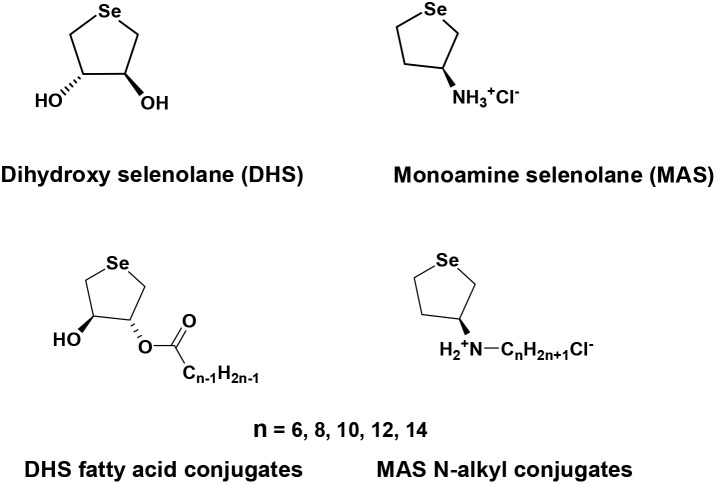
Fatty acid/alkyl group of variable chain lengths (C6–14) as a lipophilic moiety of the dihydroxy selenolane (DHS)/monoamino selenolane (MAS) conjugates not only improved their ability to incorporate antioxidant effects within cells, but also modulated their cytotoxicity.
Fatty acid/alkyl group of variable chain lengths (C6–14) as a lipophilic moiety of the dihydroxy selenolane (DHS)/monoamino selenolane (MAS) conjugates not only improved their ability to incorporate antioxidant effects within cells, but also modulated their cytotoxicity.
Abstract
A series of amphiphilic conjugates of dihydroxy selenolane (DHS) and monoamine selenolane (MAS), which we had previously reported to inhibit lipid peroxidation and assist the oxidative protein folding reaction respectively in cell free systems, were evaluated for cytotoxicity, associated mechanisms and antioxidant effects in cells. Our results indicated that a fatty acid/alkyl group of variable chain lengths (C6–14) as a lipophilic moiety of the DHS/MAS conjugates not only improved their ability to incorporate within the plasma membrane of cells but also modulated their cytotoxicity. In the concentration range of 1–50 μM, C6 conjugates were non-toxic whereas the long chain (≥C8) conjugates showed significant cytotoxicity. The induction of toxicity investigated by the changes in membrane leakage, fluidity, mitochondrial membrane potential and annexin-V–propidium iodide (PI) staining by using flow cytometry revealed plasma membrane disintegration and subsequent induction of necrosis as the major mechanism. Further, the conjugates of DHS and MAS also showed differential as well as nonlinear tendency in cytotoxicity with respect to chain lengths and this effect was attributed to their self-aggregation properties. Compared with the parent compounds, C6 conjugates not only exhibited better antioxidant activity in terms of the induction of selenoproteins such as glutathione peroxidase 1 (GPx1), GPx4 and thioredoxin reductase 1 (TrxR1) but also protected cells from the AAPH induced oxidative stress. In conclusion, the present study suggests the importance of hydrophilic–lipophilic balance (HLB) in fine tuning the toxicity and activity of bioinspired amphiphilic antioxidants.
Introduction
Design, synthesis and development of intracellular enzyme mimics have been the major thrust area of research for biochemists over the years and the latest among these are the glutathione peroxidases (GPx 1, 4 and 7) and protein disulfide isomerase (PDI) models.1,2 GPx is an important antioxidant enzyme, whose major function is to protect the cells from oxidative stress.3 To date seven different isoforms of this enzyme have been reported of which GPx1–4 and GPx6 are selenoenzymes, whereas GPx5 and GPx7 are sulfur containing enzymes.3,4 These isoforms also vary in their subcellular localization, tissue distribution, substrate specificity and apparent biological function.3,4 Among these, GPx1 is the major cytosolic enzyme accounting for most of the cellular GPx activity, which catalyses the reduction of hydroperoxide.3 GPx4 is another cytosolic GPx isoform, which specifically neutralizes the phospholipid hydroperoxides, the chain initiator of lipid peroxidation process.3 However, GPx7 is localized in the endoplasmic reticulum and cooperates with PDI in catalysing the oxidative folding of newly synthesized polypeptide chains using hydroperoxides as a cofactor.4,5 Considering the important functions played by the above enzymes in physiological protection against reactive oxygen species (ROS) and protein folding, it is believed that imitating their functions by using a synthetic molecule will be useful in developing drugs for both antioxidant therapy and protein misfolding induced diseases.1,2 Anticipating this, much effort has been made in the past few decades on the design and synthesis of functionalized organoselenium compounds to mimic GPx-like enzyme and to assist the oxidative protein folding reaction.6–9
Currently, our group is working on a similar research area and has reported the synthesis of simple water-soluble cyclic organoselenium compounds such as dihydroxy selenolane (DHS) and monoamine selenolane (MAS).10–13 Both DHS and MAS were shown to exhibit a wide range of biological activities such as free radical scavenging, mimicking the function of GPx1 and catalysing the oxidative protein folding reaction.10–18 Interestingly, a number of studies have indicated that conjugation of a drug molecule containing alcohol or amino functional group with a fatty acid/alkyl group to yield an ester and/or amide as a pro-drug can be used as a strategy to take advantage of the metabolic enzymes (like esterase) involved in lipid metabolism to increase the membrane affinity, uptake and bioactivity of active principle or the drug.19–21 On similar lines, it was hypothesised that incorporating lipophilicity into the structures of DHS and MAS might allow them to localize in the membranes and catalyse the reduction of lipid hydroperoxide as a GPx4 mimic.17,18 Additionally, such structural modulations can increase their specificity towards the hydrophobic domain of denatured proteins in catalysing oxidative folding reactions like a PDI-GPx7 hybrid system of the cells.13 Keeping these considerations in view, attaching DHS or MAS to a lipophilic moiety such as fatty acids or alkyl groups of variable chain lengths, hereafter referred as the conjugates of DHS or MAS, appeared to be the right strategy to achieve this.19–23 Indeed employing cell free systems, we showed that these conjugates of DHS and MAS could inhibit the accumulation of lipid hydroperoxide and catalyse the folding of denatured proteins respectively.13,17,18 Therefore, such conjugates were projected as better antioxidants compared to the parent compounds such as DHS and MAS.13,17,18 In continuation to these studies, herein DHS and MAS conjugates were evaluated for cytotoxicity, associated mechanisms and antioxidant effects in cells in order to explore them for future biological applications. The chemical structures of DHS, MAS and their conjugates used in the present study are presented in Scheme 1.
Scheme 1. Chemical structures of DHS, MAS and their fatty acid/alkyl conjugates.
Materials and methods
Chemicals
The synthesis and characterisation of parent compounds (DHS and MAS) and their conjugates of varying chain lengths (C6–14) were reported previously,10,13,17,18 except for MAS-C6 conjugate, which was synthesized from MAS and hexanoic acid by following the similar scheme applied for the synthesis of other conjugates of MAS. Spectral data of MAS-C6 are provided as Methods S1.† Butylated hydroxytoluene (BHT), 2,2′-dinitrophenyl hydrazine (DNPH), 1,6-diphenyl-1,3,5-hexatriene (DPH), dimethyl sulfoxide (DMSO), glutathione (GSH), β-nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt hydrate (NADPH), glutathione reductase, cumene hydroperoxide, guanidine hydrochloride, (4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT), thiobarbituric acid (TBA), trichloroacetic acid (TCA), diethyl pyrocarbonate (DEPC), 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH), Cellytic M reagent, Tri reagent, 10× SYBR green polymerase chain reaction (PCR) mix, thioredoxin reductase (TrxR) assay kit, protease inhibitor cocktail, and amplification grade DNase were purchased from Sigma Chemical Company (St. Louis, MO, USA). 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolo-carbocyanine iodide (JC-1) was obtained from Molecular Probes, USA. The lactate dehydrogenase (LDH) assay kit was obtained from Roche, Switzerland. Dulbecco modified Eagle's medium (DMEM), fetal calf serum (FCS), penicillin and streptomycin were purchased from Himedia, India. cDNA synthesis kit was obtained from Thermo Scientific, USA. Annexin-V labeling assay kit was purchased from Abcam, USA. The Bradford protein assay kit was purchased from Bangalore Genei, India. The gene specific primers for RT-PCR were custom synthesized from local agents. All other chemicals with maximum available purity were purchased from reputed local manufacturers/suppliers.
Cell culture and treatment with selenium compounds
Chinese hamster ovary (CHO) and human breast carcinoma (MCF7) cells obtained from National Centre for Cell Sciences (Pune, India) were cultured in DMEM medium supplemented with 10% fetal calf serum, 100 μg ml–1 streptomycin and 100 U ml–1 penicillin and maintained at 37 °C under 5% CO2 and humidified air. The stock solutions of DHS and MAS were prepared in DMEM culture medium and their conjugates in DMSO and then added to the culture medium to obtain the desired concentrations. The hydrolytic stability of the conjugates was confirmed by recording 1H NMR spectra of DHS-C14 (a representative molecule) in deuterated water as a function of time (Fig. S1†). The concentration of DMSO was kept constant within permissible limits of toxicity (0.25%). The cells treated with selenium compounds were incubated under a humidified atmosphere with 5% CO2 for the desired time points prior to assay.
Cytotoxicity assay
Cytotoxicity was estimated by a colorimetric MTT assay as described previously.24 Briefly, cells (0.2, 0.5 and 1 × 104) incubated with increasing concentrations of selenium compounds for 24, 48 and 72 h respectively in triplicates were treated with MTT solution (0.5 mg ml–1 in PBS) for 4 h at 37 °C. The formazan metabolites formed from the reduction of MTT by the living cells were solubilized using 10% SDS in 0.01 N HCl and detected by measuring the absorbance at 550 nm. The percentage (%) cytotoxicity was calculated from the decrease in absorbance of treated samples as compared to that of control cells.
Cell death characterization
For quantifying the cell death types, cells (1 × 105 cells per ml) treated with selenium compounds for 16 h were labeled using an apoptosis assay kit (Abcam, USA) as per the manufacturer's instructions. The labeled cells were acquired on flow cytometer and characterized using FlowJo® software into four groups: healthy, dead due to loss of membrane integrity, apoptotic and necrotic cells. The following staining criteria were adopted for characterization: cells that did not stain for either Annexin-V or Propidium Iodide (PI) as healthy, which stained only with Annexin-V as apoptotic, both PI and Annexin-V as necrotic and only PI as dead cells with ruptured plasma membrane.25
Mitochondrial membrane potential (MMP) assay
MMP was analyzed using an aggregate-forming lipophilic dye JC-1 as described previously.26 In brief, cells (1 × 104) treated with selenium compounds for 2, 4 and 8 h in quadruplicates were incubated with JC-1 (10 μg ml–1, final concentration) for 20 min at 37 °C in the dark. Further, the cells were rinsed twice with ice cold PBS and fluorescence emission at 535 and 610 nm was recorded after excitation at 485 and 565 nm respectively using the multimode microplate reader (Synergy H1, BioTek, USA). The representative images showing green emission and red emission were captured using an Olympus fluorescence microscope (Model no. CKX41, Japan) equipped with ProgRes® camera.
Membrane leakage
Cells (1 × 104) cultured in 96-well plates with selenium compounds for 2, 4, 6 and 24 h in quadruplicates were assayed for membrane leakage by determining the activity of LDH leaking out of the cells into the culture medium, according to the manufacturer's instructions (LDH detection kit, Roche, Switzerland).
Measurement of hemolysis
Blood was collected in heparinised tube by venipuncture from healthy volunteers with strict adherence to the ethical guidelines laid down by the institutional ethics committee of Bhabha Atomic Research Centre. The subject completed the informed consent process prior to participation. The blood samples were processed to obtain a hematocrit or RBCs suspension of 5% in PBS as described previously,27 stored at 4° C and was used within 6 h. The effect of selenium compounds on hemolysis was evaluated by mixing their varying concentrations with the 5% suspension of RBCs in PBS and incubating this reaction mixture at 37 °C with gentle shaking. The aliquots from this reaction mixture were used in a time course manner for a total time of 2.5 h to determine hemolysis by measuring the absorbance at 540 nm. For reference, RBCs were treated with distilled water and the absorbance of the hemolysate at 540 nm was used as 100% hemolysis.
Measurement of membrane fluidity
Cell membrane fluidity was measured by estimating the fluorescence anisotropy value of a lipophilic fluorophore, DPH.28,29 The decrease in anisotropy is indicative of the loss of membrane integrity and/or increase in membrane fluidity.28–31 In brief cells (5 × 106) grown in a culture flask were labeled with DPH at a final concentration of 1 μM at 37 °C for 30 min.32 Following this, selenium compounds were added to the cells and cultured for 2 and 4 h in a humidified incubator at 37 °C with 5% CO2. Upon incubation, cells were harvested by scraping, washing twice with PBS, and then suspending into 1 ml of PBS. Steady-state fluorescence anisotropy measurements were performed on a Jasco FR-6300 spectrofluorometer equipped with excitation and emission polarizers. The excitation and emission wavelengths were set at 365 and 430 nm, respectively.32 Fluorescence anisotropy (r) was calculated using eqn (1):
 |
1 |
where IVV and IVH are the fluorescence intensities determined at vertical and horizontal orientations of the emission polarizer, respectively, when the excitation polarizer is set in the vertical position. The G factor, which compensates for differences in detection efficiency for vertically and horizontally polarized light, was calculated from the fluorescence intensity ratio of vertical and horizontal emissions when the excitation polarizer is set in the horizontal position (IHV/IHH). The spectral bandwidth of the excitation and emission monochromator was set at 2.5 nm.
Estimation of selenium incorporation/uptake by cells
Cells (5 × 106) in 5 ml of culture medium were treated with selenium compounds (25 μM) for 1 h and/or 16 h, harvested by scraping, washing three times with PBS, and suspending into one ml of PBS (pH 7.4). The cell lysate was prepared by disrupting cells five times using Branson Sonifier® (Branson Ultrasonics, USA) at 20% amplitude for 2 seconds each. Further the cell lysate was digested with concentrated nitric acid which oxidises selenide (Se–2) to selenite ion Se+4 and the amount of selenium was estimated by graphite furnace atomic absorption spectrometry (906AA with PAL 3000, GBC Scientific Equipment, Australia) at 197 nm.33 The total amount (membrane + cellular) of selenium quantified from the cell lysate was normalized with respect to the amount of selenium added to the cells and expressed as percent (%) incorporation/uptake.
Monitoring interaction of DHS and MAS conjugates with cells
For this, cells (5 × 106) in one ml of PBS were labeled with DPH (1 μM, final concentration) as described in previous sections.32 In order to remove the unbound DPH molecules, the cells were centrifuged at 2000 rpm for 5 min, washed twice with PBS, and resuspended into 1 ml of PBS. To this long chain (C14) conjugates of DHS and MAS were added at desired concentration and their interaction with the plasma membrane of the cells was monitored by following the changes in the fluorescence emission intensity of DPH (λem = 430 nm) as a function of time (0–45 min) on a Jasco FR-6300 spectrofluorometer after excitation at 365nm. The spectral bandwidth of the excitation and emission monochromator was similar to that of anisotropy studies.
Measurement of aggregation properties
The aggregation properties of the conjugates of DHS and MAS were studied using fluorescence enhancement34,35 of DPH. The aqueous solutions of selenium compounds of varying concentrations were incubated with DPH (1 μM, final concentration) for 30 min at 37 °C. Following this, fluorescence spectra of the above solutions were recorded. The fluorescence enhancement was calculated as the ratio of the fluorescence emission intensity of DPH at λem = 430 nm in the presence (If) and absence (Io) of the selenium compounds. The spectral bandwidth of the excitation and emission monochromator was similar to that of anisotropy studies. It should also be noted here that in the above system DPH molecules will be exclusively excited at 365 nm, because selenium compounds used in the study show negligible absorption at this wavelength.
Measurement of GPx and TrxR activities in cells
Cells (5 × 106) in DMEM were treated with selenium compounds for 16 h in a humidified incubator at 37 °C with 5% CO2, harvested by trypsinization, washed twice with PBS and lysed in cellytic M® containing protease inhibitors cocktail. The lysate was subjected to centrifugation at 10 000g for 10 min and the supernatant obtained was estimated for TrxR activity using a commercially available kit as per the manufacturer's instructions and GPx activity was estimated according to a method described previously.36 The protein content in the cell lysate was determined using the Bradford protein assay kit according to the manufacturer's instructions and results are presented as U mg–1 of protein.
Gene expression studies
Following treatment with selenium compounds for 16 h as in the case of antioxidant enzyme studies, total RNA was isolated from the cells (1 × 106) using TRI reagent (Sigma Chemical Company, St. Louis, MO, USA) according to the manufacturer's instructions. Four micrograms of total RNA was used for the synthesis of cDNA by reverse transcription (cDNA synthesis kit, Thermo Scientific, USA) and real-time PCR was carried out using the template (cDNA), SYBR green master mix and gene specific primers in a Rotor Gene 3000 (Corbett Life Science) machine as described previously. The threshold cycle (CT) values obtained from the above runs were used for calculating the relative expression levels of genes as per the method described previously.37 The expressions of genes were normalized against a housekeeping gene, β-actin. The primers (forward and reverse) used for cDNA amplification are shown in Table 1.
Table 1. List of RT-PCR primers used in the gene expression studies.
| Name of gene | Primer sequence |
| β-actin | 5′-GGCTGTATTCCCCTCCATCG-3′ |
| 5′-CCAGTTGGTAACAATGCCATGT-3′ | |
| GPx1 | 5′-AGTCCACCGTGTATGCCTTCT-3′ |
| 5′-GAGACGCGACATTCTCAATGA-3′ | |
| GPx4 | 5′-TGTGCATCCCGCGATGATT-3′ |
| 5′-CCCTGTACTTATCCAGGCAGA-3′ | |
| TrxR1 | 5′-CCCACTTGCCCCAACTGTT-3′ |
| 5′-GGGAGTGTCTTGGAGGGAC-3′ |
AAPH induced lipid peroxidation and protein carbonylation
Cells (5 × 106) treated with selenium compounds for 16 h were further incubated with AAPH (30 mM) for 6 h in a humidified incubator at 37 °C with 5% CO2 and the cell lysate was prepared as described in the previous section. Lipid peroxidation and protein carbonylation in the cell lysate were assayed according to TBARS and DNPH methods as described previously.36 The amount of TBARS was calculated from a standard plot generated using 1,1,3,3-tetraethoxypropane and expressed as nmol of TBARS per mg of protein. The amount of protein carbonyls was calculated using the extinction coefficient of DNPH (ε370 = 22 000 M–1 cm–1) and expressed as nmol carbonyls per mg of protein.
Statistical analysis
All the experiments were carried out in triplicate and repeated at least two times. The results are presented as means ± SEM, n = 3 from an independent experiment. The data were analyzed by one-way ANOVA using Origin (version 6.1) software to confirm the variability of the data. The P values <0.05 were considered as statistically significant.
Results
Effect of chain length on the cytotoxicity of DHS & MAS conjugates
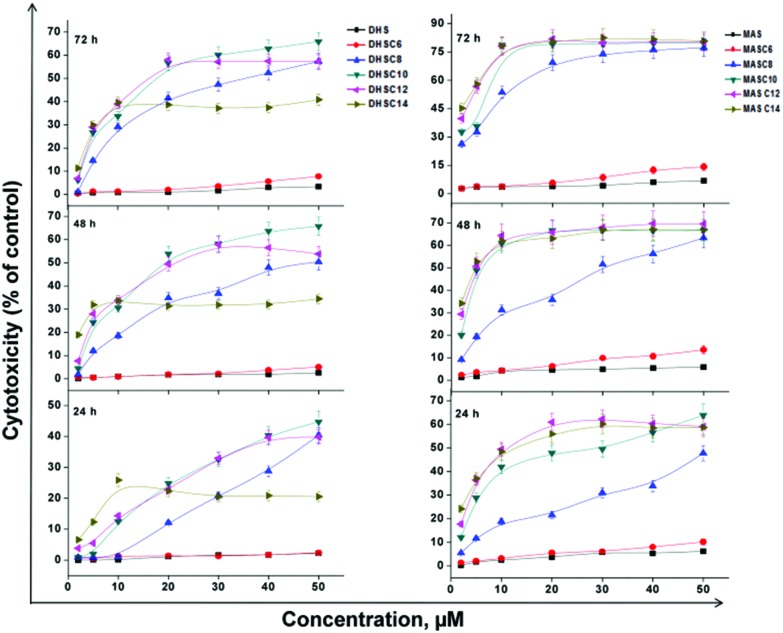
The cytotoxic effects of DHS, MAS and their conjugates (C6–14) in CHO cells evaluated using the MTT assay are shown in Fig. 1. The results indicate that the parent compounds, DHS and MAS in the concentration range (1–50 μM) did not exhibit significant cytotoxicity even after 72 hours of their addition into the cells. The shortest chain (C6) conjugates of DHS and MAS did not show cytotoxicity up to a treatment concentration of 30 μM. Further increase in treatment concentration up to 50 μM showed a concentration and time dependent marginal increase (∼8–15%) in the cytotoxicity. Longer chain (>C6) conjugates of DHS and MAS exhibited significantly higher cytotoxicity compared to the parent compounds or C6 conjugates at all treatment concentrations and time points (Fig. 1). At an identical treatment concentration, the cytotoxicity effects of DHS conjugates followed the order C6 < C8 < C10 ∼ C12 > C14, whereas for MAS conjugates it was seen as C6 < C8 < C10 ∼ C12 ∼ C14 (Fig. S2A and S2B†). The conjugates of intermediate chain lengths (C8–10 of DHS and C8 of MAS) exhibited a concentration (1–50 μM) and time (24–72 h) dependent increase in cytotoxicity. However, the conjugates of longer chain lengths (C12–14 of DHS and C10–14 of MAS) showed a saturation effect (Fig. 1). Between the DHS and MAS conjugates, the former showed significantly lesser cytotoxicity than the latter at each chain length, treatment concentration, when evaluated up to 48 h time point (Fig. 1).
Fig. 1. Cytotoxic effects of DHS, MAS and their conjugates (C6–14) in CHO cells. Cytotoxicity was evaluated by the MTT assay at different time points (24, 48 and 72 h) after the addition of the varying concentrations (1–50 μM) of DHS, MAS and their conjugates (C6–14). Cytotoxicity is expressed as percentage of the control cells (DMSO, 0.25%). Results are presented as mean ± SEM, n = 3.
In continuation to this study, the cytotoxic effects of the above compounds were also evaluated in a tumor cell type, MCF7. As in the case of CHO cells, the parent compounds DHS and MAS were not toxic to MCF7 cells in the concentration range (1–50 μM) tested (Fig. S3†). However, the conjugates exhibited similar trends of cytotoxicity with respect to the lipophilic chain lengths (C6–14), treatment concentrations (1–50 μM) and time points (24–72 h) (Fig. S3†). Additionally at an identical treatment concentration, DHS conjugates exhibited comparable toxicity between the CHO and MCF7 cells, whereas MAS conjugates showed marginally higher toxicity in MCF7 cells compared to CHO cells (Fig. 1, S2 and S3†). In a control experiment, treatment with the fatty acids of variable chain lengths (C6 to C14) without any selenide moiety for 72 hours in the 1–50 μM concentration range did not induce significant toxicity in either of the cell types (Fig. S4†).
Further to characterize the nature of cell death induced by the conjugates, CHO cells treated with the shortest (C6) and longest (C14) chain conjugates of DHS and MAS at an identical concentration of 25 μM were subjected to Annexin-V–PI staining. The representative dot plots and bar graphs are shown in Fig. 2A and B respectively. The results indicated that the parent compounds DHS and MAS and their C6 conjugates neither induced apoptosis nor necrosis confirming the non-toxic nature of these compounds. However, the C14 conjugates of DHS and MAS showed a significant decrease in the counts of viable cells. The major mechanism of cell death was identified to be membrane disruption leading to necrosis as seen by the significant increase in the number of AnnexinV–vePI+ve and Annexin V+vePI+ve cells in these groups (Fig. 2A and B). In line with previous results, the number of viable cells was significantly lower in the MAS-C14 treated group as compared to DHS-C14 suggesting the higher toxicity of former than the latter (Fig. 2A and B).
Fig. 2. Characterization of cell death induced by the conjugates (C6 and C14) of DHS and MAS by Annexin-V and PI staining in CHO cells. The assay was performed at 16 h after addition of the conjugates (C6 and C14) of DHS and MAS to CHO cells at a concentration of 25 μM. (A) Representative dot plots showing distribution of cells under different treatment conditions after flow cytometry acquisition. (B) Bar graph showing percentage (%) live, apoptotic, necrotic and dead cells (membrane disintegration) under different treatment conditions. Results are presented as mean ± SEM, n = 3. *p < 0.05 as compared to the DMSO control group and #p < 0.05 as compared to the DHS-C14 treated group.
Since necrosis is also marked by the acute mitochondrial depolarization, the MMP was estimated using a fluorescent probe, JC-1 under similar experimental conditions and the results are shown in Fig. 3A and B. Treatment of cells with C14 conjugates of DHS and MAS showed a much faster decrease in MMP (estimated as the ratio of red and green fluorescence emission of JC-1 at 535 and 610 nm respectively) as a function of time compared to C6 conjugates or the parent compounds (DHS and MAS) and vehicle (DMSO) control, suggesting acute mitochondrial depolarization by C14 conjugates leading to necrosis (Fig. 3A and B). Here also, MAS-C14 in comparison with DHS-C14 was more effective in reducing the MMP in a time dependent manner (Fig. 3A and B). For example at the end of 8 h, the ratios of red and green fluorescence emission intensity were observed to be 0.13 and 0.01 respectively for cells treated with C14 conjugates of DHS and MAS as compared to 1.21 of control cells (Fig. 3A).
Fig. 3. Effect of the treatment of 25 μM of the conjugates (C6 and C14) of DHS and MAS on the mitochondrial membrane depolarization in CHO cells. The mitochondrial membrane potential was determined by JC-1 staining in CHO cells: (A) Quantitative analysis of red and green fluorescence intensity ratio at 2, 4 and 8 h after addition of selenium compounds to cells. (B) Representative photographs of red and green fluorescence emission at 8 h after addition of selenium compounds to cells. Results are presented as mean ± SEM, n = 3. *p < 0.05 as compared to the DMSO control group and #p < 0.05 as compared to the DHS-C14 treated group.
Effect of chain length on membrane disruption/integrity by DHS & MAS conjugates
The leakage of the intracellular enzyme LDH from cells is considered as a marker of membrane disruption/toxicity. Organochalcogens are known to inhibit LDH,38 therefore it is important to know the suitability of LDH assay to be used in the present study. In order to address this, the effect of the treatment with DHS or MAS on the activity of LDH, freshly isolated from the cells, was evaluated. The results indicated that neither DHS nor MAS affected the activity of LDH (Fig. S5†). Based on this, the effect of DHS, MAS and their conjugates on the plasma membrane integrity was evaluated at an identical treatment concentration of 25 μM by monitoring the leakage of an intracellular enzyme LDH from cells to the culture medium. The results are shown in Fig. 4A and B. It can be seen from the figure that parent compounds DHS and MAS did not induce much leakage of LDH from the cells. Treatment with conjugates (C6–14) of DHS and MAS led to the time dependent increase in the leakage of LDH from the cells compared to the respective parent compounds, and this effect was significant for conjugates with chain lengths longer than C6 suggesting their ability to cause plasma membrane disruption (Fig. 4A and B). DHS conjugates showed a biphasic response with regard to the effect of chain length on LDH leakage at each time point. For example, LDH leakage increased with increasing chain lengths from C6 to C10, saturated at C12 and then decreased at C14. In comparison, MAS conjugates exhibited a chain length dependent increase in LDH release until C12 and the saturation effect at C14 at each time point.
Fig. 4. Effect of the treatment of DHS, MAS and their conjugates (C6–14) on membrane integrity in CHO cells. (A) & (B) Membrane leakage measured as % LDH release induced by the DHS and MAS series of compounds (1–50 μM) respectively at 2, 4, 6 and 24 h after their addition to the cells. *p < 0.05 as compared to the DHS or MAS treated group (C) percent (%) hemolysis in human RBCs induced under different treatment conditions for a total time of 2.5 h. (D) Plasma membrane fluidity measured as the changes in the anisotropy value of a membrane bound fluorophore, DPH at 2 and 4 h after addition of different selenium compounds at 25 μM to the cells. λex = 365 nm, λem = 430 nm. Results are presented as mean ± SEM, n = 3. *p < 0.05 as compared to the DMSO control group and #p < 0.05 as compared to DHS-C14.
Among the DHS and MAS conjugates the former was less effective in causing LDH leakage than the latter at each chain length.
The effect of lipophilic chain length on plasma membrane disruption was revalidated using RBC hemolysis as a model system wherein treatments with 25 μM of parent compounds (DHS and MAS) and their C6 conjugates did not cause hemolysis as time progressed(0.5 to 2.5 h) (Fig. 4C). Whereas treatments with longest conjugates (DHS-C14 and MAS-C14) at identical concentration showed a time dependent increase in hemolysis and this effect was found to be significant for MAS-C14 compared to DHS-C14 supporting our earlier observations of differential effects by these two compounds (Fig. 4C).
Since plasma membrane disruption is marked by the changes in its fluidity, this parameter was evaluated in CHO cells as an anisotropy value of a fluorophore, DPH is known to be localized in the plasma membrane.29,32 The results shown in Fig. 4D indicate that the control cells exhibited a maximum anisotropy value of 0.17. Treatments with parent compounds (DHS and MAS) at 25 μM did not affect the anisotropy value of DPH even after 4 h of their addition to cells, suggesting that these compounds did not cause change in fluidity of the plasma membrane (Fig. 4D). Treatments with C6 and C14 conjugates of DHS and MAS at identical concentration showed a time dependent decrease in anisotropy of DPH and this effect was more prominent at longer chain lengths (C14). The anisotropy value of DPH in cells treated with DHS-C14 and MAS-C14 for 4 h was 0.13 and 0.09 respectively (Fig. 4D). These results thus suggested that the conjugates of DHS and MAS caused membrane disruption resulting in increase in membrane fluidity.
Effect of chain length on the incorporation of DHS & MAS conjugates within membranes
From the above studies, it was anticipated that the conjugation of the lipophilic moiety of variable chain lengths (C6–14) with DHS and MAS might be affecting their ability to incorporate within membranes and/or cells. In order to address this, the incorporation of DHS, MAS and their conjugates within 1 h after addition to cells (CHO) at a treatment concentration of 25 μM was estimated in terms of the selenium level. The bar graph representing the percent loading under different treatment conditions is shown in Fig. 5A. From the figure, it is clear that the basal selenium level in control cells and those treated with parent compounds such as DHS and MAS was undetectable (<10 ng). Treatment with the conjugates of DHS and MAS led to a significant increase in the percent of selenium incorporated into the cells compared to that of the amount present in the control cells (Fig. 5A). The MAS conjugates showed significantly higher loading compared to the DHS conjugates at each chain length (Fig. 5A). The effect of lipophilic chain length on the cellular incorporation of both DHS and MAS conjugates was observed to be biphasic. For example the percent incorporation increased with increasing chain length up to C12 and a further increase in chain length to C14 led to the decrease in loading. The uptake studies performed at an early time point (1 h) may be indicative of the incorporation of conjugates mainly into the plasma membrane of cells. Therefore, the above studies suggested that between DHS and MAS conjugates the latter exhibited greater affinity for cellular membranes and for each of these two series of compounds, such affinity increased up to a length of C12.
Fig. 5. Studies on the affinity of DHS, MAS and their conjugates towards plasma membrane in CHO cells. (A) Effect of alkyl chain length on the incorporation/uptake of selenium into membranes/cells following treatment with DHS, MAS and their conjugates (C6–14) at 25 μM for an hour. The amount of selenium in the cells was determined as described in the materials and methods section and normalized with respect to the treated amount of selenium. Results are presented as mean ± SEM, n = 3. CN – untreated control cells. *p < 0.05 as compared to the DMSO control group and #p < 0.05 as compared to the DHS conjugates at each chain length. (B) & (C) Overlapped fluorescence spectra of CHO cells stained with a membrane bound fluorophore, DPH recorded soon after the addition of DHS-C14 and MAS-C14 respectively to the cell suspension in a time course manner (0–45 min). The excitation was performed at 365 nm. Insets of (B) & (C) show the interaction/binding of DHS-C14 and MAS-C14 respectively with the plasma membrane monitored in terms of the changes in the fluorescence emission (λem = 430 nm) intensity of DPH.
In order to revalidate the above conclusion, the binding/interaction of the longest C14 conjugates of DHS and MAS to the plasma membrane of CHO cells was studied employing DPH as a probe. The fluorescence of DPH is highly sensitive to the changes in polarity of the membrane microenvironment.28,29,32 Earlier it has been shown that time resolved changes in the fluorescence intensity of DPH can be used as a means to understand the binding of a hydrophobic drug to the plasma membrane of cells.39 In the present study, addition of DHS-C14 to the cells at 25 μM did not cause much change in the fluorescence intensity of DPH during the initial 30 min of interaction but decreased at later time points (Fig. 5B). Whereas treatment with MAS-C14 at identical concentration led to a sharp increase in DPH fluorescence by 15 min and then decreased in a time dependent manner (Fig. 5C).
The binding of a lipophilic conjugate to plasma membrane is expected to increase the hydrophobic environment around the DPH molecules resulting in the increase in its fluorescence intensity. However, membrane disruption by the conjugates can cause a decrease in DPH fluorescence. Therefore, our results confirmed that DHS-C14 exhibited lesser affinity towards cells membranes compared to MAS-C14.
Effect of chain length on the self-aggregation properties of DHS and MAS conjugates
The self-aggregation behaviour of the conjugates (C6–14) of DHS and MAS was monitored by measuring the fluorescence intensity (λem = 430 nm) of DPH as a function of their increasing concentrations (2–50 μM) in aqueous solution. DPH shows weak fluorescence in aqueous solution, however incorporation of this molecule into micellar structure or the aggregates, causes a significant increase in the fluorescence intensity.32 The representative emission spectrum and fluorescence enhancement ratio of DPH under different treatment conditions are shown in Fig. 6A–D.
Fig. 6. Aggregation studies of DHS, MAS and their conjugates (C6–14) using fluorescence enhancement of a lipophilic fluorophore DPH. (A) & (B) Overlapped fluorescence spectra of DPH in aqueous solutions of the increasing concentrations (2–50 μM) of DHS and MAS series of compounds respectively containing 0.25% DMSO. (C) & (D) Enhancement in the fluorescence intensity of DPH induced by DHS and MAS series of compounds respectively. If – Fluorescence intensity in the presence of selenium compounds. Io – Fluorescence intensity in the absence of selenium compounds. λex = 365 nm, λem = 430 nm. Results are presented as mean ± SEM, n = 3.
Our results indicated that the fluorescence intensity of DPH did not change much as a function of concentration of DHS or MAS conjugates up to a chain length of C8 and C10 respectively (Fig. 6A–D). However, C10–14 conjugates of DHS and C12–14 conjugates of MAS exhibited a concentration and chain length dependent increase in the fluorescence intensity of DPH suggesting formation of aggregates by the long chain conjugates at higher concentrations (Fig. 6A–D). Between the longer conjugates (≥C10) of DHS and MAS of identical chain length and concentration, the former showed significantly higher enhancement in the fluorescence emission of DPH compared to the latter. For example at a concentration of 25 μM, the longest chain conjugates, DHS-C14 and MAS-C14 showed enhancement in the fluorescence intensity of DPH by ∼20 and ∼8 folds respectively (Fig. 6A–D). This confirmed that longer chain (≥C10) conjugates of DHS exhibited higher tendency of forming aggregates compared to MAS conjugates of identical chain length.
Effect of chain length on the antioxidant activity of DHS & MAS conjugates in the cells
The nontoxic C6 conjugates of DHS and MAS screened from the above experiments were further evaluated for antioxidant effects in CHO cells in terms of their ability to modulate the expression of important antioxidant selenoenzymes (such as GPx1, GPx4 and TrxR1) and also to protect the cells from AAPH induced oxidative damage like lipid peroxidation and protein carbonylation. The results were compared with those of the parent compounds (DHS and MAS). Since the incorporation/uptake of parent compounds into cells was undetectable for a treatment time of 1 h, we increased the treatment time to 16 h and then estimated the uptake. The results indicated that the uptake of DHS and MAS increased only in nanograms, which corresponded to 0.15 ± 0.01% and 0.23 ± 0.01% respectively. The uptake of C6 conjugates of DHS and MAS at 16 h was higher in comparison with parent compounds and showed a saturation effect with respect to early detection (1 h). Furthermore, the results on the antioxidant activities as shown in Fig. 7A revealed that treatments with DHS and MAS at a concentration of 25 μM led to a moderate increase in TrxR activity but a significant increase in GPx activity. The compound MAS was more effective than DHS in inducing the GPx activity. In agreement with the above results, DHS and MAS showed significantly higher induction in the expressions of GPx isoforms (GPx1 and GPx4) than TrxR1 at the mRNA level (Fig. 7B). Whereas the expression of GPx1 was higher in MAS treated cells, another important isoform GPx4 was induced more with DHS (Fig. 7B). The C6 conjugates of DHS and MAS showed even higher induction in the expressions of GPx and TrxR both at mRNA and activity levels compared to their respective parent compounds (Fig. 7A and B). Further, pretreatment of cells with DHS or MAS caused significant reduction in the levels of malondialdehyde in cells exposed to AAPH indicating their ability to protect from the lipid peroxidation (Fig. 7C). The C6 conjugates of DHS and MAS showed an increase in the protection of cells from AAPH induced lipid peroxidation and protein carbonylation compared to the respective parent compounds (Fig. 7C). With regard to the antioxidant enzymes, the exposure of cells to AAPH did not affect the activity of GPx, but led to a significant increase in the activity of TrxR (Fig. 7D). The pretreatment with DHS or MAS did not show much change in the activities of GPx and TrxR compared to the AAPH group (Fig. 7D). On contrary pretreatment with C6 conjugates of DHS and MAS showed significantly elevated GPx activity and no change in TrxR activity compared to the AAPH group (Fig. 7D). Taken together, these results suggested that C6 conjugates are better than the parent compounds in exhibiting antioxidant effects in the cell.
Fig. 7. Antioxidant effects of 25 μM of DHS, MAS and their C6 conjugates in CHO cells. (A) Modulation in the activities of GPx and TrxR at 16 h after addition of the compounds. (B) Modulation in the expression of genes such as GPx1, GPx4, and TrxR1 at 16 h after addition of the compounds. The expressions of above genes in different treatment groups were normalized against the control group and the relative expression changes have been plotted. Actin expression was used as internal control for all the genes. (C) Protective effect of the pretreatment with compounds against the AAPH (30 mM) induced lipid peroxidation and protein carbonylation estimated at 6 h post exposure by TBARS and DNPH assays respectively. (D) Effect of the pretreatment with compounds on activities of GPx and TrxR at 6 h post exposure of AAPH (30 mM). Results are presented as means ± SEM, n = 3. *p < 0.05 as compared to the control group, #p < 0.05 as compared to respective parent compounds DHS and/or MAS, $ p < 0.05 as compared to the AAPH alone group.
Discussion
With an aim of designing new selenium based antioxidants we had earlier established that combining the fatty acid/alkyl group as a lipophilic unit with the redox active hydrophilic selenide moiety such as DHS and MAS is an effective approach.13,17,18 For example, the amphiphilic fatty acid conjugates of DHS were shown to inhibit the lipid peroxidation in the liposomal model system through the GPx4 like catalytic mechanism involving 2e– reduction, whereas the N-alkylated conjugates of MAS catalysed the oxidative folding of misfolded/denatured proteins like a PDI-GPx7 hybrid model.13,17,18 These different activities of the conjugates of DHS and MAS were also reported to be dependent on the chain length of the lipophilic moiety. Since the lipophilicity of a compound is often associated with biological functions as well as the toxicity,40–43 the first parameter that is necessary to be evaluated prior to biological application of the conjugates of DHS and MAS as GPx4 and PDI-GPx7 mimics respectively is their toxicity to the cells.
In order to address this, in the present study, we used two different cell lines CHO and MCF7 representing the normal model and tumor cell type respectively for the cytotoxicity evaluation.40–44 Our results indicated that neither the parent compounds (DHS and MAS) nor the free fatty acids (C6 to C14) in the concentration range of 1–50 μM were toxic to CHO and MCF7 cells. However, the conjugates (≥C8) of DHS and MAS in a similar concentration range were significantly toxic to both the cell types. This prompted us to believe that the amphipathic character resulting from the combination of a hydrophilic head as selenide and lipophilic tail as a fatty acid/alkyl group makes the conjugates membrane active, which finally dictates the cytotoxicity.41,45–48 Interestingly, the fatty acid conjugates of similar chain length containing oxygen in place of selenium in the ring structure (furan fatty acids) have been reported to be antioxidants and non-toxic to cells, confirming that the observed cytotoxicity is indeed due to the selenium moiety.49,50 Further, a recent report indicated that the polarity of the hydrophilic head group affects the surface properties of the amphipathic surfactants.51 Taken together, it can be inferred that selenium by influencing the polarity of the hydrophilic head might be controlling the surface properties and in turn the cytotoxicity of DHS and MAS conjugates. As expected the affinity of the conjugates of DHS and MAS for the plasma membrane was evidenced in terms of their ability to enhance the incorporation of selenium in the membranes per cell within 1 h of the treatment. The nature of cell death induced by the conjugates of DHS and MAS was identified to be necrosis as supported by the increase in the number of PI+veAnnxinV+ve stained cells through flow cytometry.25 The plausible mechanisms of cytotoxicity could be the vertical insertion of the conjugates of DHS and MAS into plasma membrane with their selenide and fatty acid/alkyl chain groups facing towards the polar head and hydrophobic tail of lipid bilayer respectively.46,48 Since the lipophilic chains in conjugates are saturated, it may further allow them to pack together with the hydrophobic tails of the lipids in membrane through hydrophobic interactions to form microcluster or aggregates.46,48,49 Such aggregates can finally cause local disturbance in the dynamics and packing order of lipids and proteins in the membrane resulting in disintegration or pore formation followed by leakage of intracellular constituents, acute depolarisation of mitochondria (the power house of cell) and necrosis.47,48,52–54 Supporting this hypothesis, the conjugates of DHS and MAS were observed to cause an increase in the fluidity (as a drop in anisotropy value of DPH) of plasma membrane, leakage of intracellular proteins like LDH in CHO cells and haemoglobin in RBCs and finally membrane disintegration (PI+ve cells). These findings are also in agreement with the previous studies wherein similar mechanism of membrane disintegration and subsequent cytotoxicity has been proposed for surface active amphipathic drugs like N-alkylated imino sugars and antimicrobial peptides such as magainin and cecropins.41,47,48
Further, MAS conjugates exhibited significantly higher toxicity than DHS conjugates at each chain length. Additionally, the effect of chain length (C6–14) on the cytotoxic effect of the conjugates of DHS and MAS were observed to be nonlinear, where the maximum toxicity was seen at C10. It is well known that the plasma membranes of the mammalian cells are negatively charged.55 Since the conjugates of MAS and DHS are cationic and neutral in nature respectively, the insertion of the former into the plasma membranes is expected to be higher compared to the latter and thus accounting for their differential toxicity. This is in concurrence with previous reports wherein cationic surface active drugs have been shown to be more toxic than the neutral ones.48,56 Moreover, the membranes of transformed (tumor) cells have been shown to be more negatively charged than that of normal cells55 and that is why we observed MAS conjugates but not the DHS conjugates exhibiting higher toxicity in MCF7 compared to CHO cells. In addition to the charge differences, DHS and MAS conjugates being amphiphilic in nature may also differ in their surface properties contributing to their differential cytotoxicity.47,48 In line with this, our results on fluorescence enhancement of DPH indicated that the long chain (≥C10) conjugates of DHS and MAS formed aggregates as a function of concentration and this effect was prominent in the case of the DHS conjugates. Such differences can be justified by the explanation that the aggregation of MAS conjugates being cationic in nature would be less favorable due to repulsive forces. Since DHS conjugates showed higher aggregation behavior, it can be understood that due to this supramolecular formation there would be lesser availability of free molecules to interact with the cell membrane causing lesser cytotoxicity.46 This was indeed supported by the fact that in all our studies, the C14 conjugates of DHS and MAS exhibited most notable differences in terms of cellular effects (such as cytotoxicity, membrane disruption, incorporation). Further, the nonlinear relationship observed between the cytotoxicity and chain length (C6–14) of the lipophilic moiety of DHS and MAS conjugates could also be attributed to their self-aggregation properties.23 It is important to note here that the conjugates of DHS and MAS in the concentration range of 1–50 μM did not exhibit the point of inflection (concentration of the compound at which a dramatic increase in DPH fluorescence occurred) suggesting that concentrations at which these compounds evoked significant toxicity were much lower than their critical micelle concentrations (CMCs).33,35,41 This is in agreement with our observation on the mechanism of cell death induced by these compounds through necrosis as amphiphilic compounds at concentrations higher than the CMC cause solubilization of cellular lipids and proteins instead of membrane disintegration.41
The results of the present study and those of our previous studies are in agreement that increasing the lipophilicity of DHS and MAS through long chain alkylation increased their affinity for the membrane beyond any doubt.13,17,18 In these studies, increased membrane affinity was attributed to be reason for the ability of the conjugates of DHS and MAS to mimic the functionality of GPx4 and PDI-GPx7 respectively in a cell free system.13,17,18 However, the same very reason of membrane affinity became the cause of toxicity and thus a major concern in the biological applications of long chain conjugates of DHS and MAS. Taken together, the cytotoxic effects of DHS and MAS conjugates appear to be independent of their abilities to act as GPx4 and PDI-GPx7 mimics in cell free systems. It is also important to note here that the toxicity of DHS, MAS and their C6 conjugate is extremely low when compared to other known organochalcogens,57 making them suitable prototypes for new drug design.
At this stage it was felt necessary to evaluate the antioxidant effect of parent compounds and the nontoxic C6 conjugates in normal CHO cells. We restricted our study to the estimation of the levels of selenoproteins exhibiting antioxidant activities in the cells such as GPx1, GPx4 and TrxR1. Like the above GPx isoforms, TrxR1 is also an important cytosolic selenoenzyme that is required to maintain thioredoxin (endogenous antioxidant) in the reduced state.58 In this study parent compounds DHS and MAS significantly induced the expressions of all the above antioxidant selenoenzymes both at mRNA and activity levels and also provided protection against AAPH induced lipid peroxidation.27,36 Interestingly, the C6 conjugates of DHS and MAS were even better than the respective parent compounds in imparting the above activities confirming the role of HLB in improving the antioxidant activity. The mechanisms by which DHS and MAS led to the induction of selenoproteins remain to be understood. Interestingly, the antioxidant genes like GPx1, GPx4 and TrxR1 are the transcriptional targets of a redox sensitive transcription factor, Nrf2 (nuclear factor-E2-related factor 2), which has been shown to be induced by organochalcogens including ebselen and diphenyl diselenide.59–61 Therefore similar mechanisms may also account for the antioxidant activity of DHS, MAS and their C6 conjugates in cells. It is also worth mentioning here that DHS-C6 was less active than MAS-C6 in inducing the selenoenzymes and in protecting from AAPH mediated oxidative stress. The reason for this could be the probable cleavage of DHS-C6 (which contains an ester linkage) into the parent compound by the esterase present in the plasma membrane of the cells. In the absence of any evidence for this cleavage, we can conclude based on the fact that DHS-C6 could significantly increase the incorporation of selenium into the membranes per cell justifies its application as a pro-drug.19–21 In contrast MAS-C6, which contains an amide linkage may be a model compound for fine-tuning the toxicity and other biological application. The observed cellular effects of the conjugates of DHS and MAS are summarized in Scheme 2.
Scheme 2. Schematic representation of the cellular effects of the conjugates of DHS and MAS.
Conclusions
In conclusion the amphiphilic conjugates of DHS and MAS mimicked surface active compounds in causing cytotoxicity through membrane disintegration and necrosis. Conjugating a fatty acid/alky group as a lipophilic unit with a hydrophilic antioxidant moiety has been an effective approach to enhance the antioxidant activities. However, HLB is the important consideration in converting a nontoxic compound to a toxic one. Among DHS and MAS conjugates of varying chain lengths, C6 conjugates appear to be the appropriate bioinspired prototypes of selenium antioxidants.
Supplementary Material
Acknowledgments
Miss Prachi Verma acknowledges Homi Bhabha National Institute for financial assistance in the form of senior research fellowship. The authors also wish to thank Dr (Smt) S. Suvarna, Analytical Chemistry Division, Chemistry Group, BARC for providing help in the selenium estimation in the cells.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c5tx00331h
References
- Bhowmick D., Srivasstava S., D'Silva P., Mugesh G. Angew. Chem., Int. Ed. 2015;54:8449–8453. doi: 10.1002/anie.201502430. [DOI] [PubMed] [Google Scholar]
- Kersteen E. A., Raines R. T. Antioxid. Redox Signaling. 2003;5:413–424. doi: 10.1089/152308603768295159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flohe R. B., Maiorino M. Biochim. Biophys. Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Bosello-Travain V., Conrad M., Cozza G., Negro A., Quartesan S., Rossetto M. Biochim. Biophys. Acta. 2013;1830:3846–3857. doi: 10.1016/j.bbagen.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang L., Niu Y., Sitia R., Wang C. Antioxid. Redox Signaling. 2014;20:545–556. doi: 10.1089/ars.2013.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees W. J. Curr. Opin. Chem. Biol. 2008;12:740–745. doi: 10.1016/j.cbpa.2008.08.032. [DOI] [PubMed] [Google Scholar]
- Beld J., Woycechowsky K. J., Hilvert D. Biochemistry. 2008;47:6985–6987. doi: 10.1021/bi8008906. [DOI] [PubMed] [Google Scholar]
- Mugesh G., Singh H. B. Chem. Soc. Rev. 2000;29:347–357. [Google Scholar]
- Bhabak K. P., Mugesh G. Acc. Chem. Res. 2010;43:1408–1419. doi: 10.1021/ar100059g. [DOI] [PubMed] [Google Scholar]
- Iwaoka M., Takahashi T., Tomoda S. Heteroat. Chem. 2001;12:293–299. [Google Scholar]
- Kumakura F., Mishra B., Priyadarsini K. I., Iwaoka M. Eur. J. Org. Chem. 2010:440–445. [Google Scholar]
- Arai K., Dedachi K., Iwaoka M. Chem. – Eur. J. 2011;17:481–485. doi: 10.1002/chem.201002742. [DOI] [PubMed] [Google Scholar]
- Arai K., Moriai K., Ogawa A., Iwaoka M. Chem. – Asian J. 2014;12:3464–3471. doi: 10.1002/asia.201402726. [DOI] [PubMed] [Google Scholar]
- Singh B. G., Thomas E., Kumakura F., Dedachi K., Iwaoka M., Priyadarsini K. I. J. Phys. Chem. A. 2010;114:8271–8277. doi: 10.1021/jp103727e. [DOI] [PubMed] [Google Scholar]
- Chakraborty S., Yadav S. K., Subramanian M., Priyadarsini K. I., Iwaoka M., Chattopadhyay S. Free Radical Res. 2012;46:1378–1386. doi: 10.3109/10715762.2012.718766. [DOI] [PubMed] [Google Scholar]
- Arai K., Kumakura F., Takahira M., Sekiyama N., Kuroda N., Suzuki T. J. Org. Chem. 2015;80:5633–5642. doi: 10.1021/acs.joc.5b00544. [DOI] [PubMed] [Google Scholar]
- Iwaoka M., Sano N., Lin Y. Y., Katakura A., Noguchi M., Takahashi K. ChemBioChem. 2015;16:1226–1234. doi: 10.1002/cbic.201500047. [DOI] [PubMed] [Google Scholar]
- Iwaoka M., Katakura A., Mishima J., Ishihara Y., Kunwar A., Priyadarsini K. I. Molecules. 2015;20:12364–12375. doi: 10.3390/molecules200712364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguerre M., Bayrasy C., Lecomte J., Chabi B., Decker E. A., Wrutniak-Cabello C., Cabello G., Villeneuve P. Biochimie. 2013;95:20–26. doi: 10.1016/j.biochi.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Bayrasy C., Chabi B., Laguerre M., Lecomte J., Jublanc E., Villeneuve P., Wrutniak-Cabello C., Cabello G. Pharma Res. 2013;30:1979–1989. doi: 10.1007/s11095-013-1041-4. [DOI] [PubMed] [Google Scholar]
- Lambert D. M. Eur. J. Pharm. Sci. 2000;(Suppl 2):S15–S27. doi: 10.1016/s0928-0987(00)00161-5. [DOI] [PubMed] [Google Scholar]
- Mellor H. R., Nolan J., Pickering L., Wormald M. R., Platt F. M., Dwek R. A. Biochem. J. 2002;366:225–233. doi: 10.1042/BJ20020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R., Comelles F., Alcantara D., Maldonado O. S., Curcuroze M., Parra J. L. J. Agric. Food Chem. 2010;58:8021–8026. doi: 10.1021/jf1009928. [DOI] [PubMed] [Google Scholar]
- Mosmann T. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Van Engeland M., Ramaekers F. C., Schutte B., Reutelingsperger C. P. Cytometry. 1996;24:131–139. doi: 10.1002/(SICI)1097-0320(19960601)24:2<131::AID-CYTO5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Cossarizza A., Baccaranicontri M., Kalashnikova G., Franceschi C. Biochem. Biophys. Res. Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- Kunwar A., Mishra B., Barik A., Kumbhare L. B., Pandey R., Jain V. K. Chem. Res. Toxicol. 2007;20:1482–1487. doi: 10.1021/tx700137a. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholzb Y. Biochim. Biophys. Acta. 1978;515:367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Royce L. A., Liu P., Stebbins M. J., Hanson B. C., Jarboe L. R. Appl. Microbiol. Biotechnol. 2013;97:8317–8327. doi: 10.1007/s00253-013-5113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande M. B., Donovan J. M., Zeidel M. L. J. Gen. Physiol. 1995;106:67–84. doi: 10.1085/jgp.106.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. B. Annu. Rev. Pharmacol. Toxicol. 1984;24:43–64. doi: 10.1146/annurev.pa.24.040184.000355. [DOI] [PubMed] [Google Scholar]
- Plasek J., Jarolim P. Gen. Physiol. Biophys. 1987;6:425–437. [PubMed] [Google Scholar]
- Chaurasia R. K., Balakrishnana S., Kunwar A., Yadav U., Bhata N., Anjariaa K. Mutat. Res. 2014;774:8–16. doi: 10.1016/j.mrgentox.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Stuart M. C. A., van de Pas J. C., Engberts J. B. F. N. J. Phys. Org. Chem. 2005;18:929–934. [Google Scholar]
- Wolszczak M., Miller J. J. Photochem. Photobiol. 2002;147:45–54. [Google Scholar]
- Kunwar A., Narang H., Priyadarsini K. I., Krishna M., Pandey R., Sainis K. B. J. Cell. Biochem. 2007;102:1214–1224. doi: 10.1002/jcb.21348. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lugokenski T. H., Müller L. G., Taube P. S., Rocha J. B., Pereira M. E. Drug Chem. Toxicol. 2011;34:66–76. doi: 10.3109/01480541003782294. [DOI] [PubMed] [Google Scholar]
- Carfagna M. A., Muhoberac B. B. Mol. Pharmacol. 1993;44:129–141. [PubMed] [Google Scholar]
- Mckarns S. C., Hansch C., Caldwell W., Morgan W. T., Moore S. K., Doolite A. D. J. Fundam. Appl. Toxicol. 1997;36:62–70. doi: 10.1006/faat.1996.2252. [DOI] [PubMed] [Google Scholar]
- Mellor H. J., Platt F. M., Dwek R. A., Butters T. D. Biochem. J. 2003;374:307–314. doi: 10.1042/BJ20030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier M. A., Simard P., Zhang Z., Zhu X. X. J. R. Soc., Interface. 2007;4:1145–1150. doi: 10.1098/rsif.2007.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga S., Jimbo M., Gill M. B., Wyhe L. L. L., Murata M., Nonomura K. ChemBioChem. 2011;12:2191–2200. doi: 10.1002/cbic.201100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday D. L., Speirs V. Breast Cancer Res. 2011;13:215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond M., Attard G., Postle A. D. J. R. Soc., Interface. 2008;5:1371–1386. doi: 10.1098/rsif.2008.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inacio A. S., Mesquita K. A., Baptista M., Santos J. R., Vaz W. L. C., Vieira O. V. PLoS One. 2011;6:1–15. doi: 10.1371/journal.pone.0019850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier S., Malheiros S. V. P., Paula E. Biochim. Biophys. Acta. 2000;1508:210–234. doi: 10.1016/s0304-4157(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Bechinger B., Lohner K. Biochim. Biophys. Acta. 2006;1758:1529–1539. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Lengler I., Buhrke T., Scharmach E., Lampen A. Lipids. 2012;47:1085–1097. doi: 10.1007/s11745-012-3713-y. [DOI] [PubMed] [Google Scholar]
- Teixeira A., Cox R. C., Egmond M. R. Food Funct. 2013;4:1209–1215. doi: 10.1039/c3fo60094g. [DOI] [PubMed] [Google Scholar]
- Borse M., Sharma V., Aswal V. K., Goyal P. S., Devi S. J. Colloid Interface Sci. 2005;284:282–288. doi: 10.1016/j.jcis.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Brown D. A., London E. J. Biol. Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Lima T. M., Cury M. F., Giannocco G., Nunes M. T., Curi R. Clin. Sci. 2006;111:307–317. doi: 10.1042/CS20060064. [DOI] [PubMed] [Google Scholar]
- Zong W. X., Thompson C. B. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- Ambrose E. J., Easty D. M., Jones P. C. T. Br. J. Cancer. 1958;12:439–447. doi: 10.1038/bjc.1958.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. E., Broadbent J. R. J. Food Sci. 2009;74:R12–R15. doi: 10.1111/j.1750-3841.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- Caeran D. B., Meinerz D. F., Allebrandt J., Waczuk E. P., dos Santos D. B., Mariano D. O., Rocha J. B. Biomed. Res. Int. 2013;2013:537279. doi: 10.1155/2013/537279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustacich D., Powis G. Biochem. J. 2000;346:1–8. [PMC free article] [PubMed] [Google Scholar]
- Tamasi V., Jeffries J. M., Arteel G. E., Falkner K. C. Arch. Biochem. Biophys. 2004;431:161–168. doi: 10.1016/j.abb.2004.07.030. [DOI] [PubMed] [Google Scholar]
- de Bem A. F., Fiuza B., Calcerrada P., Brito P. M., Peluffo G., Dinis T. C., Trujillo M., Rocha J. B., Radi R., Almeida L. M. Nitric Oxide. 2013;31:20–30. doi: 10.1016/j.niox.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang G., Nitteranon V., Guo S., Qiu P., Wu X., Li F., Xiao H., Hu Q., Parkin K. L. Chem. Res. Toxicol. 2013;26:456–464. doi: 10.1021/tx300515j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.