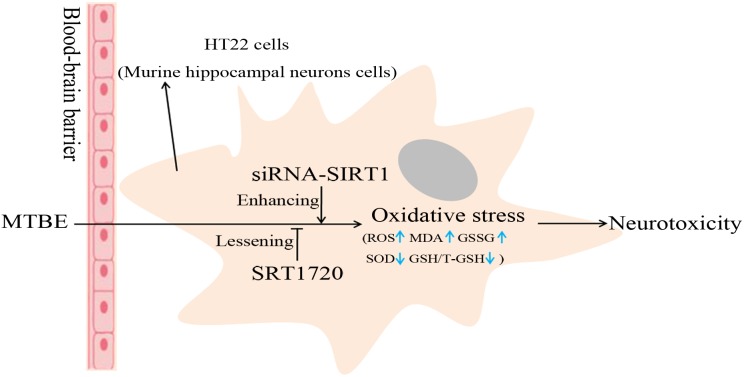
 Methyl tertiary-butyl ether (MTBE), an unleaded gasoline additive, can lead to oxidative stress and then injury to the nervous system after long-term exposure. SIRT1, a NAD+-dependent histone deacetylase, can play a neuroprotective role in brain injury induced by MTBE.
Methyl tertiary-butyl ether (MTBE), an unleaded gasoline additive, can lead to oxidative stress and then injury to the nervous system after long-term exposure. SIRT1, a NAD+-dependent histone deacetylase, can play a neuroprotective role in brain injury induced by MTBE.
Abstract
Methyl tertiary-butyl ether (MTBE), an unleaded gasoline additive, can lead to oxidative stress, thus injuring the nervous system after long-term exposure. SIRT1, a NAD+-dependent histone deacetylase, can play a neuroprotective role in brain injury. However, the mechanism is unclear. This present study intended to define the role of SIRT1 during the process of MTBE-induced oxidative stress in mouse hippocampal neurons (HT22 cells). Our data showed that MTBE could directly trigger oxidative stress in HT22 cells by decreasing the activity of superoxide dismutase (SOD) and GSH/T-GSH level while increasing ROS, lipid peroxidation product malondialdehyde (MDA) and GSSG level. Similarly, the expression of SIRT1, an antioxidant, decreased in a dose-dependent manner. To further explore whether SIRT1 plays a key role during the process of oxidative stress, HT22 cells were transfected with siRNA-SIRT1 and preconditioned with the agonist of SIRT1 (SRT1720) for 2 h. The levels of oxidative stress (ROS, SOD, MDA, GSH/GSSG) were detected again after siRNA-SIRT1 HT22 cells and SRT1720 HT22 cells were exposed to MTBE for 6 h. In contrast to the non-pretreated group, levels of oxidative stress were tonic in siRNA-SIRT1 HT22 cells and attenuated in SRT1720 HT22 cells. Our results indicate that MTBE could directly cause oxidative stress in HT-22 cells, and SIRT1 might be an important antioxidant during MTBE-induced oxidative stress.
1. Introduction
Methyl tertiary-butyl ether (MTBE) is an oxygenated compound, currently in use worldwide, that is added to gasoline to increase oxygen content and reduce tailpipe emission of carbon monoxide. With widespread use of MTBE, its health effects on animals and humans have expanded. Exposure to MTBE can lead to acute reversible central nervous system (CNS) depression and impairment of spatial learning and memory in rat.1,2 Epidemiological studies show that many people exposed to MTBE-blended fuels experienced neurotoxic illnesses,3 such as headache, dizziness, nausea, fatigue, and mood swings.4–6 But to date, detailed scientific study regarding the mechanism of neural injury induced by MTBE is lacking.
MTBE is a fat-soluble substance that has a strong affinity for the nervous system and can cause acute toxic effects on brain tissue through the blood–brain barrier.7 After pregnant mice were exposed to MTBE during gestation by gavage, the level of MDA in brain tissues of mice offspring was increased (P < 0.05), while the Na+, K+-ATPase, Ca2+-ATPase, Mg2+-ATPase, SOD and GSH activities decreased (P < 0.05), and the data showed a dose-effect correlation.8,9 In addition, the ultrastructural changes of brain tissue included edema and degeneration of the nerve and neuroglial cells and damage to the mitochondria and cell membrane.8 The above research showed that MTBE could cause oxidative damage to the brain tissue of mice offspring by decreasing the activity of ATPase, SOD and GSH, leading to lipid peroxidation. Other works have reported that MTBE exposure could induce oxidative stress in human blood lymphocytes, rat Sertoli cells, and rat spermatogenic cells.10–12 MTBE also induced oxidative damage and caused mitochondrial dysfunctions in rats.13 Silent information regulator two ortholog 1 (SIRT1) is a conserved nicotinamide adenine dinucleotide (NAD+)-dependent deacylase involved in a variety of cellular pathways, such as oxidative stress response, apoptosis, axonal neurodegeneration, and so on.14,15 We suspected that MTBE could induce oxidative stress in neural cells and that SIRT1 might play an important role during the process. Here, we chose the HT22 cell, a cell line from mouse hippocampal neurons, as the subject, and the indexes of oxidative stress (ROS, SOD, MDA, GSH/GSSG) were detected respectively in HT22 cells and HT22 cells preconditioned with SRT1720 or transfected with siRNA-SIRT1 then exposed to different concentrations of MTBE.
2. Materials and methods
Chemicals
Methyl tert-butyl ether (>99.8%) was obtained from Sigma-Aldrich (St Louis, MO, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay (MTT assay), lysis buffer and BCA Protein Assay Kit were purchased from Beyotime Biotechnology (Jiangsu, China). Dulbecco's modified Eagle minimal essential medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco (USA). The antibody SIRT1 (2028s), GAPDH (2118L), and anti-rabbit IgG horseradish peroxidase-linked antibody (7074s) were brought from Cell Signaling Technology. Reactive oxygen species (ROS) assay kit, superoxide dismutase (SOD) assay kit, malondialdehyde (MDA) assay kit, total glutathione and oxidized glutathione (T-GSH/GSSG) assay kit were acquired from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Cell culture and treatment
HT22 hippocampal neurons were obtained from School of Life Sciences and Biotechnology, Shanghai Jiao Tong University, and cultured in DMEM (supplemented with 10% FBS, 50 μg ml–1 penicillin and 100 μg ml–1 streptomycin, pH = 7.4) at 37 °C in a humidified air atmosphere containing 5% CO2. All cells utilized were in the exponential growth phase.
Cells in logarithmic growth phase were inoculated into culture plates for 12 h and directly exposed to MTBE solution with concentrations of 0.125, 0.25, 0.5, 1.0 and 2.0 mmol L–1 for 6 h,10 except for SRT1720 group cells, which were pretreated with SRT1720 solution (10 μmol L–1) for 2 hours, and cells treated with 0.1 ‰ DMSO as the control.
MTT assay
Cell viability was detected by MTT assay (Beyotime Biotechnology, China). In brief, HT22 cells were treated with different concentrations of MTBE (i.e., 0, 0.125, 0.25, 0.5, 1.0, 2.0, 2.5, 5.0, 10.0 mmol L–1) for 6 h. Analysis was performed on a microplate reader (Thermo) at 490 nm wavelength. Three independent experiments were carried out with three replicate wells for each analysis. The data were presented as mean ± SD, expressed as percentages of the cell viability in treated groups relative to the control.
Western blotting
After treatment, HT22 cells were harvested washed twice with ice-cold PBS. Sample cells were disrupted in lysis buffer (P0013B) containing 1% protease inhibitor cocktail on ice for 30 min. Then, cell lysates from whole cells were centrifuged for 10 min at 140 000g. The supernatant contained the total proteins, and their concentrations were determined by BCA protein assay kit (P0009). 35 μg total proteins per lane were added to SDS polyacrylamide gels, and proteins were detected by WB. Antibodies used were anti-SIRT1 (1 : 1000), anti-GAPDH (1 : 1000), and anti-rabbit IgG horseradish peroxidase-linked antibody (1 : 5000). Finally, protein bands were scanned and quantified by using Tanon-5200 (Beijing YuanPing Hao Biotech Co, Ltd).
Transient transfection
Lentivirus vectors (hU6-MCS-CMV-Puromycin) and SIRT1 siRNA were purchased from Genechem (Shanghai, China). We chose the optimal infection conditions according to pre-experimental results. 4 × 104 HT22 cells per well were seeded into a 6-well plate for 12 hours, and then were exposed to lentivirus (4 × 106 transducing units) with 500 μl enhanced infection solution and polybrene (5 μg mL–1) according to the manufacturer's instructions (Genechem). SIRT1 silencing efficiency was detected by western blotting and reverse transcription-polymerase chain reaction. The sequences of Sirt116 and GAPDH17 primers were according to reported literature.
ROS measurement
One microliter of 2′,7′-dichlorofluorescein diacetate (DCFH-DA, 10 mmol L–1) (Nanjing Jian Cheng Institute, China) was added before the end of MTBE exposure (30 min at 37 °C) to assess the ROS-mediated oxidation. Total fluorescence intensity was measured at an excitation wavelength of 485 nm and emission wavelength of 538 nm. Average fluorescence intensity is equal to total intensity fluorescence divided by number of cells.
Measurement of SOD, MDA and GSH/GSSG
After treatment, the supernatant was collected and assessed for SOD, MDA and GSH/GSSG content using commercially available kits (S0101, A003-2 and S0053, Nanjing Jian Cheng Institute, China).
Statistical analysis
All experiments were performed in triplicate and assessed using SPSS 18.0 software (SPSS Inc., USA). All results were presented as means ± SD, and P-value < 0.05 was considered statistically significant. Origin 8.5 (OriginLab, USA) was used to prepare the figures.
3. Results
Cell viability
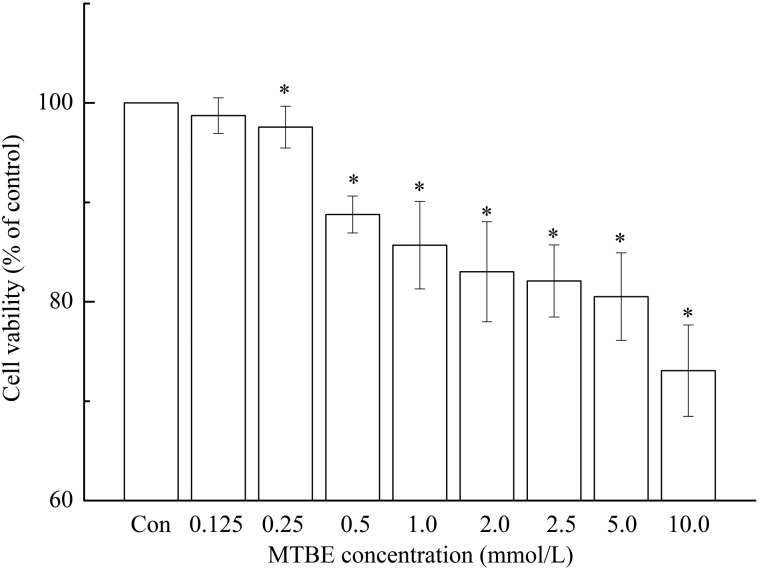
The viabilities of HT22 cells exposed to different concentration of MTBE were 98.72 ± 1.79% (0.125 mmol L–1), 97.57 ± 2.1% (0.25 mmol L–1), 88.78 ± 1.86% (0.5 mmol L–1), 85.69 ± 2.40% (1.0 mmol L–1), 83.02 ± 5.03% (2.0 mmol L–1), 82.09 ± 3.63% (2.5 mmol L–1), 80.51 ± 4.40% (5.0 mmol L–1) and 73.07 ± 4.60% (10.0 mmol L–1) of the control respectively (Fig. 1). Aside from cells exposed to 0.125 mmol L–1, cell viability decreased significantly compared to the control (P < 0.05). Accordingly, the dose groups (0.125, 0.25, 0.5, 1.0 and 2.0 mmol L–1) with higher cell viability were used in the subsequent experiment.
Fig. 1. Viability of HT22 cells after exposure to MTBE for 6 h. Viability of HT22 cells was assessed by MTT. The data was derived from triplicate experiments and expressed as percentages of surviving cells in treated groups relative to the control. Based on one-way ANOVA, *P < 0.05 indicates that the differences are considered significant versus the control.
Oxidative stress in HT22 cells
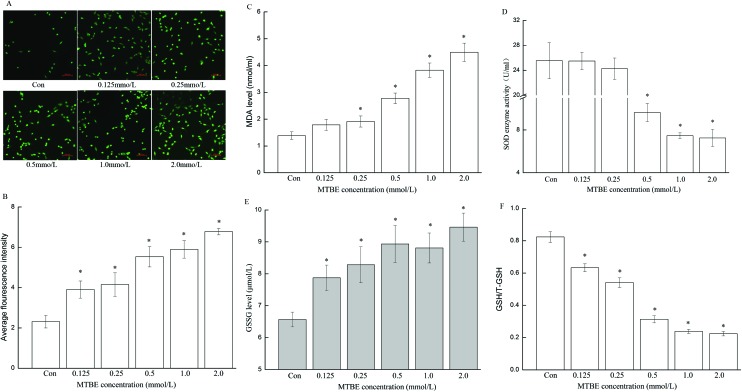
ROS, MDA and GSSG levels showed apparent dose–response relationships, which significantly increased with increasing dose of MTBE compared to the control (P < 0.05) (Fig. 2A–C and E). In contrast, the GSH level decreased with increasing dose of MTBE compared to the control (P < 0.05) (Fig. 2F). The activity of SOD enzyme obviously decreased only in the high-dose group (0.5, 1.0 and 2.0 mmol L–1) compared to the control (P < 0.05) (Fig. 2D).
Fig. 2. Oxidative stress was induced by MTBE in HT22 cells. After cells were exposed to 0 (Con), 0.125, 0.25, 0.5, 1.0 and 2.0 mM MTBE for 6 h, respectively, fluorescence microscopy images of ROS (A), average fluorescence intensity of ROS (B), and levels of other parameters of oxidative stress including MDA (C), SOD (D), GSSG (E), and ratio of GHS/T-GSH (F) were graphed. (A) was captured by a fluorescent microscope under 200× magnification. Data are expressed as mean ± SD, *P < 0.05.
Effect of MTBE on SIRT1 expression
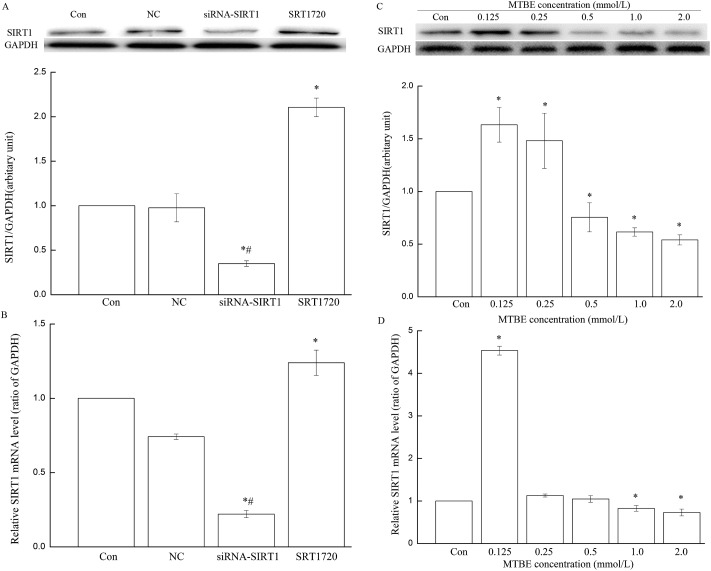
Levels of SIRT1 mRNA and protein significantly increased in 0.125 and 0.25 mmol L–1 MTBE but obviously decreased in 2.0 mmol L–1 MTBE compared to the control (P < 0.05) (Fig. 3C and D).
Fig. 3. SIRT1 expression in three cell models and HT22 cells exposed to different doses of MTBE. Normal HT22 cells, HT22 cells pretreated with SRT1720 for 2 h, and HT22 cells transfected with siRNA-SIRT1 are the three cell models. SIRT1 protein (A) and mRNA (B) in three cell models were assessed with GAPDH as the internal control. In HT22 cells, after cells were treated with 0 (Con), 0.125, 0.25, 0.5, 1.0 and 2.0 mM MTBE for 6 h, respectively, expressions of SIRT1 protein (C) and mRNA (D) were detected by WB and PCR. *P < 0.05 versus control, and #P < 0.05 versus NC (empty lentiviral vector).
Oxidative stress in SRT1720 HT22 cells and siRNA-SIRT1 HT22 cells
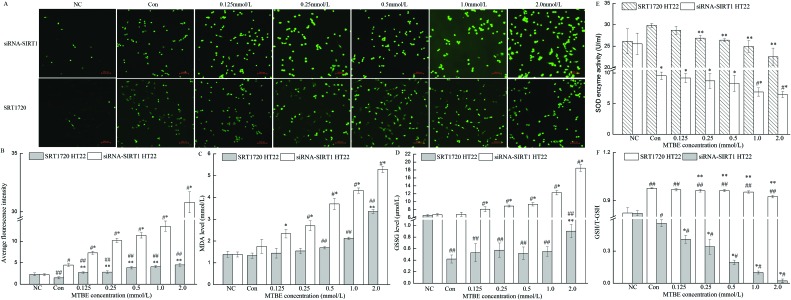
To further observe the role of SIRT1 in oxidative stress induced by MTBE, cells were preconditioned with agonist (SRT1720) and suppressant (transfected with SIRT1 siRNA), respectively, and then exposed to MTBE. Compared to the control, levels of SIRT1 mRNA and protein in siRNA-SIRT1 HT22 cells significantly reduced to 22.12% and 34.96% (P < 0.05), and they advanced to 210.57% and 123.94% in SRT1720 HT22 cells (P < 0.05), but there was no significant change in NC (empty lentiviral vectors) (Fig. 3A and B). The trends of oxidative stress indexes (ROS, MDA, GSSG, GSH and SOD) in SRT1720 HT22 cells and siRNA-SIRT1 HT22 cells were similar with their variances in HT22 cells (Fig. 2A–F and 4A–F). However, the increment of ROS, MDA, and GSSG and the decrement of GSH and SOD in siRNA-SIRT1 HT22 cells were much more obvious compared to HT22 cells or SRT1720 HT22 cells.
Fig. 4. Role of SIRT1 in MTBE-induced oxidative stress. Four oxidative stress indexes, including ROS (A–B), MDA (C), GSSG (D), SOD (E), and the ratio of GHS/T-GSH (F) were detected in HT22 cells pretreated with SRT1720 for 2 h and HT22 cells transfected with siRNA-SIRT1, after exposure to 0 (Con), 0.125, 0.25, 0.5, 1.0 and 2.0 mM MTBE for 6 h, respectively. (A) was captured by a fluorescent microscope under 200× magnification. Data are expressed as mean ± SD. In siRNA-SIRT1 HT22 cells, *P < 0.05 versus control, and #P < 0.05 versus NC (empty lentiviral vector); while in SRT1720 HT22 cells, **P < 0.05 versus control, and ##P < 0.05 versus NC (not pretreated with SRT1720).
4. Discussion
In the present study, we investigated the protective effect of SIRT1 on oxidative stress induced by MTBE in HT22 cells. By detecting oxidative stress, we found that MTBE-induced oxidative stress was significantly alleviated by SRT1720 pretreatment. The protective effect of SIRT1 was further confirmed by SIRT1 siRNA transfection. Higher oxidative stress was observed in siRNA-SIRT1 HT22 cells compared with untreated HT22 cells and SRT1720 HT22 cells. Interestingly, the protection of SIRT1 might be associated with GSH. These results indicate that SIRT1 exhibits a protective effect against MTBE-induced oxidative stress in HT22 hippocampal cells.
MTBE has been shown to cause brain oxidative injuries and neurological damage.2,8,9 To our knowledge, no other comprehensive research has so far been conducted to evaluate the possible toxicity of MTBE on hippocampal neuronal cells and its mechanisms. Oxidative stress induced by MTBE was observed in rat hepatocytes,18 Sertoli cells,12 spermatogenic cells11 and human blood lymphocytes.10 It was suspected that oxidative stress could be measured in HT22 hippocampal cells exposed to MTBE. Oxidative stress, one major cause of neurological diseases, occurs when ROS level exceeds the endogenous antioxidant capacity in the cells. In addition, oxidative stress has been shown to play a vital role in to contributing ROS19 because excessive amounts of ROS can cause further damage to the mitochondrial respiratory chain and exacerbate ROS production, forming a vicious cycle.20 Thus, it is important to control the balance between oxidation and antioxidation in the progression of neurodegenerative disease.21 We observed that MTBE caused statistically obvious decrease in cell viability (Fig. 1). Previous studies have also shown that MTBE could have cytotoxic effects on several cell lines.10–12,18 Our data regarding oxidative stress parameters indicated that MTBE cytotoxicity was associated with the formation of ROS, MDA and oxidized glutathione as well as the depletion of antioxidant enzymes SOD and glutathione (GSH) (Fig. 2). Thus, it can be considered that oxidative stress contributes to the cytotoxic mechanism of neurological diseases triggered by MTBE and mitochondrial injury by increasing the protein expression of SOD2 (MnSOD).22
Reduced glutathione (GSH) has been described as a defensive reagent involved in cell protection from excess oxidant stress, both directly and as a cofactor of glutathione peroxidases.23 Generally, 90–95% of total glutathione is reduced glutathione. As an antioxidant, GSH can prevent intracellular ROS formation and lipid peroxidation by oxidizing to GSSG. Therefore, GSH depletion is a marker of cellular oxidative stress and is attributed to GSSG expulsion. Our results demonstrated that when HT22 cells were incubated with MTBE, the ratio of GSH to total GSH reduction occurred as a consequence of ROS formation and lipid peroxidation, causing GSSG increase (Fig. 2A, B and F). These results are consistent with a previous study18 indicating that MTBE-induced toxicity was related to the decrease in GSH content. In addition, GSH was involved in the metabolic activation of MTBE,18 which might be another cause of GSH depletion.
SIRT1 has been reported to play protective roles in several neurodegenerative diseases, which might relate to its functions in stress resistance.24 SIRT1 belongs to the deacetylase family and can reduce oxidative stress by deacetylating its substrates, such as Foxo3, p53, NF-κ.25 Furthermore, another research also showed that SIRT1-specific activator SRT1720 reduces oxidative stress and promotes mitochondrial function in neuronal cells.26 In our study, SIRT1 expression was enhanced by MTBE in low dose (0.125 and 0.25 mmol L–1) but alleviated in high dose (Fig. 3C and D). It was supposed that the decrement of SIRT1 expression might be related to the involvement of oxidative stress triggered by MTBE. To further elucidate the role of SIRT1 during oxidative stress, the variations of oxidative stress levels were observed in the SRT1720-pretreated group and the SIRT1 siRNA-transfected group. Compared to HT22 cells, oxidative stress was obviously attenuated in SRT1720 HT22 cells and enhanced in siRNA-SIRT1 HT22, accompanied by selective improvements in antioxidant enzyme expression (Fig. 4). These results are agreement with studies22,27–29 suggesting that SIRT1 plays an important role in MTBE-induced oxidative stress. Gano et al. reported that SIRT1 activator SRT1720 could reverse vascular superoxide and superoxide suppression of EDD and selectively increase antioxidant enzymes in old mice.27 Consistently, our present study also demonstrated that SIRT1720 could ameliorate oxidative stress induced by MTBE via enhanced level of the antioxidant enzyme SOD. SOD is a pivotal antioxidative enzyme located downstream of Sirt1 and possessing strong antioxidant effects.28 A recent study showed that CsV rescued H2O2-induced SH-SY5Y cells from oxidative damage by upregulating Mn-SOD mRNA expression.29 Pengyun Li also found that polydatin could restore decreased deacetylase activity and protein expression of SIRT1 and suppress oxidative stress-induced lysosomal instability.22
Based on our findings, we believe that MTBE decreases cell viability directly, associated with the increase of ROS in HT22 cells. These mentioned effects trigger glutathione depletion and SOD enzyme and lipid peroxidation. Moreover, MTBE-triggered oxidative stress was attenuated in SIRT1 activator SRT1720-pretreated cells and aggravated in SIRT1-silenced cells. It is therefore concluded that MTBE is capable of inducing oxidative stress and that SIRT1 exerts a protective effect during the process.
Conflict of interest
The authors declare no conflicts of interest associated with this work.
Acknowledgments
This work was financially supported by the Funding Project for National Natural Science Foundation of China (81502790) and Beijing Natural Science Foundation (7154184).
References
- Daughtrey W. C., Gill M. W., Pritts I. M., Douglas J. F., Kneiss J. J., Andrews L. S. J. Appl. Toxicol. 1997;17(Suppl 1):S57–S64. doi: 10.1002/(sici)1099-1263(199705)17:1+<s57::aid-jat411>3.3.co;2-1. [DOI] [PubMed] [Google Scholar]
- Zheng G., Zhang W., Zhang Y., Chen Y., Liu M., Yao T., Yang Y., Zhao F., Li J., Huang C., Luo W., Chen J. Toxicol. Appl. Pharmacol. 2009;236:239–245. doi: 10.1016/j.taap.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Mehlman M. A. Toxicol. Ind. Health. 1996;12:613–627. doi: 10.1177/074823379601200502. [DOI] [PubMed] [Google Scholar]
- Nihlen A., Walinder R., Lof A., Johanson G. Toxicol. Appl. Pharmacol. 1998;148:281–287. doi: 10.1006/taap.1997.8342. [DOI] [PubMed] [Google Scholar]
- Fiedler N., Kelly-McNeil K., Mohr S., Lehrer P., Opiekun R. E., Lee C., Wainman T., Hamer R., Weisel C., Edelberg R., Lioy P. J. Environ. Health Perspect. 2000;108:753–763. doi: 10.1289/ehp.00108753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar R. L., Hefflin B. J., Ashley D. L., Middaugh J. P., Etzel R. A. Arch. Environ. Health. 1994;49:402–409. doi: 10.1080/00039896.1994.9954993. [DOI] [PubMed] [Google Scholar]
- Yun Z., Wenjing L., Ting Y., Gang Z., Mingchao L., Yaoming C., Jingyuan C. J. Environ. Health. 2007;24:987–989. [Google Scholar]
- Shufang D., Suyun L., Xingpeng H. Chin. J. Public Health. 2010;26:877–878. [Google Scholar]
- Shufang D., Suyun L., Xingpeng H. J. Nanhua Univ., Med. Ed. 2007;35:7–9. [Google Scholar]
- Salimi A., Vaghar-Moussavi M., Seydi E., Pourahmad J. Environ. Sci. Pollut. Res. Int. 2016;23:8556–8564. doi: 10.1007/s11356-016-6090-x. [DOI] [PubMed] [Google Scholar]
- Li D., Yin D., Han X. J. Appl. Toxicol. 2007;27:10–17. doi: 10.1002/jat.1178. [DOI] [PubMed] [Google Scholar]
- Li D., Liu Q., Gong Y., Huang Y., Han X. Reprod. Toxicol. 2009;27:170–176. doi: 10.1016/j.reprotox.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Saeedi A., Omidi M., Khoshnoud M. J., Mohammadi-Bardbori A., Xenobiotica, 2015. 10.3109/00498254.2015.1125040 , , [Epub ahead of print] . [Google Scholar]
- Khan R. S., Fonseca-Kelly Z., Callinan C., Zuo L., Sachdeva M. M., Shindler K. S. Front. Cell. Neurosci. 2012;6:63. doi: 10.3389/fncel.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Sauve A. A. AAPS J. 2006;8:E632–E643. doi: 10.1208/aapsj080472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Lee C. E., Bookout A. L., Lee S., Williams K. W., Anderson J., Elmquist J. K., Coppari R. J. Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A., Dobersch S., Dammann R. H., Bellusci S., Ilinskaya O. N., Braun T., Barreto G. Int. J. Mol. Sci. 2015;16:4492–4511. doi: 10.3390/ijms16034492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourahmad J., Eskandari M. R., Alavian G., Shaki F. Toxicol. Environ. Chem. 2012;94:281–293. [Google Scholar]
- Ismail N., Ismail M., Imam M. U., Azmi N. H., Fathy S. F., Foo J. B., Abu Bakar M. F. BMC Complementary Altern. Med. 2014;14:467. doi: 10.1186/1472-6882-14-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov D. B., Juhaszova M., Sollott S. J. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Chen Y., Yang A., Chen C., Liao L., Li S., Ying M., Tian J., Liu Q., Ni J. Int. J. Mol. Sci. 2016;17:469. doi: 10.3390/ijms17040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Wang X., Zhao M., Song R., Zhao K. S. Expert Opin. Ther. Targets. 2015;19:997–1010. doi: 10.1517/14728222.2015.1054806. [DOI] [PubMed] [Google Scholar]
- Pompella A., Visvikis A., Paolicchi A., De Tata V., Casini A. F. Biochem. Pharmacol. 2003;66:1499–1503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- Herskovits A. Z., Guarente L. Neuron. 2014;81:471–483. doi: 10.1016/j.neuron.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Lei M., Hu T., Yu F., Xiao D. M., Kang H. J. Huazhong Univ. Sci. Technol., Med. Sci. 2016;36:350–355. doi: 10.1007/s11596-016-1590-y. [DOI] [PubMed] [Google Scholar]
- Valero T. Curr. Pharm. Des. 2014;20:5507–5509. doi: 10.2174/138161282035140911142118. [DOI] [PubMed] [Google Scholar]
- Gano L. B., Donato A. J., Pasha H. M., Hearon Jr. C. M., Sindler A. L., Seals D. R. Am. J. Physiol.: Heart Circ. Physiol. 2014;307:H1754–H1763. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Emidio G., Falone S., Vitti M., D'Alessandro A. M., Vento M., Di Pietro C., Amicarelli F., Tatone C. Hum. Reprod. 2014;29:2006–2017. doi: 10.1093/humrep/deu160. [DOI] [PubMed] [Google Scholar]
- Wan J., Deng L., Zhang C., Yuan Q., Liu J., Dun Y., Zhou Z., Zhao H., Liu C., Yuan D., Wang T. Can. J. Physiol. Pharmacol. 2016;94:919–928. doi: 10.1139/cjpp-2015-0262. [DOI] [PubMed] [Google Scholar]