 Dioxin and dioxin-like compounds are known as a class of highly toxic and persistent environmental contaminants threatening human and animal health.
Dioxin and dioxin-like compounds are known as a class of highly toxic and persistent environmental contaminants threatening human and animal health.
Abstract
Dioxin and dioxin-like compounds are known as a class of highly toxic and persistent environmental contaminants threatening human and animal health. In the present study, the protective effects of Coenzyme Q10 (CoQ10) and Resveratrol (RSV) against 2,3,7,8-TCDD, 1,2,3,7,8-PeCDD, 1,2,3,7,8,9-HxCDD, and 1,2,3,4,6,7,8,9-OCDD induced acute toxicity and measurement of oxidative stress were studied. Lethal doses of these chemicals were determined. Transheterozigot larvae of Drosophila melanogaster were treated using either dioxins (10 × 10–7 μg mL–1) or dioxins + CoQ10 (10 × 10–7 μg mL–1 + 150 μg mL–1) and dioxins + RSV (10 × 10–7 μg mL–1 + 100 μM). After dioxin treatment, antioxidant combination therapy with dioxins and CoQ10 or dioxins and RSV resulted in indicators of acute toxicity including a decrease in total oxidant status as compared to dioxins alone (p < 0.05). The combination treatment also produced a significant increase in total antioxidant status as compared to dioxins only (p < 0.05). Results indicate a potential role of dioxins for oxidative stress with acute toxicity and the protective performance of CoQ10 and RSV in the overall toxicity of dioxins including measuring oxidative stress.
Introduction
Recently, advancing technology and new production techniques have led to biochemical contamination. These newly produced substances have many toxic effects on the environment and living organisms; toxic dioxin and dioxin-like substances are among the most pervasive and toxic substances.1,2 According to Hutzinger and Fiedler (1989),3 due to having similar chemical structures and effects on living organisms, chemical compounds called dioxin or dioxin-like chemicals are produced by burning certain chemical wastes in ovens, by producing polyvinyl chloride in industry, and from manufacturing certain pesticides or herbicides used on farms and in fruit gardens. Since polychlorinated dibenzo-p-dioxins, a subgroup of halogenated aromatic hydracarbons, are water-insoluble, they are resistant to environmental degradation, difficult to get rid of from the body, highly toxic, and common substances in nature.4
In this study, the protective effect of coenzyme Q10 and resveratrol, natural antioxidants currently under investigation due to their important biological activities, against oxidative stress induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 1,2,3,7,8-pentachlorodibenzo-p-dioxin (PeCDD), 1,2,3,7,8,9-hexachlorodibenzo-p-dioxin (HxCDD), and 1,2,3,4,6,7,8,9-octachlorodibenzo-p-dioxin (OCDD) on transheterozigot larvae of Drosophila melanogaster were investigated.
Materials and methods
Chemicals
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), 1,2,3,7,8-pentachlorodibenzo-p-dioxin (PeCDD), 1,2,3,7,8,9-hexachlorodibenzo-p-dioxin (HxCDD), and 1,2,3,4,6,7,8,9-octachlorodibenzo-p-dioxin (OCDD) were obtained from AccuStandard (USA). Coenzyme Q10 (CoQ10) and Resveratrol (RSV) were obtained from MP Biomedicals (France). Prior to use, all the dioxins were dissolved in 1% dimethyl sulphoxide (DMSO).
Strains
In our study, mwh (mwh/mwh) and flr3 (flr3/In (3LR) TM3 BdS) mutant strains of Drosophila, used in the somatic mutation and recombination test (SMART) to determine genotoxic potentials of the chemicals to which they are exposed, were used. This stock had been maintained for many years in the Laboratory at the Department of Biology of the Atatürk University in Erzurum, Turkey. Therefore, it is highly inbred with little genetic variation. Chemical applications were made with transheterozigote larvae (72 ± 4 h after the beginning of oviposition) of the mutant strains. Imaginal discs of Drosophila were one of the early systems in which the importance of patterned cell division, cell shape changes, and cell rearrangements to morphogenesis was determined. Imaginal disc cells proliferate through larval stages and begin to differentiate at the end of their larval stage and develop into most of the adult body structures during the pupal period.5 For this reason, the third instar larvae were used in our study. Flies and larvae were reared on standard Drosophila medium at 25 ± 1 °C containing agar–agar, maize powder, sugar, yeast, and propionic acid.
Experimental procedures
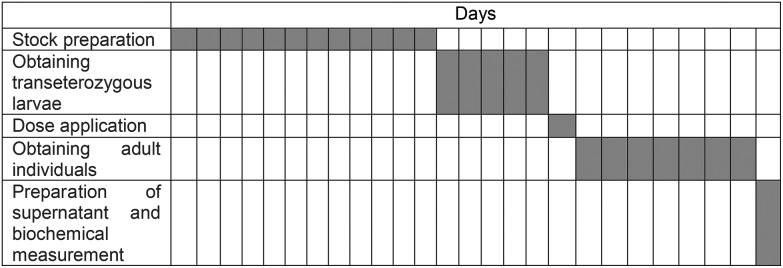
Table 1 provides the timetable of study assessments. Biochemical analyses were carried out to determine whether these possible toxic effects are associated with detoxification of antioxidants systems in male adult (approximately 500 male individuals for each group) subjects of Drosophila melanogaster due to using growing concentrations of dioxins, identified as genotoxic profiles in our preliminary study, and on transheterozigot larvae. All experiments were performed in compliance with relevant laws and institutional guidelines, and also state institutional committee(s) that have approved these experiments. First, lethal doses of the dioxins were determined to be 15 × 10–7 μg mL–1. In accordance with this data, two separate sets of experiments were prepared as dioxins and dioxins + antioxidant application groups. Experimental groups were set as follows: 10 × 10–7 μg mL–1 for all dioxin groups, 150 μg mL–1 for coenzyme Q10 and 100 μM for resveratrol. The transheterozigote larvae exposed to chemicals remained in the vials until emergence of surviving adult flies. For extraction, adult male flies within two or three hours after eclosion of pupa were collected, washed and dechorionated, and immediately homogenized in cold 0.1 M phosphate buffer (pH 7.4) containing 0.15 M KCl. The homogenate was filtered and centrifuged at 20 000 rpm for 10 min at 0 °C. Supernatants were used for biochemical analysis.
Table 1. Experimental schedule.
|
Measurement of total oxidant status (TOS) and total antioxidant status (TAS) of tissues
Total oxidant and total antioxidant status of tissues were measured using a novel automated colorimetric measurement method for TOS and TAS developed by Erel.6 Both parameters were measured according to the manufacturer's protocol (TOS and TAS Assay Kits) (Rel Assay Diagnostics, Turkey).
Determination of oxidative stress index (OSI)
The ratio of TOS level to TAS was accepted as the oxidative stress index (OSI).7
Statistical analysis
Results were presented as mean ± standard deviation (SD) or percentages, when applicable. To be able to determine the statistical significance of the results, Duncan's one-way range test was applied. Data were analyzed using SPSS (ver. 15 for Windows™) software.
Results
Total oxidant status (TOS), total antioxidant status (TAS) and oxidative stress index (OSI) values of adult D. melanogaster were examined to determine oxidative parameters by following the dioxins (TCDD + CoQ10, PeCDD + CoQ10, HxCDD + CoQ10 and OCDD + CoQ10) and dioxins + antioxidant (TCDD + RSV, PeCDD + RSV, HxCDD + RSV, and OCDD + RSV) applications to the transheterozigot larvae. We determined that in all dioxin treated groups, the TOS value was higher and TAS value was lower than in the control groups (Table 2). Measured levels of TOS and TAS in dioxin applied groups were statistically different from the control group (p < 0.05). Furthermore, the high value of OSI, obtained by dividing the total oxidant status to total antioxidant status, indicated the presence of toxic effects (Table 2).
Table 2. Oxidative parameters data obtained from D. melanogaster (mwhxflr3) for experimental groups with the dioxins and dioxins + antioxidant.
| Experimental groups |
TOS (μmol H2O2 equiv. L–1) | TAS (mmol trolox eqvui. L–1) | OSI (AU) | |
| Control groups | Distilled water | 6.37 ± 0.01ab | 0.98 ± 0.10f | 6.64 ± 0.70bc |
| DMSO | 7.61 ± 0.16b | 0.93 ± 0.12ef | 8.42 ± 0.94c | |
| CoQ10 | 4.13 ± 0.60a | 1.83 ± 0.08h | 2.23 ± 0.22a | |
| RSV | 5.32 ± 0.62a | 1.56 ± 0.10g | 3.38 ± 0.16ab | |
| Application groups | TCDD | 28.81 ± 1.62g | 0.34 ± 0.00a | 84.62 ± 3.35l |
| PeCDD | 27.18 ± 1.55fg | 0.36 ± 0.04a | 76.47 ± 4.35k | |
| HxCDD | 25.63 ± 1.17f | 0.47 ± 0.02ab | 54.63 ± 0.85j | |
| OCDD | 25.01 ± 1.21f | 0.60 ± 0.05bc | 42.07 ± 2.04i | |
| TCDD + CoQ10 | 16.87 ± 1.55de | 0.68 ± 0.07bcd | 24.91 ± 0.46gh | |
| PeCDD + CoQ10 | 15.04 ± 1.68cde | 0.72 ± 0.04cde | 20.75 ± 1.00efg | |
| HxCDD + CoQ10 | 14.11 ± 0.64cd | 0.74 ± 0.04cde | 19.08 ± 0.17def | |
| OCDD + CoQ10 | 12.77 ± 0.99c | 0.86 ± 0.06def | 14.83 ± 0.06d | |
| TCDD + RSV | 17.81 ± 0.61e | 0.64 ± 0.04bcd | 27.98 ± 1.06h | |
| PeCDD + RSV | 15.42 ± 1.28cde | 0.69 ± 0.07cd | 22.47 ± 0.58fg | |
| HxCDD + RSV | 14.63 ± 1.00cde | 0.71 ± 0.05cde | 20.61 ± 0.09efg | |
| OCDD + RSV | 13.98 ± 0.67cd | 0.80 ± 0.02cdef | 17.45 ± 0.34de | |
The levels of TAS were increasing while the amounts of TOS were decreasing when we compared RSV and CoQ10 with only the dioxin groups and these differences were statistically important (p < 0.05). In the dioxins + antioxidant applied groups that used the exogenous antioxidant source, like RSV and CoQ10, the levels of TAS increased while TOS levels decreased, whereas with dioxins only applied groups the results were vice versa and also those differences were statistically significant (p < 0.05) (Table 2). However, there were no significant differences between the dioxins + CoQ10 and dioxins + RSV application groups according to TOS, TAS, and OSI values (p > 0.05) (Table 2).
Discussion
In the literature, genotoxic effects were not observed after in vivo and in vitro exposure to dioxin but DNA damage was observed with secondary effects caused by oxidative stress.8 As a result of an increase in the synthesis of P450 enzymes by dioxins, molecular oxygen transport increases and this leads to excessive formation of reactive oxygen species and lipid peroxidation.8 In TCDD treated rodents, increased oxidative stress due to increased lipid peroxidation and superoxide generation and also single-stranded DNA fracture was determined.9,10
Protective and healing effects of endogenous and exogenous antioxidants on oxidative stress caused by dioxins and other toxic effects, plus many studies on the antioxidant effects of CoQ10 and RSV against various xenobiotics are also available. Antioxidant therapy has protective effects against a variety of toxic effects of dioxins such as TCDD.11,12 Vitamin A, vitamin C, carotenoids, and ellagic acid, a natural plant phenolic compound, have also been reported to be protective against oxidative effects of TCDD.13 In a study that investigated the protective role of lycopene on the effects of various doses of TCDD on oxidant–antioxidant systems of rats, the protective role of lycopene on liver microsomal toxicity was found by reducing lipid peroxidation caused by TCDD.14
In a study showing the effects of antioxidant ASTA on rat liver cells viability induced by TCDD, ASTA decreased the toxic effect of TCDD to the minimum on liver cells by decreasing the TOS, increasing the TAS, and also caused a decrease in the rate of micronucleus.15 In fact, numerous studies have demonstrated the protective ability of RSV and CoQ10 to scavenge free radicals.16 In a study in which CoQ10 (150 mg per kg per day) was added to the diet of rats for 4–13 weeks, CoQ10 formed a protective effect against oxidative stress by increasing potential antioxidants in liver, kidney, heart, skeletal muscle, and brain tissue of the rats.17 In a study conducted on rats using an antioxidant antiinflammatory neuroprotective drug that is used for Parkinson disease treatment (and containing the CoQ10 inside), it was shown that CoQ10 has a strong protective effect against the disease by preventing oxidative stress without causing any change in dopamine levels and number of neurons.18
Tomasetti et al. (1999)19 suggested that by using the DNA damage Comet test, DNA damage in human being's lymphocytes caused by H2O2-induced oxidation, showed that CoQ10 was not directly involved in DNA damage repair, but prevented oxidative stress. Also, the protective role of endogenous and exogenous CoQ10 was shown with oxidative stress and tissue damage in brain tissue of Parkison's patients.20
In another study of resveratrol, where vitamins C and/or E were added to the cell culture medium during oxidative stress, it was shown that the combination of resveratrol and vitamins C and/or E were more effective in protecting cells than any of these three antioxidants alone.21 Also, Orallo et al. (2002)22 demonstrated that trans-resveratrol could play an important role in the cardioprotective effect in rat aortas caused by inhibition of vascular NADH/NADPH oxidase.
In another study, the antiproliferative activity of resveratrol on a panel of cell lines of various histogenetic origins (including normal rat fibroblasts and mouse mammary epithelial cells compared to human breast, colon, and prostate cancer cells) was evaluated. It was found that resveratrol is able to prevent an increase in reactive oxygen species (ROS) following exposure to oxidative agents.23
Dutra et al. (2009)24 investigated the effects of trans-resveratrol on energetic metabolism in adult D. melanogaster. Both sexes of flies treated with 1 mM of trans-resveratrol or DMSO showed different values for cholesterol, glycogen, and total protein as compared to control flies. These results indicate that both treatments can modulate energy metabolism and lipoperoxidation in this strain of D. melanogaster in both sexes. In other studies of the effects of resveratrol on D. melanogaster, positive effects on lifespan of flies were reported.25,26
In conclusion, CoQ10 and RSV prevent dioxins-induced toxicity by increasing inhibition of lipid peroxidation and antioxidant status in D. melanogaster. The protective influence of CoQ10 and RSV was partly associated with its free radical scavenging and antioxidant actions. Also, antioxidants taken from outside sources considerably increase the total antioxidant activity of endogenous antioxidants, showing the support property of an antioxidant defense system.
Conflict of interest
There are no conflicts of interest to declare.
Acknowledgments
This study was supported by the Atatürk University Research Foundation (Project Code: 2009/79). The authors would like to thank Atatürk University for financial support for the project.
References
- Schwarz M., Appel K. E., Regul. Toxicol. Pharmacol., 2005, 43 , 19 –34 , . [PubMed: 16054739] . [DOI] [PubMed] [Google Scholar]
- Lee V. K., Cheung W. H., McKay G., Chemosphere, 2008, 704 , 682 –688 , . [PubMed: 17706744] . [DOI] [PubMed] [Google Scholar]
- Hutzinger O., Fiedler H. Chemosphere. 1989;18:23–32. doi: 10.1016/0045-6535(89)90102-1. [DOI] [Google Scholar]
- Diaz-Ferrero J., Rodriguez-Larena M. C., Cornellas L., Jimenez B. TrAC, Trends Anal. Chem. 1997;16(10):563–573. doi: 10.1016/S0165-9936(97)00058-7. [DOI] [Google Scholar]
- Cohen S. M., Imaginal disc development, in The development of Drosophila melanogaster, ed. M. Bate and A. Martinez Arias, Cold Spring Harbor Laboratory Press, Plainview, 1993, pp. 747–841. [Google Scholar]
- Erel O., Clin. Biochem., 2005, 38 , 1103 –1111 , . [PubMed: 16214125] . [DOI] [PubMed] [Google Scholar]
- Demirbag R., Gur M., Yilmaz R., Int. J. Cardiol., 2007, 116 , 14 –19 , . [PubMed: 16824626] . [DOI] [PubMed] [Google Scholar]
- Kern P. A., Fishman R. B., Song W., Brown A., Fonseca V., Toxicology, 2002, 1712–3 , 117 –125 , . [PubMed: 11836018] . [DOI] [PubMed] [Google Scholar]
- Wahba Z. Z., Lawson T. A., Stohs S. J., Cancer Lett., 1988, 39 , 281 –286 , . [PubMed: 3359422] . [DOI] [PubMed] [Google Scholar]
- Bagchi M., Stohs S. J. Free Radicals Biol. Med. 1993;14(1):11–18. doi: 10.1016/0891-5849(93)90504-N. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C., Mathur P. P., J. Appl. Toxicol., 2002, 225 , 345 –351 , . [PubMed: 12355564] . [DOI] [PubMed] [Google Scholar]
- Alsharif N. Z., Hassoun E. A., Basic Clin. Pharmacol. Toxicol., 2004, 953 , 131 –138 , . [PubMed: 15447737] . [DOI] [PubMed] [Google Scholar]
- Hassoun E. A., Al-Ghafr M., Abushaban A., Free Radicals Biol. Med., 2003, 35 , 1028 –1036 , . [PubMed: 14572606] . [DOI] [PubMed] [Google Scholar]
- Aly H. A., El-Shitany N. A., El-Beshbishy H. A., Ashour O. M., Toxicol. Ind. Health, 2015, 3110 , 938 –950 , . [PubMed: 23572394] . [DOI] [PubMed] [Google Scholar]
- Turkez H., Geyikoglu F., Yousef M. I., Toxicol. Ind. Health, 2014, 302 , 101 –112 , . [PubMed: 22778115] . [DOI] [PubMed] [Google Scholar]
- de la Lastra C. A., Villegas I., Biochem. Soc. Trans., 2007, 35 , 1156 –1160 , . [PubMed: 17956300] . [DOI] [PubMed] [Google Scholar]
- Kwong L. K., Kamzalov S., Rebrin I., Free Radicals Biol. Med., 2002, 335 , 627 –638 , . [PubMed: 12208349] . [DOI] [PubMed] [Google Scholar]
- Faust K., Gehrke S., Yang Y., Yang L., Beal M. F., Lu B., BMC Neurosci., 2009, 10 , 109 , . [PubMed: 19723328] . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti M., Littarru G. P., Stocker R., Alleva R., Free Radicals Biol. Med., 1999, 279–10 , 1027 –1032 , . [PubMed: 10569635] . [DOI] [PubMed] [Google Scholar]
- Ebadi M., Govitrapong P., Sharma S., Biol. Signals Recept., 2001, 103–4 , 224 –253 , . [PubMed: 11351130] . [DOI] [PubMed] [Google Scholar]
- Chanvitayapongs S., Draczynska-Lusiak B., Sun A. Y., NeuroReport, 1997, 86 , 1499 –1402 , . [PubMed: 9172162] . [DOI] [PubMed] [Google Scholar]
- Orallo F., Alvarez E., Camiña M., Leiro J. M., Gómez E., Fernández P., Mol. Pharmacol., 2002, 612 , 294 –202 , . [PubMed: 11809853] . [DOI] [PubMed] [Google Scholar]
- Sgambato A., Ardito R., Faraglia B., Boninsegna A., Wolf F. I., Cittadini A., Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2001, 4961–2 , 171 –180 , . [PubMed: 11551493] . [DOI] [PubMed] [Google Scholar]
- Dutra B. K., Fernandes F. A., Luis da Cunha G., Oliveira G. T. Rev. Bras. Biocienc. 2009;7(4):387–394. [Google Scholar]
- Bauer J. H., Goupil S., Garber G. B., Helfand S. L., Proc. Natl. Acad. Sci. U. S. A., 2004, 10135 , 12980 –12985 , . [PubMed: 15328413] . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass T. M., Weinkove D., Houthoofd K., Gems D., Partridge L., Mech. Ageing Dev., 2007, 12810 , 546 –552 , . [PubMed: 17875315] . [DOI] [PubMed] [Google Scholar]


