 Carpesii Fructus, the dried fruit of Carpesium abrotanoides L., has been used as a traditional Chinese medicine for centuries to kill intestinal parasites in children.
Carpesii Fructus, the dried fruit of Carpesium abrotanoides L., has been used as a traditional Chinese medicine for centuries to kill intestinal parasites in children.
Abstract
Carpesii Fructus, the dried fruit of Carpesium abrotanoides L., has been used as a traditional Chinese medicine for centuries to kill intestinal parasites in children. It has been recorded as a mildly toxic medicine in the Chinese pharmacopoeia. However, little proof of its toxicology has been reported in modern pharmacology. This study investigated for the first time its developmental toxicity on zebrafish embryos/larvae from 6 to 96 h post-fertilization (hpf). In addition, the enzymes and genes associated with oxidative stress and apoptosis were tested to investigate the potential toxicologic mechanism preliminarily. The observation of toxicologic endpoints showed the developmental toxicity of Carpesii Fructus. Pericardial edema, yolk sac edema, bleeding tendency, and enlarged yolk were the most commonly occurring morphological changes observed in our study. According to the results of acridine orange staining and morphological observation, the developing heart was speculated to be the target organ of toxicity. Furthermore, Carpesii Fructus exposure changed the activities of defense enzymes, increased malondialdehyde (MDA) content, decreased caspase-3 activity, and altered mRNA levels of related genes (ogg1, p53, Cu/Zn-Sod, Mn-Sod, and Cat↓; Gpx↑) in zebrafish larvae, indicating that oxidative stress and additional apoptosis should have roles in the developmental toxicity of Carpesii Fructus. This is the first study that provides proof of modern pharmacology on the teratogenicity and possible toxicologic mechanism of Carpesii Fructus.
Introduction
Carpesii Fructus, the dried fruit of Carpesium abrotanoides L., has been used as a traditional Chinese medicine to kill parasitic intestinal worms in children. This herb is very effective in killing roundworms and tapeworms. The anti-diarrheal, anti-inflammatory, abirritation and antibacterial effects of Carpesii Fructus have been reported in recent years.1 It is worth noting that this herb was recorded as a mildly toxic medicine in the Chinese pharmacopoeia.2 However, there is little proof in modern pharmacology regarding its toxicity. Therefore, to promote its clinical safety, our aim is to perform a toxicologic assay of Carpesii Fructus to discover its potential toxicity, including developmental toxicity.
The zebrafish embryo has become a promising alternative model to screen the developmental toxicity of compounds. The characteristics of the zebrafish, such as its small size, transparency, rapid in vitro development, considerable fecundity, and economic husbandry requirements, make it suitable for the studies of developmental toxicity.3–5 One pair of adult zebrafish commonly produces more than 200 fertilized eggs in each mating. The 2 to 3 mm body length of the larva makes it possible to perform the tests in multi-well plates. In addition, the zebrafish embryo/larvae tests require few samples. More importantly, many previous studies using the zebrafish prove its applicability to screen the potential toxicity of drugs on humans.6 Because of their transparency, the in vitro development process of zebrafish embryos/larvae can be observed easily and directly with a stereomicroscope.7 This feature is much more in line with animal welfare because there is no need for euthanizing the mother fish.8 In addition, zebrafish embryo tests are considered to be in vivo tests, with no pain inflicted on the fish.9
Redox regulation and apoptosis play crucial roles in the developmental process. Their disturbances can lead to deformity and death.10 Apoptosis can occur naturally during embryogenesis or as a result of cell damage or stress. It regulates many developmental processes including morphogenesis, removal of vestigial structures, cell number regulation, and elimination of dangerous cells as the broad functions of cell death during development. These categories illustrate that apoptosis has great influence on the developing embryo. Caspases are crucial mediators of apoptosis. Among them, caspase-3 is a frequently activated death protease. Furthermore, caspase-3 is essential for certain processes associated with the dismantling of the cell and the formation of apoptotic bodies.11 Reactive oxygen species (ROS) are byproducts of normal life activities. However, the disorder of ROS regulation may cause damage to DNA, protein, or lipid. Subsequently, the organelles will be damaged. Thus, exogenous toxicants can alter the redox environment to disrupt development and cause teratogenesis. Published evidence has shown that oxidative stress is a toxicologic mechanism of a drug.10,12,13 To our knowledge, few articles have been published to date pertaining to the relationship between the potential toxicity of Carpesii Fructus and oxidative stress or apoptosis. Therefore, the present article discusses the potential mechanism of Carpesii Fructus-induced toxicity in accordance with the disturbance of redox regulation and apoptosis.
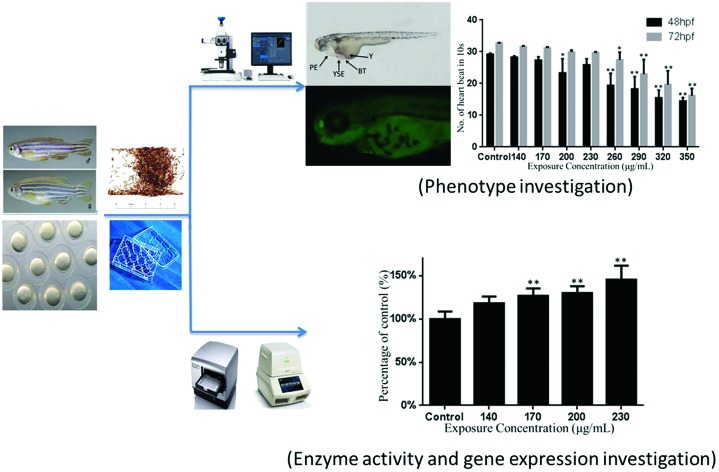
We performed an assay to find the potential developmental toxicity of Carpesii Fructus on the embryo–larval stages of zebrafish. The analyses of mortality, malformations, spontaneous movement, heart rate, hatching rate, and body length were performed to examine the developmental toxicity of Carpesii Fructus. Furthermore, activities of anti-oxidative enzymes, effect on malondialdehyde (MDA) content, and caspase-3 activity were examined. In addition, the gene expression related to antioxidant proteins, the oxidative-DNA repair system, and apoptosis was tested to determine the underlying toxicologic mechanism of Carpesii Fructus preliminarily.
Materials and methods
Chemicals and reagents
Carpesii Fructus was purchased from Beijing Tong Ren Tang Medicinal Materials Co., Ltd (Beijing, China) and authenticated by professor Rui-chao Lin at the Beijing University of Chinese Medicine. Sodium chloride (NaCl), calcium chloride (CaCl2), sodium hydrogen carbonate (NaHCO3), potassium chloride (KCl), potassium dihydrogen phosphate (KH2PO4) and disodium hydrogen phosphate (Na2HPO4) were purchased from Beijing Chemical Reagent Co., Ltd (Beijing, China). All these reagents were of analytical grade. The total superoxide dismutase (T-SOD) assay kit (A001-1), catalase (CAT) assay kit (A007-1), glutathione peroxidase (GPX) assay kit (A005), malondialdehyde (MDA) assay kit (A003-1), and caspase-3 activity assay kit (G015) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Preparation of Carpesii Fructus extracts
Carpesii Fructus was refluxed with 10-fold water for 1 h. Then, filtrates were collected with suction filtering and the residues were refluxed twice as previously stated. The mixed filtrates were vacuum evaporated in a rotary evaporator. Then, they were dried in a 60 °C water bath. Finally, the concentrated solution was vacuum dried at 60 °C in a vacuum drying oven. The extract rate of Carpesii Fructus in this study was calculated as 11.70%. The zebrafish exposure solutions were prepared by dissolving the extracts in fish water (13.675 mmol L–1 NaCl, 0.537 mmol L–1 KCl, 0.025 mmol L–1 Na2HPO4, 0.044 mmol L–1 K2HPO4, 1.3 mmol L–1 CaCl2, 1.0 mmol L–1 MgSO4, and 0.417 mmol L–1 NaHCO3) and were further diluted to the exposure concentrations.
Zebrafish husbandry
Adult AB strain zebrafish were purchased from the China Zebrafish Resource Center (Wuhan, China). In our laboratory, the adult fish were maintained at 28 ± 0.5 °C with a 14 : 10-hour light–dark cycle in an automatic zebrafish housing system (ESEN, China). The system water was prepared by charcoal-filtered tap water supplemented with a salt solution to reach a pH and conductivity range of 6.8 to 7.2 and 450 to 520 μS, respectively. The zebrafish housing system was set to aerate the system water continuously. Meanwhile, it automatically replaced approximately one-third of the water daily to maintain the system water quality. The fish were fed with live brine shrimp three times a day. On the night before spawning, two male and one female zebrafish were separated in a spawning box with an isolation plate. In the morning, the isolation plate was removed as the light went on. After 1 hour, the fertilized eggs were collected and washed three times with fish water. Then, the fertilized eggs were maintained in a light incubator at 28 °C until 6 hours post-fertilization (hpf). The animal experiment protocols were approved by the Animal Ethics Committee of Beijing University of Chinese Medicine according to the requirements of the National Act on the Use of Experimental Animals (Beijing, China).
Teratogenic and lethal assay
The teratogenic and lethal assay tests were performed as described in previous studies with a little change. In brief, fertilized eggs were manually selected under a stereomicroscope (Nikon) at 6 hpf. Next, they were randomly transferred to pre-conditioned 12-well plates (Corning, NY, USA) refilled with either exposure solutions (treatment groups) or fish water (control group) to reach a final volume of 3 mL per well.6 Twenty embryos were incubated per well of 12-well plates. The chemicals could be absorbed by ingestion and transdermal absorption. We planned a range-finding experiment to determine the no adverse effect concentration of the exposure solution and the highest concentration at which all embryos/larvae are killed. Then, eight concentration groups between no adverse effect concentration and 100% lethal concentration were set up to perform the developmental toxicity assay. Finally, 140, 170, 200, 230, 260, 290, 320, and 350 mg L–1 of Carpesii Fructus extracts were selected as the exposure concentrations. The sub-lethal concentration groups (140, 170, 200, and 230 mg L–1) were used to test the activities of enzymes and gene expression. Twenty embryos were used per treatment. Each treatment was repeated three times. Additional plates were set for acridine orange (AO) staining and gene expression analysis, respectively. To ensure the stability of chemical constituents of the exposure solutions, the plates were wrapped in tinfoil. They were placed in an illumination incubator (Yiheng DHP-9082, China) at 28 °C. The exposure solutions were replaced at 24, 48, and 72 hpf, to ensure water quality and the appropriate concentrations of Carpesii Fructus extracts. We checked the abnormal development as per the description in the OECD guidelines and removed the dead embryos/larvae when we replaced exposure solutions.14–16
Antioxidative enzyme assay
At 96 hpf, 60 larvae from the same groups were pooled in a centrifuge tube with 380 μL cold saline (0.65% sodium chloride solution). Then, the larvae were homogenized on ice using a homogenizer (IKA T10 basic ULTRA, Germany). The homogenates were centrifuged at 3500 rpm for 15 min at 4 °C, and the supernatants were collected for various assays.
The activities of total superoxidate dismutase, CAT and GPX were measured according to the manufacturer's recommendations (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). T-SOD activity was assayed using the xanthine/xanthine oxidase method based on the production of O2– anions.17 The CAT activity was measured based on the hydrolysis reaction of hydrogen peroxide (H2O2) with CAT, which could be terminated by molybdenum acid (MA) to produce a yellow MA–H2O2 complex. The CAT activity was calculated by the decrease in absorbance at 405 nm due to the degradation of H2O2.18 The GPX activity was determined by the velocity method using a reduced glutathione (GSH)-Px kit. The change in absorbance during the conversion of GSH to oxidized glutathione (GSSG) was recorded spectrophotometrically at 412 nm.19–21
Lipid peroxidation assay
MDA reacts with thiobarbituric acid (TBA) to produce a colored mixture. The colored mixture can be analyzed by spectrofluorometry to reflect lipid peroxidation.22 In this article, we detected the MDA content with a malondialdehyde assay kit (TBA method). All the procedures were conducted as described in the manufacturer's recommendations (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The level of MDA was presented as nmol mg–1 protein.
Acridine orange staining analysis
Embryo cell apoptosis was identified using AO staining as described in ref. 23. In that study, AO staining was performed at 96 hpf. Although melanin had appeared at this point, the disturbance of apoptosis observation was at an acceptable level for a qualitative analysis. Moreover, the plates were wrapped in tinfoil in our test. Without light stimulation, the melanin released slowly.
In brief, 10 larvae from each group (n = 3) were washed twice in fish water at 96 hpf, then transferred to 5 μg mL–1 of AO dissolved in fish water for 20 minutes at room temperature. The larvae were then washed with fish water three times for 5 minutes each. Finally, after being anesthetized with 0.03% tricaine for 3 minutes, apoptotic cells were observed and photographed under a stereoscopic fluorescence microscope (AXIO Zoom. V16, Zeiss, Germany) equipped with a microscope camera (Axiocam 503 color, Zeiss, Germany). In this study, we set three independent replicate plates of embryos for AO staining analysis.
Caspase activity assay
In this study, a kit was used to test caspase-3 activity as described in the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The tests were repeated three times.
Gene expression analysis
At the end of exposure, 60 embryos from other independent plates were pooled together for gene expression analysis. We used a trizol reagent (Invitrogen, USA) to isolate the total RNA as described in the manufacturer's protocol. Then, we evaluated the RNA quality by the OD260/OD280 ratio and the banding patterns on a 1.2% agarose gel. A reverse transcriptase kit (CWBiotech, Beijing, China) was used for cDNA synthesis. Quantitative real-time polymerase chain reaction (RT-PCR) amplifications were performed on an RT-PCR system (Biorad, CA, USA) using the SYBR green system (Takara, Dalian, China).
In the present study, Cu/Zn superoxide dismutase (Cu/Zn-Sod), manganese superoxide dismutase (Mn-Sod), catalase (Cat), glutathione peroxidase (Gpx), and DNA repair gene (Ogg1) were detected. Activation of the p53 protein, which can lead to a variety of outcomes, including cell cycle arrest and apoptosis, has been known as an effective indicator to show that the cell has entered an apoptotic state.23,24 Therefore, the mRNA level of p53 was also investigated in this study. Table 1 lists the primer pair sequences.
Table 1. The sequences of primer pairs used in RT–PCR.
| Gene | Primer sequence (5′–3′) | Accession number | Ref. |
| Mn-Sod | F: CCGGACTATGTTAAGGCCATCT | AY195857 | 23 |
| R: ACACTCGGTTGCTCTCTTTTCTCT | |||
| Cu/Zn-Sod | F: GTCGTCTGGCTTGTGGAGTG | Y12236 | 23 |
| R: TGTCAGCGGGCTAGTGCTT | |||
| Cat | F: AGGGCAACTGGGATCTTACA | AF170069 | 23 |
| R: TTTATGGGACCAGACCTTGG | |||
| Gpx | F: AGATGTCATTCCTGCACACG | AW232474 | 23 |
| R: AAGGAGAAGCTTCCTCAGCC | |||
| Ogg1 | F: CGGATTGTATTAGCCC | AY355149 | 9 |
| R: AAACCCAAAGATGGAGG | |||
| P53 | F: GGGCAATCAGCGAGCAAA | AB021961 | 9 |
| R: ACTGACCTTCCTGAGTCTCCA | |||
| β-Actin | F: CGAGCAGGAGATGGGAACC | AF057040.1 | 23 |
| R: CAACGGAAACGCTCATTGC |
Statistical analysis
In this study, we used SPSS17.0 (USA) for data processing. Statistical differences were determined by one-way analysis of variance (ANOVA). The values in this study were expressed as mean ± standard deviation (SD). When p was less than 0.05 or 0.01, the values were considered to have a significant difference.
Results
Embryonic development toxicity of Carpesii Fructus
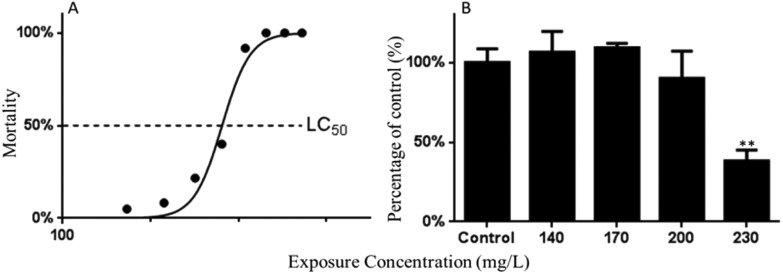
The mortality of embryos/larvae at 96 hpf is shown in Fig. 1A. The LC50 value of Carpesii Fructus to zebrafish embryos was calculated as 230.40 mg L–1. Carpesii Fructus induced a suite of abnormalities in zebrafish embryos. Hatching inhibition, increased spontaneous movement, heartbeat inhibition, pericardial edema, yolk-sac edema, bleeding tendency, yolk malformation, enlarged yolk, and shortened body length were observed in this study (Fig. 2 & 3). The malformations were widespread at 24, 48, 72, and 96 hpf at high concentrations. Pericardial edema, yolk-sac edema, bleeding tendency, and an enlarged yolk were the most commonly occurring morphological changes. Teratogenic and lethal effects were not found in the control group.
Fig. 1. The mortality of embryos or larvae at 96 hpf (A); and the changes in caspase-3 activity (B) in the zebrafish larvae exposed to various concentrations of Carpesii Fructus until 96 hpf. The values are expressed as mean ± SD (n = 3). *Represents p value less than 0.05 and **represents p value less than 0.01.
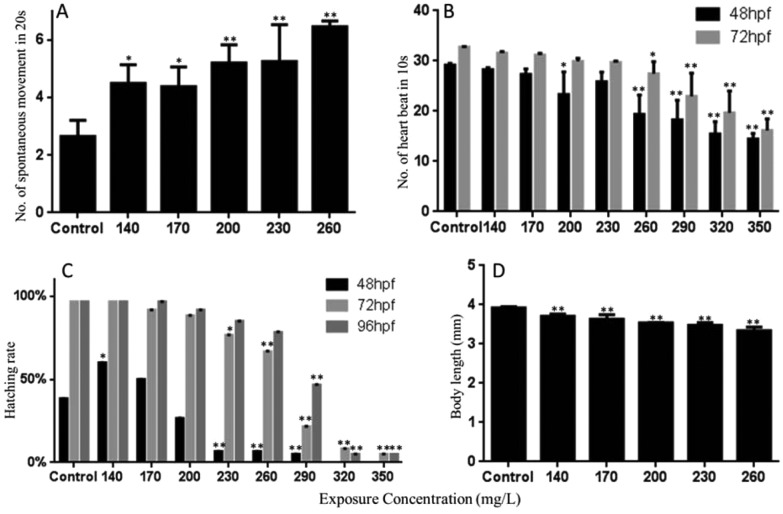
Fig. 2. The spontaneous movement at 24 hpf (A); heartbeat of zebrafish during each observation time (B); hatching rates of zebrafish embryos for different periods (C); body length of the hatched larvae at 96 hpf (D). The values are expressed as mean ± SD (n = 3). *Represents p value less than 0.05 and **represents p value less than 0.01.
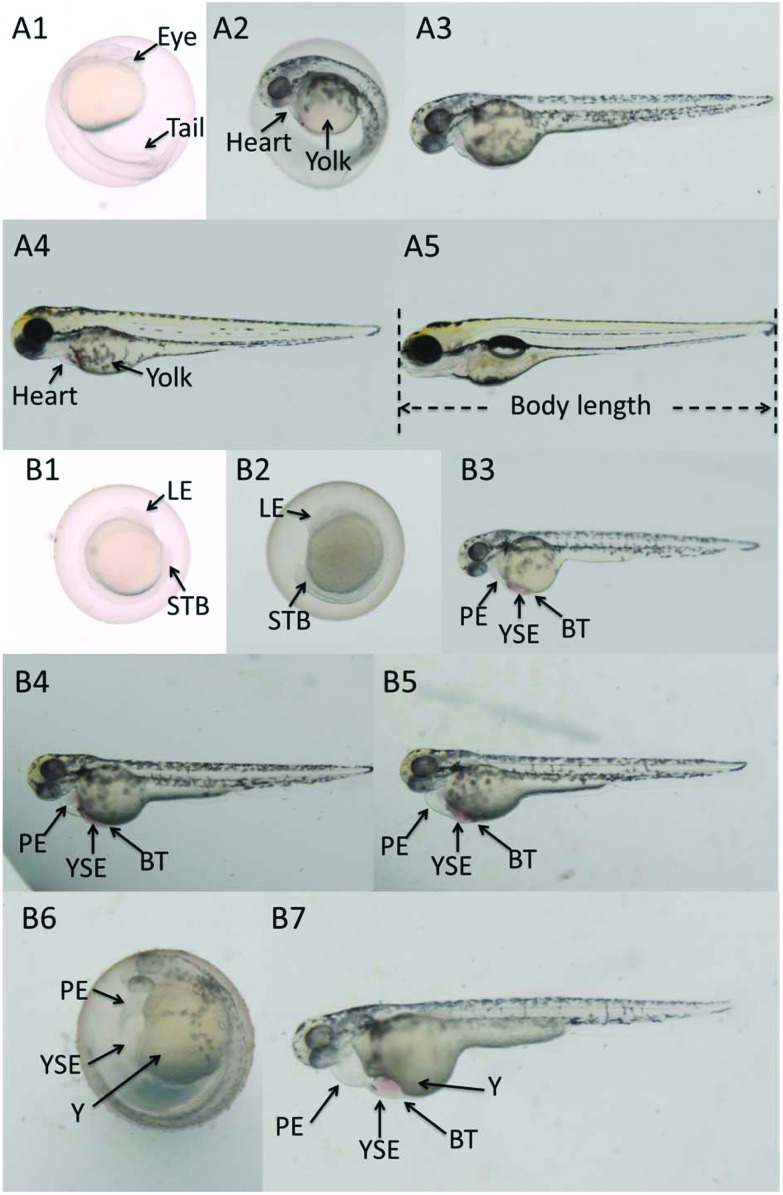
Fig. 3. Microscopy images of zebrafish embryos or larvae at 24, 48, 72, 96 hpf. A1–A5: the normal embryos or larvae in the control group at 24, 48, 48, 72 and 96 hpf. B1–B7: exposed to 320 mg L–1 Carpesii Fructus at 24 hpf (B1, with growth retardation (lack of eyes, short tail bud)); 320 mg L–1 Carpesii Fructus at 48 hpf (B2, with growth retardation (lack of eyes, short tail bud)); 140 mg L–1 Carpesii Fructus at 72 hpf (B3, with pericardial edema, yolk sac edema, bleeding tendency); exposed to 170 mg L–1 Carpesii Fructus at 96 hpf (B4, with pericardial edema, yolk sac edema, bleeding tendency, enlarged yolk); 200 mg L–1 Carpesii Fructus at 96 hpf (B5, with pericardial edema, yolk sac edema, bleeding tendency, enlarged yolk); 200 mg L–1 Carpesii Fructus at 96 hpf (B6, with pericardial edema, yolk sac edema, yolk malformation, big yolk); 230 mg L–1 Carpesii Fructus at 96 hpf (B7, with pericardial edema, yolk sac edema, bleeding tendency, yolk malformation, big yolk). YSE: yolk sac edema; PE: pericardial edema; BT: bleeding tendency; Y: yolk malformation; LE: lack of eyes; STB: short tail bud.
The spontaneous movements (spontaneous contractions characterized by side-to-side contractions of the tail) of embryos at 24 hpf were significantly increased at 140, 170, 200, 230, and 260 mg L–1 of Carpesii Fructus (Fig. 2A).
Heart rate is a crucial endpoint reflecting cardiac developmental toxicity. In this study, the number of heart beats in 10 seconds was recorded at 48 and 72 hpf. Significant inhibitions of heart beat were observed in greater or equal to 200 mg L–1 Carpesii Fructus treated groups at 48 hpf. At 72 hpf, significant heartbeat inhibition was found at concentrations of 260 mg L–1 and greater. As the concentrations increased, the heart beat continued to decrease (Fig. 2B).
The zebrafish larvae usually hatch out from the chorion at 48 to 72 hpf at 28 °C. The rate and time of hatching were sensitive to poison. In the control group of the present study, a few embryos hatched at 48 hpf, and most of them completed hatching at 96 hpf (Fig. 2C). Compared with the control group, the hatching rate at 140 mg L–1 at 48 hpf was significantly increased, which may be caused by a long time severe spontaneous movement. The hatching rate at 230 mg L–1 and greater concentrations was significantly reduced. At 72 hpf, the hatching rates were significantly decreased in a dose-dependent manner. A dose-dependent decrease in hatching rates was also observed at 96 hpf.
Furthermore, a photo of each larva was taken at 96 hpf for its body length measurement. The significant dose-dependent inhibition of body length was found in 140, 170, 200, 230, and 260 mg L–1 of Carpesii Fructus treated groups (Fig. 2D).
Effect of Carpesii Fructus on antioxidant enzymes
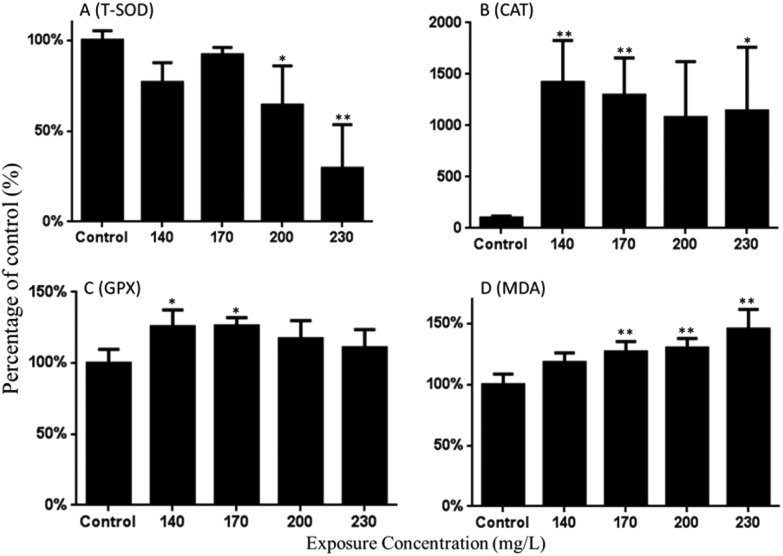
As shown in Fig. 4A, the SOD activity was decreased in this study. Compared with the control group, 0.64- and 0.29-fold decreased activities were found in 200 and 230 mg L–1 of the Carpesii Fructus treated groups. Fig. 4B shows that the CAT activity was significantly increased by 14.20-, 12.95- and 11.42-fold after exposure to 140, 170, and 230 mg L–1 Carpesii Fructus. GPX activities were significantly increased by 1.26-fold relative to the control group after exposure to 140 mg L–1 Carpesii Fructus (Fig. 4C).
Fig. 4. The changes in activities of T-SOD, CAT, GPX and MDA content in the zebrafish larvae exposed to various concentrations of Carpesii Fructus until 96 hpf. The values are expressed as mean ± SD (n = 3). *Represents p value less than 0.05 and **represents p value less than 0.01.
Effect of Carpesii Fructus on lipid peroxidation
As shown in Fig. 4D, the MDA content in zebrafish embryos/larvae was significantly increased in a concentration-dependent manner after exposure to Carpesii Fructus. As the calculation results show, 1.18-, 1.27-, 1.30- and 1.45-fold increased MDA content were detected in 140, 170, 200, and 230 mg L–1 Carpesii Fructus treated groups, compared with the control group.
Additional apoptosis induced by Carpesii Fructus
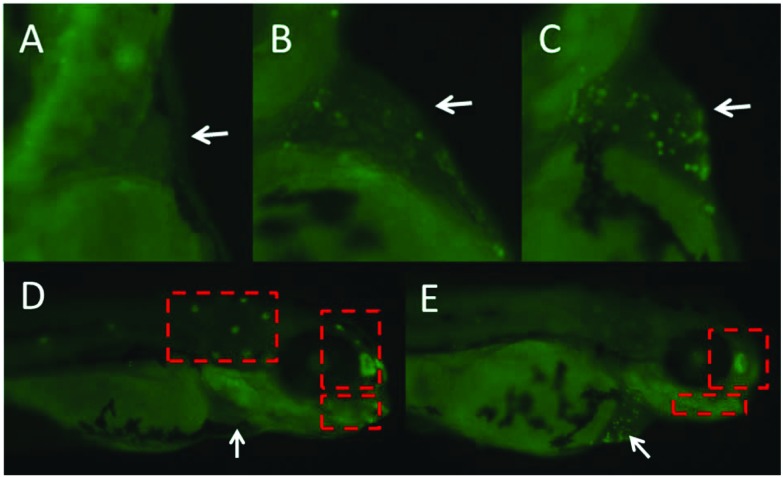
Apoptotic cells were universal in the heart area in the treated groups. To show the appearance of apoptotic cells in the heart area, we chose pictures from the control and treated groups randomly and these are shown in Fig. 5. As shown in Fig. 5, the normal apoptotic cells were observed in the trunk and head area in control larvae, while they were absent in the treated larvae. This could be because of abnormal or slow development induced by Carpesii Fructus. Moreover, under the microscope, we could observe the green fluorescent dots beating in synchronization with the heart beat. So, we can say that Carpesii Fructus induced cardiotoxicity in the embryos/larvae of zebrafish.
Fig. 5. The fish exposed to Carpesii Fructus until 96 hpf were stained with acridine orange (AO). Additional apoptotic cells stained with AO appeared mainly in the heart region (white arrow). Control (A); 140 mg L–1 (B); 170 mg L–1 (C). The normal apoptotic cells were absent in treated larvae (red box): control (D); 170 mg L–1 (E).
Result of caspase-3 activity test
The activity of caspase-3 was detected to confirm Carpesii Fructus induced apoptosis by the caspase pathway. Results showed that a 0.38-fold decreased activity of caspase-3 was detected in the 230 mg L–1 Carpesii Fructus treated group when compared with the control group (Fig. 1B).
Effect of Carpesii Fructus on mRNA expression
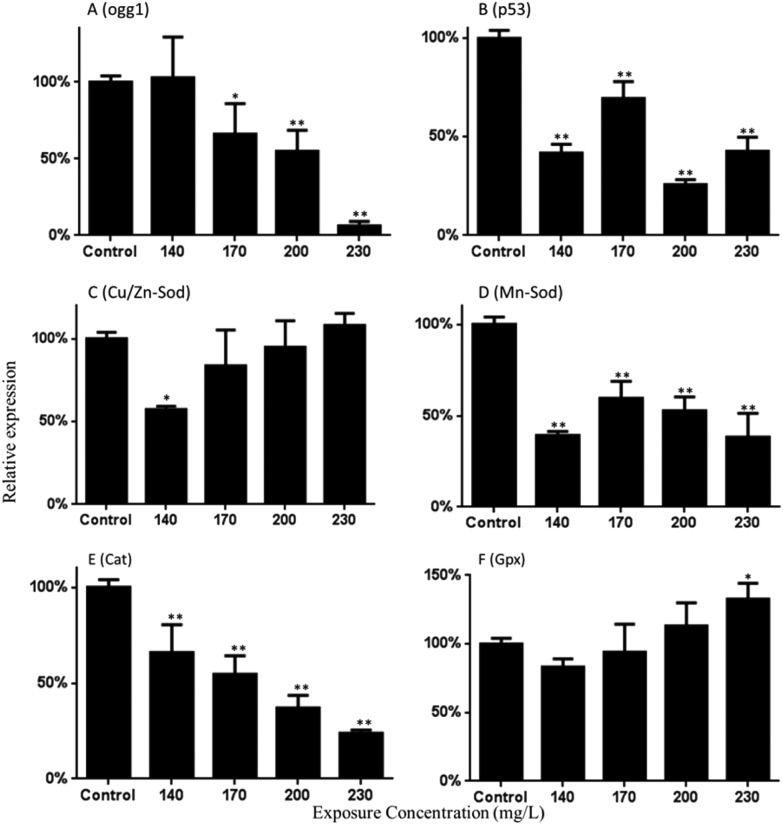
As shown in Fig. 6A, the expression of Ogg1 gene was down-regulated. Approximately 0.66-, 0.55- and 0.06-fold down-regulated activities were found in the 170, 200, and 300 mg L–1 Carpesii Fructus treated groups. Moreover, the results of this study show that the p53 gene was down-regulated 0.42-, 0.70-, 0.26- and 0.43-fold relative to the control group in the 140, 170, 200, and 230 mg L–1 Carpesii Fructus treated groups (Fig. 6B).
Fig. 6. Expression of the mRNA of Ogg1 (A), P53 (B), Cu/Zn-Sod (C), Mn-Sod (D), Cat (E) and Gpx (F) in the zebrafish larvae exposed to various concentrations of Carpesii Fructus until 96 hpf. The values are expressed as mean ± SD (n = 3). *Represents p value less than 0.05 and **represents p value less than 0.01.
The expression of Cu/Zn-Sod was significantly down-regulated. Decreases of approximately 0.57-fold were found in the 140 mg L–1 Carpesii Fructus treated groups (Fig. 6C). Mn-Sod mRNA levels decreased significantly in the 140, 170, 200, and 230 mg L–1 Carpesii Fructus treatment groups, with decreases of approximately 0.39-, 0.60-, 0.53- and 0.38-fold (Fig. 6D). Fig. 6E shows that Carpesii Fructus significantly reduced the mRNA levels of Cat in a dose-dependent manner. Decreases of approximately 0.66-, 0.55-, 0.37- and 0.24-fold were found in the 140, 170, 200, and 230 mg L–1 Carpesii Fructus treated groups. Gpx mRNA levels increased as significantly as 1.33-fold in 230 mg L–1 Carpesii Fructus (Fig. 6F).
Discussion
Carpesii Fructus has been used as a traditional Chinese medicine for centuries to kill intestinal parasites in children. It was recorded as a mildly toxic medicine in the Chinese pharmacopoeia. However, little proof of its toxicology in modern pharmacology has been reported. To promote its clinical safety, we performed a toxicity assay, including developmental toxicity, of Carpesii Fructus to reflect its potential toxicity. Thus far, there are no reports on the toxicologic mechanism of Carpesii Fructus to our knowledge. This is the first study on developmental toxicity of Carpesii Fructus. Considering that redox regulation and apoptosis are essential for physiological development, activities of anti-oxidative enzymes, effect on MDA content, and caspase-3 activity were examined. Furthermore, gene expression patterns related to antioxidant proteins, the oxidative-DNA repair system and apoptosis were also used to elucidate the potential mechanism of Carpesii Fructus-induced toxicity.
In this study, based on the zebrafish lethality curves of the 96 hpf toxicity assay, the LC50 value of Carpesii Fructus to zebrafish embryos was calculated as 230.40 mg L–1. In addition, a suite of developmental abnormalities were observed in the embryonic developmental process after exposure to Carpesii Fructus. Hatching inhibition, abnormal spontaneous movement, depressed heart rates, pericardial edema, yolk sac edema, bleeding tendency, yolk malformation, enlarged yolk, and shortened body length were observed in Carpesii Fructus treated groups. Pericardial edema, yolk sac edema, bleeding tendency, and enlarged yolk were the most commonly occurring morphological changes observed. Enlarged yolk might be caused by liver injury or developmental retardation. These remarkable malformations caused by Carpesii Fructus were reported for the first time.
The balance between endogenous and exogenous ROS can be destroyed by toxic drugs. The unbalanced ROS level usually gives rise to a variety of pathological reactions.25–27 T-SOD, CAT, and GPX are usually used to evaluate the levels of ROS.28,29 In the present study, a significant increase in CAT and GPX activities was found in the serial Carpesii Fructus concentration treated groups. According to these results, we can infer that the organism attempted to eliminate redundant ROS to repair the balance. The down-regulated activities of T-SOD might decrease the capacity of the elimination of ROS. Finally, the MDA content in the treated zebrafish embryos/larvae increased significantly compared to the control group in a dose-dependent manner, indicating that a large amount of lipid peroxidation had occurred. This may generate lethality and diverse malformations in the zebrafish development. At the same time, the mRNA levels of genes encoding antioxidant proteins also had significantly changed in the zebrafish embryos after Carpesii Fructus exposure. The expression of the Gpx was up-regulated. However, expressions of the mRNA of Cu/Zn-Sod, Mn-Sod and Cat were down-regulated. The protein levels always have the same trend of change with the mRNA expression. However, the trends of CAT activities tested by kits and Cat gene expression were opposite in this study. This may be caused by many factors. The level of gene expression is unequal to the activity of the corresponding enzyme. Post-translational protein modification plays a key role in enzyme activation as well.30–32 The dysfunction of enzyme activation may be one of the factors resulting in the opposite trends of CAT activities and Cat gene expression.33–35 In addition, down-regulation of gene expression may be caused by the feedback inhibition from overly high protein activity.36–38 In this study, the overly high CAT activities may give rise to the down-regulation of Cat gene expression by the negative feedback mechanism. Moreover, two triplicate embryos or larvae were used to test CAT activities and Cat gene expression, respectively. The difference of samples may be another factor. However, we have tested in triplicate to reduce the experimental error. The real reasons for this phenomenon will be discussed in further experiments. The expression of Ogg1, an important DNA repair gene to protect against oxidative damage, has also been tested.9,39,40 The result showed that the expression of mRNA of Ogg1 was down-regulated at 170, 200, and 230 mg L–1 of Carpesii Fructus, which might show a decreased repair capacity.
AO staining showed that additional apoptosis appeared primarily in the heart area. The apoptosis cells were observed clearly, and even the melanin had appeared at this point. Furthermore, as described previously, heart rate inhibition and pericardial edema were observed as well. These results implied that the developing heart might be a key developmental toxic target organ of Carpesii Fructus. The heart rate inhibition and pericardial edema might be caused by the additional apoptosis. As a consequence, the developmental cardiotoxicity of Carpesii Fructus might have led to a series of developmental toxicities in zebrafish embryos/larvae. The cell is usually deemed to enter the progress of apoptosis if the p53 protein is activated. Although additional apoptosis appeared in the heart area in this study, a significant decrease of p53 gene expression was detected in the Carpesii Fructus treatment groups. This may be associated with the apoptosis level in the whole body of embryos/larvae. A caspase-way is an important pathway of cell apoptosis and the activity of caspase-3 is considered to be a key marker.41,42 The results of this study showed that the activity of caspase-3 was significantly down-regulated at high concentrations. These results indicate that the apoptosis level of the whole larvae might be decreased, although apoptosis signs were increased in the heart area. However, the role of cardiotoxicity on the upregulation or downregulation of p53 or caspase-3 is unclear according to the present data. Further experiments about the cardiotoxicity and the toxic effect on p53 expression or caspase-3 activity should be performed.
Taken together, this study proved that Carpesii Fructus has developmental toxicity to embryos/larvae of zebrafish. Carpesii Fructus significantly changed the hatching time and rate of embryos and increased malformations. The developing heart is speculated to be the toxic target organ and the cardiotoxicity eventually led to a series of developmental toxicities in zebrafish embryos/larvae. Furthermore, this study demonstrated that Carpesii Fructus exposure altered the activities of defense enzymes (SOD, CAT, and GPX), increased the MDA content, decreased the caspase-3 activity, and altered the mRNA levels of related genes in zebrafish larvae. All these results confirmed the developmental toxicity of Carpesii Fructus. Moreover, oxidative stress and additional apoptosis should be responsible for abnormal development after Carpesii Fructus exposure. In the future, more experiments should be performed to research the mechanism of developmental toxicity of Carpesii Fructus. In addition, the profile of the compounds in the extracts of Carpesii Fructus should be analyzed. Definite components, especially their relevance to toxicity, will be helpful to the quality control of this herb.
Conclusion
In the present study, the developmental toxicity of Carpesii Fructus was investigated on zebrafish embryos/larvae from 6 to 96 hpf. This is the first study on the teratogenicity and possible mechanism of Carpesii Fructus. Our results showed the developmental toxicity of Carpesii Fructus, and the developing heart was speculated as the target organ. Moreover, oxidative stress and additional apoptosis should be responsible for the abnormal development. This is the first study to provide proof of modern pharmacology on the teratogenicity and possible mechanism of Carpesii Fructus.
Conflict of interest
There are no conflicts of interest to declare.
Acknowledgments
This work was supported by the Beijing joint project of science research with postgraduate education–Key technology research and application of safety evaluation of toxic Chinese medicinal materials based on the chemical composition and the characteristics of zebrafish (no. 2050205).
References
- Qin F., He X., Zhang J., Xie E., Chen L. Asia-Pac. Trad. Med. 2008;4:136–137. [Google Scholar]
- Editorial Committee of Chinese Pharmacopoeia, Chinese Pharmacopoeia, China Medical Science Press, Beijing, China, 2015 edn, 2015. [Google Scholar]
- Xu Z., Williams F. E., Liu M. C. J. Appl. Toxicol. 2011;31:157–163. doi: 10.1002/jat.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath P., Li C. Q. Drug Discovery Today. 2008;13:394–401. doi: 10.1016/j.drudis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Strecker R. T., Macrae C. A. Annu. Rev. Pharmacol. Toxicol. 2012;52:433–453. doi: 10.1146/annurev-pharmtox-010611-134751. [DOI] [PubMed] [Google Scholar]
- Peterson R. T., Macrae C. A. Annu. Rev. Pharmacol. Toxicol. 2012;52:433–453. doi: 10.1146/annurev-pharmtox-010611-134751. [DOI] [PubMed] [Google Scholar]
- Tropepe V., Sive H. L. Genes, Brain Behav. 2003;2:268–281. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Lu Z. G., Li M. H., Wang J. S., Wei D. D., Liu Q. W., Kong L. Y. Reprod. Toxicol. 2014;47:33–41. doi: 10.1016/j.reprotox.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Shi X., Gu A., Ji G., Li Y., Di J., Jin J. Chemosphere. 2011;85:1010–1016. doi: 10.1016/j.chemosphere.2011.07.024. [DOI] [PubMed] [Google Scholar]
- Wang X., Martínez M. A., Dai M., Chen D., Ares I., Romero A. Environ. Res. 2016;149:86–104. doi: 10.1016/j.envres.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Jänicke R. U. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Kupsco A., Schlenk D. Int. Rev. Cell Mol. Biol. 2015;317:1–66. doi: 10.1016/bs.ircmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deavall D. G., Martin E. A., Horner J. M., Roberts R. J. Toxicol. 2012;2012:645460. doi: 10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD, OECD guidelines for the testing of chemicals 236–Fish Embryo Acute Toxicity (FET) Test, OECD Observer Organisation for Economic Co Operation & Development, 2013. [Google Scholar]
- Selderslaghs I. W. T., Blust R., Witters H. E. Reprod. Toxicol. 2012;33:142–154. doi: 10.1016/j.reprotox.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Weigt S., Huebler N., Strecker R., Braunbeck T., Broschard T. H. Reprod. Toxicol. 2012;33:133–141. doi: 10.1016/j.reprotox.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Zhang J. Q., Shen M., Zhu C. C., Yu F. X., Liu Z. Q., Ally N. PLoS One. 2014;9:e86589. doi: 10.1371/journal.pone.0086589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. X., Xiao Y., Cao L. L., Yan X., Li C., Shi H. Y. PLoS One. 2013;8:e73380. doi: 10.1371/journal.pone.0073380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Qi H. P., Wei S. Q., Gao X. C., Yu N. N., Hu W. Z., Bi S. Mol. Vision. 2012;18:151–160. [PMC free article] [PubMed] [Google Scholar]
- Li J., Shen F., Guan C., Wang W., Sun X., Fu X. PLoS One. 2014;9:e100685. doi: 10.1371/journal.pone.0100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Zhu L., Mu X., Wang K., Chai T., Yang Y., Qiu L. Environ. Pollut. 2015;203:40–49. doi: 10.1016/j.envpol.2015.03.044. [DOI] [PubMed] [Google Scholar]
- Shen Y., White E. Adv. Cancer Res. 2001;82:55–84. doi: 10.1016/s0065-230x(01)82002-9. [DOI] [PubMed] [Google Scholar]
- Taplin D., Meinking T. L. Arch. Dermatol. 1990;126:213–221. [PubMed] [Google Scholar]
- Kushnareva Y., Murphy A. N., Andreyev A. Biochem. J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G. Int. J. Biochem. Cell Biol. 2002;34:1372–1381. doi: 10.1016/s1357-2725(02)00077-8. [DOI] [PubMed] [Google Scholar]
- Valavanidis A., Vlahogianni T., Dassenakis M., Scoullos M. Ecotoxicol. Environ. Saf. 2006;64:178–189. doi: 10.1016/j.ecoenv.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Zhang X., Xie P., Li D., Tang R., Lei H., Zhao Y. Bull. Environ. Contam. Toxicol. 2009;82:574–578. doi: 10.1007/s00128-009-9671-2. [DOI] [PubMed] [Google Scholar]
- Cesaro L., Pinna L. A., Salvi M. Curr. Genomics. 2015;16:128–138. doi: 10.2174/1389202916666150216221038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold F. Annu. Rev. Biochem. 1981;50:783–814. doi: 10.1146/annurev.bi.50.070181.004031. [DOI] [PubMed] [Google Scholar]
- Wani W. Y., Boyer-Guittaut M., Dodson M., Chatham J., Darley-Usmar V., Zhang J. Lab. Invest. 2015;95:14–25. doi: 10.1038/labinvest.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcu B. MOJ Cell Sci. Rep. 2016;3:00047. [Google Scholar]
- Thompson D. N., Reed D. W., Thompson V. S., Lacey J. A. and Apel W. A., WO, 2015, US8969033.
- Waszczak C. J. Exp. Bot. 2015;66:2923–2934. doi: 10.1093/jxb/erv084. [DOI] [PubMed] [Google Scholar]
- Pershina E. V., Arkhipov V. I. Life Sci. 2016;153:50–54. doi: 10.1016/j.lfs.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Liu X. H., Bauman W. A., Cardozo C. Biochem. Biophys. Res. Commun. 2015;464:208–213. doi: 10.1016/j.bbrc.2015.06.118. [DOI] [PubMed] [Google Scholar]
- Novoa I., Zeng H., Harding H. P., Ron D. J. Cell Biol. 2001;153:1011–1021. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra T. K., Das A., Das S., Choudhury S., Kow Y. W., Roy R. DNA Repair. 2007;6:470–480. doi: 10.1016/j.dnarep.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokan H. E., Standal R., Slupphaug G. Biochem. J. 1997;325:1–16. doi: 10.1042/bj3250001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Yu K., Shi X., Wang J., Lam P. K., Wu R. S. Aquat. Toxicol. 2007;82:135–143. doi: 10.1016/j.aquatox.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Zeng C., Sun H., Xie P., Wang J., Zhang G., Chen N. Aquat. Toxicol. 2014;149:25–32. doi: 10.1016/j.aquatox.2014.01.021. [DOI] [PubMed] [Google Scholar]