 Arecoline is the key component of areca nut and has been suggested as a carcinogenic agent.
Arecoline is the key component of areca nut and has been suggested as a carcinogenic agent.
Abstract
Arecoline is the key component of areca nut and has been suggested as a carcinogenic agent. In the present study, the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg9 were allowed to feed on a diet having 5, 10, 20, 40 and 80 μM arecoline for 24 h. After the completion of 24 h, the larvae were subjected to ONPG assay, X-gal staining, trypan blue exclusion test, oxidative stress markers, and apoptotic and comet assays. A dose-dependent increase in the β-galactosidase activity, tissue damage, glutathione-S-transferase (GST) activity, lipid peroxidation assay, monoamine oxidase (MAO), caspase-9 and 3, protein carbonyl content (PCC), apoptotic index, and DNA damage and decrease in glutathione (GSH) content, delta aminolevulinic acid dehydrogenase (δ-ALA-D), and acetylcholinesterase (AChE) activity were observed in the larvae exposed to 20, 40 and 80 μM arecoline. The results suggest that arecoline is toxic at 20, 40, and 80 μM toward the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg9. Arecoline did not show any toxic effects at 5 and 10 μM.
1. Introduction
Arecoline (natural alkaloid) is obtained from the betel nut (areca nut) as dried seeds of Areca catechu found in India and many other Asian countries. Betel nut is chewed as a psychostimulant and also for its digestive properties.1 In India and some other countries, betel nuts are consumed with catechin, tobacco and betel leaves in the form of paan.2 According to one study, 10% of the world population chew areca nut and it is the fourth most widely used addictive substance in the world.3 The post-consumption effect of areca nut includes alertness, tachycardia, cholinergic activation and euphoria.4 It also increases the skin temperature and plasma concentration of adrenaline and noradrenaline. Regular long-term areca users were found to have cardiac arrhythmias, acute psychosis, asthma exacerbation and oropharyngeal tumors.5 Studies on human population have shown that areca nut is a major etiological factor of oral submucous fibrosis and oral cancer.6 Arecoline is a parasympathomimetic tertiary amine which acts on both nicotinic and muscarinic receptors and therefore has been used against the symptoms of schizophrenia.7 It has been reported to be genotoxic in both mammalian as well as bacterial test systems.8 It induced unscheduled DNA synthesis, as well as DNA breaks leading to submucous fibrosis, leukoplakia, and squamous cell carcinoma.9 It induced cancer of the oral cavity, pharynx, esophagus and liver.2 Reactive oxygen species (ROS) are produced when areca nut constituents are auto-oxidized under alkaline conditions.10 The toxic effects of arecoline on the central nervous system are still unknown even though arecoline has shown an apprehension enhancing the effect.11 It induced oxidative stress and affects the antioxidant system and induces liver toxicity and cell death.12 Cytotoxicity has also been demonstrated in oral KB epithelial cells and buccal epithelial cells by the exposure of arecoline.9,13 It enters into the body through the buccal and sublingual mucosa.14 Earlier studies have indicated that arecoline treatment also results in the reduction of superoxide dismutase (SOD) activities and glutathione (GSH) levels.15
Drosophila melanogaster is widely used for the toxicological evaluation of various chemicals due to the presence of about 50% homology with mammalian proteins. Nowadays there has been a world-wide effort to reduce the use of higher animals in toxicological research and testing. Drosophila melanogaster is a well-established alternative in vivo model and is being successfully used for screening of various environmental agents. Stress response (heat shock protein) and the antioxidant defence system are the primary protective responses that are highly conserved components of cellular stress responses found in all phyla from bacteria to man.16 Among the stress gene family, hsp70 is one of the highly conserved genes and first to be induced in Drosophila and other organisms.17 It has been reported that hsp-70 induction by certain environmental chemicals is generally correlated with cytotoxic events.17 Elevated levels of hsp70 have been reported in the cells of transgenic larvae exposed to several chemicals.17 In the present study we decided to evaluate the toxic potential of arecoline toward the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg9. Before performing the experiments the LC 50 was calculated for the third instar larvae of Drosophila by exposing them to 50, 100, 200, 300, 400, 500 and 600 μM of arecoline in a similar way to that performed by Fatima et al. (2017)18 for cyclophosphamide and tangeritin. 50% mortality was observed at 500 μM of arecoline. In our present study, the highest studied dose is less than ¼th of the LC 50 value.
2. Materials and methods
2.1. Fly strain
A transgenic Drosophila melanogaster line that expresses bacterial β-galactosidase as a response to stress was used in the present study.19 In this strain of flies, the transformation vector is inserted with a P-element, i.e. the line contains a wild-type hsp70 sequence up to the lacZ fusion point. The flies and larvae were cultured on standard Drosophila food containing agar, corn meal, sugar and yeast at 24 ± 1 °C.20 Arecoline hydrobromide (Sigma, USA) was dissolved in the diet and final concentrations of 5, 10, 20, 40 and 80 μM were established. The third instar larvae were allowed to feed on it for 24 h before performing the assays.
2.2. Soluble O-nitrophenyl-β-d-galactopyranoside (ONPG) assay
The expression of hsp70 provides a measurement of cytotoxicity.21 The method described by Nazir et al. (2003) was used in this study.22 After washing in phosphate buffer, the larvae were placed in microcentrifuge tubes (30 larvae per tube, 5 replicates per group), permeabilized for 10 min with acetone, and incubated overnight at 37 °C in 600 μl of ONPG staining buffer. Following incubation, the reaction was stopped by adding 300 μl of Na2CO3. The extent of the reaction was quantified by measuring the absorbance at 420 nm.
2.3. In situ histochemical β-galactosidase activity
The larvae (10 larvae per treatment; 5 replicates per group) were dissected out in Poels’ Salt Solution (PSS) and X-gal staining was performed using the method as described by Chowdhuri et al. (1999).21 The tissue explants were fixed in 2.5% glutaraldehyde, washed in 50 mM sodium phosphate buffer (pH 8.0) and stained overnight in X-gal staining solution at 37 °C in the dark.
2.4. Trypan blue exclusion test
The extent of tissue damage in larvae caused by the exposure to different concentrations of arecoline was assayed by a dye exclusion test.22,23 Briefly, the internal tissues of the larvae were explanted in a drop of Poel's salt solution (PSS), washed in phosphate buffer saline (PBS), stained in trypan blue (0.2 mg ml–1 in PBS) for 30 min, washed thoroughly in PBS, and scored immediately for dark blue staining. The scoring for the trypan blue staining was done on an average composite index per larvae: no color = 0; any blue = 1; darkly stained = 2; large patches of darkly stained cells = 3; or complete staining of most cells in the tissue = 4.23
2.5. Preparation of homogenate for biochemical assays
The larvae (50 larvae per treatment; 5 replicates per group) were homogenized in 1 ml of cold homogenizing buffer (0.1 M phosphate buffer containing 0.15 M KCl; pH 7.4). The supernatant after centrifugation at 9000g was used for the determination of glutathione content (GSH), protein carbonyl content (PCC), lipid peroxidation assay (LPO), glutathione-S-transferase (GST) activity, acetylcholinesterase (AChE), monoamine oxidase (MAO) and delta-aminolevulinic acid dehydrogenase (ALA-D) activities.
2.5.1. Estimation of glutathione (GSH) content
The glutathione (GSH) content was estimated colorimetrically using Ellman's reagent according to the procedure described by Jollow et al. (1974).24 The supernatant was precipitated with 4% sulphosalicylic acid (4%) in the ratio of 1 : 1. The samples were kept at 4 °C for 1 h and then subjected to centrifugation at 4200 rpm for 10 min at 4 °C. The assay mixture consisted of 550 μl of 0.1 M phosphate buffer, 100 μl of the supernatant and 100 μl of 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB). The OD was read at 412 nm and the results were expressed as μ moles of GSH/gram tissue.
2.5.2. Determination of glutathione-S-transferase (GST) activity
The glutathione-S-transferase (GST) activity was determined by the method of Habig et al. (1974).25 The reaction mixture consists of 500 μl of 0.1 M phosphate buffer, 150 μl of 10 mM 1-chloro-2-4,dinitrobenzene (CDNB), 200 μl of 10 mM reduced glutathione and 50 μl of the supernatant. The OD was taken at 340 nm and the enzyme activity was expressed as μmoles of CDNB conjugatesper min per mg per protein.
2.5.3. Lipid peroxidation assay
Lipid peroxidation was measured according to the method described by Ohkawa et al. (1978).26 The reaction mixture consisted of 5 μl of 10 mM butyl-hydroxy toluene (BHT), 200 μl of 0.67% thiobarbituric acid, 600 μl of 1% O-phosphoric acid, 105 μl of distilled water and 90 μl of the supernatant. The resultant mixture was incubated at 90 °C for 45 min and the OD was measured at 535 nm. The results were expressed as μ moles of TBARS formed/h/gram tissue.
2.5.4. Protein carbonyl content
The protein carbonyl content was estimated according to the protocol described by Hawkins et al. (2009).27 The homogenate was diluted to a protein concentration of approximately 1 mg ml–1. About 250 μl of each diluted homogenate was taken in eppendorf centrifuge tubes separately. To it 250 μl of 10 mM 2,4-dinitrophenyl hydrazine (dissolved in 2.5 M HCl) was added, vortexed and kept in the dark for 20 min. About 125 μl of 50% (w/v) trichloroacetic acid (TCA) was added, mixed thoroughly and incubated at –20 °C for 15 min. The tubes were then centrifuged at 4 °C for 10 min at 9000 rpm. The supernatant was discarded and the pellets obtained were washed twice with ice-cold ethanol : ethylacetate (1 : 1). Finally the pellets were re-dissolved in 1 ml of 6 M guanidine hydrochloride and the absorbance was read at 370 nm.
2.5.5. Determination of acetylcholinesterase activity
Acetylcholinesterase activity was estimated according to the method of Ellman et al. (1961).28 The reaction mixture consisted of 650 μl of 0.1 M phosphate buffer (pH 7.4), 100 μl of 10 mM 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) and 100 μl of the supernatant. The content was mixed thoroughly and the absorbance was measured at 412 nm. After obtaining a stable absorbance value, 10 μl of 0.075 M acetylthiocholine was added and the change in the absorbance per minute was recorded to calculate the enzyme activity.
2.5.6. Estimation of monoamine oxidase (MAO)
The method described by McEwen (1965) was used to estimate the monoamine oxidase activity.29 The assay mixture consisted of 400 μL of 0.1 M phosphate buffer (pH 7.4.), 1300 μL of distilled water, 100 μL of benzylamine hydrochloride and 200 μL of brain homogenate. The assay mixture was incubated for 30 min at room temperature and then 1 mL of 10% perchloric acid was added and centrifuged at 1500g for 10 min. The OD was taken at 280 nm.
2.5.7. Determination of delta-aminolevulinic acid dehydrogenase (δ-ALA-D)
The δ-ALA-D activity was assayed using the method as described by Abolaji et al. (2014), by measuring the rate of porphobilinogen formation.30 The reaction mixture consisting of 10 μl of 0.5 M Tris-glycine (pH 8.5), 7 μl of distilled water, 25 μl of the homogenate and 8 μl of 31.25 mM 5-aminolevulinic acid hydrochloride was incubated at 37 °C for 3 h. Subsequently, 10% tricarboxylic acid containing 20 mM CuSO4 was added to each reaction mixture and centrifuged at 4500g for 10 min. After this, 100 μl from each of the supernatant obtained was added to 100 μl of Ehrlich's reagent and incubated for 30 min at room temperature. The absorbance was read at 555 nm.
2.6. Assays to detect apoptosis
2.6.1. Spectrophotometric assay for caspase-9 (Dronc) and caspase-3 (Drice) activities
The assay was performed according to the manufacturer's protocol with some modification (Bio-Vision, CA, USA). The assay was based on the spectrophotometric detection of the chromophore p-nitroanilide (pNA) obtained after the specific action of caspase-3 and caspase-9 on tetrapeptide substrates, DEVD-pNA and LEHD-pNA, respectively. The assay mixture consisted of 50 μl of cell suspension and 50 μl of chilled cell lysis buffer incubated on ice for 10 min. After incubation, 50 μl of 2× reaction buffer (containing 10 mM DTT) with 200 μM substrate (DEVD-pNA for Drice and IETD-pNA for Dronc) was added and incubated at 37 °C for 1.5 h. The reaction was quantified at 405 nm.
2.6.2. Assay to detect apoptosis in midgut cells of the larvae
The apoptotic cells were analyzed by staining with ethidium bromide (EB) and acridine orange (AO). The midgut of the larvae was explanted in PSS. The PSS was replaced with 300 μl of collagenase (0.5 mg ml–1) and kept for 15 min at 25 °C. The collagenase was removed and the pellet was washed three times with PBS with gentle shaking.31 Finally, the pellet was suspended in 80 μl of PBS. About 25 μl of cell suspension was mixed with 2 μl of EB/AO dye. The staining dye was prepared by dissolving 100 μg ml–1 AO and 100 μg ml–1 EB in PBS. About 100 cells were scored per treatment (5 replicates per group) for estimating the apoptotic index and expressed in percent.
2.6.3. Acridine orange staining of eye imaginal discs
For the detection of apoptosis in eye discs, the eye discs from the third instar larvae were dissected in Poels’ salt solution (PSS) and stained according to the method described by Lakhotia and Tapadia (1998).32 Eye discs were incubated in 1 μg ml–1 acridine orange (Sigma) solution in PBS. After 3 min of incubation the eye discs were washed three times with PSS and were immediately observed under a fluorescence microscope (Optika, Italy).
2.7. Analysis of DNA damage by comet assay
The comet assay was performed according to Mukhopadhyay et al. (2004).33 The midgut from 20 larvae per treatment (3 replicates per group) was explanted in PSS. PSS in the microcentrifuge tube was replaced with 300 μl of collagenase (0.5 mg ml–1 in PBS, pH 7.4) and kept for 15 min at 25 °C. The cell suspension was prepared by washing three times in PBS and finally the cells were suspended in 80 μl of PBS. The cell viability was checked by performing trypan blue assay before beginning the experiment.34 About 75 μl of cell suspension was mixed with 80 μl of 1.5% low melting agarose and layered on top of the precoated slides with 1% normal melting point agarose. The slides were then immersed in freshly prepared chilled lysing solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris pH 10.0 and 1% Triton X-100, pH 10) for 2 h at 4 °C. The slides were then transferred to the chilled electrophoresis solution (1 mM Na2 EDTA and 300 mM NaOH, pH > 13). The slides were left in this solution for 10 min to allow DNA unwinding. Electrophoresis was conducted for 15 min at 0.7 V cm–1 and 300 mA at 4 °C. Following electrophoresis, the slides were washed with neutralizing buffer (0.4 M Tris buffer) three times. The slides were then stained with ethidium bromide (20 μg ml–1; 75 μl per slides) for 10 min in the dark. The slides were then dipped in chilled distilled water to remove the excess of stain and subsequently cover slips were placed over them. Each experiment was performed in triplicate and the slides were prepared in duplicate. Twenty-five cells per slide were randomly captured at a constant depth of the gel, and the mean tail length (a.u.) was calculated to measure DNA damage by using comet score 1.5 software (Comet Score™ v1.5 Software, TriTek Corporation, Sumerduck).
2.8. Statistical analysis
All data were expressed as mean ± standard error and the Dunnet ANOVA test was used for the analysis by using software SPSS 16. The statistical significance was considered at 5% level.
3. Results
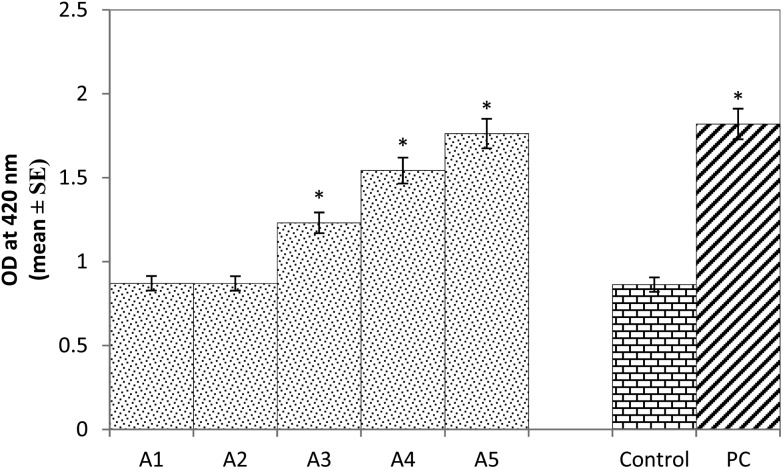
The results obtained for β-galactosidase activity showed a 1.42, 1.78 and 2.04 fold significant dose-dependent increase in the activity of β-galactosidase compared to the control in the larvae exposed to 20, 40, and 80 μM of arecoline (Fig. 1; p < 0.05). No significant increase in the activity of β-galactosidase was observed in the larvae exposed to 5 and 10 μM of arecoline compared to the control (Fig. 1; p < 0.05). The positive control was associated with a 2.10 fold of significant increase in the activity of β-galactosidase compared to the control (Fig. 1; p < 0.05).
Fig. 1. β-Galactosidase activity measured in the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg9 exposed to various doses of arecoline for 24 h [A1 = 5 μM; A2 = 10 μM; A3 = 20 μM; A4 = 40 μM, A5 = 80 μM; A = arecoline; PC = positive control (0.001 μl ml–1 methyl methanesulfonate)] *significant at p < 0.05 compared to the control.
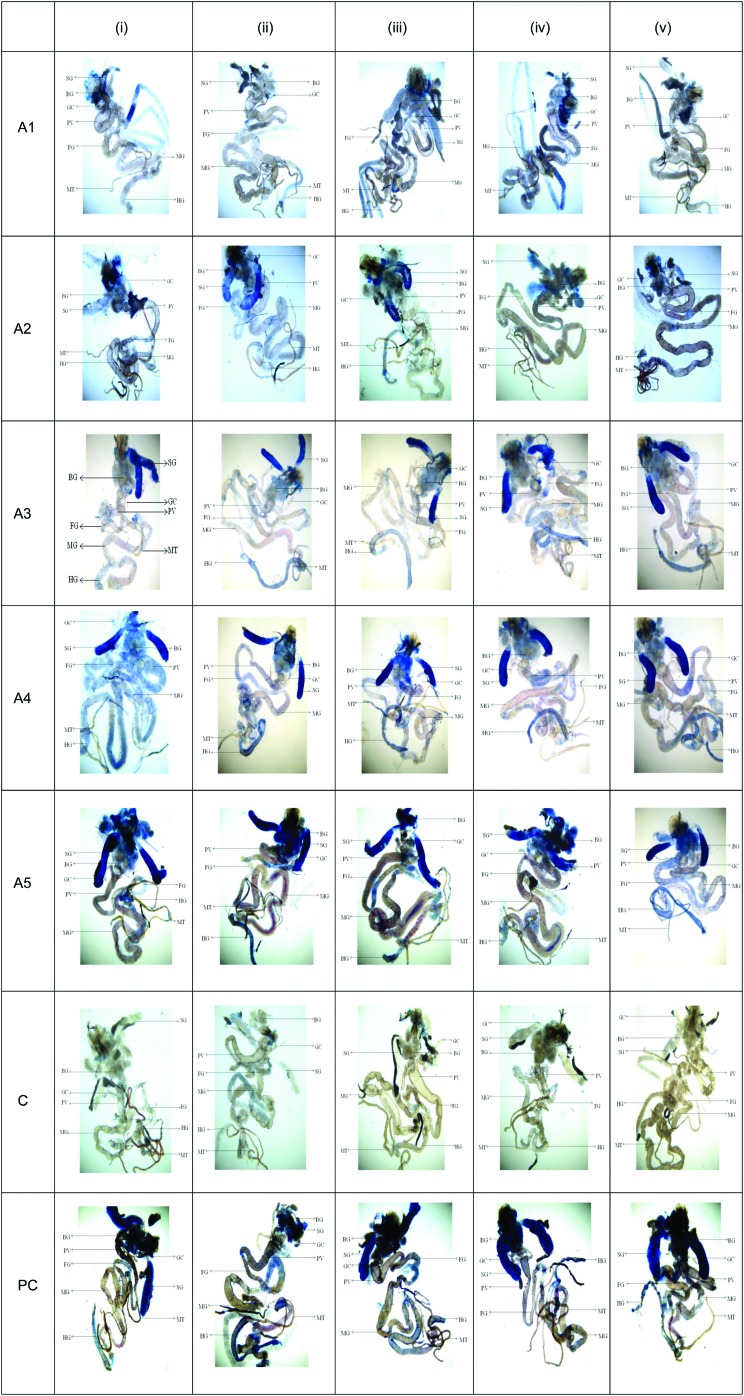
The results obtained for X-gal staining showed a clear dose-dependent increase in the expression of β-galactosidase in the larvae exposed to 20, 40 and 80 μM of arecoline Fig. 2, (A3–A5) compared to the control (Fig. 2, C). The larvae exposed to 20 μM of arecoline showed the β-galactosidase activity in the proventriculus and the midgut region (Fig. 2, A3). The larvae exposed to 40 μM of arecoline showed the β-galactosidase activity in the proventriculus, foregut and midgut (Fig. 2, A4). The larvae exposed to 80 μM of arecoline showed more intense staining in the entire gut region compared to the control (Fig. 2, A5). Intense staining was observed in the gut region (foregut, midgut and hindgut). The larvae did not show significant staining at the exposure of 5 and 10 μM of arecoline compared to the positive control (Fig. 2, A1 & A2).
Fig. 2. X-gal staining in the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg9 after the exposure to various doses of arecoline for 24 h. [A1 = 5 μM; A2 = 10 μM; A3 = 20 μM; A4 = 40 μM; A5 = 80 μM; C = control; PC = positive control (0.001 μl ml–1 methyl methanesulfonate)] [BG – brain ganglia, SG – salivary gland, PV – proventriculus, FG – foregut, MG – midgut, HG – hindgut, MT – Malpighian tubules, and GC – gastric caeca].
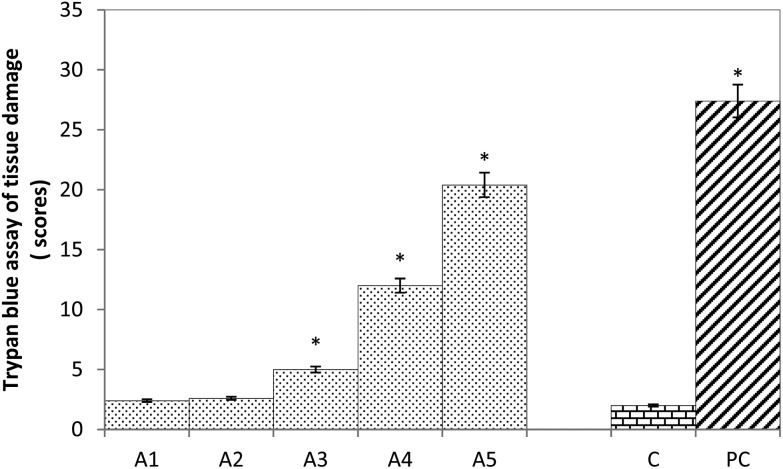
The results obtained for trypan blue staining are shown in Fig. 3. The larvae exposed to 5 and 10 μM of arecoline showed no significant tissue damage, (Fig. 3; A1, i–v & A2, i–v). It is also evident by the quantification of tissue damage (Fig. 4). The tissue damage in the larvae was in concordance with an increase in the dose of arecoline (Fig. 3; A3, i–v and A4, i–v) compared to the control (Fig. 3; C, i–v). The maximum damage was observed in the salivary glands, brain ganglia, midgut, hindgut and Malpighian tubules (Fig. 3; A5, i–v). The larvae exposed to 20, 40 and 80 μM of arecoline showed 2.5, 6.0 and 10.2 fold significant tissue damage compared to the control (Fig. 4; p < 0.05).
Fig. 3. Trypan blue staining in the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg9 after the exposure to various doses of arecoline for 24 h. [A1 = 5 μM; A2 = 10 μM; A3 = 20 μM; A4 = 40 μM; A5 = 80 μM; C = control; PC = positive control (0.001 μl ml–1 methyl methanesulfonate)] [BG – brain ganglia, SG – salivary gland, PV – proventriculus, FG – foregut, MG – midgut, HG – hindgut, MT – Malpighian tubules, and GC – gastric caeca].
Fig. 4. Scoring of trypan blue (Tb) positive areas. The graph represents scores for tissue damage in the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg9 exposed to various doses of arecoline for 24 h [A1 = 5 μM; A2 = 10 μM; A3 = 20 μM; A4 = 40 μM, A5 = 80 μM; A = Aarecoline; C = control; PC = positive control (0.001 μl ml–1 methyl methanesulfonate)] *significant at p < 0.05 compared to the control.
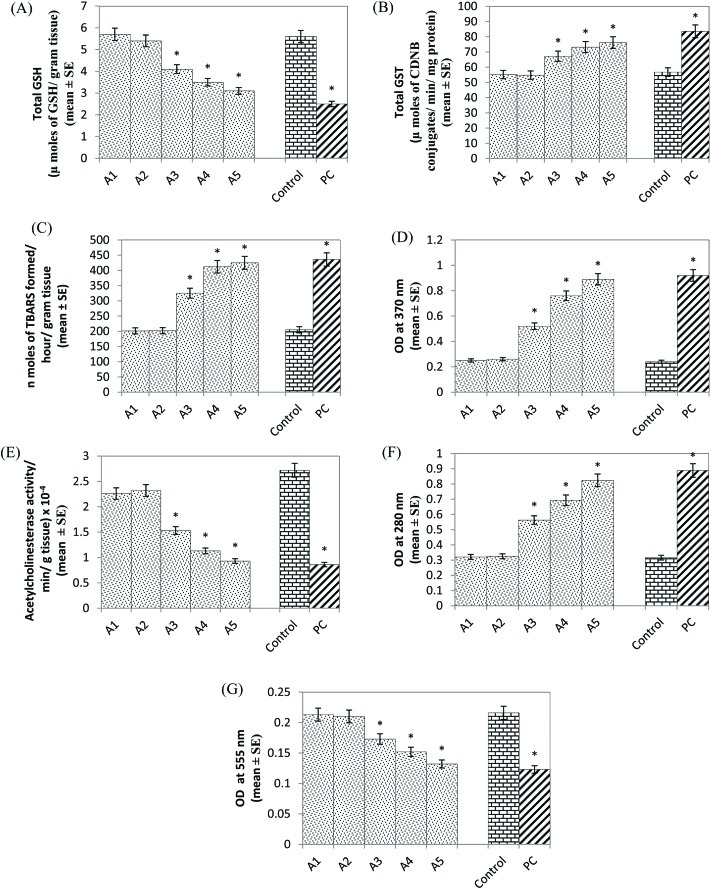
A significant decrease of 1.36, 1.60 and 1.80 fold GSH content was observed in the larvae exposed to 20, 40 and 80 μM of arecoline, respectively, compared to the control (Fig. 5A, p < 0.05). No significant decrease in the GSH content was observed in the larvae exposed to 5 and 10 μM of arecoline (Fig. 5A; p < 0.05). A significant dose-dependent increase of 1.18, 1.28 and 1.34 fold was observed in the GST activity in the larvae exposed to 20, 40 and 80 μM of arecoline, respectively, compared to the control (Fig. 5B; p < 0.050). No significant increase in the GST activity was observed in the larvae exposed to 5 and 10 μM of arecoline (Fig. 5B; p < 0.05). The results obtained for lipid peroxidation showed a significant dose-dependent increase of 1.58, 2.0 and 2.07 fold in the larvae exposed to 20, 40 and 80 μM arecoline, respectively, compared to the control (Fig. 5C; p < 0.05). The LPO was not increased significantly in the larvae exposed to 5 and 10 μM arecoline (Fig. 5C; p < 0.05). A significant dose-dependent increase of 2.16, 3.16 and 3.70 fold in the protein carbonyl content was observed in the larvae exposed to 20, 40 and 80 μM arecoline, respectively, compared to the control (Fig. 5D; p < 0.05). No significant increase in protein carbonyl content was observed in the larvae exposed to 5 and 10 μM of arecoline (Fig. 5D; p < 0.05). The larvae exposed to 20, 40 and 80 μM arecoline showed a significant dose-dependent decrease of 1.77, 2.40 and 2.92 fold in the acetyl cholinesterase activity, respectively, compared to the control (Fig. 5E; p < 0.05). No significant decrease in the activity of acetyl cholinesterase was observed in the larvae exposed to 5 and 10 μM arecoline (Fig. 5E; p < 0.05). A significant increase of 1.78, 2.19 and 2.60 fold was observed in the activity of monoamine oxidase in the larvae exposed to 20, 40 and 80 μM arecoline, respectively, compared to the control (Fig. 5F; p < 0.05). Monoamine oxidase activity was not increased significantly in the larvae exposed to 5 and 10 μM of arecoline (Fig. 5F; p < 0.05). A significant dose-dependent decrease of 1.24, 1.42 and 1.63 fold was observed in the activity of δ-aminolevulinic acid (ALA-D) in the larvae exposed to 20, 40 and 80 μM arecoline, respectively, compared to the control (Fig. 5G; p < 0.05). No significant decrease in the activity was observed in the larvae exposed to 5 and 10 μM arecoline (Fig. 5G; p < 0.05).
Fig. 5. Effect of arecoline on the glutathione (GSH) content (A), glutathione-S-transferase (GST) activity (B), lipid peroxidation (C), protein carbonyl content (D), acetylcholinesterase activity (E), monoamine oxidase activity (F) and ALA-D (delta-aminolaevulinic acid dehydratase) activity (G) measured in the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg9 exposed to various doses of arecoline for 24 h [A1 = 5 μM; A2 = 10 μM; A3 = 20 μM; A4 = 40 μM; A5 = 80 μM; A = arecoline; PC = positive control (0.001 μl ml–1 methyl methanesulfonate)] *significant at p < 0.05 compared to the control.
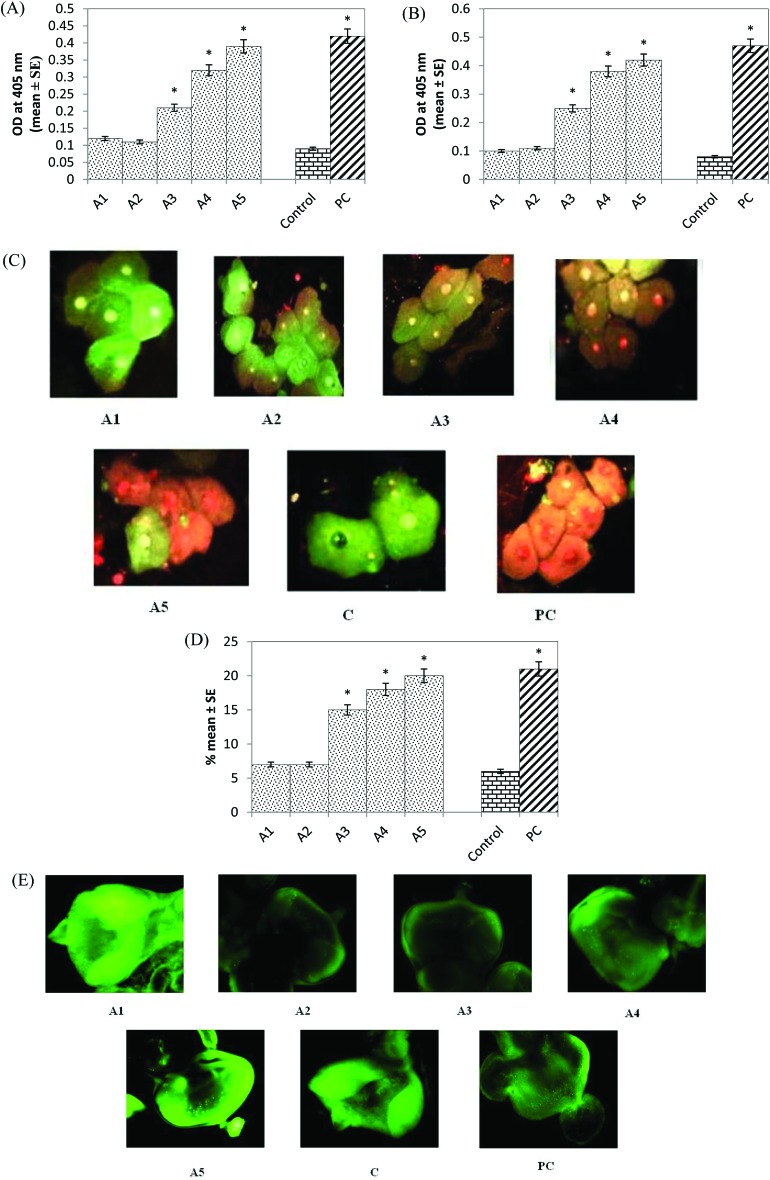
Caspase-9 activity was significantly increased by 2.33, 3.55 and 4.33 fold in the larvae exposed to 20, 40 and 80 μM arecoline, respectively, compared to the control (Fig. 6A; p < 0.05). No significant increase in the activity of caspase-9 was observed in the larvae exposed to 5 and 10 μM arecoline (Fig. 6A; p < 0.05). Caspase-3 activity also showed a dose-dependent increase of 3.12, 4.75 and 5.25 fold in the larvae exposed to 20, 40 and 80 μM arecoline, respectively, compared to the control (Fig. 6B; p < 0.05). No significant increase of caspase-3 activity was observed in the larvae exposed to 5 and 10 μM arecoline (Fig. 6B; p < 0.05). The normal and apoptotic midgut cells are shown in Fig. 6C (A1-PC), and the results obtained for the apoptotic index showed a significant dose-dependent increase of 2.5, 3 and 3.33 fold in the larvae exposed to 20, 40 and 80 μM arecoline, respectively, compared to the control (Fig. 6D; p < 0.05). No significant increase in the apoptotic index was observed in the larvae exposed to 5 and 10 μM arecoline (Fig. 6D; p < 0.05). The results obtained for the apoptosis in the larval eye imaginal disc are shown in Fig. 6E (A1-PC). The larvae exposed to 20, 40 and 80 μM arecoline showed fluorescent green spots throughout the disc in a dose-dependent manner, (Fig. 6; A3–A5) compared to the control (Fig. 6E, C). No apoptotic bodies were detected in the eye imaginal discs after the treatment with 5 and 10 μM arecoline (Fig. 6E; A1 & A2). The positive control showed the maximum number of green spots throughout the disc (Fig. 6E; PC).
Fig. 6. (A) Caspase-9 activity; (B) caspase-3 activity; (C) apoptosis in the midgut cells exposed to various doses of arecoline; (D) apoptotic index estimated in the midgut cells of larvae; (E) acridine orange staining of the eye imaginal disc exposed to various doses of arecoline [A1 = 5 μM; A2 = 10 μM; A3 = 20 μM; A4 = 40 μM; A5 = 80 μM; A = arecoline; C = control; PC = positive control (0.001 μl ml–1 methyl methanesulfonate)] *significant at p < 0.05 compared to the control.
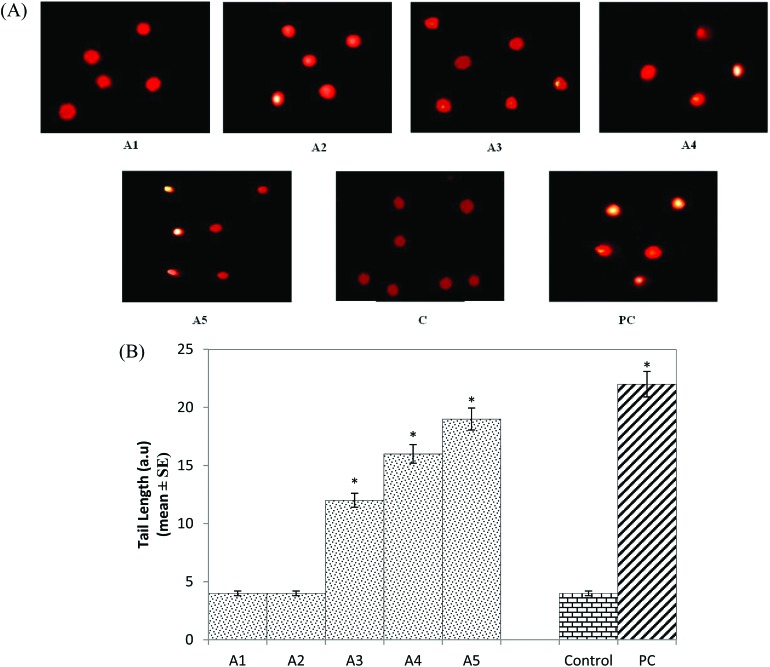
The results obtained for the DNA damage in the midgut cells are shown in Fig. 7A (A1-PC). The larvae exposed to 20, 40 and 80 μM arecoline showed a significant dose-dependent increase of 3, 4 and 4.75 fold in the comet tail length, respectively, compared to the control (Fig. 7B; p < 0.05). No significant increase in the comet tail length was observed in the larvae exposed to 5 and 10 μM arecoline (Fig. 7B; p < 0.05).
Fig. 7. (A) Comet assay performed on the midgut cells exposed to various doses of arecoline; (B) comet tail length measured in the midgut cell after performing the comet assay exposed to various doses of arecoline [A1 = 5 μM; A2 = 10 μM; A3 = 20 μM; A4 = 40 μM; A5 = 80 μM; A = arecoline; C = control; PC = positive control (0.001 μl ml–1 methyl methanesulfonate)] *significant at p < 0.05 compared to the control.
4. Discussion
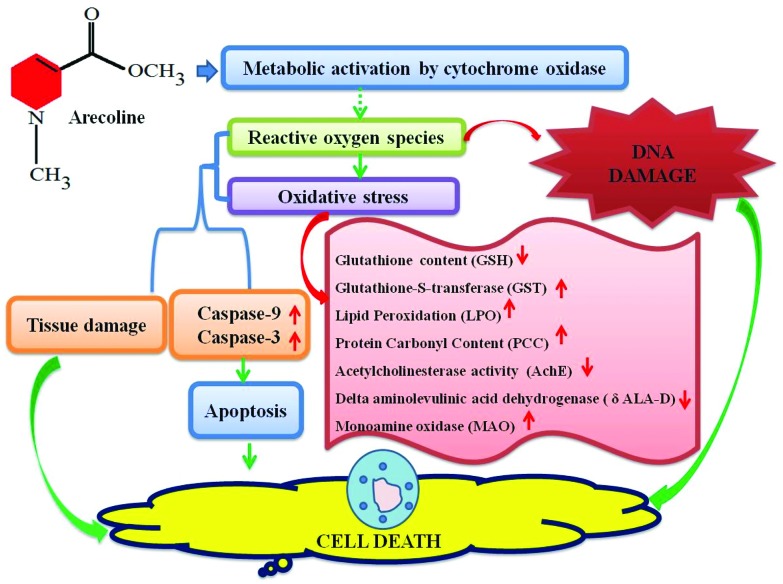
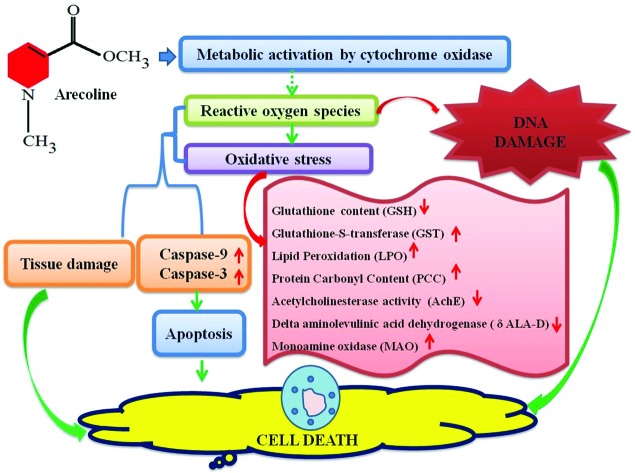
The results of our present study suggest that arecoline showed the toxic effect at 20, 40 and 80 μM in the third instar larvae of Drosophila melanogaster (hsp70-lacZ) Bg9. β-Galactosidase activity (both qualitative and quantitative) was used as an indicator of the expression of hsp70. Elevated levels of hsp70 have been reported in Drosophila melanogaster exposed to pesticides, fungicides, municipal solid wastes, antibiotics, steroids, and nanoparticles.20,24,25,35,36 Due to its conserved nature of stress response across species the expression of hsp70 has been proposed as a sensitive indicator of adverse biological effects.31 In our study with arecoline, a clear dose-dependent increase in the activity of β-galactosidase was observed (both qualitative and quantitative). The same strategy of studying hsp70 expression was used in transgenic soil nematodes C. elegans for studying the toxic effects of heavy metals.37 The exposure of arecoline results in the reduction of GSH content and increased GST activity. GSH is a tripeptide and is involved in many biological phenomena, including enzymatic reactions, molecular transport, protein and nucleic acid biosynthesis, microtubule formation, signal transduction, gene expression and protection of cells against oxidative damage.38 Arecoline is known to react readily with free thiols such as GSH to produce an alkylating adduct with the lost methyl ester group. Hence depletion in the GSH content was observed in the third instar larvae exposed to arecoline. Cellular GSH is the principal detoxifying system, capable of scavenging reactive oxygen species (ROS) and maintaining the redox state of cellular thiols.39 The depletion of cellular thiols may also potentially lead to cellular oxidative stress.13 As a result, an increase in the activity of GST, LPO, and PCC was also observed. Every living organism possesses a detoxification enzyme system to overcome the harmful effects of the generated toxic species via two pathways consisting of Phase I and Phase II.40 Although the mammalian system of cytochromes may be entirely different from the insect system, yet the basic function of the cytochromes may have a similarity in the mode of action. Midgut tissues of the insects were reported to be rich in cytochrome P450 species and high microsomal oxidase activity.41 To date, the metabolites of arecoline have not been properly studied, but it has been reported to be cytotoxic as well as genotoxic in various in vivo as well as in vitro studies.38,42 Although the global organization of mammalian and insect guts is not conserved yet similar functional sequences can be observed.43 The Drosophila's gut exhibits many similarities in both structure and function with the human gastrointestinal tract.43 The development of the foregut, midgut, and hindgut is similar in both i.e. the ectoderm, endoderm and ectoderm, respectively.44 Malpighian tubules are functional analogs of the mammalian kidney present at the junction of the mid and hindgut. The results obtained for lipid peroxidation and protein carbonyl content showed a dose-dependent increase in the larvae exposed to 20, 40 and 80 μM of arecoline. Lipid peroxidation is a measure of oxidative stress resulting from the production of reactive oxygen species that damage the cell membranes, proteins and DNA.45 The major reactive aldehyde resulting from the peroxidation of biological membranes is malonaldehyde (MDA). It can react with DNA, proteins, RNA and other biological molecules.46 The production of MDA was estimated by thiobarbituric acid reactive substance assay (TBARS). The larvae exposed to arecoline showed a dose-dependent increase in TBARS. The oxidation of protein can be measured by estimating the protein carbonyl content.47 The increase in the protein carbonyl content clearly demonstrates the toxic effect of arecoline at 20, 40 and 80 μM. The activity of acetylcholinesterase (AchE) was also estimated among the exposed and control groups. AchE hydrolyses the neurotransmitter acetylcholine to terminate the synaptic transmission. Any decrease in the level is a good marker for neural pathology.48 In our study, a decrease in the AchE activity was observed at 20, 40 and 80 μM of arecoline which suggests its neurotoxic role at these doses. However, there are reports of an increase in AchE activity upon the exposure of arecoline.38 But in our study, the decrease in AchE may be due to the neuronal damage. A significant inhibition in AchE enzyme activity in the brain ganglia of larvae that were temperature shocked and treated with a higher concentration of the certain chemicals was concurrent with the significant induction of hsp70.31 Monoamine oxidase is an iron-containing enzyme that catalyses bioactive monoamines.49 However, the physiological functions of monoamines are still unknown, but in our study, an increase in the activity of MAO was observed in a dose-dependent manner.38 In some cases MAO can generate toxic reactive oxygen species (ROS) i.e. in the metabolism of dopamine.38 The δ-Aminolevulinic Acid Dehydratase (δ-ALA-D) is a metalloenzyme that catalyses the asymmetric condensation of two aminolevulinic acid (δ-ALA) molecules to yield porphobilinogen, a heme precursor.38 The active sites of δ-aminolaevulinic acid dehydrase contain 3 thiol groups that coordinate Zn(ii) ions. The special proximity of these thiols makes the enzyme particularly sensitive to oxidation.50 In our study, the exposure of arecoline showed a dose-dependent decrease in the activity of δ-ALA-D. This inhibition might impair heme biosynthesis and lead to the accumulation of δ-ALA-D. δ-ALA itself has been reported as a pro-oxidant and can disrupt aerobic metabolism processes.51 To study the effect of arecoline on apoptosis, the apoptotic assays were performed on the midgut cells (as these cells have high microsomal oxidation activities) of larvae (caspase-9 and caspase-3, apoptotic index) and eye imaginal discs. The exposure of larvae to arecoline showed a dose-dependent increase in caspase-9 and caspase-3 activities in the larvae exposed to 20, 40 and 80 μM arecoline. The cleavage of procaspase-9 induced the cleavage of the downstream effector caspase-3 to induce the cell death.52 In our study, the midgut cells of the larvae exposed to arecoline also showed an increased number of apoptotic cells at a higher dose of arecoline (80 μM). Caspase plays an essential role in apoptosis, necrosis and inflammation.53 Caspase-9 is an initiator caspase and caspase-3 interacts with caspase-8 and caspase-9.54 The colorimetric estimation of caspase-9 and caspase-3 can give us an indication of the initiation of apoptosis. The exposure of arecoline to the third instar larvae results in the increased activity of caspase-9 and caspase-3. For estimating the genotoxic activity of arecoline the comet assay was performed on the midgut of the larvae exposed to arecoline. A dose-dependent increase in the tail length was observed in the larvae exposed to 20, 40 and 80 μM arecoline compared to the control. The larval eye imaginal disc also clearly demonstrates a dose-dependent increase in the number of apoptotic cells. There is substantial evidence which shows that arecoline is potent in inducing apoptosis, chromosomal aberration, sister chromatid exchange and DNA breaks.54 It also exhibits cytotoxicity and inhibits the growth of oral fibroblasts and keratinocytes.38 Arecoline itself can form a conjugate with GSH and lead to intracellular GSH depletion as reported in the studies performed on fibroblasts and keratinocytes.55 The toxicity of arecoline is exhibited through oxidative stress-induced apoptotic signalling with increased ROS and decreased antioxidants.52 The depletion in GSH content has been reported to activate the apoptotic signalling in the cells and eventually lead to cell death.56 The results obtained in our study for GSH, GST, LPO, PCC and δ-ALA-D support the imbalance in the cellular homeostasis and increased oxidative stress. An increased level of monoamine oxidase and a decrease in the level of antioxidant enzymes (GSH) were found in the liver of rats exposed to 4 to 20 mg kg–1 d–1 of arecoline for seven consecutive days.57 On the basis of the results obtained in our study, we have proposed a mechanism for the toxicity of arecoline (Fig. 8).
Fig. 8. Possible mechanism of toxicity by arecoline.
5. Conclusion
Arecoline is toxic at 20, 40, and 80 μM to the third instar larvae of transgenic Drosophila melanogaster. These doses not only affect the anti-oxidant defense system but also induce DNA damage. However, at the exposure of 5 and 10 μM of arecoline, no adverse effects were observed.
Conflicts of interest
There is no conflict of interest to declare.
Acknowledgments
We are thankful to the Chairman, Department of Zoology for providing laboratory facilities and UGC for providing a non-Net fellowship to Ms Barkha Shakya, Department of Zoology, Faculty of Life Sciences, Aligarh Muslim University, Aligarh, Uttar Pradesh, India. We are also thankful to Dr Smita Jyoti, Rahul and Falaq Naz for their support and technical help.
References
- Chandra P. S., Carey M. P., Carey K. B., Jairam K. R. Drug Alcohol Depend. 2003;69:311–316. doi: 10.1016/s0376-8716(02)00329-0. [DOI] [PubMed] [Google Scholar]
- Bhisey R. A., Boucher B. J., Chen T. H., Gajalakshmi V., Gupta P. C. and Hecht S. S., IARC working group on the evaluation of carcinogenic risk to humans: Betel-quid and Areca-nut chewing and some Areca-nut-derived nitrosamines. IARC Press, Lyon, 2004. [PMC free article] [PubMed] [Google Scholar]
- Boucher B. J., Mannan N. Addict. Biol. 2002;7:103–110. doi: 10.1080/13556210120091464. [DOI] [PubMed] [Google Scholar]
- Deng J. F., Ger J., Tsai W. J., Kao W. F., Yang C. C. Clin. Toxicol. 2001;39:355–360. doi: 10.1081/clt-100105155. [DOI] [PubMed] [Google Scholar]
- Chu N. S. Addict. Biol. 2002;7:111–114. doi: 10.1080/13556210120091473. [DOI] [PubMed] [Google Scholar]
- Walvekar R. R., Chaukar D. A., Deshpande M. S., Pai P. S., Chaturvedi P., Kakade A., Kane S. V., D'Cruz A. K. Oral Oncol. 2009;45:47–51. doi: 10.1016/j.oraloncology.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Sullivan R. J., Allen J. S., Otto C., Tiobech J., Nero K. Br. J. Psychiatry. 2000;177:174–178. doi: 10.1192/bjp.177.2.174. [DOI] [PubMed] [Google Scholar]
- Jeng J. H., Chang M. C., Hahn L. J. Oral Oncol. 2001;37:477–492. doi: 10.1016/s1368-8375(01)00003-3. [DOI] [PubMed] [Google Scholar]
- Sundqvist K., Liu Y., Nair J., Bartsch H., Arvidson K., Grafstrom R. C. Cancer Res. 1989;49:5294–4298. [PubMed] [Google Scholar]
- Stich H. F., Anders F. Mutat. Res. 1989;214:47–61. doi: 10.1016/0027-5107(89)90197-8. [DOI] [PubMed] [Google Scholar]
- Bales A., Peterson M. J., Ojha S., Upadhaya K., Adhikari B., Barrett B. Psychiatr. Res. 2009;169:203–211. doi: 10.1016/j.psychres.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Thangjam G. S., Kondaiah P. J. Periodontal Res. 2009;44:673–682. doi: 10.1111/j.1600-0765.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- Chang M. C., Ho Y. S., Lee P. H., Chan C. P., Lee J. J., Hahn L. J., Wang Y. J., Jeng J. H. Carcinogenesis. 2001;22:1527–1535. doi: 10.1093/carcin/22.9.1527. [DOI] [PubMed] [Google Scholar]
- Winstock A. Addict. Biol. 2002;7:133–138. doi: 10.1080/13556210120091509. [DOI] [PubMed] [Google Scholar]
- Sorce S., Krause K. H. Antioxid. Redox Signaling. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- Lindquist S., CraiG E. A. Annu. Rev. Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Singh M. P., Reddy M. P., Mathur N., Saxena D. K., Chowdhuri D. K. Toxicol. Appl. Pharmacol. 2009;235:226–243. doi: 10.1016/j.taap.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Fatima A., Khanam S., Jyoti S., Naz F., Rahul, Beg T., Siddique Y. H. J. Diet. Suppl. 2017:1–17. [Google Scholar]
- Lis J. T., Simon J. A., Sutton C. A. Cell. 1983;35:403–410. doi: 10.1016/0092-8674(83)90173-3. [DOI] [PubMed] [Google Scholar]
- Nazir A., Mukhopadhyay I., Saxena D. K., Siddiqui M. S., Chowdhuri D. K. J. Biochem. Mol. Toxicol. 2003;17:98–107. doi: 10.1002/jbt.10066. [DOI] [PubMed] [Google Scholar]
- Chowdhuri D. K., Saxena D. K., Viswanathan P. N. Pestic. Biochem. Physiol. 1999;63:15–25. [Google Scholar]
- Nazir A., Saxena D. K., Chowdhuri D. K. Biochim. Biophys. Acta. 2003;1621:218–225. doi: 10.1016/s0304-4165(03)00060-6. [DOI] [PubMed] [Google Scholar]
- Krebs R. A., Feder M. E. J. Exp. Biol. 1997;200:2007–2015. doi: 10.1242/jeb.200.14.2007. [DOI] [PubMed] [Google Scholar]
- Jollow D. J., Mitchell J. R., Zampaglione N. A., Gillette J. R. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Fleischner G., Gatmaitan Z., Arias I. M., Jakoby W. B. Proc. Natl. Acad. Sci. U. S. A. 1974;71:3879–3882. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. J. Lipid Res. 1978;19:1053–1057. [PubMed] [Google Scholar]
- Hawkins C. L., Morgan P. E., Davies M. J. Free Radical Biol. Med. 2009;46:965–988. doi: 10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Ellman G. L., Courtney K. D., Andres V., Featherstone R. M. Biochem. Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- McEwen C. M. J. Biol. Chem. 1965;240:2003–2010. [PubMed] [Google Scholar]
- Abolaji A. O., Kamdem J. P., Lugokenski T. H., Nascimento T. K., Waczuk E. P., Farombi E. O., Loreto E. L., Rocha J. B. T. Free Radicals Biol. Med. 2014;71:99–108. [Google Scholar]
- Mukhopadhyay I., Saxena D. K., Chowdhuri D. K. Environ. Health Perspect. 2003;111:1926. doi: 10.1289/ehp.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhotia S. C., Tapadia M. G. Chromosoma. 1998;107:127–135. doi: 10.1007/s004120050288. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay I., Chowdhuri D. K., Bajpayee M., Dhawan A. Mutagenesis. 2004;19:85–90. doi: 10.1093/mutage/geh007. [DOI] [PubMed] [Google Scholar]
- Siddique Y. H., Ara G., Afzal M. J. Insect Sci. 2012;12:92. doi: 10.1673/031.012.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique Y. H., Ara G., Beg T., Gupta J., Afzal M. Exp. Toxicol. Pathol. 2010;62:503–508. doi: 10.1016/j.etp.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Siddique Y. H., Fatima A., Jyoti S., Naz F., Khan W., Singh B. R., Naqvi A. H. PLoS One. 2013;8(12):e80944. doi: 10.1371/journal.pone.0080944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringham E. G., Candido E. P. M. Environ. Toxicol. Chem. 1994;13:1211–1220. [Google Scholar]
- Jeng J. H., Tsai C. L., Hahn L. J., Yang P. J., Kuo Y. S., Kuo M. Y. P. Food Chem. Toxicol. 1999;37:751–756. doi: 10.1016/s0278-6915(99)00050-2. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D., Chattopadhyay A. Bull. Environ. Contam. Toxicol. 2014;93(1):64–70. doi: 10.1007/s00128-014-1271-0. [DOI] [PubMed] [Google Scholar]
- Giri S., Idle J. R., Chen C., Zabriskie T. M., Krausz K. W., Gonzalez F. J. Chem. Res. Toxicol. 2006;19:818–827. doi: 10.1021/tx0600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Zhang J., Niu J., Zhou X., Li J., Li B. PLoS One. 2015;10:e0120165. doi: 10.1371/journal.pone.0120165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Osman D., David F. P., Fang H. Y., Boquete J. P., Deplancke B., Lemaitre B. Cell Rep. 2013;3:1725–1738. doi: 10.1016/j.celrep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Hakim R. S., Baldwin K., Smagghe G. Annu. Rev. Entomol. 2010;55:593–608. doi: 10.1146/annurev-ento-112408-085450. [DOI] [PubMed] [Google Scholar]
- Ryter S. W., Kim H. P., Hoetzel A., Park J. W., Nakahira K., Wang X., Choi A. M. Antioxid. Redox Signaling. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- Vaca C. E., Wilhelm J., Harms-Ringdahl M. Mutat. Res., Rev. Genet. Toxicol. 1988;195:137–149. doi: 10.1016/0165-1110(88)90022-x. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I., Rossi R., Giustarini D., Milzani A., Colombo R. Clin. Chim. Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Siddique Y. H., Haidari M., Khan W., Fatima A., Jyoti S., Khanam S., Naz F., Rahul, Ali F., Singh B. R., Beg T. Toxicol. Mech. Methods. 2015;25:425–432. doi: 10.3109/15376516.2015.1045653. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. Int. J. Neurosci. 1993;70:75–84. doi: 10.3109/00207459309000563. [DOI] [PubMed] [Google Scholar]
- Saraiva R. A., Bueno D. C., Nogara P. A., Rocha J. B. T. J. Toxicol. Environ. Health, Part A. 2012;75:1012–1022. doi: 10.1080/15287394.2012.697810. [DOI] [PubMed] [Google Scholar]
- Bechara E. J., Medeiros M. J., Monteiro H. P., Hermes-Lima M., Pereira B., Demasi M., Costa C. A., Abdalla D. S., Onuki J., Wendel C. M., Di Mascio P. Quim. Nova. 1993;16:385–392. [Google Scholar]
- Shih Y. T., Chen P. S., Wu C. H., Tseng Y. T., Wu Y. C., Lo Y. C. Free Radicals Biol. Med. 2010;49:1471–1479. doi: 10.1016/j.freeradbiomed.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Alnemri E. S., Livingston D. J., Nicholson D. W., Salvesen G., Thornberry N. J., Wong W. W., Yuan J. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- Tseng S. K., Chang M. C., Su C. Y., Chi L. Y., Chang J. Z. C., Tseng W. Y., Yeung S. Y., Hsu M. L., Jeng J. H. Clin. Oral Invest. 2012;16(4):1267–1273. doi: 10.1007/s00784-011-0604-1. [DOI] [PubMed] [Google Scholar]
- Jeng J. H., Kuo M. J., Hahn L. J., Kuo M. Y. P. J. Dent. Res. 1994;73:1043–1049. doi: 10.1177/00220345940730050501. [DOI] [PubMed] [Google Scholar]
- Lie A. A., Becker A., Behle K., Beck H., Malitschek B., Conn P. J., Kuhn R., Nitsch R., Plaschke M., Schramm J., Elger C. E. Apoptosis. 2000;15:16. [PubMed] [Google Scholar]
- Run-mei X., Jun-jun W., Jing-ya C., Li-juan S., Yong C. Toxicol. Sci. 2014;39:609–614. doi: 10.2131/jts.39.609. [DOI] [PubMed] [Google Scholar]