 The interpretation of high T-2 toxin detection rate and amount in endemic areas on Kashin–Beck disease prevalence and development.
The interpretation of high T-2 toxin detection rate and amount in endemic areas on Kashin–Beck disease prevalence and development.
Abstract
To reveal the influence of T-2 toxin detection rate and detection amount in food samples on Kashin–Beck disease (KBD), and define a linking mechanism between T-2 toxin induced chondrocytes or cartilage damage and KBD pathological changes, seven electronic databases were searched to obtain epidemiological and experimental studies. For epidemiological studies, subgroup analyses of the positive detection rate (PDR) of the T-2 toxin and PDR of the T-2 toxin with concentrations (PDRC of T-2) >100 ng g–1 were carried out, together with a histogram of the T-2 toxin concentrations in different food types in KBD and non-KBD areas. For experimental studies, a systematic review of a variety of chondrocyte and cartilage changes and damage induced by the T-2 toxin was performed. As a result, in epidemiological studies, meta-analysis demonstrated that the T-2 toxin PDR and the overall PDRC of T-2 toxin >100 ng g–1 showed a slightly significant increase in KBD areas than that in non-KBD areas separately. From the histogram, T-2 toxin accumulation was more serious in endemic areas, especially in wheat flour samples. In experimental studies, the T-2 toxin could induce damage of chondrocytes and cartilage, and inhibit cell proliferation by promoting apoptosis and catabolism as well as intracellular injuries, which is similar to the characteristics of KBD. In conclusion, the amount of T-2 toxin detected has a more significant influence on KBD prevalence and development as compared to the T-2 toxin detection rate. Besides, the T-2 toxin induces chondrocyte and cartilage damage through apoptosis, catabolism promotion and intracellular impairment, which is similar to the KBD change.
1. Introduction
T-2 toxin, a kind of trichothecene mycotoxin, is produced by the Fusarium fungus.1 In 1968, the T-2 toxin was separated and purified for the first time by Bamburg et al.2 With a wide range of distribution in many parts of the world,3 the T-2 toxin can be detected in approximately 20% of the food samples from 12 European Union countries.4 Meanwhile, it has been reported that the T-2 toxin was found in up to 65% of corn samples in New Zealand.5 Dietary ingestion is claimed as the most common route for human exposure to the T-2 toxin. Moreover, T-2 toxin contamination shows no specificity to food samples, and can occur in a number of field crops (wheat, maize, barley and oats) and processed grains (malt, beer and bread).1 The T-2 toxin is demonstrated to have a variety of toxic effects on both experimental animals and humans, including dermal toxicity, lethal effects with disruption of the central nervous system, inhibition of protein, DNA and RNA synthesis6 as well as damage of chondrocytes and cartilage.
Kashin–Beck disease (KBD), an endemic, chronic and deformed osteoarthropathic disease, was first reported in 1849.7 KBD mostly occurs from northeastern to southwestern China, south-eastern Siberia and North Korea.8 In China, there are about 0.7 million patients and 105 million residents living in endemic areas that are at risk.9 It is reported that KBD can affect the growth of articular cartilage, and further lead to apoptosis and necrosis of chondrocytes. The common syndromes of KBD are joint pain, stiffness in the morning, motion restriction of the elbow and finger joint, joint enlargement and joint space narrowing.10 The etiology of KBD is still unclear. In China, the proposed risk factors include selenium deficiency, organic acid contamination in drinking water, and fungal contamination of staple grains.11
Previous epidemiological studies have confirmed that the concentration of the T-2 toxin in endemic food samples remains at a high level (2.0–1549.4 ng g–1, with an average of 468.7 ng g–1).8 In addition, it is also reported that the pathologic changes of the cartilage in chicks fed with food containing the T-2 toxin are quite similar to KBD patients in animal studies.8 However, it is still difficult to confirm that the T-2 toxin is one of the important etiological factors for KBD, because discrepancies exist in the detection rate and the amount of T-2 toxin detected from the staple food in KBD endemic and non-endemic areas (in China, national criteria of WS/T 207-2010 (; http://www.moh.gov.cn/zwgkzt/s9500/201006/47920.shtml) and GB 16395-2011 (; http://www.moh.gov.cn/zwgkzt/s9500/201207/55322.shtml) were applied for the diagnosis of KBD and the determination and classification of KBD endemic areas respectively). Since lots of experimental studies have been performed to investigate the mechanism of T-2 toxin in chondrocytes or cartilage damage at present, a comprehensive and systematic review is really needed for better understanding the effects of T-2 toxin on the prevalence and development of KBD.
Therefore, a meta-analysis and systematic review of the effects of T-2 toxin on the prevalence and development of KBD are carried out in the present study. This review will focus on the influence of T-2 toxin detection rate and detection amount in food samples on KBD prevalence and development, as well as the role of the T-2 toxin on chondrocyte or cartilage damage in human or animal subjects and its mechanisms.
2. Materials and methods
2.1. Search strategy
With respect to the search strings: for epidemiological studies, search strings of “KBD” or “Kashin–Beck disease”, “T-2 toxin” and “Endemic detection” were used; and for experimental studies, search strings of “cartilage” or “chondrocyte” and “T-2 toxin” were applied. Seven electronic databases: MEDLINE, Web of Knowledge, EMBASE, Google Scholar, CNKI (Chinese National Knowledge Infrastructure), CBM (Chinese Biomedical Literature Database), and the Wan Fang database were used independently for the search process together with other relevant published studies. There were no restrictions on the language, date, design and publication of the studies. The last update search was conducted on May 29th, 2015.
2.2. Included/excluded criteria
All studies following the search strategy could be divided into epidemiological studies and experimental studies and both of them could be initially included in this article if: (1) they were written in English or Chinese; (2) they had original data and results; (3) for epidemiological studies, they should be related to KBD and the T-2 toxin, the specimens should be food samples, positive detection rates (PDRs) or average content of T-2 toxin should be obtained from KBD endemic and non-endemic areas (intervention and control groups) without any other interventions; (4) for experimental studies, they should address only the effect of T-2 toxin on chondrocyte or cartilage damage, and the research studies on T-2 toxin plus other interventions would be excluded. Studies would be excluded if they failed to meet any one of the criteria.
2.3. Study selection
Firstly, all included titles were screened by three reviewers (LDY, HJ and YFF) in order to remove duplicate studies. Then the abstracts of the selected studies were reviewed if they met the selection criteria. Any articles that did not match the standards were excluded. And after full-text articles were assessed for eligibility, some of them were eliminated because of data duplication or nonconformity to the criteria.
2.4. Methodical evaluation
For the epidemiological studies, after being carefully reviewed, all the included studies were found to be cross-sectional studies. Thus the AHRQ (Agency for Healthcare Research and Quality) standard12 was applied for assessing the studies. According to the standard, 11 items (Table 1) were evaluated by answering with “Yes”, “No” or “Unclear” respectively, including the source of information, the character of the subjects, the quality assessment of the articles and so on.
Table 1. Methodological quality of cross-sectional studies according to the AHRQ standard.
| Luo et al. 199217 | Yang et al. 199518 | Sun et al. 199719 | Feng et al. 200420 | Liu et al. 200421 | Bao et al. 200522 | Sun et al. 201223 | |
| (1) Define the source of information (survey, record review) | Y | Y | Y | Y | Y | Y | Y |
| (2) List inclusion and exclusion criteria for exposed and unexposed subjects (cases and controls) or refer to previous publications | Y | Y | Y | Y | Y | Y | Y |
| (3) Indicate time period used for identifying patients | Y | Y | Y | U | Y | Y | Y |
| (4) Indicate whether or not subjects were consecutive if not population-based | Y | Y | Y | Y | Y | Y | Y |
| (5) Indicate if evaluators of subjective components of study were masked to other aspects of the status of the participants | U | U | U | U | U | U | U |
| (6) Describe any assessments undertaken for quality assurance purposes (e.g., test/retest of primary outcome measurements) | U | Y | Y | Y | U | U | U |
| (7) Explain any patient exclusions from analysis | U | U | U | U | U | U | U |
| (8) Describe how confounding was assessed and/or controlled | U | U | U | U | U | U | Y |
| (9) If applicable, explain how missing data were handled in the analysis | U | U | U | U | U | U | U |
| (10) Summarize patient response rates and completeness of data collection | Y | Y | Y | Y | Y | Y | Y |
| (11) Clarify what follow-up, if any, was expected and the percentage of patients for which incomplete data or follow-up was obtained | U | U | U | U | U | U | U |
Experimental studies were divided into in vitro studies and in vivo studies. Due to the lack of an agreed evaluation standard at present, the “Evidence Pyramid”13 and the grading system of the previous studies14,15 were used. For the in vitro studies, the articles were evaluated according to the following standards: (A) systematic reviews (including meta-analyses) of studies in vitro; (B) with comparable baseline; (C) baseline unknown; and (D) no comparable baseline. For the in vivo studies, the evaluation standards used were the following: (A) systematic reviews (including meta-analyses) of studies in animals; (B) randomized controlled studies, or inbred animal studies; (C) controlled studies; and (D) non-controlled studies.
2.5. Data extraction and collection
For the epidemiological studies, data were extracted from cross-sectional studies after all the selected articles had been reviewed, including study design, location, total number of food samples, types of investigated food in each area, the number of samples with detectable T-2 toxin, T-2 toxin content >100 ng g–1 and the distribution (i.e., medians, means) of T-2 toxin in different types of food samples.
For the experimental studies, because of the heterogeneity across the data, descriptive methods and data extraction tables were used for extracting experimental data from every study following PICO (P: sources, I: interventions, C: control study, O: outcomes) standards. Data extraction was performed by two independent reviewers (LDY and HJ); any disagreement was resolved by consensus.
2.6. Data analysis
In epidemiological studies of selected cross-sectional articles, meta-analysis (subgroup analysis) of the PDR of T-2 toxin and PDR of T-2 toxin with concentrations (PDRC of T-2 toxin) >100 ng g–1 in KBD and non-KBD areas was performed according to food types by using Stata 12.0, the relative risks (RRs) with 95% confidence intervals (CIs) were estimated. The heterogeneity was quantified by the I2 statistic among different studies. A “Fixed-effect” model was used when the heterogeneity was statistically insignificant, otherwise a “Random-effect” model was used (when P < 0.05) to pool RRs. Low, moderate and high heterogeneity were considered when I2 = 25%, 50%, 75% separately. In addition, a histogram of the T-2 toxin concentrations in various food types from endemic and non-endemic regions was shown by using Microsoft Excel 2003.
In the experimental studies, we reviewed the effects of T-2 toxin on chondrocytes and cartilage from humans and animals. In in vitro studies, the discrepancies of the morphological and ultrastructural changes of chondrocytes, cell viability and proliferative activity discrepancies, as well as the metabolism, apoptosis of chondrocytes and other changes in chondrocytes were estimated. Furthermore, the morphological and radiological changes of chondrocytes and cartilage, intracellular changes of chondrocytes and metabolism of the extracellular matrix in cartilage were investigated as well. The supposed toxic mechanism of the T-2 toxin on the prevalence and development of KBD, including chondrocytes and cartilage damage through apoptosis, catabolism promotion and intracellular impairment, was proposed by drawing a conclusion from the extracted data.
3. Results
3.1. Search results and study quality
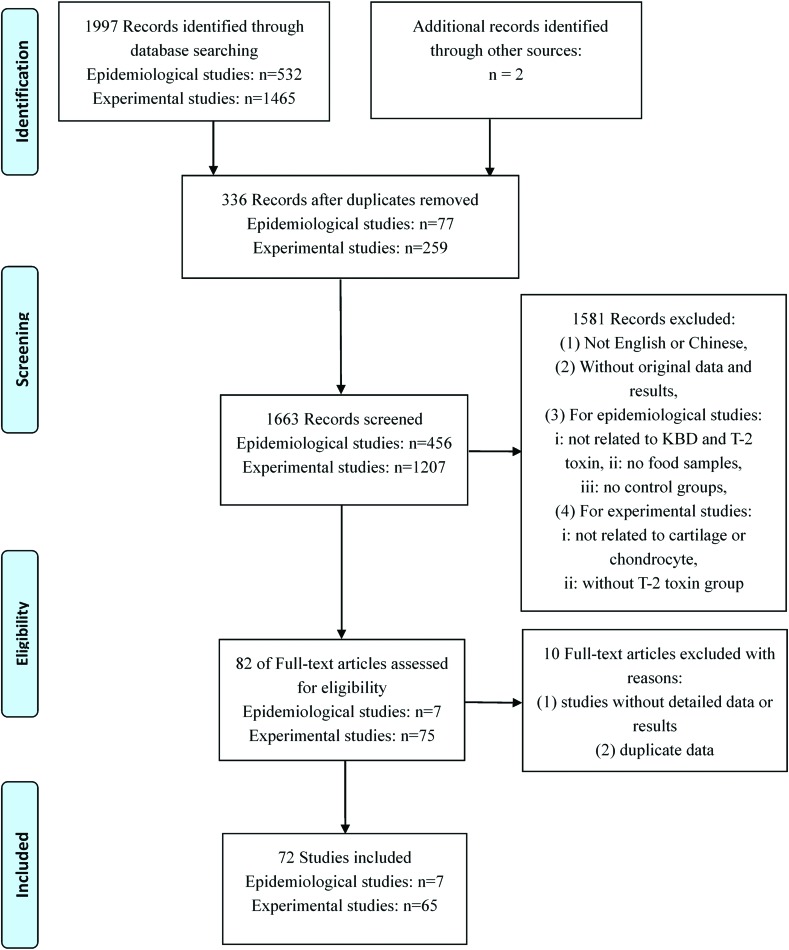
A total of 1999 citations were initially included in this article. After the titles or abstracts were reviewed, 82 articles were enrolled for full text reviewing. Finally, 72 articles were selected and assessed against the exclusion criteria, including seven epidemiological articles and 65 experimental articles [33 in vitro studies and 33 in vivo studies (one article covers both the in vitro and in vivo studies)16] (Fig. 1).
Fig. 1. Flow chart of the study selection process.
The methodological quality of all included cross-sectional studies of the epidemiological studies were basically in accordance with the selection requirements, as most of the studies were assessed as having five or six “Yes” answers to the items of the AHRQ standard (Table 1). Meanwhile, for experimental studies, all the in vitro studies were evaluated as grade B with a comparable baseline according to the previously mentioned criteria. Additionally, 29 of the in vivo studies were randomized controlled studies (RCTs), and four were controlled studies.
3.2. Accumulation of T-2 toxin in food samples of epidemiological studies
3.2.1. Characteristics of epidemiological studies
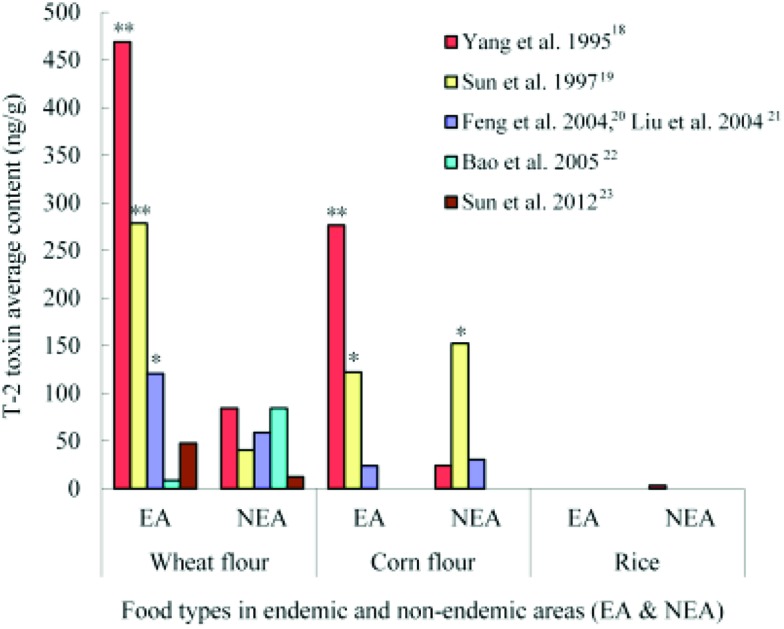
The characteristics of all included 15 epidemiological studies in seven articles17–23 are shown in Table 2. Most of the investigations were performed from 1990 to 2010 in the Northwest and Northeast of China. Four kinds of food including wheat flour (six studies), wheat (two studies), corn flour (five studies) and rice (two studies), were investigated in these studies. Ten food studies showed the results of the PDR of the T-2 toxin with a maximum rate of 100% in five KBD and one non-KBD areas.19,22 The highest content of T-2 toxin in the average of wheat flour samples in endemic regions was 468.7 ng g–1 23 and 152.1 ng g–1 in the control regions,19 respectively.
Table 2. Baseline characteristics of included cross-sectional studies of T-2 toxin exposure in food samples.
| Ref. | Sites | Food type | Endemic areas |
Non-endemic areas |
||||||||
| Number of samples |
T-2 toxin (ng g–1) |
Number of samples |
T-2 toxin (ng g–1) |
|||||||||
| Total | Positive | PDR (%) | PDRC > 100 | Average | Total | Positive | PDR (%) | PDRC > 100 | Average | |||
| Luo et al. 199217 | Xi'an city, Shanxi, Shandong, Jilin, Qinghai and Neimenggu provinces | Wheat | 16 | 0 | 0 | — | — | 7 | 0 | 0 | — | — |
| Corn flour | 67 | 0 | 0 | — | — | 10 | — | — | — | — | ||
| Yang et al. 199518 | Sichuan and Shaanxi provinces | Wheat flour | 15 | 10 | 66.67 | 8 | 468.7 | 15 | 10 | 66.67 | 3 | 84.2 |
| Corn flour | 8 | 4 | 50 | 3 | 276.3 | 7 | 4 | 57.14 | 0 | 23.9 | ||
| Rice | 3 | 0 | 0 | — | 0 | 15 | 5 | 33.33 | — | 3.1 | ||
| Sun et al. 199719 | Fuyu and Shuangcheng counties | Wheat flour | 10 | 10 | 100 | — | 278.4 | 5 | 5 | 100 | — | 40.3 |
| Corn flour | 5 | 5 | 100 | — | 122.0 | 5 | 4 | 80 | — | 152.1 | ||
| Feng et al. 200420 | Heilongjiang province and Fuyu village | Wheat flour | 27 | 21 | 77.78 | 7 | 120.64 | 130 | 80 | 61.53 | 8 | 58.74 |
| Corn flour | 25 | 13 | 52.00 | — | 23.73 | 130 | 43 | 33.07 | — | 30.41 | ||
| Rice | 130 | 9 | 6.92 | — | 17.2 | — | — | — | — | — | ||
| Liu et al. 200421 | Fengtian and Linmao villages, North East and North China areas | Wheat flour | 27 | 21 | 77.78 | 8 | 120.64 | 130 | 80 | 61.53 | 12 | 58.74 |
| Corn flour | 25 | 13 | 52.00 | — | 23.73 | — | — | — | — | — | ||
| Bao et al. 200522 | Nenjiang county and Shitougou village | Wheat flour | 16 | 16 | 100 | — | 8.58 | 15 | 10 | 66.67 | — | 84.2 |
| Sun et al. 201223 | Xinghai and Tongde counties | Wheat flour | 171 | 171 | 100 | 19 | 47.47 | 30 | — | — | — | 12.23 |
| Wheat | 153 | 153 | 100 | 41 | 78.91 | — | — | — | — | — | ||
3.2.2. Meta-analysis of PDR of T-2 toxin in epidemiological studies
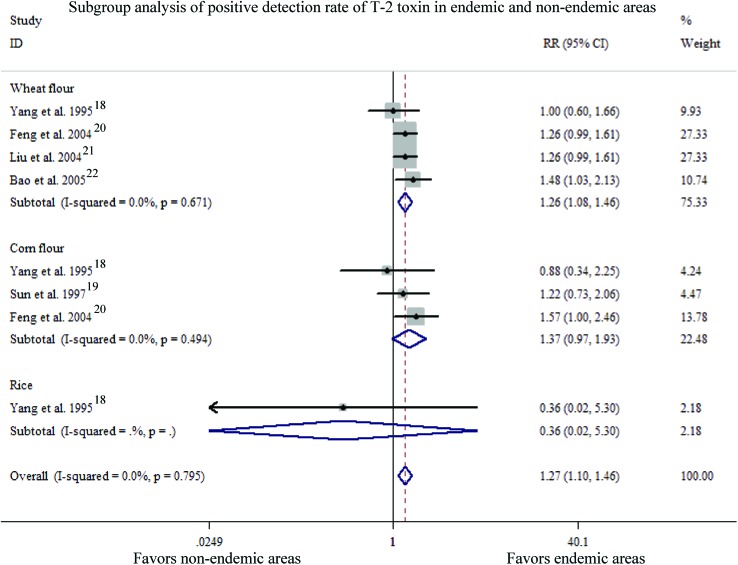
Subgroup analysis of eight studies in five articles18–22 was pooled to measure the difference of PDR of the T-2 toxin between endemic and normal areas (Fig. 2). The heterogeneity of the studies was examined with the “Fixed-effect model”, which showed no statistically significant differences in the heterogeneity of the studies within the different subgroups (overall: P = 0.795, I2 = 0.0%; wheat flour: P = 0.671, I2 = 0.0%; corn flour: P = 0.494, I2 = 0.0%; rice: only one study). The overall PDR of the T-2 toxin in endemic regions was slightly higher than that in control regions [Pooled RR = 1.27, 95% CI (1.10, 1.46)] indicating a significant difference in efficacy (Z = 3.26, P = 0.001). In addition, the T-2 toxin detection rate in wheat flour was a bit higher in KBD areas than that in control areas, but no obvious differences were observed in the T-2 toxin detection rate in the corn flour or rice in KBD areas when compared with that in control areas [wheat flour: RR = 1.26, 95% CI (1.08, 1.46); corn flour: RR = 1.37, 95% CI (0.97, 1.93); rice: RR = 0.36, 95% CI (0.02, 5.30)]. Furthermore, the efficacy showed a significant difference in wheat flour between KBD areas and control areas (wheat flour: Z = 3.03, P = 0.002; corn flour: Z = 1.81, P = 0.070; rice: Z = 0.74, P = 0.459).
Fig. 2. Subgroup analysis of the positive detection rate of the T-2 toxin in endemic and non-endemic areas.
3.2.3. Meta-analysis of PDRC of T-2 toxin >100 ng g–1 in epidemiological studies
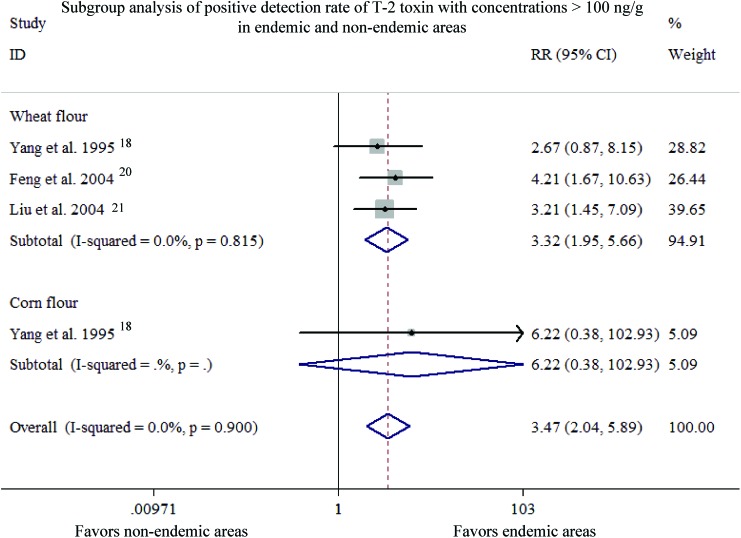
A total of four studies in three articles20,21,23 were included for assessing the PDRC of T-2 toxin >100 ng g–1 in different subgroups for meta-analysis (Fig. 3). Since the heterogeneity of studies was insignificant within different subgroups (overall: P = 0.900, I2 = 0.0%; wheat flour: P = 0.815, I2 = 0.0%; corn flour: only one study), the “Fixed-effect model” was applied. The overall PDRC of T-2 toxin >100 ng g–1 was much higher in KBD areas than that in normal areas with pooled RR = 3.472, 95% CI (2.045, 5.895), which indicated a significant difference in efficacy (Z = 4.61, P < 0.001), meanwhile, the PDRC of the T-2 toxin >100 ng g–1 was significantly higher in wheat flour than that in corn flour between endemic regions and non-endemic regions [wheat flour: RR = 3.32, 95% CI (1.95, 5.66); corn flour: RR = 6.22, 95% CI (0.38, 102.93)] with a significant difference in efficacy (wheat flour: Z = 4.43, P < 0.001; corn flour: Z = 1.28, P = 0.202).
Fig. 3. Subgroup analysis of the positive detection rate of the T-2 toxin with concentrations >100 ng g–1 in endemic and non-endemic areas.
3.2.4. Difference of T-2 toxin average contents in epidemiological studies
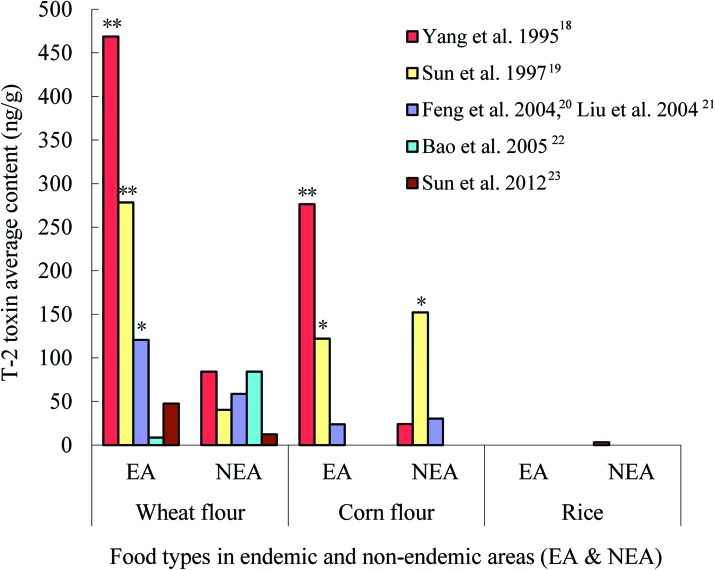
The differences of T-2 toxin contents in different groups were compared with a histogram made from the nine studies in six articles (Fig. 4).18–23 Almost in every study, the average contents of T-2 toxin were much higher in endemic areas than that in normal areas. According to the Food and Agriculture Organization (FAO) standard related to food contamination with the T-2 toxin (the maximum detection of T-2 toxin <100 ng g–1),24 the average contents of the T-2 toxin in five studies were above 100 ng g–1 (three wheat flour samples and two corn flour samples in endemic areas, and one corn flour sample in a non-endemic area) in all nine studies. More seriously, the average contents of the T-2 toxin in three food samples (two wheat flour samples and one corn flour sample) from endemic areas were more than 200 ng g–1,18,19 which exceeded the human tolerance per day based on the standard.25 The T-2 toxin contamination in food samples, especially in the wheat flour samples was obviously existent in the endemic areas.
Fig. 4. Histogram of the T-2 toxin content in endemic and non-endemic areas (EA: endemic areas; NEA: non-endemic areas; *: T-2 toxin average content >100 ng g–1; **: T-2 toxin average content >200 ng g–1).
3.3. Effects of T-2 toxin on chondrocytes or cartilage in experimental studies
3.3.1. Effects of T-2 toxin on chondrocytes in in vitro studies
Morphological observations of chondrocyte damage and cell proliferation
A total of 12 in vitro studies26–37 were involved in the assessment of the damage effects of the T-2 toxin on chondrocyte morphology. As shown in Table 3, the T-2 toxin in different doses could induce the damage of the cell structure in the human fetus, Wistar rats and rabbits with a decrease in cell density and increase of cell separation, and incomplete cytomembrane when observed by an inverted/light microscope. Scanning electron microscopy (SEM) images showed that collagen microfibrils and cytoskeleton were decreased in chondrocytes from a chicken embryo treated with the T-2 toxin. Furthermore, the results of transmission electron microscopy showed that the nucleus, cytoplasmic and endoplasmic reticulum damage could be found in most chondrocytes of the human fetus, Wistar rat and rabbit after the co-culturing of chondrocytes with different doses of T-2 toxin for 4–5 days. Membrane damage could also be detected in rabbit and chicken chondrocytes from these three studies.33–35 The same inhibitory effect on the cell viability and proliferative activity of chondrocytes could be seen in the 14 in vitro studies (Table 4).27,28,38–49 This effect was independent of the concentration of the T-2 toxin.
Table 3. Morphological damage in chondrocytes.
| Ref. | Sources | Interventions |
Outcomes |
|||||||
| T-2 toxin | Time | LM | SEM | TEM (Damage of) |
||||||
| Membrane | Mitochondria | Endoplasmic reticulum | Nucleus | Cytoplasm | ||||||
| Chen et al. 200526 | Human fetus | 1, 10, 20 μg L–1 | 5 d | Y | Y | Y | Y | |||
| Chen et al. 200627 | Human fetus | 10 ng mL–1 | 5 d | Y | Y | |||||
| Chen et al. 200628 | Human fetus | 1, 10, 20 ng mL–1 | 5 d | Y | Y | Y | Y | |||
| Li et al. 200829 | Human fetus | 0.01 μg mL–1 | 18 d | Nucleus fragmentation↑, integral cytomembrane↓, cell ghosts↑ | ||||||
| Huo et al. 199830 | Wistar rat | 0.0005, 0.001, 0.005 mg L–1 | 2 d, 4 d | Cell density↓ | Y | Y | Y | |||
| Wang et al. 200531 | Wistar rat | 0.5, 1.0 μg L–1 | 1 d | Cell falls off↑ | Y | Y | Y | Y | ||
| Cao et al. 199432 | Rabbit | 0.005, 0.01, 0.02 μg mL–1 | 2 d, 4 d | Cell density↓, cell falls off↑ | ||||||
| Cao et al. 199533 | Rabbit | 0.005, 0.01, 0.02 μg mL–1 | 4 d | Cell proliferation↓, cell density↓ | Y | Y | Y | Y | Y | |
| Cao et al. 199534 | Rabbit | 0.005, 0.0 l, 0.02 μg mL–1 | 4 d | Cell density↓, cytoplasmic granules↑, irregular cells↑ | Y | Y | Y | Y | ||
| Li et al. 199335 | Chick embryo | 0.01 ppm | 5 d | Collagen microfibrils↓, cytoskeleton↓ | Y | |||||
| Li et al. 199336 | Chick embryo | 0.01, 0.04 ppm | 4 d | Collagen microfibrils↓, cytoskeleton↓ | ||||||
| Lin et al. 199437 | Chick embryo | 0.01, 0.04 ppm | 4 d | Collagen microfibrils↓, cytoskeleton↓ | ||||||
Table 4. Cell viability and proliferative activity of chondrocytes.
| Ref. | Sources | Interventions |
Outcomes |
||
| T-2 toxin | Time | Cell viability (MTT assay) | Proliferation (Cell counting) | ||
| Wang et al. 201238 | Human (C28/I2) | 1.5625–400 ng ml–1 | 2–5 d | ↓ | |
| Han et al. 201339 | Human | 1–500 ng ml–1 | 2–5 d | ↓ | |
| Liu et al. 201440 | Human | 1–100 ng ml–1 | 3–5 d | ↓ | |
| Yang et al. 200141 | Human fetus | 1–8 μg l–1 | 3–7 d | ↓ | |
| Yang et al. 200142 | Human fetus | 5, 10, 20, 40 μg l–1 | 3–7 d | ↓ | |
| Chen et al. 200627 | Human fetus | 1, 10, 20 ng ml–1 | 3–5 d | ↓ | |
| Chen et al. 200643 | Human fetus | 0.001–8 mg l–1 | 3–5 d | ↓ | |
| Chen et al. 200628 | Human fetus | 1–8000 ng ml–1 | 2–5 d | ↓ | |
| Chen et al. 200844 | Human fetus | 1–8000 ng ml–1 | 3–5 d | ↓ | |
| Chen et al. 201145 | Human fetus | 1–8000 ng ml–1 | 3–5 d | ↓ | |
| He et al. 201146 | Broiler chicken | 10, 100, 1000 nm/5, 50, 500, 5000 nmol l–1 | 3, 6, 9 d/48, 72 h | ↓ | |
| Liu et al. 200847 | Zelanian rabbit | 1, 10, 20, 100 μg l–1 | 1–5 d | ↓ | |
| Liu et al. 201148 | Zelanian rabbit | 1, 10, 20, 100 μg l–1 | 1–5 d | ↓ | |
| Tian et al. 201249 | Murine (ATDC5) | 10, 20, 40, 80 μg l–1 | 6, 12, 24 h | ↓ | |
Apoptosis of chondrocytes
The results of 10 studies26–28,39,40,42,44,46,50,51 were included in the analysis of apoptosis of chondrocytes, and shown in Table 5. In less than five days of T-2 toxin intervention, the apoptotic rate of chondrocytes in humans, human fetus and broiler chicken was significantly increased in a concentration-dependent manner, when analyzed by flow cytometry (FCM) analysis. The mRNA and protein levels of Fas and p53 were increased in human or human fetus chondrocytes after being treated with T-2 toxin. In the Bcl-2 family, Bax mRNA and protein expression were up-regulated, whereas Bcl-xL expression was down-regulated after treatment with the T-2 toxin. The ratio of Bcl-2/Bcl-xL at the protein level was consistent in different studies. Moreover, both caspase-9 and caspase-3 at the protein and mRNA levels increased after T-2 toxin treatment. In addition, JNK, p38 and mitochondrial pathways were involved in mediating the apoptosis by the T-2 toxin.
Table 5. Apoptosis in chondrocytes.
| Ref. | Sources | Interventions |
Outcomes |
|||||
| T-2 toxin | Time | Apoptosis (FCM) | Fas, P53 | Bcl-2 family | Caspases | Others | ||
| Yang et al. 200142 | Human fetus | 5, 10, 20, 40 μg l–1 | 16 h | Y | Apoptosis according to TUNEL staining↑ | |||
| Chen et al. 200526 | Human fetus | 1, 10, 20 μg /l/10 μg l–1 | 5 d/1, 3, 5 d | Y | Bcl-2 (P)↑, Bax (P)↑, Bax/Bcl-2 (P)↑ | |||
| Chen et al. 200627 | Human fetus | 1, 10, 20 ng ml–1 | 5 d | Y | Bcl-2 (P)↑, Bcl-2 (R) (–), Bax (P, R)↑, Bax/Bcl-2 (P)↑ | |||
| Chen et al. 200628 | Human fetus | 1, 10, 20 ng ml–1 | 5 d | Y | Fas (P)↑ | NO↑, iNOS↑ | ||
| Chen et al. 200844 | Human fetus | 1, 10, 20 ng ml–1 | 5 d | Fas (P, R)↑ | Bcl-xL (P, R)↓, Bcl-2 (P, R) (–), | Procaspase-3 (P)↑ | ||
| P53 (P, R)↑ | Bax (P, R)↑, | Caspase-3 (P, R)↑ | ||||||
| Bax/Bcl-2 (P)↑, | ||||||||
| Bax/Bcl-xL (P)↑ | ||||||||
| Yang et al. 200850 | Human fetus | 1, 10, 20 μg l–1 | 5 d | P53 (P, R)↑ | Bcl-xL (P)↓, Bcl-xL (R) (–) | Caspase-3 (P, R)↑ | ||
| Yang et al., 200951 | Human fetus | 1, 10, 20 μg l–1 | 5 d | P53 (P, R)↑ | Bcl-xL (P)↓, Bcl-xL (R) (–) | Caspase-3 (P, R)↑ | ||
| Han et al. 201339 | Human | 20 ng ml–1 | 3 d/24 h | Y | AFT2, JNK and p38↑ | |||
| Liu et al. 201440 | Human | 1, 10, 20 ng ml–1 | 5 d | Y | Caspase-3, 9 (P)↑ | Cytochrome c release↑ | ||
| He et al. 201146 | Broiler chicken | 5, 50, 500 nmol l–1 | 48 h | Y | Caspase-3 (P, R)↑ | Mitochondrial membrane potential↓, pathological aggregation of calcium↑, ROS↑, GPx↑ | ||
Metabolism of chondrocytes
The metabolic inhibition of T-2 toxin-treated chondrocytes was found in the 13 in vitro studies (Table 6).16,29,38,41,43,45–47,49,52–55 After T-2 toxin intervention, the expression of matrix metalloproteinases (MMPs, MMP-1, 3, 13) at the gene and protein levels, aggrecanase-1, 2 mRNAs and a disintegrin and metalloproteinase with thrombospondin motifs 4, 5 (ADAMTS 4, 5) proteins, and pro-inflammatory factors such as IL-1β, IL-6 and TNF-α were increased. Meanwhile, tissue inhibitors of metalloproteinase 1–3 (TIMP 1–3), alpha-2-Macroglobulin (α2M), collagens (total collagen, type I, II, IX), proteoglycan (PG) and aggrecan were reduced both at the protein and mRNA levels, while collagen X expression at the mRNA and protein levels was still controversial. Additionally, other factors such as CD44, hyaluronan synthetase 2 (HAS2) and integrins at the mRNA and protein levels were also changed.
Table 6. Metabolism of chondrocytes.
| Ref. | Sources | Interventions |
Outcomes |
||||||
| T-2 toxin | Time | MMPs, aggrecanase | TIMPs,α2M | ILs, TNFs | Collagens | PG, aggrecan | Others | ||
| Yang et al. 200141 | Human fetus | 8 μg l–1 | 2 d | IL-1β↑, IL-6↑ | |||||
| Li et al. 200452 | Human fetus | — | 5 d/15 d | CD44 (R, P)↓ | |||||
| Chen et al. 200643 | Human fetus | 1, 10, 20 μg l–1 | 5 d | Type II (P, R)↓ | Aggrecan (P, R)↓ | ||||
| Li et al. 200829 | Human fetus | 0.01 μg ml–1 | 5 d | Aggrecanase-2 (R)↑ | IL-1β↑, TNF-α↑ | Aggrecan (R)↓, HA (P)↓ | CD44 (R, P)↓, sCD44 (P)↑, HAS-2 (R)↓ | ||
| Chen et al. 201145 | Human fetus | 1, 10, 20 ng ml–1/10 ng ml–1 | 5 d/14 d | MMP-1 (P, R)↑, MMP-13 (P, R)↑ | TIMP1–2 (R)↓, a2M (P, R)↓ | Type II (P)↓ | |||
| Yu et al. 201253 | Human fetus | l, 10, 20 μg l–1 | 5 d | Aggrecanase-1, 2 (R)↑ | Aggrecan (P)↓ | ||||
| Lu et al. 201254 | Human fetus | 0.01 μg ml–1 | 21 d | MMP1, 3 (P)↑ | TIMP1, 3 (P)↓, α2M (P)↓ | Type II (P)↓, type X (P)↑ | Aggrecan (P)↓ | ||
| Wang et al. 201238 | Human (C28/I2) | 1, 6, 12 ng ml–1 | 3 d | Integrins αv↑, β1↑, α2↓α5↓, β5↓, α1, α3, α6, α10, β3 (R) (–) | |||||
| Chen et al. 201416 | Human (C28/I2) | 20, 40 μg l–1 | 24 h | MMP-13 promoter↑ | |||||
| Cao et al. 200755 | Wistar rat | 0.4, 0.8, 1.6, 3.2 μg l–1 | 24 h | MMP-13 (P)↑ | |||||
| Tian et al. 201249 | Murine (ATDC5) | 20 μg l–1/10–80 μg l–1 | 24 h/1–48 h | MMP-3, 9, 12, 13 (P)↑, ADAMTS4, 5 (P)↑ | Type I, II, IX, X (P)↓ | Aggrecan (P)↓ | HIF-2α (P, R)↑, IκB-α (P)↓, SOX9, Runx2, HIF-1α (R) (–) | ||
| He et al. 201146 | Broiler chicken | 1, 10, 100, 1000 nmol l–1 | 3, 6, 9 d | Total collagen (P)↓ type X (R)↓ | PG (P)↓ | VEGF, Runx2 (R)↓ | |||
| Liu et al. 200847 | Zelanian rabbit | 1, 10, 20, 100 μg l–1 | 5 d | MMP-3 (R)↑ | Aggrecan (R)↓ | ||||
Other intracellular changes in chondrocytes (Table 7)
Table 7. Other intracellular changes in chondrocytes.
| Ref. | Sources | Interventions |
Outcomes | |
| T-2 toxin | Time | |||
| Alteration of DNA and proteins | ||||
| Li et al. 200829 | Human fetus | 0.01 μg ml–1 | 5 d | DNA content↓ |
| Cao et al. 199432 | Rabbit | 0.005, 0.01, 0.02 μg ml–1 | 4 d | DNA content↓, GLcUA content in matrix↓ |
| Huo et al. 199830 | Rabbit | 0.0005, 0.001, 0.005 mg l–1 | 4 d | DNA content↓, protein content ↓ |
| Wang et al. 200656 | Wistar rat | 1, 10, 100 μg l–1 | 24 h | DNA damage↑ |
| Mitochondria damage | ||||
| Liu et al. 201440 | Human | 1, 10, 20 ng ml–1 | 5 d | Citrate synthase (–), complexes I, II (–), III–V↓, ΔΨm↓, ATP↓, ROS↑, GSH↓, GPx↓ |
| Li et al. 199336 | Chick embryo | 0.004, 0.01, 0.04 ppm | 5 d | H+-ATP enzyme↓, cytochrome C oxidase↓, succinate dehydrogenase (–) |
| Li et al. 199335 | Chick embryo | 0.01 ppm | 5 d | H+-ATP enzyme↓, cytochrome C oxidase↓, succinate dehydrogenase (–) |
| Lin et al. 199437 | Chick embryo | 0.004, 0.01, 0.04 ppm | 4 d | H+-ATP enzyme↓, cytochrome C oxidase↓, succinate dehydrogenase (–) |
| Oxidative stress | ||||
| He et al. 201146 | Broiler chicken | 5, 50, 500 nmol l–1 | 48 h | ROS↑, MDA↑, CAT↑, SOD↑, ALP↓, GSH↓ |
| Tian et al. 201249 | Murine (ATDC5) | 10, 20, 40 μg l–1 | 1–24 h | ROS↑ |
| NO synthesis | ||||
| Chen et al. 200628 | Human fetus | 1, 10, 20 ng ml–1 | 2 d, 5 d | NO↑, iNOS↑ |
| Yang et al. 200857 | Human fetus | 1, 10, 20 μg l–1 | 2 d, 5 d | NO↑, iNOS↑ |
Alteration of DNA and proteins. A total of four studies29,30,32,56 related to DNA and protein alteration showed that the T-2 toxin caused DNA damage and the content reduction of DNA, matrix proteins and glucuronic acid (GLcUA) in a concentration-dependent manner (Table 7).
Mitochondrial damage. All four in vitro studies35–37,40 referred to the damage of mitochondria and showed that the T-2 toxin destroyed the antioxidant defense system, including the inhibition of glutathione peroxidase (GPx) activity and intracellular glutathione (GSH) content. The T-2 toxin increased the reactive oxygen species (ROS), but reduced the levels of mitochondrial transmembrane potential (ΔΨm) and cellular adenosine triphosphate (ATP) in a dose-dependent manner. Furthermore, the activities of complexes III–V, H+-ATP enzyme and cytochrome C oxidase rather than complexes I, II, citrate synthase and succinate dehydrogenase were restrained by the T-2 toxin in chondrocytes from the human and chick embryo.
Oxidative stress. The two studies46,49 related to oxidative stress indicated that the levels of ROS and malondialdehyde (MDA) were increased after exposure to the T-2 toxin, while the activities of alkaline phosphatase (ALP) and GSH were decreased. Simultaneously, up-regulated activities of catalase (CAT) and superoxide dismutase (SOD) (two important antioxidases) by the T-2 toxin were observed.
Nitric oxide (NO) synthesis. As shown in studies by Yang et al. and Chen et al.,28,57 NO was increased in a time-dependent manner after the exposure to the T-2 toxin. The expression of inducible nitric oxide synthase (iNOS) also had a significant promotion when treated with the T-2 toxin.
3.3.2. Effects of T-2 toxin on cartilage in in vivo studies
Morphological observation in cartilage
Morphological changes in cartilage after T-2 toxin treatment were investigated in 25 in vivo studies (Table 8),58–82 which were mainly histological and radiological changes. The histological changes included: damage of the epiphyseal growth plate, articular cartilage and chondrocyte necrosis in cartilage after 7-day exposure to T-2 toxin, which could be classified as a short term toxic effect of the T-2 toxin; and the injury of the epiphyseal growth plate, articular cartilage and chondrocyte in 1–6 months, which would be the consequences of the subchronic toxicity effect of the T-2 toxin. However, no effects of T-2 toxin treatment have been found in two studies, which showed no damage on the epiphyseal growth plate after T-2 toxin treatment.73,78 In addition, a study by Pang et al.72 reported a reduction of the bone mineralization rate after 4 week exposure to the T-2 toxin in SD rats’ cartilage. On the other hand, the radiological changes involved in all four studies66,68–70 showed that T-2 toxin treatment caused significant damage of the epiphyseal growth plate in the cartilage of Wistar rats after eight weeks exposure.
Table 8. Morphological and radiological changes in cartilage.
| Ref. | Sources | Interventions |
Outcomes |
||||
| T-2 toxin | Time | Damage of epiphyseal growth plate | Damage of articular cartilage | Chondrocyte necrosis | Retardation of bone mineralization | ||
| Histology changes | |||||||
| Wang et al. 200758 | Wistar rats | 10 μg per kg BW per d/0.1, 0.6 μg per kg BW per d | 7/90 d | Y | Y | ||
| Kang et al. 200959 | Wistar rats | 1 mg per kg BW per d | 2, 4 w | Y | Y | ||
| Wang et al. 200960 | Wistar rats | 100 ng g–1 | 3, 6 m | Y | |||
| Yao et al. 201061 | Wistar rats | 1 mg per kg BW per d | 2, 4 w | Y | |||
| Yao et al. 201062 | Wistar rats | 10 mg per kg BW per d | 4 w | Y | Y | ||
| Yan et al. 201063 | Wistar rats | 0.04 mg per kg BW per d | 1, 2, 4 w | Y | Y | ||
| Meng et al. 201164 | Wistar rats | 100, 200, 300 μg kg–1 | 6 m | Y | Y | ||
| Wang et al. 201165 | Wistar rats | 100 ng g–1 | 6, 10 m | Y | Y | ||
| Yan et al. 201166 | Wistar rats | 0.04 mg per kg BW per d | 4, 8, 12 w | Y | |||
| Sa et al. 201267 | Wistar rats | 100 ng kg–1 | 3, 5 m | Y | Y | ||
| Kang et al. 201368 | Wistar rats | 0.1 mg per kg BW per d | 8, 12 w | Y | |||
| Yan et al. 201469 | Wistar rats | 0.04 mg per kg BW per d | 4, 8, 12 w | Y | |||
| Liao et al. 201470 | Wistar rats | — | 12 w | Y | |||
| Sa et al. 201571 | Wistar rats | 100 ng kg–1 | 5 m | Y | |||
| Pang et al. 200072 | SD rats | 0.267 mg per kg BW per d | 31 d | Y | |||
| Chen et al. 201073 | SD rats | 100, 200 ng per g BW per d | 12 w | N | Y | ||
| Chen et al. 201274 | SD rats | 100, 200 ng per g BW per d | 4 w | Y | |||
| Guan et al. 201375 | SD rats | 100, 200 ng per g BW per d | 4 w | Y | Y | ||
| Zhou et al. 201476 | SD rats | 100, 200 ng per g BW per d | 4 w | Y | |||
| Yang et al. 199477 | Chicks | 100 μg per kg BW per d | 5 w | Y | |||
| Bai et al. 199678 | Chicks | 100 μg per kg BW per d | 30 d | N | |||
| Sun 199779 | Chicks | 100 μg per kg BW per d | 5 w | Y | Y | ||
| Liu et al. 199880 | Chicks | 1.0 mg per kg BW per d | 7 d | Y | |||
| Wang et al. 200681 | Chicks | 100, 600 μg per kg BW per d | 5 w | Y | Y | ||
| Peng et al. 199382 | Chick embryos | 0.1, 0.5 μg | 8 d | Y | |||
| Radiology changes | |||||||
| Yan et al. 201166 | Wistar rats | 0.04 mg per kg BW per d | 8, 12 w | Y | |||
| Kang et al. 201368 | Wistar rats | 0.1 mg per kg BW per d | 8, 12 w | Y | |||
| Yan et al. 201469 | Wistar rats | 0.04 mg per kg BW per d | 8, 12 w | Y | |||
| Liao et al. 201470 | Wistar rats | — | 12 w | Y | |||
Intracellular changes in cartilage (Table 9)
Table 9. Intracellular damage in cartilage.
| Ref. | Sources | Interventions |
Outcomes | |
| T-2 toxin | Time | |||
| Cell growth and metabolism | ||||
| Sun et al. 199583 | Chicks | 100 μg per kg BW per d | 8 w | DNA content↓, protein content↓, DNA fragmentation (–) |
| Sun 199779 | Chicks | 100 μg per kg BW per d | 5 w | DNA content↓, protein content↓, DNA fragmentation (–) |
| Liu et al. 199880 | Chicks | 1.0 mg per kg BW per d | 7 d | DNA fragmentation↑ |
| Liu et al. 199884 | Chicks | 1.0, 2.0 mg per kg BW per d | 1 w | DNA fragmentation↑ |
| Oxidative stress | ||||
| Chen et al. 201274 | SD rats | 100, 200 ng per g BW per d | 4 w | TBARS↑, T-AOC↓, SOD↓, CAT↓, GPX↓, SOD mRNA↓, CAT mRNA↓, GPX mRNA↓ |
| Xue et al. 201385 | SD rats | 100, 200 ng per g BW per d | 30 d | MDA↑, T-AOC↓, SOD↓, CAT↓, GSH-Px↓, SOD mRNA↓, CAT mRNA↓, GPX mRNA↓ |
| Xue et al. 201486 | SD rats | 100, 200 ng per g BW per d | 4 w | MDA↑, T-AOC↓, SOD↓, CAT↓, GSH-Px↓ |
| Apoptosis | ||||
| Yang et al. 201187 | SD rats | 200 ng per g BW per d | 30 d | P53 mRNA↑, Bax mRNA↑, Bcl-2 mRNA↓, caspase-3 mRNA↑ |
Cell growth and metabolism. The inhibition effects of the T-2 toxin on cell growth and metabolism79,80,83,84 were confirmed in four studies (Table 9). The contents of DNA and protein were decreased by 100 μg per kg BW per d T-2 toxin exposure for 5 or 8 weeks. During the exposure of 1.0 mg per kg BW per d T-2 toxin for 1 week on the cartilage of chicks, DNA fragmentation was increased. However, the results were still controversial, and the studies by Sun and Sun et al.79,83 showed an insignificant change of DNA fragmentation after five or eight weeks of 100 μg per kg BW per d T-2 toxin intervention.
Oxidative stress. As shown in Table 9, oxidative stress response was changed with an increase of MDA and thiobarbituric acid reactive substance (TBARS) contents in the cartilage of SD rats fed with 100, 200 ng per g BW per d T-2 toxin in four weeks. Glutathione peroxidase (GSH-Px), glutathione peroxidase (GPX), SOD and CAT at the protein and mRNA levels were decreased.
Apoptosis. With 200 ng per g BW per d T-2 toxin treatment for 30 days, Bax (an apoptosis regulator) at mRNA levels was up-regulated, whereas Bcl-2, as an anti-apoptotic protein was down-regulated. The expression of p53 and caspase-3 was increased in the costal cartilage of SD rats after T-2 toxin treatment.
Metabolism of the extracellular matrix in cartilage
Changes in the cartilage matrix metabolism16,59–64,68,73,75,76,81,88,89 induced by the T-2 toxin are listed in Table 10. In SD rat cartilage, the T-2 toxin at a concentration of 100–200 ng per g BW per d promoted the expression of MMP-13, IL-6, IL-lβ and TNF-α in four weeks. In cartilage from Wistar rats, different doses of the T-2 toxin significantly decreased the total collagen at the beginning of the first week. Meanwhile, changes of collagens with the increase, breakage and desquamation of collagen fibers were observed in the cartilage from Wistar rats after 6 months, but fibrils appeared at 3 months for SD rats. Furthermore, type II collagen was reduced, while type I collagen was increased in the cartilage ECM of chicks when exposed to 100–600 μg per g BW per d T-2 toxin. Proteoglycan and its components (sulfate groups, hexosamine and glucuronic acid) were decreased in the cartilage of Wistar rats after 3–6 months of T-2 toxin intervention. Similarly in the cartilage from SD rats and chicks fed with T-2 toxin, total PG, sulfated glycosaminoglycan (sGAG), keratan sulfate and chondroitin sulfate were also decreased in 4–9 weeks.
Table 10. Metabolism of the cellular matrix in cartilage.
| Ref. | Sources | Interventions |
Outcomes |
||||
| T-2 toxin | Time | MMPs | ILs, TNFs | Collagens | PG, PG components | ||
| Mo et al. 199488 | Wistar rats | 0.2 mg per kg BW per 2 d | 100 d | Total collagen↓ (SP) | Sulfate groups↓ (SP) , hexosamine↓ (SP), glucuronic acid↓ (SP) | ||
| Kang et al. 200959 | Wistar rats | 1 mg per kg BW per d | 2, 4 w | Total collagen↓ (MS) | |||
| Wang et al. 200960 | Wistar rats | 100 ng g–1 | 3, 6 m | Collagen fibers appear↑ (W/VG), collagen fibers breakage and desquamation↑ (SEM) | PG↓ (SEM) | ||
| Yan et al. 201063 | Wistar rats | 0.04 mg kg–1 d–1 | 1, 2, 4 w | Total collagen↓ (MS) | |||
| Yao et al. 201061 | Wistar rats | 1 mg per kg BW per d | 2, 4 w | Total collagen↓ (MS) | |||
| Yao et al. 201062 | Wistar rats | 10 mg per kg BW per d | 4 w | Total collagen↓ (MS) | |||
| Meng et al. 201164 | Wistar rats | 100, 200, 300 μg kg–1 | 6 m | Collagen fibers breakage↑ (SEM) | PG↓ (SEM) | ||
| Kang et al. 201368 | Wistar rats | 0.1 mg per kg BW per d | 8, 12 w | Total collagen↓ (MS) | |||
| Chen et al. 201073 | SD rats | 100, 200 ng per g BW per d | 12 w | Fibrils appear↑ (HE) | |||
| Guan et al. 201375 | SD rats | 100, 200 ng per g BW per d | 4 w | sGAG↓ (TB) | |||
| Chen et al. 201416 | SD rats | 100 μg per kg BW per d | 30 d | MMP-13↑ (IH) | |||
| Zhou et al. 201476 | SD rats | 100, 200 ng per g BW per d | 4 w | IL-6↑, IL-lβ↑, TNF-α↑, IL-6 mRNA↑, IL-lβ mRNA↑ TNF-α mRNA↑ | sGAG↓ (TB) | ||
| Hu et al. 199689 | Chicks | 0.4 mg per kg BW | 9 w | Type I↑, type II↓ (IH) | Keratan sulfate↓, chondroitin sulfate↓ (HC) | ||
| Wang et al. 200681 | Chicks | 100, 600 μg per kg BW per d | 5 w | Type II↓ (W/VG) | PG↓ (AB) | ||
4. Discussion
4.1. Interpretation of the discrepancy of T-2 toxin detection rate and amount
In general, subgroup meta-analysis showed that the overall PDR of T-2 toxin and PDRC of T-2 toxin >100 ng g–1 in food samples was higher in endemic areas, especially in wheat powder. Moreover, T-2 toxin contamination in wheat flour was more serious in KBD endemic areas as compared to non-endemic areas.
A recent study by the meta-analysis of community-based trials of changing grains has demonstrated its benefits for the prevention and treatment of KBD in China,90 which verified that local food might be one of the factors for KBD incidence. As T-2 toxin contamination was the most investigated food contamination in KBD regions, more attention should be paid to the causes of accumulating T-2 toxin as well as the methods of controlling and reducing T-2 toxin in staple food. First of all, because of the climate and soil situation in KBD areas, local residents preferred to cultivate wheat and corn,8,91 and use wheat flour as their main staple food. However, these areas were marked by cold temperature and a humid environment,8,91,92 which provided suitable conditions for T-2 toxin synthesis.93–95 Thus, it would be better for local people to use rice for their staple food, which was also proposed in the study by Sun et al.96 Secondly, in local endemic areas, inadequate food farming, harvesting and processing procedures also increased the opportunity of T-2 toxin propagation.97–99 When most of the cereals and foodstuffs were placed in a moist storage environment and bad sanitary conditions, they might induce more production of the poisoned T-2 toxin.92,97–101 Therefore, the environment for grain processing and storage should be improved such as by improving hygienic conditions, increasing ventilation and reducing wheat flour storage.23
In addition to KBD areas, Yang et al.102 reported that up to 80% of wheat samples from seven provinces in China were contaminated by T-2 toxins in 1992. Our present results indicated that the PDR of the T-2 toxin was up to 60% in most non-endemic survey sites, and PDRC above 100 ng per g T-2 toxin was found in food samples from three non-endemic regions. This phenomenon suggested that the T-2 toxin might easily be generated in food, not only in KBD areas, but also in non-KBD areas. However, there were many standards for evaluating T-2 toxin contamination. When assessed by the FAO standard, the PDRC of the T-2 toxin at 100 ng g–1 in food was claimed as heavy T-2 toxin pollution. While according to the World Health Organization (WHO) standard, the maximum tolerable daily intake of the T-2 toxin was less than 60 ng per kg of body weight per day (which equaled a daily consumption of 500 g staple food containing 7.2 ng per g T-2 toxin for an 60 kg adult).24 Thus, due to the difference between the above two standards, a more reliable standard should be formulated in order to determine T-2 toxin contamination for further steps.
4.2. Interpretation of the results from in vitro, in vivo and KBD studies
4.2.1. Comparison of morphological and ultrastructure damage
The effects of the T-2 toxin in both in vitro and in vivo studies including the damage of the chondrocyte morphology, nucleus, cytoplasm, organelle, and membrane were investigated. The T-2 toxin caused a short term and subchronic toxicity to chondrocytes and induced damage at the subcellular, cellular and tissue levels without species specificity. When compared with the characteristics of KBD patients, some changes of chondrocytes and cartilages induced by the T-2 toxin were quite similar such as focal chondronecrosis in the hypertrophic zone of the growth plate and in the deep zone of the articular cartilage,103,104 suggesting that the T-2 toxin-induced chondrocyte and cartilage damage was probably one of the pathological factors of KBD. Therefore, understanding the complexities of the toxic mechanism should be crucial for the prevention and treatment of KBD. In addition, the mechanism of chondrocyte and cartilage damage induced by the T-2 toxin could be associated with apoptosis, metabolism alteration and intracellular changes.
4.2.2. Comparison of proliferation and alterations of antioxidant capacity
The results from MTT and cell counting showed a restriction effect of the T-2 toxin on the viability and proliferation of chondrocytes. Both in the chondrocytes and cartilage, the contents of DNA and proteins were suppressed in a time and dose-dependent behavior, indicating inhibition of chondrocyte proliferation and metabolism. Besides, the increase of superoxide with decreased antioxidant ability might be responsible for oxidative stress. ROS, MDA, and TBARS were the factors mediating lipid peroxidation activated by the T-2 toxin. In contrast, antioxidants such as GSH and T-AOC were restrained, which reflected the loss of antioxidant capacity. The antioxidases such as CAT, SOD and GSH-Px were restrained in in vivo studies, while CAT and SOD were increased in in vitro studies, which is probably due to the difference of the oxidative stress extent in different chondrocytes and cartilage. In KBD patients, it was reported that TBARS was elevated, while antioxidant enzymes such as T-AOC, SOD, CAT and GPX, were suppressed in the serum,74,105 which were similar to the changes in the T-2 toxin-intervened chondrocyte or cartilage. Meanwhile, ROS was increased as one of the mitochondrial apoptotic factors by T-2 toxin treatment. The T-2 toxin restrained the activities of complexes, H+-ATP enzyme and cytochrome C oxidase, a manifestation of mitochondrial respiratory chain repression. A previous study has demonstrated that mitochondrial damage played an important role in the pathogenesis of KBD.106 Therefore, all the consequences mentioned above indicated the connection of chondrocytes change between T-2 toxin exposure and KBD.
4.2.3. Comparison of apoptosis changes
As mentioned above, the T-2 toxin induced apoptosis in chondrocytes from humans and animals. The T-2 toxin was able to up-regulate Fas and p53 as a pro-apoptotic factor.107,108 The expression of factors of the Bcl-2 family as an important regulator of apoptosis was altered,109,110 especially the expression of Bax in the mRNA and protein levels as well as the ratio of Bax/Bcl-2 and Bax/Bcl-xL at the protein level. A previous study has shown that the ratio of pro-apoptotic and anti-apoptotic proteins in the Bcl-2 family might be the core factor of the apoptosis process,111 so the increase of heterodimerization of the Bcl-2 family indicated chondrocytes apoptosis induced by the T-2 toxin. Under the condition of Bcl-2 family changes, the activity of caspases, especially caspase-3, was finally enhanced to mediate apoptosis indispensably.112,113 As concluded, the T-2 toxin might induce Fas and p53 up-regulation following Bcl-2 family and caspase alteration, which results in chondrocyte apoptosis. In KBD patients, previous studies have demonstrated that the expression of Fas, Bax, Bcl-2 and caspases in chondrocytes also rose,114–117 thus, the mechanism of chondrocyte apoptosis induced by the T-2 toxin is linked to KBD pathogenesis. Besides, the T-2 toxin also caused other mechanisms related to apoptosis such as NO and mitochondrial-related pathways which needed more experiments to confirm. Furthermore, the NO content and iNOS expression were elevated in the serum of KBD patients as well as in the chondrocytes after exposure to the T-2 toxin.118
4.2.4. Comparison of metabolism and ECM degradations
The cartilage matrix consists of several PGs, glycoproteins and collagens, most of which are secreted by chondrocytes. Based on our results, the T-2 toxin perturbs the synthesis of PG and collagens, especially total collagen and type II collagen in in vitro and in vivo studies, thereby promoting an excessive catabolism over anabolism. In the cartilage, the collagen changed after exposure to the T-2 toxin, which demonstrated a metabolic disturbance in the ECM. MMPs, aggrecanases, and ADAMTSs are the most important enzymes of matrix proteolysis. As reported, the degradation of type II collagen and aggrecan was accelerated as a result of the elevated expression of MMP-13 induced by the T-2 toxin.119 Simultaneously, the T-2 toxin triggered up-regulation of aggrecanase-1, 2 activities, which could directly affect the aggrecan degradation. TIMPs and α2M are both inhibitors of the MMPs. After T-2 toxin treatment, cartilage degradation was accelerated because of decreased TIMP 1–3 and α2M expression. Moreover, the T-2 toxin enhanced pro-inflammatory factors including TNF-α, IL-1β and IL-6. All of them act as a kind of catabolic cytokine resulting in matrix degradation. Some other molecules such as CD44 and integrins related to the chondrocyte metabolism were also influenced by the T-2 toxin, as certified in chondrocyte catabolism promotion. In summary, after the cartilage or chondrocytes being exposed to the T-2 toxin, MMPs and α2M were increased while TIMPs and aggrecanases were decreased, which caused the degradation of collagens and PG in ECM as a result. Interestingly, matrix degradation was also found in the development of KBD, including a low type II collagen expression120,121 and decreased PG.10,122 MMP-13 was elevated in the articular cartilage of both KBD121 and OA.123 Pro-inflammatory factors were also increased in the synovial fluid124 and serum of KBD patients.125 All of them showed similar alterations in the chondrocytes and cartilage in both KBD and T-2 toxin intervention.
4.3. Suggestions for further studies
Nevertheless, there are still some limitations to be addressed. For epidemiological studies, data collection among these papers was insufficient. The overall methodological quality of the included studies needed to be improved. So far, all studies on the T-2 toxin were cross-sectional studies, which lacked continuous and systemic investigation, although most of them could be traced back to at least 10 years in the Northeast of China. Therefore, high-quality and well-designed experiments are required. It is suggested that survey locations could be expanded in more KBD regions and focus more on T-2 toxin concentration in different food types with a unified measurement control condition. In addition, the results may be limited by potential bias in that few studies referred to the evaluation of confounding factors. Thus, some information, such as the effect of evaluators of subjective components, and the handling of missing data from analysis should be revealed in further studies. As is known, KBD may be influenced by many factors such as low selenium, iodine of the grains and other mycotoxins such as moniliformin (MON) and deoxynivalenol (DON). More details should be provided when measuring the T-2 toxin content in food. In addition, the relationship between the T-2 toxin and other factors still needs to be investigated in future studies.
For experimental studies included in this article, they were almost at the B level with regard to quality but the evaluation standard was insufficient. Further standards need to be improved to assess the relevant experimental studies accurately. According to our results, the T-2 toxin could destroy the chondrocytes and cartilage through a variety of pathways including apoptosis, changes of metabolism, DNA and protein, oxidative stress, mitochondrial damage and NO synthesis. Some of these pathways are linked to each other, such as the connection of mitochondrial dysfunction and apoptosis,109,126,127 matrix destruction128,129 as well as apoptosis and metabolism degradation.130,131 Additionally, some factors, such as ROS and pro-inflammatory factors are thought to have effects on different pathways. ROS can play an important role in apoptosis,132,133 matrix degradation,133 and is considered a mediating factor of intracellular regulation. Other studies demonstrated that pro-inflammatory factors were able to enhance NO134 and iNOS57 production and induce chondrocyte apoptosis as well.135,136 However, whether the T-2 toxin has direct or indirect effects on these connected pathways and the involved factors are not completely confirmed yet. Moreover, since the T-2 toxin in the body is metabolized to HT-2,137 some results could be different between in vitro and in vivo experiments with T-2 toxin exposure. Hence, it is necessary to clarify different toxic effects of T-2 and HT-2 toxins on in vitro experiments as well. Finally, cartilage is not the only targeted organ of the T-2 toxin, some articles83,137 reported that the T-2 toxin could result in damage in other organs such as the heart, liver, etc. causing diseases such as Keshan disease, alimentary toxic aleukia (ATA)138 and osteoarthritis.79 Thus, an overall review of the effect of the T-2 toxin on these organs and diseases is also needed to be investigated in further studies.
In order to confirm the etiology of KBD, the most convincing evidence is in accordance with the results from the cohort and case-control studies in epidemiology. But no studies have directly shown the causality of the T-2 toxin and KBD at present. Further confirmation of the etiologic relationship is needed in subsequent epidemiological investigations. Moreover, with further investigations resulting in the definition of clear clinical signs of the T-2 toxin detection rate in KBD patients, we may draw a more reasonable conclusion about the effects of the T-2 toxin on KBD prevalence. However, no data on the T-2 toxin concentration in the human body has been obtained in any of the studies yet. This review indicates a high-degree of similarity in the pathology and mechanism of the T-2 toxin and KBD. Combining the summarized results of cross-sectional studies and experimental studies, the T-2 toxin is a likely cause for KBD prevalence. But to some extent, the conclusion is still preliminary. Current experimental studies have only provided a possible explanation for the effect of the T-2 toxin on the pathogenesis of KBD based on similar comparison results, and a correlation between KBD and T-2 toxin is simply presented in cross-sectional, in vitro and in vivo studies, which excludes population-based studies due to ethical constraints. Our present results may provide a new insight for better understanding the effect of the T-2 toxin on the etiology and pathogenesis of KBD.
Conflict of interests
The authors declare no conflicts of interest. The author's affiliation is as shown on the cover page. The authors are solely responsible for the writing and content of the paper.
Acknowledgments
We thank Professor Eng San Thian from the National University of Singapore for his assistance in the editing of this manuscript. This study was supported by the National Natural Science Foundation of China (81402639, 81472924), the China Postdoctoral Science Foundation (2014M562423) and the Shaanxi Province Natural Science Basic Research Program for Youths (2015JQ8310).
Biographies

Danyang Li
Danyang Li, Female, China. Postgraduate of the College of Public Health, Xi'an Jiaotong University Health Science Center. Research interests: epidemiology and pathogenesis of Kashin–Beck disease. E-mail address: 121819866@qq.com.

Jing Han
Jing Han, Female, China. Lecturer in the Department of Public Health and the Key Laboratory of Trace Elements and Endemic Diseases, Health Science Center, Xi'an Jiaotong University. Areas of research work include (but not limited to) pathogenesis of selenium deficiency and T-2 toxin in the occurrence and development of Kashin–Beck disease (KBD); selenium supplements for the prevention and treatment of KBD; scaffolds for cartilage and bone tissue remolding. E-mail address: bbbishop@126.com.

Xiong Guo
Xiong Guo, Male, China. Professor in the Department of Public Health, and director of the Key Laboratory of Trace Elements and Endemic Diseases, Health Science Center, Xi'an Jiaotong University. Research interests: clinical diagnosis, molecular pathogenesis and prevention of Kashin–Beck disease (KBD), the relationship between low Se as well as other elements, toxins and KBD, and the prevention effect of the supplement Se on KBD; chondrocyte apoptosis, cell differentiation, environmental factors and gene interaction, differentially expressed proteins for KBD. E-mail address: guox@mail.xjtu.edu.cn.

Chengjuan Qu
Chengjuan Qu, Female, Finland. Department of Integrative Medical Biology, Umeå University, Umeå, Sweden. A senior researcher with more than 10-year experience in cartilage tissue engineering with primary chondrocytes, and mesenchymal and pluripotent stem cells. Teaching experience in anatomy and stem cell biology. Docent in Tissue Engineering, Department of Applied Physics, the University of Eastern Finland, Kuopio, Finland. Doctor's thesis on Articular Cartilage Proteoglycan and Biosynthesis, Department of Anatomy, the University of Eastern Finland, Kuopio, Finland. Eight-year clinical experience in diagnosis and examination in Radiology, Tianjin, China. E-mail address: chengjuan.qu@gmail.com.

Fangfang Yu
Fangfang Yu, Male, China. PhD candidate in occupational and environmental health at Xi'an Jiaotong University. Research interests: epidemiology and pathogenesis of Kashin–Beck disease. E-mail address: 844481760@qq.com

Xiaofang Wu
Xiaofang Wu, female, China. Postgraduate of Xi'an Jiaotong University. Major in public health. Research interest is cell toxicity. E-mail address: 1028294561@qq.com.
References
- Lv S. Q., Wang Z. L., Lv S. M., Pang W., Chin. J. Control Endem. Dis., 1996, 11 , 282 –285 , (in Chinese) . [Google Scholar]
- Bamburg J. R., Riggs N. V., Strong F. M. Tetrahedron. 1968;24:3329–3336. doi: 10.1016/s0040-4020(01)92631-6. [DOI] [PubMed] [Google Scholar]
- World Health Organization and International Programme on Chemical Safety, Selected Mycotoxins: Ochratoxins, Trichothecenes, Ergot, World Health Organization, Geneva, 1990. [Google Scholar]
- Schothorst R. C., van Egmond H. P. Toxicol. Lett. 2004;153:133–143. doi: 10.1016/j.toxlet.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Hussein H. M., Franich R. A., Baxter M., Andrew I. G. Food Addit. Contam. 1989;6:49–57. doi: 10.1080/02652038909373738. [DOI] [PubMed] [Google Scholar]
- Huo Y. T., Cao J. L., Foreign Med. Sci., Sect. Medgeogr., 1997, 18 , 55 –58 , , 65 (in Chinese) . [Google Scholar]
- Zhang S. Y. and Mo X. Y., Biochemistry of cartilage and bone cartilage disease, Shaanxi Science and Technology Press, Xi'an, 1996. (in Chinese). [Google Scholar]
- Yang J. B., Chin. J. Endemiol., 1995, 14 , 201 –204 , (in Chinese) . [Google Scholar]
- An Y. H., Jia X. F., Li X. F., He J., Han S. B., Zhang H., Geol. China., 2010, 37 , 539 –563 , (in Chinese) . [Google Scholar]
- Cao J., Li S., Shi Z., Yue Y., Sun J., Chen J., Fu Q., Hughes C. E., Caterson B. Osteoarthritis Cartilage. 2008;16:680–688. doi: 10.1016/j.joca.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Guo X., J. Xi'an Jiaotong Univ., Med. Sci., 2008, 29 , 481 –488 , (in Chinese) . [Google Scholar]
- Rostom A., Dube C., Cranney A., Saloojee N., Sy R., Garritty C., Sampson M., Zhang L., Yazdi F., Mamaladze V., Pan I., McNeil J., Moher D., Mack D. and Patel D., Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms, http://www.ncbi.nlm.nih.gov/books/NBK35156.
- Center SDM: EBM Evidence Pyramid, http://library.downstate.edu/ebmdos/2100.htm.
- Xiao Z., Li C. W., Shan J., Luo L., Feng L., Lu J., Li S. F., Long D., Li Y. P. Am. J. Nephrol. 2011;33:558–566. doi: 10.1159/000328584. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Shan J., Li C. W., Luo L., Lu J., Li S. F., Long D., Li Y. P. Am. J. Nephrol. 2013;37:30–40. doi: 10.1159/000345988. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Cao J. L., Wang Z. L., Ma T. Y., Wang M. Y., He Y., Yang Z. T., Chen C., Chin. J. Endemiol., 2014, 33 , 357 –362 , (in Chinese) . [Google Scholar]
- Luo Y., Zheng J. S., Yang J. S., Liu F., Yoshizawa T., Zhang S. Y., Zhang B. J., Liu K. C., Zhai S. S., Sha R., Wen H., Chin. J. Control Endem. Dis., 1992, 7 , 71 –75 , , 127 (in Chinese) . [Google Scholar]
- Yang J. B., Sun D. J., Wang Z. W., Chin. J. Endemiol., 1995, 14 , 146 –149 , (in Chinese) . [Google Scholar]
- Sun D. J., Liu Y. Q., Li Q. W., Chin. J. Endemiol., 1997, 16 , 207 –209 , (in Chinese) . [Google Scholar]
- Feng J., Cao Y. H., Wang S. P., Gao B., Sun X. N., Chin. J. Endemiol., 2004, 23 , 560 –561 , (in Chinese) . [Google Scholar]
- Liu N., Bao W. S., Li D. A., Feng J., Gao B., Sun X. N., Deng Q., Chin. J. Endemiol., 2004, 23 , 237 –239 , (in Chinese) . [Google Scholar]
- Bao W. S., Feng J., Cao Y. H., Sun X. N., Deng Q. Chin. J. Endemiol. 2005;24:318–319. [Google Scholar]
- Sun L. Y., Li Q., Meng F. G., Fu Y., Zhao Z. J., Wang L. H. Biol. Trace Elem. Res. 2012;150:371–375. doi: 10.1007/s12011-012-9469-7. [DOI] [PubMed] [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives (2001: Geneva, Switzerland), World Health Organization and International Programme on Chemical Safety, Evaluation of certain mycotoxins in food, Fifty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives, World Health Organization, Geneva, 2002. [PubMed]
- Thuvander A., Möller T., Barbieri H. E., Jansson A., Salomonsson A. C., Olsen M. Food Addit. Contam. 2001;18:696–706. doi: 10.1080/02652030121353. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Chu Y. L., Yang Z. T., Cao J. L., Shi Z. L., Li S. Y., Guo X., Wang Z. L., J. Xi'an Jiaotong Univ., Med. Sci., 2005, 26 , 130 –134 , (in Chinese) . [Google Scholar]
- Chen J. H., Chu Y. L., Cao J. L., Yang Z. T., Guo X., Wang Z. L. Food Chem. Toxicol. 2006;44:567–573. doi: 10.1016/j.fct.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Chu Y. L., Cao J. L., Yang Z. T., Shi Z. L., Guo X., Wang Z. L., J. Sichuan Univ., Med. Sci. Edn, 2006, 37 , 583 –586 , (in Chinese) . [PubMed] [Google Scholar]
- Li S. Y., Cao J. L., Shi Z. L., Chen J. H., Zhang Z. T., Hughes C. E. J. Zhejiang Univ., Sci., B. 2008;9:22–33. doi: 10.1631/jzus.B071322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y. T., Cao J. L., Guo X., Chin. J. Endemiol., 1998, 17 , 143 –146 , (in Chinese) . [Google Scholar]
- Wang L. H., Zhang L., Chin. J. Endemiol., 2005, 24 , 291 –293 , (in Chinese) . [Google Scholar]
- Cao J. L., Xiong Y. M., Zhang S. Y., Chin. J. Endemiol., 1994, 13 , 268 –270 , (in Chinese) . [Google Scholar]
- Cao J. L., Xiong Y. M., Zhang S. Y., Mo D. X., Chin. J. Control Endem. Dis., 1995, 10 , 69 –71 , (in Chinese) . [Google Scholar]
- Cao J. L., Xiong Y. M., Zhang S. Y., Mo D. X., J. Xi'an Jiaotong Univ., Med. Sci., 1995, 16 , 249 –251 , (in Chinese) . [Google Scholar]
- Li S. G., Wu L. Y., Sun S., Hong J., Ji H. F., Lu Q. W., Lin Z. H., Yang F. Y., Chin. Biochem. J., 1993, 9 , 81 –86 , (in Chinese) . [Google Scholar]
- Li S. G., Sun S., Wu L. Y., Ji H. F., Hong J., Lin Z. H., Prog. Biochem. Biophys., 1993, 20 , 364 –368 , (in Chinese) . [Google Scholar]
- Lin Z. H., Li S. G., Wu L. Y., Sun S., Lu Q. W. J. Clin. Biochem. Nutr. 1994;17:119–132. [Google Scholar]
- Wang J. L., Luo M. X., Li J., Chen J. H., Fu Q., Wang W., Zhang Z. T., Cao J. L., J Xi'an Jiaotong Univ., Med. Sci., 2012, 33 , 271 –275 , (in Chinese) . [Google Scholar]
- Han J., Guo X. J. Nanopart. Res. 2013;15:1–8. [Google Scholar]
- Liu J. T., Wang L. L., Guo X., Pang Q. J., Wu S. X., Wu C. Y., Xu P., Bai Y. D. PLoS One. 2014;9:e108394. doi: 10.1371/journal.pone.0108394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. F., Jia Z. Q., Shen B., Chin. J. Endemiol., 2001, 20 , 84 –86 , (in Chinese) . [Google Scholar]
- Yang T. F., Zhao B. C., Wang G. L., Chin. J. Endemiol., 2001, 20 , 322 –324 , (in Chinese) . [Google Scholar]
- Chen J. H., Cao J. L., Chu Y. L., Yang Z. T., Shi Z. L., Wang H. L., Guo X., Wang Z. L., J. South Med. Univ., 2006, 26 , 381 –385 , (in Chinese) . [PubMed] [Google Scholar]
- Chen J. H., Cao J. L., Chu Y. L., Wang Z. L., Yang Z. T., Wang H. L. J. Zhejiang Univ. Sci. B. 2008;9:455–463. doi: 10.1631/jzus.B0820013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Chu Y. L., Cao J. L., Wang W., Liu J. Y., Wang J. L. Toxicol. In Vitro. 2011;25:492–499. doi: 10.1016/j.tiv.2010.12.001. [DOI] [PubMed] [Google Scholar]
- He S. J., Study on the mechanism of toxicity of T-2 toxin on primary cultured chondrocytes from chicken tibial growth plate, Nanjing Agricultural University, 2011. (in Chinese). [Google Scholar]
- Liu B., Lu C. Y., Wang Q., Gong X. Y., Chen D. C. Bone. 2008;43:S113–S114. [Google Scholar]
- Liu B., Chen D. C., China Pharm., 2011, 20 , 12 –13 , (in Chinese) . [Google Scholar]
- Tian J., Yan J. D., Wang W., Zhong N. N., Tian L. F., Sun J., Min Z. X., Ma J., Lu S. M. Toxicol. In Vitro. 2012;26:1106–1113. doi: 10.1016/j.tiv.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Yang Z. T., Wang Z. L., Chen J. H., Tan X. W., Cao J. L., Guo X., Xiong Y. M., Chin. J. Control Endem. Dis., 2008, 23 , 412 –415 , (in Chinese) . [Google Scholar]
- Yang Z. T., Chen J. H., Wang Z. L., Cao J. L., Guo X., Xiong Y. M., Tan X. W., J. Environ. Health, 2009, 26 , 283 –286 , (in Chinese) . [Google Scholar]
- Li S. Y., Cao J. L., Shi Z. L., Cao P. H., Li W. B., Chin. J. Endemiol., 2004, 23 , 527 –529 , (in Chinese) . [Google Scholar]
- Yu B. Q., Cao J. L., Chen J. H., Shi Z. L., Wang W. Y., Yang Z. T., Ma T. Y., Wang S. J., Chin. J. Endemiol., 2012, 31 , 46 –50 , (in Chinese) . [Google Scholar]
- Lu M. L., Cao J. L., Liu F. Q., Li S. Y., Chen J. H., Fu Q., Zhang Z. T., Liu J. Y., Luo M. X., Wang J. L., Li J., Caterson B. Cells Tissues Organs. 2012;196:241–250. doi: 10.1159/000335046. [DOI] [PubMed] [Google Scholar]
- Cao Y. H., Wang S. P., Hui Y., Liu N., Chin. J. Endemiol., 2007, 26 , 599 –602 , (in Chinese) . [Google Scholar]
- Wang L. H., Yao H. J., Yang J. B., Chin. J. Control Endem. Dis., 2006, 21 , 212 –214 , (in Chinese) . [Google Scholar]
- Yang Z. T., Guo X., Chen J. H., Wang Z. L., Cao J. L., Xiong Y. M., Tan X. W., Shaanxi Med. J., 2008, 37 , 1115 –1117 , , 1146 (in Chinese) . [Google Scholar]
- Wang L. H., Wang W. G., Yang J. B., Chin. J. Endemiol., 2007, 26 , 596 –598 , (in Chinese) . [Google Scholar]
- Kang P. D., Yan D. L., Yao Y. F., Li X. B., Yang J., Shen B., Zhou Z. K., Pei F. X., Chin. J. Bone Joint Surg., 2009, 2 , 404 –410 , (in Chinese) . [Google Scholar]
- Wang L. H., Shi Y. X., Fu Y., Ma W. C., Jia Q., Contemp. Med., 2009, 15 , 1 –3 , (in Chinese) . [Google Scholar]
- Yao Y. F., Kang P. D., Li X. B., Yang J., Shen B., Zhou Z. K., Pei F. X. Int. Orthop. 2010;34:1351–1356. doi: 10.1007/s00264-010-0966-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y. F., Kang P. D., Li X. B., Yang J., Shen B., Zhou Z. K., Pei F. X., Chin. J. Endemiol., 2010, 29 , 475 –479 , (in Chinese) . [Google Scholar]
- Yan D. L., Kang P. D., Yang J., Shen B., Zhou Z. K., Duan L. J., Deng J. Y., Huang H., Pei F. X. Int. J. Rheum. Dis. 2010;13:266–272. doi: 10.1111/j.1756-185X.2010.01530.x. [DOI] [PubMed] [Google Scholar]
- Meng F. G., Ma W. C., Wang L. H., Chin. J. Endemiol., 2011, 30 , 498 –501 , (in Chinese) . [Google Scholar]
- Wang L. H., Fu Y., Shi Y. X., Wang W. G. Toxicol. Pathol. 2011;39:502–507. doi: 10.1177/0192623310396902. [DOI] [PubMed] [Google Scholar]
- Yan D. L., Kang P. D., Li Y. S., Yang J., Shen B., Zhou Z. K., Deng J. Y., Pei F. X. Int. J. Rheum. Dis. 2011;14:92–97. doi: 10.1111/j.1756-185X.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- Sa R. L., Man W. W., Wang L. H., Chin. J. Endemiol., 2012, 313 , 292 –295 , (in Chinese) . [Google Scholar]
- Kang P., Yao Y., Yang J., Shen B., Zhou Z., Pei F. Osteoarthritis Cartilage. 2013;21:1108–1115. doi: 10.1016/j.joca.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Yan D. L., Song Y. C., Shen B., Kang P. D., Pei F. X. J. Orthop. Surg. Res. 2014;9:39. doi: 10.1186/1749-799X-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J. C., Yang X. B., Li Y., Pei F. X., Kang P. D., Gao F. B. Jt., Bone, Spine. 2014;81:267–268. doi: 10.1016/j.jbspin.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Sa R. L., Wang L. H., Chin. J. Public Health, 2015, 31 , 603 –605 , (in Chinese) . [Google Scholar]
- Pang W., Wang L. J., Wang Z. L., Bi H. Y., Chin. J. Control Endem. Dis., 2000, 15 , 263 –265 , (in Chinese) . [Google Scholar]
- Chen J. H., Wang Z. L., Yang H. J., Xue S. H., Song D. Q., Dong L., Yang Z. T., Tan X. W., Wang W., Yu B. Q., Ma T. Y., Chin. J. Control Endem. Dis., 2010, 25 , 98 –101 , (in Chinese) . [Google Scholar]
- Chen J. H., Xue S. H., Li S. Y., Wang Z. L., Yang H. J., Wang W., Song D. Q., Zhou X. R., Chen C. J. Orthop. Res. 2012;30:1229–1237. doi: 10.1002/jor.22073. [DOI] [PubMed] [Google Scholar]
- Guan F., Li S. Y., Wang Z. L., Yang H. J., Xue S. H., Wang W., Song D. Q., Zhou X. R., Zhou W., Chen J. H., Caterson B., Hughes C. Rheumatol. Int. 2013;33:157–166. doi: 10.1007/s00296-011-2335-7. [DOI] [PubMed] [Google Scholar]
- Zhou X. R., Wang Z. L., Chen J. H., Wang W., Song D. Q., Li S. Y., Yang H. J., Xue S. H., Chen C. Rheumatol. Int. 2014;34:995–1004. doi: 10.1007/s00296-013-2862-5. [DOI] [PubMed] [Google Scholar]
- Yang J. B., Sun D. J., Jin L., Chin. J. Endemiol., 1994, 13 , 1 –2 , (in Chinese) . [Google Scholar]
- Bai X. W., Lv S. M., Bai S., Zhang S. Y., Bi H. Y., Zheng B., Zhang F. J., Wang Z. L., Lv S. Q., Chin. J. Control Endem. Dis., 1996, 11 , 149 –151 , (in Chinese) . [Google Scholar]
- Sun D. J. J. Rheumatol. 1997;2:20–24. [Google Scholar]
- Liu N., Ren Z. H., Chin. J. Endemiol., 1998, 17 , 238 –240 , (in Chinese) . [Google Scholar]
- Wang L. H., Wang W. G., Yang J. B., Chin. J. Endemiol., 2006, 25 , 271 –274 , (in Chinese) . [Google Scholar]
- Peng S. Q., Yu X. L., Wang B. Z., Yang Y., Zheng Z. F., Yang J. S., Chin. J. Control Endem. Dis., 1993, 8 , 258 –259 , , 319 (in Chinese) . [Google Scholar]
- Sun D. J., Yang J. B., Zhang Y. H., Ji Q. W., Chin. J. Endemiol., 1995, 14 , 363 –365 , (in Chinese) . [Google Scholar]
- Liu N., Ren Z. H., Chin. J. Endemiol., 1998, 17 , 72 –74 , (in Chinese) . [Google Scholar]
- Xue S. H., Wang Z. L., Chen J. H., Yang H. J., Song D. Q., Zhao C. H., Acta Nutr. Sin., 2013, 35 , 64 –67 , (in Chinese) . [Google Scholar]
- Xue S. H., Wang Z. L., Chen J. H., Yang H. J., Song D. Q., Zhao C. H., Chin. J. Control Endem. Dis., 2014, 29 , 256 –257 , (in Chinese) . [Google Scholar]
- Yang H. J., Wang Z. L., Chen J. H., Song D. Q., Xue S. H., Zhou X. R., Chen Q., Tan X. W., Yang Z. T., Ma T. Y., J. Xi'an Jiaotong Univ., Med. Sci., 2011, 32 , 272 –278 , (in Chinese) . [Google Scholar]
- Mo X. Y., Peng S. Q., Yang J. S., Zhang F. Y., Chin. J. Endemiol., 1994, 13 , 83 –85 , (in Chinese) . [Google Scholar]
- Hu M. S., Yuan B. L., Yu S. Z., Yang J. S., Bull. Acad. Mil. Med. Sci., 1996, 10 , 26 –29 , (in Chinese) . [Google Scholar]
- Han J., Yu F. F., Chang Z. P., Yang B., Qu C. J., Zhou T. T., Liu R. Y., Guo X. Biomed. Environ. Sci. 2015;28:308–311. doi: 10.3967/bes2015.043. [DOI] [PubMed] [Google Scholar]
- Yang J. B., Chin. J. Endemiol., 1998, 17 , 201 –206 , (in Chinese) . [Google Scholar]
- Yang J. B., Chin. J. Endemiol., 2004, 23 , 3 –6 , (in Chinese) . [Google Scholar]
- Edwards S. G., Investigation of Fusarium mycotoxins in UK wheat production, HGCA Project Report No. 413, London, 2007.
- Muller H. M., Reimann J., Schumacher U., Schwadorf K. Food Addit. Contam. 1998;15:801–806. doi: 10.1080/02652039809374713. [DOI] [PubMed] [Google Scholar]
- Li Q. W., Li D. A., Meng X. Q., Li X. D., Chin. J. Endemiol., 1998, 17 , 355 –358 , (in Chinese) . [Google Scholar]
- Sun D. J., Yang J. B., Li X. D., J. Harbin Med. Univ., 1995, 29 , 283 –285 , (in Chinese) . [Google Scholar]
- Li Q. W., Li D. A., Tang X. B., Li X. D., Jiang G., Chin. J. Endemiol., 1999, 18 , 30 –31 , (in Chinese) . [Google Scholar]
- Xie Y., Sun G. J., Xiong C. L., Wang S. K., Wang H., Wang J. S., Chin. J. Food Hyg., 2005, 17 , 157 –159 , (in Chinese) . [Google Scholar]
- Meng F. G., Li Q., Fu Y., Zhao Z. J., Zhou L. W., Wang H., Liu H., Li D. A., Wang L. H., Chin. J. Endemiol., 2012, 31 , 426 –429 , (in Chinese) . [Google Scholar]
- Wang X. C., Liu X. D., Liu J. C., Wang G., Wan K. Y. Bull. Environ. Contam. Toxicol. 2012;88:396–400. doi: 10.1007/s00128-011-0478-6. [DOI] [PubMed] [Google Scholar]
- Fu Y., Meng F. G., Deng J. Y., Fu X. Y., Huang H., Li D. A., Wang L. H., Chin. J. Endemiol., 2010, 29 , 325 –329 , (in Chinese) . [Google Scholar]
- Yang C. H., Luo X. Y., Ji R., Liu C. Acta Microbiol. Sin. 1992;32:450–455. [PubMed] [Google Scholar]
- Xiong G. Int. Orthop. 2001;25:147–150. doi: 10.1007/s002640100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F. L., Guo X., Zhang R. J., Wang S. J., Zuo H., Zhang Z. T., Geng D., Yu Y., Su M. Osteoarthritis Cartilage. 2007;15:1171–1177. doi: 10.1016/j.joca.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Wang W., Wei S., Luo M., Yu B., Cao J., Yang Z., Wang Z., Goldring M. B., Chen J. Osteoarthritis Cartilage. 2013;21:1781–1789. doi: 10.1016/j.joca.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Zhang F., Guo X., Wang W., Yan H., Li C. PLoS One. 2011;6:e22983. doi: 10.1371/journal.pone.0022983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M., Macdonald K., Chan S. W., Luzio J. P., Simari R., Weissberg P. Science. 1998;282:290–293. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- Waring P., Müllbacher A. Immunol. Cell Biol. 1999;77:312–317. doi: 10.1046/j.1440-1711.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- Hengartner M. O. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Yang E., Korsmeyer S. J. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- Porter A. G., Jänicke R. U. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Na K. S., Park B. C., Jang M., Cho S., Lee do. H., Kang S., Lee C. K., Bae K. H., Park S. G. Mol. Cells. 2007;24:261–267. [PubMed] [Google Scholar]
- Liu J. T., Guo X., Ma W. J., Zhang Y. G., Xu P., Yao J. F., Bai Y. D. Osteoarthritis Cartilage. 2010;18:1218–1226. doi: 10.1016/j.joca.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Wang Y., Guo X., Zhang Z. T., Wang M., Wang S. J., J. South Med. Univ., 2011, 31 , 1314 –1317 , (in Chinese) . [PubMed] [Google Scholar]
- Wang S. J., Guo X., Ren F. L., Zhang Y. G., Zhang Z. T., Zhang F. J., Geng D., Zhongguo Yi Xue Ke Xue Yuan Xue Bao, 2006, 28 , 267 –270 , (in Chinese) . [PubMed] [Google Scholar]
- Wang S. J., Guo X., Zuo H., Zhang Y. G., Xu P., Ping Z. G., Zhang Z., Geng D. J. Rheumatol. 2006;33:615–619. [PubMed] [Google Scholar]
- Zhang B. D., Guo X., Bai G. L., Ping Z. G., Zuo H., Reng F. L., Xu G. Y., Geng D., Chin. J. Endemiol., 2004, 23 , 172 –175 , (in Chinese) . [Google Scholar]
- Visse R., Nagase H. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Wu C. Y., Lei R. H., Tiainen M., Wu S. X., Zhang Q., Pei F. X., Guo X. Exp. Cell Res. 2014;326:240–250. doi: 10.1016/j.yexcr.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Wang W., Guo X., Chen J. H., Xu P., Lammi M. J. J. Rheumatol. 2008;35:696–702. [PubMed] [Google Scholar]
- Li S., Cao J., Caterson B., Hughes C. E. Glycoconjugate J. 2012;29:241–248. doi: 10.1007/s10719-012-9421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeberg L., Nagase H. Biochim. Biophys. Acta. 2012;1824:133–145. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W. S., Yang T. F. Chin. J. Control Endem. Dis. 2000;15:71–72. [Google Scholar]
- Yan D. L., Kang P. D., Shen B., Yang J., Zhou Z. K., Duan L. J. Int. J. Rheum. Dis. 2010;13:406–411. doi: 10.1111/j.1756-185X.2010.01550.x. [DOI] [PubMed] [Google Scholar]
- Estaquier J., Vallette F., Vayssiere J. L., Mignotte B. Adv. Exp. Med. Biol. 2012;942:157–183. doi: 10.1007/978-94-007-2869-1_7. [DOI] [PubMed] [Google Scholar]
- Wang C., Youle R. J. Annu. Rev. Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. N., Wilson G., Pearsall A., Grishko V. I. Mol. Cell. Biochem. 2014;397:195–201. doi: 10.1007/s11010-014-2187-z. [DOI] [PubMed] [Google Scholar]
- Cillero-Pastor B., Rego-Pérez I., Oreiro N., Fernandez-Lopez C., Blanco F. J. BMC Musculoskeletal Disord. 2013;14:235. doi: 10.1186/1471-2474-14-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. M., Fuller C. J., Whittles C. E., Sharif M. Osteoarthritis Cartilage. 2006;15:27–34. doi: 10.1016/j.joca.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Aigner T., Kim H. A. Arthritis Rheum. 2002;46:1986–1996. doi: 10.1002/art.10554. [DOI] [PubMed] [Google Scholar]
- Circu M. L., Aw T. Y. Free Radicals Biol. Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrotin Y. E., Bruckner P., Pujol J. P. Osteoarthritis Cartilage. 2003;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- Schuerwegh A. J., Dombrecht E. J., Stevens W. J., Van Offel J. F., Bridts C. H., De Clerck L. S. Osteoarthritis Cartilage. 2003;11:681–687. doi: 10.1016/s1063-4584(03)00156-0. [DOI] [PubMed] [Google Scholar]
- López-Armada M. J., Caramés B., Lires-Deán M., Cillero-Pastor B., Ruiz-Romero C., Galdo F., Blanco F. J. Osteoarthritis Cartilage. 2006;14:660–669. doi: 10.1016/j.joca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Fernandes J. C., Martel-Pelletier J., Pelletier J. P. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- Li Y. S., Wang Z. H., Beier R. C., Shen J. Z., De Smet D., De Saeger S., Zhang S. X. J. Agric. Food Chem. 2011;59:3441–3453. doi: 10.1021/jf200767q. [DOI] [PubMed] [Google Scholar]
- Mo D. X., Chin. J. Control Endem. Dis., 1995, 10 , 294 –298 , (in Chinese) . [Google Scholar]