 Grape seed proanthocyanidin extract (GSPE) is a rich source of proanthocyanidins with multiple biological activities and potential health benefits.
Grape seed proanthocyanidin extract (GSPE) is a rich source of proanthocyanidins with multiple biological activities and potential health benefits.
Abstract
Grape seed proanthocyanidin extract (GSPE) is a rich source of proanthocyanidins with multiple biological activities and potential health benefits. In the present study, we investigated the protective effect of GSPE against liver injury caused by perfluorooctanoic acid (PFOA) in mice and its possible mechanisms of action. Simultaneous treatment with GSPE for 14 consecutive days attenuated the functional and morphological changes in the liver of PFOA-exposed mice. Furthermore, simultaneous supplementation of GSPE reduced the production of inflammatory cytokines IL-6 and TNF-α, increased the expression of Nrf2 and its target antioxidant genes superoxide dismutase and catalase, and decreased the production of malondialdehyde and hydrogen peroxide in the liver of mice exposed to PFOA. Moreover, GSPE supplementation up-regulated the expression of anti-apoptotic protein Bcl-2 and down-regulated the expression of pro-apoptotic proteins p53 and Bax, with a decreased activity of caspase-3 in the liver of PFOA-treated mice. These findings suggest that GSPE ameliorates PFOA-induced inflammatory response, oxidative stress and apoptosis in the liver of mice.
1. Introduction
In recent years, there has been a growing interest in plant polyphenols because of their potential health benefits. Polyphenols are naturally occurring compounds found largely in fruits, vegetables, cereals and beverages. Grape seeds, a by-product of the winery and grape juice industry, contain a large amount of polyphenolic compounds, such as (+)-catechins, (–)-epicatechin, (–)-epicatechin-3-O-gallate, procyanidin dimers (B1–B5), procyanidin C1, and procyanidin B5-3′-gallate.1,2 Grape seed proanthocyanidin extract (GSPE) is derived from grape seeds, and has been suggested to possess a broad spectrum of biological, pharmacological and therapeutic properties, such as anti-inflammatory, antibacterial, antiviral, anti-carcinogenic, anti-hypertensive, hypolipidemic, cardioprotective, hepatoprotective, and neuroprotective properties.2–5 Most of the beneficial effects of GSPE are attributed to the antioxidant activity and free radical scavenging ability, which provide excellent protection against oxidative stress and free radical-mediated tissue injury under pathological conditions.6
Perfluorooctanoic acid (PFOA), a member of stable organic perfluorinated compounds, is mainly used as an emulsifier in the production of fluoropolymers and fluoroelastomers. Due to the resistance to biodegradation and bioaccumulation in food chains, PFOA has been detected extensively in the environment, wildlife and humans.7 The data from NHANES 2007–2010 showed that the geometric mean serum PFOA concentration was 3.5 ng mL–1 in the U.S. population.8 In occupational exposure population, the median serum PFOA level could reach 775 ng mL–1.9 Food intake and packaging, water, house dust, and airborne particles are all potentially significant sources of PFOA.10
Exposure to PFOA has been reported to elicit a variety of toxicities.7 Epidemiologic evidence has shown that PFOA exposure is positively associated with cardiovascular disease,11 chronic kidney disease,12 thyroid disease,13 and hepatocellular damage.14 The liver serves as the primary target organ for PFOA-induced toxicity in the body. Numerous studies have reported that PFOA exposure exerts toxic effects on the liver.15–18 Because oxidative stress plays an important role in PFOA-induced hepatic toxicity, antioxidants may be useful in the prevention and treatment of PFOA-induced liver damage. As a more potent scavenger of oxygen free radicals than vitamin C and V,19 GSPE exhibits strong antioxidant activity in disease prevention and treatment.6,20,21 A clinical study has reported that grape seed extract improves the liver function in patients with nonalcoholic fatty liver disease.22 In animal experiments, GSPE has been shown to exert a protective effect against the hepatotoxicity triggered by lipopolysaccharide through the anti-inflammatory and antioxidant mechanisms.23 Moreover, GSPE has been demonstrated to attenuate acetaminophen-induced DNA damage and apoptotic and necrotic cell death in the liver.24 Although studies have suggested the hepatoprotective action of GSPE, the protection of GSPE against PFOA-induced hepatotoxicity has not yet been elucidated. Therefore, the present study was designed to investigate the protective effects of GSPE on PFOA-induced liver damage and to analyze the possible hepatoprotective mechanisms of GSPE in mice at the morphological, biochemical and molecular levels.
2. Materials and methods
2.1. Animals and chemicals
Male Kunming mice weighing 20–22 g were obtained from the Laboratory Animal Center of Nanchang University. GSPE (purity ≥ 95%) was purchased from Sciphar Biotechnology (Xi'an, China), and was stored in a sealed container at 4 °C to maintain its stability. PFOA (purity 96%) was purchased from Sigma-Aldrich (St. Louis, USA); GSPE and PFOA were dissolved in water. The mice were housed at room temperature (22 °C) under a standard 12 h light/dark cycle and given ad libitum access to food and water. All experimental procedures followed the Guidelines for Care and Use of Laboratory Animals of Nanchang University. The protocols were approved by the Animal Ethics Committee of Nanchang University (no. 20130916).
After a week of adaptation to laboratory conditions, the animals were divided into four groups as follows: (1) control group: mice received vehicle only. (2) PFOA group: mice received PFOA 10 mg per kg per day. (3) GSPE group: mice received GSPE 150 mg per kg per day. (4) PFOA + GSPE group: mice received PFOA 10 mg per kg per day and GSPE 150 mg per kg per day. All animals were treated once daily by oral gavage for 14 consecutive days. Chemicals from a single lot were used for all of the animal experiments.
At the end of treatment period, the mice were fasted for 12 h, anesthetized with sodium pentobarbital (50 mg kg–1 ip), and sacrificed by cervical dislocation. The blood samples were collected by cardiac puncture and then centrifuged at 13 000 rpm at 4 °C for 30 min to separate the serum. The liver samples were excised and frozen in liquid nitrogen or fixed in 4% paraformaldehyde for subsequent measurements. The serum samples were stored at –80 °C until analysis.
2.2. Measurement of serum enzymes
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) activities were measured using the Hitachi 7180 automatic biochemical analyzer.
2.3. Histopathological examination
The fixed liver tissues were dehydrated in an ascending series of alcohol, cleared in xylene, embedded in paraffin, and sectioned at 5 μm. The sections were stained with hematoxylin and eosin. The morphological changes were observed under the Olympus IX71 microscope.
2.4. Measurement of inflammatory markers
The frozen liver tissues were homogenized in ice-cold normal saline. The levels of inflammatory response markers interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) in liver tissue homogenates were measured using commercially available ELISA kits (Westang Biotechnology, Shanghai, China), in accordance with the manufacturers’ instructions.
2.5. Oxidative stress analysis
The concentrations of malondialdehyde (MDA) and hydrogen peroxide (H2O2) and activities of superoxide dismutase (SOD) and catalase (CAT) in liver homogenates were measured using commercially available kits (Jiancheng Institute of Biotechnology, Nanjing, China), in accordance with the manufacturers’ instructions.
2.6. Quantitative real-time PCR assay
Total RNA was extracted from the frozen liver tissue using the Trizol reagent (Invitrogen, CA), and was reverse transcribed into cDNA using the RT reagent kit with the gDNA eraser (TaKaRa, China). Quantitative real-time PCR was performed using the ABI Prism 7500 sequence detection system (PE Applied Biosystems) with SYBR Green Mix (TaKaRa, China). The sequences of the specific primers are listed in Table 1. The relative expression levels of the target genes were determined using GAPDH mRNA as an internal control, and the relative fold change in the mRNA expression was calculated using the 2–ΔΔCT.
Table 1. Specific primers for real-time PCR analysis.
| Gene | Description | Primer sequence (5′–3′) | Product length (bp) |
| Nrf2 | Forward | TGAAGCTCAGCTCGCATTGA | 108 |
| Reverse | TGCTCCAGCTCGACAATGTT | 108 | |
| SOD1 | Forward | ATCCACTTCGAGCAGAAGGC | 96 |
| Reverse | CTGATGGACGTGGAACCCAT | 96 | |
| CAT | Forward | TTTTGCCTACCCGGACACTC | 154 |
| Reverse | GGGGTAATAGTTGGGGGCAC | 154 | |
| GAPDH | Forward | GGCAAATTCAACGGCACAGT | 84 |
| Reverse | GTCTCGCTCCTGGAAGATGG | 84 |
2.7. Western blot analysis
The liver tissues were lysed in lysis buffer and then lysates were centrifuged for 10 min at 14 000g at 4 °C. The supernatants were collected for the immunoblot assay. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. The immunoblots were subsequently blocked with 5% non-fat dry milk and 0.1% Tween 20 in Tris-buffered saline (TBST). The membranes were incubated overnight at 4 °C with the primary antibodies against Nrf2, p53, Bcl-2, Bax or GAPDH (Santa Cruz Biotechnology Inc.). After washing with TBST, the membranes were incubated with the secondary horseradish peroxidase-conjugated antibodies (Santa Cruz Biotechnology Inc.) for 1 hour at room temperature. Immunoreactive bands were visualized by using the enhanced chemiluminescence kit (Thermo Fisher Scientific Inc.), and chemiluminescent signals were collected on autoradiography films.
2.8. Measurement of caspase-3 activity
The activity of caspase-3 in liver homogenates was determined using a commercially available kit (KeyGEN Biotechnology, Nanjing, China), in accordance with the manufacturers’ instructions.
2.9. Statistical analysis
The experiment was repeated three times. Statistical analysis was performed using SAS software version 8.2 (SAS Institute Inc.). Data were presented as the mean ± standard deviation of 4 mice and evaluated by one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
3. Results
3.1. Effect of GSPE on PFOA-induced hepatic dysfunction
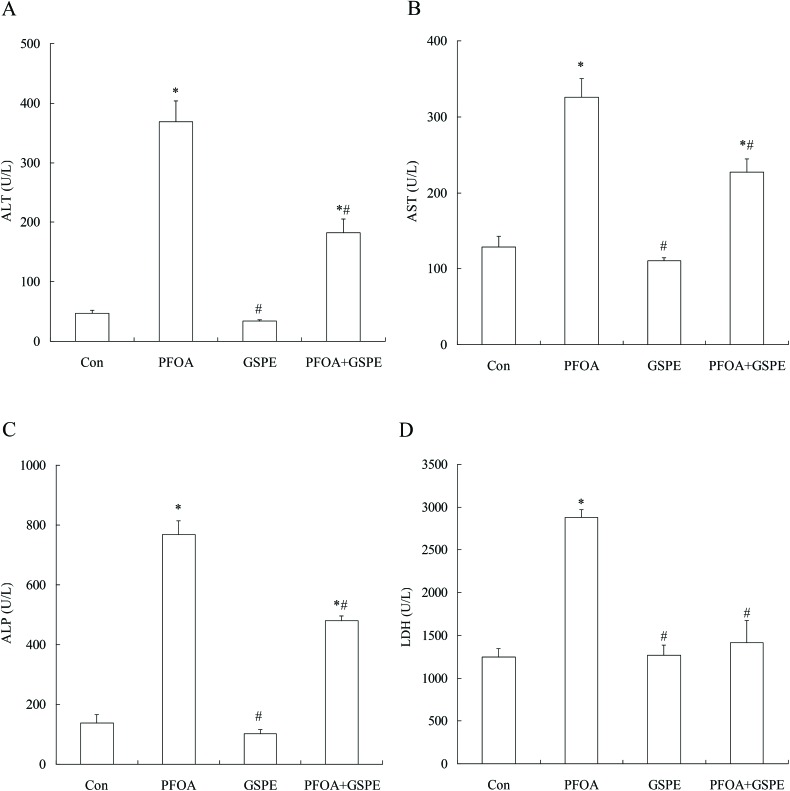
In order to investigate the protective effect of GSPE on PFOA-induced hepatic damage, serum liver function markers AST, ALT, ALP and LDH were measured. As shown in Fig. 1, exposure to PFOA for 14 consecutive days induced a marked increase in serum ALT, AST, ALP and LDH levels compared with the control group (P < 0.05). However, simultaneous supplementation of GSPE significantly decreased the levels of these biological markers in the mice exposed to PFOA (P < 0.05). Especially, LDH was restored to the control levels by GSPE treatment. No significant differences in serum ALT, AST, ALP and LDH levels were observed between the control group and the GSPE group (P > 0.05).
Fig. 1. Effect of GSPE on the serum levels of ALT (A), AST (B), ALP (C) and LDH (D) in PFOA-treated mice. Values are expressed as mean ± SD of 4 mice. * P < 0.05 versus control group; #P < 0.05 versus PFOA group.
3.2. Effect of GSPE on PFOA-induced changes in the liver morphology
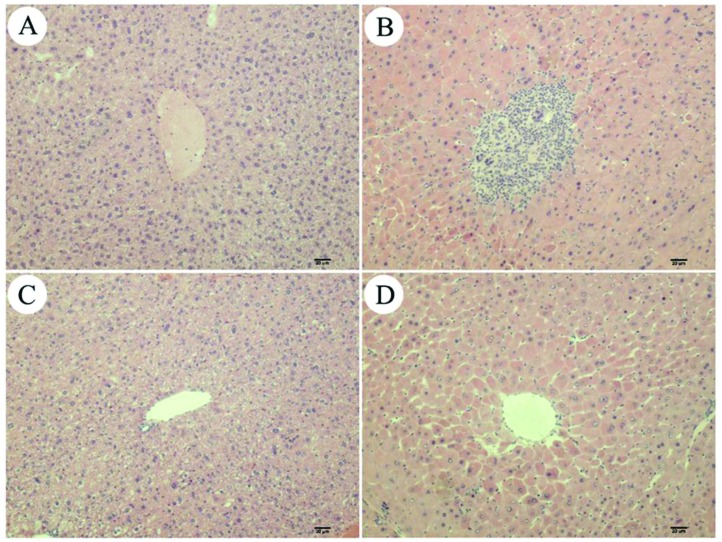
As shown in Fig. 2, oral administration of PFOA for 14 consecutive days caused extensive morphological changes in the liver of mice, which were characterized by architectural disorganization, marked edema, vacuolar degeneration, hepatocellular necrosis and inflammatory cell infiltration. However, all of these adverse histological changes induced by PFOA were significantly attenuated by simultaneous treatment with GSPE. The mice treated with GSPE alone displayed the same normal liver morphology as the control mice.
Fig. 2. Histopathological staining. (A) Control group; (B) PFOA group; (C) GSPE group; (D) PFOA + GSPE group. Magnification: 100×.
3.3. Effect of GSPE on PFOA-induced liver inflammation
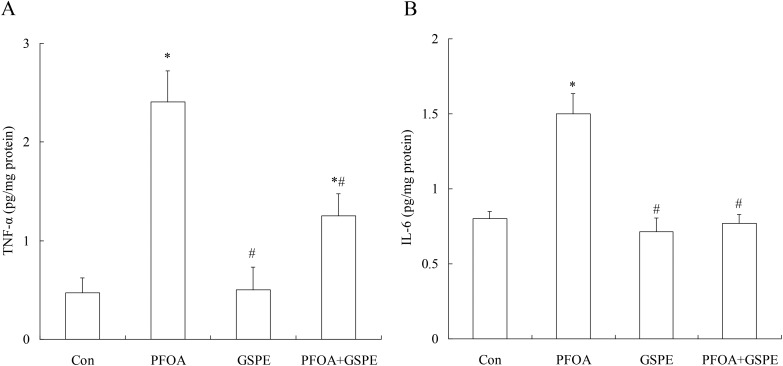
In order to investigate the anti-inflammatory effect of GSPE on PFOA-induced liver inflammation, inflammatory markers TNF-α and IL-6 were measured in the liver tissue. As shown in Fig. 3, exposure to PFOA for 14 consecutive days resulted in a significant increase in hepatic TNF-α and IL-6 levels compared with the control group (P < 0.05). However, simultaneous supplementation of GSPE markedly decreased the hepatic levels of these two markers for inflammatory response in mice exposed to PFOA (P < 0.05). Especially, IL-6 was returned to the control levels after simultaneous GSPE treatment. No significant differences in hepatic TNF-α and IL-6 levels were found between the control group and the GSPE group (P > 0.05).
Fig. 3. Effect of GSPE on the hepatic levels of TNF-α (A) and IL-6 (B) in PFOA-treated mice. Values are expressed as mean ± SD of 4 mice. * P < 0.05 versus control group; #P < 0.05 versus PFOA group.
3.4. Effect of GSPE on PFOA-induced liver oxidative stress
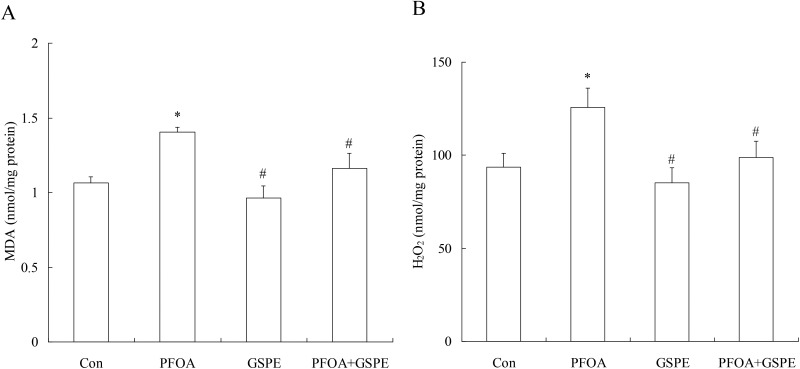
In order to investigate the antioxidative effect of GSPE on PFOA-induced liver oxidative stress, the production of MDA and H2O2 were measured in the liver tissue. As shown in Fig. 4, exposure to PFOA for 14 consecutive days significantly increased the hepatic levels of MDA and H2O2 compared with the control group (P < 0.05). However, simultaneous treatment with GSPE significantly reduced the hepatic levels of these two markers for oxidative stress to normal levels in mice exposed to PFOA (P < 0.05). Compared with the control group, GSPE treatment alone had no significant effect on the production of MDA and H2O2 in the liver of mice (P > 0.05).
Fig. 4. Effect of GSPE on the hepatic levels of MDA (A) and H2O2 (B) in PFOA-treated mice. Values are expressed as mean ± SD of 4 mice. * P < 0.05 versus control group; #P < 0.05 versus PFOA group.
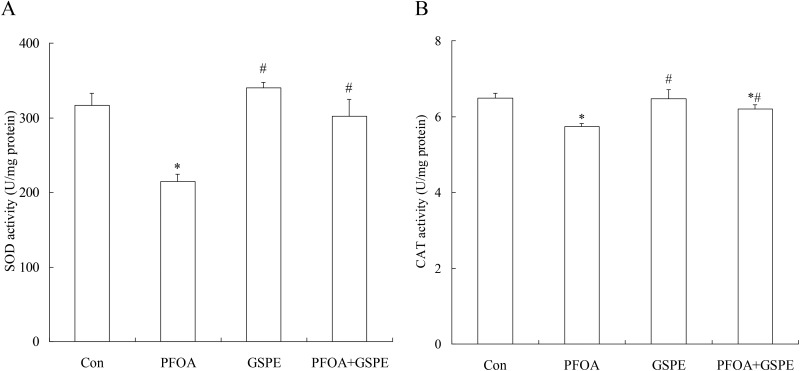
3.5. Effect of GSPE on antioxidant enzymes in the liver of mice exposed to PFOA
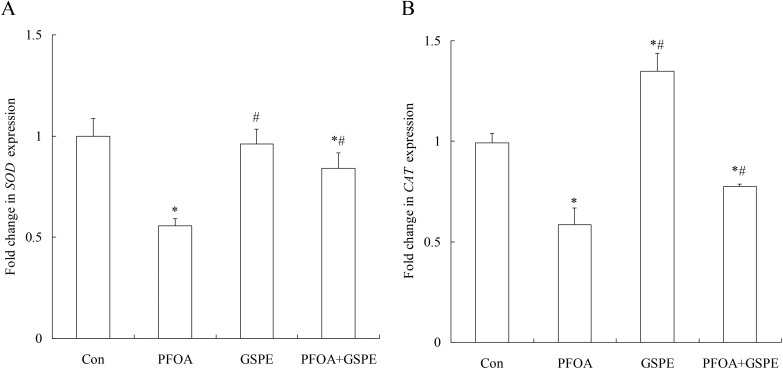
Compared with the control group, PFOA treatment for 14 consecutive days significantly decreased the activities of SOD and CAT in the liver (Fig. 5) (P < 0.05). Furthermore, quantitative real-time PCR revealed a marked down-regulation of SOD and CAT expression in the liver of mice exposed to PFOA (Fig. 6) (P < 0.05). However, the activities of SOD and CAT, as well as the expression of SOD and CAT, were significantly increased by treatment with GSPE in the liver of PFOA-exposed mice (Fig. 5 and 6) (P < 0.05). Compared with the control group, GSPE treatment alone had no significant effect on the activities of SOD and CAT in the liver of mice (P > 0.05).
Fig. 5. Effect of GSPE on the hepatic activities of SOD (A) and CAT (B) in PFOA-treated mice. Values are expressed as mean ± SD of 4 mice. * P < 0.05 versus control group; #P < 0.05 versus PFOA group.
Fig. 6. Effect of GSPE on mRNA expression of SOD (A) and CAT (B) in the liver of mice exposed to PFOA. Values are expressed as mean ± SD of 4 mice. * P < 0.05 versus control group; #P < 0.05 versus PFOA group.
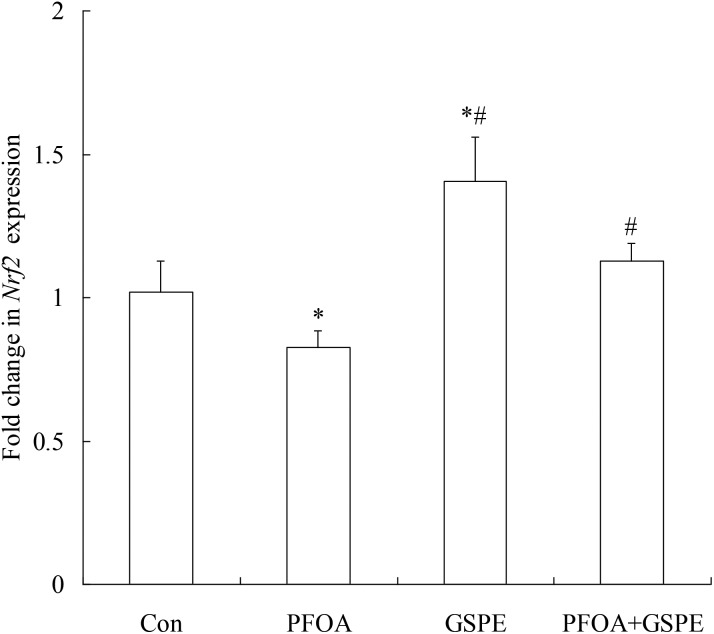
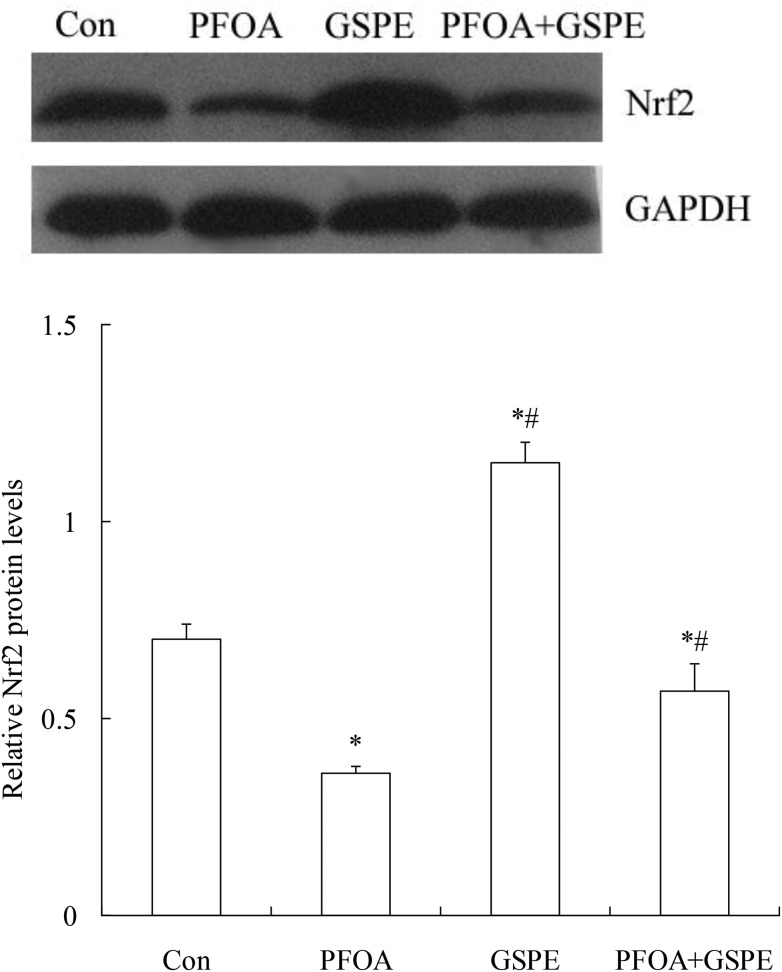
3.6. Effect of GSPE on Nrf2 expression in the liver of mice exposed to PFOA
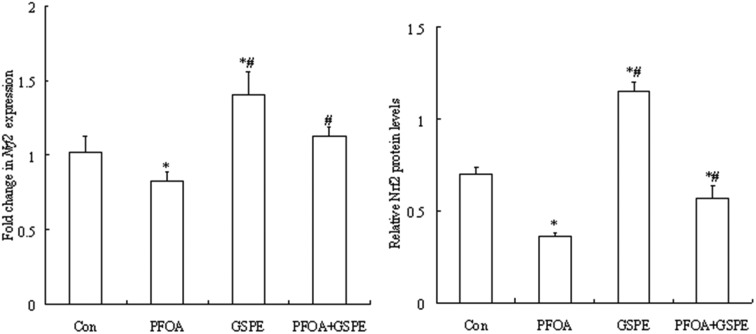
As shown in Fig. 7 and 8, oral administration of PFOA for 14 consecutive days significantly down-regulated the mRNA and protein expression of Nrf2 in the liver of mice (P < 0.05). However, the down-regulation of Nrf2 expression induced by PFOA was significantly restored by simultaneous GSPE treatment (P < 0.05). Compared with the control group, the mRNA and protein expression of Nrf2 in the liver was markedly up-regulated by GSPE treatment alone (P < 0.05).
Fig. 7. Effect of GSPE on Nrf2 mRNA expression in the liver of mice exposed to PFOA. Values are expressed as mean ± SD of 4 mice. * P < 0.05 versus control group; #P < 0.05 versus PFOA group.
Fig. 8. Effect of GSPE on Nrf2 protein expression in the liver of mice exposed to PFOA. Values are expressed as mean ± SD of 4 mice. * P < 0.05 versus control group; #P < 0.05 versus PFOA group.
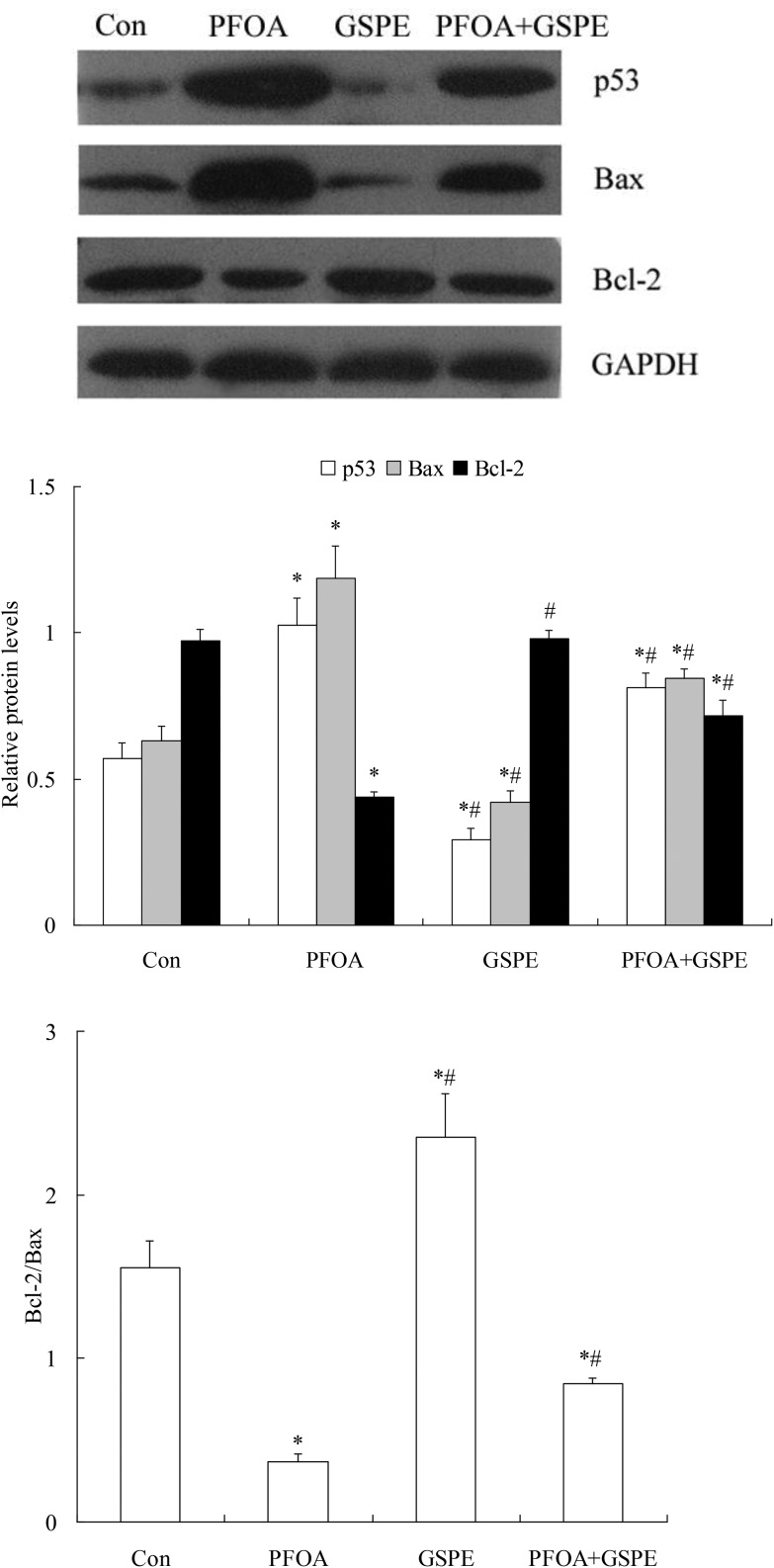
3.7. Effect of GSPE on PFOA-induced hepatocellular apoptosis
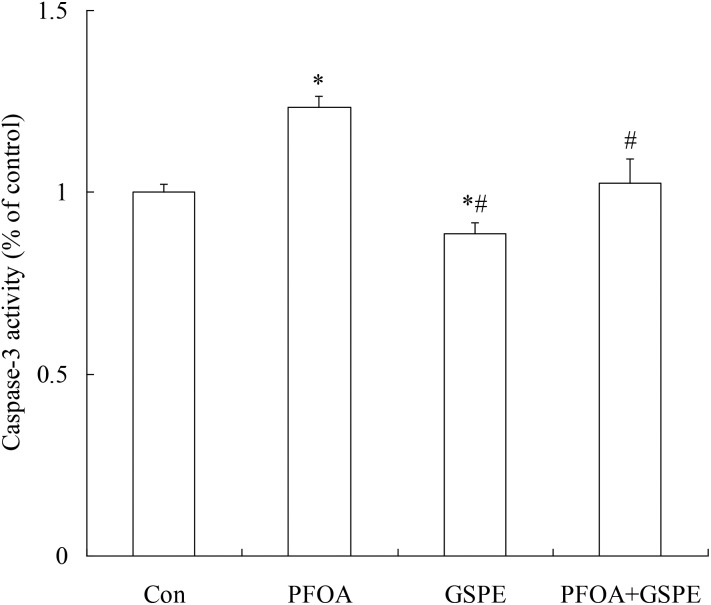
As shown in Fig. 9, western blot analysis revealed a significant increase in the pro-apoptotic proteins p53 and Bax expression and a marked decrease in the anti-apoptotic protein Bcl-2 expression in the liver of mice exposed to PFOA (P < 0.05). However, the increase in p53 and Bax expression and the decrease in Bcl-2 expression induced by PFOA were significantly inhibited by simultaneous GSPE treatment (P < 0.05). Furthermore, compared with the control group, a significant increase in caspase-3 activity was found in the liver of PFOA-exposed mice (P < 0.05). However, hepatic caspase-3 activity was restored to control levels by simultaneous GSPE treatment (P < 0.05). Moreover, compared with the control group, the expression of p53 and Bax and the activity of caspase-3 in the liver were markedly decreased by GSPE treatment alone (Fig. 10) (P < 0.05).
Fig. 9. Effect of GSPE on protein expression of p53, Bax and Bcl-2 in liver of mice exposed to PFOA. Values are expressed as mean ± SD of 4 mice. * P < 0.05 versus control group; #P < 0.05 versus PFOA group.
Fig. 10. Effect of GSPE on caspase-3 activity in the liver of mice exposed to PFOA. Values are expressed as mean ± SD of 4 mice. * P < 0.05 versus control group; #P < 0.05 versus PFOA group.
4. Discussion
There is increasing evidence that environmental pollutants exert adverse effects on the animal and human health. As a stable compound widely used in various industrial processes, PFOA has been found not only in occupationally exposed workers but also general populations.25 The liver is particularly susceptible to injury following systemic exposure to xenobiotics because of its central role in drug metabolism.26 It has been reported that exposure to PFOA is positively associated with the hepatic injury in humans.14 Animal experiments also demonstrated the hepatotoxicity of PFOA.15–18 It has been suggested that grape seed extract exhibits the hepatoprotective effect on liver damage induced by different chemicals in experimental animals.24,27,28 GSPE is considered to be a rich source of proanthocyanidins with potential health benefits. In the present study, the ameliorating effect of GSPE on PFOA-induced hepatotoxicity was assessed in mice. In a previous study, the serum PFOA concentration in mice exposed to PFOA 20 mg per kg per day for 28 days reached 105 μg mL–1,29 which was similar to the highest serum PFOA levels reported in occupational workers (114.1 ppm).30 Therefore, the dose of PFOA tested in this experiment is relevant to occupational exposure in humans.
Serum transaminases are the most universally important indicators for the diagnosis of hepatic injury. Hepatocellular damage with the subsequent disruption of the plasma membrane allows the leakage of intracellular enzymes such as ALT or AST into the bloodstream, thereby leading to an elevation of serum transaminase levels.26 LDH is another leakage enzyme used as the potential marker of hepatocellular toxicity. ALP is widely employed to detect the impaired bile flow, and the elevated serum ALP activity is mostly attributed to hepatobiliary origin. In this study, the serum levels of AST, ALT, ALP, and LDH were significantly elevated after oral administration of PFOA. However, the elevated levels of serum hepatic enzymes were significantly decreased by simultaneous treatment with GSPE. In addition, histopathology revealed that the hepatic morphological changes induced by PFOA administration were significantly diminished by simultaneous treatment with GSPE. These results implied a protective effect of GSPE against liver injury induced by PFOA, which leads to a restoration of physiological functions and a reduction of pathological changes in the liver of mice exposed to PFOA.
Although the hepatoprotective effects of GSPE have been reported, the precise mechanism of action of GSPE is not yet fully understood. As a naturally occurring anti-inflammatory agent, GSPE has been demonstrated to reduce the production of proinflammatory cytokines and to alleviate the inflammation induced by LPS, high-fat diet and hepatic ischemia-reperfusion in rats.23,31,32 The proinflammatory cytokines play an important role in the mediation of inflammation. In the present study, PFOA treatment resulted in a significant increase in inflammatory biomarkers IL-6 and TNF-α in the liver of mice. However, the increase in the hepatic levels of proinflammatory cytokines was significantly inhibited by simultaneous treatment with GSPE. These results indicated an anti-inflammatory effect of GSPE on PFOA-induced inflammatory liver injury.
Oxidative stress is a crucial factor in the development and progression of various liver diseases. It primarily affects the proteins, lipids and DNA in the hepatocytes.33 It has been shown that natural antioxidants quercetin and gallic acid protect against sodium fluoride-induced oxidative stress in the hepatic tissue.34,35 GSPE has strong antioxidant activity and free radical scavenging ability. It can protect the cellular membrane from oxidative damage and consequently prevents protein and lipid oxidation in isolated rat hepatocytes.36 Furthermore, in vivo studies also demonstrated that GSPE decreased the generation of MDA, an end product of lipid peroxidation, and increased the activities of antioxidant enzymes in the liver.37,38 In the present study, PFOA administration increased the levels of oxidative stress markers MDA and H2O2 and decreased the activities of endogenous antioxidants SOD and CAT in the liver of mice. However, GSPE supplementation significantly attenuated hepatic oxidative stress caused by PFOA exposure through suppressing lipid peroxidation and restoring antioxidant enzyme activities, suggesting that GSPE exerts the hepatoprotective effect as an antioxidant on PFOA-induced oxidative liver injury.
The central role in defense against oxidative stress is attributed to nuclear factor-erythroid-2-related factor 2 (Nrf2), an important transcription factor that protects cells from endogenous and exogenous stresses. Nrf2 plays a crucial role in the induction of the expression of antioxidants involved in environmental oxidative stress responses.39 However, Nrf2 is down-regulated in cells or tissues exposed to an overwhelming or long-lasting oxidative stress.40 The Nrf2-knockout mice are devoid of the basal and inducible expression of phase 2 detoxification enzymes and antioxidant genes in hepatic cells.41 Furthermore, the tumor suppressor p53 is important in maintaining both basal and induced levels of Nrf2.42 It has been demonstrated that p53 suppresses the Nrf2-dependent transcription of antioxidant response genes.43 At high levels or extended exposure of ROS, p53 expression is enhanced, and the elevated p53 level inhibits the protein expression of Nrf2 and suppresses Nrf2-mediated survival response.42 In the present study, PFOA treatment induced a significant decrease in the expression of Nrf2 protein and its target antioxidant genes SOD and CAT in the liver of mice, which might be due to the increase in p53 expression mediated by oxidative damage. These results suggest that the activation of Nrf2 might be a potential strategy to ameliorate liver injury caused by PFOA. A study reported that grape seed proanthocyanidins ameliorated cadmium-induced renal injury and oxidative stress through the up-regulation of Nrf2 in rats.44 In our study, GSPE supplementation induced a significant increase in the expression of Nrf2, SOD and CAT in the liver of PFOA-exposed mice, suggesting that GSPE plays a protective role against PFOA-caused hepatotoxicity through Nrf2-mediated induction of antioxidant enzymes.
Apoptosis is a ubiquitous homeostatic process involved in numerous physiological processes. Environmental stressors are known to induce apoptosis and oxidative stress may be the central element in the regulation of the apoptotic pathways triggered by environmental stressors.45 Mitochondria play a critical role in mediating apoptotic cell death. In the liver, ROS are primarily produced in the mitochondria.33 ROS production results in an alteration in mitochondrial permeability and transition potential, which induces the release of pro-apoptotic factors and an increase in caspase-3 activation. At mitochondria, p53 protein can directly induce permeabilization of the outer mitochondrial membrane by forming complexes with the protective Bcl-2 family proteins.46 It has been documented that PFOA induces hepatic L-02 cell apoptosis through the p53-dependent mitochondrial pathway.47 In the present study, PFOA exposure up-regulated the protein expression of pro-apoptotic p53 and Bax, and down-regulated the expression of anti-apoptotic Bcl-2 in the liver of mice. Furthermore, the activity of caspase-3, a key mediator of p53-induced apoptosis, was significantly elevated in the liver of PFOA-treated mice. These results demonstrated that PFOA induced apoptosis in the mouse liver.
A previous study reported that GSPE increased in vivo Bcl-Xl expression and attenuated acetaminophen-induced DNA damage and apoptotic cell death in the mouse liver.24 Moreover, GSPE ameliorated chemotherapy-induced cytotoxicity through up-regulating Bcl-2 expression and down-regulating c-myc and p53 expression in normal human Chang liver cells.48 In the present study, the alterations in the expression of p53, Bax and Bcl-2 and activity of caspase-3 were significantly restored in the liver of PFOA-exposed mice, suggesting that GSPE attenuates PFOA-induced hepatotoxicity through inhibiting hepatic cell apoptosis.
5. Conclusion
PFOA exposure resulted in liver injury in mice through the induction of oxidative stress, inflammation and apoptosis. However, GSPE supplementation inhibited lipid peroxidation, up-regulated the expression of transcription factor Nrf2 and antioxidant enzymes SOD and CAT, reduced the production of inflammatory cytokines IL-6 and TNF-α, increased the expression of anti-apoptotic protein Bcl-2, and decreased the expression of pro-apoptotic proteins p53 and Bax and activity of caspase-3 in the liver of PFOA-exposed mice. In summary, GSPE exerted a protective effect against PFOA-induced hepatotoxicity by reducing oxidative stress, attenuating inflammatory response and inhibiting hepatocellular apoptosis. Our results suggest that GSPE may serve as a potent activator of Nrf2 to ameliorate PFOA-induced liver damage.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81560248 and 81060056).
References
- Perumalla A. V. S., Hettiarachchy S. Food Res. Int. 2011;44:827–839. [Google Scholar]
- Nassiri-Asl M., Hosseinzadeh H. Phytother. Res. 2009;23:1197–1204. doi: 10.1002/ptr.2761. [DOI] [PubMed] [Google Scholar]
- Vislocky L. M., Fernandez M. L. Nutr. Rev. 2010;68:656–670. doi: 10.1111/j.1753-4887.2010.00335.x. [DOI] [PubMed] [Google Scholar]
- Yin W., Li B., Li X., Yu F., Cai Q., Zhang Z., Cheng M., Gao H. Food Funct. 2015;6:3065–3071. doi: 10.1039/c5fo00496a. [DOI] [PubMed] [Google Scholar]
- Lan C., Ding L., Su Y., Guo K., Wang L., Kan H., Ou Y., Gao S. Food Funct. 2015;6:2179–2186. doi: 10.1039/c5fo00253b. [DOI] [PubMed] [Google Scholar]
- Bagchi D., Bagchi M., Stohs S. J., Das D. K., Ray S. D., Kuszynski C. A., Joshi S. S., Pruess H. G. Toxicology. 2000;148:187–197. doi: 10.1016/s0300-483x(00)00210-9. [DOI] [PubMed] [Google Scholar]
- Lau C., Anitole K., Hodes C., Lai D., Pfahles-Hutchens A., Seed J. Toxicol. Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Gleason J. A., Post G. B., Fagliano J. A. Environ. Res. 2015;136:8–14. doi: 10.1016/j.envres.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Emmett E. A., Shofer F. S., Zhang H., Freeman D., Desai C., Shaw L. M. J. Occup. Environ. Med. 2006;48:759–770. doi: 10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J. L. Environ. Int. 2012;40:187–195. doi: 10.1016/j.envint.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Shankar A., Xiao J., Ducatman A. Arch. Intern. Med. 2012;172:1397–1403. doi: 10.1001/archinternmed.2012.3393. [DOI] [PubMed] [Google Scholar]
- Shankar A., Xiao J., Ducatman A. Am. J. Epidemiol. 2011;174:893–900. doi: 10.1093/aje/kwr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D., Rice N., Depledge M. H., Henley W. E., Galloway T. S. Environ. Health Perspect. 2010;118:686–692. doi: 10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Leonardi G., Genser B., Lopez-Espinosa M. J., Frisbee S. J., Karlsson L., Ducatman A. M., Fletcher T. Environ. Health Perspect. 2012;120:655–660. doi: 10.1289/ehp.1104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H. Y., Kim H. Y., Shin H. I., Bae H. I., Yang J. H. Arch. Toxicol. 2008;82:239–246. doi: 10.1007/s00204-007-0246-x. [DOI] [PubMed] [Google Scholar]
- Cui L., Zhou Q. F., Liao C. Y., Fu J. J., Jiang G. B. Arch. Environ. Contam. Toxicol. 2009;56:338–349. doi: 10.1007/s00244-008-9194-6. [DOI] [PubMed] [Google Scholar]
- Eldasher L. M., Wen M., Little M. S., Bircsak K. M., Yacovino L. L., Aleksunes L. M. Toxicology. 2013;306:108–113. doi: 10.1016/j.tox.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Zou W. Y., Hu Z. Z., Liu F. M., Zhou L., Yang S. L., Kuang H. B., Wu L., Wei J., Wang J. L., Zou T., Zhang D. L. Biomed. Res. Int. 2014:409837. doi: 10.1155/2014/409837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi D., Garg A., Krohn R. L., Bagchi M., Tran M. X., Stohs S. J. Res. Commun. Mol. Pathol. 1997;95:179–189. [PubMed] [Google Scholar]
- Zhao Y., Gao L., Zhang H., Guo J., Guo P. Food Funct. 2014;5:605–611. doi: 10.1039/c3fo60486a. [DOI] [PubMed] [Google Scholar]
- Bao L., Cai X., Dai X., Ding Y., Jiang Y., Li Y., Zhang Z., Li Y. Food Funct. 2014;5:1872–1880. doi: 10.1039/c4fo00340c. [DOI] [PubMed] [Google Scholar]
- Khoshbaten M., Aliasgarzadeh A., Masnadi K., Farhang S., Tarzamani M. K., Babaei H., Kiani J., Zaare M., Najafipoor F. Saudi J. Gastroenterol. 2010;16:194–197. doi: 10.4103/1319-3767.65197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallarès V., Fernández-Iglesias A., Cedó L., Castell-Auví A., Pinent M., Ardévol A., Salvadó M. J., Garcia-Vallvé S., Blay M. Free Radicals Biol. Med. 2013;60:107–114. doi: 10.1016/j.freeradbiomed.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Ray S. D., Kumar M. A., Bagchi D. Arch. Biochem. Biophys. 1999;369:42–58. doi: 10.1006/abbi.1999.1333. [DOI] [PubMed] [Google Scholar]
- Kato K., Wong L. Y., Jia L. T., Kuklenyik Z., Calafat A. M. Environ. Sci. Technol. 2011;45:8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Amacher D. E. Regul. Toxicol. Pharm. 1998;27:119–130. doi: 10.1006/rtph.1998.1201. [DOI] [PubMed] [Google Scholar]
- Cetin A., Kaynar L., Kocyigit I., Hacioglu S. K., Saraymen R., Ozturk A., Sari I., Sagdic O. Am. J. Chinese Med. 2008;36:861–872. doi: 10.1142/S0192415X08006302. [DOI] [PubMed] [Google Scholar]
- El-Ashmawy I. M., Gad S. B., Salama O. M. Phytother. Res. 2010;24:1710–1715. doi: 10.1002/ptr.3200. [DOI] [PubMed] [Google Scholar]
- Zhang H., Lu Y., Luo B., Yan S., Guo X., Dai J. J. Proteome Res. 2014;13:3370–3385. doi: 10.1021/pr500228d. [DOI] [PubMed] [Google Scholar]
- Olsen G. W., Burris J. M., Burlew M. M., Mandel J. H. Drug Chem. Toxicol. 2000;23:603–620. doi: 10.1081/dct-100101973. [DOI] [PubMed] [Google Scholar]
- Terra X., Montagut G., Bustos M., Llopiz N., Ardèvol A., Bladé C., Fernández-Larrea J., Pujadas G., Salvadó J., Arola L., Blay M. J. Nutr. Biochem. 2009;20:210–218. doi: 10.1016/j.jnutbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Sehirli O., Ozel Y., Dulundu E., Topaloglu U., Ercan F., Sener G. Phytother. Res. 2008;22:43–48. doi: 10.1002/ptr.2256. [DOI] [PubMed] [Google Scholar]
- Cichoż-Lach H., Michalak A. World J. Gastroenterol. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S. M., Nabavi S. F., Eslami S., Moghaddamd A. H. Food Chem. 2012;132:931–935. [Google Scholar]
- Nabavi S. F., Nabavi S. M., Habtemariam S., Moghaddam A. H., Sureda A., Jafari M., Latifi A. M. Ind. Crops Prod. 2013;44:50–55. [Google Scholar]
- Valls-Belles V., Torres M. C., Muñiz P., Beltran S., Martinez-Alvarez J. R., Codoñer-Franch P. Eur. J. Nutr. 2006;45:251–258. doi: 10.1007/s00394-006-0591-1. [DOI] [PubMed] [Google Scholar]
- Shin M. O., Yoon S., Moon J. O. Arch. Pharm. Res. 2010;33:167–173. doi: 10.1007/s12272-010-2239-1. [DOI] [PubMed] [Google Scholar]
- Yousef M. I., Saad A. A., El-Shennawy L. K. Food Chem. Toxicol. 2009;47:1176–1183. doi: 10.1016/j.fct.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Limón-Pacheco J., Gonsebatt M. E. Mutat. Res., Rev. Mutat. Res. 2009;674:137–147. doi: 10.1016/j.mrgentox.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Tan Y., Dai J., Li B., Guo L., Cui J., Wang G., Shi X., Zhang X., Mellen N., Li W., Cai L. Toxicol. Lett. 2011;200:100–106. doi: 10.1016/j.toxlet.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Ma Q., Battelli L., Hubbs A. F. Am. J. Pathol. 2006;168:1960–1974. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Jiang T., Wang H., Tao S., Lau A., Fang D., Zhang D. D. Antioxid. Redox Signaling. 2012;17:1670–1675. doi: 10.1089/ars.2012.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraonio R., Vergara P., Di Marzo D., Pierantoni M. G., Napolitano M., Russo T., Cimino F. J. Biol. Chem. 2006;281:39776–39784. doi: 10.1074/jbc.M605707200. [DOI] [PubMed] [Google Scholar]
- Nazima B., Vaihundam M., Selvaraj M. P. Biochem. Cell Biol. 2014;93:210–226. [Google Scholar]
- Franco R., Sánchez-Olea R., Reyes-Reye E. M., Panayiotidis M. I. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2009;674:3–22. doi: 10.1016/j.mrgentox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Mihara M., Erster S., Zaika A., Petrenko O., Chittenden T., Pancoska P., Moll U. M. Mol. Cells. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Huang Q., Zhang J., Martin F. L., Peng S., Tian M., Mu X., Shen H. Toxicol. Lett. 2013;223:211–220. doi: 10.1016/j.toxlet.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Joshi S. S., Kuszynski C. A., Bagchi D. Curr. Pharm. Biotechnol. 2001;2:187–200. doi: 10.2174/1389201013378725. [DOI] [PubMed] [Google Scholar]