 The present study was planned to evaluate the protective role of resveratrol (Res) against subchronic malathion exposure in rats over four weeks.
The present study was planned to evaluate the protective role of resveratrol (Res) against subchronic malathion exposure in rats over four weeks.
Abstract
The present study was planned to evaluate the protective role of resveratrol (Res) against subchronic malathion exposure in rats over four weeks. In total, 48 Wistar rats were used and divided equally into six groups. The groups were designed as the control group (received only a rodent diet and tap water), the corn oil group (0.5 ml corn oil by the oral route), and the malathion group (100 mg kg–1 day–1 by the oral route). Other three groups received malathion (100 mg kg–1 day–1) plus Res (5, 10, and 20 mg kg–1 day–1, respectively) by the oral route. Malathion increased malondialdehyde and 8-OHdG levels, whereas it decreased glutathione levels. Also, acetylcholinesterase, superoxide dismutase, and catalase activities were found to be low in the blood, liver, kidney, heart, and brain tissues. Biochemical parameters were not notably changed in all groups. In contrast, Res treatment inverted malathion-induced oxidative stress, lipid peroxidation, and activity of enzymes. Additionally, malathion-induced histopathological changes in the liver, kidney, heart, and brain were ameliorated by Res treatment. These results demonstrate that malathion increases oxidative stress and decreases the antioxidant status while Res has a protective function against malathion toxicity in rats.
Introduction
Organophosphorus pesticides (OPs) are the most widely-used pest control agents in many countries. However, OPs have caused severe environmental pollution, which results in a significant health hazard to target and non-target organisms alike, including humans.1,2 Malathion [O,O-dimethyl-S-(1,2-dicarcethoxyethyl) phosphorodithioate] is an OP, used to eradicate or control disease-inducing pests, that has been targeted by public health programs.3 Several studies have suggested the hazardous effects of malathion on humans and animals while other studies have shown that OPs have produced reactive oxygen species (ROS) by causing damage to various membranous cell components. Treatment with antioxidants or mineral substances can decrease lipid peroxidation (LPO) and oxidative stress related to OP-induced toxicity.4–12
Polyphenols, such as flavonoids, anthocyanins, and phenolic acids, are antioxidant substances used for this purpose. Resveratrol (3,4′,5-trihydroxystilbene; Res) is a subgroup of stilbenes and is a polyphenolic compound found in grapes, wine, peanuts, and blueberries. Res was originally discovered in 1976 as phytoalexin in grapes. In 1982, Res was called Kojo-kon and also Itadori tea in China and Japan, where it was used to treat skin infections, fungal infections and heart, liver, and vascular diseases.13,14 Since then, many studies have shown that Res can prevent the progression of many diseases, including cancer, cardiovascular disease, and ischemic injuries, as well as increase stress resistance and prolong the life span of various organisms.15,16 The natural antioxidant role of Res can be explained by its various effects. One of them is that it reduces the oxidative chain complex at the site of ROS formation. The other is through the capture of superoxide radicals, which are formed by mitochondria, and the inhibition of lipid peroxidation that has been induced by the products of the Fenton reaction. Numerous studies have shown that Res has the ability to capture both superoxide and hydroxyl radicals.17
Until now, according to our knowledge, the protective role of Res against malathion has not been examined, so this study aimed to evaluate the protective role of Res against malathion-induced oxidative damage. Therefore, malondialdehyde (MDA) as a LPO marker, acetylcholinesterase (AChE), superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), and histopathological changes in tissues were evaluated in rats.
Materials and methods
Materials and experimental protocol
Malathion and Res were purchased from Agrobest A.S. (Izmir, Turkey) and Terraternal (Santa Clara, CA, USA), respectively. All the other chemicals which are analytical reagent grade were obtained from commercial sources. Wistar male rats (200–250 g and 12–16 weeks of age) were obtained from the Experimental Animal Research and Application Center (Afyonkarahisar, Turkey). The animals were kept at 25 °C and 50–55% humidity with ad libitum access to rodent diet and water.
The animals were separated into 6 groups consisting of 8 animals each. The rodent diet and fresh water were given to the control and treated groups. Corn oil (0.5 ml) was given by gastric gavage to the corn oil group for a period of 28 days. Malathion (100 mg kg–1 day–1; dissolved in corn oil) was given by gastric gavage to the malathion group for a period of 28 days. Three different doses of Res (5, 10, and 20 mg kg–1 day–1; dissolved in corn oil) were given by gastric gavage to the other groups (Res + malathion) throughout the entire period of 28 days. In this study, doses of malathion (toxic and adverse effects) and Res (cell protective effects) were chosen according to the previous studies.6,18 Also, Res was given to the animals one hour before malathion administration and the experimental protocols were also approved by the Animal Care and Use Committee at Afyon Kocatepe University (2013/59269667).
Blood collection and erythrocyte preparation
Blood samples from each group were collected by cardiac puncture into heparinised and non-heparinised tubes under light ether anesthesia at the end of 28 days. Within 30 min of blood collection, the erythrocytes were precipitated by centrifugation at 600g for 15 min at 4 °C, and the plasma and serum were removed. The erythrocytes were washed three times with isotonic saline and the puffy coat was discarded. Then, the same volumes of isotonic saline and erythrocytes were added into vials and stored at –20 °C in a deep freeze. When used, the erythrocyte suspension was destroyed by osmotic pressure, using five times cold deionised water. The erythrocyte lysate was stored at 4 °C until measurements within 3 days.19
Preparation of homogenate
Animals were sacrificed by cervical dislocation and the liver, kidney, and brain tissues were washed immediately with ice cold 0.9% NaCl. Each tissue was trimmed free of extraneous tissue and rinsed in chilled 0.15 M Tris–HCl buffer (pH 7.4). These tissues were blotted dry, and homogenized in 0.15 M Tris–HCl buffer (pH 7.4) to yield a 10% (w/v) homogenate. Then, they were centrifuged at 2100g for 10 min at 4 °C. The pellets represented the nuclear fraction and the supernatants were subjected to centrifugation at 18 600g for 20 min at 4 °C. The resultant pellets and the supernatants represented the mitochondrial fraction and the cytosolic (including the microsomal fraction) fraction, respectively. Reactive oxygen species generation was observed in all the fractions as well as the whole homogenate.
Preparation of tissues for histopathological analysis
At the end of the experimental period, 48 male rats were sacrificed. Then, animals were dissected and the liver, kidney, and heart tissues from each animal were collected. Tissues were fixed into 10% formalin solution for 48 h and then dehydrated through graded alcohol series (70 to 100%), cleared in xylene and embedded in paraffin. 5 to 6 μm thick paraffin sections were cut and stained with haematoxylin-eosin (H&E) and analyzed under a light microscope (Olympus Bx51 model, Tokyo, Japan) equipped with a camera (Olympus DP20, Tokyo, Japan).
Measurement of 8-OHdG, AchE, and biochemical parameters in serum
The serum samples were examined for their concentration of 8-hydroxy-2′-deoxyguanosine (8-OHdG) using a competitive enzyme immunoassay (EIA) kit (Cayman Chemical Company, Ann Arbor, MI, USA)20,21 and intra-assay and inter-assay CVs were found to be 5.3% and 8.2%, respectively. AchE activity was also measured using a quantitative sandwich EIA kit (Cusabio Chemical Company, Wuhan, Hubei Province, P. R. China) and intra-assay and inter-assay CVs were found to be 6.4% and 8.1%, respectively. ELISA measurements were performed by using an ELx800 Absorbance Microplate Reader (BIOTEK, Bad Friedrichshall, Germany). Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), urea, creatinine, cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride, and glucose levels were also determined using COBAS test kits (Roche Diagnostics Systems, Istanbul, Turkey) according to the manufacturers’ instructions in the Biochemistry Laboratory, Faculty of Medicine, University of Afyon Kocatepe (Turkey).
Measurement of LPO and reduced GSH in whole blood and tissue homogenates
Malondialdehyde, as a marker of LPO, was determined by the methods of Draper and Hadley22 in whole blood and of Ohkawa et al.23 in tissue homogenates. The principle of the methods is based on the spectrophotometric measurement of the colour produced during the reaction of thiobarbituric acid with MDA and its absorbance was measured spectrophotometrically at 532 nm. The concentration of MDA was expressed in nmol per ml blood and nmol per g protein. Reduced GSH concentration was measured using the method described by Beutler et al.24 in whole blood and tissue homogenates. The optical density was measured at 412 nm using a spectrophotometer. The results were expressed as nmol per ml blood and nmol per g protein. Spectrophotometric measurements were performed by using a Shimadzu 1601 UV–VIS spectrophotometer (Tokyo, Japan).
Measurement of SOD and CAT activities in erythrocyte lysate and tissue homogenates
The antioxidant enzyme activity of SOD in the erythrocyte lysate and tissue homogenate was measured according to the method of Sun et al.25 The measurement of SOD is based on the principle that xanthine reacts with xanthine oxidase as a source of substrate (superoxide) and reduced nitroblue tetrazolium (NBT) as an indicator of superoxide. In this method, xanthine–xanthine oxidase was used to generate a superoxide flux. The absorbance obtained from NBT reduction to blue formazon by superoxide was determined at 560 nm spectrophotometrically. SOD activity was expressed in U per gHb erythrocyte and U per μg protein tissue. CAT activities in the erythrocyte lysate and tissue homogenate were determined according to the methods of Luck26 and Aebi,27 respectively. The method is based on the decomposition of H2O2 by catalase. The reaction mixture was composed of 50 mM phosphate buffer (pH 7.0), 10 mM H2O2 and the sample. The reduction rate of H2O2 was followed at 240 nm for 45 s at room temperature. One unit of catalase is the amount of catalase decomposing 1.0 μmol H2O2 per min at pH 4.5 at 25 °C, and the catalase activity (k, nmol min–1) was expressed in k per gHb erythrocyte and k per μg protein tissue.
Measurement of hemoglobin (Hb) and protein concentrations
Hb was determined using a colorimetric cyanomethemoglobin method according to Drabkin and Austin,28 and the tissue protein content was assayed according to the colorimetric method of Lowry et al.29
Analysis of malathion and malaoxon concentration
The concentrations of malathion and malaoxon which is a malathion metabolite in the diet, water and tissue samples were measured by liquid chromatography/tandem mass spectrometry (LC-MS/MS). For malathion and malaoxon determination, sample extractions were carried out according to the validated methods reported by Hogendoorn and van Zoonen.30 Briefly, 1 g sample was put into a 50 ml centrifuge tube, followed by adding 15 ml acetonitrile containing 1% acetic acid. After shaking for 5 min, 2.5 g anhydrous MgSO4 plus sodium acetate was added into the tube. The mixed solution was centrifuged for 5 min at 1650g and then 6 ml supernatant was transferred into a 15 ml tube (containing 300 mg primary secondary amine sorbent plus 1.8 g anhydrous MgSO4, which constituted a cleanup procedure called dispersive solid-phase extraction). Similarly, the solution was centrifuged for 5 min at 1650g and then 1 ml supernatant was transferred into auto-sampler vials for analysis by LC-MS/MS. The LC-MS/MS system used in this work consisted of an Agilent 1200 series including a vacuum solvent degassing unit, a binary high-pressure gradient pump, an automatic sample injector, a column thermostat and a photodiode array detector. Mass spectrometry was performed using an Agilent 6460 LC-MS Triple Quadrupole instrument equipped with an ESI source (Agilent Technologies, Waldbronn, Germany). The detection limit for malathion and malaoxon was 0.01 μg kg–1.
Statistical analyses
Data obtained from experimental animals were expressed as means and standard deviation of means (±SD) and analysed using one-way analysis of variance (ANOVA), followed by Duncan post-hoc tests on the SPSS (20) software computer program. A difference in the mean values of p < 0.05 was considered to be significant.
Results
Malaoxon levels in tissue samples
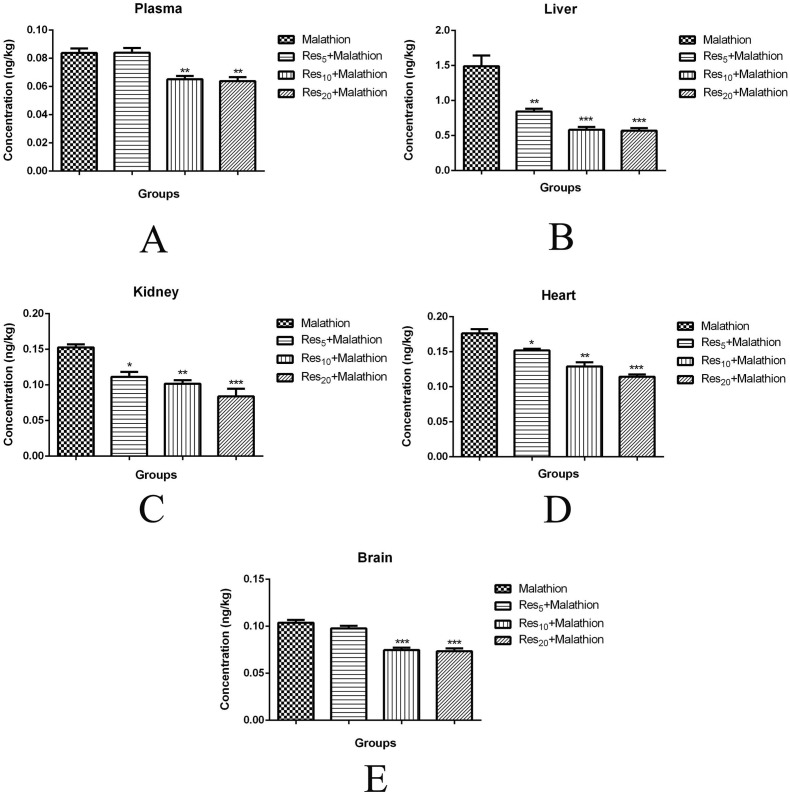
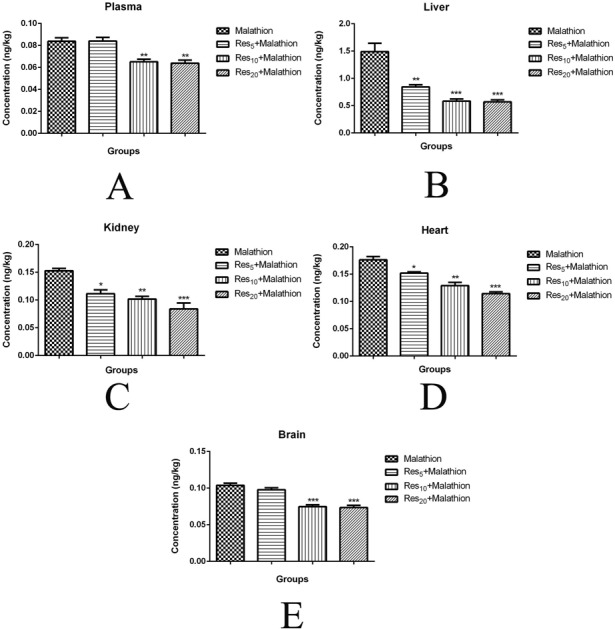
The malaoxon concentration of plasma (0.08 ± 0.005 ng kg–1; Fig. 1A), liver (1.49 ± 0.26 ng kg–1; Fig. 1B), kidney (0.15 ± 0.007 ng kg–1; Fig. 1C), heart (0.17 ± 0.01 ng kg–1; Fig. 1D), and brain (0.08 ± 0.005 ng kg–1; Fig. 1E) tissues was found to be at high levels in malathion groups compared to Res groups (p < 0.05). Malaoxon concentrations of Res5, Res10, and Res20 were found to be 0.08 ± 0.005, 0.06 ± 0.005 (p < 0.01), and 0.06 ± 0.004 ng kg–1 (p < 0.01) in plasma, respectively. Malaoxon concentrations of Res5, Res10, and Res20 were found to be 0.84 ± 0.06 (p < 0.01), 0.58 ± 0.07 (p < 0.001), and 0.56 ± 0.06 ng kg–1 (p < 0.001) in the liver, respectively. Malaoxon concentrations of Res5, Res10, and Res20 were found to be 0.11 ± 0.01 (p < 0.05), 0.10 ± 0.008 (p < 0.01), and 0.08 ± 0.01 ng kg–1 (p < 0.001) in the kidney, respectively. Malaoxon concentrations of Res5, Res10, and Res20 were found to be 0.15 ± 0.004 (p < 0.05), 0.12 ± 0.01 (p < 0.01), and 0.11 ± 0.005 ng kg–1 (p < 0.001) in the heart, respectively. Malaoxon concentrations of Res5, Res10, and Res20 were found to be 0.09 ± 0.004, 0.07 ± 0.004 (p < 0.001), and 0.07 ± 0.005 ng kg–1 (p < 0.001) in the brain, respectively. Res treatment in a dose dependent manner significantly decreased the concentration of malaoxon in the plasma, liver, kidney, heart, and brain. Also, the malathion residue was not detected in either the diet or water sample.
Fig. 1. Malaoxon levels in the plasma (A), liver (B), kidney (C), heart (D), and brain (E) of rats treated with malathion at a dose of 100 mg kg–1 day–1, orally. Values are expressed as the mean ± SD of 8 samples per group. Statistical significance: *p < 0.05, ** p < 0.01, ***p < 0.001 versus the control group.
Effect of AChE activity and 8-OHdG level
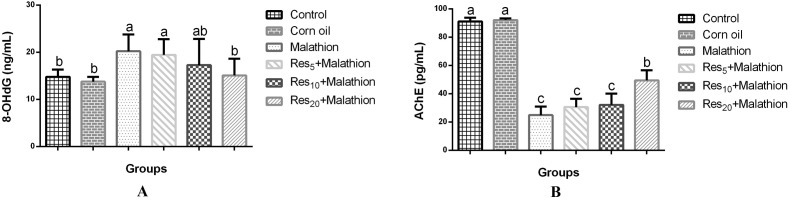
AChE activity and 8-OHdG level in the evaluation of malathion toxicity are shown in Fig. 2. There was a significant decrease of AChE activity in the serum compared to the control group (Fig. 2A). By contrast, the 8-OHdG level in the serum was found to be higher (p < 0.05) in the malathion group than in the control group (Fig. 2B). In a dose-dependent manner, Res administration reversed the malathion-induced alterations, AChE activity and 8-OHdG levels, in rat serum.
Fig. 2. 8-OHdG (A) and AchE activity (B) levels in serum of rats treated with malathion at a dose of 100 mg kg–1 day–1, orally. Values are expressed as the mean ± SD of 8 samples per group. Statistical significance: *p < 0.05 versus the malathion group.
Effects of markers of liver and kidney functions
The hepatic biochemical data for the evaluation of malathion toxicity are summarized in Table 1. There was a significant increase in the serum ALT, AST, and ALP (p < 0.05) levels of the malathion-exposed group compared to the control group. However, the administration of Res at doses of 5, 10, and 20 mg kg–1 day–1 reversed the malathion-induced alteration of hepatic biochemical parameters in a dose-dependent manner. The serum urea level was found to be low in the malathion-exposed group compared to the control group whereas serum creatinine levels did not change in the groups.
Table 1. Effects of malathion (100 mg kg–1) and resveratrol (Res: 5, 10, and 20 mg kg–1) + malathion (100 mg kg–1) on alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), urea and creatinine levels in serum of rats.
| Experimental design | ALT (U l–1) | AST (U l–1) | ALP (U l–1) | Urea (mg dl–1) | Creatinine (mg dl–1) |
| Control | 38.63 ± 9.73c | 40.05 ± 8.87c | 0.88 ± 0.43c | 53.54 ± 4.23a | 0.39 ± 0.06 |
| Corn oil | 41.03 ± 10.11c | 44.85 ± 6.30c | 0.70 ± 0.39c | 48.88 ± 3.28a | 0.38 ± 0.08 |
| Malathion | 64.33 ± 10.67a | 60.35 ± 10.06a | 7.05 ± 1.03a | 45.12 ± 4.96b | 0.33 ± 0.04 |
| Res5 + malathion | 51.37 ± 10.29b | 113.53 ± 11.52a | 6.33 ± 1.07a | 44.45 ± 3.42b | 0.34 ± 0.06 |
| Res10 + malathion | 50.22 ± 8.62bc | 112.85 ± 7.31a | 6.05 ± 1.27ab | 43.40 ± 6.06b | 0.33 ± 0.04 |
| Res20 + malathion | 49.50 ± 10.53bc | 77.88 ± 12.03b | 3.47 ± 1.60b | 41.67 ± 5.05b | 0.32 ± 0.04 |
Effects of markers of lipid profile
The lipid profile values for the evaluation of malathion toxicity are summarized in Table 2. There were no significant changes in the serum cholesterol, HDL, LDL, triglyceride, and glucose (p > 0.05) levels in the malathion-exposed and Res + malathion groups compared to the control group.
Table 2. Effects of malathion (100 mg kg–1) and resveratrol (Res: 5, 10, and 20 mg kg–1) + malathion (100 mg kg–1) on cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), triglyceride and glucose levels in the serum of rats.
| Experimental design | Cholesterol (mg dl–1) | HDL-cholesterol (mg dl–1) | LDL-cholesterol (mg dl–1) | Triglyceride (mg dl–1) | Glucose (mg dl–1) |
| Control | 62.94 ± 9.24 | 14.76 ± 2.84 | 57.45 ± 8.00 | 144.42 ± 11.69 | 81.12 ± 13.91 |
| Corn oil | 63.89 ± 8.72 | 11.91 ± 1.40 | 49.76 ± 8.00 | 149.11 ± 13.50 | 84.70 ± 12.10 |
| Malathion | 58.67 ± 3.92 | 10.52 ± 1.19 | 52.68 ± 3.39 | 161.19 ± 19.57 | 106.28 ± 7.02 |
| Res5 + malathion | 59.72 ± 10.55 | 13.13 ± 4.98 | 55.70 ± 15.71 | 140.28 ± 15.31 | 96.63 ± 12.10 |
| Res10 + malathion | 54.10 ± 7.17 | 13.05 ± 2.33 | 51.48 ± 18.74 | 131.51 ± 12.91 | 93.84 ± 13.55 |
| Res20 + malathion | 52.91 ± 9.54 | 12.89 ± 1.78 | 50.99 ± 9.13 | 132.82 ± 12.15 | 89.74 ± 9.88 |
Effects on LPO and reduced GSH levels
Highly significant elevation in the MDA levels of whole blood, liver, kidney, heart, and brain (p < 0.05) tissues was observed in the malathion group compared to the control group. However, MDA levels of whole blood and tissues were found to be lower in the malathion + Res groups than in the malathion group (Table 3). Reduced GSH levels in whole blood, liver, kidney, and heart (p < 0.05) tissues were found to be low in the malathion group compared to the control group. However, the administration of Res in a dose-dependent manner showed significantly increased GSH levels compared to the malathion group (Table 4).
Table 3. Effects of malathion (100 mg kg–1) and resveratrol (Res: 5, 10, and 20 mg kg–1) + malathion (100 mg kg–1) on malondialdehyde levels in whole blood, liver, kidney, heart and brain tissue homogenates of rats.
| Experimental design | Blood (nmol ml–1) | Liver (nmol per g tissue) | Kidney (nmol per g tissue) | Heart (nmol per g tissue) | Brain (nmol per g tissue) |
| Control | 3.76 ± 0.62b | 4.45 ± 0.86b | 3.82 ± 2.20b | 3.34 ± 0.40c | 3.64 ± 0.74b |
| Corn oil | 3.83 ± 0.83b | 4.30 ± 0.54b | 4.14 ± 1.27b | 3.26 ± 0.68c | 3.57 ± 0.75b |
| Malathion | 7.27 ± 1.80a | 7.68 ± 0.99a | 6.98 ± 1.00a | 6.19 ± 1.08a | 7.41 ± 1.14a |
| Res5 + malathion | 4.78 ± 0.69ab | 6.04 ± 1.76ab | 5.53 ± 1.52ab | 6.05 ± 1.25a | 5.66 ± 2.52ab |
| Res10 + malathion | 4.41 ± 0.48ab | 5.25 ± 1.32b | 5.47 ± 1.22ab | 4.87 ± 0.51b | 4.51 ± 0.62b |
| Res20 + malathion | 4.14 ± 0.70b | 4.30 ± 0.70b | 5.07 ± 1.24b | 3.89 ± 0.49c | 4.11 ± 1.07b |
Table 4. Effects of malathion (100 mg kg–1) and resveratrol (Res: 5, 10, and 20 mg kg–1) + malathion (100 mg kg–1) on reduced glutathione levels in whole blood, liver, kidney, heart and brain tissue homogenates of rats.
| Experimental design | Blood (nmol ml–1) | Liver (nmol per g tissue) | Kidney (nmol per g tissue) | Heart (nmol per g tissue) | Brain (nmol per g tissue) |
| Control | 21.19 ± 2.37a | 23.94 ± 5.86a | 25.43 ± 1.78a | 20.83 ± 6.47a | 21.21 ± 5.32 |
| Corn oil | 21.22 ± 3.23a | 22.44 ± 4.37a | 20.13 ± 1.76a | 17.10 ± 2.57ab | 20.81 ± 3.26 |
| Malathion | 14.01 ± 1.26b | 14.93 ± 1.18b | 14.64 ± 2.78c | 14.44 ± 2.49b | 16.48 ± 1.74 |
| Res5 + malathion | 14.81 ± 2.54b | 15.85 ± 1.59b | 15.48 ± 2.25c | 16.68 ± 2.33ab | 17.84 ± 1.21 |
| Res10 + malathion | 16.67 ± 1.50ab | 16.16 ± 1.73b | 16.04 ± 0.50bc | 16.85 ± 1.66a | 17.46 ± 1.61 |
| Res20 + malathion | 19.23 ± 2.56ab | 21.25 ± 2.14a | 16.27 ± 2.27bc | 21.91 ± 7.47a | 18.41 ± 4.65 |
Effects on antioxidant enzymes
Antioxidant enzymes SOD and CAT activities were determined in the erythrocyte, liver, kidney, heart and brain tissues of rats, as shown in Tables 5 and 6, respectively. In the malathion group, SOD and CAT activities were found to be low in erythrocytes and all tissues (p < 0.05) compared to the control group. By contrast, Res administration before malathion treatment was observed to reverse the malathion-induced alterations of SOD and CAT activities.
Table 5. Effects of malathion (100 mg kg–1) and resveratrol (Res: 5, 10, and 20 mg kg–1) + malathion (100 mg kg–1) on superoxide dismutase activity in erythrocyte, liver, kidney, heart and brain tissue homogenates of rats.
| Experimental design | Erythrocyte (U per gHb) | Liver (U per μg protein) | Kidney (U per μg protein) | Heart (U per μg protein) | Brain (U per μg protein) |
| Control | 62.66 ± 10.85a | 4.93 ± 1.21a | 4.94 ± 1.40 | 5.57 ± 0.77a | 4.26 ± 0.53a |
| Corn oil | 68.44 ± 11.70a | 4.56 ± 0.82a | 4.87 ± 1.45 | 5.11 ± 0.66a | 4.15 ± 0.47a |
| Malathion | 18.41 ± 6.41b | 3.43 ± 0.54b | 3.26 ± 0.71 | 3.68 ± 0.81b | 0.77 ± 0.13d |
| Res5 + malathion | 26.83 ± 13.01b | 3.48 ± 0.77b | 3.52 ± 1.16 | 3.37 ± 1.18b | 0.84 ± 0.29d |
| Res10 + malathion | 30.00 ± 7.64b | 3.51 ± 1.05b | 3.60 ± 0.69 | 3.51 ± 0.62b | 1.72 ± 0.32c |
| Res20 + malathion | 62.42 ± 10.29a | 4.47 ± 0.69ab | 3.79 ± 0.79 | 3.92 ± 1.04b | 3.13 ± 0.27b |
Table 6. Effects of malathion (100 mg kg–1) and resveratrol (Res: 5, 10, and 20 mg kg–1) + malathion (100 mg kg–1) on catalase activity in erythrocyte, liver, kidney, heart and brain tissue homogenates of rats.
| Experimental design | Erythrocyte (k per gHb) | Liver (k per μg protein) | Kidney (k per μg protein) | Heart (k per μg protein) | Brain (k per μg protein) |
| Control | 60.77 ± 15.65a | 707.61 ± 63.93a | 581.27 ± 50.28a | 265.95 ± 43.98 | 36.94 ± 3.86a |
| Corn oil | 60.10 ± 16.70a | 704.16 ± 64.50a | 664.48 ± 54.26a | 217.95 ± 31.55 | 38.58 ± 3.71a |
| Malathion | 22.47 ± 7.34b | 259.59 ± 78.44b | 163.51 ± 39.12c | 202.99 ± 47.11 | 12.60 ± 2.28d |
| Res5 + malathion | 27.93 ± 8.07b | 271.52 ± 39.21b | 222.40 ± 55.10c | 208.41 ± 38.96 | 13.16 ± 2.52d |
| Res10 + malathion | 26.89 ± 7.51b | 265.90 ± 79.17b | 316.53 ± 47.44c | 228.35 ± 46.75 | 17.58 ± 2.37c |
| Res20 + malathion | 42.12 ± 9.13ab | 427.06 ± 98.07ab | 450.21 ± 36.97b | 278.97 ± 28.24 | 30.53 ± 5.77b |
Histopathological examination
This study also evaluated the histopathological changes in brain, heart, liver, and kidney tissues. Focal gliosis and neuronal degenerations in the brain (Fig. 3A3), hyaline degenerations in the heart (Fig. 3B3), sinusoidal dilatation and degenerations of hepatocytes in the liver (Fig. 3C3), and degenerations in the tubule and shrinkage of Bowman's capsule in the kidney (Fig. 3D3) were observed in the malathion group.
Fig. 3. The effect of resveratrol (Res) on malathion induced damage in the brain (A), heart (B), liver (C), and kidney (D) of rats. Representative figures were stained with H&E. The original magnification was ×20 and the scale bars represent 100 μm. Arrows and arrow heads indicate focal gliosis and neuronal degenerations in the brain (A3), hyaline degenerations in the heart (B3), sinusoidal dilatation and degenerations of hepatocytes in the liver (C3), and degenerations in the tubule and shrinkage of Bowman's capsule in the kidney (D3) of rats, respectively. (1) The control group, (2) the corn oil group, (3) animals treated with 100 mg kg–1 day–1 malathion, (4) animals treated with 5 mg kg–1 day–1 Res and 100 mg kg–1 day–1 malathion, (5) animals treated with 10 mg kg–1 day–1 Res and 100 mg kg–1 day–1 malathion, and (6) animals treated with 20 mg kg–1 day–1 Res and 100 mg kg–1 day–1 malathion.
In the malathion + Res groups, mild sinusoidal dilatation in the liver (Fig. 3C4–C6) and slight degenerations in the kidney (Fig. 3D4–D6) were observed, as was mild focal gliosis in the brain (Fig. 3A4–A6), and slight degenerations in the heart (Fig. 3B4–B6). In the control (Fig. 3A1, B1, C1, and D1) and corn oil (Fig. 3A2, B2, C2, and D2) groups, no significant histopathological changes were observed in the liver, kidney, heart, and brain tissues. According to histopathological findings, Res has obviously preventive effects on malathion-induced toxicity, especially at high doses (Res10 and Res20).
Discussion
Prior to this investigation, malathion distributions in tissues were examined by Rashid et al.31 in rats that had been orally treated with malathion (10, 25, and 50 ml per kg bw). The concentration was the highest in the liver of these rats. Coban et al.6 also reported that when malathion was orally administered at 100 mg kg–1 to rats, the malaoxon levels in the liver of rats were high. Richardson and Seiber32 reported on 3 mg kg–1 diazinon and 0.6 mg kg–1 parathion that were given to pigeons. In this study, OP levels were detected at 0.06 mg kg–1 diazinon and 0.07 mg kg–1 parathion in the kidney tissue by gas chromatography. Similarly, malaoxon levels in the tissues were found to be quite high as well. However, malaoxon levels were found to be low in the Res group, which indicates that Res may exhibit a preventive effect against malathion toxicity. This situation may demonstrate that Res enhances the biotransformation of malaoxon or that Res causes an increase in malaoxon excretion from organisms.
Serum AChE activity was found to occur at a low level in the case of OP toxicity;6 likewise, this study showed that malathion inhibited the AChE activities in the serum of rats. By contrast, Res administered in a dose-dependent manner can improve and sustain AChE activity. 8-OHdG as a biomarker of oxidative stress shows the mutagenic damage.33 In this study, the treatment of malathion caused enhanced levels of 8-OHdG in the serum of rats. Moore et al.34 reported that even a low level of malathion exposure caused cytotoxic effects in human liver carcinoma (hepG2) cells, and exposure to high levels resulted in genotoxic effects within rats. Nevertheless, Res decreased the 8-OHdG levels in rats that had been administered with Res compared with those that had been given malathion. As such, Res might alleviate the mutagenic damage and oxidative stress created by malathion.
Liver damage was evident from the increased serum levels of ALP, ALT, and AST although OP insecticides can also increase these serum enzymes.35 In this study, ALP, ALT, and AST levels were higher in the malathion-treated groups than in the control group, which is consistent with the hepatic tissue damage observed in malathion-treated rats. In contrast, Res was effective at protecting the rats against malathion-induced hepatotoxicity, as proven by a dose-dependent decrease in ALP, ALT, and AST activities.36 Serum urea levels were found to be low in the malathion-exposed group compared to the control group. Also Res has not been shown to have a positive effect on the serum urea levels in rats. Similarly, El-Sebae et al.37 suggested that OP compounds caused hypoglycaemia in rats and also reduced their blood urea concentration.
Numerous research studies have demonstrated that malathion causes oxidative damage,38 as well as increases in the levels of MDA, depletion of GSH, and decreased activities of antioxidant enzymes, such as SOD and CAT.39 In this study, the increased blood and tissue LPO that was observed aligned with the results of other studies. Res has been shown to act as a scavenger of superoxide, hydroxyl radicals, and singlet molecular oxygen.40,41 Consistent with our results, the Res treatment administered in a dose-dependent manner significantly decreased lipid peroxides in comparison with the malathion treatment. Coban et al.6 reported that malathion intoxication reduced GSH levels in the blood, liver, kidney, and brain. Also, Alp et al. and Bhatti et al. showed that free radicals produced by malathion could cause a dramatic drop in GSH, but Res reduced the peroxidative activity in cells, so it resulted in normal GSH levels.
SOD and CAT activities were determined since they are important markers of the cellular defence systems against oxidative stress. SOD turns superoxide into hydrogen peroxide (H2O2), and CAT disintegrates H2O2 into H2O and O2 to protect the tissues from highly reactive hydroxyl radicals.42 In this study, malathion administration decreased the SOD and CAT activities in tissues and erythrocytes, a finding that is in accordance with Possamai et al.43 and Coban et al.6 Similar to the OP toxicity in the malathion group, low levels of SOD and CAT might be related to the utilization of these enzymes. SOD and CAT activities increased in the Res groups, which suggested that Res could encourage and sustain the activity of these enzymes.44,45
Malathion (100 mg kg–1 day–1) produced noticeable histopathological changes in the livers, kidneys, hearts, and brains of the rats. Focal gliosis and neuronal degenerations in the brain were observed; degenerations in the kidney and heart were evident, and sinusoidal dilatation and liver degeneration were seen in the malathion group of rats. Kalender et al.35 administered 27 mg kg–1 of malathion to rats for 28 days, and mononuclear cell infiltration, haemorrhage, calcification, degeneration of the vacuoles, dilation of sinusoids, vascular congestion, and necrosis were observed. Tos-Luty et al.46 also reported that dermal exposure to malathion at 16 mg kg–1 caused swollen mitochondria in cardiomyocytes. Moreover, Alp et al.47 reported that malathion (200 mg kg–1) evoked necrosis and inflammatory cell infiltration in the kidneys. Sharma et al.48 reported that dimethoate, an OP compound, caused lymphocytic infiltration that may have resulted in chronic, mild meningeal changes in the brain. On the other hand, Res, administered using a dose-dependent approach, protected the liver, kidney, heart, and brain tissue of the rats against malathion-induced cellular damage.
Conclusion
The results of this study demonstrate that Res, given in a dose-dependent manner, successfully prevented malathion-induced toxicity in rats. These results suggest that Res increases the activity of the antioxidant defence system, as well as inhibits LPO, thus enhancing tissue regeneration in rats.
Conflicts of interest
The authors declare that there is no conflict of interest associated with their contribution to this manuscript.
Acknowledgments
EA is grateful to Uşak University Scientific Research Council, Uşak, Turkey for providing financial support (Project no: BAPK-2012/MF003). IK thanks Afyon Kocatepe University, Faculty of Veterinary Medicine, Department of Biochemistry for providing laboratory facilities. Also, this study was orally presented at the 2nd International Congress on Advances in Veterinary Sciences and Technics, Skopje, Macedonia.
References
- El-Demerdash F. M., Nasr H. M. J. Trace Elem. Med. Biol. 2014;28:89–93. doi: 10.1016/j.jtemb.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Gupta V. K., Sharma B. Anatomy Physiol. Biochem. Int. J. 2016;1:555558. [Google Scholar]
- Suresh B. N., Malik J. K., Rao G. S., Aggarwal M., Ranganathan V. Environ. Toxicol. Pharmacol. 2006;22:167–171. doi: 10.1016/j.etap.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Ince S., Arslan-Acaroz D., Demirel H. H., Varol N., Ozyurek H. A., Zemheri F., Eryavuz A. Biomed Pharmacother. 2017;96:263–268. doi: 10.1016/j.biopha.2017.09.141. [DOI] [PubMed] [Google Scholar]
- El-Demerdash F. M. Food Chem. Toxicol. 2011;49:1346–1352. doi: 10.1016/j.fct.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Coban F. K., Ince S., Kucukkurt I., Demirel H. H., Hazman O. Drug Chem. Toxicol. 2015;38:391–399. doi: 10.3109/01480545.2014.974109. [DOI] [PubMed] [Google Scholar]
- Jaiswal S. K., Gupta V. K., Siddiqi N. J., Sharma B. Cell. Mol. Biol. 2017;63:12–17. doi: 10.14715/cmb/2017.63.6.3. [DOI] [PubMed] [Google Scholar]
- Jaiswal S. K., Gupta V. K., Ansari M. D., Siddiqi N. J., Sharma B. Toxicol. Rep. 2017;4:265–273. doi: 10.1016/j.toxrep.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D., Sharma B., Rizvi S. I. Prog. Health Sci. 2016;6:148. [Google Scholar]
- Jaiswal S. K., Sharma A., Gupta V. K., Singh R. K., Sharma B. Biochem. Res. Int. 2016;1:1–7. doi: 10.1155/2016/7637931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S. K., Siddiqi N. J., Sharma B., Saudi J. Biol. Sci., 2016. 10.1016/j.sjbs.2016.03.002 , , in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S. K., Gupta V. K., Siddiqi N. J., Pandey R. S., Sharma B. Chin. J. Biol. 2015:1–10. [Google Scholar]
- Sayın O., Arslanand N., Güner G. Turk J.Biochem. 2008;33:117–121. [Google Scholar]
- Ince S., Arslan-Acaroz D., Neuwirth O., Demirel H. H., Denk B., Kucukkurt I., Turkmen R. Food Chem. Toxicol. 2014;72:147–153. doi: 10.1016/j.fct.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Baur J. A., David A. Nat. Rev. Drug Discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Arslan-Acaroz D., Zemheri F., Demirel H. H., Kucukkurt I., Ince S., Eryavuz A. Environ. Sci. Pollut. Res. 2018;25:2614–2622. doi: 10.1007/s11356-017-0391-6. [DOI] [PubMed] [Google Scholar]
- Yazir Y., Utkan T., Gacar N., Aricioglu F. Physiol. behav. 2015;138:297–304. doi: 10.1016/j.physbeh.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Sharma M., Gupta Y. K. Life Sci. 2002;71:2489–2498. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Hawkins R. E., Brian M., Carrell R. W. J. Lab. Clin. Med. 1975;55:337–341. [PubMed] [Google Scholar]
- Devries M. C., Hamadeh M. J., Glover A. W., Raha S., Samjoo I. A., Tarnopolsky M. A. Free Radicals Biol. Med. 2008;45:503–511. doi: 10.1016/j.freeradbiomed.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Michoulas A., Tong V., Teng X. W., Chang T. K., Abbott F. S., Farrell K. J. Pediatr. 2006;149:692–696. doi: 10.1016/j.jpeds.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Draper H. H., Hardley M. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi Y. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Beutler E. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Sun Y., Oberley L. W., Li Y. Clin. Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- Luck H., Catalase, in Methods in analysis, ed. H. U. Bergmeyer, Academic Press, London, 1955. [Google Scholar]
- Aebi H. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Drabkin D. L., Austin J. H. J. Biol. Chem. 1935;112:51–65. [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Hogendoorn E., van Zoonen P. J. Chromatogr. A. 2000;892:435–453. doi: 10.1016/s0021-9673(00)00151-5. [DOI] [PubMed] [Google Scholar]
- Rashid R. A., Osman K., Ismail M. I., Zuha R. M., Hassan R. A. Trop. Biomed. 2008;25:184–190. [Google Scholar]
- Richardson E. R., Sieber J. N. J. Agric. Food Chem. 1993;41:416–422. [Google Scholar]
- Salmon A. B., Richardson A., Perez V. I. Free Radicals Biol. Med. 2010;48:642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Yedjou C. G., Tchounwou P. B. Environ. Toxicol. 2010;25:221–226. doi: 10.1002/tox.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalender S., Uzun F. G., Durak D., Demir F., Kalender Y. Food Chem. Toxicol. 2010;48:633–638. doi: 10.1016/j.fct.2009.11.044. [DOI] [PubMed] [Google Scholar]
- Eraslan G., Kanbur M., Silici S. Food Chem. Toxicol. 2009;47:86–91. doi: 10.1016/j.fct.2008.10.013. [DOI] [PubMed] [Google Scholar]
- El-Sebae A. H., Enan E. E., Soliman S. A., El-Fiki S., Khamees E. J. Environ. Sci. Health, Part B. 1981;16:475–491. doi: 10.1080/03601238109372273. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Yedjou C. G., Tchounwou P. B. Environ. Toxicol. 2010;25:221–226. doi: 10.1002/tox.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince S., Keles H., Erdogan M., Hazman O., Kucukkurt I. Drug Chem. Toxicol. 2012;35:285–292. doi: 10.3109/01480545.2011.607825. [DOI] [PubMed] [Google Scholar]
- Attia S. M. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2012;741:22–31. [Google Scholar]
- Zhang Z., Gao L., Cheng Y., Jiang J., Chen Y., Jiang H., Hongxiang Y., Anshan S., Baojing C. Biomed. Res. Int. 2014:1–7. [Google Scholar]
- Ince S., Kucukkurt I., Cigerci I. H., Fidan A. F., Eryavuz A. J. Trace Elem. Med. Biol. 2010;24:161–164. doi: 10.1016/j.jtemb.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Possamai F. P., Fortunato J. J., Feier G., Agostinho F. R., Quevedo J., Wilhelm Filho D., Dal-Pizzol F. Environ. Toxicol. Pharmacol. 2007;23:198–204. doi: 10.1016/j.etap.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Alp H., Aytekin I., Hatipoglu N. K., Alp A., Ogun M. Eur. Rev. Med. Pharmacol. Sci. 2012;16:144–148. [PubMed] [Google Scholar]
- Bhatti G. K., Sidhu I. P. S., Bhatti J. S. J. Basic Appl. Sci. 2013;9:438–446. [Google Scholar]
- Toś-Luty S., Obuchowska-Przebirowska D., Latuszyńska J., Tokarska-Rodak M., Haratym-Maj A. Ann. Agric. Environ. Med. 2003;10:101–106. [PubMed] [Google Scholar]
- Alp H., Aytekin I., Esen H., Alp A., Buyukbas S., Basarali K., Hatipoglu N. K., Kul S. Revue Méd. Vét. 2011;162:333–340. [Google Scholar]
- Sharma Y., Bashir S., Irshad M., Nag T. C., Dogra T. D. Toxicol. 2005;215:173–181. doi: 10.1016/j.tox.2005.06.029. [DOI] [PubMed] [Google Scholar]