METH exposure activated GSK3β mediating tau and α-syn hyperphosphorylation, autophagy–lysosomal impairment, and α-syn accumulation and aggregation, causing METH neurotoxicity.
METH exposure activated GSK3β mediating tau and α-syn hyperphosphorylation, autophagy–lysosomal impairment, and α-syn accumulation and aggregation, causing METH neurotoxicity.
Abstract
Methamphetamine (METH) is well-known as a potent psychostimulant of abuse worldwide. METH administration can cause neurotoxicity and neurodegenerative injury, which are similar to the two prevalent neurodegenerative disorders Alzheimer's disease (AD) and Parkinson's disease (PD). Recent results suggested that METH exposure increased the level of α-synuclein (α-syn) that could be a possible cause of neurotoxicity. However, the mechanism of METH-induced neurodegeneration remains unclear. This study was aimed at examining the effects of glycogen synthase kinase3β (GSK3β), α-syn, and tau on METH-induced neurotoxicity. Our results indicated that P-GSK3β (Tyr216), P-Tau (Ser396), α-syn, and P-α-syn (Ser129) levels were increased after METH administration in dose- and time-dependent manners. Upon inhibiting the GSK3β activity with LiCl or GSK3β-siRNA, these protein expressions were significantly decreased. We observed that LiCl protected the cells from METH-caused cytotoxicity by weakening the cell morphological damage and preventing cell apoptosis and death. We also found that P-GSK3β colocalized with P-Tau and α-syn by the immunofluorescence method. Further, METH disrupted the cellular autophagy by upregulation of LC3-II and P62 proteins, and the cellular autophagy was restored by LiCl and GSK3β-siRNA. The expressions of the α-syn-specific degradative enzyme glucocerebrosidase (GCase) with its regulator lysosomal integral membrane protein type-2 (LIMP-2) decreased inversely with the doses of METH treatment. The GCase inhibitor conduritol-β-epoxide (CβE) increased the α-syn levels, and LiCl restored GCase and LIMP-2 expressions disrupted by the METH treatment. In summary, we conclude that GSK3β plays key roles in METH-induced neurotoxicity and neurodegenerative injury by promoting abnormal protein phosphorylation and α-syn accumulation, blocking the autophagy–lysosomal degradation pathway, and finally leading to cell apoptosis and death. GSK3β may be a potential target to prevent METH-induced neurodegeneration.
Introduction
Methamphetamine (METH), developed as an amphetamine derivative, is a highly addictive psychostimulant drug that is widely abused in the world, whose hydrochloride is called Ice.1 METH has adverse effects on each organ, especially on the central nervous system. A number of evidences indicate that METH abuse leads to significant neurotoxicity via multiple events, including oxidative stress, hyperthermia, neuroinflammatory responses, mitochondrial dysfunction, endoplasmic reticulum stress, and so on, which converge to mediate METH-induced terminal degeneration and neuronal apoptosis.2–4 Epidemiological studies suggest that METH abuse is associated with an increased risk of PD and may predispose users to develop PD.5,6 METH abuse can result in the formation of PD-like Lewy bodies (LBs) in the substantia nigra and striatum of rats.7,8 Thus, one of the major outcomes of METH toxicity is neurodegenerative injury, but the underlying mechanism is unclear.
Pathological accumulation of α-syn in the brain that results in the formation of Lewy bodies is a typical neuropathological hallmark of Parkinson's disease (PD),9 and increasing evidences suggest that α-syn is a common pathogenic molecule in several neurodegenerative diseases, referred as synucleinopathies.10 Previous studies in our laboratory have manifested METH exposure-induced overexpression of α-syn that leads to oxidative stress in vivo and in vitro, and suppression of α-syn expression can significantly attenuate METH-induced toxicity.11–13 Although α-syn aggregation is observed in METH-treated PC12 cells, the mechanism of α-syn abnormal modification/accumulation remains unclear.13 Cystoskeleton destruction is another important mechanism of neurodegeneration. The microtubule-associated protein tau has been classically linked to Alzheimer's disease (AD), which also plays important roles in the pathogenesis of PD and other related disorders, known as tauopathies.14,15 In AD and PD, hyperphosphorylation of tau leads to intracellular accumulation of this protein and the formation of neurofibrillary tangles (NFTs). Glycogen synthase kinase 3 (GSK3) proteins are multifunctional serine/threonine kinases originally identified as key enzymes in glycogen metabolism.16 In mammals, there are two closely related isoforms: GSK3α and GSK3β; the latter isoform is highly enriched in the nervous system.17 Researches show that absence or misregulation of GSK3 functions has been involved in several CNS diseases such as AD and PD.18,19 GSK3β can phosphorylate a majority of sites on tau; especially, Ser396 hyperphosphorylation seems to play a pivotal role for its function and, in particular, destabilizes microtubules.20,21 Alpha-syn may act as a connecting mediator for GSK3β and tau, resulting in GSK3β-mediated tau phosphorylation.22–24 Thus, GSK3β may act as an important signaling molecule for regulating α-syn and tau through phosphorylation during the pathogenic process of neurodegenerative diseases. Related research showed that METH-induced neurotoxicity in PC12 cells was associated with the Akt/GSK3β/mTOR pathway.25 Increased GSK3β activity was found in the NAc core after chronic METH administration.26 Herein, we hypothesized that GSK3β could mediate METH-induced neurotoxicity via regulating α-syn and tau in our model. Therefore, one of the objectives of this study was to investigate the variety of GSK3β activity and its regulation effects on α-syn and tau, and the role of GSK3β in METH-induced cytotoxicity in PC12 cells.
Recently, impairment of autophagy-lysosomal pathways (Alps) is considered as another important pathogenic event in neurodegenerative diseases. Since α-syn can be degraded by the enzyme GCase and autophagy-lysosomal pathway27,28 and GSK3β is also involved in autophagy regulation,29–31 we have proposed our second presumption: whether GSK3β regulates α-syn expression through ALPs? Our next goal was to investigate the roles of GSK3β in lysosomal GCase/α-syn and autophagy pathways during METH-induced neurotoxicity.
Experimental
Cell culture and treatment
Differentiated PC12 cells, a rat adrenal medulla pheochromocytoma cell line, obtained from the Cell Bank of Shanghai Institute for Biological Science, Chinese Academy of Science, were cultured in high glucose DMEM containing 10% fetal bovine serum and incubated at 37 °C in a humidified atmosphere containing 5% CO2. The cells were passaged every 2–3 days. Once cell cultures reached 70% confluency in 6-well plates or 96-well plates, cells were treated with METH at different concentrations of 0, 0.5, 1.0, 1.5, and 2.0 mM for 24 h or exposed to 2.0 mM METH for 0 h, 2 h, 6 h, 12 h, and 24 h or pretreated with 10 mM LiCl for 30 min and 100 μM CβE for 2 h prior to being treated with METH (2.0 mM). After treatment, cell morphological characteristics were observed using an inverted microscope. Cell culture media were obtained, and total protein was isolated from the cells and used for further analysis.
Cell viability assay
Cell viability was measured by the CCK8 assay. Briefly, PC12 cells were seeded in 96-well culture plates at a density of 5 × 103 cells per well; at the end of exposure, 10 μl CCK-8 was added to each well, and then, cells were sulfated at 37 °C in a humidified atmosphere of 5% CO2 for 2 h. An enzyme standard instrument was used to determine the absorbance plate values at 450 nm.
Annexin V apoptosis staining
Flow cytometry analysis was performed to detect early and late apoptotic cells. According to the instructions of the Annexin V-FITC apoptosis detection kit (Keygen, Nanjing, China), PC12 cells were seeded on six well plates at a density of 5 × 105/well. Cells were treated with METH and LiCl, as described before, and harvested using trypsin. Then, cells were centrifuged at 2000 rpm for 5 min to remove the medium, washed twice with 4 °C PBS, and stained with Annexin V-FITC and propidium iodide (PI). The percentage of apoptotic cells was quantified via flow cytometry (FACSCalibur, BD Biosciences, San Jose, CA, USA).
siRNA and transient transfection
Pieces of siRNA sequences targeting GSK3β and α-syn were designed by GenePharma (Shanghai, China) as shown below: GSK3β siRNA: No. 1 (Rat: 5′-GGAGAGCCCAAUGUUUCAUTT-3′), No. 2 (Rat: 5′-GGGAGCAAAUUAGAGAAAUTT-3′), and No. 3 (Rat: 5′-GGGACCCAAAUGUCAAA CUTT-3′). α-Syn siRNA: No. 1 (Rat: 5′-GCUGUACAGUGUAUUUCAATT-3′), No. 2 (Rat: 5′-CCCU AGCAGUGAGGCUUAUTT-3′), and No. 3 (Rat: 5′-AGAACAAGUGACAAAUGUUTT-3′). Scrambled siRNA sequence (5′-UUCUCCGAACGUGUCACGUTT-3′) was used as a control (siNC). siRNAs were dissolved in DEPC water at a concentration of 20 μM. Cells were placed on a 6-well plate at a density of 4 × 105 cells per well. When cells reached 70% confluence, 5 μl Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA) and 20 μmol siRNA were added to Opti-MEM medium (Gibco BRL, Paisley, UK). The mixed solution was incubated at room temperature for 20 min, and then, the siRNA/Lipofectamine 2000 complex was added to the cells. This medium was replaced after 6 h incubation at 37 °C with the same volume of the fresh DMEM. After 48 h, cells were changed to a non-serum medium prior to treatment with 2.0 mM METH for another 24 h. Total protein was extracted from PC12 cells, and protein expressions were determined by western blot.
Western blot analysis
PC12 cells exposed to vehicle or METH were lysed in an ice-cold RIPA buffer with protease inhibitors and phosphatase inhibitors. Protein concentrations were determined using the BCA-100 Protein Quantitative Analysis kit (Biocolors, Shanghai, China). Protein samples were separated by 8–12% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, USA). The membranes were incubated overnight at 4 °C or at room temperature for 2 h in a blocking buffer (5% nonfat dry milk or 5% BSA in a TBST buffer). After blocking, membranes were incubated with primary antibodies (1 : 1000 for all): anti-GSK3β, anti-P-GSK3β (Tyr216), anti-P-Tau (Ser396), anti-P-α-syn (Ser129), and anti-GCase (Abcam, Cambridge, UK); anti-tau, anti-P62, and anti-LC3-II (CST, Boston, MA, USA); anti-α-syn and anti-LIMP-2 (Santa Cruz, California, USA); and anti-β-actin overnight at 4 °C. Membranes were washed three times with TBST followed by incubation with a horseradish peroxidase-conjugated secondary antibody (1 : 10 000) for 1 h at room temperature. The blot membranes were washed as abovementioned and developed with Chemiluminescence ECLPlus Western Blotting detection reagents (Thermo Scientific, Waltham, MA, USA). Proteins of interest were quantified using the Gel-Pro analyzer software.
Immunofluorescence
PC12 cells were placed on slides, fixed with 4% paraformaldehyde in PBS for 30 min at room temperature, and then washed three times in PBS. The cells were permeabilized in 0.1% Triton-X-100 for 10 min, blocked in 3% goat serum for 1 h at room temperature, and subsequently labeled with P-GSK3β (Tyr216), P-Tau (Ser396), and α-syn antibodies (1 : 200 for all). After washing three times with PBS, the cells were incubated with Cy3-goat anti-rabbit IgG or FITC-goat anti-mouse IgG (1 : 00) for 1 h at room temperature, followed by mounting with DAPI (4′,6-diamidino-2-phenylindole) for nuclear counter staining. Fluorescently stained cells were analyzed using a confocal laser scanning microscope (Nikon, Tokyo, Japan).
Statistical analysis
Data were summarized as the mean ± SD of three independent replicates at least. Statistical analyses were performed using the SPSS 20.0 software. The parametric test contained one-way ANOVA, and the post hoc test was conducted using the Tukey HSD method. The value of p < 0.05 was considered statistically significant.
Results
METH exposure increased the expressions of P-GSK3β, P-Tau, P-α-syn, and α-syn
The activity of GSK3β was evaluated by examining the level of phosphorylated tyrosine 216 residue (Tyr216) of this protein. As shown in Fig. 1A, B and 2A, B, there was time-dependent and dose-dependent elevation of P-GSK3β expression levels after 0.5 mM–2.0 mM METH treatment, but the expressions of total GSK3β remained unchanged. The P-GSK3β expression level (P-GSK3β/GSK3β protein ratio) was about 2.8-fold higher in the 2.0 mM METH-treated group than that in the control group. After treatment with 2.0 mM METH for 2 h, the activation of GSK3β was observed, which lasted for up to 24 h with a significant increase (p < 0.01, Fig. 2A and B). Similarly, P-Tau, α-syn, and P-α-syn expression ratios were increased in a dose-dependent manner after METH exposure, with the maximal elevation of about 2-fold, 5-fold, and 2.7-fold, respectively (p < 0.01) (Fig. 1A, C, and D). After exposure to 2.0 mM METH for various durations, we observed the gradually elevating levels of α-syn, whereas the P-α-syn levels, similar to the expression trend of P-Tau, increased at 6 h and lasted for up to 24 h (Fig. 2A, C, and D). Overall, these results reveal that METH exposure can activate GSK3β, induce α-syn accumulation, and upregulate tau and α-syn phosphorylation.
Fig. 1. METH exposure increased the protein expressions of P-GSK3β, P-Tau, P-α-syn, and α-syn in a concentration-dependent manner. PC12 cells were treated with METH at concentrations ranging from 0.5 to 2.0 mM for 24 h. Western blot (A) and quantitative analyses (from B to D) were performed to determine GSK3β, Tau, P-α-syn, and α-syn protein expressions (relative to β-actin) and P-GSK3β and P-Tau protein expressions (relative to total GSK3β and Tau, respectively). β-Actin was used as a loading control. Data were expressed as means ± SD (n = 3/group). Data were analyzed with one-way ANOVA followed by the Tukey HSD post-hoc test. #,*p < 0.01 vs. control group.
Fig. 2. METH exposure increased the expressions of P-GSK3β, P-Tau, P-α-syn, and α-syn in a time-dependent manner. PC12 cells were treated with 2.0 mM METH for 0 h, 2 h, 6 h, 12 h, and 24 h. Western blot (A) and quantitative analyses (B to D) were performed to determine GSK3β, Tau, P-α-syn, and α-syn protein expressions (relative to β-actin) and P-GSK3β and P-Tau protein expressions (relative to total GSK3β and Tau, respectively). β-Actin was used as a loading control. Data were expressed as means ± SD (n = 3/group). Data were analyzed with one-way ANOVA followed by the Tukey HSD post-hoc test. #,*p < 0.01 vs. control group.
Inhibition of GSK3β activity-attenuated METH-induced protein overexpression and phosphorylation
To assess the effects of GSK3β activation on the expressions of α-syn and tau and their phosphorylation levels, PC12 cells were pretreated with LiCl, an inhibitor of GSK3β. Results indicated that LiCl effectively inhibited GSK3β activity and decreased the ratio of P-GSK3β/GSK3β by ∼35% in PC12 cells exposed to LiCl alone (Fig. 3A and B). After pretreatment with LiCl, METH-mediated phosphorylations of GSK3β, tau, and α-syn were significantly prevented (p < 0.01, Fig. 3). In the same way, total tau and GSK3β expressions had no changes. Simultaneously, α-syn overexpression induced by METH was also observably attenuated by LiCl (Fig. 3A and D).
Fig. 3. LiCl inhibited P-GSK3β, P-Tau, α-syn, and P-α-syn expressions induced by METH. PC12 cells were treated with 2.0 mM METH with or without 10 mM LiCl. Western blot (A) and quantitative analyses (B–D) were performed to determine the protein expression. GSK3β, Tau, P-α-syn, and α-syn were quantified relative to β-actin, and P-GSK3β and P-Tau were quantified relative to GSK3β and Tau, respectively. β-Actin was used as a loading control. Data were expressed as means ± SD (n = 3/group). Data were analyzed with one-way ANOVA followed by the Tukey HSD post-hoc test. *p < 0.05 vs. control group. #p < 0.01 vs. METH group.
We further used RNA knock-down technique to silence GSK3β expression and detected these protein levels again. Compared with the case of the negative control group, transfection with siRNA-1/2/3 led to significant decreases in GSK3β expression, and siRNA-1 inhibited it most efficiently (Fig. 4A and B). Thus, we selected siRNA-1 to carry out our next experiment. As shown in Fig. 4C–F, the results were consistent with those of the LiCl treatment. Knocking down GSK3β also significantly decreased α-syn expression and effectively prevented hyperphosphorylation of tau and α-syn caused by METH. These data demonstrate that both LiCl and siGSK3β block GSK3β activity, and inhibition of GSK3β attenuates METH-induced α-syn accumulation and phosphorylation of tau and α-syn.
Fig. 4. Silencing GSK3β expression inhibited METH-mediated phosphorylation of GSK3β, Tau, α-syn, and α-syn overexpression. PC12 cells were transfected with GSK3β siRNA-1, siRNA-2, siRNA-3, or empty vector as a control. All three types of siRNA effectively inhibited GSK3β protein expression, especially siRNA-1 (A and B). Then, siRNA-1 was selected for next experiments. Cells were exposed to vehicle, GSK3β siRNA, METH, and METH + GSK3β siRNA. Western blot (C) and quantitative analyses (D–F) showed that METH-induced upregulations of P-GSK3β, P-Tau, α-syn, and P-α-syn were significantly blocked by siGSK3β. β-Actin was used as a loading control. Data were expressed as means ± SD (n = 3/group). All data were analyzed with one-way ANOVA followed by the Tukey HSD post-hoc test. *p < 0.01 vs. control group. #p < 0.01 vs. METH group.
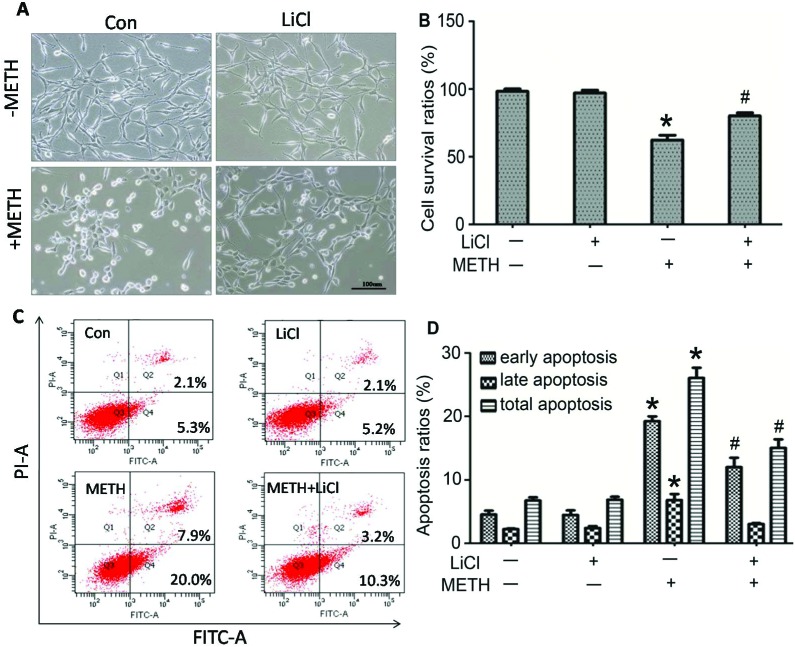
LiCl protected the cells from METH-induced cytotoxicity
Cell morphological alteration, cell survival rates, and cell apoptosis were examined to further explore the role of GSK3β in METH-induced cytotoxicity. As shown in Fig. 5A, slender dendrites and neuron-like reticular formation in the normal PC12 cells and the LiCl-only treatment cells can be observed. After METH exposure, cell bodies shrinked and became round with the disruption of the dendrites and disappearance of cell reticular formation. By adding LiCl to METH-treated cells, we found that the abovementioned cell morphological damages were weakened, and part of slender dendrites could still be seen clearly. Cell apoptosis is one of the important mechanisms of METH-induced neurotoxicity. The flow cytometry results indicated that 2.0 mM METH exposure alone significantly increased the percentage of PC12 cell apoptosis as compared to the case of the control group (26.07 ± 1.63 vs. 6.73 ± 0.51, p < 0.01), whereas the percentage of cell apoptosis was reduced to 15.03 ± 1.36 after pretreatment with LiCl (Fig. 5C and D, p < 0.01). Similarly, LiCl reversed the METH-caused decrease of cell survival rates (62.45 ± 3.48 vs. 80.19 ± 2.30, p < 0.01) (Fig. 5B). Overall, these data suggest that LiCl plays a protective role in METH-induced cytotoxicity, and inhibition of GSK3β can prevent cell apoptosis and death.
Fig. 5. LiCl protected the cells from METH-induced cytotoxicity. PC12 cells were incubated in drug free, LiCl, 2.0 mM METH, and 2.0 mM METH + LiCl media for 24 h. (A) There were no morphological changes in the normal control group and LiCl group. After exposure to METH, cells became round with the disruption of the dendrites and disappearance of cell reticular formation, and these morphological damages were attenuated by LiCl co-exposure. (B) Cell viability was assessed by the CCK8 assay. LiCl reversed the METH-induced decrease in cell survival rates. Date are expressed as means ± SD (n = 5/group). (C) Flow cytometry was performed to assess apoptosis changes. Top right quadrant and bottom right quadrant show quantification of the percentage of late and early apoptosis, respectively. Panel D represents quantitative results of the percentage of apoptotic PC12 cells in panel C. The data are expressed as means ± SD (n = 3 group). LiCl prevented METH-induced cell apoptosis. All the abovementioned data were analyzed by one-way ANOVA followed by the Tukey HSD post-hoc test. *p < 0.01 vs. control group, #p < 0.01 vs. METH group.
P-GSK3β colocalized with P-Tau and α-syn after METH exposure
To further investigate the possible interaction between P-GSK3β, α-syn, and P-Tau in our METH model, we used the double labeling immunofluorescence method with confocal microscopy to observe the subcellular localizations. Our results indicated both P-GSK3β and P-Tau expressions localized in the cytoplasm, but increased levels of them with no changes in subcellular localization after treatment with 2.0 mM METH (Fig. 6(A2-B4)). On the contrary, α-syn expression is mainly localized in the cell nuclei of the control group, whereas upon exposure to METH for 24 h, it translocated into the cytoplasm and colocalized with P-GSK3β obviously (Fig. 6(C2-D4)). These results indicate that these three proteins may interact with each other in the PC12 cells, and METH administration may promote their interaction. GSK3β phosphorylation may play a role as an intermediary agent in α-syn accumulation and aggregation after METH injury.
Fig. 6. Colocalization of P-GSK3β, P-Tau, and α-syn in PC12 cells exposed to METH. Cells were treated with drug free and 2.0 mM METH media for 24 h. Increased levels of P-GSK3β and P-Tau colocalized in the cytoplasm in the METH group without any changes in subcellular localization (B2-B4). Expression of α-syn in the nucleus was not colocalized with P-GSK3β in the control (C2-C4), but colocalization with P-GSK3β was seen in the cytoplasm after METH exposure (D2-D4).
GSK3β involved in the METH-induced α-syn autophagy dysregulation
Autophagy is suggested to be important for α-syn clearance and degradation. To assess whether GSK3β regulates autophagy degradation, the expressions of the autophagy makers LC3-II and P62 (representing respectively autophagosomal number and autophagy flux) have been measured. Our results showed that METH induced an increase in the levels of these two proteins in a dose-dependent manner (Fig. 7A and B). The levels of LC3-II and P62 proteins were respectively about 3.5-fold and 2.7-fold higher in the 2.0 mM METH-treated group than those in the control group. It could be explained that METH triggered autophagy, but inhibited the autophagy flux. Then, we evaluated the relationship between α-syn and the induction of autophagy. As seen from Fig. 7C–F, α-syn siRNA-3 inhibited α-syn expression most effectively, and after pretreatment with α-syn siRNA-3, METH-induced LC3-II overexpression was significantly downregulated; this implied that the induction of autophagy was associated with the triggering of α-syn degradation. When cells were exposed to 2.0 mM METH in the presence of LiCl, upregulation of LC3-II and P62 caused by METH was partly inhibited (METH group vs. METH + LiCl group, p < 0.01, Fig. 7G and H). In agreement with the abovementioned results, exposure to METH combined with GSK3β siRNA obviously restored the disruption of autophagy induced by METH via downregulation of LC3-II and P62 (Fig. 7I and J). These data manifest that GSK3β participates in the disruption of α-syn autophagy mediated by METH.
Fig. 7. GSK3β involved in the METH-induced α-syn autophagy dysregulation. Cells were exposed to METH at different concentrations ranging from 0.5 to 2.0 mM for 24 h and increasing levels of LC3-II and P62 were determined (A and B). Next, PC12 cells were transfected with α-syn siRNA-1, siRNA-2, siRNA-3 or empty vector as a control. All three types of siRNA effectively inhibited α-syn protein expression, especially siRNA-3 (C and D). Then, siRNA-3 was selected for next experiments. Western blot (E) and quantitative analyses (F) showed that METH-induced LC3-II overexpressions were significantly inhibited by siα-syn. When PC12 cells were pretreated with LiCl or siGSK3β, increased levels of LC3-II and P62 were inhibited (G–J). β-Actin was used as a loading control. Data were expressed as means ± SD (n = 3/group) and analyzed with one-way ANOVA followed by the Tukey HSD post-hoc test. *p < 0.01 vs. control group. #p < 0.01 vs. METH group.
LiCl rescued the α-syn degradative molecules of GCase and LIMP-2
We have detected the decreasing levels of GCase and LIMP-2, which are α-syn-specific degradative molecules, after METH treatment in a dose-dependent manner (Fig. 8A and B). Compared with the case of the control group, both GCase and LIMP-2 expressions were reduced by ∼56% in the 2.0 mM METH group. In contrast with the decreasing levels of GCase, the accumulation of α-syn increased significantly in the METH or CβE group as compared to that in the control group. After co-exposure of CβE, there were the lowest levels of GCase and highest levels of α-syn (Fig. 8C and D). LiCl also restored the decreases in GCase and LIMP2 caused by METH (Fig. 8E and F). From these data, it can be inferred that GSk3β is involved in METH-induced GCase dysfunction, disturbing α-syn clearance.
Fig. 8. GCase, LIMP-2, and α-syn expressions after treatment with METH, and disruption of GCase or GSK3β in PC12 cells. Cells were exposed to METH at different concentrations ranging from 0.5 to 2.0 mM for 24 h and pretreated with CβE (100 μM) or LiCl. Western blot and quantitative analyses were performed to determine the protein expression. β-Actin was used as a loading control. METH exposure downregulated the GCase and LIMP-2 expressions (A and B). GCase regulated the α-syn expression (C and D). LiCl reversed the decrease in GCase and LIMP2 caused by METH (E and F). Data were expressed as means ± SD (n = 3/group) and analyzed with one-way ANOVA followed by the Tukey HSD post-hoc test. *p < 0.05 vs. control group. #p < 0.05 vs. METH group.
Discussion
Our results showed that METH exposure induced GSK3β phosphorylation, which led to an increase in the levels of P-Tau, P-α-syn, and α-syn. LiCl and GSK3β siRNA blocked GSK3β activity and attenuated METH-induced protein phosphorylation and cytotoxicity. P-GSK3β colocalized with α-syn and P-Tau in the cytoplasm of PC12 cells after METH treatment. Both α-syn autophagy and GCase degradative pathways were disrupted by METH administration, and inhibition of GSK3β could restore these effects. These findings demonstrate that GSK3β is critical for α-syn abnormal phosphorylation and accumulation in METH-induced neurotoxicity.
GSK3β is a multifunctional serine/threonine kinase enriched in the nervous system. GSK3β is highly regulated by phosphorylation, and phosphorylation of tyrosine 216 residue (Tyr216) leads to GSK3β activation, but phosphorylation of Serine 9 residue (Ser9) inhibits its activity.17,32 Lithium is a known inhibitor of GSK3β. Relative studies show that increased GSK3β activity is found in the NAc core after chronic METH administration, which is associated with behavioral sensitization or hyperlocomotor activity, but lithium treatment inhibits GSK3β activity in the NAc and blocks locomotor sensitization induced by METH.26,33 Lithium is also reported to protect against METH-induced neurotoxicity via phosphorylation of the Akt/GSK3β/mTOR pathway.25 In our present results, METH exposure increased phosphorylated-GSK3β at tyrosine 216 residue in PC12 cells in a dose-dependent and time-dependent manner, but total GSK3β had no change. When the cells were pretreated with LiCl, METH-induced GSK3β phosphorylation was inhibited. Thus, it is confirmed that METH administration indeed can activate GSK3β, which may be involved in a further process during toxicity injury.
METH is the second most widely used illicit drug worldwide, which is a highly addictive drug causing neurodegeneration with unclear mechanism. Tau and α-syn have been classically linked to AD and PD, respectively. Tau activity is regulated by phosphorylation/dephosphorylation cycles. Abnormal tau phosphorylation is thought to prevent tau from binding to microtubules; this causes an accumulation of hyperphosphorylated tau that leads to PHF formation, microtubule instability, and neurodegeneration.14 GSK3β is a primary kinase for tau phosphorylation, which can also directly phosphorylate α-syn at a single site, Ser129.32 In the PD model induced by rotenone, GSK3β phosphorylates α-syn at Ser129, and lithium decreases the phosphorylation of α-syn.24
Our results showed that there were time-dependent and dose-dependent increases in P-Tau (Ser396) and P-α-syn (Ser129) after METH exposure along with P-GSK3β overexpression. It was worth noting that an increased expression of P-GSK3β was examined at 2 h, whereas both P-Tau (Ser396) and P-α-syn (Ser129) were found to increase at 6 h, which was later than the case of P-GSK3β. When GSK3β activity was inhibited by LiCl or GSK3β siRNA, both P-Tau and P-α-syn expressions induced by METH were significantly down-regulated. Therefore, we speculate that METH induces tau and α-syn phosphorylation via activating GSK3β. As is known, the major function of tau is stabilization and regulation of microtubule (MT) dynamics necessary for neurite outgrowth, morphogenesis, axonal transport, and normal neuronal functions. Since the regulation of tau phosphorylation is closely related to microtubule stability and cytoskeleton maintenance and LiCl inhibits P-Tau expression and weakens cell morphological damage caused by METH, we infer that the microtubule and cytoskeleton instability may be the possible mechanism of METH-caused cell morphological changes. In our previous studies, we confirmed that METH induced α-syn expression, abnormal aggregation mediating oxidative stress, and apoptosis.11–13 GSK3β inhibition-decreased α-syn protein expression has been reported previously.19,24 In the present METH-induced injury model, we detected α-syn overexpression in agreement with our previous studies. Notably, we have found that GSK3β can regulate not only phosphorylation of α-syn but also α-syn accumulation as METH-induced increase of α-syn is significantly down-regulated while inhibiting GSK3β with LiCl and GSK3β siRNA. Abnormally aggregated α-syn is the main component of LBs, and the predominated α-syn phosphorylated at serine 129 (Ser129) in LBs suggests its important pathological role.32 Evidences indicated that phosphorylation at Ser129 enhanced α-syn toxicity, promoting its mis-folding, aggregation, and accumulation.34 To sum up, it is well concluded that GSK3β mediates α-syn and tau toxicity of abnormal phosphorylation, aggregation, and accumulation induced by METH.
Increasing evidences show that there is a strong association between α-syn and hyperphosphorylation of tau. Tau phosphorylation increased at Ser396, Ser202/Thr205, and Thr231 in α-syn-injected brains.35 Neuronal colocalization of tau and α-syn inside LBs has been reported in brains of sporadic PD and dementia with Lewy bodies.36,37 Alpha-syn forms a heterotrimeric complex with phosphorylated tau and GSK3β,19,22 and P-GSK3β (Tyr216) colocalizes with P-Tau and P-α-syn (Ser129) in TH+ DA-neurons of the midbrain.32 To further investigate how GSK3β interacts with α-syn and tau during METH toxicity, we observed their subcellular localizations using confocal microscopy. The immunofluorescence results showed that P-GSK3β and P-Tau colocalized to the cytosol regardless of whether the cells were exposed to METH or not; however, α-syn translocated from the cell nucleus into the cytosol and colocalized with P-GSK3β after METH exposure. Therefore, we conclude that there is interaction between P-GSK3β, α-syn, and P-Tau, and the increased interaction among them stimulated by METH may facilitate its cytotoxicity. However, it is unclear and remains to be investigated that whether α-syn is essential for METH-induced protein phosphorylation and whether they interact with each other by forming a heterotrimeric complex in our model.
It is reported that GSK3β can regulate cell survival via modulation of autophagy and cell death.30 PD mimetics, such as 6-OHDA (6-hydroxydopamine), Aβ (amyloid β peptide), and MPTP (1-methyl 4-phenyl-1,2,3,6-tetrahydropyridine), induce neuronal apoptosis in a GSK3β-dependent manner in SH-SY5Y cells, PC12 cells, and dopaminergic neurons.38–40 Thus, GSK3β may be an important apoptosis modulator. Our previous studies have revealed that METH can induce cell apoptosis by different signal pathways such as the caspase-related pathway and Nupr1/Chop signal axis.4,41 Cell death and apoptosis are usually the terminal events in METH toxicity injury. Our results showed that PC12 cell morphology was severely changed and damaged, with increased cell apoptosis and decreased cell viability after exposure to 2.0 mM METH for 24 h. After inhibition of GSK3β with LiCl, both cell apoptosis and cell survival rate were restored significantly. We consider GSK3β as an important signal regulator in METH-caused cell death and apoptosis, and LiCl functions as a neuroprotective agent. GSK3β-mediated protein phosphorylation and α-syn expression participate in the process of METH toxicity injury.
Impairment of autophagy-lysosomal pathways (Alps) is increasingly regarded as a major pathogenic event in neurodegenerative diseases including PD.42,43 Aggregating and nonaggregating α-syn species can be degraded by autophagy,28 and impaired ALP in the diseased brain limits intracellular degradation of misfolded proteins and subsequent accumulation of abnormal α-syn species. LC3-II is the autophagosome marker for measure of autophagosomal number, and P62 is a marker for autophagy flux.44,45 There was an increasing trend for LC3-II levels in PD.46,47 Inhibition of autophagy correlates with increased levels of P62; this suggests that steady state levels of this protein reflect the autophagic status.44 GSK3β is also associated with regulation of autophagy.29,31 In the present study, we observed the increased protein levels of LC3-II; this suggested that METH treatment triggered or induced autophagy. However, the expression of P62 was also enhanced; this meant autophagy flux was obstructed, and the process of autophagy was incomplete (a complete process of autophagy including the delivery of cargo to lysosomes, its subsequent breakdown, and recycling). Therefore, increased LC3-II levels also represented inhibition of autophagosome clearance. Then, we silenced α-syn expression and found that METH-induced LC3-II expression decreased; this hinted that autophagy could not be induced; this suggested that autophagy was important for METH-related α-syn degradation. Owing to the obstruction of autophagy flux, α-syn could not be cleared and finally accumulated in the cells. While blocking GSK3β with LiCl and siRNA, both LC3-II and P62 levels decreased, and autophagy capabilities were restored. Together, METH inhibited the autophagy process by GSK3β activation, then reserved excessive α-syn, and finally led to α-syn aggregation and accumulation in the cytoplasm. Therefore, we conclude that GSK3β is involved in autophagy dysfunction, which destroys the balance of α-syn homeostasis after METH treatment.
Except for the autophagy pathway to α-syn degradation, there is a specific enzyme, glucocerebrosidase (GCase), that makes clearance of α-syn in the lysosome. Accordingly, GCase deficiency and abnormal accumulation of α-syn were found in neurodegenerative diseases.27,48 There was new observation of inhibitions between GCase and α-syn through unique domains in the lysosome.49 GCase is encoded by glucocerebrosidase gene (GBA), whose mutation causes the lysosomal storage disease named Gaucher disease.50 It is interesting that many PD patients carry GBA gene mutation, and Gaucher disease patients have a high risk of developing PD; this suggests a close relationship between these two diseases.51,52 We thus hypothesized that the lysosomal GCase/α-syn metabolic pathway was critical not only in neurodegenerative disease but also in our METH-induced model. In agreement with the prediction, the expression of both GCase and its trafficking receptor LIMP-2 decreased inversely with the doses of METH treatment. GCase is transported from the endoplasmic reticulum to the lysosome by LIMP-2. Thus, METH may disturb the process of GCase delivery by affecting LIMP-2; this leads to lysosomal dysfunction. Next, the GCase inhibitor CβE enhanced both upregulation of α-syn and downregulation of GCase levels induced by METH. Furthermore, LiCl restored the decrease of GCase and LIMP-2 disrupted by the METH treatment. Moreover, immunofluorescence results showing METH-caused α-syn accumulation in the cytoplasm was in agreement with the outcome of distribution imbalance of α-syn due to GCase dysfunction.50 Therefore, we consider that GSK3β acts as an upstream of GCase/α-syn pathway and disruptes α-syn degradation after METH injury.
Conclusion
In conclusion, our results demonstrate that the GSK3β/α-syn axis plays critical roles in METH-induced neurotoxicity. A schematic depicting the novel mechanism is provided in Fig. 9. METH exposure induced GSK3β activation and phosphorylation at the tyrosine 216 residue and targeted its substrates tau and α-syn by phosphorylation. GSK3β also mediated cell morphological alteration, apoptosis, and cell death after METH treatment. On the other hand, GSK3β/α-syn axis disturbed lysosomal autophagy and GCase degradative pathways, leading to abnormal α-syn accumulation and aggregation, which might explain the neurodegenerative injury induced by METH. Significantly, as the inhibitor of GSK3β, LiCl exerted protective effects on METH neurotoxicity. It is possible that GSK3β blockers, including LiCl, may be potential candidates for further therapeutic application of drug abuse although it needs more convincing evidences.
Fig. 9. Schematic of the GSK3β/α-syn axis in METH-induced neurotoxicity. METH treatment induced GSK3β activation and hyperphosphorylation, which targeted its substrate tau and α-syn by phosphorylation. GSK3β disturbed α-syn autophagy and GCase degradative pathways and finally caused abnormal α-syn accumulation and aggregation. These all mediated METH-induced cell apoptosis and death. All the abovementioned effects were rescued by LiCl or GSK3β siRNA treatment.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81501628) and Natural Science Foundation of Guangdong Province (Grant No. 2015A030310434).
References
- Vearrier D., Greenberg M. I., Miller S. N., Okaneku J. T., Haggerty D. A. Dis. Mon. 2012;58:38–89. doi: 10.1016/j.disamonth.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Krasnova I. N., Cadet J. L. Brain Res. Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D., An M., Gou H., Liu X., Liu L., Ma C., Cong B. Neurotoxicology. 2016;57:31–38. doi: 10.1016/j.neuro.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Cai D., Huang E., Luo B., Yang Y., Zhang F., Liu C., Lin Z., Xie W. B., Wang H. Cell Death Dis. 2016;7:e2161. doi: 10.1038/cddis.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow B. A., Roth R. H., Redmond D. E., Elsworth J. D. Neuroscience. 2011;189:277–285. doi: 10.1016/j.neuroscience.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan R. C., Cunningham J. K., Sykes J., Kish S. J. Drug Alcohol Depend. 2012;120:35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Fornai F., Lenzi P., Gesi M., Ferrucci M., Lazzeri G., Capobianco L., de Blasi A., Battaglia G., Nicoletti F., Ruggieri S., Paparelli A. Ann. N. Y. Acad. Sci. 2004;1025:162–170. doi: 10.1196/annals.1316.021. [DOI] [PubMed] [Google Scholar]
- Lazzeri G., Lenzi P., Busceti C. L., Ferrucci M., Falleni A., Bruno V., Paparelli A., Fornai F. J. Neurochem. 2007;101:1414–1427. doi: 10.1111/j.1471-4159.2006.04429.x. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Bae E. J., Lee S. J. Nat. Rev. Neurol. 2014;10:92–98. doi: 10.1038/nrneurol.2013.275. [DOI] [PubMed] [Google Scholar]
- Burré J., Sharma M., Südhof T. C. Cold Spring Harbor Perspect. Med. 2017 doi: 10.1101/cshperspect.a024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Huang E., Wang H., Qiu P., Liu C. Brain Res. 2013;1520:59–67. doi: 10.1016/j.brainres.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Tai Y., Chen L., Huang E., Liu C., Yang X., Qiu P., Wang H. Neural Regener. Res. 2014;9:951–958. doi: 10.4103/1673-5374.133146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. F., Wang A. F., Chen L., Huang E. P., Xie W. B., Liu C., Huang W. Y., Chen C. X., Qiu P. M., Wang H. J. Toxicol. Lett. 2014;230:19–27. doi: 10.1016/j.toxlet.2014.07.026. [DOI] [PubMed] [Google Scholar]
- Šimić G., Babić Leko M., Wray S., Harrington C., Delalle I., Jovanov-Milošević N., BaŽadona D., Buée L., de Silva R., Di Giovanni G. Biomolecules. 2016;6:6. doi: 10.3390/biom6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K., Liu F., Gong C. X. Nat.Rev. Neurol. 2016;12:15–27. doi: 10.1038/nrneurol.2015.225. [DOI] [PubMed] [Google Scholar]
- Embi N., Rylatt D. B., Cohen P. Eur. J. Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- Seira O., Del Río J. A. Mol. Neurobiol. 2014;49:931–944. doi: 10.1007/s12035-013-8571-y. [DOI] [PubMed] [Google Scholar]
- Hernandez F., de Barreda E. G., Fuster-Matanzo A., Goni-Oliver P., Lucas J. J., Avila J. Brain Res. Bull. 2009;80:248–250. doi: 10.1016/j.brainresbull.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Duka T., Duka V., Joyce J. N., Sidhu A. FASEB J. 2009;23:2820–2830. doi: 10.1096/fj.08-120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Tsuchiya A., Nishizaki T. Behav. Brain Res. 2014;274:302–306. doi: 10.1016/j.bbr.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Bian H., Bian W., Lin X., Ma Z., Chen W., Pu Y. Neurochem. Res. 2016;1:2470–2480. doi: 10.1007/s11064-016-1960-7. [DOI] [PubMed] [Google Scholar]
- Kawakami F., Suzuki M., Shimada N., Kagiya G., Ohta E., Tamura K., Maruyama H., Ichikawa T. FEBS J. 2011;278:4895–4904. doi: 10.1111/j.1742-4658.2011.08389.x. [DOI] [PubMed] [Google Scholar]
- Gąssowska M., Czapski G. A., Pająk B., Cieślik M., Lenkiewicz A. M., Adamczyk A. PLoS One. 2014;9:e94259. doi: 10.1371/journal.pone.0094259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y. H., Yan W. F., Sun J. D., Huang J. Y., Mu Z., Chen N. H. Toxicol. Lett. 2014;233:163–171. doi: 10.1016/j.toxlet.2014.11.029. [DOI] [PubMed] [Google Scholar]
- Wu J., Zhu D., Zhang J., Li G., Liu Z., Sun J. Biochem. Biophys. Res. Commun. 2015;465:368–373. doi: 10.1016/j.bbrc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Xu C. M., Wang J., Wu P., Xue Y. X., Zhu W. L., Li Q. Q., Zhai H. F., Shi J., Lu L. J. Neurochem. 2011;2118:126–139. doi: 10.1111/j.1471-4159.2011.07281.x. [DOI] [PubMed] [Google Scholar]
- Murphy K. E., Gysbers A. M., Abbott S. K., Tayebi N., Kim W. S., Sidransky E., Cooper A., Garner B., Halliday G. M. Brain. 2014;137:834–848. doi: 10.1093/brain/awt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehler A. M., Xiang W., Spitzer P., May V. E., Meixner H., Rockenstein E., Chutna O., Outeiro T. F., Winkler J., Masliah E., Klucken J. Autophagy. 2014;10:2171–2192. doi: 10.4161/auto.36436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C. M., Garcia-Haro L., Sparks C. A., Guertin D. A. Cold Spring Harbor Perspect. Biol. 2012;4:1–17. doi: 10.1101/cshperspect.a008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Takahashi Y., Cheng E., Liu J., Terranova P. F., Zhao B., Thrasher J. B., Wang H. G., Li B. J. Cell Sci. 2010;123:861–870. doi: 10.1242/jcs.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie T., Yang S., Ma H., Zhang L., Lu F., Tao K., Wang R., Yang R., Huang L., Mao Z., Yang Q. Cell Death Dis. 2016;7:e2563. doi: 10.1038/cddis.2016.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Credle J. J., George J. L., Wills J., Duka V., Shah K., Lee Y. C., Rodriguez O., Simkins T., Winter M., Moechars D., Steckler T., Goudreau J., Finkelstein D. I., Sidhu A. Cell Death Differ. 2015;22:838–851. doi: 10.1038/cdd.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B., Liang X. P., Liu P., Zhao Y., Chu Z., Dang Y. H. PLoS One. 2015;10:e0128068. doi: 10.1371/journal.pone.0128068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oueslati A., Fournier M., Lashuel H. A. Prog. Brain Res. 2010;183:115–145. doi: 10.1016/S0079-6123(10)83007-9. [DOI] [PubMed] [Google Scholar]
- Khandelwal P. J., Dumanis S. B., Feng L. R., Maguire-Zeiss K., Rebeck G. Mol. Neurodegener. 2010;5:1–13. doi: 10.1186/1750-1326-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom-Cadena E. M., Gelpi S., Charif O., Belbin R., Blesa M. J., Martí J., Clarimón A. J. Neuropathol. Exp. Neurol. 2013;72:1203–1212. doi: 10.1097/NEN.0000000000000018. [DOI] [PubMed] [Google Scholar]
- Ishizawa T., Mattila P., Davies P., Wang D., Dickson D. W. J. Neuropathol. Exp. Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- Li Y., Luo F., Wei L., Liu Z., Xu P. Neurosci. Lett. 2011;487:41–46. doi: 10.1016/j.neulet.2010.09.070. [DOI] [PubMed] [Google Scholar]
- Liang J., Liu L., Xing D. Free Radicals Biol. Med. 2012;53:1459–1467. doi: 10.1016/j.freeradbiomed.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Ren Z. X., Zhao Y. F., Cao T., Zhen X. C. Acta Pharmacol. Sin. 2016;37:1315–1324. doi: 10.1038/aps.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. Y., Xie W. B., Qiao D., Qiu P., Huang E., Li B., Chen C., Liu C., Wang Q., Lin Z., Wang H. Toxicol. Sci. 2015;145:68–79. doi: 10.1093/toxsci/kfv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay B., Martinez-Vicente M., Caldwell G. A., Caldwell K. A., Yue Z., Cookson M. R., Klein C., Vila M., Bezard E. Mov. Disord. 2013;28:725–732. doi: 10.1002/mds.25462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenovic M., Krainc D. Autophagy. 2012;8:987–988. doi: 10.4161/auto.20256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Jen N., Wu L., Lee J., Fang K., Quigley K., Lee K., Wang S., Zhou B., Vergnes L., Chen Y. R., Li Z., Reue K., Ann D. K., Hsiai T. K. Antioxid. Redox Signaling. 2015;23:1207–1219. doi: 10.1089/ars.2014.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Abdalla F. C., Abeliovich H., Abraham R. T., Acevedo-Arozena A., Adeli K., Agholme L., Agnello M., Agostinis P., Aguirre-Ghiso J. A., Ahn H. J. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filomeni G., Graziani I., De Zio D., Dini L., Centonze D., Rotilio G., Ciriolo M. R. Neurobiol. Aging. 2012;33:767–785. doi: 10.1016/j.neurobiolaging.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Ryu H. W., Oh W. K., Jang I. S., Park J. Biochem. Biophys. Res. Commun. 2013;433:121–126. doi: 10.1016/j.bbrc.2013.02.053. [DOI] [PubMed] [Google Scholar]
- Ginns E. I., Mak S. K., Ko N., Karlgren J., Akbarian S., Chou V. P., Guo Y., Lim A., Samuelsson S., LaMarca M. L., Vazquez-DeRose J., Manning-Boğ A. B. Mol. Genet. Metab. 2014;111:152–162. doi: 10.1016/j.ymgme.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Yap T. L., Jiang Z., Heinrich F., Gruschus J. M., Pfefferkorn C. M., Barros M., Curtis J. E., Sidransky E., Lee J. C. J. Biol. Chem. 2015;290:744–754. doi: 10.1074/jbc.M114.610584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert M., Sidransky E., Westbroek W. Brain. 2014;137:1304–1322. doi: 10.1093/brain/awu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli J. R., Xu Y. H., Sun Y., Knight A. L., McLean P. J., Caldwell G. A., Sidransky E., Grabowski G. A., Krainc D. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi S. P., Clarke J., Viel C., Chan M., Tamsett T. J., Treleaven C. M., Bu L., Sweet J., Passini M. A., Dodge J. C., Yu W. H., Sidman R. L., Cheng S. H., Shihabuddin L. S. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3537–3542. doi: 10.1073/pnas.1220464110. [DOI] [PMC free article] [PubMed] [Google Scholar]