2-deficient cells and SN4741 were kept in complete medium (C) or starved of serum for 24 h in the absence (St) or in the presence of NH4Cl or NH4Cl and leupeptin (leup). Panel A shows the results obtained in MEF Wt cells and Lamp-2-deficient (Lamp-2-/y) cells. Panel B shows the results obtaine from N2a shRNA scrmbl cells and Lamp-2-deficient N2a shRNA Lamp-2 cells. Panel C shows the resutls obtained with SN4741 Total cell lysates were analysed by Western and immunoblot with the corresponding specific antibodies: as indicated. Anti-tubulin antibodies were used as total protein loading control. Graphs show the quantification of the levels of the different proteins analysed respect to the levels in cells kept in complete growth medium, controls. Values are expressed as mean ± s.e.m. from three different experiments. Significant differences between the indicated pairs analyzed by Student t-test are indicated by ** at p<0.01. and * p<0.05.
(TIF)
Acknowledgments
Special thanks to Drs. Judith Blanz and Paul Saftig from Institute of Biochemistry, Christian-Albrechts-Universität zu Kiel, Olshausenstrasse 40, D-24098 Kiel, Germany for providing us the MEF and N2a cell lines used in the present work and for critical reading of the manuscript. We thank José Luis Zugaza from Department of Genetics, Physical Anthropology and Animal Genetics, Faculty of Science and Technology, University of the Basque Country, UPV/EHU and Achucarro Basque Center for Neuroscience, Bilbao, Spain for providing us with the SN4741 cell line.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from MINECO SAF-2012-34556 and CIBERNED to JGC.
References
- 1.Nuytemans K, Theuns J, Cruts M, van Broeckhoven C Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum Mutat 2010; 31: 763–780. 10.1002/humu.21277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez DG, Reed X, Singleton AB Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J Neurochem 2016; 139 Suppl 1: 59–74. 10.1111/jnc.13593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao X, Tong L Crystal structure of human DJ-1, a protein associated with early onset Parkinson's disease. J Biol Chem 2003; 278: 31372–31379. 10.1074/jbc.M304221200 [DOI] [PubMed] [Google Scholar]
- 4.Honbou K, Suzuki NN, Horiuchi M, Niki T, Taira T, Ariga H et al. The crystal structure of DJ-1, a protein related to male fertility and Parkinson's disease. J Biol Chem 2003; 278: 31380–31384. 10.1074/jbc.M305878200 [DOI] [PubMed] [Google Scholar]
- 5.Wilson MA, Collins JL, Hod Y, Ringe D, Petsko GA The 1.1-A resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early onset Parkinson's disease. Proc Natl Acad Sci U S A 2003; 100: 9256–9261. 10.1073/pnas.1133288100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huai Q, Sun Y, Wang H, Chin LS, Li L, Robinson H et al. Crystal structure of DJ-1/RS and implication on familial Parkinson's disease. FEBS Lett 2003; 549: 171–175. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Kim SJ, Kim IK, Ko J, Jeong CS, Kim GH et al. Crystal structures of human DJ-1 and Escherichia coli Hsp31, which share an evolutionarily conserved domain. J Biol Chem 2003; 278: 44552–44559. 10.1074/jbc.M304517200 [DOI] [PubMed] [Google Scholar]
- 8.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A et al. Proteomics. Tissue-based map of the human proteome. Science 2015; 347: 1260419 347/6220/1260419 [pii]; 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- 9.Macedo MG, Anar B, Bronner IF, Cannella M, Squitieri F, Bonifati V et al. The DJ-1L166P mutant protein associated with early onset Parkinson's disease is unstable and forms higher-order protein complexes. Hum Mol Genet 2003; 12: 2807–2816. 10.1093/hmg/ddg304 [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Castelao B, Munoz C, Sanchez I, Goethals M, Vandekerckhove J, Castano JG Reduced protein stability of human DJ-1/PARK7 L166P, linked to autosomal recessive Parkinson disease, is due to direct endoproteolytic cleavage by the proteasome. Biochim Biophys Acta 2012; 1823: 524–533. S0167-4889(11)00312-0 [pii]; 10.1016/j.bbamcr.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 11.Moore DJ, Zhang L, Dawson TM, Dawson VL A missense mutation (L166P) in DJ-1, linked to familial Parkinson's disease, confers reduced protein stability and impairs homo-oligomerization. J Neurochem 2003; 87: 1558–1567. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Cai Z, Tao K, Zeng W, Lu F, Yang R et al. Essential control of mitochondrial morphology and function by chaperone-mediated autophagy through degradation of PARK7. Autophagy 2016; 1–14. 10.1080/15548627.2015.1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuervo AM, Wong E Chaperone-mediated autophagy: roles in disease and aging. Cell Res 2014; 24: 92–104. cr2013153 [pii]; 10.1038/cr.2013.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez-Lanzas R, Alvarez-Castelao B, Bermejo T, Ayuso T, Tunon T, Castano JG Protein degradation in a LAMP-2-deficient B-lymphoblastoid cell line from a patient with Danon disease. Biochim Biophys Acta 2016; 1862: 1423–1432. S0925-4439(16)30091-6 [pii]; 10.1016/j.bbadis.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R et al. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 2000; 406: 902–906. 10.1038/35022595 [DOI] [PubMed] [Google Scholar]
- 16.Eskelinen EL, Illert AL, Tanaka Y, Schwarzmann G+, Blanz J, von Figura K et al. Role of LAMP-2 in Lysosome Biogenesis and Autophagy. Molecular Biology of the Cell 2002; 13: 3355–3368. 10.1091/mbc.E02-02-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothaug M, Stroobants S, Schweizer M, Peters J, Zunke F, Allerding M et al. LAMP-2 deficiency leads to hippocampal dysfunction but normal clearance of neuronal substrates of chaperone-mediated autophagy in a mouse model for Danon disease. Acta Neuropathol Commun 2015; 3: 6 s40478-014-0182-y [pii]; 10.1186/s40478-014-0182-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmittgen TD, Livak KJ Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 19.Endo Y, Furuta A, Nishino I Danon disease: a phenotypic expression of LAMP-2 deficiency. Acta Neuropathol 2015; 129: 391–398. 10.1007/s00401-015-1385-4 [DOI] [PubMed] [Google Scholar]
- 20.Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J et al. Global quantification of mammalian gene expression control. Nature 2011; 473: 337–342. 10.1038/nature10098 [DOI] [PubMed] [Google Scholar]
- 21.Cambridge SB, Gnad F, Nguyen C, Bermejo JL, Kruger M, Mann M Systems-wide proteomic analysis in mammalian cells reveals conserved, functional protein turnover. J Proteome Res 2011; 10: 5275–5284. 10.1021/pr101183k [DOI] [PubMed] [Google Scholar]
- 22.Lau E, Cao Q, Ng DC, Bleakley BJ, Dincer TU, Bot BM et al. A large dataset of protein dynamics in the mammalian heart proteome. Sci Data 2016; 3: 160015 sdata201615 [pii]; 10.1038/sdata.2016.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McShane E, Sin C, Zauber H, Wells JN, Donnelly N, Wang X et al. Kinetic Analysis of Protein Stability Reveals Age-Dependent Degradation. Cell 2016; 167: 803–815. S0092-8674(16)31248-X [pii]; 10.1016/j.cell.2016.09.015 [DOI] [PubMed] [Google Scholar]
- 24.Wisniewski JR, Mann M A Proteomics Approach to the Protein Normalization Problem: Selection of Unvarying Proteins for MS-Based Proteomics and Western Blotting. J Proteome Res 2016; 15: 2321–2326. 10.1021/acs.jproteome.6b00403 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total lysates from exponentially growing control and Lamp2-deficient MEF (A, B) and N2a (C, D) cells were prepared and the expression levels of Lamp-2A (A, C) and Lamp-1 (B, D) membrane lysosomal proteins were analysed by Western and immunoblot with the corresponding specific antibodies, as indicated. Anti-tubulin antibodies were used as protein loading control. Below each panel is shown the graph of quantification of the corresponding immunoblots. Data are mean ± s. e. m. from at least three different experiments.
(TIF)
Exponentially growing control and Lamp-2-deficient MEF, N2a, B-LCLs cells were cultured in complete medium and total RNA was isolated and analyzed by qRT-PCR, as described under "Materials and methods". Graph shows the relative fold change using β-actin mRNA levels, as reference. Data are average from two experiments assayed by triplicate (technical replica), upper and lower values are represented by horizontal lines.
(TIF)
Exponentially growing HeLa (A), HEK (B) and SN4741 (C) cells were treated with cycloheximide (CHX) for the times indicated. Total cell lysates were prepared and DJ-1 protein levels were analyzed by Western and immunoblot with specific antibodies. Anti-tubulin antibodies were used as total protein loading control. Right graph shows the quantification of the levels of DJ-1 protein. Values are expressed as mean ± s.e.m. from three different experiments.
(TIF)
Exponentially growing cells from control and Lamp-2-deficient cells were treated with cycloheximide (CHX) for the times indicated. Total cell lysates were prepared and IKappaBα (Iκbα) protein levels were analysed by Western and immunoblot with specific antibodies. Anti-tubulin antibodies were used as total protein loading control. Panels show the results obtained with MEF, N2a, and B-LCLs and SN4741 cell lines, Graphs on the right side show the quantification of the levels of IKappaBα protein respect to their corresponding untreated cells as controls (time 0 h). Values are expressed as mean ± s.e.m. from three different experiments, no significant differences in degradation was found.
(TIF)
Exponentially growing control and Lamp-2-deficient cells and SN4741 were kept in complete medium (C) or starved of serum for 24 h in the absence (St) or in the presence of NH4Cl or NH4Cl and leupeptin (leup). Panel A shows the results obtained in MEF Wt cells and Lamp-2-deficient (Lamp-2-/y) cells. Panel B shows the results obtaine from N2a shRNA scrmbl cells and Lamp-2-deficient N2a shRNA Lamp-2 cells. Panel C shows the resutls obtained with SN4741 Total cell lysates were analysed by Western and immunoblot with the corresponding specific antibodies: as indicated. Anti-tubulin antibodies were used as total protein loading control. Graphs show the quantification of the levels of the different proteins analysed respect to the levels in cells kept in complete growth medium, controls. Values are expressed as mean ± s.e.m. from three different experiments. Significant differences between the indicated pairs analyzed by Student t-test are indicated by ** at p<0.01. and * p<0.05.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.