Modern optical microscopy1, including confocal microscopy, two-photon microscopy and optical coherence tomography (OCT), has revolutionized life sciences by providing detailed information of biological samples with cellular and subcellular resolutions, and has become an essential tool for biomedical research labs. However, optical microscopy typically has a limited penetration depth of ~1 mm in biological tissue due to strong optical scattering2, 3. Moreover, with respective contrast mechanisms, confocal and two-photon microscopy usually rely on fluorescent labeling of the samples and OCT still lacks sensitivity to many biological functions. In contrast, optical-resolution photoacoustic microscopy (OR-PAM) has emerged over the last decade as a complementary imaging tool to the existing optical microscopy by taking advantage of its unique optical absorption contrast4. By acoustically detecting the optical absorption in the tissue, OR-PAM has been proven a powerful tool for anatomical, functional and molecular imaging with endogenous or exogenous contrast agents. In particular, using hemoglobin as the endogenous optical absorber, OR-PAM currently represents the most sensitive blood detector and has been widely used for in vivo imaging of the blood perfusion and oxygenation, especially for cancer and brain studies. Nevertheless, the acoustic detection in OR-PAM is a double-edged sword; on the one hand, it provides a relatively deep penetration with one-way optical attenuation and negligible acoustic attenuation, but on the other hand, the acoustic detection typically needs a coupling medium, such as water and ultrasound gel, between the tissue surface and the ultrasound transducer. The need for acoustic coupling has become one of the major factors that has hindered the wide adoption of OR-PAM by biomedical researchers whenever the biological samples are not compatible with an aquatic environment. Therefore, contact-free detection of the photoacoustic signals (that is, without the need for acoustic coupling) has captured the attention of the photoacoustic imaging community and resulted in many exciting advancements. If successful, the contact-free photoacoustic technologies will free up the working space and greatly expand the territory of PAM applications.
As optical detection of acoustic waves in ultrasound imaging, optical interferometry is the most popular approach in contact-free PAM5. Briefly, the detection light beam and the reference light beam are split from the same light source and then mixed. The mixed signal is perturbed by a photoacoustically generated thermal and pressure rise, which is then detected either locally (for example, via an OCT-based method) or at the sample’s surface (for example, via a displacement-based method). However, the interferometry-based methods often suffer from the rather complex signal generation progress that is sensitive to phase noise and artifact induced by the light source, the target and the acoustic propagation environment, and thus have met only limited success in well-controlled lab settings.
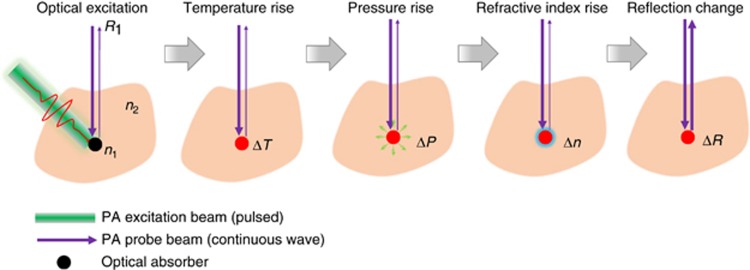
To address the above issues, Hajireza et al.6 have reported a novel contact-free OR-PAM technology that is (1) free of a coupling medium for acoustic propagation, (2) free of interferometry that is vulnerable to phase noise, (3) maximally sensitive to the local photoacoustic pressure rise and (4) extremely easy to implement and highly compatible with other optical imaging modalities. The reported remote-sensing PAM (PARS) uses a low-coherence probe light beam (coherence length: ~40 μm) to detect the local initial pressure rise (pressure magnitude: ~100 MPa) following pulsed light excitation (pulse energy: ~1 nJ; Figure 1). The local pressure rise can substantially magnify the existing difference in refractive indices of the local optical absorber and the surrounding environment and thus change the reflectance of the probe beam. The change in the probe beam reflection can then be remotely recorded and is proportional to the original optical energy deposition inside the imaged sample.
Figure 1.
Remote-sensing photoacoustic microscopy. Hajireza et al., have developed a contact-free OR-PAM system that has achieved remote detection of local pressure rise by using a low-coherence probe light beam. Upon excitation by a pulsed laser light, the temperature of the local optical absorber increases, inducing a local pressure rise that changes the absorber’s refractive index and the reflection of the probe beam.
Compared with the previous OR-PAM technologies7, Hajireza’s work has a striking advantage: A substantially simplified imaging system. The all-optical excitation and detection configuration avoids the complex optical–acoustic combiners that are typically bulky and lossy and has paved the way for seamless combination of OR-PAM with other pure optical imaging modalities with complementary contrast mechanisms. Moreover, the detected optical signals are truly wideband and thus do not suffer from acoustic attenuation along the pathway. This advantage, however, is contaminated by a major drawback that acoustic flight-time cannot be recorded by the non-interferometry method and thus multiple targets at different depths will be mixed at the detector. In other words, the inherent acoustically determined depth resolution, as exists in traditional OR-PAM, is lost. This drawback could potentially be resolved by depth-gated or coherence-gated detection, at the cost of imaging speed or sensitivity.
The remote-sensing PAM technology has without a doubt opened a new window into many exciting technology breakthroughs and corresponding biomedical applications. (1) Reflection-mode OR-PAM with a high-NA objective (>1.0) is now possible, providing a lateral resolution of ~200 nm. The high-NA objective typically has a working distance of less than a few millimeters, so the all-optical detection configuration is well suited for the reflection-mode system. Super-resolution PAM can then be developed on top of the high-NA system. (2) Biological applications that are not water friendly can now be performed without damaging the samples (for example, C. elegans or Drosophila) or complicating the accompanying procedures (for example, intraoperative delineation of tumor margin). Moreover, mid-IR wavelengths that are strongly attenuated by water can now be used, allowing their corresponding endogenous absorbers such as water, lipids and glucose to be better imaged.
Footnotes
The author declares no conflict of interest.
References
- Conchello JA, Lichtman JW. Optical sectioning microscopy. Nat Methods 2005; 2: 920–931. [DOI] [PubMed] [Google Scholar]
- Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods 2010; 7: 603–614. [DOI] [PubMed] [Google Scholar]
- Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol 2005; 23: 313–320. [DOI] [PubMed] [Google Scholar]
- Wang LV, Yao JJ. A practical guide to photoacoustic tomography in the life sciences. Nat Methods 2016; 13: 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong BQ, Sun C, Zhang HF. Optical detection of ultrasound in photoacoustic imaging. IEEE Trans Biomed Eng 2017; 64: 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajireza P, Shi W, Bell K, Paproski RJ, Zemp RJ. Non-interferometric photoacoustic remote sensing microscopy. Light Sci Appl 2017; 6: e16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JJ, Wang LV. Photoacoustic microscopy. Laser Photonics Rev 2013; 7: 758–778. [DOI] [PMC free article] [PubMed] [Google Scholar]