 Exposure to heavy metals during pregnancy is an important risk factor for adverse birth outcomes.
Exposure to heavy metals during pregnancy is an important risk factor for adverse birth outcomes.
Abstract
Background: Exposure to heavy metals during pregnancy is an important risk factor for adverse birth outcomes. We aimed to investigate the current heavy metal exposure levels in cord blood from healthy pregnant women residing in the Huaihe River Basin, China, and examined the association between heavy metal levels and dietary habits and lifestyle factors. In this study, we measured the exposure levels of five heavy metals in the umbilical cord blood from 350 healthy pregnant women and administered 350 self-reported questionnaires regarding the general characteristics and dietary habits of those women. Methods: This study was undertaken in Shenqiu county, Henan province, which is in the area of the Huai River Basin, in a cohort of pregnant women and newborn babies in 2013–2014. We recruited a sample of 1000 pregnant women among those receiving prenatal examination, measured the real individual newborn exposure to heavy metals in serum by ICP-MS, collected information regarding the pregnant women with a questionnaire survey and obtained data on environmental quality from environmental protection agencies and the available literature. We estimated the daily individual exposure to heavy metals of all the 1000 participants throughout the pregnancy and recorded their birth outcomes after delivery. Then we analyzed the association between birth outcome and individual exposure to heavy metals. Results: 54 newborn children had birth defects. The geometric means of cord blood levels of As, Cd, Cr, Pb and Hg were measured at 0.92 ± 1.01 ng mL–1, 0.11 ± 0.17 ng mL–1, 4.57 ± 5.02 ng mL–1, 3.37 ± 3.81 ng mL–1 and 0.89 ± 1.69 ng mL–1 for subjects (n = 54) who gave birth to infants with birth defects and 0.43 ± 0.88 ng mL–1, 0.52 ± 3.86 ng mL–1, 1.94 ± 2.92 ng mL–1, 4.38 ± 4.96 ng mL–1 and 0.43 ± 0.91 ng mL–1 for subjects (n = 296) with healthy infants, respectively. The contents of all five heavy metals in the whole blood of both of these two groups were higher than the reference values of the Chinese general population (P < 0.001). Conclusions: The occurrence of birth defects was 15.4% in this cohort, and was correlated to exposure of parents to environments containing heavy metal contaminants in Shenqiu county in the Huai River Basin. The heavy metal exposure situation of the investigated population had serious effects in terms of reproductive defects in children. The specific link between newborn defects and environmental heavy metal contaminants suggested that contamination in pregnant women persisted over time, and that the exposure may have a long term effect.
Introduction
The term “heavy metals” generally refers to metal elements with relative density ≥25 in the chemical literature. Heavy metals exist widely in nature, but because of their exploitation, smelting, processing and commercial manufacture by human beings, increasing amounts of heavy metals, such as lead and cadmium, have entered the atmosphere, water and soil, resulting in environmental pollution. Heavy metals usually enter the body through the respiratory tract, the digestive tract and skin. Most heavy metals, such as cadmium, lead, and their relatives, are not necessary for biological processes, and heavy metals over a certain concentration are harmful to the human body. Excessive heavy metals may accumulate in some organs of the human body and interact with proteins and enzymes, making them inactive or damaged. In general, the toxic range of heavy metals is about 1–10 mg L–1, while for highly toxic metals such as mercury, the toxic mass concentration range is between 0.01 and 0.001 mg L–1, and even trace concentrations can cause poisoning and become enriched through the food chain, accumulating in more advanced organisms and causing chronic poisoning. Heavy metal pollution events have occurred frequently in recent years. According to incomplete statistics, 11 heavy metal pollution incidents occurred in China from January to August of 2011. A cadmium pollution incident occurred in the Longjiang river in Guangxi in 2012. There were 20 unexpected environmental events involving heavy metals in 2011 and 2012 leading to a serious threat to the ecological environment and the health of the population, resulting in negative social impacts. The chronic harm caused by heavy metal pollution in the environment is gradual, and the harmful effects are not easily detected or perceived. Once the more obvious symptoms appear, the body is often irreversibly damaged.
The Huaihe River Basin, one of the most populated areas in East China, has been reported to suffer environmental problems, including heavy metal contamination. Even though the Chinese government has made a great effort to control the pollution in the Huaihe river, serious environmental problems still exist. Recently, the cancer incidences of children and adolescents have increased rapidly in Shenqiu county, which has aroused widespread concern among researchers in China. Data on polluting enterprises (including existing enterprises and those discontinued since 1985) in Shenqiu county and the Shaying river upstream region have been collected. The results show that the major polluting industries among current and historical enterprises were leather, paper, chemicals, printing and plastics, which discharged large volumes of waste water containing various organic pollutants and heavy metals. The irresponsible use of sewage irrigation and chemical fertilizers and pesticides during agricultural production is an important source of heavy metal pollution. Cadmium (Cd), arsenic (As) and chromium (Cr) have been classified as first class carcinogens by the International Agency for Research on Cancer (IARC). The biological half-life of Cd in the human body is 10–25 years, so it is possible for long-term toxic accumulation in the body to cause damage to the bones, kidney, and liver, including obvious effects on reproductive toxicity and endocrine disruption. The element As can harm many systems and organs, such as the liver, kidney, nervous system, cardiovascular system, etc. Drinking water contaminated with chromium-containing industrial waste water can cause abdominal discomfort, diarrhea and other toxic symptoms due to the presence of six-valent chromium. Chromium is also a skin irritant that can cause allergic dermatitis. Mercury (Hg) is listed as a global pollutant by the United Nations Environment Programme (UNEP) and every year nearly 5000 tons of various forms of mercury are discharged into the environment. It is then converted into organic mercury, including methyl mercury, dimethyl mercury etc. Methyl mercury is fat soluble, highly accumulable and can enter the fetus through the placental barrier and affect fetal development. Lead (Pb) affects the human nervous system, the blood system, the gastrointestinal system, the cardiovascular system and the kidney system. The number of deaths caused by Pb exposure is about 143 000 cases per year, accounting for 0.6% of the global burden of disease. Children are especially susceptible to the neurotoxicity of Pb and even low levels of exposure may cause irreversible nerve damage. Inorganic Pb has been identified by the IARC and the Department of Health and Human Services (DHHS) as a probable carcinogen for humans (USEPA, 2011b).
A large number of data shows that As, Cd, and Pb can fully or partially pass through the placental barrier into the fetus, to influence fetal growth and health,1–9 and can be harmful to the nervous system even at low levels of exposure.10–13 The effects of Pb exposure during pregnancy on birth outcomes include lower birth weight,14 lower birth crown-heel length and head circumference,15–17 and preterm birth.18,19 Also, several studies have reported that methyl mercury can easily cross the placenta and affect cognitive development.3,4,20–22 Although the placenta acts as a barrier, protecting the fetus from Cd exposure by increasing metallothionein expression,23 the presence of Cd in cord blood has been associated with decreased birth weight24 and increased incidence of preterm delivery.25 However, so far there appears to be little or no available information regarding current heavy metal exposure levels among pregnant women. This study was undertaken in Shenqiu county, which has been reported to suffer serious environmental problems with high cancer incidences in children and adolescents. Rather than focusing on the cancer, we aimed to find the association between birth outcomes and individual exposure to heavy metal pollutants (As, Cd, Cr, Pb and Hg). We hypothesized that the incidence of reproductive defects may be associated with the exposure levels of pregnant women to various environmental heavy metal pollutants during gestation and even the preconceptional period.
Heavy metal poisoning has the characteristics of long-term build-up, accumulation, difficulty of detection, and irreversibility. The serious difficulty of detection necessitates the use of professional laboratory instruments to identify the condition. The term “individual heavy metal exposure” refers to the internal exposure levels of affected persons. Human biomonitoring of heavy metals is of great significance for evaluating the public health status and developing medical diagnostic standards and environmental hygiene standards. Against this background, the development of techniques for the effective determination of heavy metals and their speciation in biological matrices is an area which has been attracting increasing attention in recent years. Since its introduction in 1980, inductively coupled plasma mass spectrometry (ICP-MS) has developed into an accurate and sensitive technique for multi-element determinations in a range of sample matrices. Compared with flame atomic absorption spectroscopy (FAAS), graphite furnace atomic absorption spectroscopy (GFAAS) and ICP-optical emission spectroscopy (ICP-OES), ICP-MS has the advantages of lower detection limit, high sensitivity, and simultaneous multi-element analysis, making it suitable for human biomonitoring of heavy metals. To evaluate the potential health risks of heavy metals (Cr, Cd, Pb, As and Hg) in terms of birth defects (BD), we investigated (i) the concentrations of those five metals in umbilical cord blood using ICP-MS and (ii) related lifestyle and dietary intake factors among pregnant women of the Huaihe River Basin in China. The aim was to obtain useful practical experience for the method development of heavy metal biomonitoring and to test for correlations between the concentrations of heavy metals and the occurrence of human birth defects.
Methods
Study design and scope
The study was conducted between November 2013 and June 2014, and a total of 371 pregnant women were enrolled from a local hospital in the Huaihe River Basin. Responses to 371 questionnaires were obtained, and 349 umbilical cord blood samples were collected. None of the participants had been occupationally exposed to heavy metals. Women with chronic illnesses (diabetes, renal, cardiovascular, hepatobiliary, thyroid-related, and pulmonary diseases, HIV, etc.) and pregnancy complications (pregnancy-induced hypertension, urinary tract infection, etc.) were excluded. Exclusions were also made if they had resided for less than one year in the study area. Before umbilical cord blood was obtained, the pregnant women were required to sign a consent form after receiving a detailed explanation of the study. Trained personnel interviewed both parents at their home to complete an epidemiological questionnaire (socio-demographic factors, environmental and occupational exposures). Participants provided details regarding age, education level, monthly household income, smoking habits, alcoholic beverage consumption, length of residency, parity, history of disease, and dietary habits through a questionnaire. Dietary information during the period of gestation was gathered from a food frequency questionnaire (FFQ). The diet items of the FFQ included staple foods (rice, steamed bread, noodles, corn), red meat (pork, beef, lamb), poultry (chicken, duck, goose), fish, seafood (shrimp, shellfish, sea cucumbers, crab), fruit, bean products, milk, yogurt, eggs, dry fruits, tubers (potato, sweet potato), vegetables, pickles, and tea. The responses of dietary intake habits were divided into the following categories: never or less than once a month, 1–3 times per month, 1–6 times per week, and ≥1 time per day. All mothers were surveyed by a trained interviewer. Approximately 10 mL of umbilical cord blood was collected in EDTA vials at the time of delivery, and the centrifuged plasma samples were stored at –80 °C before use. This study was reviewed and approved by the Ethics Committee of the National Institute for Occupational Health and Poison Control, Chinese Center for Disease Control and Prevention (IRB no. 201310).
Study area and sampling sites
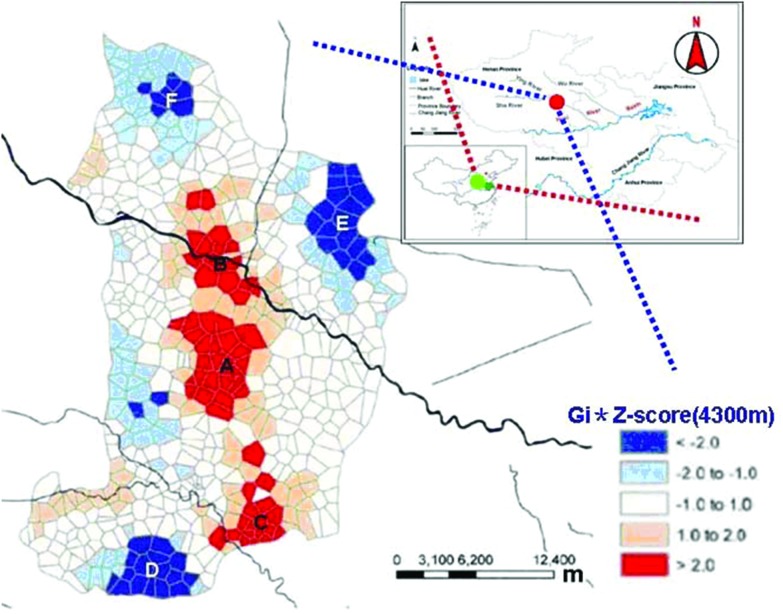
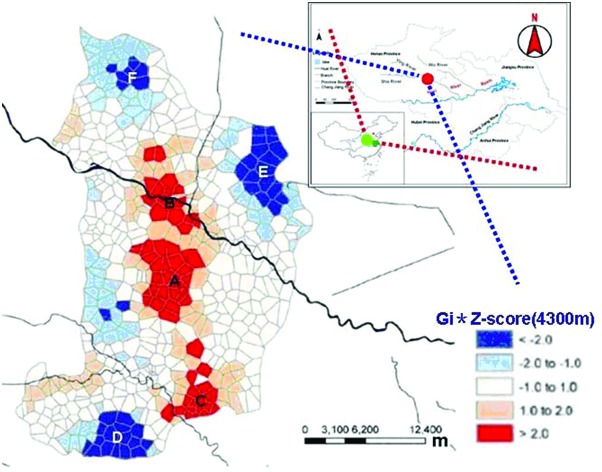
The study sites were located in Shenqiu county of Henan province (110°21′–116°39′E, 31°23′–36°22′N) in East Mid China, which has a population of 1.22 million. The Huai River runs through the area. The northwest of Shenqiu county is an area of higher terrain, 42 meters above sea level, while the elevation is slightly lower in the southeast, 36 meters above sea level. The Shaying river is a major river of the Huai river basin. Shenqiu county experiences a warm temperate continental monsoon climate, with an annual average temperature of 14.5 °C, average annual precipitation of about 700 mm, and annual frost free period of about 200 days. In addition to rapid urbanization and industrialization, Shenqiu adopted electric power, electronics, machinery, chemicals, textiles, food, building materials, and the leather industry as the pillars of its economy. The study area has experienced economic development during the last few decades: the county achieved a total GDP of ¥1845 billion in 2013, an increase of 10.1% compared to the previous year. The level of local public finance reached a historic high of ¥106 billion, an increase of 26.3% over the previous year (China NBoSo, 2013). This growth is likely to have been accompanied by unprecedented environmental changes. The sampling sites were primarily chosen from the Shenqiu section of the Shaying river, which is a high incidence region of cancer (Fig. 1A–F). Areas A–C are high exposure areas and E–F are low exposure areas. On the basis of a preliminary environmental investigation focused on the area of the Huaihe River Basin, including the river direction, local conditions and an epidemiological investigation, the sampling points were set up to include 1 per every 20000 people. A total of 350 newborn cord blood samples were collected in 22 health centers in small towns in Shenqiu county during the study period.
Fig. 1. The sampling sites were selected according to the distribution of cancer incidence in Shenqiu County: A–C are high cancer incidence areas (aggregate index > 2.0 with red color); E–F are low cancer incidence areas (aggregate index ≤ 2.0 with deep blue color).
ICP-MS analysis
All samples were analyzed for Cd, Cr, As, Hg and Pb using an inductively coupled plasma mass spectrometer (ICP-MS, Thermo X Series 2). Digested samples were typically diluted 20× with 2% nitric acid for analysis. The composition of the digestion reagent was 500 μL nitric acid and 200 μL hydrogen peroxide. After heating in a boiling water bath for 2 hours, sample digestion was complete and the measurement result was satisfactory. A quantitative method was developed for the analysis of Cr, Cd, As, Hg, and Pb in the plasma samples. The correlation coefficients of the calibration curves were larger than 0.999 in the range of 0.43–10 μg L–1. The limits of detection (LODs) were 0.2 (Cd), 2.5 (Pb), 5 (Cr), 5 (Hg), 25 (As) μg kg–1. The spiked recoveries were in the range of 83.1%–111.7% with relative standard deviations less than 7.9%, which meets the basic requirements of determination.
Quality assurance and quality control
Quantification of the targeted compounds was performed by the internal standard addition method with 115In. Each batch of 20 samples analyzed included one quality control plasma sample and one method blank sample in this study. Each sample was measured in duplicate. The calibration curves were plotted in the range of 1.0–200 ng mL–1 for each analyte, with R2 greater than 0.996. The limit of detection (LOD) and the limit of quantitation (LOQ) of the targeted compounds were defined as 3 times the ratio of signal-to-noise (S/N = 3) and 10 times the ratio of S/N (S/N = 10), respectively. The corresponding LOD and LOQ of As, Cd, Cr, Pb, and Hg were 25, 0.2, 5, 2.5, 5 ng mL–1 and 50, 0.5, 10, 5, 10 ng mL–1, respectively. The recoveries of all compounds ranged from 83.1% to 111.7% with relative standard deviations (RSDs) <7.9%.
Data analysis
In all statistical analyses, concentrations below the LOD were set at half of the LOD. The concentrations of all heavy metals were expressed as nanogram per milliliter (ng mL–1). The heavy metal levels showed nonnormal distributions and were log-transformed. To evaluate the association between heavy metals and related factors such as age, education levels, smoking status, monthly household income, parity, and dietary intake, a Spearman rank correlation was conducted. Multiple linear regression analyses with a stepwise approach were used to assess the effect of potential confounders, based on the Spearman rank correlation results, on the heavy metal levels in cord plasma. The analysis via Spearman rank correlation and multiple linear regression models in our study found that all heavy metals had a detection frequency >50%. All statistical analyses were conducted with SPSS version 19.0 for Windows, and two-sided p < 0.05 was considered statistically significant.
Results
The general characteristics of the selected pregnant women are presented in Table 1. Because of missing demographic information and cord blood samples, only 350 of the 365 pregnant women were included in the final statistical analyses. The mean age of the 350 pregnant women who participated in the study was 26.05 ± 3.84 years. Most of the women (95.1%) and their spouses (98.0%) had middle and high school education levels. Most of the women (90.3%) had a length of residency less than 10 years, and the mean length of residency was 7.0 ± 2.1 years. The monthly family household income was subdivided into less than 1000 yuan (14.3%), between 1000 and 2999 yuan (22.9%), between 3000 and 4999 yuan (41.7%), and more than 5000 yuan (21.1%). Among all subjects, 3 women (0.9%) had a history of smoking, 22 women (6.3%) passively smoked, and 9 women (2.6%) consumed alcoholic beverages during pregnancy. More than half (57.4%) of the pregnant women were primipara, and the mean menarche age of all pregnant women was 14.2 ± 1.1 years.
Table 1. General characteristics of the pregnant women participating in the study.
| Characteristics | N | Mean ± S.D or % |
| Age (years) | 350 | 26.05 ± 3.84 |
| Menarche age (years) | 350 | 14.1 ± 1.1 |
| Education level of the pregnant women | ||
| None or elementary school | 17 | 4.9 |
| Middle and high school | 312 | 89.1 |
| College and university or above | 21 | 6.0 |
| Education level of their spouses | ||
| None or elementary school | 7 | 2.0 |
| Middle and high school | 326 | 93.1 |
| College and university or above | 17 | 4.9 |
| Length of residency (years) | 350 | 7.0 ± 2.1 |
| <10 | 316 | 90.3 |
| ≥10 | 34 | 9.7 |
| Monthly household income (Yuan) | ||
| <1000 | 50 | 14.3 |
| 1000–2999 | 80 | 22.9 |
| 3000–4999 | 146 | 41.7 |
| ≥5000 | 74 | 21.1 |
| Smoking history of the pregnant women | ||
| Yes | 3 | 0.9 |
| No | 347 | 99.1 |
| Passive smoking of the pregnant women | ||
| Yes | 22 | 6.3 |
| No | 328 | 93.7 |
| Alcoholic beverage consumption of the pregnant women | ||
| Yes | 9 | 2.6 |
| No | 341 | 97.4 |
| Smoking status of their spouses | ||
| Yes | 142 | 40.6 |
| No | 208 | 59.4 |
| Alcohol consumption of their spouses | ||
| Yes | 328 | 93.7 |
| No | 22 | 6.3 |
| Parity | ||
| 1 | 201 | 57.4 |
| 2 | 132 | 37.7 |
| ≥3 | 17 | 4.9 |
The results of the concentrations of heavy metals in cord blood are shown in Table 2. The detection frequencies of all analytes were between 54.32% and 93.83%, among which Cr, Pb, Hg, Cd and As had detection frequencies of 93.83%, 93.52%, 93.21%, 59.26%, and 54.32%, respectively. Cr, Pb and Hg were the dominant heavy metals, with mean levels of 0.23 ± 1.29, 1.13 ± 19.73 and 0.07 ± 0.81 ng mL–1, respectively, as well as As and Cd with mean concentrations of 0.07 ± 1.26 and 0.03 ± 0.44 ng mL–1 respectively.
Table 2. Cord blood concentrations of heavy metals in newborns with birth defects (ng mL–1; n = 54).
| Analytes | Mean | SD | Median | Range | Frequency (%) |
| As | 0.92 | 1.01 | 0 | ND to 4.09 | 54.32 |
| Cd | 0.03 | 0.44 | 0.43 | ND to 2.49 | 59.26 |
| Cr | 0.23 | 1.29 | 0.92 | ND to 10.02 | 93.83 |
| Pb | 1.13 | 19.73 | 19.27 | ND to 19.96 | 93.52 |
| Hg | 0.07 | 0.81 | 0.85 | ND to 1.08 | 93.21 |
Table 3 shows the associations among As, Cd, Cr, Pb and Hg in cord blood. There were significant Spearman rank correlation coefficients (p < 0.01) in cord blood among the dominant heavy metals.
Table 3. The results of Spearman rank correlation analyses: associations among As, Cd, Cr, Pb, Hg.
| As | Cd | Cr | Pb | Hg | |
| As | 1.00 | ||||
| Cd | 0.794** | 1.00 | |||
| Cr | 0.491** | 0.463** | 1.00 | ||
| Pb | –0.256** | –0.135** | 0.393** | 1.00 | |
| Hg | 0.379** | 0.424** | 0.657** | 0.512** | 1.00 |
The associations between the levels of major heavy metals, general characteristics, and dietary intake habits are shown in Table 4. According to the results of the Spearman rank correlation, we found statistically significant but weak correlations between the levels of major heavy metals, general characteristics and dietary intake habits, with the absolute value of the correlation coefficients ranging from –0.231 to 0.990. The cord blood concentration of the heavy metal As was significantly associated with the level of the monthly household income (rs = –0.137, p < 0.05), passive smoking (rs = 0.118, p < 0.05), and consumption of bean products (rs = 0.150, p < 0.01); the level of Cd was correlated with the monthly household income level (rs = –0.179, p < 0.01), passive smoking (rs = 0.116, p < 0.05), and consumption of bean products (rs = 0.143, p < 0.01); the level of Cr was correlated with the monthly household income level (rs = –0.231, p < 0.01) and consumption of vegetables (rs = 0.139, p < 0.01); the level of Pb was correlated with age (rs = –0.115, p < 0.05), monthly household income level (rs = –0.207, p < 0.01), passive smoking (rs = 0.120, p < 0.05), and consumption of red meat (rs = –0.194, p < 0.01), fish (rs = –0.124, p < 0.05), bean products (rs = –0.198, p < 0.01), milk (rs = –0.143, p < 0.01), eggs (rs = –0.109, p < 0.05) and vegetables (rs = –0.113, p < 0.05); the level of Hg was correlated with the monthly household income level (rs = –0.227, p < 0.01), passive smoking (rs = 0.141, p < 0.01), and consumption of red meat (rs = –0.130, p < 0.05) and vegetables (rs = –0.114, p < 0.01) with statistically significant differences.
Table 4. Correlation coefficients of selected heavy metals, general characteristics, and dietary intake habits.
| Variables | As |
Cd |
Cr |
Pb |
Hg |
|||||
| r s | p | r s | p | r s | p | r s | p | r s | p | |
| Age | –0.073 | 0.175 | –0.040 | 0.455 | –0.101 | 0.059 | –0.115 | 0.032* | –0.069 | 0.195 |
| Education level of the pregnant women | 0.104 | 0.652 | 0.068 | 0.208 | 0.061 | 0.257 | –0.048 | 0.373 | 0.061 | 0.255 |
| Length of residency | –0.036 | 0.502 | 0.019 | 0.720 | 0.050 | 0.349 | –0.032 | 0.551 | 0.030 | 0.573 |
| Monthly household income | –0.137 | 0.011* | –0.179 | 0.001** | –0.231 | 0.000** | –0.207 | 0.000** | –0.227 | 0.000** |
| Smoking history of the pregnant women | 0.113 | 0.035 | 0.65 | 0.226 | –0.023 | 0.669 | –0.064 | 0.230 | 0.104 | 0.053 |
| Passive smoking of the pregnant women | 0.118 | 0.027* | 0.116 | 0.030* | 0.076 | 0.115 | 0.120 | 0.024* | 0.141 | 0.008** |
| Alcoholic beverage consumption of the pregnant women | 0.015 | 0.773 | 0.031 | 0.560 | 0.025 | 0.639 | –0.070 | 0.189 | –0.040 | 0.453 |
| Parity | 0.012 | 0.826 | 0.079 | 0.138 | –0.055 | 0.309 | –0.058 | 0.280 | 0.009 | 0.870 |
| Smoking status of their spouses | –0.024 | 0.656 | –0.092 | 0.086 | 0.011 | 0.842 | –0.005 | 0.921 | –0.019 | 0.728 |
| Staple food (rice, steamed bread, noodle, corn) | 0.084 | 0.115 | –0.035 | 0.519 | –0.060 | 0.264 | –0.124 | 0.021 | –0.087 | 0.104 |
| Red meat (pork, beef, lamb) | 0.059 | 0.270 | –0.011 | 0.834 | –0.058 | 0.281 | –0.194 | 0.000** | –0.130 | 0.015* |
| Poultry (chicken, duck, goose) | –0.099 | 0.063 | –0.067 | 0.212 | –0.076 | 0.158 | –0.048 | 0.374 | –0.063 | 0.237 |
| Fish | 0.038 | 0.477 | 0.000 | 0.993 | –0.048 | 0.369 | –0.124 | 0.021* | –0.102 | 0.056 |
| Fruit | –0.060 | 0.262 | –0.063 | 0.238 | –0.081 | 0.130 | –0.063 | 0.242 | –0.054 | 0.316 |
| Bean product | 0.150 | 0.005** | 0.143 | 0.007** | –0.053 | 0.319 | –0.198 | 0.000** | 0.045 | 0.396 |
| Milk | 0.092 | 0.084 | 0.056 | 0.300 | –0.098 | 0.066 | –0.143 | 0.008** | –0.082 | 0.125 |
| Eggs | 0.076 | 0.157 | 0.071 | 0.185 | –0.043 | 0.419 | –0.109 | 0.042* | –0.001 | 0.990 |
| Vegetables | 0.073 | 0.173 | –0.044 | 0.142 | –0.139 | 0.009** | –0.113 | 0.034* | –0.114 | 0.006** |
| Pickles | 0.010 | 0.849 | 0.053 | 0.319 | 0.020 | 0.712 | 0.014 | 0.799 | 0.096 | 0.074 |
| Folic acid | –0.083 | 0.119 | –0.079 | 0.142 | –0.005 | 0.926 | 0.014 | 0.789 | –0.042 | 0.429 |
| Mulvital | –0.003 | 0.950 | 0.013 | 0.803 | 0.018 | 0.733 | 0.051 | 0.342 | 0.088 | 0.099 |
The results of multiple linear regression analyses of As, Cd, Cr, Pb and Hg are given in Table 5. The concentration of As in cord blood was significantly and positively correlated with passive smoking (β = 0.189, p < 0.01) and bean products consumption (β = 0.219, p < 0.01), while the association between As level and monthly household income was negative (β = –0.086, p < 0.05). The cord blood Cr level was significantly higher than its constant (β = 3.665, p < 0.01) and negatively correlated with monthly household income (β = –0.252, p < 0.05). The concentration of Pb in cord blood was significantly higher than its constant (β = 7.719, p < 0.01) and positively correlated with passive smoking (β = 0.752, p < 0.01) while there were negative associations with monthly household income (β = –0.490, p < 0.05) and red meat consumption (β = –0.659, p < 0.01). The cord blood Hg level was significantly higher than its constant (β = 0.586, p < 0.01) and positively correlated with monthly household income (β = 0.087, p < 0.01). The concentration of Cd in cord blood was correlated with monthly household income, passive smoking and bean products consumption but the associations were not significant.
Table 5. Multiple linear regression analyses of As, log Cd, log Cr, log Pb, and log Hg.
| Unstandardized coefficients |
Standardized coefficients | t | Sig. | 95% Confidence interval for β |
|||
| β | Std. error | β | Lower bound | Upper bound | |||
| As constant | –0.068 | 0.240 | –0.285 | 0.776 | –0.626 | 0.513 | |
| Monthly household income | –0.086 | 0.037 | –0.122 | –2.320 | 0.034* | –0.159 | –0.006 |
| Passive smoking | 0.189 | 0.081 | 0.123 | 2.344 | 0.003** | 0.083 | 0.301 |
| Bean product | 0.219 | 0.067 | 0.172 | 3.287 | 0.003** | 0.072 | 0.351 |
| Cd constant | 0.472 | 0.687 | 0.687 | 0.493 | –0.269 | 1.868 | |
| Monthly household income | 0.018 | 0.106 | 0.009 | 0.165 | 0.707 | –0.048 | 0.136 |
| Passive smoking | 0.048 | 0.231 | 0.011 | 0.206 | 0.396 | –0.066 | 0.104 |
| Bean product | –0.282 | 0.191 | –0.079 | –1.474 | 0.510 | –1.093 | 0.110 |
| Cr constant | 3.665 | 0.546 | 6.716 | 0.000** | 2.406 | 5.028 | |
| Monthly household income | –0.252 | 0.093 | –0.148 | –2.718 | 0.014* | –0.442 | –0.070 |
| Vegetables | –0.349 | 0.233 | –0.081 | –1.497 | 0.185 | –1.008 | 1.105 |
| Pb constant | 7.719 | 1.902 | 4.059 | 0.003** | 2.982 | 13.264 | |
| Age | –0.022 | 0.066 | –0.018 | –0.339 | 0.769 | –0.208 | 0.117 |
| Monthly household income | –0.490 | 0.153 | –0.186 | –3.192 | 0.031* | –0.856 | –0.100 |
| Passive smoking | 0.752 | 0.304 | 0.130 | 2.476 | 0.014* | –0.046 | 2.475 |
| Bean product | –0.260 | 0.273 | –0.054 | –0.955 | 0.399 | –0.968 | 0.284 |
| Milk | –0.440 | 0.217 | –0.108 | –2.029 | 0.043 | –0.779 | 0.009 |
| Egg | 0.102 | 0.292 | 0.019 | 0.348 | 0.664 | –0.372 | 0.537 |
| Fish | 0.260 | 0.217 | 0.069 | 1.197 | 0.232 | –0.247 | 0.861 |
| Red meat | –0.659 | 0.310 | –0.119 | –2.128 | 0.006** | –1.066 | –0.271 |
| Vegetables | –0.180 | 0.364 | –0.027 | –0.496 | 0.590 | –0.741 | 0.502 |
| Hg constant | 0.586 | 0.151 | 3.869 | 0.000** | 0.309 | 0.878 | |
| Monthly household income | –0.032 | 0.026 | –0.069 | –1.231 | 0.231 | –0.077 | 0.021 |
| Passive smoking | 0.087 | 0.055 | 0.086 | 1.590 | 0.003** | 0.034 | 0.127 |
| Red meat | 0.047 | 0.053 | 0.048 | 0.875 | 0.382 | –0.070 | 0.203 |
| Vegetables | –0.066 | 0.064 | –0.057 | –1.026 | 0.306 | –0.226 | 0.084 |
In China, especially in the Huaihe River Basin, few studies have evaluated prenatal exposure to heavy metals. In this study, we observed 65 children with birth defects among all 350 birth outcomes. The corresponding incidence was 185.7‰ in Shenqiu county in the Huaihe River Basin, which was much higher than that of other areas in China. For example, Wang et al. observed 295 children with birth defects among all 4856 birth outcomes, representing an incidence of 60.75‰, in Ma'anshan.26 In our study, birth defects occurred in about 14.28‰ of twin pregnancies, compared to 171.43‰ in singletons. Of the 350 newborns, 203 were boys and 147 were girls. The incidences of birth defects were 94.28‰ and 71.43‰ for boys and girls, respectively. 64 newborns had a single defect and 1 had two defects, representing incidences of 182.86‰ and 2.86‰, respectively.
This study in a Shenqiu birth cohort reported a total of 3 nervous system congenital malformations, 22 eye, ear, face and neck congenital malformations, 4 circulatory system congenital malformations, 4 cleft lip and cleft palate cases, 2 genital organ congenital malformations, 2 urinary system congenital malformations, 7 musculoskeletal system congenital malformations, and 21 skin system congenital malformations. Their incidences were 8.59‰, 63.04‰, 11.46‰, 11.46‰, 5.73‰, 5.73‰, 20.06‰ and 60.17‰ respectively. According to the composition ratio, the order of birth defects from highest to lowest was eye, ear, face and neck congenital malformations 40.74%, skin system congenital malformations 38.89%, musculoskeletal system congenital malformations 12.96%, circulatory system congenital malformations and cleft lip and cleft palate cases both 7.41%, nervous system congenital malformations 5.55%, and genital organ congenital malformations and urinary system congenital malformations both 3.70%.
To analyze the correlations among heavy metal exposure levels and specific birth defects, a Spearman correlation analysis was conducted between the 5 kinds of heavy metal and the different birth defect groups. The results are shown in Table 6. The correlation analysis identified a number of high correlations among Cr and/or Cd levels and birth defect samples. However, the only statistically significant correlations were between Cr and eye, ear, face and neck congenital malformations (p < 0.05) and musculoskeletal system congenital malformations (p < 0.01), and between Cd and skin system congenital malformations (p < 0.01).
Table 6. Correlations among heavy metals in cord blood of newborns with birth defects from Shenqiu county.
| EEFNCM | SYCM | MSCM | CSCM | CLCP | NSCM | GOUCM | |
| As | 0.131 | 0.079 | 0.268 | 0.192 | 0.115 | 0.231 | 0.014 |
| Cd | 0.179 | 0.375** | 0.119 | 0.058 | 0.019 | 0.221 | 0.113 |
| Cr | 0.290* | 0.095 | 0.382** | 0.231 | 0.211 | 0.043 | 0.182 |
| Pb | 0.093 | 0.259 | 0.198 | 0.048 | 0.019 | 0.231 | 0.117 |
| Hg | 0.072 | 0.173 | 0.287* | 0.115 | 0.163 | 0.067 | 0.141 |
To analyze the correlations between heavy metal exposure and the occurrence of any birth defects a one-way ANOVA and T-test analysis was conducted between the 5 kinds of heavy metal and the different birth outcome groups. The results are shown in Table 7. Compared to the healthy newborns group, the levels of As, Cd, Cr and Hg were all higher in the group of newborns with birth defects (p < 0.01).
Table 7. Cord blood concentrations of heavy metals in newborns with birth defects and healthy newborns.
| Analytes | Birth defect newborns | Health newborns | t | p |
| As | 0.92 ± 1.01** | 0.43 ± 0.88 | 27.22 | <0.001 |
| Cd | 0.11 ± 0.17** | 0.52 ± 3.86 | 42.27 | <0.001 |
| Cr | 4.57 ± 5.02** | 1.93 ± 2.92 | 5.28 | <0.001 |
| Pb | 3.37 ± 3.81 | 4.38 ± 4.96 | 1.40 | >0.05 |
| Hg | 0.89 ± 1.69** | 0.43 ± 0.91 | 2.81 | <0.01 |
Discussion
This study found evidence that maternal environmental exposure to heavy metals is a risk factor for newborn birth defects, and that life style during pregnancy also had a potential influence on the incidence. These results of our study can be considered as robust due to the use of various procedures, including confirmation of the clinical diagnosis of BD and its severity by two practitioners, and an extensive interviewer-based questionnaire involving the use of a validated internal exposure index for heavy metals. Most previous studies on BD have been based on routinely collected registry data with limited information on potential risk factors, no information on internal exposure levels and covariates and varying levels of quality control and completeness. Based on the measured concentrations and detection frequencies, Cd, Pb, and Hg were the most dominant heavy metals, with median concentrations of 0.92, 19.27, and 0.85 ng mL–1, respectively, and detection frequencies ranging from 93.21% to 93.83%, among the five heavy metals from the 350 pregnant women included in this study. The high frequencies indicated that the method is stable and only minimal data loss occurred during ICP-MS detection. The measured concentration data of heavy metals also provide good evidence that the internal exposure index from blood samples is reliable for tracing heavy metals in vivo.
Moreover, the specific heavy metal elements and their sources could also provide information regarding the contamination history of the environment. With the Huai river valley's rapid urbanization and industrialization, an increasing volume of waste discharge has led to environmental contamination by heavy metals. Mass produced goods and non-treated sewage have directly entered the river via industrial discharge, and waste has also been dumped into the river. In particular, the area has suffered from the development of a number of small paper mills, leather mills, chemical plants, and other industrial enterprises which have huge sewage outputs with deleterious social impacts. These factors have all subjected the Huai river to extremely serious ecological and environmental pollution. A substantial number of severe pollution events have occurred in the Huai river Area in recent years. Pollution has significantly degraded the water quality in about 2/3 of the river and this has already threatened the safety of drinking water in some areas. The drinking water, soil, leafy vegetables and wheat have been polluted in the Shenqiu section of Shaying river via horizontal spread and vertical permeability. Heavy metals are non-biodegradable and persistent. This causes changes in the pH, oxidation–reduction potential and organic matter composition of the sludge that is applied to cultivated fields and incorporated into the soil, a possible route by which heavy metals could transfer into the food chain.27–34 Airborne heavy metal pollutants have been found in street dust,35,36 resuspended dust37 and other airborne sources38–40 and accumulated in plants grown in topsoil, posing potential risks to human health.41,42 For heavy metals that are shown to be carcinogens, their accumulation in vegetables and fruits may increase the risk of cancer in individuals who consume these foods.31 The pollution status of heavy metals in sediments and soils from the Shenqiu section of Shaying river was investigated by Li43–45 and the levels of As, Cr, Hg, Cd and Pb were detected and the potential ecological risks of these metals were calculated using the Hakanson potential ecological risks index. The level of As was 9.206–11.641 mg kg–1 in sediments and 8.52–80.31 mg kg–1 in soil. The excessive rate of As was 57.8%. The excessive rates of Cr, Hg, Cd and Pb were 32.8%, 59.4%, 67.2% and 39.1%, respectively. The total potential ecological risks of heavy metals in sediments and soils from the Shenqiu section of Shaying river were mainly posed by Hg and As (Table 8). These data provide evidence that the environmental heavy metal pollution along the river is very severe and has raised the internal exposure levels of Pb, Hg, and Cd in the residents of Shenqiu county.
Table 8. Comparison of investigated mean levels of heavy metals (mg kg–1 ) in different environmental mediums in Shenqiu county, Huaihe river area.
| Heavy metals | Soil (mg kg–1) |
Drinking Water (μg L–1) |
Grain (mg kg–1) |
Sediment (mg kg–1) |
||||
| Shenqiu | SL a | Shenqiu | SL b | Shenqiu | SL c | Shenqiu | SL | |
| As | 34.67 ± 14.37 | 30 | 17.28 ± 4.46 | 10 | 0.27 ± 0.09 | 0.15 | 11.64 ± 2.99 | NA |
| Cd | 0.55 ± 0.30 | 0.45 | 5.58 ± 2.14 | 5 | 0.24 ± 0.11 | 0.20 | 0.73 ± 0.15 | NA |
| Cr | 184.80 ± 97.12 | 200 | 25.24 ± 4.42 | 50 | 1.01 ± 0.19 | 1.00 | 101.17 ± 30.72 | NA |
| Pb | 90.00 ± 55.89 | 80 | 17.20 ± 8.30 | 10 | 0.33 ± 0.16 | 0.20 | 143.54 ± 52.43 | NA |
| Hg | 1.14 ± 0.83 | 0.70 | 0.81 ± 5.16 | 1 | 0.03 ± 0.02 | 0.02 | 1.10 ± 0.41 | NA |
aSL: safety limits (GB 15618-2008).
bSL: safety limits (GB 5749-2006).
cSL: safety limits (GB 2762-2005).
The association observed between maternal age and Pb exposure may be related to exposure time. More advanced parental age was also identified as a risk factor for BD, as reported in some studies.46,47 Monthly household income was also found to be negatively correlated with exposure to all five heavy metals, which indicated that the family economic condition played an important role in the life style of the pregnant women. Families with high monthly household income could purchase relatively safe food and water without heavy metal pollution. Passive smoking by the pregnant women was also correlated with exposure to four of the heavy metals but not Cd, indicating polluted indoor air to be a risk factor for BD because Cr, As, Pb and Hg are the main components of cigarette smoke, while Cd entered the body mainly through the digestive tract and skin. The correlations of bean product consumption with markedly high As, Cd and Pb levels and of vegetable consumption with high Cr, Pb and Hg exposure also indirectly showed that heavy metals in the environment were enriched in the food chain through contaminated water and soil. However, the correlation between paternal smoking status and heavy metal exposure was lower, which indicated that the life style of the mother, but not the father, had a decisive impact on newborn birth defects. These results were also identified by the multiple linear regression analyses of the five heavy metals with general characteristics and dietary intake habits.
Considerable efforts have been made over recent years to test and identify the effects of heavy metals on the ecological environment, but their effects on newborns remain difficult to determine. Cases were included prospectively in this study to evaluate the influence of foetal exposure to heavy metals on the incidence of BD in the Huai River Basin. We were specifically interested in certain environmental heavy metal pollutants in order to identify ways of preventing maternal exposure during pregnancy. After ruling out 22 pregnant women with whom we lost contact, we observed 54 children with birth defects among all 349 birth outcomes. Birth defects thus occurred in 15.47% of cases. The 3 most common categories of birth defects in Shenqiu accounted for 92.50% of the total 54 birth defects, indicating that the distribution of defect types was very concentrated. Therefore, the government should focus on the three-level prevention of these defects in the aspects of capital, manpower, and material resources and scientific research.
However, our study has some limitations. The quality of data concerning exposure during the first trimester could have been affected by the retrospective nature of the questionnaire because data about exposure were collected at birth, as well as by the fact that some mothers of newborns with BD may have deliberately or unconsciously overestimated or underestimated their exposures, and the fact that the number of women who declined to take part in the study was not recorded. In case of overestimated exposures, this bias could have resulted in a false positive association and overestimation of the risk related to exposure, which would constitute a confounder. We can also assume that mothers omitted some items of the questionnaire simply because they failed to notice the questions. As this assumption cannot be confirmed, an absence of response to any question was classified as missing data. Data were also considered to be missing when the answer was “do not know”, which explains the high rate of missing data for certain questions and which represents a significant bias in our study, and so the results of this study therefore need to be interpreted cautiously. Until the effects of heavy metals have been more clearly determined, the precautionary principle should apply to pregnant women.
Conclusion
This study was the first retrospective study in the Huaihe River Basin of China aiming to examine the effects of environmental heavy metal exposure and internal heavy metal exposure in cord blood on birth defects during pregnancy by following a birth cohort in Shenqiu county. In our cohort, birth defects occurred in 15.4% of cases. This unusually high rate could be related to improved medical awareness of these birth defects as a result of recent publications. Heavy metals such as Cr are strongly suspected to participate in the pathophysiology of eye, ear, face and neck congenital malformations and musculoskeletal system congenital malformations, Cd with skin system congenital malformations and Hg with musculoskeletal system congenital malformations. Our results support this hypothesis. This study also provides a new research method allowing accurate quantification of the incidence of birth defects in Shenqiu from 2013 to 2014. The establishment of the Shenqiu birth cohort provides a technical platform to research the influences of environmental factors on aristogenesis and provide data to determine the relation between environmental exposure before and during pregnancy and birth defects or adverse pregnancy outcomes. Meanwhile, it also lays a theoretical basis to help the government introduce policies to enact birth defect prevention measures.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
The protocol and consent forms were approved by the institutional review boards at the National Key Technology R&D Program of China and each participating trial site. All participants provided written informed consent. A data and safety monitoring board monitored data in an unblinded fashion every 3 months. The investigators developed the protocol with assistance from the CDC of China. Professor Xin Sun, the funder of the trial, participated in the design or conduct of the trials, review, or reporting of the data before the manuscript was submitted for publication. All the authors participated in the design and conduct of the trials. Trial statisticians performed all data analyses. The first author wrote the first draft of the manuscript, and all the authors contributed to subsequent drafts. The authors want to thank participators who volunteered to participate in this study and permit the collection of umbilical cord blood, as well as medical staff who provide technical assistance and dietary intake survey.
References
- National Scientific Council on the Developing Child, Early Exposure to Toxic Substances Damages Brain Architecture, Working Paper No. 4. Center on the Developing Child at Harvard University, Cambridge, MA, USA, 2006. [Google Scholar]
- Johnston J. E., Valentiner E., Maxson P., Miranda M. L., Fry R. C. PLoS One. 2014;10(9):1–9. doi: 10.1371/journal.pone.0109661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julvez J., Grandjean P. Ind. Health. 2014;47:459–468. doi: 10.2486/indhealth.47.459. [DOI] [PubMed] [Google Scholar]
- Pan J., Song H., Pan X. C. Zhonghua Liuxingbingxue Zazhi. 2007;28:1215–1218. [PubMed] [Google Scholar]
- Bose-O Reilly S., McCarty K. M., Steckling N., Lettmeier B. Curr. Probl. Pediatr. Adolesc. Health Care. 2010;40:186–215. doi: 10.1016/j.cppeds.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau K. C., Li Z., Farzan S., Koestler D., Robbins D., Fei D. L. Clin. Immunol. 2014;155:188–197. doi: 10.1016/j.clim.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A., Vahter M., Ekstrom E. C., Rahman M., Golam Mustafa A. H., Wahed M. A. Am. J. Epidemiol. 2007;165:1389–1396. doi: 10.1093/aje/kwm025. [DOI] [PubMed] [Google Scholar]
- Benett R. W., Persaud T. V., Moore K. L. Anat. Anz. 1975;138:365–378. [PubMed] [Google Scholar]
- Domingo J. L., Gomez M., Colomina M. T. Contrib. Sci. 2000;1:479–487. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Arsenic; Agency for Toxic Substances and Disease Registry, Atlanta, GA, USA, 2007. [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (EPA), Implementation Guidance for the Arsenic Rule-Drinking Water Regulations for Arsenic and Clarifications to Compliance and New Source Contaminants Monitoring, U.S. Environmental Protection Agency, Washington, DC, USA, 2002. [Google Scholar]
- Bellinger D. C. Curr. Opin. Pediatr. 2008;20:172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- Ha M., Kwon H. J., Lim M. H., Jee Y. K., Hong Y. C., Leem J. H. Neurotoxicology. 2009;30:31–36. doi: 10.1016/j.neuro.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Bellinger D. C. Birth Defects Res. A Clin. Mol. Teratol. 2005;73:409–420. doi: 10.1002/bdra.20127. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila M., Peterson K. E., Gonzalez-Cossio T., Sanin L. H., Aro A., Schnaas L. Arch. Environ. Health. 2002;57:482–488. doi: 10.1080/00039890209601441. [DOI] [PubMed] [Google Scholar]
- Osman K., Akesson A., Berglund M., Bremme K., Schutz A., Ask K. Clin. Biochem. 2000;33:131–138. doi: 10.1016/s0009-9120(00)00052-7. [DOI] [PubMed] [Google Scholar]
- Ballew C., Khan L. K., Kaufmann R., Mokdad A., Miller D. T., Gunter E. W. J. Pediatr. 1999;134:623–630. doi: 10.1016/s0022-3476(99)70250-7. [DOI] [PubMed] [Google Scholar]
- Falcon M., Vinas P., Luna A. Toxicology. 2003;185:59–66. doi: 10.1016/s0300-483x(02)00589-9. [DOI] [PubMed] [Google Scholar]
- Torres-Sanchez L. E., Berkowitz G., Lopez-Carrillo L., Torres-Arreola L., Rios C., Lopez-Cervantes M. Environ. Res. 1999;81:297–301. doi: 10.1006/enrs.1999.3984. [DOI] [PubMed] [Google Scholar]
- Roosli M. Prog. Biophys. Mol. Biol. 2011;107:315–322. doi: 10.1016/j.pbiomolbio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Sanders T., Liu Y., Buchner V., Tchounwou P. B. Rev. Environ. Health. 2009;24:15–45. doi: 10.1515/reveh.2009.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. T., Bellinger D. C., Shaywitz B. A. Am. J. Prev. Med. 2005;29:353–365. doi: 10.1016/j.amepre.2005.06.007. [DOI] [PubMed] [Google Scholar]
- McAleer M. F., Tuan R. S. In Vitr. Mol. Toxicol. 2001;14:25–42. doi: 10.1089/109793301316882522. [DOI] [PubMed] [Google Scholar]
- Zhang Y. L., Zhao Y. C., Wang J. X., Zhu H. D., Liu Q. F., Fan Y. G. J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. 2004;39:2507–2515. doi: 10.1081/ese-200026331. [DOI] [PubMed] [Google Scholar]
- Nishijo M., Nakagawa H., Honda R., Tanebe K., Saito S., Teranishi H. Occup. Environ. Med. 2002;59:394–396. doi: 10.1136/oem.59.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Establishment of Ma'anshan birth cohort and its birth outcomes, Master's thesis, Anhui Medical University, 2013. [Google Scholar]
- Hai D. M., Qiu X., Xu H., Honda M., Yabe M., Kadokami K. Bull. Environ. Contam. Toxicol. 2017;99(1):131–137. doi: 10.1007/s00128-017-2081-y. [DOI] [PubMed] [Google Scholar]
- Antonkiewicz J., Koodziej B., Bielińska E. J. Int. J. Phytorem. 2017;19(4):309–318. doi: 10.1080/15226514.2016.1225283. [DOI] [PubMed] [Google Scholar]
- Ben Fredj F., Han J., Irie M., Funamizu N., Ghrabi A., Isoda H. Ecotoxicol. Environ. Saf. 2012;84:54–62. doi: 10.1016/j.ecoenv.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Peralta-Videa J. R., Lopez M. L., Narayan M., Saupe G., Gardea-Torresdey J. Int. J. Biochem. Cell Biol. 2009;41(8–9):1665–1677. doi: 10.1016/j.biocel.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Dong W., The Pollution and Risk Assessment of Heavy Metals in Soil of the High Cancer area of Huai River Basin, Master's thesis, Henan University, 2014. [Google Scholar]
- Orisakwe O. E., Nduka J. K., Amadi C. N., Dike D., Bede O. Chem. Cent. J. 2012;6(1):77. doi: 10.1186/1752-153X-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong T. T., Leveque T., Austruy A., Goix S., Schreck E., Dappe V. Environ. Geochem. Health. 2014;36(5):897–909. doi: 10.1007/s10653-014-9607-6. [DOI] [PubMed] [Google Scholar]
- Liu W. Y., Li Y., Ba Y., Cheng X. M., Zuo Q. T., Xue Y. T. J. Zhengzhou Univ., Med. Sci. 2015;3(50):410–412. [Google Scholar]
- Kim K. H., Shon Z. H., Mauulida P. T., Song S. K. Chemosphere. 2014;111:312–319. doi: 10.1016/j.chemosphere.2014.03.138. [DOI] [PubMed] [Google Scholar]
- Mandal P., Sarkar R., Mandal A., Patel P., Kamal N. Bull. Environ. Contam. Toxicol. 2016;97(6):798–805. doi: 10.1007/s00128-016-1944-y. [DOI] [PubMed] [Google Scholar]
- Lee P. K., Youm S. J., Jo H. Y. Chemosphere. 2013;91(7):1018–1025. doi: 10.1016/j.chemosphere.2013.01.074. [DOI] [PubMed] [Google Scholar]
- Cayir A., Coskun M., Coskun M. Bull. Environ. Contam. Toxicol. 2007;79(4):367–370. doi: 10.1007/s00128-007-9232-5. [DOI] [PubMed] [Google Scholar]
- Lei T., Gao P., Jia L., Chen X., Lu B., Yang L., Feng Y. Ecotoxicol. Environ. Saf. 2016;130:214–223. doi: 10.1016/j.ecoenv.2016.03.031. [DOI] [PubMed] [Google Scholar]
- Liu C., Zhou P., Fang Y. Bull. Environ. Contam. Toxicol. 2016;96(5):638–644. doi: 10.1007/s00128-016-1777-8. [DOI] [PubMed] [Google Scholar]
- Spliethoff H. M., Mitchell R. G., Shayler H., Marquez-Bravo L. G., Russell-Anelli J., Ferenz G. Environ. Geochem. Health. 2016;38(4):955–971. doi: 10.1007/s10653-016-9790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampio E., Salo T., Rintala J. J. Environ. Manage. 2016;169:293–302. doi: 10.1016/j.jenvman.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Li S., Zhu J., Cui L., Cheng X., Zuo Q. Asian J. Ecotoxicol. 2013;2(8):275–279. [Google Scholar]
- Fregonezi P. A., Silva T. G., Simes R. T., Moreau P., Carosella E. D., Klay C. P. Am. J. Otolaryngol. 2012;33(2):193–198. doi: 10.1016/j.amjoto.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Kuwae M., Tsugeki N. K., Agusa T., Toyoda K., Tani Y., Ueda S. Sci. Total Environ. 2013;442:189–197. doi: 10.1016/j.scitotenv.2012.10.037. [DOI] [PubMed] [Google Scholar]
- Morera A. M., Valmalle A. F., Asensio M. J., Chossegros L., Chauvin M. A., Durand P. J. Pediatr. Urol. 2006;2:169–177. doi: 10.1016/j.jpurol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Nørgaard M., Wogelius P., Pedersen L., Rothman K. J., Sørensen H. T. Urology. 2009;74:583–587. doi: 10.1016/j.urology.2009.04.034. [DOI] [PubMed] [Google Scholar]