 Fluorescent nanocrystals embedded in liposomes for bioimaging applications.
Fluorescent nanocrystals embedded in liposomes for bioimaging applications.
Abstract
The use of fluorescent nanocrystals (NCs) as probes for bioimaging applications has emerged as an advantageous alternative to conventional organic fluorescent dyes. Therefore their toxicological evaluation and intracellular delivery are currently a primary field of research. In this work, hydrophobic and highly fluorescent CdSe@ZnS NCs were encapsulated into the lipid bilayer of liposomes by the micelle-to-vesicle transition (MVT) method. The obtained aqueous NC-liposome suspensions preserved the spectroscopic characteristics of the native NCs. A systematic study of the in vitro toxicological effect on HeLa cells of these red emitting NC-liposomes was then carried out and compared to that of empty liposomes. By using liposomes of different phospholipid composition, we evaluated the effect of the lipid carrier on the cytotoxicity towards HeLa cells. Surprisingly, a cell proliferation and death study along with the MTT test on HeLa cells treated with NC-liposomes have shown that the toxic effects of NCs, at concentrations up to 20 nM, are negligible compared to those of the lipid carrier, especially when this is constituted by the cationic phospholipid DOTAP. In particular, obtained data suggest that DOTAP has a dose- and time-dependent toxic effect on HeLa cells. In contrast, the addition of PEG to the liposomes does not alter significantly the viability of the cells. In addition, the ability of NC-liposomes to penetrate the HeLa cells was assessed by fluorescence and confocal microscopy investigation. Captured images show that NC-liposomes are internalized into cells through the endocytic pathway, enter early endosomes and reach lysosomes in 1 h. Interestingly, red emitting NCs co-localized with endosomes and were positioned at the limiting membrane of the organelles. The overall results suggest that the fluorescent system as a whole, NCs and their carrier, should be considered for the development of fully safe biological applications of CdSe@ZnS NCs, and provide essential indications to define the optimal experimental conditions to use the proposed system as an optical probe for future in vivo experiments.
Introduction
In the past decade, fluorescent nanoparticles (NPs) have proved effective tools for the investigation of biological systems at the molecular scale, with several applications in the study of complex processes in cells and tissues.1 The application of fluorescent NPs appears particularly appealing in the field of bio- and medical-imaging when small amounts of target biomolecules are present and long-term imaging is required, cases where conventional fluorescent probes (fluorescent proteins and organic dyes) show several limitations, such as scarce in vitro and in vivo stability, poor photostability and low quantum yield.2,3
Among the NPs, metal chalcogenide nanocrystals (NCs), also known as Quantum Dots (QDs), show unique and superior optical properties such as broadband excitation, narrow bandwidth emission, high quantum yield, resistance to quenching and high photochemical stability. Single photon, 2-photon, and recently 3-photon excitation of QDs have been utilized for bio-imaging applications.1,4 These NCs are typically made from combinations of zinc(ii), cadmium(ii), selenide, sulfide and several additional components (e.g. surface coatings) and dopants.1 The presence of a core containing heavy metals as an inorganic component raised intense concerns regarding the potential cytotoxicity of fluorescent NCs as soon as they appeared in the scientific limelight. In fact, several studies have demonstrated the dangerousness of these NCs related to the release of toxic ions (e.g. Cd2+) which may result in potential in vitro and in vivo toxicity.5,6 Actually other studies have highlighted that, in addition to the release of toxic ions, other effects should be considered to explain the observed cytotoxic effects including the size, the shape and the charge of NCs, the nature of the capping agents, the presence of additional structures or functionalization on the NC surface aimed at modulating their solubility or bioavailability.5–8 Therefore the difficulty in comparing the NC toxicity data from different studies appears dramatically evident due to the use of a variety of NCs, surface coatings and ancillary structures, as well as because of the diversity of the bio- and/or chemical assays employed and the cellular target chosen.5 Overall, these considerations suggest that, although many efforts are made in order to estimate the toxicity of inorganic NCs in vivo and in vitro, the system as a whole (the NC and its capping agent, inorganic/organic carrier, and any possible functionalization) should be considered for the development of fully safe biological and biomedical applications of NCs in imaging, diagnostics and therapy. This aspect is very important when one considers that the overall toxicity of the system could be significantly different from the sum of the toxicity of the individual components separately tested, due to possible synergistic effects.
Among the possible carriers useful for the incorporation of both hydrophobic and hydrophilic NCs and their delivery in biological environments, phospholipid based liposomes represent the most versatile and popular choice.9,10 Indeed, liposomes mimic the lipid assembly of biological membranes, e.g. by forming a double-layer structure and by trapping an aqueous volume in their core. Moreover, these lipid vesicles offer exceptional engineering versatility because their physicochemical characteristics such as lipid vesicle size, lamellarity, surface charge and coverage can be easily modified with several established methodologies.11–13 All these features make the liposomes ideal carriers for biomedical imaging, drug delivery, targeted therapy, and biosensing as well as suitable carriers for transferring hydrophobic NCs in an aqueous environment, changing their affinity phase, and, at the same time, providing a biocompatible shield against the biological environment.9,10,14 Among the liposomes, cationic vesicles are widely employed in gene and drug (especially anticancer) delivery applications,15 but the proposed uses are broader, spanning from use as vaccine delivery systems and adjuvants16 to employment with bacterial biofilm targets for effective antimicrobial therapy.17 Nevertheless, in spite of the numerous advantages arising from the capability in binding anionic molecules and in interacting with negatively charged biomembranes, cationic lipids for cationic liposome development may lead to toxic effects both in vitro and in vivo.18 Therefore, when cationic drug delivery systems are developed, the contribution of cationic lipids to the toxicity of the system should be taken into account.
In this work, hydrophobic and highly fluorescent CdSe@ZnS NCs were encapsulated into the lipid bilayer of cationic, small unilamellar vesicles (SUVs) by the micelle-to-vesicle transition (MVT) method. N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate (DOTAP) was chosen as a cationic lipid for cationic liposome formulation. The cytotoxicity of these red-emitting NC-loaded liposomes (NC-liposomes) was investigated on HeLa cells and compared to that of empty liposomes. By using liposomes of different phospholipid composition, we evaluated the effect of the charge and of the PEG surface coating, which greatly affect the vesicle–cell interaction and response, on the cytotoxicity towards HeLa cells. In addition, the ability of NC-liposomes to penetrate HeLa cells was qualitatively assessed by fluorescence and confocal microscopy investigations.
Materials and methods
Chemicals
All chemicals of the highest purity available were purchased and were used without further purification. Cadmium oxide (CdO, powder 99.5%), selenium (Se, powder 99.99%), oleic acid (OLEA, technical grade 90%), trioctylphosphine oxide (TOPO, 99% and technical grade), tributylphosphine (TBP, 99%), trioctylphosphine (TOP, technical grade), tert-butylphosphonic acid (TBPA), diethylzinc (1.0 M solution in heptane), 1-dodecanethiol (DDT), hexamethyldisilathiane (HMDT), reagent grade salts for 50 mM K-phosphate, 100 mM KCl (pH 7.0) buffer solutions, sodium cholate (SC), phosphatidylcholine (PC), and hexadecylamine (HDA) were purchased from Sigma-Aldrich (St Louis, USA). 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (16:0 PEG-2000-PE) and 1,2-dipalmitoyl-3-trimethylammonium-propane (DOTAP) were purchased from Avanti Polar Lipids (Alabaster, USA). All aqueous solutions were prepared by using water obtained by using a Milli-Q Gradient A-10 system (Millipore, 18.2 MΩ cm, organic carbon content ≤4 μg L–1).
Synthesis of CdSe@ZnS core–shell NCs
CdSe@ZnS colloidal core–shell NCs were prepared under an anhydrous environment under nitrogen flux using a Schlenk line. CdO (1 mmol) was dissolved in a TOPO : HDA : TBPA mixture (15 : 25 : 1), heated at 290 °C and 2 mL of TBP was added. CdSe nucleation was promoted at 300 °C upon the injection of Se (5 mmol) dissolved in 20 mmol of TBP. CdSe NC growth was carried out at 270 °C for 10 min and then the temperature was lowered to 110 °C. After one hour the temperature was raised to 150 °C and an appropriate amount of a Zn(C2H5)2 : HMDT (1 : 1) stock solution in TBP (50 mmol) was added to allow ZnS shell growth until a maximum in the PL spectra was observed. The as prepared core–shell NCs were precipitated by methanol and finally dissolved in CHCl3. Capping exchange of TOPO with DDT was accomplished by adding the latter to the chloroform solution of CdSe@ZnS NCs (usually in a molar ratio of 2000 : 1). The mixture was vigorously stirred for 24 h at RT; the NCs were then precipitated with methanol and dissolved in CHCl3 in order to remove the excess DDT.
Encapsulation of the CdSe@ZnS core–shell NCs in liposomes
NC-liposomes were prepared by the MVT method as previously described.9,19 Cationic liposomes were tested with or without the presence of PEG2000-PE to confer steric stabilization and stealth property. Control liposomes were made with pure PC while cationic liposomes were made with a PC/DOTAP 2 : 1 mol/mol mixture. Lipids, PEG 2000 PE and CdSe@ZnS NCs were dissolved in CHCl3 to give the desired ratio and dried on the walls of a conical glass tube. After that, 0.5 mL of 4% SC, in 50 mM K-phosphate and 100 mM KCl (pH 7.0) were added and after sonication (20 shots with a Branson Sonicator 250) a clear, translucent mixed micelle solution was formed. The latter was loaded into a glass column (20 × 1 cm) packed with G-50 Sephadex Superfine equilibrated with 50 mM K-phosphate and 100 mM KCl (pH 7.0) for detergent removal. NC-liposomes eluted in a 1 mL fraction after a void volume of about 1.5 mL. In all cases the final lipid concentration was 6 mg mL–1. Dynamic light scattering measurements (DLS) on liposomes were performed using an HORIBA Dynamic Light Scattering Particle Size Analyzer LB-550 instrument (Horiba Jobin Yvonne), equipped with a laser diode source (wavelength 650 nm, 5 mW). Measurements were performed at 25 °C with 100 sampling. The determination of the zeta-potential was performed using laser Doppler anemometry (ZetasizerNanoZS, ZEN 3600, Malvern, UK) after dilution in KCl 1 mM.20 Absorption measurements were performed by a UV/vis/NIR Cary 5000 spectrophotometer (Varian). The photoluminescence (PL) spectra were recorded by using an Eclypse Spectrofluorimeter (Varian). The relative PL quantum yield (QY) was estimated by using rhodamine 101 as the reference dye as previously reported.9
Cell culture and transfection
Cell culture reagents were purchased from Sigma-Aldrich (St Louis, USA). HeLa cells (human cervix carcinoma) were cultured in DMEM (Dulbecco's Modified Eagle's Medium) supplemented with 10% FBS (fetal bovine serum), 2 mM l-glutamine, 100 U mL–1 penicillin and 10 mg mL–1 streptomycin in an humidified incubator at 37 °C, under 5% CO2. For all experiments, only cells at passage number <20 were used. Cells were confirmed to be contamination-free.
When needed, HeLa cells were transfected with the pEGFP-Rab5Q79L plasmid. Transfection was performed using Metafectene Pro (Biontex; Karlsruhe, Germany) according to the manufacturer's instructions, using the ratio DNA (μg)/transfection reagent (μL) 1 : 4.
Biocompatibility assay
For cell growth and death assays, 300 μL cells were plated on 24 well plates (8 × 104 cells per well) and, the day after, were incubated with 10, 20, 40, 80, and 160 μg mL–1 of NC-liposomes and empty liposomes (control liposomes) for 24 h or 48 h. Cells incubated with the culture medium without liposomes represented the basic control. After incubation, cells were detached from the plates using trypsin 0.05%–EDTA 0.02% solution (Sigma-Aldrich; St Louis, USA), treated with 0.4% Trypan Blue (MP Biomedicals; Santa Ana, USA) in order to check for cell deaths, and counted using the Bürker chamber. Trypan Blue stained cells were counted as dead cells. The data presented come from three independent experiments.
In viable cells MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenol tetrazolium bromide) is reduced into a purple formazan product by mitochondrial succinate dehydrogenase resulting in a colorimetric change measured at 570 nm. This method was used as an indicator of the viability of the cells. To each well of a 96-well plate 100 μL of cell suspension (1 × 104 cells per mL) was added. After overnight incubation, cells were treated with 10, 20, 40, 80, and 160 μg mL–1 of NC-liposomes (and control liposomes) for different incubation times (1, 2, 4, 8, 24, 48 and 72 h). Cells incubated with culture medium without liposomes represented the basic control. After the treatment, the medium was removed and cells were washed twice with phosphate-buffered saline (PBS). Next, 90 μL of DMEM w/o phenol red (Gibco; Waltham, USA) and 10 μL of MTT (Sigma-Aldrich; St Louis, USA) stock solution (5 mg mL–1, prepared in PBS 1× and filtered) were added to each well and incubated for 4 h at 37 °C. Then, the medium with MTT was removed and 100 μL of DMSO (Sigma-Aldrich; St Louis, USA) were added to each well. One well (without cells) with the same amount of MTT and DMSO represented the sample blank to measure the background absorbance. The plates were subsequently read on a Multilabel Plate Reader (Victor X5, PerkinElmer, USA) at 570 nm. The cell survival percentage was calculated as the absorbance ratio of treated to untreated cells. The data presented come from three independent experiments with 6 replicate wells for each point.
Immunofluorescence microscopy
HeLa cells grown on 11 mm diameter coverslips in 24 multiwell plates were incubated with NC-liposomes or control liposomes for 6 h, washed once with PBS and subsequently fixed with 3% paraformaldehyde. When transfected cells were used, they were treated with different amounts of NC- and control liposomes the day after transfection. Cells were then permeabilized with 0.25% saponin (Sigma-Aldrich; St Louis, USA) and subsequently incubated with mouse monoclonal anti LAMP-1 at 1 : 250 dilution (H4A3 antibody deposited with the Developmental Studies Hybridoma Bank by J. T. August21) and mouse monoclonal anti-TfR1 (transferrin receptor-1) at a 1 : 100 dilution (Boehringer Mannheim GmbH; Mannheim, Germany) primary antibodies for 20 minutes at room temperature. After washes in 0.25% saponin, cells were incubated with the anti-mouse AlexaFluor488 (1 : 500, Invitrogen, Carlsbad, USA) secondary antibody for 20 minutes in the dark, at room temperature and then with DAPI dye (1 μg mL–1) (Calbiochem; Darmstadt, Germany). Coverslips were then mounted with Mowiol 4-88 (Calbiochem; Darmstadt, Germany) and examined using a “NIKON Super High pressure mercury lamp supply power” microscope or the Zeiss confocal LSM710. Image processing was carried out with Adobe Photoshop CS4 extended version 11.0.
Results and discussion
Preparation and characterization of NC-liposomes
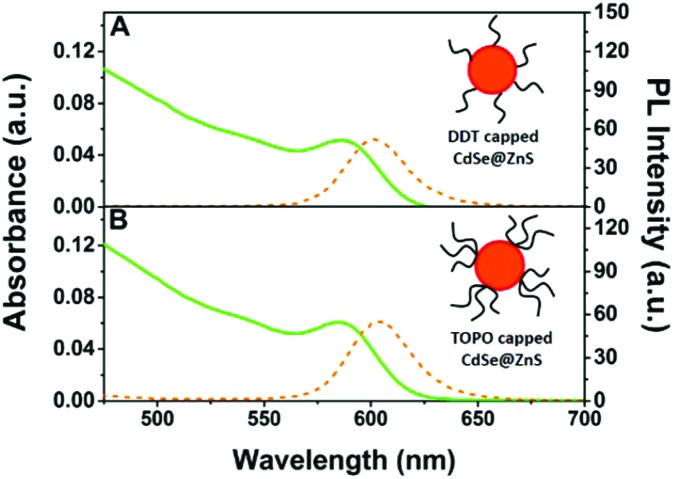
NC-liposomes were prepared starting from 1-dodecanethiol (DDT)-capped CdSe@ZnS NCs, obtained by exchanging the pristine capping layer of trioctylphosphine oxide (TOPO) at the CdSe@ZnS NC surface. The linear alkyl chains of DDT are required for an optimal hydrophobic interaction with the phospholipid tails in the lipid bilayer of liposomes and for preserving a high quantum yield of fluorescence (QY) for CdSe@ZnS NCs, about 40% relatively to rhodamine 101, as previously evidenced.9Fig. 1 shows that the capping exchange procedure negligibly affects the spectroscopic characteristics of NCs, suggesting that no NC aggregation occurs under the realized conditions.
Fig. 1. Absorption (green line) and photoluminescence (orange dot line) spectra (λex = 400 nm) of CdSe@ZnS core–shell NCs in chloroform after (A) and before (B) the capping exchange procedure. Schematic sketches of DDT (1-dodecanethiol)- and TOPO (trioctylphosphine oxide)-capped NCs are merely qualitative and the dimensions of the different components are not to scale.
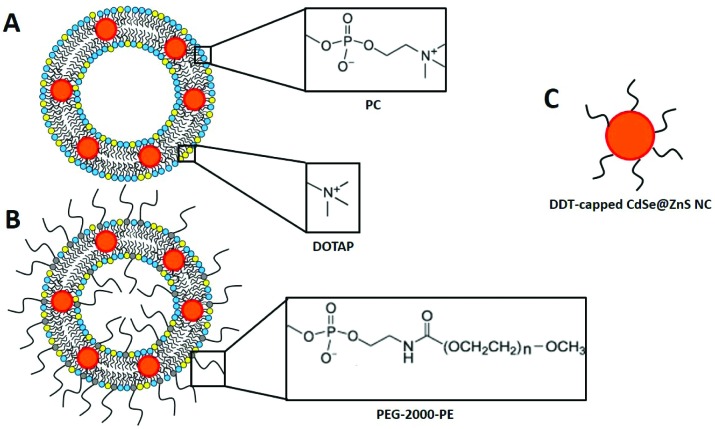
NC-liposomes were obtained by the MVT method, starting from mixed micelles composed of detergent and phospholipid molecules. PC, DOTAP and PEG-modified lipids (PEG) were selected to achieve different lipid vesicles with a comparable size and with different surface charge, which are parameters affecting the liposome–cell interaction, cellular uptake and definitely cell toxicity.10 The sonication of a lipid–CdSe@ZnS NC thin film added with a high critical micellar concentration (CMC) detergent solution leads to the formation of a mixed micelle solution, with the CdSe@ZnS NCs trapped within the hydrophobic region of the micelles. The removal of detergent molecules from mixed micelles by means of a size-exclusion chromatography leads to the merging of bilayer fragments until closed vesicles containing NCs are formed. Some examples of liposomes assembled in this work are shown in Fig. 2.
Fig. 2. Schematic depiction of PC/DOTAP- (A) and PC/DOTAP/PEG-liposomes (B) containing DDT-capped CdSe@ZnS NCs (C). This sketch is merely qualitative. The dimensions of the different components are not to scale.
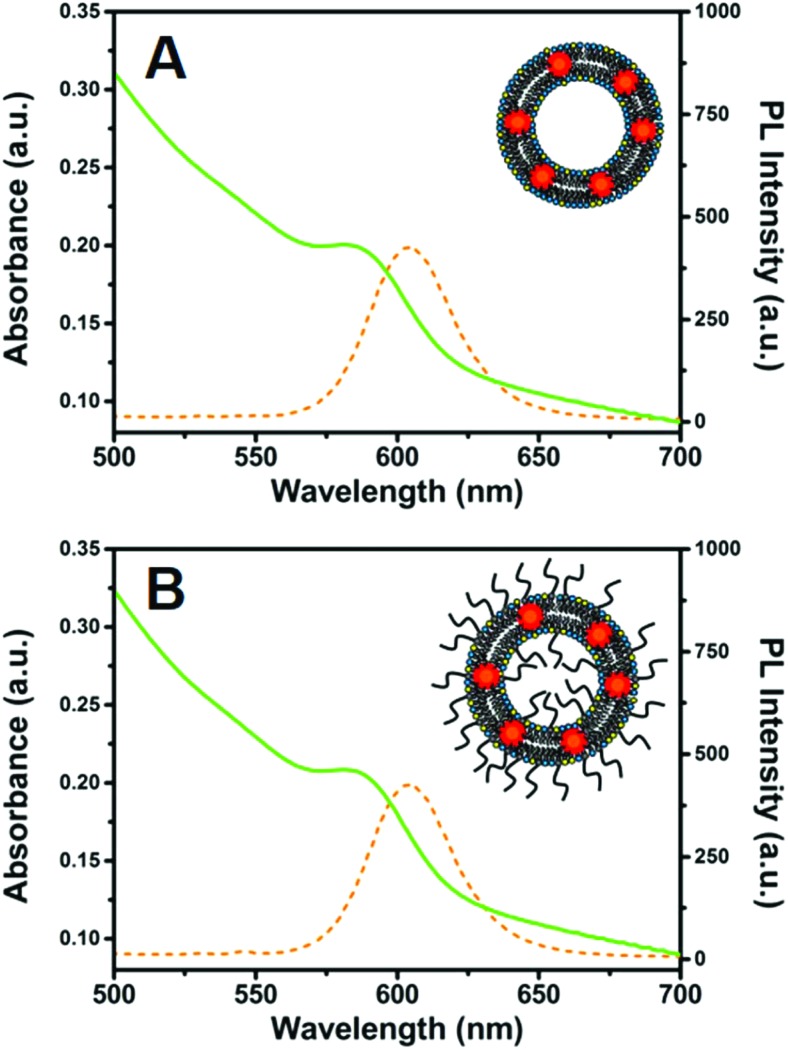
Fig. 3 shows that the UV-vis absorbance and fluorescence spectra of NCs embedded in PC/DOTAP and PC/DOTAP/PEG liposomes closely resemble those of CdSe@ZnS NCs in chloroform solution, keeping a narrow emission peak at λ = 605 nm. The weak red-shift observed in the NC–liposome spectra with respect to signals recorded for NC chloroform solution arises from the rearrangement of the surface charge carriers due to the modified chemical surrounding. The light scattering contribution of the NC-liposome suspensions is barely visible in their absorption spectra, thanks to the small size of the liposomes obtained by means of the MVT method. Similar results were obtained for the other NC-liposomes (data not shown).
Fig. 3. Absorbance (green line) and fluorescence spectra (orange dotted line, λex = 400 nm) of NC-PC/DOTAP-liposome (A) and NC-PC/DOTAP/PEG-liposomes (B). In both samples the NC concentration was 0.1 μM.
NC-liposomes and control liposomes were characterized by DLS analysis and ζ-potential measurements in order to get information on vesicle diameter, surface charge and colloidal stability (Table 1). It should be noted that two types of NC-liposomes were prepared, with 0.1 μM and 1 μM NC concentrations, corresponding to lipid/NC ratios of 80 000 : 1 and 8000 : 1 respectively. A QY of about 8% was measured for 0.1 μM NCs embedded in liposomes, a value much higher than that obtained by other authors for this kind of preparation. This parameter strongly indicates the goodness of the liposome preparation method, the good interaction (interdigitation) between the NC organic coating and the phospholipid hydrophobic tails and ultimately a good shield effect of the bilayer from the surrounding aqueous environment. The QY decreases to 3% for a 1 μM NC-liposome suspension. In a previous work, we demonstrated that the lowering of the QY value with the increasing concentrations of NCs in liposomes is mainly ascribed to self-quenching phenomena coming from NC crowding within the lipid bilayer.9
Table 1. Characterization of NC-liposomes and control liposomes by DLS analysis and ζ-potential measurements. Errors are expressed as standard deviations (n = 3).
| Liposome sample | NC conc. (μM) | Lipid/PEG ratio (mol/mol) | Lipid/NC ratio (mol/mol) | Hydrodynamic diameter (nm) | ζ potential (mV) |
| PC/DOTAP 2 : 1 | — | — | 45 ± 5 | +37 ± 5 | |
| NC-PC/DOTAP 2 : 1 | 0.1 | 80 000 : 1 | 52 ± 5 | +35 ± 5 | |
| NC-PC/DOTAP 2 : 1 | 1 | 8000 : 1 | 71 ± 7 | +40 ± 8 | |
| PC/DOTAP/PEG 2 : 1 : 0.05 | — | 60 : 1 | — | 77 ± 3 | +15 ± 7 |
| NC-PC/DOTAP/PEG 2 : 1 : 0.05 | 0.1 | 60 : 1 | 80 000 : 1 | 80 ± 5 | +12 ± 8 |
| NC-PC/DOTAP/PEG 2 : 1 : 0.05 | 1 | 60 : 1 | 8000 : 1 | 98 ± 10 | +11 ± 7 |
| PC | — | — | 40 ± 2 | –12 ± 2 | |
| PC/PEG 3 : 0.05 | — | 60 : 1 | — | 61 ± 5 | –2.5 ± 0.3 |
Assessment of NC-liposome toxicity on the cell viability of HeLa cells
In vitro cytotoxicity tests are mainly used for screening compounds with a potentially toxic effect and for establishing whether these molecules affect cell proliferation or lead to cell death. Toxicity assays are also used for monitoring markers of cellular activity.22 The toxicological effects of NC-liposomes were assessed in vitro by cultures of HeLa cells, one of the most commonly used cellular model for this kind of assays.23,24 Indeed, HeLa cells, the first human cell line established in culture,25 are robust and characterized by short doubling time allowing the rapid detection of biological activity and/or toxic effect of the molecules of interest.23,24
The first set of experiments was carried out to investigate the effect of the cationic NC-liposomes on cell proliferation and death. Proliferation assays are used to monitor the growth and division of a cell population and are performed by measuring the number of viable cells, cellular divisions or DNA synthesis.26 The easiest assay for the estimation of cell proliferation and cell death is based on the use of Trypan Blue. Trypan Blue is a dye that allows discrimination between viable cells with an intact plasma membrane and dead cells with a damaged plasma membrane. Indeed, the dye will penetrate only disrupted membranes and, thus, dead cells will turn blue.27 Therefore, using this dye exclusion assay it is possible, by counting only viable cells, to measure the rate of proliferation.27 Furthermore, the number of blue cells will give information on the percentage of dead cells.27
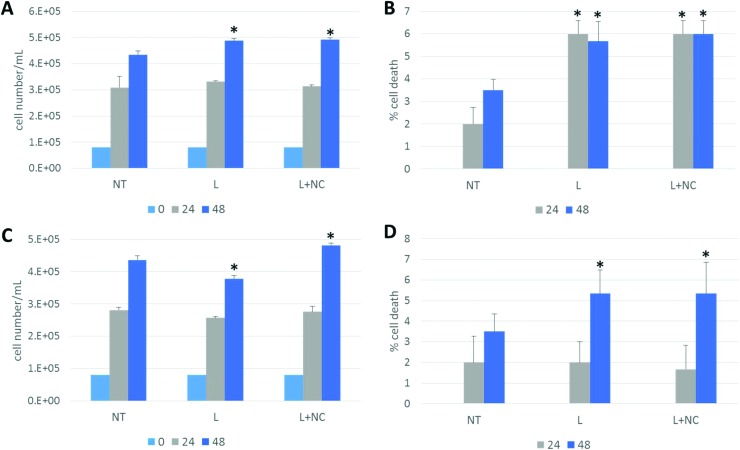
In order to discriminate the toxicity contribution ascribed to the inorganic NCs from that due just to the organic carrier, NC-liposomes made of different lipid blends were tested: cationic NC-PC/DOTAP-liposomes and cationic NC-PC/DOTAP/PEG-liposomes, as well as their respective control liposomes consisting of the empty carriers. Indeed, the potential toxicity of NCs depends on the carrier composition, the surface coating, their size and surface charge that significantly affect the NC internalization across the cell membrane and then the intracellular distribution.28 To study the effect of NC-liposomes on cell proliferation and death, HeLa cells were seeded into 24 multiwell plates (8 × 104 cells per well) and treated for 24 h and 48 h with 10, 20, 40, 80, and 160 μg mL–1 of empty PC/DOTAP-liposomes (L) and NC-PC/DOTAP-liposomes (L + NCs) concentration. For the NC-liposomes tested in these assays, the lipid/NC ratio was set at 80 000 : 1; thus the final concentrations of NCs corresponding to the previous lipid concentrations were 0.17, 0.34, 0.68, 1.36, and 2.7 nM. HeLa cells incubated with DMEM without liposomes (NT) were used as the control. After treatment, the cells were collected and stained with Trypan Blue. Then, the unstained viable cells and stained dead cells were counted. In Fig. 4A only data obtained with 160 μg mL–1 liposome treatment are shown. No significant changes in the number of viable cells were observed following the exposure of HeLa cells to the different amounts of PC/DOTAP-liposomes ± NCs for 24 h compared to untreated cells (NT) (Fig. 4A and data not shown). Conversely, after 48 h of treatment with the highest liposome concentrations tested, i.e. 160 μg mL–1 of the PC/DOTAP-liposomes and NC-PC/DOTAP-liposomes, a small but significant increase of the number of viable cells of (12 ± 3)% (P ≤ 0.05) and (14 ± 5)% (P ≤ 0.05), respectively, was observed (Fig. 4A).
Fig. 4. Effect of PC/DOTAP-liposomes ± NCs, and PC/DOTAP : PEG-liposomes ± NCs on HeLa cell proliferation and death. HeLa cells were treated with liposomes (L) or liposomes containing NCs (L + NCs), as indicated, for 24 h and 48 h. Cells incubated with DMEM without liposomes were used as control (NT). Viable (A) or dead (B) cells treated with PC/DOTAP-liposomes ± NCs were counted to determine the rate of proliferation expressed as cell number per mL (A) or the percentage of cell death expressed as the ratio between dead cells and total cell number (B). Viable (C) or dead (D) cells treated with PC/DOTAP/PEG-liposomes ± NCs were counted to determine the rate of proliferation expressed as cell number per mL (C) or the percentage of cell death expressed as the ratio between blue dead cells and total cell number (D). Bars represent mean ± S.D. of three independent experiments in triplicate. Statistical significance was measured by comparing values with values of control untreated cells at the same time point (*P ≤ 0.05 and **P ≤ 0.01 ***P ≤ 0.001). For all treatments in the figure, the liposome concentration was 160 μg mL–1 and the NC concentration was 2.7 nM.
In order to establish whether liposomes and/or NCs alter cell death, HeLa cells treated with 10, 20, 40, 80, and 160 μg mL–1 of PC/DOTAP-liposomes ± NCs were harvested after 24 h and 48 h and dead cells were counted. The percentage of the dead cells was calculated as the ratio between blue dead cells and total cell number. Control cells after 24 h of incubation in culture medium show a dead cell percentage of 2% and this percentage increased to about 3.5% after 48 h, although this change is not statistically significant (Fig. 4B, NT). Treatment for 24 h with 160 μg mL–1 of PC/DOTAP-liposomes determined an increase of the death percentage of about 3-fold (P ≤ 0.05) while treatment for 48 h determined an increase of 1.6-fold (P ≤ 0.05) compared to untreated cells (NT) (Fig. 4B). Interestingly, similar results were obtained after the incubation with the PC : DOTAP-liposomes containing NCs (Fig. 4B). In fact, the inclusion of NCs caused a significant increase of the percentage of death of about 3-fold (P ≤ 0.05) after 24 h and of about 1.7-fold (P ≤ 0.05) after 48 h compared to control untreated cells (Fig. 4B). An increase of death percentage after exposure with liposomes was also observed in HeLa cells treated with 6, 12 and 24 μg of PC : DOTAP-liposomes ± NCs for 24 h or 48 h compared to control cells, while no change in cell death was observed after treatment with 3 μg of liposomes (data not shown).
These data suggest that the toxicity observed is due to the liposome carriers and not due to the presence of the DDT capped CdSe@ZnS NCs in the liposomes.
The effect of PEG liposome coating on the cell toxicity was subsequently evaluated. PEG is non-ionic, low fouling and possesses high solubility in both aqueous and organic solvents, thus enabling the formulation of stealth liposomes. PEG indeed inhibits non-specific and specific protein interactions on the liposome surface, including those involved in the immune response, increasing the duration of the persistence of liposomes in blood circulation.29 PEG is also characterized by excellent biocompatibility, lack of toxicity, low immunogenicity, antigenicity and good excretion kinetics, and can be used for developing liposomes with fusogenic properties.29,30
HeLa cells were thus treated for 24 h and 48 h with stepwise amounts 10, 20, 40, 80 and 160 μg L–1 of PC/DOTAP/PEG-liposomes ± NCs. No significant change was observed in the viable cell number after treatment with all doses of PC/DOTAP/PEG-liposomes for 24 h compared to untreated cells (Fig. 4C and data not shown). Similar results were obtained after 24 h of the treatment with NC-PC/DOTAP/PEG-liposomes. Treatment for 48 h with 10, 20, 40, and 80 μg mL–1 of PC/DOTAP/PEG-liposomes did not affect proliferation (data not shown) while a decrease of viable cell number of (13 ± 6)% (p ≤ 0.01) was observed after treatment with 160 μg mL–1 (Fig. 4C). In contrast, a small but a significant increase in the viable cell number was observed after treatment for 48 h with 10, 20, 40 and 80 μg mL–1 (10 ± 5)% (P ≤ 0.05) of NC-PC/DOTAP/PEG-liposomes (Fig. 4C and data not shown).
The percentage of cell death was also evaluated for cells treated with PC/DOTAP/PEG-liposomes ± NCs for 24 h and 48 h, with 10, 20, 40, 80 and 160 μg mL–1 amounts. Cells were then stained with Trypan Blue and counted. Control cells after 24 h of incubation in culture medium show 2.7% of cell death and this percentage increases to about 3.7% after 48 h, although the difference between the two samples is not statistically significant. No change was observed in death percentage for cells treated with increasing amounts of PC/DOTAP/PEG-liposomes ± NCs for 24 h compared to untreated cells (Fig. 4D and data not shown). In contrast, an increase of 1.4-fold (P ≤ 0.05) was observed after 48 h of treatment with 160 μg mL–1 of PC/DOTAP/PEG-liposomes and NC-PC/DOTAP/PEG-liposomes, respectively (Fig. 4D).
Overall, the experiments conducted with PEG coated liposomes indicate that only minor changes occur with respect to cell proliferation, but a marked decrease of cell death is observed after 24 h, if compared with the outcome of the corresponding PEG-less liposomes (Fig. 4B). After 48 h, however, the results become similar.
MTT assay
To further investigate the toxic effect of NC-liposomes, an MTT viability assay was performed. Cell viability assays are often used to assess cell health by measuring markers of cellular metabolic activity such as enzyme activity, or by monitoring the trafficking of cellular components and organelle function.22 In particular, the MTT assay is based on the capacity of mitochondrial succinate dehydrogenase to reduce MTT into a formazan product in metabolically active cells. The amount of formazan produced is directly proportional to cell viability.31 Therefore the MTT assay can be used to discriminate metabolically active cells that do not divide and cells that proliferate.32
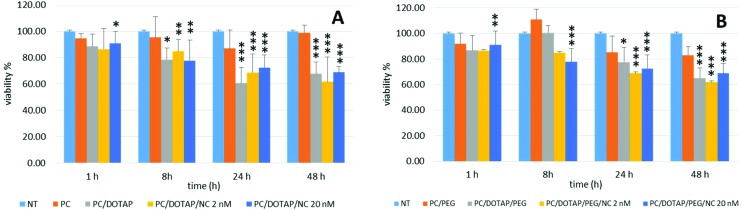
HeLa cells were seeded into 96 multiwell plates (1 × 104 cells per mL) and treated for 1, 2, 4, 8, 24, 48 and 72 h with PC-liposomes and PC/DOTAP-liposomes ± NCs at NC concentrations of 2 and 20 nM, thus significantly increasing the NC concentration compared to previous experiments. Untreated cells were used as the control. The percentage of cell viability was evaluated by the MTT assay, in which the decrease of viability was calculated by setting the value obtained with no treatment at 100%. In Fig. 5A is shown the effect caused by incubation with 120 μg mL–1 of liposomes for 1, 8, 24 and 48 h. No significant change was observed after treatment with empty PC-liposomes compared with control cells. Conversely, in cells treated with empty PC/DOTAP-liposomes a decrease of viability was observed compared to untreated cells (Fig. 5A). Indeed, there was a decrease of viability of (11 ± 9)%, (22 ± 9)% (P ≤ 0.05), (39 ± 12)% (P ≤ 0.001) and (32 ± 9)% (P ≤ 0.001), after 1, 8, 24 and 48 h of treatment, respectively. Similar results were observed after the incubation of NC-PC/DOTAP-liposomes with 2 nM of NCs that determined a time-dependent cell viability decrease of (14 ± 16)%, (15 ± 9)% (P ≤ 0.01), (31 ± 14)% (P ≤ 0.001) and (38 ± 18)% (P ≤ 0.001), compared to untreated cells, after 1, 8, 24 and 48 h of treatment, respectively. A decrease of viability was also observed after treatment with NC-liposomes containing 20 nM of NCs by (9 ± 9)%, P ≤ 0.05; (22 ± 15)%, P ≤ 0.05; (27 ± 9)%, P ≤ 0.001; and (31 ± 4)%, P ≤ 0.001, after 1, 8, 24 and 48 h of treatment, respectively (Fig. 5A). These data again indicate that DOTAP has a toxic effect on cells as viability decreases after treatment with cationic vesicles but not incubating cells with PC-liposomes without DOTAP. Furthermore, the viability of cells treated with liposomes containing NCs, at all concentrations, was similar to the viability of cells treated with PC/DOTAP-liposomes suggesting that no toxic effect is due to DDT capped NCs in the concentration range tested.
Fig. 5. HeLa cell viability assessed by the MTT assay. (A) Cells were treated with 120 μg mL–1 of PC-liposomes (PC), PC/DOTAP-liposomes (PC/DOTAP), PC/DOTAP-liposomes containing NCs at the concentration of 2 nM (PC/DOTAP/NC 2 nM) and 20 nM (PC/DOTAP/20 nM). Cells incubated with DMEM without liposomes were used as control (NT). (B) Cells were treated with 120 μg mL–1 of PC/PEG-liposomes (PC/PEG), PC/DOTAP/PEG-liposomes (PC/DOTAP/PEG), PC/DOTAP/PEG-liposomes containing NCs at the concentration of 2 nM (PC/DOTAP/PEG/NC 2 nM) and 20 nM (PC/DOTAP/PEG/NC 20 nM). Cells incubated with DMEM without liposomes were used as the control (NT). The cell viability was calculated by setting the value obtained with no treatment at 100%. The data are the mean ± S.D. of three independent experiments. Statistical significance was measured by comparing values with values of control untreated cells (*P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001).
To investigate the effect of PEG on toxicity, the MTT assay was also performed in cells treated with PC/DOTAP/PEG-liposomes ± NCs at the concentration of 2 nM and 20 nM for 1, 2, 4, 8, 24, 48 and 72 h. In Fig. 5B the results after the treatment for 1, 8, 24 and 48 h are shown. Treatment of cells with PC/PEG-liposomes determined no change in cell viability (Fig. 5B). Treatment with PC/DOTAP/PEG-liposomes decreased cell viability compared with untreated cells by (13 ± 11)%, (22 ± 11)% (P ≤ 0.05), and (35 ± 8)% (P ≤ 0.001), after 1, 24 and 48 h, respectively. A decrease of the cell viability was also observed after treatment with NC-PC/DOTAP/PEG-liposomes containing NCs at the concentration of 2 nM by (18 ± 22)%, (12 ± 18)%, (21 ± 8)% (P ≤ 0.001) and (30 ± 14)% (P ≤ 0.001) for 1, 8, 24 and 48 h, respectively. Similar results were observed after incubation with NC-PC/DOTAP/PEG-liposomes with 20 nM of NCs. The viability decreased by (18 ± 10)% (P ≤ 0.01), (37 ± 10)% (P ≤ 0.001); (37 ± 10)% (P ≤ 0.001); and (40 ± 7)% (P ≤ 0.001) after treatment for 1, 8, 24 and 48 h, respectively. These data indicate that, as expected, the addition of PEG in the composition of liposomes does not have any toxic effect and confirms that the decrease in cell viability observed is due to the presence of DOTAP into liposomes.
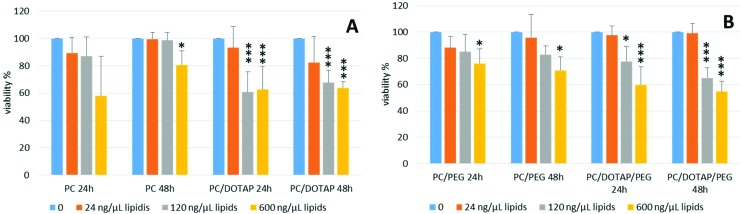
To further study the role of PEG and DOTAP in cytotoxicity, cells were treated with PC-, PC/DOTAP- and PC/DOTAP/PEG-liposomes at a stepwise concentration (24, 120 and 600 μg mL–1) for 24 h and 48 h and subjected to the MTT assay. Data obtained indicate that the treatment with PC-liposomes determines a decrease of viability compared to untreated cells of about (40 ± 29)% at 24 h, although not statistically significant, and (20 ± 10)% at 48 h (P ≤ 0.05) but only at 600 ng μL–1 (Fig. 6A). The addition of DOTAP to the liposomes caused a strong decrease of cell viability compared to untreated cells, both at 24 h and 48 h (Fig. 6A). In particular, viability decreased by (39 ± 12)% (P ≤ 0.001) and (37 ± 13)% (P ≤ 0.001) after 24 h of incubation with 120 μg mL–1 and 600 μg mL–1 of PC/DOTAP-liposomes, respectively, while treatment for 48 h determined a decrease of cell viability of (32 ± 9)% (P ≤ 0.001) at 120 μg mL–1 and (36 ± 5)% (P ≤ 0.001) at 600 μg mL–1 (Fig. 6A). Finally, cells were treated with PC/PEG-liposomes with or without DOTAP at stepwise concentrations of 24, 120 and 600 μg mL–1 for 24 h and 48 h. No significant change was observed after 24 h and 48 h of treatment at the concentrations of 24 μg mL–1 and 120 μg mL–1 compared to untreated cells. A viability decrease was observed only after treatment with PC/PEG-liposomes at 600 μg mL–1 after 24 h (24 ± 10)%, (P ≤ 0.05) and 48 h (30 ± 10)%, (P ≤ 0.05). The addition of DOTAP to the PC/PEG-liposomes caused a significant dose-dependent decrease of cell viability compared to untreated cells. In fact, cell viability remained unchanged after 24 h and 48 h of treatment with PC/DOTAP/PEG-liposomes at a concentration of 24 μg mL–1 while it strongly decreased by (22 ± 11)% (P ≤ 0.05) and (40 ± 13)% (P ≤ 0.001) after treatment for 24 h at 120 μg mL–1 and 600 μg mL–1, respectively. Cell viability further decreased at 48 h to (35 ± 8)% (P ≤ 0.001) and (56 ± 8)% (P ≤ 0.001) at the concentrations of 120 μg mL–1 and 600 μg mL–1, respectively. These data suggest that DOTAP has a dose- and time-dependent toxic effect while the addition of PEG to the liposomes does not alter significantly the viability of the cells.
Fig. 6. Cytotoxic effect of DOTAP and PEG in liposomes assessed by the MTT assay. (A) Cells were treated with 0 (NT), 24, 120 and 600 μg mL–1 of PC-liposomes (PC), PC/DOTAP-liposomes (PC/DOTAP) for 24 h and 48 h. (B) Cells were treated with 0 (NT), 24, 120 and 600 μg mL–1 of PC/PEG-liposomes ± DOTAP (PC/PEG and PC/DOTAP/PEG) for 24 h and 48 h. Cell viability was calculated by setting the value obtained with no treatment at 100%. Data are the mean ± S.D. of three independent experiments. Statistical significance was measured by comparing values with values of control untreated cells (*P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001).
Evaluation of cellular uptake of NC-liposomes by fluorescence and confocal microscopy
Red emitting NCs are considered a suitable tool in cell and tissue imaging applications under visible light, as they allow limiting the issues related to the native autofluorescence of biological samples. Furthermore, a fluorescence QY between 3% and 8% was measured for our vesicles whereas a QY ranging from 1% to 5% has been reported to be sufficient for cell-labeling studies.9
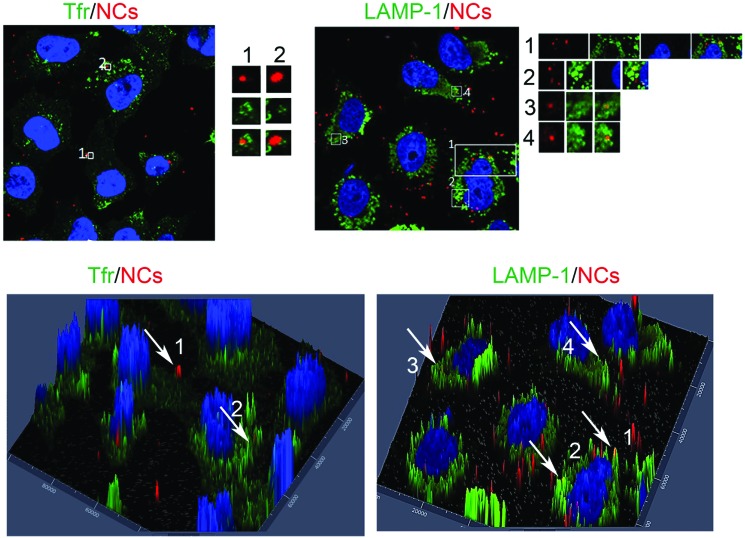
The cellular uptake and localization of 40 μg mL–1 cationic PC/DOTAP-liposomes containing 20 nM DDT-capped CdSe@ZnS NCs was firstly investigated by fluorescence microscopy and confocal microscopy. As early endosomes can be reached in 15 min while late endosomes and lysosomes are reachable in about an hour, cells were incubated with NC-liposomes for 15 min or 1 h and stained with specific monoclonal antibodies against different cellular antigens. In particular, transferrin receptor, a marker of early endosomal compartments, and LAMP-1, a marker of late endosomes and lysosomes, were respectively used (Fig. 7).
Fig. 7. Immunofluorescence analysis of intracellular localization of red emitting NCs. HeLa cells treated with cationic PC/DOTAP-liposomes for 15 min or 1 h were fixed and subjected to immunofluorescence analysis using anti-TfR (A) and anti-LAMP-1 (B) antibodies, as indicated, followed by FITC-secondary antibodies (green). For each image, magnifications of the boxed areas are shown in the respective lower insets. (C) 3D surface plots of the images presented in A. (D) 3D surface plots of the images presented in B. Experiments were conducted with 40 μg mL–1 of NC-liposomes containing 20 nM DDT-capped CdSe@ZnS NCs.
These immunofluorescence experiments showed that NC-liposomes colocalized with both transferrin receptor and LAMP-1, indicating that NC-liposomes are internalized into cells through the endocytic pathway, enter early endosomes and reach lysosomes in 1 h (Fig. 7).
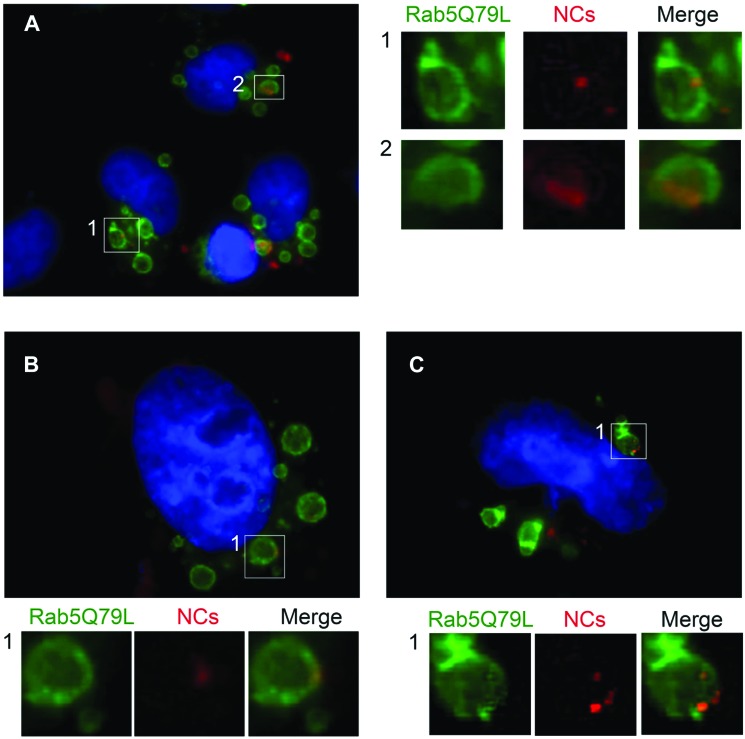
Rab5 is a marker of early endosomes and it was demonstrated that the expression of the constitutively active mutant Rab5Q79L increases the size of early endosomes.33,34 Larger endosomes could allow a more precise localization of NCs as it is easier to distinguish the confining membrane of the organelle from the lumen. Therefore, the intracellular localization of NC-liposomes was studied by fluorescence microscopy using HeLa cells transfected with the pEGFP-Rab5Q79L plasmid. This plasmid expresses the Rab5Q79L protein tagged with the Enhanced Green Fluorescent Protein (EGFP). After transfection, cells were treated with 40 μg mL–1 of PC/DOTAP/PEG-liposomes (Fig. 8A) and PC/DOTAP-liposomes (Fig. 8B and C) containing 20 nm DDT-capped CdSe@ZnS NCs for 6 h, fixed and analysed. Interestingly, red-emitting NCs colocalized with Rab5-containing endosomes and were positioned at the limiting membrane of the organelles (Fig. 8). This observation suggests that hydrophobic CdSe@ZnS NCs are retained in the phospholipid palisade of liposomes, which is integrated in the lipid membrane of endosomes after the uptake event. To the best of our knowledge, it is the first time that a similar behaviour is highlighted. Even if our experiments do not allow us to follow the ultimate fate of vesicles such as degradation and efflux, the fluorescence images have shown a poor release of NCs from the lipid carrier in the cytosol, suggesting a greater stability of our liposomes with respect to those previously reported.35
Fig. 8. Fluorescence microscopy analysis of the intracellular localization of red emitting NCs loaded into PC/DOTAP/PEG-liposomes (A) and PC/DOTAP-liposomes (B–C). HeLa cells were transfected with GFP-Rab5Q79L (green) for 24 h and treated with NC-liposomes for 6 h. Experiments were conducted with 40 μg mL–1 of NC-liposomes containing 20 nM DDT-capped CdSe@ZnS NCs. Cells were then fixed and subjected to fluorescence microscopy analysis. For each image, magnifications of the boxed areas are shown in the respective lower insets.
Conclusions
In this work, we showed that MVT is an efficient method for encapsulating hydrophobic, fluorescent NCs in liposomes for intracellular delivery purposes, with very good photochemical properties, stability and dispersibility in biological media.
A systematic investigation into the in vitro response of HeLa cells exposed to luminescent CdSe@ZnS NCs embedded in liposomes provided insights relevant to their toxicity. The results provide clear evidence that the toxic effects of NCs, in the concentration range tested, are negligible compared to those of the lipid carrier. In particular, obtained data suggest that the cationic phospholipid DOTAP has a dose- and time-dependent toxic effect on HeLa cells. In contrast, the addition of a PEG coating to the liposomes does not alter significantly the viability of the cells.
Finally, using the MVT method and dodecanethiol-capped QDs, we were able to obtain NC-liposomes with diameter <100 nm and to preserve a very good QY. Fluorescence micrographs showed that NC-liposomes are internalized into cells through the endocytic pathway, enter early endosomes and reach lysosomes in 1 h. Interestingly, CdSe@ZnS NCs colocalized with endosomes and were early positioned at the limiting lipid membrane of these organelles. Fluorescence micrographs showed also a poor NC release into the cytosol indicating greater stability of our liposomes. All these findings suggest that bright, dodecanethiol-capped NCs embedded in small unilamellar liposomes at very low concentrations could be used not only for in vitro but also for in vivo experiments.
Summarizing, the overall results of the work confirm that the fluorescent NCs, their carrier and any surface functionalization should be considered for the development of fully safe bioimaging in vitro and in vivo applications of CdSe@ZnS NCs.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
This work was partially supported by AIRC (IG2016 N. 19068 to C. B.), Telethon Italy (GGP16037 to C. B.) and supported by PRIN 2010–2011 “Organizzazione Funzionale a Livello Nanoscopico di (Bio)Molecole e Ibridi per Applicazioni nel Campo della Sensoristica, della Medicina e delle Biotecnologie”.
References
- Wolfbeis O. S. Chem. Soc. Rev. 2015;44:4743–4768. doi: 10.1039/c4cs00392f. [DOI] [PubMed] [Google Scholar]
- Medintz I. L., Uyeda H. T., Goldman E. R., Mattoussi H. Nat. Mater. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- Zhou J., Yang Y., Zhang C.-y. Chem. Rev. 2015;115:11669–11717. doi: 10.1021/acs.chemrev.5b00049. [DOI] [PubMed] [Google Scholar]
- Yu J. H., Kwon S.-H., Petrášek Z., Park O. K., Jun S. W., Shin K., Choi M., Park Y. I., Park K., Na H. B., Lee N., Lee D. W., Kim J. H., Schwille P., Hyeon T. Nat. Mater. 2013;12:359–366. doi: 10.1038/nmat3565. [DOI] [PubMed] [Google Scholar]
- Chen N., He Y., Su Y., Li X., Huang Q., Wang H., Zhang X., Tai R., Fan C. Biomaterials. 2012;33:1238–1244. doi: 10.1016/j.biomaterials.2011.10.070. [DOI] [PubMed] [Google Scholar]
- Hardman R. Environ. Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovrić J., Bazzi H. S., Cuie Y., Fortin G. R. A., Winnik F. M., Maysinger D. J. Mol. Med. 2005;83:377–385. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]
- Oh E., Liu R., Nel A., Gemill K. B., Bilal M., Cohen Y., Medintz I. L. Nat. Nanotechnol. 2016;11:479–486. doi: 10.1038/nnano.2015.338. [DOI] [PubMed] [Google Scholar]
- De Leo V., Catucci L., Falqui A., Marotta R., Striccoli M., Agostiano A., Comparelli R., Milano F. Langmuir. 2014;30:1599–1608. doi: 10.1021/la404160b. [DOI] [PubMed] [Google Scholar]
- Depalo N., De Leo V., Corricelli M., Gristina R., Valente G., Casamassima E., Comparelli R., Laquintana V., Denora N., Fanizza E., Striccoli M., Agostiano A., Catucci L., Curri M. L. J. Mater. Chem. B. 2017;5:1471–1481. doi: 10.1039/c6tb02590k. [DOI] [PubMed] [Google Scholar]
- Al-Jamal W. T., Al-Jamal K. T., Tian B., Lacerda L., Bomans P. H., Frederik P. M., Kostarelos K. ACS Nano. 2008;2:408–418. doi: 10.1021/nn700176a. [DOI] [PubMed] [Google Scholar]
- Depalo N., Catucci L., Mallardi A., Corcelli A., Agostiano A. Bioelectrochemistry. 2004;63:103–106. doi: 10.1016/j.bioelechem.2003.09.031. [DOI] [PubMed] [Google Scholar]
- De Leo V., Mattioli-Belmonte M., Cimmarusti M. T., Panniello A., Dicarlo M., Milano F., Agostiano A., De Giglio E., Catucci L. Colloids Surf., B. 2017;158:387–396. doi: 10.1016/j.colsurfb.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Malam Y., Loizidou M., Seifalian A. M. Trends Pharmacol. Sci. 2009;30:592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Knudsen K. B., Northeved H., Kumar Ek P., Permin A., Gjetting T., Andresen T. L., Larsen S., Wegener K. M., Lykkesfeldt J., Jantzen K., Loft S., Møller P., Roursgaard M. Nanomedicine. 2015;11:467–477. doi: 10.1016/j.nano.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Christensen D., Korsholm K. S., Andersen P., Agger E. M. Expert Rev. Vaccines. 2011;10:513–521. doi: 10.1586/erv.11.17. [DOI] [PubMed] [Google Scholar]
- Rukavina Z., Vanić ž. Pharmaceutics. 2016;8:18. doi: 10.3390/pharmaceutics8020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H., Zhang S., Wang B., Cui S., Yan J. J. Controlled Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Catucci L., De Leo V., Milano F., Giotta L., Vitale R., Agostiano A., Corcelli A. J. Bioenerg. Biomembr. 2012;44:487–493. doi: 10.1007/s10863-012-9447-y. [DOI] [PubMed] [Google Scholar]
- Bloise E., Carbone L., Colafemmina G., D'Accolti L., Mazzetto S. E., Vasapollo G., Mele G. Molecules. 2012;17:12252. doi: 10.3390/molecules171012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. W., Murphy T. L., Willingham M. C., Pastan I., August J. T. J. Cell Biol. 1985;101:85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riss T. L., Moravec R. A., Niles A. L., Duellman S., Benink H. A., Worzella T. J. and Minor L., Cell Viability Assays, http://www.ncbi.nlm.nih.gov/books/NBK144065/.
- Rajeshwar P. V., Corwin H. Curr. Med. Chem. 2006;13:423–448. [Google Scholar]
- Ekwall B., Silano V., Paganuzzi-Stammati A. and Zucco F., in Short-term Toxicity Tests for Non-genotoxic Effects, ed. P. Bourdeau, et al., John Wiley & Sons Ltd, Chichester, New York, 1990, ch. 7, pp. 75–97. [Google Scholar]
- Gey G. O., Coffman W. D., Kubicek M. T. Cancer Res. 1952;12:264–265. [Google Scholar]
- Yadav K., Singhal N., Rishi V. and Yadav H., in eLS, John Wiley & Sons, Ltd, 2001, 10.1002/9780470015902.a0002566. [DOI] [Google Scholar]
- Strober W., in Current Protocols in Immunology, John Wiley & Sons, Inc., 2001, 10.1002/0471142735.ima03bs21. [DOI] [Google Scholar]
- Latronico T., Depalo N., Valente G., Fanizza E., Laquintana V., Denora N., Fasano A., Striccoli M., Colella M., Agostiano A., Curri M. L., Liuzzi G. M. PLoS One. 2016;11:e0153451. doi: 10.1371/journal.pone.0153451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag O. K., Awasthi V. Pharmaceutics. 2013;5:542–569. doi: 10.3390/pharmaceutics5040542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira R. B., Seabra M. A. B. L., Matos A. L. L., Vasconcelos J. V., Bezerra D. P., de Paula E., Santos B. S., Fontes A. J. Mater. Chem. B. 2013;1:4297–4305. doi: 10.1039/c3tb20245c. [DOI] [PubMed] [Google Scholar]
- Mosmann T. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Gerlier D., Thomasset N. J. Immunol. Methods. 1986;94:57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Parton R. G., Steele-Mortimer O., Lütcke A., Gruenberg J., Zerial M. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arrigo A., Bucci C., Toh B. H., Stenmark H. Eur. J. Cell Biol. 1997;72:95–103. [PubMed] [Google Scholar]
- Li Y., Wang J., Gao Y., Zhu J., Wientjes M. G., Au J. L. S. AAPS J. 2011;13:585–597. doi: 10.1208/s12248-011-9298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]