 Ciguatoxins are marine biotoxins that induce the human poisoning syndrome known as ciguatera fish poisoning (CFP).
Ciguatoxins are marine biotoxins that induce the human poisoning syndrome known as ciguatera fish poisoning (CFP).
Abstract
Ciguatoxins are marine biotoxins that induce the human poisoning syndrome known as ciguatera fish poisoning (CFP). In humans, different kinds of neurological symptoms have been reported after CFP, including anxiety, depression and memory loss. Repetitive exposures to sub-threshold levels of ciguatera toxins may cause irreversible sub-clinical damage, and eventually cause more severe illness. Our previous study has shown that an acute single dose of Pacific ciguatoxin-1 (P-CTX-1) induced synaptic facilitation and blockage of the induction of electrical stimulation-induced long-term potentiation in the medial thalamus–anterior cingulate cortex pathway. Reactive astrogliosis was detected in acute ciguatera poisoning. Despite the reports of complex and prolonged neurological symptoms in patients, few studies have been conducted in animal models to investigate the emotional and cognitive deficits after chronic exposure to ciguatoxin. In the present study, using a rat model with repeated exposures to low dosage of P-CTX-1, we observed development of anxiety-like behavior by open field test and elevated plus maze test, and learning and memory deficits by the Morris water maze; further, decision-making impairment was determined in the chronic P-CTX-1-treated rats by the rats gambling task. We conclude that chronic ciguatera poisoning leads to anxiety, and to impairment of spatial reference memory and decision-making behavior.
Introduction
Ciguatera fish poisoning (CFP) is a common seafood-borne illness caused by consumption of reef fishes. In humans, the risk of fish poisoning is broadly recognized by resident populations in endemic regions in remote island nations and territories of the Pacific.1 Symptoms of CFP have long been recognized as indicative of central and peripheral nervous system injury, and a wide range of neurobehavioral and neuropsychological symptoms were reported following ciguatera poisoning, including cold allodynia,2 visceral pain,3 fatigue, anxiety, depression, memory disturbance and mental inefficiency.1,4 Additionally, repeated exposure to ciguatoxin (CTX) results in more severe clinical signs than observed in patients experiencing ciguatera for the first time.5,6 Persons who have previously had ciguatera may suffer a recurrence of typical ciguatera symptoms after eating fish that do not cause symptoms in other persons. Such sensitization can occur many months or even years after the attack of ciguatera;5,7 and a residual/cumulative toxic effect was considered as an underlying mechanism of this sensitization.6 There is a cumulative effect with repeated exposures inasmuch as lengthy persistence of CTX was previously reported in mice blood,8 in cardiac tissue, and in the rat brain.9 However, it is not known whether repeated exposures to sub-threshold levels of CTX, which cause unobvious general physiological symptoms as well as a generalized reticence to report illness, will affect emotional and cognitive functions in the long term.
The primary mechanism by which the CTX causes neurotoxicity is related to its interaction with voltage-gated sodium channels.10 Previously, using isolated and purified Pacific ciguatoxin (P-CTX-1) (State Key Laboratory of Marine Pollution in the City University of Hong Kong) we were the first group to study the neurotoxic effects of CTX in the brain by in vivo electrophysiology.3 Our published data provided direct in vivo evidence showing that a single dose of P-CTX-1 induced visceral pain,3 and anterior cingulate cortex (ACC) synaptic plasticity, and furthermore blocked the induction of electrical stimulation-induced long-term potentiation (LTP) in the medial thalamus (MT)–ACC pathway.3 Reactive astrogliosis was identified, supporting the concept that neuron and astroglia signals may play roles in acute ciguatera poisoning.3 The ACC is a major cortical component of the limbic loop system, and its functional relationship to sensory, emotional and motivational responses as well as cognitive functions has been well described.11–14 Recently, our published data of animal behavioral and electrophysiological studies suggested the role of ACC circuitry in anxiety-like behavior and memory loss in different disease models. We reported that neuronal damage and impairment of synaptic plasticity in the ACC circuitry may result in various neuropsychological signs in animals.15,16 Therefore, in the current study, a rat model with repetitive low dosage of ciguatera poisoning was established; we hypothesized that repeated exposures to low doses of P-CTX-1 cause a wide range of neurobehavioral signs including emotional and cognitive dysfunctions in rats.
Cognition refers to a set of mental processes, including attention, memory, evaluation, decision-making, etc. Making a decision under complicated and uncertain conditions is a basic cognitive process for adaption. In humans, decision-making has been accurately modeled using the Iowa gambling task (IGT) in the laboratory.17–19 In behavioral tasks for animals, the rat gambling task (RGT) based on the same principle has been developed to investigate the neurobiological mechanisms underlying IGT-like decision-making behavior.14,20,21 Recently, we have characterized decision-making deficits in different animal disease models by the RGT.15,16 However, no human or animal models have been developed to examine the effects of P-CTX-1 on decision-making behavior. In the current study we evaluated the effects of repeated exposures to low doses of P-CTX-1 on decision-making behavior using the RGT. No significant differences of food intake and overall motivation were detected between sham-treated and P-CTX-1 rats, whereas P-CTX-1 administration caused decreases in the proportion of good decision-makers and increases in the proportion of poor decision makers, suggesting impairment of decision-making in rats. In addition, open field and elevated plus maze tests were performed to evaluate rats’ anxiety-like behavior, and a water maze was used to detect learning and spatial memory capacities in rats following chronic CTX treatment. Rats showed no significant change in locomotor functions; however, development of anxiety-like behavior was clearly detected after chronic CTX treatment. Further, spatial learning and memory deficits were characterized in rats after 5–6 weeks of toxin poisoning. To the best of our knowledge, this is the first study using an animal model of chronic ciguatera poisoning mimicking clinically prolonged repetitive CFP to explore changes of emotions, learning and memory, and decision-making functions caused by chronic CFP.
Materials and methods
Animals
Adult male Sprague–Dawley rats (280–300 g) were used in the present study. Before experiments, rats were kept in plastic cages in groups with a supply of food and water ad libitum. The animal holding room was maintained in 12:12 h light and dark cycle with a constant room temperature of 25 °C. All the experimental procedures were approved by the Committee on the Use and Care of Animals at City University of Hong Kong and the Department of Health of Hong Kong [reference no. (14-42) in DH/HA&P/8/2/5 Pt.2] and all methods were carried out in accordance with the approved guidelines.
Chronic ciguatoxin treatment
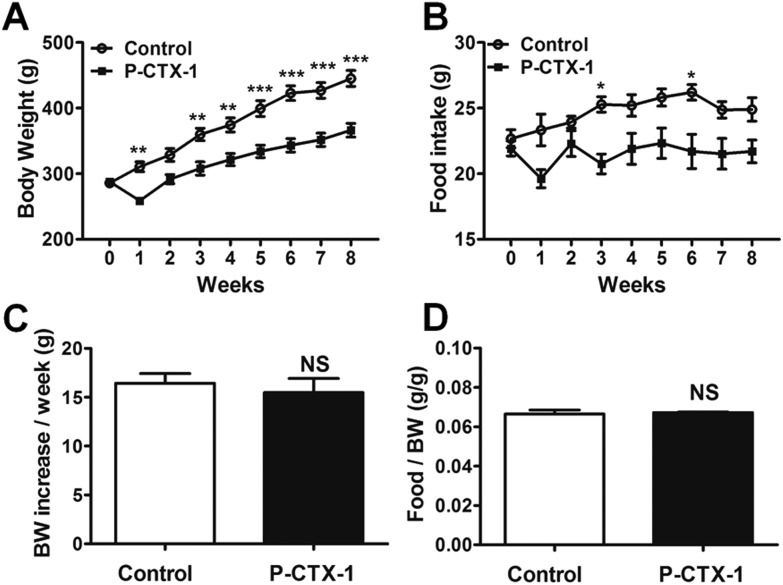
Pacific ciguatoxin-1 (P-CTX-1, purity ≥95%22) was isolated and purified from the viscera of moray eels collected from the Republic of Kiribati using methods described previously.22 Rats were weighed and randomly divided into a chronic P-CTX-1 group and a control group. P-CTX-1 was dissolved in saline with 1% Tween 60. An initial dosage of P-CTX-1 (0.26 ng g–1) was delivered to the rats intragastrically via gavage. This dosage has been used for acute ciguatera studies by our own group and others.3,9 Then, the P-CTX-1 rats were further treated with chronic repeated toxin exposure with one-quarter of the initial dosage (0.065 ng g–1) every 72 hours for 8 weeks. This low dosage was chosen because it did not affect the average food intake per unit body weight and the average body weight increase (Fig. 1C and D). The control group was treated with saline containing 1% Tween 60 of the same dosage and at the same time points as the chronic P-CTX-1 group. Body weight and food intake were monitored and reported every week. Behavioral tests were performed between week 5 and week 8 during chronic ciguatoxin exposure.
Fig. 1. Body weight and food intake in control and chronic P-CTX-1 rats. (A) Body weight measured every week during chronic low dosage of P-CTX-1 or sham treatment. (B) Food intake measured every week during the treatment. (C) Average body weight (BW) increase per week. (D) Average food intake per unit body weight for 8 weeks. Chronic P-CTX-1 rats showed decreased body weight and food intake compared with control rats. However, no obvious difference was found in the average food intake per unit body weight and average body weight increase per week between the two groups. Data are presented as mean ± SEM. NS, no significance; *p < 0.05, **p < 0.01, ***p < 0.001; n = 5 for control rats, and n = 5 for chronic P-CTX-1 rats.
Open field test
The open field test is a typical rodent behavioral test that assesses locomotor function and anxiety-like exploratory behavior. It was performed in chronic P-CTX-1 rats and control rats at week 5 of the treatment. An area of 40 cm × 40 cm inside an 80 cm × 80 cm × 40 cm (L × W × H) square plastic box was defined as the center region while the peripheral area was defined as the marginal region. Rodents tend to stay close to walls (thigmotaxis) when first exposed to an open field, and the tendency should decrease gradually with time; the degree of thigmotaxis during this period of decrease can be considered as the anxiety index of rodents. The test was started by placing the rat in a corner of the box in a dim room, and allowing it to explore the field freely for 5 min. The maze was cleaned between rats using 70% ethanol. A CCD camera was set above the chamber to track the movement. The horizontal activity of the rats was recorded to measure (1) total distance travelled, (2) mean speed, (3) time spent in center area, and (4) center entry times, using ANY-maze software (SD instrument Co., US).
Elevated plus maze
The elevated plus maze is a widely used behavioral test for anxiety-like behavior and is thought to result from natural aversion of rats to explore elevated and open areas.23 The apparatus was made of brown acrylic plastic with two sets of opposing arms (open arms: 50 × 10 cm; closed arms: 50 × 10 × 40 cm), 50 cm from the ground. Rats were placed individually and then allowed to start exploring the maze freely from the junction of the two sets of arms (10 × 10 cm), facing one open arm, in a 5-minute test. The maze was cleaned between rats using 70% ethanol. Time spent in each arm was recorded using the ANY-maze software with entry being defined as 85% of the area of the animal being present in the area entered. Time spent in open arms, especially the percentage of open arm time versus closed arm time, was evaluated to assess anxiety.
Morris water maze
The Morris water maze test is a classical behavioral assessment to test the spatial learning and reference memory ability of rodents. It was performed in chronic P-CTX-1 rats and control rats during week 6 of the treatment. A circular tank (diameter 1.5 m) was used as the swimming pool. The temperature of the water was maintained at 22–25 °C to reduce the stress response of the rats to cold water during training. Visual cues of different shapes were placed on the walls around the swimming pool. In this study, the whole procedure was divided into two parts: hidden platform training and probe test. First, rats were consecutively trained for 3 days (2 trails per session and 2 sessions per day) to find a submerged (2.5 cm) platform located at a constant position in one of the quadrants of the pool using the distal spatial cues provided. In each trail, the rat was placed in the water facing the wall at the start position and given 60 s to find the platform. Then the rat was forced to stay on the platform for 15 s. The start position was randomly designed in each trail. The time that each rat spent to find the platform in each trail was measured as escape latency. A probe test was performed 24 hours after the last hidden platform training trail. In the probe test, the platform was removed and the rat was allowed to swim to search the tank freely for 1 min. The percentage of the time the rat spent searching in the target quadrant and the percentage of the time the rat spent in the removed platform region in the probe test were measured. Data were collected and analyzed by ANY-maze software.
Rat gambling task
The rat gambling task (RGT) was conducted during week 7 to 8 of the chronic P-CTX-1 or sham treatment to detect decision-making behavior. Detailed training and test procedures were described in our previous publications.15,16 Briefly, the RGT was performed in a polyvalent conditioning box (28 × 30 × 34 cm) with 4 nose-poke holes on the front curved wall and a food dispenser at the back wall (Imetronic, Pessac, France), with a transparent central opening partition (7 × 7 cm) dividing the box into two chambers at the middle. Infrared detectors were in the holes detected the nose-pokes and were connected to the food dispenser.
Before the training session, food was restricted for 3 days following 1 day fasting. Simultaneously, 50 food pellets (45 mg per pellet; Test Diet, USA) per rat were put inside the cage every day to make sure the rats became habituated to their taste. During the training phase, daily food was restricted for each rat to maintain its body weight at 90% of free feeding weight, and rats usually spent 5–7 days making the association between nose-pokes in illuminated holes and food rewards in the food dispenser. In order to guarantee that the selection of the nose-poke was a conscious choice, the rats were trained to associate a single nose-poke with one food pellet delivery according to a criterion of at least 100 pellets obtained within a 30-min session, followed by two consecutive nose-pokes with one food pellet delivery with the same criterion. Two final 5-min training sessions were conducted to habituate the rats with the quantity of pellets that could be obtained during the test. The first session was set by two nose-pokes with two pellets at a time (maximum 30 pellets) after making a choice and the other with one pellet (maximum 15 pellets).
The 60-min test was performed one day after the training session. Rats were free to make choices among the four holes (A–D). However, different choices triggered different outcomes: choices A or B related to two pellets each time as immediate reward, but had separately 1/2 probability of triggering a long penalty time-out (222 s) or 1/4 probability for a very long penalty time-out (444 s), during which period no pellet could be obtained; choices C or D were associated with one pellet immediate reward, and smaller penalty (1/4 chance for 12 s time-out, or 1/2 chance for 6 s time-out). Although the immediate reward of choices A and B was twice that of choices C and D, in the long run choice C or D would allow rats to obtain more food reward than choice A or B. Thus, choices C and D are defined as advantageous choices, while choices A and B are disadvantageous choices. The percentage of advantageous choices (number of nose-pokes (C + D)/number of nose-pokes (A + B + C + D) × 100%) was used as a criterion to distinguish the good (>70% preference of advantageous choices during the last 20 minutes of RGT test), undecided (30%–70% preference) and poor (<30% preference) decision-makers.
Ciguatoxicity quantification
Rats were sacrificed at different time intervals (24 h, 48 h and 72 h) after the final exposure to the low dosage of P-CTX-1 at week 8. Blood and brain were collected and stored at –80 °C for further toxin analysis. The blood and brain were treated according to the published method.24 Briefly, whole blood and brain were sonicated in an ultrasonic water bath for 15 min at room temperature with acetonitrile, then stored at –20 °C for 15 min and centrifuged for 15 min at 4 °C. Supernatants were collected and the extraction procedure was repeated twice. All supernatants were pooled together and dried using rotary evaporation.
The ciguatoxicity of the extracts was quantified by mouse neuroblastoma (Neuro-2a) assay according to the methods described previously.22 Neuro-2a cells (ATCC CCL131; ATCC, Manassas, VA, USA) were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Life Technologies, Carlsbad, CA, USA) at 37 °C in 5% CO2. The RPMI-1640 was supplemented with 10% fetal bovine serum (BD Biosciences, San Jose, CA, USA), 2 g L–1 Na2CO3 and antibiotic solution (50 units per ml penicillin, 50 μg ml–1 streptomycin and 2.5 μg ml–1 Fungizone® (Gibco, Life Technologies, Carlsbad, CA, USA)). Cells were seeded at a density of 2.5 × 105 cells per mL in 96-well plates. After 24 h, medium was renewed with complete RPMI-1640 containing 0.1 mM ouabain and 0.01 mM veratridine. Cells were dosed with 10 μL per well P-CTX-1 standards and extracts in three replicates. Cell proliferation was measured by MTT [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay after 18 h incubation. Absorbance was measured using a microplate reader (Molecular Devices Spectra Max 340 PC) at 595 nm with a reference wavelength of 655 nm. The optical density acquired for each well was then normalized to the MTT blank. The toxicity of the extracts was determined based on the standard curve of P-CTX-1, which was plotted at seven concentrations ranging from 9.77 pg mL–1 to 312.5 pg mL–1. The assays were conducted twice, and the toxicity results are reported as mean P-CTX-1 equivalents between the two assays.
Statistical analysis
All data in the results are presented as mean ± SEM. Statistical comparisons were performed in SPSS v19.0 (SPSS, Chicago, IL, USA) or Prism 5.0 (GraphPad, La Jolla, CA). Comparisons between control and chronic P-CTX-1-treated rats were analyzed with unpaired Student's t-test or two-way ANOVA followed by Bonferroni's post-tests where appropriate. Comparisons of changes of proportions of decision-makers between the two groups were treated as a single ordinal contingency table and the nonparametric Mann-Whitney U test was used. Differences between values were considered statistically significant when p < 0.05.
Results
Normal body weight increase and food intake during chronic low dosage ciguatoxin exposure
The body weight and food intake of chronic P-CTX-1 rats were markedly decreased after the initial dosage (0.26 ng g–1) of toxin administration compared with those of sham-treated rats, which were consistent with our previous report.3 The body weight gradually increased when the higher dose was changed to low dosage P-CTX-1 exposure (0.065 ng g–1). Then, the rats gained weight consistently during the 8-week chronic repeated low dosage P-CTX-1 treatment (Fig. 1A). Similar trends of food intake were observed in the chronic P-CTX-1 rats (Fig. 1B). However, owing to the significant drops in food intake and body weight during the first week, the animals were unable to fully recover and reach the control levels. The average food intake per unit body weight was not significantly different between the two groups (0.066 ± 0.002 vs. 0.067 ± 0.001 in control and chronic P-CTX-1 rats, respectively; p > 0.05; Fig. 1D). Also, no significant difference was found in average body weight increase per week between the two groups (15.53 ± 0.50 vs. 14.09 ± 0.55 in control and chronic P-CTX-1 rats, respectively; p > 0.05; Fig. 1C). These data indicate that chronic exposure of low dosage of P-CTX-1 did not obviously affect the general status of the rats as regards body weight increase and food intake.
Chronic P-CTX-1 rats showed similar locomotor activity in the open field test
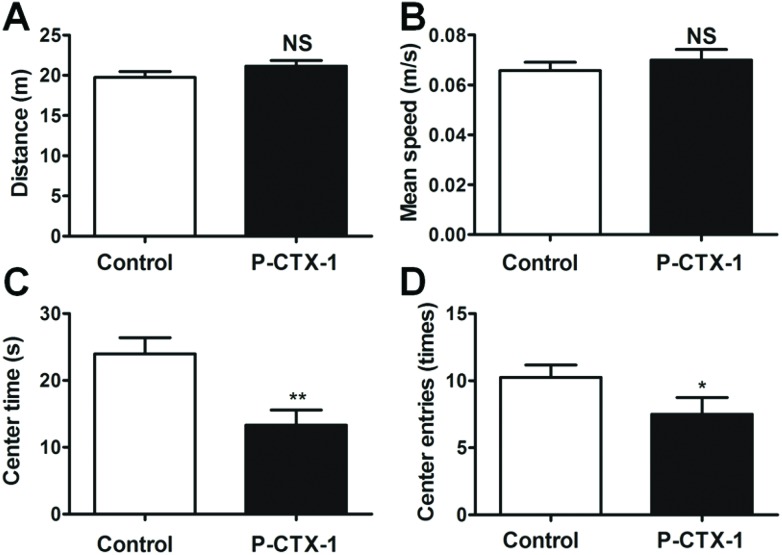
In the open field test, the locomotor function of rats was examined by the total distance moved and mean speed. The total distance moved was similar between control and chronic P-CTX-1 rats (19.73 ± 0.74 vs. 21.15 ± 0.69 m in control and chronic P-CTX-1 rats, respectively; p > 0.05; Fig. 2A). Also, no difference was found in mean speed between the two groups (0.066 ± 0.003 vs. 0.071 ± 0.004 m s–1 in control and chronic P-CTX-1 rats, respectively; p > 0.05; Fig. 2B). Spontaneous exploratory activity in chronic P-CTX-1-treated rats was suppressed compared with control rats, as indicated by a reduced time spent in the center area (23.95 ± 2.43 vs. 13.31 ± 2.26; p < 0.01; Fig. 2C), and a decreased number of entries into the center area (10.25 ± 0.91 vs. 7.50 ± 1.24; p < 0.05; Fig. 2D) during the open field test. These results indicate that chronic low dosage P-CTX-1 exposure did not affect the locomotor function of rats; however, the decreases in the center entry times and center duration in chronic P-CTX-1 rats compared with control rats suggest that chronic low dosage P-CTX-1 exposure leads to increased anxiety-like behavior in rats.
Fig. 2. Chronic P-CTX-1 rats showed normal locomotor function in open field test. (A) Total distance travelled in the open field was similar between control and chronic P-CTX-1 rats. (B) No difference was found in the mean speed between the two groups. (C) Chronic P-CTX-1 rats showed decreased center duration compared with control rats. (D) Chronic P-CTX-1 rats showed a decrease in the number of times they entered the center area compared with control rats. Data are presented as mean ± SEM. Comparison between the two groups was made by means of Student's t-test. NS, no significance, *p < 0.05, **p < 0.01; n = 20 for control rats, and n = 17 for chronic P-CTX-1 rats.
Anxiety-like behavior following chronic ciguatoxin exposure
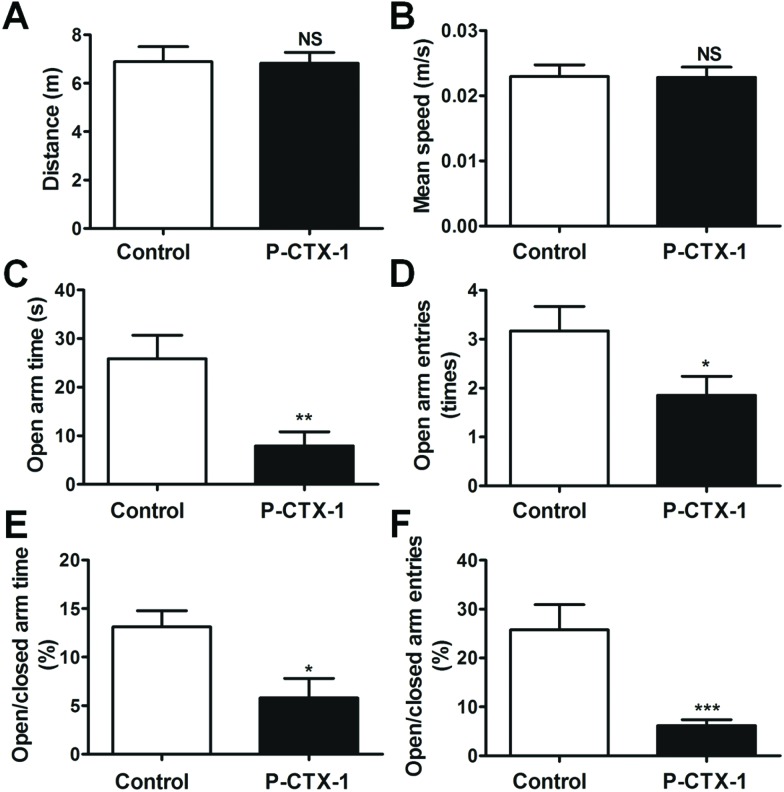
A more specific test for evaluating anxiety-related behavior, the elevated plus maze, was further used in the current study. Chronic P-CTX-1-treated rats exhibited normal locomotor activity in the elevated plus maze compared with control rats, as indicated by the total distance travelled (6.89 ± 0.62 vs. 6.83 ± 0.45; p > 0.05; Fig. 3A) and mean speed (0.023 ± 0.002 vs. 0.023 ± 0.002; p > 0.05; Fig. 3B) during the 5 min testing session. However, the chronic P-CTX-1-treated rats spent less time in the open arms (25.84 ± 4.85 vs. 7.92 ± 2.88; p < 0.01, Fig. 3C) and made fewer entries into the open arms (3.17 ± 0.50 vs. 1.85 ± 0.39; p < 0.05, Fig. 3D) compared with the controls. Furthermore, the percentage of time spent in open arms/closed arms (13.10 ± 1.67 vs. 5.79 ± 2.01; p < 0.05; Fig. 3E) and the percentage of entries into open arms/closed arms (25.74 ± 5.17 vs. 6.15 ± 1.23; p < 0.001; Fig. 3F) were reduced in chronic P-CTX-1-exposed rats. These observations indicate that an increase in anxiety-like behavior existed in the chronic low dosage P-CTX-1-treated rats.
Fig. 3. Anxiety-like behavior of chronic P-CTX-1-treated rats in the elevated plus maze. (A) Total distance travelled in the elevated plus maze was similar between control and chronic P-CTX-1 rats. (B) No difference was found in the mean speed between the two groups. (C) Chronic P-CTX-1 rats showed a decrease of time spent in the open arms compared with control rats. (D) Numbers of entries into open arms were decreased in the chronic P-CTX-1 group. (E) Percentage of time spent into open arms versus closed arms. (F) Percentage of the number of times that the rats entered the open arms versus closed arms was also reduced in chronic P-CTX-1 rats. Data are presented as mean ± SEM. Comparison between the two groups was made by Student's t-test. NS, no significance, *p < 0.05, **p < 0.01, ***p < 0.001; n = 20 for control rats, and n = 17 for chronic P-CTX-1 rats.
Chronic P-CTX-1 rats showed impaired spatial learning and reference memory in the Morris water maze
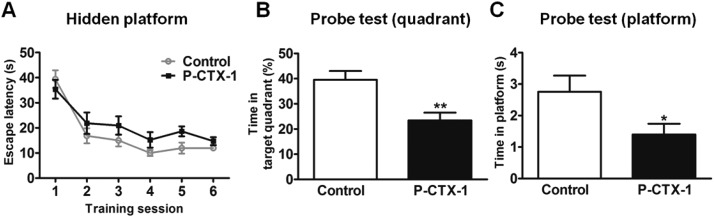
Spatial learning and reference memory were assessed in the Morris water maze in chronic P-CTX-1 rats and control rats at week 6 during the treatment. Both groups of rats showed gradually decreased escape latency during 3-day hidden platform training. However, chronic P-CTX-1 rats showed longer escape latency than control rats during the training process (F(1, 35) = 4.74; p < 0.05; Fig. 4A), suggesting spatial learning ability was affected in these rats. Furthermore, in the probe test, chronic P-CTX-1 rats showed significantly shorter duration in the target quadrant (39.57 ± 3.46 vs. 23.46 ± 3.01 s; p < 0.01; Fig. 4B) and shorter duration in the platform region (2.76 ± 0.51 vs. 1.40 ± 0.34 s; p < 0.05; Fig. 4C) compared with control rats. These results suggest that chronic low dosage P-CTX-1 exposure impaired spatial learning and reference memory in rats.
Fig. 4. Chronic P-CTX-1 rats showed impaired spatial reference memory in the Morris water maze. (A) Escape latency in the hidden platform training measured at week 6. The chronic P-CTX-1 rats showed longer escape latency, suggesting impairment of spatial learning. (B) Time spent in the target quadrant in the probe test. (C) Time spent in the removed platform region in the probe test. Total time spent in the target quadrant and in the removed platform region in the probe test was shorter in the chronic P-CTX-1 rats compared with control rats, suggesting impaired spatial reference memory. Data are presented as mean ± SEM. Comparison between the two groups was made by Student's t-test. NS, no significance, *p < 0.05, **p < 0.01; n = 20 for control rats, and n = 17 for chronic P-CTX-1 rats.
Decision-making deficit in chronic P-CTX-1-treated rats
In the rats gambling task, two control rats and two P-CTX-1 rats developed a spatial preference behavior for apertures on one side during training without sampling the different options, and maintained this side preference for the whole 60 min test. These rats were discarded from final analysis as their preferred choices in the test were not dependent on the decision-making process based on the different outcomes.
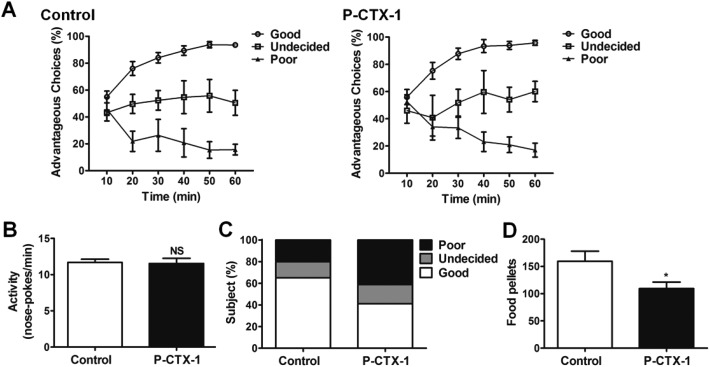
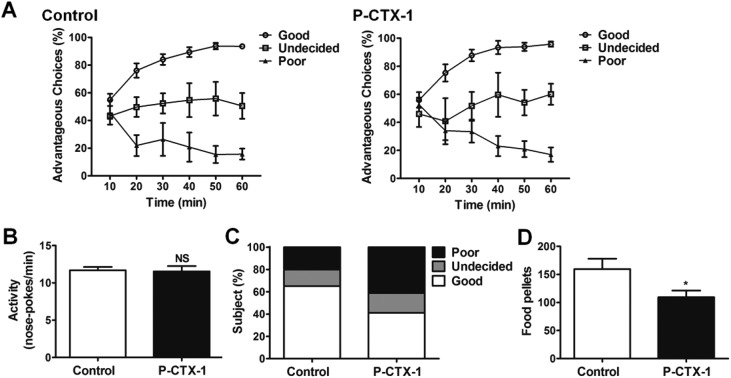
The performances of sham- and chronic P-CTX-1-treated rats during the RGT test are shown in Fig. 5A. Three types of decision-making behaviors were identified in both groups. Good decision-makers were those that first chose randomly and then progressively orientated their preference toward the more advantageous options, and ended up making more than 70% advantageous choices during the last 20 min of the test. Undecided rats did not show any stable preference for either option across the whole test. Poor decision-makers sampled the different options early, and then developed a stable preference for the adverse options. They made fewer than 30% advantageous selections during the last 20 min of the test. These types of decision-making performance have been described and defined in previous studies.14–16 In rats, after chronic low dosage P-CTX-1 treatment, the proportion of good decision-makers decreased (65.0% vs. 41.2%), and the proportion of poor decision-makers increased (15.0% vs. 41.2%) compared with controls. Similar results in the proportion of undecided rats were detected between the two groups (20.0% vs. 17.6%; Table 1). The difference in the proportions of the three types of decision-making behaviors between the two groups was significant (Mann-Whitney U test, U = 102.5; p < 0.05; Fig. 5C). The mean food reward obtained during the RGT by CTX rats was significantly lower than controls (159.6 ± 18.3 vs. 109.3 ± 12.0; p < 0.05, Fig. 5D). Moreover, no difference was detected in the general activity (numbers of nose-pokes per minute) (11.69 ± 0.44 vs. 11.54 ± 0.51; p > 0.05; Fig. 5B) between the two groups. These data indicate that rats developed a decision-making deficit after chronic low dosage P-CTX-1 treatment.
Fig. 5. Deficit of decision-making behavior in chronic P-CTX-1 rats. (A) The ratios of advantageous choices across the 60 min test identify three types of decision-makers both in sham- and in chronic P-CTX-1-treated rats. (B) The general activity (number of nose-pokes in the four holes per minute in the last training session) showed no difference between control and chronic P-CTX-1 rats. (C) The different proportions of three types of decision-makers in control and chronic P-CTX-1 rats. (D) The number of food pellets obtained during the test in control and chronic P-CTX-1 rats. NS, no significance, *p < 0.05; n = 20 for control group, n = 17 for chronic P-CTX-1 group.
Table 1. Number and percentage of subjects in the RGT for control and chronic P-CTX-1 groups.
| Number of subjects | Control group (n = 20) n (%) | P-CTX-1 group (n = 17) n (%) |
| Good decision-makers | 13 (65%) | 7 (41.2%) |
| Undecided decision-makers | 3 (15%) | 3 (17.6%) |
| Poor decision-makers | 4 (20%) | 7 (41.2%) |
P-CTX-1 concentrations in blood and brain samples of chronically poisoned rats
To determine quantity of P-CTX-1 present in blood and brain of rats after chronic repetitive administration of the toxin, toxicokinetic analysis was conducted by ciguatoxicity quantification. As a result of prolonged repetitive treatment of low dosage P-CTX-1, the P-CTX-1 concentration in blood samples ranged from 0.0065 to 0.015 ng mL–1 at 24 h, 48 h and 72 h after the final exposure to the toxin at week 8, and no difference was detected among these three time points (F = 2.72, p > 0.05, n = 4 rats for each time point). Higher concentrations were detected in the brain samples of rats after repetitive administration of P-CTX-1 (0.034 ± 0.010, 0.030 ± 0.009 and 0.035 ± 0.005 ng g–1 brain sample at 24 h, 48 h and 72 h, respectively). No difference was detected among these three time points (F = 0.11, p > 0.05, n = 4 rats for each time point). In contrast, there was no detectable P-CTX-1 concentration in either blood or brain samples of rats after the control treatment. Notably, in our experimental protocol repetitive low dosage P-CTX-1 was administrated every 72 h; thus the toxin level at 72 h was the baseline concentration for the next toxin administration. These data suggest that accumulative quantities of the toxin are present in the brain and blood of rats as a result of chronic low dose P-CTX-1 treatment, and the persistent level of toxin remaining during our behavioral testing period may play a causative role in the emotional and cognitive declines observed in the present study.
Discussion
Using a rat model of chronic low dosage P-CTX-1 poisoning, in this study we thoroughly investigated neurobehavioral features of prolonged low doses of ciguatera toxin. The noteworthy observations in the present study are as follows. The general activities were similar in rats after chronic low dosage of P-CTX-1 treatment compared with controls; however, the development of anxiety-like behavior was seen in open field and elevated plus maze tests. Further, learning and memory deficits were detected by the Morris water maze and decision-making impairment was observed using a rats gambling task in rats after chronic exposure to sub-clinical doses of P-CTX-1. Notably, rats were treated with a single sublethal dose of P-CTX-1 followed by repeated low dosage P-CTX-1 to mimic clinical recurrence of the poisoning in endemic regions which may induce irreversible sub-clinical damage, and may eventually cause more severe clinical illness because of the cumulative effect.1,6,9,25 Consistent with previous studies, we found that the body weight and food intake of rats were markedly decreased after the initial sublethal dosage P-CTX-1 administration compared with sham-treated rats;3,9 however, rats gained weight and food intake increased consistently during the 8-week prolonged low dosage P-CTX-1 exposure, suggesting that the behavioral alterations observed are not due to changes of basic physiological states of rats. Thus we called the ciguatoxin poisoning in the current study chronic sub-clinical (or low dosage) to distinguish it from the acutely sublethal dose of P-CTX-1 poisoning rat model used in our previous study.3
CFP has been ranked by worldwide public health institutions as one of the most common food-borne illnesses and adversely affects an estimated 50 000–500 000 people globally on an annual basis.26,27 Consumption of coral reef fishes containing ciguatoxin levels >0.01 ng may result in human ciguatera fish poisoning.4,28 Moreover, ciguatoxins are heat/acid-stable; therefore, normal food preparation does not allow them to be detected or eliminate them. In humans, CFP is associated with gastrointestinal (GI), cardiovascular, neurological and neuropsychiatric symptoms and signs.1,28,29 Neuropsychiatric symptoms may include anxiety,30 depression and memory loss,4 which generally appear within the first few days in CFP, often becoming prominent after the GI symptoms. Clinical studies suggested that symptoms are rarely chronic in a single-dosage CFP case.28,30 Repetitive exposure to sub-threshold levels of ciguatera toxins may cause irreversible sub-clinical damage,28 and eventually cause more severe illness.6,9,25 Our previously published animal study showed that treatment with a single sublethal dose of P-CTX-1t induced visceral pain response, facilitation of synaptic transmission, impairment of LTP in the MT-ACC synapse, and astrogliosis in the ACC region; however, all these observed phenomena had gone at day 7 after P-CTX-1 treatment.3 Further, toxicokinetic data also showed that P-CTX-1 was measurable in blood and brain samples of rats after chronic low dosage P-CTX-1 treatment, whereas it was undetectable at day 7 after a single dose treatment. Thus, it appears that the neuropsychiatric declines observed in the current study may be the prolonged cumulative effect of P-CTX-1 instead of long-term effects of the initial single dosage administration.
Using cultured neural cells, a large body of research has elucidated the primary mechanism by which CTX causes neurotoxicity as activation of voltage-gated sodium channels,10 elevation of intracellular calcium levels,31 and blockade of voltage-gated potassium channels,32 which result in oscillations in membrane potential and alter action potential firing, and are believed to be the potential cause of the array of neurological signs. As we reported previously, P-CTX-1 directly causes damage in both neurons and astrocytes in the ACC.3 The ACC is a major cortical area of the limbic loop system, integrating emotion and cognition. Recently attention has been given to a causal role of ACC in anxiety-like behavior in both human and animal studies.33–35 In humans, brain imaging studies have revealed an increase of ACC neuronal activity in patients with anxiety disorders,36 and pharmacological inactivation or surgical lesions of the ACC produced anxiolytic effects in humans37 and animals.38 However, it is absolutely unclear whether ACC functional disturbances induced by P-CTX-1 poisoning cause emotional and cognitive outcomes in animal models. Thus in the present study a series of behavioral experiments were designed to investigate changes of emotion and cognition induced by chronic low dosage P-CTX-1 treatment. In both open field and elevated plus maze tests, general activity as represented by travelled distance and mean speed was similar in rats after chronic P-CTX-1 treatment compared with controls, suggesting undetectable changes of basic physiological status of these rats. However, the development of anxiety-like behavior was characterized by less time spent in and number of entries into the center area in the open field test as well as open arms in the elevated plus maze test. These results indicate emotional signs occurred in chronic low dosage P-CTX-1-poisoned rats.
Using a visceral hypersensitive rat model our series of investigations have demonstrated that ACC hypersensitivity can be observed up to 7 weeks after the initiation of colonic anaphylaxis independently of mucosal inflammation, suggesting triggering of pain memory in the ACC neuronal circuitry.11,12,39–41 Recently, we also showed that ACC neuronal activities play a key role in aversive memory processing by employing a conditional place avoidance test.13 Despite the hippocampus having been believed to play a critical role in spatial memory formation, a growing body of evidence has demonstrated the important contribution of the ACC to expression of spatial memory.42,43 In the present study, the chronic P-CTX-1-treated rats showed longer escape latency in their search for the hidden platform during 6 trials of training session in the water maze test compared with control rats, suggesting a learning deficit in week 6. Further, the P-CTX-1 rats spent less time in the platform quadrant compared with control rats, suggesting impaired spatial reference memory. These observations suggest chronic low dosage P-CTX-1 treatment causes interruptions of learning and spatial reference memory.
Decision-making has recently emerged as a central theme in neurophysiological studies of cognition. Impaired decision-making has been demonstrated to represent a key symptom in many mental disorders,44,45 and results in an inability to make profitable long-term decisions that incorporate expectations of future outcomes.20 In humans, decision-making has been investigated by the Iowa gambling task (IGT) in the laboratory setting,17,19 which is used to model everyday life choices through a conflict between immediate gratification and long-term outcomes.20 Rodents are individuals that can exhibit human-like cognitive characteristics; thus, the RGT was designed to test decision-making behavior. It is particularly valuable as experimental conditions can be controlled to investigate the neurobiological mechanisms underlying IGT-like decision-making.14,21 In the present study the RGT was performed on the rats after chronic low dosage P-CTX-1 treatment. We observed that all three kinds of decision-making behaviors were detected by the RGT in both control and chronic P-CTX-1-treated rats, supporting the idea that a continuum may be present across these two groups of rats. However, our data showed that chronic ciguatera poisoning induced behavioral changes in decision-making. Chronic P-CTX-1 significantly reduced the proportion of good decision-makers compared with sham-treated rats, and significantly increased the poor decision-maker population. Further, the mean food reward obtained during the RGT test by chronic P-CTX-1-treated rats was significantly less than that of controls. Notice that chronic P-CTX-1-treated rats showed no general effect on the motivation to actively retrieve a food reward (duration of the last training session before reaching the criterion). Also, no difference was detected in the general activity (total number of nose-pokes in the four holes in the last training session). These observations suggested that chronic low dosage P-CTX-1 treatment-induced impairment of decision-making behavior was not due to a direct effect on the motivational processes and general status of rats.
In summary: this study, using a rat model of chronic low dosage P-CTX-1 poisoning, demonstrated that chronic low dosage CTX treatment did not alter basic physiological status and general activity in rats; whereas emotional and cognitive dysfunctions were clearly present in chronic P-CTX-1-poisoned rats. The clinical significance of these findings is compelling. These data provide, we believe, the first evidence in animals that repetitive exposure to sub-threshold levels of P-CTX-1 may cause irreversible sub-clinical damage, and eventually cause more severe illness characterized by cognitive deficits.
Conflict of interest
There are no conflicts of interest to declare.
Acknowledgments
This work was supported by the Research Grants Council of Hong Kong [11101315, 11100914, 160811, 160812, and 160713 to Y. Li], the Health and Medical Research Fund of Hong Kong [9211056 (CityU 01122006) to Y. Li], City University of Hong Kong Neuroscience Research Infrastructure Grant [9610211 to Y. Li], and the City University of Hong Kong Centre for Biosystems, Neuroscience, and Nanotechnology Grant [9360148 to S. Pang and Y. Li]. This work was also supported by the National Science Foundation of China [81170353 to Y. Li, 21307102 to J. Wu], the Shenzhen Knowledge Innovation Program on Basic Research Project [JCYJ20130401145617275 to Y. Li], and the Shenzhen Strategic Emerging Industry Development Special Project [CXZZ20130517151212424].
References
- Dickey R. W., Plakas S. M. Toxicon. 2010;56:123–136. doi: 10.1016/j.toxicon.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Vetter I., Touska F., Hess A., Hinsbey R., Sattler S., Lampert A., Sergejeva M., Sharov A., Collins L. S., Eberhardt M., Engel M., Cabot P. J., Wood J. N., Vlachova V., Reeh P. W., Lewis R. J., Zimmermann K. EMBO J. 2012;31:3795–3808. doi: 10.1038/emboj.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Cao B., Wang J., Liu J., Tung V. O. V., Lam P. K. S., Chan L. L., Li Y. NeuroMol. Med. 2013;15:310–323. doi: 10.1007/s12017-013-8220-7. [DOI] [PubMed] [Google Scholar]
- Arena P., Levin B., Fleming L. E., Friedman M. A., Blythe D. Harmful Algae. 2004;3:51–60. [Google Scholar]
- Glaziou P., Martin P. M. Toxicon. 1993;31:1151–1154. doi: 10.1016/0041-0101(93)90130-b. [DOI] [PubMed] [Google Scholar]
- Bottein Dechraoui M. Y., Rezvani A. H., Gordon C. J., Levin E. D., Ramsdell J. S. Toxicology. 2008;246:55–62. doi: 10.1016/j.tox.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Bagnis R. Hawaii Med. J. 1968;28:25–28. [PubMed] [Google Scholar]
- Bottein Dechraoui M. Y., Wang Z., Ramsdell J. S. Toxicon. 2007;49:100–105. doi: 10.1016/j.toxicon.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Bottein M. Y., Wang Z., Ramsdell J. S. Toxicology. 2011;284:1–6. doi: 10.1016/j.tox.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Dechraoui M. Y., Naar J., Pauillac S., Legrand A. M. Toxicon. 1999;37:125–143. doi: 10.1016/s0041-0101(98)00169-x. [DOI] [PubMed] [Google Scholar]
- Cao Z., Wu X., Chen S., Fan J., Zhang R., Owyang C., Li Y. Gastroenterology. 2008;134:535–543. doi: 10.1053/j.gastro.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Gao J., Wu X., Owyang C., Li Y. J. Physiol. 2006;570:169–183. doi: 10.1113/jphysiol.2005.096073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yan N., Cao B., Xu J., Hao C., Zhang X., Li Y. Neurobiol. Learn. Mem. 2012;97:156–164. doi: 10.1016/j.nlm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Rivalan M., Coutureau E., Fitoussi A., Dellu-Hagedorn F. Front. Behav. Neurosci. 2011;5:22. doi: 10.3389/fnbeh.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu L., Wang J., Cao B., Jelfs B., Chan R. H., Xu X., Hasan M., Zhang X., Li Y. Mol. Brain. 2015;8:32. doi: 10.1186/s13041-015-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Cao B., Wang J., Yu T., Li Y. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;60:26–35. doi: 10.1016/j.pnpbp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Tranel D., Damasio A. R. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A. R., Lee G. P. J. Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Tranel D., Damasio H. Brain. 2000;123(Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- de Visser L., Homberg J. R., Mitsogiannis M., Zeeb F. D., Rivalan M., Fitoussi A., Galhardo V., van den Bos R., Winstanley C. A., Dellu-Hagedom F. Front. Neurosci. 2011;5:109. doi: 10.3389/fnins.2011.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivalan M., Ahmed S. H., Dellu-Hagedorn F. Biol. Psychiatry. 2009;66:743–749. doi: 10.1016/j.biopsych.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Wu J. J., Mak Y. L., Murphy M. B., Lam J. C., Chan W. H., Wang M., Chan L. L., Lam P. K. Anal. Bioanal. Chem. 2011;400:3165–3175. doi: 10.1007/s00216-011-4977-4. [DOI] [PubMed] [Google Scholar]
- Komada M., Takao K., Miyakawa T. J. Visualized Exp. 2008 doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak Y. L., Wu J. J., Chan W. H., Murphy M. B., Lam J. C., Chan L. L., Lam P. K. Anal. Bioanal. Chem. 2013;405:3331–3340. doi: 10.1007/s00216-013-6766-8. [DOI] [PubMed] [Google Scholar]
- Chan T. Y., Kwok T. C. Hum. Exp. Toxicol. 2001;20:426–428. doi: 10.1191/096032701682692928. [DOI] [PubMed] [Google Scholar]
- Robertson A., Garcia A. C., Quintana H. A., Smith T. B., Castillo 2nd B. F., Reale-Munroe K., Gulli J. A., Olsen D. A., Hooe-Rollman J. I., Jester E. L., Klimek B. J., Plakas S. M. Mar. Drugs. 2014;12:88–97. doi: 10.3390/md12010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehane L., Lewis R. J. Int. J. Food Microbiol. 2000;61:91–125. doi: 10.1016/s0168-1605(00)00382-2. [DOI] [PubMed] [Google Scholar]
- Friedman M. A., Fleming L. E., Fernandez M., Bienfang P., Schrank K., Dickey R., Bottein M. Y., Backer L., Ayyar R., Weisman R., Watkins S., Granade R., Reich A. Mar. Drugs. 2008;6:456–479. doi: 10.3390/md20080022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearn J. J. Neurol., Neurosurg. Psychiatry. 2001;70:4–8. doi: 10.1136/jnnp.70.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. A., Arena P., Levin B., Fleming L., Fernandez M., Weisman R., Bernstein J., Schrank K., Blythe D., Backer L., Reich A. Arch. Clin. Neuropsychol. 2007;22:545–553. doi: 10.1016/j.acn.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Molgo J., Gaudry-Talarmain Y. M., Legrand A. M., Moulian N. Neurosci. Lett. 1993;160:65–68. doi: 10.1016/0304-3940(93)9000-4. [DOI] [PubMed] [Google Scholar]
- Birinyi-Strachan L. C., Gunning S. J., Lewis R. J., Nicholson G. M. Toxicol. Appl. Pharmacol. 2005;204:175–186. doi: 10.1016/j.taap.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Koga K., Descalzi G., Chen T., Ko H. G., Lu J., Li S., Son J., Kim T., Kwak C., Huganir R. L., Zhao M. G., Kaang B. K., Collingridge G. L., Zhuo M. Neuron. 2015;85:377–389. doi: 10.1016/j.neuron.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C., Hen R. Nat. Rev. Neurosci. 2004;5:545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz A. C., Burgos-Robles A., Bhagat N. D., Leppla C. A., Tye K. M. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuch E. A., Ketter T. A., Kimbrell T. A., George M. S., Benson B. E., Willis M. W., Herscovitch P., Post R. M. Biol. Psychiatry. 2000;48:1020–1023. doi: 10.1016/s0006-3223(00)00920-3. [DOI] [PubMed] [Google Scholar]
- Hay P., Sachdev P., Cumming S., Smith J. S., Lee T., Kitchener P., Matheson J. Acta Psychiatry Scand. 1993;87:197–207. doi: 10.1111/j.1600-0447.1993.tb03356.x. [DOI] [PubMed] [Google Scholar]
- Kim S. S., Wang H. S., Li X. Y., Chen T., Mercaldo V., Descalzi G., Wu L. J., Zhuo M. Mol. Brain. 2011;4:6. doi: 10.1186/1756-6606-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Gao J., Yan J., Fan J., Owyang C., Li Y. Am. J. Physiol.: Gastrointest. Liver Physiol. 2008;294:G918–G927. doi: 10.1152/ajpgi.00452.2007. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang X., Cao B., Liu J., Li Y. Cereb. Cortex. 2015;25:859–868. doi: 10.1093/cercor/bht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Wu X., Cao Z., Chen S., Owyang C., Li Y. Gastroenterology. 2009;136:1732–1740. doi: 10.1053/j.gastro.2024.01.004. [DOI] [PubMed] [Google Scholar]
- Teixeira C. M., Pomedli S. R., Maei H. R., Kee N., Frankland P. W. J. Neurosci. 2006;26:7555–7564. doi: 10.1523/JNEUROSCI.1068-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsson E. O., Nader K. Learn. Mem. 2012;19:449–452. doi: 10.1101/lm.027227.112. [DOI] [PubMed] [Google Scholar]
- Rahman S., Sahakian B., Cardinal R., Rogers R., Robbins T. Trends Cognit. Sci. 2001;5:271–277. doi: 10.1016/s1364-6613(00)01650-8. [DOI] [PubMed] [Google Scholar]
- Paulus M. P. Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]