 The aging phenomenon is associated with oxidative stress damage in biomolecules, especially DNA.
The aging phenomenon is associated with oxidative stress damage in biomolecules, especially DNA.
Abstract
The aging phenomenon is associated with oxidative stress damage in biomolecules, especially DNA. 5-Methyltetrahydrofolate (5-MTHF), the active folate form, plays a pivotal role in maintaining genomic integrity. However, recently it was associated with cancer development. In Brazil, there are folic acid enriched foods, such as flour, making the general population chronically exposed to folates. Therefore, the aim of this study was to investigate whether erythrocytes 5-MTHF levels were associated with age-related DNA damage in two groups (elderly and young subjects). Additionally, a study in Caenorhabditis elegans, an in vivo alternative model, was performed to verify if 5-MTHF presents a pro-oxidant effect. A total of 50 elderly and 25 young subjects participated in this study, which analyzed whole blood DNA damage, plasma carbonyl proteins (PCO), and erythrocytes 5-MTHF levels. In addition, ROS and RNS production, survival rate, and lifespan were performed in C. elegans exposed to 5-MTHF. Blood 5-MTHF levels and DNA damage were increased in the elderly compared to the young group. A positive association was found between 5-MTHF levels and DNA damage, and between DNA damage and PCO levels, suggesting an oxidative cause of damage associated with the active folate form. In an experimental study it was observed that 5-MTHF increased ROS production in C. elegans, in a dose dependent manner, while survival rate and life span were not affected at the test doses. These findings suggest that 5-MTHF, the active folate form, may be involved in DNA damage in the elderly. This damage could be a result of oxidative stress, as observed in the in vivo alternative model; however, more studies are necessary to prove our present results.
Introduction
For millennia, the aging phenomenon has been extensively studied but particular characteristics remain incompletely understood to date. No single mechanism is able to explain aging, however, it is known that oxidative stress has a relevant involvement in this process.1 Reactive oxygen species (ROS) trigger accumulating damage in aging cells, especially in biomolecules like DNA, thus causing loss of function or even cell death.2 We have demonstrated the influence of both oxidative stress and inflammation in aging.3 In fact, the elderly with lower antioxidant status were found to be more vulnerable to oxidative stress, which may have negative consequences on the quality and duration of life.3
Some vitamins are important in preventing common diseases in old age. It is now established that micronutrient deficiencies are associated with impaired immune responses.4 Folate, a water-soluble B9 vitamin occurring naturally in food, is essential for cell growth and reproduction.5 Folates occur mainly as 5-methyltetrahydrofolate (5-MTHF) in red blood cells, which present higher 5-MTHF levels than in serum.6 5-MTHF, the actual active folate form,7 is generally correlated to folate nutritional status.6
Folate acts in the body as a one-carbon unit carrier,6 and thus is essential for nucleotide biosynthesis and DNA replication, as well as being involved in many methylation reactions. In brief, 5-MTHF is involved in the regulation of methionine metabolism, which is the precursor of S-adenosylmethionine, the primary methyl donor molecule, including for DNA.8 DNA methylation is implicated in control of gene expression, cell proliferation, and differentiation, among a variety of important functions.9,10 Therefore, its deregulation is a contributing factor to carcinogenesis.11
Although 5-MTHF plays a pivotal role in maintaining genomic integrity11 and may have a protective role against cancer,12 recent evidence suggests that high levels of folates intake may also have detrimental effects. In fact, some studies reported neoplastic growth13 and particularly risk of colorectal cancer.14 Besides aberrant DNA methylation, several mechanisms may underlie cancer development, including DNA strand breaks and impaired DNA repair.15,16
Moreover, depending on their concentration, some vitamins can exert pro-oxidant properties that can lead to DNA damage. The quantification of ROS production in human blood has some difficulties due to ROS instability. Because of this, the use of an experimental model to explore ROS production is an interesting tool. The use of Caenorhabditis elegans as an alternative in vivo model has emerged as a main tool to study the biology of aging, being widely used to evaluate life span.17 Moreover, due to its simple genetic and its short life cycle, it is suitable and able to simplify studies of toxicity,18 longevity,19 oxidative stress,20 age-related diseases,21 and micronutrients effects.22
In the present study, the initial aim was to investigate whether 5-MTHF levels were associated with age-related DNA damage in the elderly. Additionally, assays were performed in an alternative experimental model to study the 5-MTHF pro-oxidative effect.
Materials and methods
Study population
This study was approved by the ethics committee of the Federal University of Rio Grande do Sul (No. 15146) and of the Clinical Hospital of Porto Alegre (No. 110171). All volunteers gave their written informed consent.
The study was performed with 50 elderly people (ages 76.06 ± 1.29 years) and 25 health young subjects (ages 26.48 ± 0.69 years) from Porto Alegre, Brazil. The exclusion criteria adopted were diagnoses of diseases such cancer, congenital, neurological, or psychiatric pathologies, advanced neurological disease, gastrectomy, vitamin B12 deficiency, and use of parenteral nutrition. Moreover, all volunteers were non-smokers and not taking any antioxidant supplementation (vitamins or minerals).
All subjects answered an investigator-administered questionnaire to assess general health, lifestyle, and demographic data. Anthropometric measurements were determined. Body mass index (BMI) was calculated as body weight (kg) divided by the square of body height (m).
Sample collection
Venous blood samples were collected from all subjects under fasting conditions into heparinized tubes and EDTA-containing tubes. The EDTA tube was immediately centrifuged at 1500g for 10 min at 4 °C, and the plasma obtained was used to determine protein carbonyls (PCO) while the erythrocytes (RBC) were used for quantification of 5-methyltetrahydrofolate (5-MTHF). All samples were kept at –80 °C until analysis and protected from light when necessary. The whole blood with heparin was transferred to amber microtubes and protected from light until comet assay, which was performed immediately.
5-Methyltetrahydrofolate (5-MTHF)
Quantification of 5-MTHF levels in erythrocytes was performed according to Leeming et al. (1990) with some modifications.23 In brief, an aliquot of 0.1 mL erythrocyte sample was mixed with 0.5 mL 2% ascorbic acid and incubated at 37 °C for 120 min in the dark, then mixed with 0.1 mL 15% trichloroacetic acid for 1 min. After, that, the sample was centrifuged at 13 000g for 5.5 min, and the supernatant was mixed with 0.03 mL 7 M NaOH. Finally, the supernatant was analyzed by high-performance liquid chromatography (HPLC) with fluorescence detection by a RF-10 AXL Shimadzu® (Kyoto, Japan). For HPLC, a Phenyl column (250 mm × 4.6 mm, 7 μm) protected by a guard column was used. The mobile phase was a mixture of 0.07 M formic acid (pH 3.75) and methanol (82.5 : 17.5, v/v). The flow rate was maintained isocratically at 1.0 mL min–1, and absorbance of the eluent was monitored at 295 nm and 365 nm, as excitation and emission wavelengths, respectively. The Phenyl column used was thermostated at 40 °C. All sample preparation steps were conducted under dim light. The seven-points calibration curve included 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, and 1.0 μmol L–1 5-MTHF and the limits of detection (LOD) and quantification (LOQ), determined by standard deviation of the response and the slope from the calibration curve, were 0.01 and 0.04 μmol L–1, respectively.
Comet assay
The comet assay, or single cell gel electrophoresis, followed a standard protocol for preparation and analysis.24,25 Slides were prepared by mixing whole blood and low melting point agarose (0.75%). The mixture was poured on a frosted microscope slide coated with normal melting point agarose (1.5%). After solidification, the slides were placed in lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, 1% Triton X-100, 10% DMSO) for a minimum of 1 h and a maximum of 4 weeks. Subsequently, the slides were incubated in freshly made alkaline solution (300 mM NaOH and 1 mM EDTA, pH > 13) for 20 min and alkaline horizontal electrophoresis was carried out for 20 min at 25 V and 300 mA. After electrophoresis, the slides were washed with neutralization buffer (0.4 M Tris, pH 7.5), stained with silver nitrate, and observed under an optical microscope. Images of 100 randomly selected cells were analyzed from each sample. The damages were visually scored, according to tail size, into five classes ranging from no tail (0) to maximal (4) tail length. Therefore, a damage index (DI) could range from 0 (all cells with no tail, 100 cells × 0) to 400 (all cells with maximally long tails, 100 cells × 4).
Protein carbonyls (PCO)
Protein carbonyls were measured by a sensitive ELISA method according to Buss et al. (1997).26 Total protein concentration in plasma was measured by the Bradford method using bovine serum albumin as a standard. PCO levels were determined as follows: plasma samples were diluted with PBS buffer to a normalized concentration of 4 mg protein per mL and then samples were derivatized with 2,4-dinitro-phenylhydrazine (DNPH) and incubated in Maxisorb multiwellplates (Nunc Immuno 96 Microwell™ Maxisorp) overnight at 4 °C in the dark. Protein carbonyls were detected using a dinitrophenyl rabbit IgG-antiserum (Sigma, Deisenhofen, Germany) as the primary antibody and a monoclonal anti-rabbit immunoglobulin G peroxidase conjugate (Sigma) as the secondary antibody. Color development was performed with o-phenylenediamine and H2O2 and the reaction was stopped with H2SO4 after 15 min incubation at 37 °C. The absorbance was measured using a microplate reader (SpectraMax M2, Molecular Devices, LLC, Sunnyvale, CA, USA) with a detection wavelength of 492 nm. Each sample was analyzed in triplicate. Plasma protein carbonyl concentration was expressed as nmol mg–1 protein.
In vivo study
Chemicals
Standards of (6R,S)-5-methyl-5,6,7,8-tetrahydrofolic acid calcium salt (5-MTHF) were purchased from Schircks Laboratories® (Jona, Switzerland) and the reagents bacto-agar and bacto-peptona were obtained from Becton Dickinson BD® (New Jersey, USA) and HiMedia Laboratories® (Mumbai, India), respectively. 2′,7′-Dichlorofluorescein diacetate (DCF-DA) was supplied by Sigma-Aldrich Co. (St Louis, MO, USA). To evaluate the survival rate of the nematodes, the treatments were performed in two thousand five hundred synchronized L1 worms per concentration tested (100, 200, 500, 1000, 2000, 4000 or 8000 μg L–1). After the treatment, the worms were washed three times with M9 buffer and all worms were placed on OP50-seeded NGM plates (90 × 15 mm). The number of surviving worms was verified 24 hours after treatment and the results for all concentrations were calculated as percentage compared to the control (0 μg L–1 5-MTHF). Three independent experiments were performed.
C. elegans strain
The nematodes C. elegans were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Twin Cities, MN, USA). The C. elegans strains N2 (wild-type) were maintained on nematode growth medium (NGM) plates seeded with Escherichia coli OP50 at 20 °C.
Synchronization
Gravid C. elegans were rinsed off the plates into centrifuge tubes and were lysed with a bleaching mixture (1% NaOCl; 0.25 M NaOH), followed by flotation in a 30% sucrose solution (m/v) to separate eggs. The eggs were washed with M9 buffer (0.02 M KH2PO4, 0.04 M Na2HPO4, 0.08 M NaCl, and 0.001 M MgSO4) and allowed to hatch overnight on NGM agar plates without bacteria.27
Exposure to 5-MTHF
A stock solution of 5-MTHF was prepared by dissolving 10 mg in 1 mL 0.01 M NaOH. A 100 μL aliquot was diluted 100 times with buffer solution (0.01 M K2HPO4, pH 7.0) to determine the true content of the original 5-MTHF by measuring the absorbance of a standard solution at 290 nm (ε: 31 700 L–1 mol–1 cm–1).6 Another aliquot (100 μL) of the remaining 5-MTHF stock solution was diluted 100 times with 2% ascorbic acid, calibrated to 10 mL and stored at –80 °C as the intermediate solution (100 mg L–1). Daily working solutions were prepared by diluting the intermediate solution with 2% ascorbic acid. All operations with 5-MTHF standard were conducted in dim light.
Synchronized L1 worms were acutely treated for 30 minutes at 20 °C, by constant agitation in a rotator, with 8 different concentrations of 5-MTHF ranging from 50 to 8000 μg L–1. In addition, a control was treated only with 2% ascorbic acid, the same solvent used in the intermediate solutions.
ROS measurement
For ROS measurements, 1500 worms were treated with 5-MTHF (50, 100, 200, 500, 1000, 2000, 4000 or 8000 μg L–1) and ascorbic acid as solvent control, respectively, following the protocols detailed above. After exposure, the worms were washed three times and re-suspended in 100 μL of 0.9% NaCl solution (m/v) and transferred to 96-well plates. Following this, 100 μL of 0.05 mM 2′,7′-dichlorofluorescein diacetate (DCF-DA) was added and the fluorescence was measured for 90 minutes at 485 nm excitation and 530 nm emission using a microplate reader (Spectramax Me2; Molecular Devices LLC, Sunnyvale, CA, USA) at 20 °C. In the presence of reactive species, the DCF-DA is oxidized to the fluorescent product dichlorofluorescein (DCF). The values were expressed as percentage of fluorescence intensity relative to control wells. The experiments were run in triplicate, and at least three independent experiments were performed.
Survival rate assay
To determine survival rate of the nematodes, worms were exposed to seven concentrations of 5-MTHF (100, 200, 500, 1000, 2000, 4000 or 8000 μg L–1). L1 synchronized worms were treated with each dose at 20 °C for 30 minutes with constant agitation in a rotator. After washing three times with M9 buffer, the worms were placed on OP50-seeded NGM plates and the number of surviving nematodes on each plate was verified 24 hours after exposure. The results for all concentrations were calculated as the percentage compared to the control (0 μg L–1 5-MTHF). Three independent experiments were performed.
Life span assay
After the treatments, age synchronized young larvae (L1) were transferred to OP50-seeded NGM plates (90 × 15 mm) and incubated at 20 °C. Once the worms reached the last larval stage (L4), three days after hatching, they were transferred to new treatment plates (60 × 12 mm). This moment was considered as the first time point for counting the surviving worms. Twenty animals were transferred per plate, in duplicates, for each condition tested. These worms were transferred daily to newly prepared fresh plates (plus ingredients) to avoid overlapping generations. Moreover, ampicillin (100 mg L–1) and 5-fluoro-2′-deoxyuridine (150 mM) (Amp/FUDR) were added to the plates to prevent egg production and bacterial contamination during the egg-laying period. Vitality of the worms was analyzed daily, and the worms were considered dead when they failed to respond to external stimuli performed by gentle prods with nylon yarn. Three independent assays were carried out, totaling at least 120 nematodes for each concentration tested.
Statistical analysis
The data were analyzed using SPSS (Statistical Package for the Social Sciences, version 18). Comparisons between elderly and young groups were achieved by Student's t-test and a Mann–Whitney U-test according to variable distribution. Correlation tests also were performed according to Pearson's or Spearman's rank following the variables distribution. Data are expressed as mean ± standard error of the mean (SEM). ROS production, survival rate, and lifespan data were generated with GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). The statistical analysis of significance was carried out using Analysis of Variance (ANOVA), followed by Tukey post hoc test in the SPSS Statistics software (version 18). P-Values less than 0.05 were considered significant for all tests.
Results
Characteristics of the study population
Characteristics of the studied groups are described in Table 1. The elderly group presented higher body mass index (BMI) than young subjects, however, both groups showed results within the normal range according to their age group.28 Moreover, in both groups, male subjects corresponded to approximately 40%, whereas female subjects were around 60% of each group.
Table 1. Baseline characteristics and parameters of the studied sample (n = 75).
| Variable | Young group (n = 25) | Elderly group (n = 50) |
| Age (years) | 26.48 (0.69) | 76.06 (1.29)** |
| BMI | 23.08 (0.63) | 25.58 (0.59)* |
| Gender, frequency (%) | ||
| Male | 11 (44.0) | 21 (42.0) |
| Female | 14 (56.0) | 29 (58.0) |
Levels of 5-MTHF
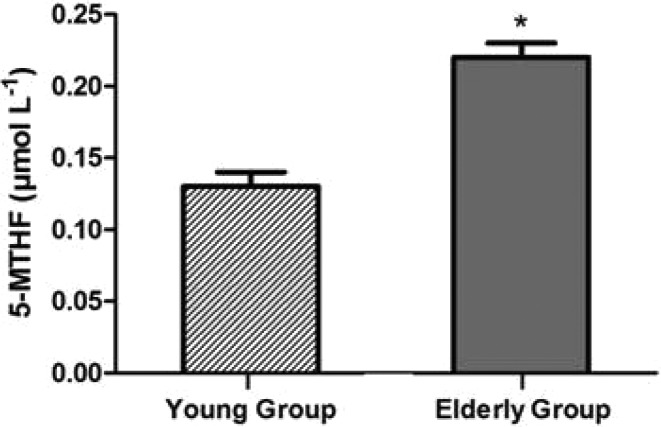
5-MTHF levels were significantly increased in the elderly group (p < 0.01, Student's t-test; Fig. 1), which presented an average concentration of 0.22 ± 0.01 μmol L–1, whereas the young group showed 0.13 ± 0.01 μmol L–1. Although measurement of serum and red blood cell folate levels has been a cornerstone of hematological diagnosis for years, the normal ranges may show wide variation. Particularly for 5-MTHF, there is no established reference value. In this line, Sobczynska-Malefora et al. (2014) aimed to determine the reference intervals of 5-MTHF in healthy adults without vitamin deficiencies.29 They evaluated 126 British subjects from different ethnicities and the interval ranged from 0.22 to 1.04 μmol L–1.29
Fig. 1. Results of 5-MTHF levels, the active folate form, in the young group (n = 25) and elderly group (n = 50). *Significantly different from young group (p < 0.05; Student's t-test).
DNA damage
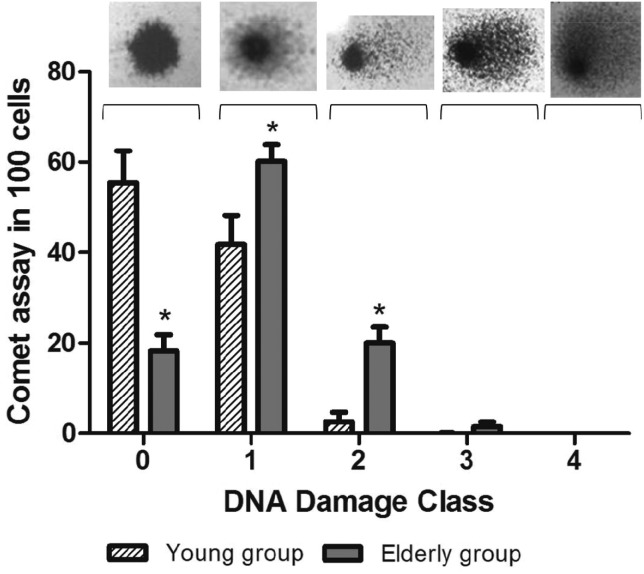
After electrophoresis, the presence of a tail in the cell nucleus, i.e. comet-shaped nuclei, indicated that the DNA had been damaged. In order to evaluate the extent of DNA damage, the tails were classified in a score from 0 (no tail/no damage) to 4 (maximal long tail), resulting in a single DNA damage score for each subject and, consequently, for each study group. The elderly group presented a high damage index when compared with young people (p < 0.01, Student's t-test); the results were 104.79 ± 6.83 and 47.27 ± 8.17, respectively. The DNA damage index was associated with advancing age (r = 0.482; p < 0.001, Pearson correlation; n = 75). According to tail size score, the damages were classified into five classes as shown in Fig. 2. The results showed significant differences between the groups for classes 0, 1 and 2 (Fig. 2).
Fig. 2. DNA damage classes in the young group (n = 25) and elderly group (n = 50). The images show how the damage is observed in each class (0, 1, 2, 3 and 4). *Significantly different from young group (p < 0.05, Student's t-test), n = 75.
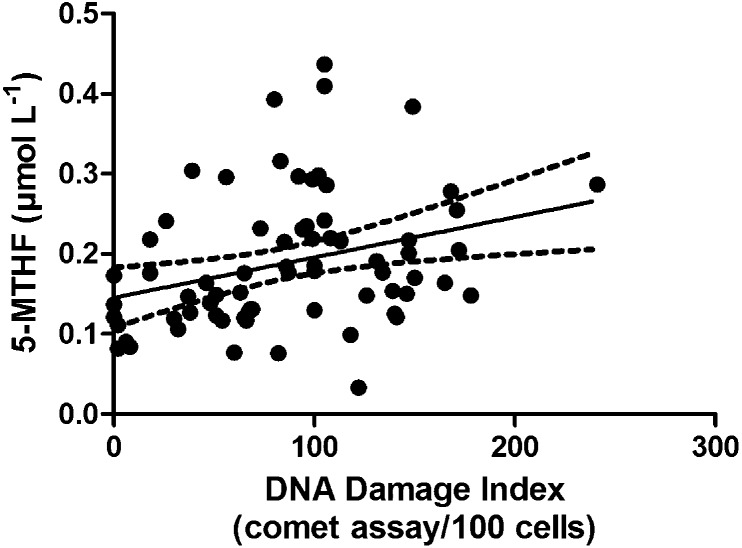
Moreover, the increase of 5-MTHF levels was associated with the increase of DNA damage as shown in Fig. 3 (r = 0.310; p < 0.01, Pearson's correlation).
Fig. 3. Pearson's correlation between DNA damage index and 5-MTHF levels (r = 0.310; p < 0.01; n = 75).
Protein oxidative damage
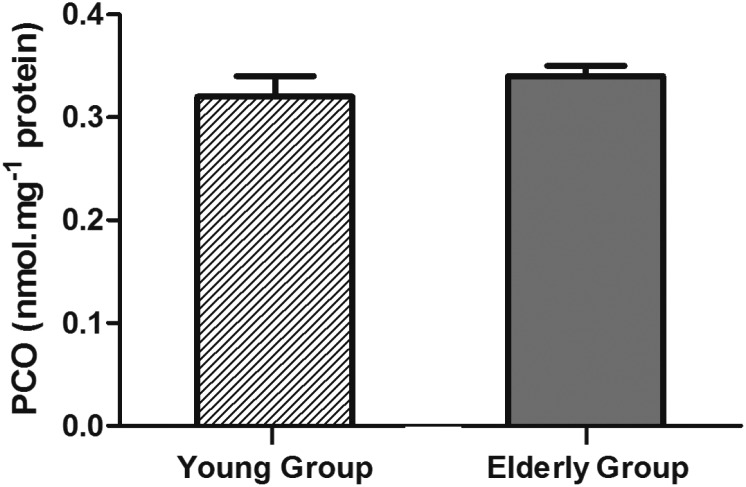
PCO levels were measured as an oxidative biomarker of protein damage and the results showed a tendency (p = 0.063, Student's t-test) to be higher in the elderly group (0.34 ± 0.01 nmol mg–1 protein) than in the young group (0.32 ± 0.02 nmol mg–1 protein) as shown in Fig. 4.
Fig. 4. Results of PCO levels in the young group (n = 25) and elderly group (n = 50) (p = 0.063, Student's t-test).
Additionally, protein damage, characterized by PCO levels, was positively associated with 5-MTHF levels (r = 0.263; p < 0.05 Pearson correlation; n = 75) and DNA damage (r = 0.281; p < 0.05, Pearson correlation; n = 75).
In vivo study in C. elegans
ROS production
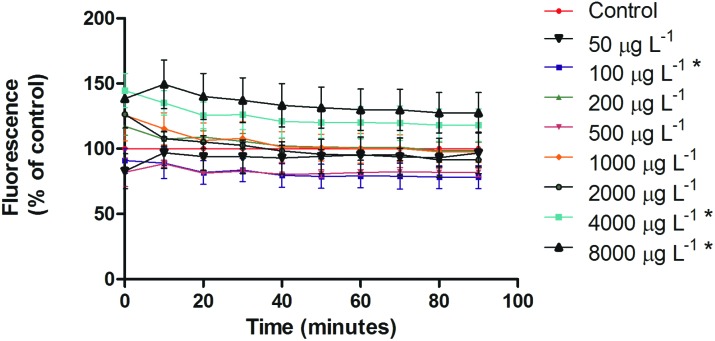
5-MTHF had an antioxidant profile at lower doses, being significant at 100 μg L–1 (p < 0.05, ANOVA/Tukey), whereas the increase of 5-MTHF concentration caused increase in DCF-DA oxidation in a dose-dependent manner (Fig. 5). However, the significantly higher ROS levels were observed only in the two highest doses tested (4000 and 8000 μg L–1) (p < 0.05, ANOVA/Tukey).
Fig. 5. ROS production in C. elegans after 5-MTHF treatment. ROS were measured by DCF-DA. The production of ROS increased at higher concentrations. *p < 0.05 compared to the control group, ANOVA/Tukey.
Survival rate
After acute treatment, none of the tested doses of 5-MTHF showed any mortality compared to the control group, which did not receive the treatment. This means that the worms, at least at the tested concentrations, had high tolerance to 5-MTHF.
Life span
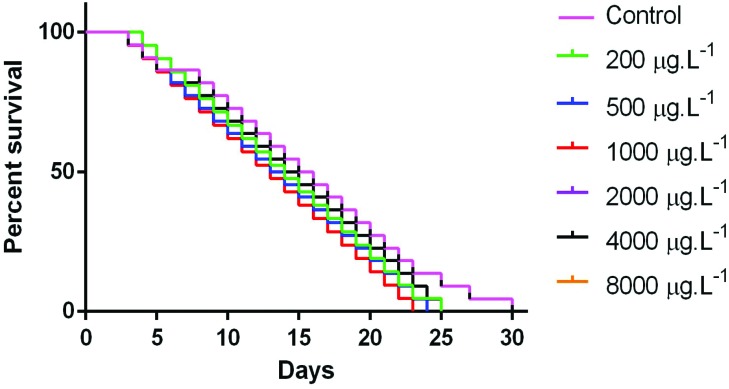
Life span after 30 min of 5-MTHF treatment, at all tested doses, was statistically indistinguishable from controls, indicating that this vitamin, at these concentrations, did not interfere in the worms’ longevity (Fig. 6).
Fig. 6. Kaplan–Meier survival curves of N2 Bristol wild-type treated with 5-MTHF.
Discussion
Adequate folate intake during the periconceptional period is important for preventing congenital anomalies, such as neural tube defects, which its supplementation often indicated.30 However, Veeranki et al. (2015) demonstrated that maternal folic acid supplementation during pregnancy was associated with development of early childhood asthma.30 On the other hand, folate deficiency is one of the causes of anemia31 in old age requiring treatment, while in other cases there is still controversy about the benefits of folate supplementation. Studies have shown its inefficacy, especially in the absence of folate deficiency.32,33 All these aspects raise the hypothesis that there is no consensus about the use of folates and the need to achieve a healthy balance may vary according to age and an individual context.
In the present study, the elderly subjects presented higher 5-MTHF levels than the young group (p < 0.05). Similarly, a previous study found a positive association between red cell 5-MTHF levels and age.29 Although there is controversy about the physiology of aging3 and the behavior of folate levels at this stage of life, it has been suggested that higher concentrations in the elderly could represent an oversupply of the vitamin from diet or it could reflect a tendency to retain folate both in plasma and red blood cells during aging.34
There are few studies reporting population-based reference intervals for 5-MTHF, the predominant form of folate in plasma and red cells.29 Many of them were performed in serum or plasma, thus hindering a comparison with present findings, which were performed in erythrocytes.35,36 Besides, differences in methodologies, interlaboratory variation, lack of the precision of some methods, and other population characteristics, such as diet, further complicate a comparison of the results.
Nevertheless, the levels found here were slightly below the values found by Sobczynska-Malefora et al. (2014).29 This might be attributed to the way that the 5-MTHF red cell folate concentration was calculated in that study. While in the present study, 5-MTHF was measured using red cell samples, in the aforementioned study it was evaluated through a formula using whole blood 5-MTHF and plasma 5-MTHF, as well as hematocrit values.29 Moreover, the authors determined a reference interval for adults with a wide range of age and these levels may differ from those physiologically required for the elderly to a healthy aging. Therefore, specific reference values still remain to be established for a healthy elderly population.
In addition, a sensitive method for measuring DNA damage, the comet assay, showed that the elderly group presented a significantly higher DNA damage index in comparison to the young group (p < 0.05). These findings are consistent with previous studies, which also showed cytogenetic damage in elderly people.37,38 Aging is linked with the progressive loss of genomic fidelity. Certainly, DNA has the most highly developed biomolecule repair process, reflecting the biological importance of preserving the integrity of this molecule.39 Based on the results, the genome damage rate appears to exceed the DNA repair capacity in the elderly subjects studied. In other words, as well as most human aging disorders, the aging itself involves defects in the repair of DNA lesions, which along with increased ROS levels result in accumulation of damage.16,40–42 Thus, the positive correlation between DNA damage and age found in this work is consistent with this observation.
There is a variety of sources of DNA damage, but lesions caused by free radicals and ROS may often be significant43 and include base modifications, single- and double-strand breaks, and inter-strand cross-links,43 which, biologically, may result in mutagenesis and genomic instability, consequently contributing to carcinogenesis and, more critically, to cell death.16
Many types of oxidative lesions by ROS have been reported26,44,45 and various indicators have been used to assess the extent of oxidative damage during aging. One common indicator is PCO content. The present findings related to PCO levels showed an increase in the elderly compared to young subjects, but not a statistically significant one. However, the positive linear correlation found between DNA damage index and the biomarker of oxidative damage, PCO, is consistent with genotoxic effects related to oxidative stress.
Furthermore, 5-MTHF was positively correlated with DNA damage. Indeed, once folate is deficient, damage to nuclear and mitochondrial DNA increases, but little is known about the role of different folate levels during aging.46 Recently, a relationship between high folate levels and cancer progression has been described,47 possibly due to its critical action in maintaining DNA.9,48 In fact, both hypomethylation and hypermethylation lead to cancer, activating oncogenes and pro-metastatic genes or silencing tumor suppressor genes, respectively.11,49 According to some studies, folate presents a dual role in carcinogenesis.9 In conditions of folate deficiency, hypomethylation is favored.46 Conversely, increasing concentrations of folic acid promoted hypermethylation of PTEN, APC, and RARbeta2 tumor suppressor genes and hence, their transcriptional activities became impaired in cell lines.9 In addition, Ebbing et al. (2009) suggested that 5-MTHF may enhance DNA replication and neoplastic growth.48
High folate levels have been associated with development of colon and breast cancer.47 In this context, Choi et al. (2005) demonstrated that supplementation of folate in elder rats at four times the basal requirement significantly increases genomic DNA methylation compared with folate-deplete status.50 However, folic acid appears to act differently in normal cells and cancer cells depending on the timing that the supplementation is performed.13 Folate administration prior to the existence of neoplastic lesions may aid in cancer prevention, but also promotes progression of pre-cancerous cells/tumors if supplied when early lesions are already established,13 probably because rapidly proliferating tissues have an increased requirement for nucleotides, whose synthesis is dependent of folates. This appears to explain the ineffectiveness or even the harmful effects of supplementation in some studies.48 Notedly, considering the difficulty of early diagnosis of cancerous lesions, it becomes a necessary caution with the use of supplements, especially in populations that receive folic acid fortification in foods.
Additionally, a positive relationship was observed between the 5-MTHF and PCO levels, supporting the hypothesis that oxidative stress may underlie the effects of 5-MTHF on DNA, besides its role in methylation reactions. Although the results demonstrated only a tendency of PCO levels to increase in the elderly compared to the young group, this could be a limitation probably due to the number of subjects in the young group, which was half of elderly group. In order to investigate possible mechanisms involved in this condition, especially related to physiological effects of different concentrations of this folate metabolite in an organism, an in vivo experiment was performed with the well-known C. elegans model.
Through a ROS production experiment a dual profile for 5-MTHF was observed, since at lower tested concentrations this vitamin acted as an antioxidant and at higher doses, it caused increase of ROS amounts in a dose-dependent manner compared to the control. Actually, 5-MTHF has high antioxidant activity which resides in the pterin core and whose antioxidant pharmacophore is 4-hydroxy-2,5,6-triaminopyrimidine.51 On the other hand, oxidative stress is involved in the toxicity mechanism of various substances.52,53 According to our knowledge, there are no data in the literature showing whether 5-MTHF can act as a pro-oxidant agent at high concentrations; however, this behaviour has already been demonstrated with other potential antioxidant vitamins, such as vitamin C.54–56 Thus, a pro-oxidant action for 5-MTHF at high concentrations can be suggested, which corroborates other findings of this study, namely the age-related DNA damage associated with erythrocyte 5-MTHF levels and both correlated with the oxidative biomarker PCO in the studied subjects.
Most of the 5-MTHF concentrations used in our treatment are certainly high when compared with the levels of this compound that might be found in human red cells and tissues. They were even higher than the levels found in the elderly and young subjects studied herein. However, it is not possible to determine the actual extent in which the molecules of the compound are incorporated by the worm,57 which could be quite low. Especially in the intermediate concentrations tested, the incorporated amount may have been insufficient to cause toxic pro-oxidant effects.57
The survival rate after acute treatment with 5-MTHF was evaluated in nematodes as an endpoint test that is easily observed under microscopic inspection.53 The nematodes were exposed to seven different concentrations of 5-MTHF and the results showed that there was no significant mortality by any of the tested concentrations. There is no study available in literature evaluating direct effects of 5-MTHF in C. elegans survival. However, a previous study reported that folic acid at 4 and 8 mg L–1 did not affect growth and viability of breast cancer cells measured by the trypan blue exclusion test.9 However, an increased number of apoptotic cells in a non-invasive MCF-7 breast cancer cell line was observed by flow cytometry, whereas invasive MDA-MB-231 cells were not responsive to folic acid as a pro-apoptotic agent.9
Like humans, C. elegans cannot synthesize folate and thus obtains this micronutrient from its diet.22 Thus, live E. coli provides to the nematodes the needed vital micronutrients. There are reports that both limited (insufficient) and excess folate might reduce lifespan.22 Interestingly, Virk et al. (2012) earlier observed that excessive synthesis of folate in E. coli limits lifespan in C. elegans.58 In accordance to literature and the high ROS production induced by 5-MTHF observed here, a shortened lifespan would be expected. However, our results demonstrated that none of the tested concentrations of 5-MTHF affected the life span of C. elegans. A possible explanation to absence of alteration in C. elegans lifespan is that low-level stressors often enhance oxidative stress resistance and increase longevity through a process termed stress-induced hormesis.19
In agreement, a recent work examining the effect of folic acid supplementation on the life span of C. elegans showed that oxidative stress resistance and longevity increased at lower concentrations of folic acid, causing a delay in aging.59 Rathor et al. (2015) showed that pretreatment with 25 μM folic acid increased survival rate and reduced ROS levels after oxidative stress induced by juglone, an oxidant agent.59 Moreover, it is suggested that folic acid inhibits a mechanistic target of rapamycin (mTOR) and insulin/insulin growth factor 1 (IGF-1), signaling pathways to control both oxidative stress levels and life span, as well as enhancing the expression levels of stress- and life span-relevant gerontogenes and oxidative enzymes.59
By the way, that quoted study is the most similar available in the literature to date focusing on direct effects of folate in C. elegans, although the influence of other micronutrients has already been described in this model.60,61 Differing from our study, those authors used folic acid to perform the treatments,59 whereas here we used 5-MTHF, the actual folate active form.
Regarding a longevity assessment, three different concentrations of folic acid (10, 25, and 50 μM) were placed uniformly over the E. coli OP50 food on NGM plates and then the life span experiment was performed.59 The lower doses (10 and 25 μM) of folic acid significantly increased the life span of C. elegans compared to the control, even after eliminating possible interference of E. coli in assays with heat-killed bacteria.59 On the other hand, exposure to higher concentrations of folic acid (250 μM, 500 μM, and 1 mM) drastically reduced the life span of C. elegans.59 However, it is not possible to compare these findings with the present study because the exact amount of 5-MTHF which was made available from these folic acid doses is not known. Accordingly, the higher doses tested by Rathor et al. (2015)59 may correspond to a 5-MTHF concentration far above our highest dose of 8000 μg L–1 (about 16 μM), which may explain our divergent outcomes.
As for limitations of the present study, the relatively small sample size of subjects, low specificity of the comet assay to detect DNA oxidative damage, and complexity of the aging process, which is a multifactorial process and takes place in combination with many other factors, may be highlighted. In this line, it is important in future studies to use more specific markers, such as the urinary excretion of 8-hydroxy-2-deoxyguanosine (8-OHdG), which is one of the predominant free radical-induced oxidative lesions in DNA.62 Another way would be to use our own comet assay in the presence of enzymes endonuclease (Endo III) and formamidopyrimidine-DNA glycosylase (FPG) that recognize oxidized pyrimidines or purines, respectively, converting them to strand breaks.63 On the other hand, the assessment of DNA damage in C. elegans after 5-MTHF supplementation would be helpful for understanding the mechanisms involved in this process.
Conclusions
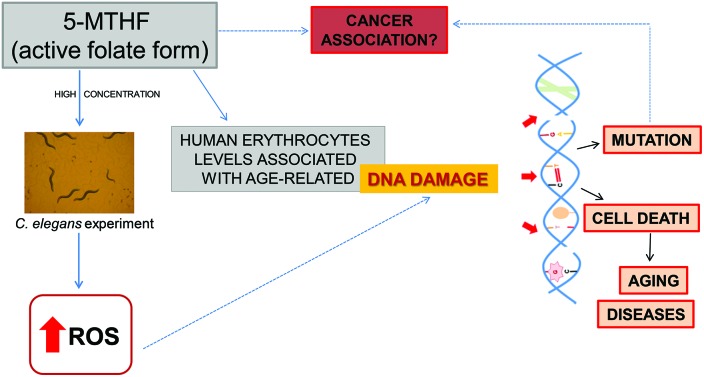
In conclusion, the present findings suggest that 5-MTHF might be involved in DNA damage observed during aging, resulting from oxidative stress. Moreover, this is the first study that assessed the direct effects of 5-MTHF treatment on ROS production in C. elegans, an alternative in vivo model, and proposed a dual profile to this vitamin acting either as antioxidant or pro-oxidant, depending on the concentration. Thus, the results contributed to gaining insights into the physiological effects of this micronutrient, which is the active form of folate. Further studies with 5-MTHF might lead to improving an understanding in relation to the optimal concentration of folate required throughout life to prevent adverse effects, e.g. DNA damage.
Conflict of interest
All authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by Probral CAPES/DAAD (granted to S. C. Garcia; process No. 352/10). M. Baierle is the recipient of a CNPq PhD scholarship (process 146950/2010-0); and S. C. Garcia is the recipient of a CNPq Research Fellowship. The authors would like to thank to Dr Nicolle Breusing for her help in the development of this work.
References
- Sohal R. S., Orr W. C. Free Radicals Biol. Med. 2012;52:539–555. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qabazard B., Li L., Gruber J., Peh M. T., Ng L. F., Kumar S. D., Rose P., Tan C., Dymock B. W., Wei F., Swain S. C., Halliwell B., Sturzenbaum S. R., Moore P. K. Antioxid. Redox Signaling. 2013:1–10. doi: 10.1089/ars.2013.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baierle M., Nascimento S. N., Moro A. M., Brucker N., Freitas F., Gauer B., Durgante J., Bordignon S., Zibetti M., Trentini C. M., Duarte M. M. M. F., Grune T., Breusing N., Garcia S. C. Oxid. Med. Cell. Longevity. 2015;2015:1–12. doi: 10.1155/2015/804198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra R. K. Eur. J. Clin. Nutr. 2012;56:S73–S76. doi: 10.1038/sj.ejcn.1601492. [DOI] [PubMed] [Google Scholar]
- Lucock M., Yates Z., Glanville T., Leeming R., Simpson N., Daskalakis I. Nutr. Res. 2003;23:1463–1475. [Google Scholar]
- Luo W., Li H., Zhang Y., Ang C. Y. W. J. Chromatogr., B: Biomed. Appl. 2002;766:331–337. doi: 10.1016/s0378-4347(01)00521-7. [DOI] [PubMed] [Google Scholar]
- Obeid R., Kirsch S. H., Kasoha M., Eckert R., Herrmann W. Metabolism. 2011;60:673–680. doi: 10.1016/j.metabol.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Shorter K. R., Felder M. R., Vrana P. B. Prog. Biophys. Mol. Biol. 2015;118:14–20. doi: 10.1016/j.pbiomolbio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Lubecka-Pietruszewska K., Kaufman-Szymczyk A., Stefanska B., Fabianowska-Majewska K. Biochem. Biophys. Res. Commun. 2013;430:623–628. doi: 10.1016/j.bbrc.2012.11.103. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gowher H., Jeltsch A. Cell. Mol. Life Sci. 2004;61:2571–2587. doi: 10.1007/s00018-004-4201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias N., Ho N., Butler S., Delaney L., Morrison J., Shahrzad S., Coomber B. L. J. Nutr. Biochem. 2015;26:818–826. doi: 10.1016/j.jnutbio.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Azeem S., Gillani S. W., Siddiqui A., Jandrajupalli S. B., Poh V., Sulaiman S. A. S. Asian Pac. J. Cancer Prev. 2015;16:5389–5396. doi: 10.7314/apjcp.2015.16.13.5389. [DOI] [PubMed] [Google Scholar]
- Ulrich C. M., Potter J. D. J. Am. Med. Assoc. 2007;297:2408–2409. doi: 10.1001/jama.297.21.2408. [DOI] [PubMed] [Google Scholar]
- Mason J. B., Dickstein A., Jacques P. F., Haggarty P., Selhub J., Dallal G., Rosenberg I. H. Cancer Epidemiol., Biomarkers Prev. 2007;16:1325–1329. doi: 10.1158/1055-9965.EPI-07-0329. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Jeon Y. J., Jang M. J., Kim J. O., Chong S. Y., Ko K. H., Hwang S. G., Oh D., Oh J., Kim N. K. Mol. Clin. Oncol. 2015;3:639–648. doi: 10.3892/mco.2015.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa S., Beckman K. B. and Muller F. L., Oxidative Stress in Aging, Humana Press, Totowa, 2008. [Google Scholar]
- Sutphin G. L., Kaeberlein M. J. Visualized Exp. 2009;27:1–7. doi: 10.3791/1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul N., Pietsch K., Stürzenbaum S. R., Menzel R., Steinberg C. E. W. J. Nat. Prod. 2011;74:1713–1720. doi: 10.1021/np200011a. [DOI] [PubMed] [Google Scholar]
- Zhou K. I., Pincus Z., Slack F. J. Aging. 2011;3:1–21. doi: 10.18632/aging.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila D. S., Benedetto A., Au C., Manarin F., Erikson K., Soares F. A., Rocha J. B. T., Aschner M. Free Radicals Biol. Med. 2012;52:1903–1910. doi: 10.1016/j.freeradbiomed.2012.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Mao L., Xing H., Xu L., Fu X., Huang L., Huang D., Pu Z., Li Q. Neurosci. Lett. 2015;3:28–33. doi: 10.1016/j.neulet.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Yilmaz L. S., Walhout A. J. M. Trends Genet. 2014;30:496–503. doi: 10.1016/j.tig.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeming R. J., Pollock A., Melville L. J., Hamon C. G. Metabolism. 1990;39:902–904. doi: 10.1016/0026-0495(90)90298-q. [DOI] [PubMed] [Google Scholar]
- Tice R. R., Agurell E., Anderson D., Burlinson B., Hartmann A., Kobayashi H., Miyamae Y., Rojas E., Ryu J. C., Sasaki Y. F. Environ. Mol. Mutagen. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Singh N. P., McCoy M. T., Tice R. R., Schneider E. L. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Buss H., Chan T. P., Sluis K. B., Domigan N. M., Winterbourn C. C. Free Radicals Biol. Med. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- Stiernagle T., in C. elegans: a Practical Approach, ed. I. A. Hope, Oxford Univ. Press, New York, 1999. [Google Scholar]
- Zortéa K., Silva M. L. B., Soc. Bras. Cardiol., 2010. , 1 , , MCMXLIII Letter to the Editor . [Google Scholar]
- SobczyNska-Malefora A., Harrington D. J., Voong K., Shearer M. J. Adv. Hematol. 2014;2014:1–7. doi: 10.1155/2014/465623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranki S. P., Gebretsadik T., Mitchel E. F., Tylavsky F. A., Hartert T. V., Cooper W. O., Dupont W. D., Dorris S. L., Hartman T. J., Carroll K. N. Epidemiology. 2015;26:1–8. doi: 10.1097/EDE.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. L. Am. Fam. Physician. 2000;62:1565–1572. [PubMed] [Google Scholar]
- Aisen P. S., Schneider L. S., Sano M., Diaz-Arrastia R., van Dyck C. H., Weiner M. F., Bottiglieri T., Jin S., Stokes K. T., Thomas R. G., Thal L. J. J. Am. Med. Assoc. 2008;300:1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaa K. H., Njolstad I., Ueland P. M., Schirmer H., Tverdal A., Steigen T., Wang H., Nordrehaug J. E., Arnesen E., Rasmussen K. N. Engl. J. Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- Selhub J., Jacques P. F., Dallal G., Choumenkovitch S., Rogers G. Food Nutr. Bull. 2008;29:S67–S73. doi: 10.1177/15648265080292S110. [DOI] [PubMed] [Google Scholar]
- Fazili Z., Pfeiffer C. M., Zhang M. Clin. Chem. 2007;53:781–784. doi: 10.1373/clinchem.2006.078451. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C. M., Fazili Z., McCoy L., Zhang M., Gunter E. W. Clin. Chem. 2004;50:423–432. doi: 10.1373/clinchem.2003.026955. [DOI] [PubMed] [Google Scholar]
- Mladinic M., Kopjar N., Milic M., Dasovic A. B., Huzak M., Zeljezic D. Mutagenesis. 2010;25:455–462. doi: 10.1093/mutage/geq027. [DOI] [PubMed] [Google Scholar]
- Humphreys V., Martin R. M., Ratcliffe B., Duthie S., Wood S., Gunnell D., Collins A. R. Age Ageing. 2007;36:521–526. doi: 10.1093/ageing/afm107. [DOI] [PubMed] [Google Scholar]
- Singh K. K., Oxidative Stress, Disease and Cancer, Imperial College Press, London, 2006. [Google Scholar]
- Guachalla L. M., Rudolph K. L. Cell Cycle. 2010;9:4058–4060. doi: 10.4161/cc.9.20.13577. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Hales C. N., Ozanne S. E. Nucleic Acids Res. 2007;35:7417–7428. doi: 10.1093/nar/gkm681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota M. P., Figueiredo P. A., Duarte J. A. Rev. Port. Ciênc. Desp. 2004;4:81–110. [Google Scholar]
- Moro A. M., Brucker N., Charao M., Bulcao R., Freitas F., Baierle M., Nascimento S., Valentini J., Cassini C., Salvador M., Linden R., Thiesen F., Buffon A., Moresco R., Garcia S. C. Mutat. Res. 2012;746:42–48. doi: 10.1016/j.mrgentox.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Garcia S. C., Grotto D., Bulcao R. P., Moro A. M., Roehrs M., Valentini J., Freitas F. A., Paniz C., Bubols G. B., Charão M. F. Drug Chem. Toxicol. 2013;36:306–312. doi: 10.3109/01480545.2012.720989. [DOI] [PubMed] [Google Scholar]
- Weber D., Kneschke N., Grimm S., Bergheim I., Breusing N., Grune T. Free Radical Res. 2012;46:276–285. doi: 10.3109/10715762.2011.652627. [DOI] [PubMed] [Google Scholar]
- Fenech M. Mech. Ageing Dev. 2010;131:236–241. doi: 10.1016/j.mad.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Deghan Manshadi S., Ishiguro L., Sohn K.-J., Medline A., Renlund R., Croxford R., Kim Y. PLoS One. 2014;9:1–10. doi: 10.1371/journal.pone.0084635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbing M., Bonaa K. H., Nygard O., Arnesen E., Ueland P. M., Nordrehaug J. E., Rasmussen K., Njolstad I., Refsum H., Nilsen D. W., Tverdal A., Meyer K., Vollset S. E. J. Am. Med. Assoc. 2009;302:2119–2126. doi: 10.1001/jama.2009.1622. [DOI] [PubMed] [Google Scholar]
- Szyf M., Pakneshan P., Rabbani S. A. Biochem. Pharmacol. 2004;68:1187–1197. doi: 10.1016/j.bcp.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Choi S., Friso S., Keyes M. K., Mason J. B. Br. J. Nutr. 2005;93:31–35. doi: 10.1079/bjn20041283. [DOI] [PubMed] [Google Scholar]
- Rezk B. M., Haenen G. R. M. M., van der Vijgh W. J. F., Bast A. FEBS Lett. 2003;555:601–605. doi: 10.1016/s0014-5793(03)01358-9. [DOI] [PubMed] [Google Scholar]
- Sies H. Exp. Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- Charão M. F., Souto C., Brucker N., Barth A., Jornada D. S., Fagundez D., Avila D. S., Eifler-Lima V. L., Guterres S. S., Pohlmann A. R., Garcia S. C. Int. J. Nanomed. 2015;10:5093–5106. doi: 10.2147/IJN.S84909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Oe T., Blair I. A. Science. 2001;292:2083. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- Deaton C. M., Marlin D. J. Clin. Tech. Equine Pract. 2003;2:278. [Google Scholar]
- Chen Q., Espey M. G., Sun A. Y., Pooput C., Kirk K. L., Krishna M. C., Khosh D. B., Drisko J., Levine M. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surco-Laos F., Cabello J., Gomez-Orte E., Gonzalez-Manzano S., Gonzalez-Paramas A. M., Santos-Buelga C., Duenasa M. Food Funct. 2011;2:445–456. doi: 10.1039/c1fo10049a. [DOI] [PubMed] [Google Scholar]
- Virk B., Correia G., Dixon D. P., Feyst I., Jia J., Oberleitner N., Briggs Z., Hodge E., Edwards R., Ward J., Gems D., Weinkove D. BMC Biol. 2012;10:1–11. doi: 10.1186/1741-7007-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathor L., Akhoon B. A., Pandey S., Srivastava S., Pandey R. Age. 2015;37:1–15. doi: 10.1007/s11357-015-9850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst I. M. A., Pallauf K., Bendall J. K., Paulsen L., Nikolai S., Huebbe P., Roeder T., Rimbach G. Ageing Res. Rev. 2013;12:365–375. doi: 10.1016/j.arr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Chen W., Rezaizadehnajafi L., Wink M. J. Pharm. Pharmacol. 2013;65:682–688. doi: 10.1111/jphp.12023. [DOI] [PubMed] [Google Scholar]
- Göethel G., Brucker N., Moro A. M., Charão M. F., Fracasso R., Barth A., Bubols G., Durgante J., Nascimento S., Baierle M., Saldiva P. H., Garcia S. C. Mutat. Res. 2014;770:61–65. doi: 10.1016/j.mrgentox.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Collins A. R., Duthie S. J., Dobson V. L. Carcinogenesis. 1993;14:1733–1735. doi: 10.1093/carcin/14.9.1733. [DOI] [PubMed] [Google Scholar]