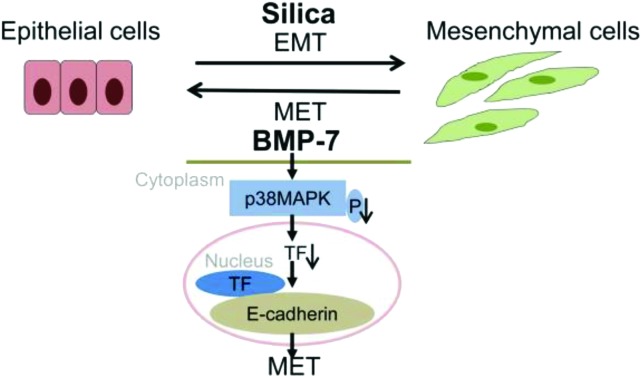
 Silica induced EMT and decreased the expression of BMP-7 in vivo and in vitro, while exogenous BMP-7 promoted MET and inhibited silica-induced EMT associated with inhibition of the p38 MAPK/transcription factor (TF) signaling pathway in RLE-6TN cells.
Silica induced EMT and decreased the expression of BMP-7 in vivo and in vitro, while exogenous BMP-7 promoted MET and inhibited silica-induced EMT associated with inhibition of the p38 MAPK/transcription factor (TF) signaling pathway in RLE-6TN cells.
Abstract
Bone morphogenetic protein-7 (BMP-7) plays an important role in the epithelial-mesenchymal transition (EMT) process, and has been identified as the most potent factor that can promote mesenchymal-epithelial transition (MET) and reduce organ fibrosis. Here we examined the important role of BMP-7 in silica-induced EMT and investigated the relationship between BMP-7 and the balance of EMT/MET. We found that silica induced EMT and decreased the expression of BMP-7 in vivo and in vitro, while silica activated the p38 MAPK/transcription factor signaling pathway in RLE-6TN cells. Lentivirus mediated transfection was used to stably upregulate the expression of BMP-7. Exogenous BMP-7 brought on MET exceeded silica-induced EMT and restrained the p38 MAPK/transcription factor signaling pathway in RLE-6TN cells. Our results revealed that BMP-7 promoted MET above EMT induced by silica associated with inhibition of the p38 MAPK/transcription factor signaling pathway, and BMP-7 was a potential target for treatment of silicosis fibrosis.
1. Introduction
Silicosis is a serious occupational disease caused by long-term inhalation of silica. People in occupations like tunneling, silica flour milling, ceramic making, sandblasting, and so on are predisposed to develop silicosis. However, exposure to silica is very common. Millions of people are occupationally exposed to silica and silicosis remains a global threat for which no specific therapy is available.1 A better understanding of the pathogenesis of silicosis is critical for the identification of new therapeutic targets.
Pulmonary fibrosis is the main pathological characteristic of silicosis. Pulmonary fibrosis is characterized by proliferation of fibroblasts and accumulation of an extracellular matrix (ECM) with subsequent scar formation resulting in impaired lung function.2 Fibroblasts play a central role in the pathogenesis of pulmonary fibrosis, and their origin has become the subject of intense investigation. There are two known origins of fibroblasts: proliferation of resident lung interstitial fibroblasts and differentiation of progenitor cells from bone marrow. But more recently, evidence suggests that injured epithelial cells may directly serve as a source of fibroblasts.3 This process has been named the epithelial-to-mesenchymal transition (EMT). EMT is a process by which fully differentiated epithelial cells can undergo transition to a mesenchymal phenotype. This transition is defined by the acquisition of a mesenchymal phenotype including vimentin and α-smooth muscle actin (α-SMA) and the loss of epithelial characteristics such as E-cadherin. EMT converts adherent, cobblestone like epithelial cells into motile mesenchymal cells with enhanced migratory capacity, invasiveness and greatly increased production of ECM components.4 Mesenchymal-epithelial transition (MET) is the reverse process of EMT.5 Many studies have shown that the balance of EMT/MET is closely associated with fibrosis diseases. When EMT is above MET, the damaged tissue would develop into fibrosis; however, if MET is above EMT, normal epithelial tissue grows, and fibrosis is reversed. MET has been represented as a significant concept in inhibiting fibrosis diseases.
The transforming growth factor-β (TGF-β) family regulates varieties of biological processes, including cell differentiation, proliferation and apoptosis. TGF-β can induce EMT in epithelial cells through various signal pathways.6 Bone morphogenetic protein-7 (BMP-7), an antagonist of TGF-β signaling, is known to be involved in the MET.7 BMP-7 has been identified as an endogenous antagonist of TGF-β mediated EMT in the kidney and other organs. Administration of recombinant BMP-7 in mice with renal fibrosis resulted in reversal of EMT and repopulation of healthy tubular epithelial cells with functional recovery.8 Our previous study has also shown that BMP-7 could reduce silicosis fibrosis in rats.9 The manipulation of impaired BMP signaling by the addition of BMP-7 has already been proven to be an effective anti-fibrotic approach in experimental models of liver, heart, and lung fibrosis.10
p38 mitogen-activated protein kinase (MAPK) is a member of the MAPK family and is essential for the regulation of many cellular processes, including inflammation, cell differentiation, cell growth and cell death. p38 MAPK mediates the signals that are relevant to the development of organ fibrosis. It is thought that p38 MAPK is a signal transducer in the underlying diabetic nephropathy pathways, and it has been proposed that the agents that inhibit the p38 MAPK signaling pathway may reduce the formation of the ECM in kidney fibrosis.11 There is also enough evidence that the activation of p38 MAPK is required in TGF-β-induced EMT in mammary epithelial cells.12
The alveolar type II (AT II) epithelial cell was regarded historically as a key target cell in initial injury by inhaled particles. However, the molecular basis for silica-induced alveolar epithelial cell injury is not fully understood. In addition, p38 MAPK was believed to be an effector of BMP-7 in various physiological processes in vertebrates.13,14 We hypothesized that silica induced EMT in vivo and AT II epithelial cells, and we decided to focus on the role of BMP-7 in silica-induced EMT and explore the relationship between BMP-7 and the balance of EMT/MET. The aim of the study is to further evaluate whether BMP-7 could be used as an MET promoter in silicosis and a new therapeutic approach for improving silicosis treatment.
2. Methods
2.1. Silica
The crystalline silica was provided by the Center of Occupational Health and Poisoning Control, Chinese Center for Diseases Control and Prevention. About 95% of the particle diameter was below 5 μm and the silica content was >99%. The silica samples were weighed and suspended in saline to a concentration of 50 mg ml–1 for animal experiments. The silica for cell experiments was suspended in phosphate-buffered saline (PBS) to a concentration of 2 mg ml–1. Then they were autoclaved to be sterilized and briefly shaken to assure dispersion of particles before use. The samples were then diluted to 12.5–100 μg ml–1 before they settled in vitro.
2.2. Animals and experimental design
All animal experiments were approved by the Laboratory Animal Care and Use Committee at Capital Medical University, and performed according to ethical guidelines of the 1975 Declaration of Helsinki. A total of 24 adult male Wistar rats (200–240 g, SPF grade) were purchased from the Vital River Laboratory Animal Technology Co. Ltd (Beijing, China). They were fed with a commercial rat chow and water. Rats were divided into control group (n = 12) and silica group (n = 12). They were anesthetized with ether and then administered intratracheally with 1 ml of saline (control group) or silica suspension (silica group). The lungs were harvested for further examination on day 15 or day 30 after instillation.
2.3. Pathological examination and immune-histochemical staining
The left lung was fixed in 4% paraformaldehyde for 48 h, embedded in paraffin and cut into 5 μm thick sections by following standard procedures. They were stained with hematoxylin and eosin (H&E) and Masson trichrome staining. Immunohistochemical staining was performed on paraffin-embedded tissue sections. Slides were incubated with BMP-7 (1 : 100 dilutions) for 1 h followed by using a DAB Envision System according to instructions from the manufacturer. A light microscope (Olympus D72, Japan) was used to examine slides with ×200 magnifications.
2.4. Cell culture and treatment
RAW264.7, mouse peritoneal macrophages, was obtained from the Cell Bank of Xiang Ya School of Medicine (Huna, China); RLE-6TN, rat AT II epithelial cells, was also from the Cell Bank of Xiang Ya School of Medicine. The cells were routinely maintained in DMEM which contains 10% fetal bovine serum, 100 IU per ml penicillin and 100 μg per ml streptomycin (purchased from KeyGEN, Nanjing, China) at 37 °C with 5% CO2 in air.
When RAW264.7 cells were grown to 70–80% confluence, culture medium was replaced by free-serum medium for 24 h. Then the cells were treated with different concentrations of silica for 24 h. The supernatant was harvested and filtered through a 0.22 μm microporous membrane for future experiments.
2.5. Reagents and antibodies
DMEM were from HyClone (Beijing, China) and bovine serum albumin (BSA) from Glenview (St Louis, USA), and fetal bovine serum (FBS) from Gibco (MD, USA). Mouse anti-α-SMA antibody was purchased from Abcam (Cambridge, UK), rabbit anti-E-cadherin antibody, anti-ZEB-2 antibody, and anti-Twist antibody from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-BMP-7, anti-vimentin, anti-Slug, anti-GAPDH, anti-p38 MAPK and anti-phosphorylation-p38 MAPK (P-p38MAPK) antibodies were purchased from Cell Signaling Technology Inc (Beverly, MA, USA). Anti-ZEB-1 antibody and anti-Snail antibody were from Abgent (San Diego, USA).
2.6. Western blot analysis
Equal amounts of protein (40 μg) were separated by 10% SDS-PAGE gel electrophoresis and transferred onto a PVDF membrane (Millipore, Billerica, MA, USA). Nonspecific binding was blocked with 5% BSA in TBST at room temperature for 1 h. The membranes were then incubated at 4 °C overnight with the primary antibodies. After three washes with TBST, the membranes were incubated with HRP-conjugated goat anti-rabbit or goat anti-mouse (Cell Signaling Technology, Beverly, MA, USA) for 1 h at room temperature and then washed, followed by enhanced chemiluminescence detection according to the manufacturer's protocol (Thermo, Rockford, USA). All blots were also immunoblotted for GAPDH to demonstrate equal loadings of protein samples. All western blots were repeated at least 3 times.
2.7. Quantitative real-time RT-PCR
Total RNA was extracted from cells (2.0 × 106) using a SV Total Isolation System, following the manufacturer's instructions (Promega, Madison, WI, USA). Aliquots of RNA were reverse transcribed to cDNA using the TransScript First-Strand cDNA Synthesis SuperMix (Transgen biotech, Beijing, China). The primers used for SYBR Green quantitative real-time PCR (qRT-PCR) were as follows: BMP-7 sense sequence, 5-GATACCACCATCGAGAGTTC-3 and antisense sequence, 5-TGGAGCACCTGATAGACTGT-3; E-cadherin sense sequence, 5-ATGAGGTCGGTGCCCGTATT-3 and antisense sequence, 5-CTCGTTGGTCTTGGGGTCTGT-3; α-SMA sense sequence, 5-CACGGCATCATCACCAACTG-3 and antisense sequence, 5-CCACGCGAAGCTCGTTATAGA-3; Slug sense sequence, 5-AGGGGAGTGGAATGGAACTGCTGA-3 and antisense sequence, 5-ACAGCGAACTGGACACACACACA-3; ZEB-2 sense sequence, 5-TGATTGAGAACCACAGCATACC-3 and antisense sequence, 5-GTTCATCAGAGTTGGGTTCCAT-3; ZEB-1 sense sequence, 5-CCAAAGCAACAGGGAGAGTTAC-3 and antisense sequence, 5-CTTGTCTTTCATCCTGGTTTCC-3; Snail sense sequence, 5-AGAAGCCTTTCTCCTGCTCC-3 and antisense sequence, 5-CACTGGTATCTCTTCACATCCGA-3; Twist sense sequence, 5-GCCGGAGACCTAGATGTCATT-3 and antisense sequence, 5-GGCCTGTCTCGCTTTCTCTT-3; β-actin sense sequence, 5-AGAGGTCTTTACGGATGTCAACGT-3 and antisense sequence, 5-GTCAGGTCATCACTATCGGCAAT-3. Gene expression was detected with the SYBR Green RT-PCR kit (Transgen biotech, Beijing, China), and was determined by normalizing to glyceraldehyde-3-phosphate dehydrogenase using the 2–ΔΔCT method. Gene transcript levels were determined using the CFX96™ real-time quantitative PCR detection system (BioRad Inc., Hercules, CA). Data are shown normalized to β-actin expression.
2.8. Establishment of RLE-6TN cell lines stably overexpressing BMP-7
Construction of the recombinant lentiviruses LV-con and BMP-7 was conducted by Neofect biotech Corporation (Beijing, China). The recombinant lentiviruses were then used to infect RLE-6TN cells. Successful overexpression of BMP-7 was verified by qRT-PCR and western blot.
2.9. Transmission electron microscopy
RLE-6TN cells were grown in 100 mm culture dishes until 85% confluence. Cells (2 × 106) were cultured with or without the supernatant of RAW264.7 treated with 50 μg per ml silica, for 48 hours. The cells were collected and fixed. Then, samples were detected with a JEM-1400 transmission electron microscope (TEM) (JEOL, Tokyo, Japan).
2.10. Statistical analysis
All experiments were performed in triplicate. The data were expressed as means ± SD. Data were analyzed by the LSD-test between any two groups. One-way ANOVA analysis of variance was used to assess the difference of means among groups. Values of P < 0.05 were considered to indicate statistical significance.
3. Results
3.1. Silica induced EMT and decreased the expression of BMP-7 associated with the activation of the p38 MAPK pathway in rats instilled with silica
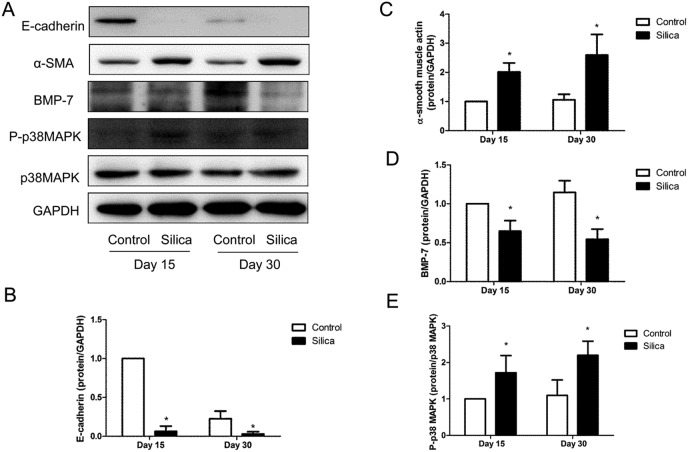
Our previous study has shown that EMT occurred in rats instilled with silica.15 We re-verified the expression of epithelial and mesenchymal markers using western blot. We found that the expression of epithelial marker E-cadherin was down-regulated and the mesenchymal marker α-smooth muscle actin (α-SMA) was up-regulated in the silica group compared with the control group (Fig. 1A–C).
Fig. 1. Silica induced EMT and the downregulation of BMP-7 associated with the activation of the p38 MAPK pathway. Western blots (n = 5) demonstrated that E-cadherin (A and B) decreased and α-SMA (A and C) increased in silica groups of 15 and 30 days, and it was also found that the levels of BMP-7 were downregulated and the levels of P-p38 MAPK were upregulated in lungs exposed with silica. GAPDH or p38 MAPK was used as a loading control. *P < 0.05 vs. control group.
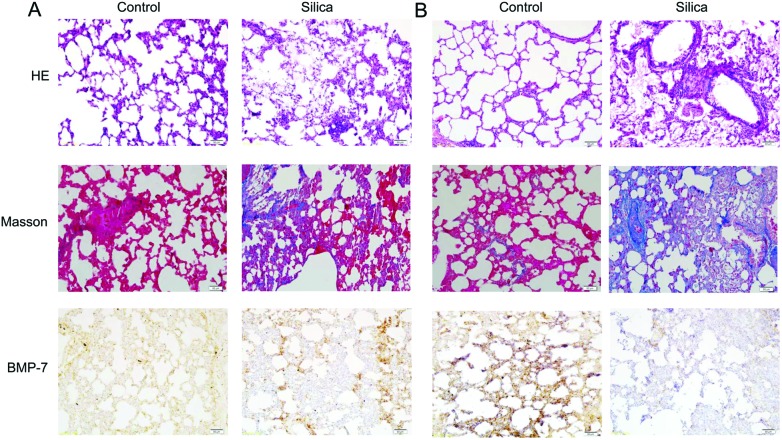
Some scholars found that BMP-7 was down-regulated in the EMT process.16 We assessed the protein expression of BMP-7 with immunohistochemistry and western blot, and we found that BMP-7 was down-regulated in silica groups compared with control groups (Fig. 1A, D and 2).
Fig. 2. Pathological changes and immunohistochemistry of BMP-7 in rats instilled with silica. Immunohistochemistry, HE and Masson staining of 15 days (A) after silica instillation showed that BMP-7 was downregulated in silicosis rats, and the staining of 30 days (B) after silica instillation displayed that there was an obvious reduction of BMP-7 in the silica group.
It has been found that the p38 MAPK pathway was activated in rats instilled with silica.15 The activity of the p38 pathway was investigated in rats instilled with silica and it was found that the protein expression of P-p38 MAPK was upregulated in silica groups compared to control groups, while the total p38 MAPK was not affected (Fig. 1A and E).
It showed that BMP-7 was down-regulated in the process of EMT associated with the activation of the p38 MAPK pathway in silicosis rats.
3.2. Exposure of RLE-6TN cells to silica induced EMT and decrease of BMP-7
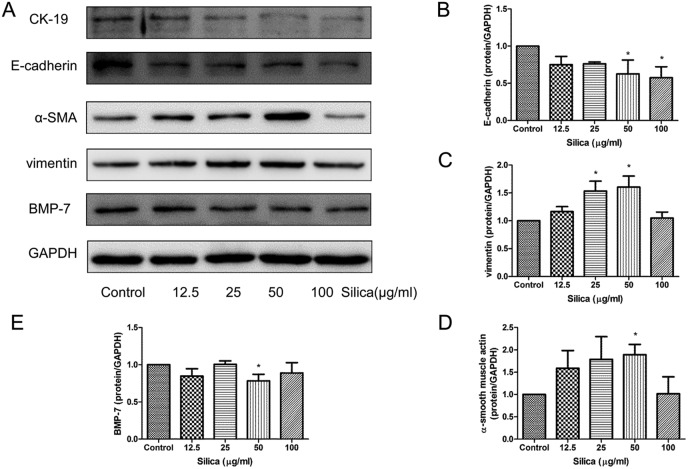
To further confirm that silica could induce EMT in the lung, we chose a type of lung cell of rats, RLE-6TN cells. The cells were treated with different concentrations of the silica supernatant of RAW264.7 for 48 hours. We sought to characterize the phenotypic differences observed among different doses of silica exposed cells. Loss of E-cadherin and cytokeratin-19 (CK-19) are considered to be universal markers for the loss of the epithelial phenotype. In this study E-cadherin levels were clearly decreased when treated with the silica supernatant (Fig. 3A and B). The protein level of CK-19 was also decreased in silica-treated cells (Fig. 3A). The expression of mesenchymal proteins associated with EMT was analyzed next. As the silica exposure doses increased, a gradual increase in vimentin (Fig. 3A and C) and α-SMA (Fig. 3A and D) was observed. Silica exposure induced a significant increase in α-SMA and vimentin levels with 50 μg ml–1.
Fig. 3. Quantification of epithelial, mesenchymal markers and BMP-7 protein expression in RLE-6TN cells exposed to silica. Representative western blots (n = 3) and densitometric analysis demonstrated decreases in the expression of E-cadherin (A and B), CK-19 (A) and BMP-7 (A and E); increases in vimentin (A and C) and α-SMA (A and D) in RLE-6TN cells treated with the increase of silica for 48 h. GAPDH was used as a loading control for all blots, and densitometric analysis was adjusted to account for relative differences in GAPDH intensity. *P < 0.05 vs. control.
To confirm the vital function of BMP-7 in silica-induced EMT in RLE-6TN cells, BMP-7 expression levels were evaluated by western blotting analysis. The protein expression levels of BMP-7 were down-regulated, especially in the 50 μg per ml group (Fig. 3E).
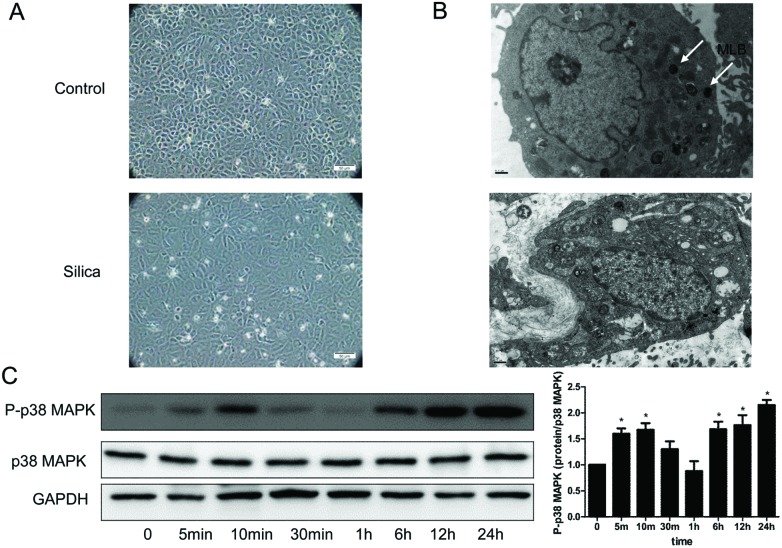
We also found that the morphology changed in RLE-6TN cells. The transformed cells lost the typical cobblestone pattern of an epithelial monolayer, and displayed a fibroblast-like morphology (Fig. 4A). Electron microscopic analysis showed multiple mature lamellar bodies, characteristics indicative of an ATII cell differentiation in unexposed RLE-6TN (Fig. 4B), while in the silica group there was substantial reduction of mature lamellar bodies (Fig. 4B).
Fig. 4. Silica changed the cell morphology, ultrastructure and activated the p38 MAPK signaling pathway in RLE-6TN cells. (A) Inverted microscope analysis of the RLE-6TN cells cultured under different conditions. A typical epithelial cuboidal shape of the RLE-6TN cells in the control group was shown, with the characteristic cobblestone morphology, while the cells became more elongated, less adhered and lost their apical-to-basal polarity after treatment with the 50 μg per ml silica supernatant. (B) Ultrastructure analysis of RLE-6TN cells. Cells in the control group showed multiple mature lamellar bodies and microphilopodia, while in the silica group there was substantial reduction of mature lamellar bodies. MLB: mature lamellar bodies. (C) P-p38 MAPK and p38 MAPK were measured by western blot of the RLE-6TN cells cultured with or without silica for 48 h. *P < 0.05 vs. control.
These results suggested that silica could induce EMT and downregulate BMP-7 in RLE-6TN cells.
3.3. Silica activated p38 MAPK/transcription factor pathway in RLE-6TN cells
The p38 MAPK signaling pathway has been found activated in A549 cells exposed with silica.15 To assess the effect of silica on the pathway in RLE-6TN cells, we examined the activation status of the p38 MAPK. Immunoblots from RLE-6TN cells stimulated by the 50 μg per ml silica supernatant of RAW264.7 displayed enhanced expression of p-p38. The analysis revealed that the silica-induced phosphorylation of the p38 MAPK was detected at 5 min after the onset of stimulation and displayed further persistent activation up to 24 hours (Fig. 4C). The abundance of p38 was not affected by silica (Fig. 4C).
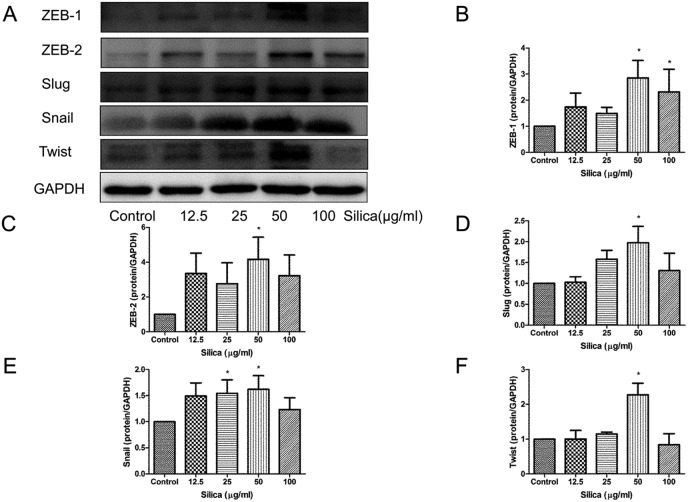
Transcription factors, including Snail, Slug, Twist, ZEB-1 and ZEB-2, can suppress the expression of E-cadherin and their stabilization emerges as features of EMT. Transcription factors were also downstream target proteins of p38 MAPK.15 As expected, silica induced dramatically the expression of Snail (Fig. 5A and E), Slug (Fig. 5A and D), Twist (Fig. 5A and F), ZEB-1 (Fig. 5A and B) and ZEB-2 (Fig. 5A and C) by 50 μg ml–1.
Fig. 5. Effects of silica on the expression of transcriptional factors in RLE-6TN cells. Snail (A and E), Slug (A and D), Twist (A and F), ZEB-1 (A and B) and ZEB-2 (A and C) were measured by western blot of the RLE-6TN cells cultured with or without silica (12.5–100 μg ml–1) for 48 h. *P < 0.05 vs. control.
These results showed that the p38 MAPK/transcription factor pathway was activated in RLE-6TN cells.
3.4. Overexpression of BMP-7 promoted MET and inhibited EMT in RLE-6TN cells
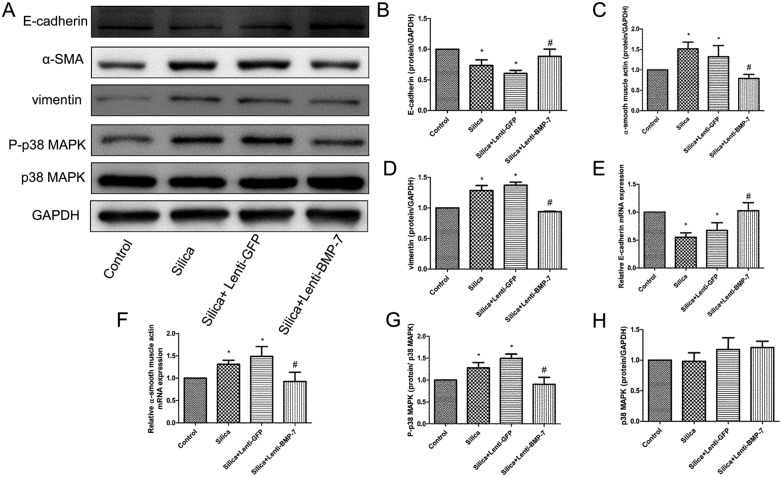
To investigate the functionality of BMP-7 induction, we examined the effect of overexpression of exogenous BMP-7 on RLE-6TN cell phenotypes. We established stable cell lines that overexpress BMP-7 by transfecting with either the expression vector of the BMP-7 or empty vector (Fig. 6A and B). Compared with the empty vector controls, after exposure to the silica supernatant of RAW264.7 for 48 h, the overexpression of BMP-7 resulted in the downregulation of vimentin (Fig. 7A and D) and α-SMA (Fig. 7A and C), whereas E-cadherin expression dramatically increased (Fig. 7A and B). Real-time PCR was performed to confirm that the EMT phenotype was inhibited in RLE-6TN cells by BMP-7. Exogenous BMP-7 induced E-cadherin mRNA expression (Fig. 7E), while α-SMA mRNA expression was markedly reduced in BMP-7-overexpressing cells (Fig. 7F).
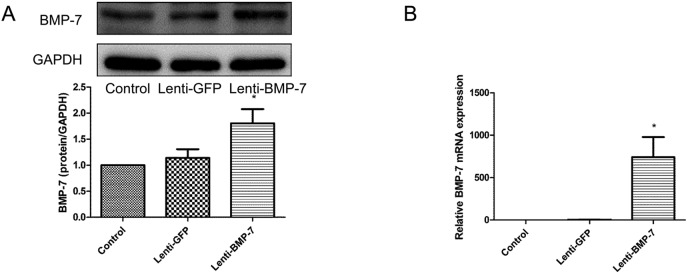
Fig. 6. Establishment of RLE-6TN cells overexpressing BMP-7 in RLE-6TN cells. (A) Western blot analysis of BMP-7 protein in lenti-BMP-7 and control vector-transfected RLE-6TN cells. (B) qRT-PCR analysis of BMP-7 mRNA in lenti-BMP-7 and control vector-transfected RLE-6TN cells. *P < 0.05 vs. lenti-GFP.
Fig. 7. Exogenous BMP-7 reduced silica-induced EMT and silica-induced phosphorylation increase of p38 MAPK in RLE-6TN. A clear protective effect of exogenous BMP-7 was demonstrated with the restoration of E-cadherin expression (A, B and E) and reduction in the expression of α-SMA and vimentin (A, C, D and F). The levels of P-p38 MAPK (G) protein were significantly reduced in lenti-BMP-7 cells compared with levels in lenti-GFP cells, whereas the protein p38 MAPK (H) was not altered. *P < 0.05 vs. control, #P < 0.05 vs. silica + lenti-GFP.
It showed that exogenous BMP-7 promoted MET and inhibited EMT.
3.5. BMP-7 inhibits the p38 MAPK/transcription factor signal pathway in RLE-6TN cells
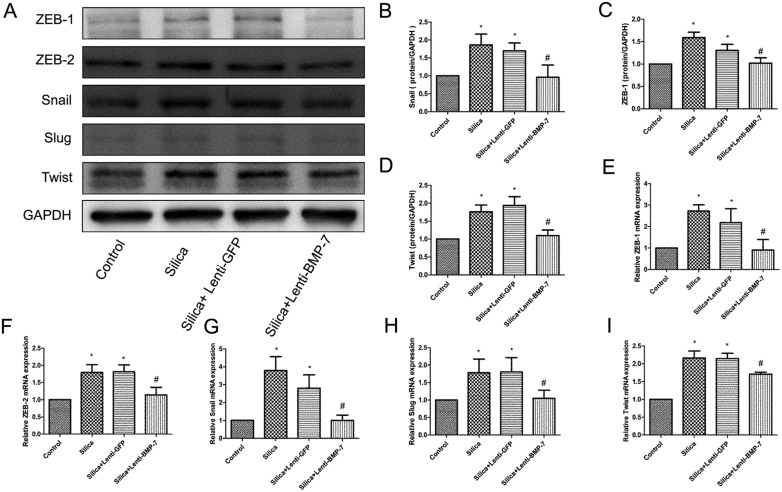
Since p38 MAPK is believed to be affected by BMP-7 in many physiological processes, we determined whether the activation of p38 MAPK and transcription factors could be regulated by BMP-7 during silica-induced EMT. As shown in Fig. 7, exogenous of BMP-7 did not increase the total amount of p38 MAPK in RLE-6TN cells (Fig. 7A and H), whereas BMP-7 decreases p38 MAPK phosphorylation (Fig. 7A and G). The decrease in p38 MAPK phosphorylation was accompanied by the down-regulation of Snail (Fig. 8A, B and G), Slug (Fig. 8A and H), ZEB-1 (Fig. 8A, C and E), ZEB-2 (Fig. 8A and F) and Twist (Fig. 8A, D and I).
Fig. 8. Exogenous BMP-7 reduced silica-induced an increase of transcription factors. The levels of Snail (A, B and G), Slug (A and H), Twist (A, D and I), ZEB-1 (A, C and E) and ZEB-2 (A and F) were significantly decreased in lenti-BMP-7 cells compared with levels in lenti-GFP cells. *P < 0.05 vs. control, #P < 0.05 vs. silica + lenti-GFP.
These results demonstrated that BMP-7 promoted MET associated with inhibition of the p38 MAPK/transcription factor pathway.
4. Discussion
Silicosis, currently the most prevalent chronic occupational lung disease in the world, usually presents after years of exposure as developing nodular fibrosing pneumoconiosis. Alveolar macrophages (AM) play a fundamental role in the pathogenesis of silicosis.17 After phagocytizing silica particles, AM are damaged. The lysosomal membranes are impaired leading to release of proteolytic enzymes into the cytoplasm and eventual death of the macrophage.1 Subsequently, continued silica exposure leads to an alteration in macrophage function. There is the generation of reactive oxygen species (ROS), release of inflammatory cytokines, such as TGF-β, TNF, IL-1 and so forth.18 This stimulates proliferation of fibroblasts and collagen synthesis that eventually lead to nodule formation and pulmonary fibrosis. Therefore, in vitro, we used the silica supernatant of RAW264.7 to stimulate RLE-6TN cells simulating the occurrence of silicosis.
The presence of fibroblast foci has been recognized as the central feature of pulmonary fibrosis pathogenesis. More recently, many researchers found that the injured epithelial cells may directly serve as a source of fibroblasts by the process of EMT. The EMT phenomenon is found in the lungs and contributes to fibrosis in pulmonary fibrosis patients. Evidence for EMT in IPF patients includes the immunostaining of lung biopsies from IPF patients which showed the expression of mesenchymal markers in epithelial cells and the EMT pathway significantly enriching with the differentially expressed IPF genes.19 We found silica induced EMT in rats instilled with silica, consistent with our previous study.15 Western blot showed that E-cadherin decreased and α-SMA increased which indicated that EMT occurred in the lungs of rats in the silica group. In our study, we also found in RLE-6TN cells that silica exposure can lead to the downregulation of epithelial proteins, changes in the cell morphology and ultrastructure, and induction of mesenchymal proteins. These results showed that silica induced EMT in vivo and in vitro.
The suppression of EMT might alleviate fibrotic progression in pulmonary fibrosis. MET is EMT regression and may be a good objective for restraining fibrosis diseases. BMP-7 is a crucial molecule to induce MET, and BMP-7 was often down-regulated in TGF-β mediated EMT in the kidney and other organs, due to its role in sustaining the epithelial phenotype.16 We found that BMP-7 decreased in the silica group in vivo and in vitro. To study the function of BMP-7 in silica induced EMT, we established RLE-6TN cell lines stably overexpressing BMP-7. Enforced expression of exogenous BMP-7 inhibited silica-mediated vimentin and α-SMA expression and partially restored E-cadherin. The results showed that BMP-7 promoted MET and inhibited silica-mediated EMT.
Many signaling pathways have all been implicated as upstream initiators of the EMT process. However, these diverse upstream signaling pathways all appear to converge on a common set of core EMT regulatory factors that includes Snail, Slug, Twist, ZEB-1 and ZEB-2.4,20 They function as regulators to suppress the expression of adhesion molecules during EMT. p38 MAPK, a central mediator of the inflammatory and stress response, plays an important role in non-inflammatory processes such as cell-cycle regulation and cell differentiation. In this study it was found that p38 MAPK was activated by silica in vivo and in vitro. Once the p38 pathway is activated, p38 phosphorylates an array of substrates in the cytoplasm and nucleus, thus regulating the gene expression, cellular polarization and cell cycle. A study into the roles of p38 MAPK in the EMT has shown that p38 MAPK up-regulated snail expression and induced EMT.21 p38 MAPK has also been shown to act as a mediator for Snail and Slug expression after UV exposure in keratinocytes.22 We hypothesized that the p38 MAPK/transcription factor signaling pathway might be involved in the silica-induced EMT in RLE-6TN cells. Here, silica increased the expression of P-p38 MAPK and Snail, Slug, Twist, ZEB1 and ZEB2, which suggested that silica activated the p38 MAPK/transcription factor signal pathway in RLE-6TN cells.
It has been reported that besides engagement of the canonical Smad signaling, BMPs may also affect cellular responses through p38 MAPK. Li RX et al. found that BMP-7 attenuates tubular pro-inflammatory responses in diabetic kidney disease by suppressing p38 MAPK signaling pathways.23 BMP-7 has been shown to contribute to cerebral IPC-induced ischemic tolerance via the p38 MAPK signaling pathway.24 Here we found that exogenous BMP-7 inhibited the activation of the p38 MAPK/transcription factor signaling pathway in cells treated with silica.
5. Conclusions
This study indicated that BMP-7 promoted MET and inhibited silica-induced EMT associated with inhibition of the p38 MAPK/transcription factor pathway, reinforcing the rationale to use BMP-7 as a therapeutic approach to improve the efficiency in silicosis fibrosis.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by grants of the National Natural Science Foundation of China (no. 81273047).
References
- Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sime P. J., O'Reilly K. M. Clin. Immunol. 2001;99:308–319. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- King Jr. T. E., Pardo A., Selman M. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Acloque H., Huang R. Y., Nieto M. A. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Ozawa Y., Kimura T., Sato Y., Kuznetsov G., Xu S., Uesugi M., Agoulnik S., Taylor N., Funahashi Y., Matsui J. Br. J. Cancer. 2014;110:1497–1505. doi: 10.1038/bjc.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhurst R. J., Hata A. Nat. Rev. Drug Discovery. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na Y. R., Seok S. H., Kim D. J., Han J. H., Kim T. H., Jung H., Lee B. H., Park J. H. Cancer Sci. 2009;100:2218–2225. doi: 10.1111/j.1349-7006.2009.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M., Hanai J., Sugimoto H., Mammoto T., Charytan D., Strutz F., Kalluri R. Nat. Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- Yang G., Zhu Z., Wang Y., Gao A., Niu P., Tian L. Toxicol. Lett. 2013;220:103–108. doi: 10.1016/j.toxlet.2013.04.017. [DOI] [PubMed] [Google Scholar]
- Myllarniemi M., Lindholm P., Ryynanen M. J., Kliment C. R., Salmenkivi K., Keski-Oja J., Kinnula V. L., Oury T. D., Koli K. Am. J. Respir. Crit. Care Med. 2008;177:321–329. doi: 10.1164/rccm.200706-945OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson D. R. Diabetologia. 1999;42:1271–1281. doi: 10.1007/s001250051439. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Fatyol K., Jin C., Wang X., Liu Z., Zhang Y. E. Mol. Cell. 2008;31:918–924. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M. C., Wasserman D., Hartwig S., Rosenblum N. D. J. Biol. Chem. 2004;279:12051–12059. doi: 10.1074/jbc.M310526200. [DOI] [PubMed] [Google Scholar]
- Motazed R., Colville-Nash P., Kwan J. T., Dockrell M. E. Pharm. Res. 2008;25:2440–2446. doi: 10.1007/s11095-008-9551-1. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang G., Zhu Z., Liang D., Niu P., Gao A., Chen L., Tian L. Exp. Mol. Pathol. 2015;98:393–402. doi: 10.1016/j.yexmp.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Wang S. N., Lapage J., Hirschberg R. J. Am. Soc. Nephrol. 2001;12:2392–2399. doi: 10.1681/ASN.V12112392. [DOI] [PubMed] [Google Scholar]
- Dostert C., Petrilli V., Van Bruggen R., Steele C., Mossman B. T., Tschopp J. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G., van den Brule S., Lo Re S., Triqueneaux P., Uwambayinema F., Yakoub Y., Couillin I., Ryffel B., Michiels T., Renauld J. C., Lison D., Huaux F. Toxicol. Sci. 2010;116:682–692. doi: 10.1093/toxsci/kfq158. [DOI] [PubMed] [Google Scholar]
- Liang H., Gu Y., Li T., Zhang Y., Huangfu L., Hu M., Zhao D., Chen Y., Liu S., Dong Y., Li X., Lu Y., Yang B., Shan H. Cell Death Dis. 2014;5:e1238. doi: 10.1038/cddis.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Weinberg R. A. Dev. Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Yang Y., Li Y., Wang K., Wang Y., Yin W., Li L. PLoS One. 2013;8:e58915. doi: 10.1371/journal.pone.0058915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler H. L., Kusewitt D. F., Colitz C. M. Vet. Ophthalmol. 2008;11:135–144. doi: 10.1111/j.1463-5224.2008.00575.x. [DOI] [PubMed] [Google Scholar]
- Li R. X., Yiu W. H., Wu H. J., Wong D. W., Chan L. Y., Lin M., Leung J. C., Lai K. N., Tang S. C. Clin. Sci. 2015;128:269–280. doi: 10.1042/CS20140401. [DOI] [PubMed] [Google Scholar]
- Guan J., Li H., Lv T., Chen D., Yuan Y., Qu S. Inflammation. 2014;37:1289–1296. doi: 10.1007/s10753-014-9856-7. [DOI] [PubMed] [Google Scholar]