 This review provides an overview of the effects of supplements containing phytoestrogens on hormone levels and potential implications for women's health.
This review provides an overview of the effects of supplements containing phytoestrogens on hormone levels and potential implications for women's health.
Abstract
Phytoestrogens are increasingly used as dietary supplements due to their suggested health promoting properties, but also by women for breast enhancement and relief of menopausal symptoms. Generally, phytoestrogens are considered to exert estrogenic activity via estrogen receptors (ERs), but they may also affect estrogen synthesis and metabolism locally in breast, endometrial and ovarian tissues. Considering that accurate regulation of local hormone levels is crucial for normal physiology, it is not surprising that interference with hormonal synthesis and metabolism is associated with a wide variety of women's health problems, varying from altered menstrual cycle to hormone-dependent cancers. Yet, studies on phytoestrogens have mainly focused on ER-mediated effects of soy-derived phytoestrogens, with less attention paid to steroid synthesis and metabolism or other phytoestrogens. This review aims to evaluate the potential of phytoestrogens to modulate local estrogen levels and the implications for women's health. For that, an overview is provided of the effects of commonly used phytoestrogens, i.e. 8-prenylnaringenin, biochanin A, daidzein, genistein, naringenin, resveratrol and quercetin, on estrogen synthesizing and metabolizing enzymes in vitro. The potential implications for women's health are assessed by comparing the in vitro effect concentrations with blood concentrations that can be found after intake of these phytoestrogens. Based on this evaluation, it can be concluded that high-dose supplements with phytoestrogens might affect breast and endometrial health or fertility in women via the modulation of steroid hormone levels. However, more data regarding the tissue levels of phytoestrogens and effect data from dedicated, tissue-specific assays are needed for a better understanding of potential risks. At least until more certainty regarding the safety has been established, especially young women would better avoid using supplements containing high doses of phytoestrogens.
Introduction
Plants contain thousands of secondary metabolites, which do not affect the normal growth and development of a plant, but reduce the palatability of the plant tissues. These compounds often consist of one or more aromatic rings bearing hydroxyl group(s) and play a role in the plants’ defense against UV radiation, fungal pathogens and/or plant-eating animals.1 Several secondary metabolites are considered to be phytoestrogens due to their structural similarity to estradiol (E2). As a result, much research focus has been on the ability of phytoestrogens to modulate estrogen receptor (ER)-signaling pathways. However, phytoestrogens may also modulate estrogen function by affecting estrogen-synthesizing enzymes such as aromatase (CYP19) or E2 metabolism. During the past few decades, phytoestrogens have gained considerable attention due to their potential beneficial effects on human and animal health, including relief of menopausal symptoms, prevention of osteoporosis, improved cardiovascular health and lowered risk of diabetes, obesity, and neurological disorders and a wide variety of cancers including breast and endometrial cancer.2 The use of dietary supplements of phytoestrogens as complementary alternative medicines is becoming increasingly popular in Western society. Generally, these plant-derived phytoestrogens are considered natural thus safe. However, supplementary intake may lead to high intake levels of bioactive compounds, yet their potential adverse effects are often not well established. As the use of these dietary supplements is much higher in women than men, especially women may be at higher risk for adverse health effects. Therefore, this review will specifically address the biological properties of phytoestrogens with respect to the modulation of estrogen synthesis and metabolism and the implications for women's health.
Phytoestrogens and uses
Phytoestrogens can be classified based on their structural similarity into flavonoids, isoflavonoids, lignans, coumestans and stilbenes.3 Clearly, phytoestrogens are commonly found in our daily diet in food and beverages containing fruits, seeds, herbs, legumes and vegetables, and the dietary intake of phytoestrogens is dependent on the food sources ingested. Typically, phytoestrogen intake in Western countries is much lower than in Asian countries, mostly due to the soy-rich Asian diet. It is estimated that typical phytoestrogen intake in Asia ranges from 20 to 100 mg day–1, while the usual consumption in the United States and Europe is less than 3 mg day–1.4,5 The high soy intake in Asian countries together with their lower prevalence of hormone-dependent cancers, like breast, ovarian and endometrium cancer, has drawn much attention to the supposed beneficial health effects of soy. Especially the estrogen-like effects of soy phytoestrogens, such as genistein and daidzein, are considered a potential mechanism to explain the protective effect of soy against female cancers in Asian countries.6 This concept has also spurred the use of soy-extracts with high levels of phytoestrogens in Western countries. Initially, these supplements were marketed for breast cancer risk reduction. Yet nowadays, soy-based supplements are commonly used for a wide variety of estrogen-associated conditions, including relief of menstrual issues and menopausal symptoms as well as stimulation of sexual behavior and fertility and breast enhancement (Table 1). A survey among over 30 000 menopausal women all over the world showed that almost 50% used complementary and alternative medicines to alleviate menopausal symptoms, of which 27% used soy-based supplements.7 With the increasing popularity of soy-based supplements, other phytoestrogen-containing plant-based supplementations also gained increasing attention for their supposed female health promoting effects. Many of these applications stem from the use of specific plant species for women's health conditions through folk medicine.8 As an overall result, plant-based supplements are nowadays a popular complementary medicine option in Europe9 and the USA with a broad spectrum of applications. Their use is also of significant economic interest with annual sales in the USA alone in the range of billions of dollars and this amount is still increasing every year.10
Table 1. Some plants used to treat women's health conditions and their major phytoestrogen(s).
| Plant origin (Latin name) | Major phytoestrogen(s) | Suggested use/condition |
| Black cohosh (Cimicifuga racemosa) | Actein, 23-epi-26-deoxyactein a | Enhanced fertility, premenstrual syndrome, menopausal symptoms; relieve menstrual cramps, aid amenorrhea and to ease labor |
| Chaste berry (Vitex agnus castus) | Casticin, isoorientin, apigenin, penduletin | Heavy menstrual bleeding, endometriosis, fertility problems |
| Citrus spp. | Naringenin, naringin | Weight gain during menopause |
| Flaxseed (Linum usitatissimum) | Enterodiol, enterolactone b | Heavy menstrual bleeding |
| Hop (Humulus lupulus) | 8-Prenylnaringenin, 6-prenylnaringenin, isoxantohumol | Breast enhancement, menopausal symptoms |
| Maca (Lepidium meyenii) | Campesterol, beta-sitosterol | Enhanced fertility and sexual behavior, menstrual problems |
| Red clover (Trifolium pratense) | Biochanin A, formononetin | Menopausal symptoms, including hot flashes |
| Soy (Glycine max) | Genistein, daidzein | Menopausal symptoms |
| Wild yam (Dioscorea villosa) | Diosgenin | Menstrual cramping, irregular menstrual cycles, pregnancy-related nausea and morning sickness |
| Ginseng (Panax ginseng) | Ginsenosides, naringenin, chlorogenic acid | Anti-ageing, cardioprotective |
| St John's wort (Hypericum perforatum) | (pseudo)Hypericin, quercetin, rutin | Premenstrual syndrome, menopausal symptoms |
| Grape (Vitis spp.), blueberry (Vaccinium spp.) | Resveratrol | Reduce hot flashes, cardioprotective, prevention of osteoporosis |
aExact phytoestrogen content is unclear. Although some earlier studies report the presence of formononetin in black cohosh extracts (BCE), most studies describe that formononetin could not be determined in BCE.11
bMammalian phytoestrogens which are formed upon conversion of plant lignans matairesinol and secoisolariciresinol by gut microbiota.12
It's the dose that makes the poison
While phytoestrogens are clearly part of our natural diet, especially the intake of phytoestrogen-containing supplements may lead to high concentrations of potential hormonally active compounds in the blood and tissues of women. Supplements can contain gram-levels of phytoestrogens and are typically taken multiple times a day for a longer period of time. It should also be recognized, that women of different age groups may use different types of phytoestrogen-based supplements, depending on the reason for treatment. For example, hop-based supplements for breast enlargement have been shown to contain up to 1000 mg 8-prenylnaringenin (8-PN), which potentially increases the 8-PN intake of many woman at the child-bearing age.13 On the other hand, supplements that are marketed to alleviate menopausal symptoms often contain gram-levels of soy or red clover-derived phytoestrogens like genistein and biochanin A, which obviously leads to exposure of peri- and postmenopausal women.14 Not only will this lead to different exposure patterns, but varying systemic hormone levels between these age groups will influence the effect of a specific phytoestrogen.15
In order to do risk assessment of phytoestrogen-containing supplements, it is crucial to consider human pharmacokinetic data. Phytoestrogens are primarily ingested as glycoside conjugates containing glucose or carbohydrate moieties.16 Several studies have shown that the form in which a phytoestrogen is ingested, for example as soymilk, tofu or as pure compound (supplement), greatly affects the bioavailability of a phytoestrogen.17–19 Upon de-conjugation by gut microbiota, the aglycone forms of the phytoestrogens can efficiently pass the intestinal barrier. It has become increasingly clear that intestinal metabolism by gut microbiota plays a crucial role in the bioavailability of phytoestrogens.20,21 Besides having a role in deconjugation, gut microbiota are also involved in the metabolic conversion of phytoestrogens. This may lead to differences in the bioactivation of phytoestrogens in specific human subpopulations, as has been demonstrated for formation of the highly estrogenic metabolite equol from the less estrogen-active daidzein22 or other weakly bio-active phytoestrogens.23 Other examples are the plant-derived lignans matairesinol and secoisolariciresinol, which are converted by gut microbiota to yield the mammalian phytoestrogens enterodiol and enterolactone.12 Generally, pharmacokinetic studies in healthy subjects show rapid absorption of phytoestrogens (Table 2). However, clear differences exist between human individuals with respect to the formation and systemic circulation of phytoestrogens and their metabolites.24 Variability in (peak) plasma levels does not appear to be caused by gender differences. Strikingly, while supplements can contain gram-levels of certain phytoestrogens that women are supposed to take for longer periods of time, human pharmacokinetic data are mostly available for low, often single, doses.
Table 2. Human pharmacokinetic data of phytoestrogens in healthy humans upon supplement intake, unless mentioned otherwise.
| Phytoestrogen | Subjects | Dosing | C max (average) | C max (peak) | T max | Ref. |
| 8-PN | Postmenopausal women | 750 mg, single oral dose | 0.07 μM | 0.2 μM | 1.5 h | 25 |
| Premenopausal women | 0.1 mg, oral intake three times per day for 5 days (soy milk) | 0.43–7.06 nM | NA | 31 | ||
| Breast tissue: 0.78–4.83 pmol g–1 | ||||||
| Biochanin A | Male/female volunteers | Promensil red clover extract containing 49 mg biochanin A, daily oral dose for 2 weeks | 0.2 μM | 0.3 μM | 3.6 h | 33 |
| Daidzein | Premenopausal women | 50 mg, single oral dose | 0.76 μM | 0.88 μM | 6 h | 23 |
| Premenopausal women | 5–10 mg, oral intake three times per day for 5 days | 0.1–1.4 μM | NA | 17 | ||
| Breast tissue: 22.2 to 770.8 pmol g–1 | ||||||
| Genistein | Premenopausal women | 50 mg, single oral dose | 1.26 μM | 1.52 μM | 9 h | 23 |
| Premenopausal women | 5–10 mg, oral intake three times per day for 5 days | 0.14–2.8 μM | NA | 17 | ||
| Breast tissue: 92.3–493.8 pmol g–1 | ||||||
| Naringenin | Male/female volunteers | 200 mg, single oral dose (grapefruit juice) | 6.0 μM | 11.4 μM | 4.8 h | 34 |
| Male/female volunteers | 135 mg, single oral dose | 7.4 μM | 10.2 μM | 4 h | 35 | |
| Resveratrol | Male/female volunteers | 500 mg, single oral dose | 0.3 μM | 0.5 μM | 1.3 h | 36 |
| Male/female volunteers | 150 mg, six times per day for 13 doses | 0.3 μM | 0.45 μM | 0.8 h | 26 | |
| Male/female volunteers | 5 g, single oral dose | 2.4 μM | 4 μM | 1.5 h | 37 | |
| Quercetin | Male/female volunteers | 500 mg, three times daily for a week | 0.05 μM | 0.13 μM | 3 h | 18 |
| Male/female volunteers | 500 mg, single oral intake (chews) | 3.5 μM | 9.4 μM | 3.3 h | 38 | |
Several pharmacokinetic studies show that plasma levels of phytoestrogens are correlated with dosage, as demonstrated for 8-PN,25 resveratrol26 and genistein.27 However, there appears to be no clear correlation between phytoestrogen plasma and tissue concentrations,28 which implies that plasma levels are not predictable for tissue levels. Indeed, efflux transporters, such as breast cancer resistance protein (BCRP), have been shown to be pivotal in determining oral bioavailability, but also in tissue-specific uptake and elimination of genistein and 8-PN, for example.27,29,30 Undoubtedly, in order to do a reliable risk assessment, it would be best to know the actual levels of phytoestrogens on the target site, i.e. breast, endometrium and ovaries. Yet, tissue concentration data are extremely limited. Only Bolca et al. (2010) showed that substantial amounts of phytoestrogens can be found in human breast tissue after drinking soy milk17 or upon intake of hop-derived supplements31 (Table 2). No studies were found that determined phytoestrogen levels in the endometrium. Also, no human data are available on the ovarian or follicular fluid levels of phytoestrogens. One study described lowered E2 and P4 levels in rat follicular fluid after soy isoflavone intake, but no direct analysis of soy isoflavones in follicular fluids was performed in this study.32 Lack of (internal) exposure data clearly poses a crucial gap in knowledge that should be addressed. Such exposure data will provide essential information that is needed to perform a risk–benefit analysis for phytoestrogens with respect to women's health. It will also aid to better interpret the many in vitro results from human relevant models and put the in vitro findings in an in vivo relevant context.
Modes of action
Considering their structural similarity to endogenous estrogens, phytoestrogens are traditionally considered to act primarily via interactions with estrogen receptors. However, many in vitro and in vivo studies, as well as some human data, have demonstrated that phytoestrogens may also modulate estrogen function by affecting estrogen synthesis and metabolism or hormonal signaling within the hypothalamus–pituitary–gonadal (HPG) axis. In addition, phytoestrogens have been ascribed many other biological activities including anti-inflammatory,39 and anti-oxidant,40 and epigenetic modulating41,42 and tyrosine kinase inhibiting43 properties. While these effects may also attribute to the reported biological actions of phytoestrogens, in this review, we primarily focus on the modulation of hormone levels in breast, endometrial and ovarian tissues via interaction with enzymes involved in estrogen synthesis and metabolism.
Estrogen receptors
The best characterized mode of phytoestrogen action is their ability to interact with estrogen receptors. The first receptor to bind estrogens was characterized in the 60s.44 This receptor was called ER-alpha (ESR1) upon the discovery of a novel, homologous estrogen-binding receptor: ER-beta (ERS2) in the 90s.45 ERα and ERβ are considered to exert opposite effects. ERα is considered to enhance estrogen-dependent cell proliferation,46 while ERβ has been shown to counteract ERα-mediated proliferation, most likely via alternative co-factor recruitment.47 Generally, phytoestrogens are found to exert weak estrogenic activity via both ERs, with 50% effect concentrations (EC50 values) that are 2 to 6 orders of magnitude lower compared to the endogenous ligand 17β-estradiol (E2).48 Also, phytoestrogens display a higher affinity for ERβ than ERα, in contrast to E2.49–51 An exception here is the hop-derived phytoestrogen 8-prenylnaringenin (8-PN), which shows a more pronounced potency for ERα- than ERβ-mediated transactivation.52 With only a 5-fold weaker potency for in vitro ERα transactivation than 17β-estradiol, 8-PN is the most potent phytoestrogen known so far.53 Its estrogenic potency, albeit somewhat lower than established in vitro, has also been demonstrated in vivo by uterine and vaginal growth assays in prepubertal rats and mice.53,54 For studies on the estrogenic potential of phytoestrogens, often the estrogen-responsive human mammary adenocarcinoma MCF-7 cell line is used. In this breast cancer cell line, several phytochemicals have been shown to possess estrogenic activity with varying potencies; for example, the potency to stimulate ER-dependent MCF-7 cell proliferation by biochanin A, genistein and naringenin was approximately 2000, 8000 and 70 000 times less, respectively, than E2.55 However, it is not uncommon that differences in estrogenic potencies are found for phytoestrogens in in vitro models of different tissue origins. Differential expression of ERs and their co-regulator proteins in breast and uterine tissue are considered to be at least partially responsible for tissue-specific responses towards estrogen receptor modulators, as was demonstrated for the ER antagonist tamoxifen.56 Although phytochemicals generally possess low estrogenic activity, studies from our lab have shown that mixtures of phytochemicals can act in vitro in an additive manner.57 This was confirmed in vivo, where phytochemical mixtures showed additive estrogenic potential in increasing uterine growth in prepubertal rats.58 Yet, in vitro potencies of phytoestrogens for ER activation do not always reflect in vivo potencies in experimental animal studies.
Besides the classical ERα and ERβ, other estrogen (-related) receptors have more recently been identified, which can also be targeted by phytoestrogens. More recent research has led to the identification of several isoforms of ERα, as well as the revelation of the estrogen-responsive transmembrane G-coupled protein estrogen receptor GPER (formerly known as GPR30).59 GPER has been shown to mediate rapid, non-genomic estrogenic effects of endogenous estrogens as well as phytoestrogens like genistein and equol.60 The activation of GPER can stimulate downstream signaling via mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3 K), and epidermal growth factor receptors (EGFRs)61 and consequently modulate the growth of hormonally responsive cancers such as endometrial,62 ovarian63 and both estrogen receptor-positive and -negative breast cancer.64,65 Another nuclear receptor family of estrogen-related receptors (ERRs) was also found to be involved in estrogen signaling.66 Even though no natural ligand has been identified for ERRs, estrogenic signaling and crosstalk with ER has been reported. Moreover, phytoestrogens like genistein, daidzein, biochanin A, and resveratrol have been described to interact with estrogen signaling via EERs (reviewed in ref. 67). Ligation of each of these estrogen receptors by estrogens leads to specific signaling events and subsequent cellular effects. Moreover, the relative ratio of ERα and ERβ in a cell as well as background estrogen levels strongly influence the estrogenic potential of a phytoestrogen. Knowledge of the relative ligand binding affinities of phytoestrogens, tissue distribution and developmental pattern of expression of estrogen receptors may help to explain the tissue-specific actions of phytoestrogens.
Estrogen synthesis and metabolism
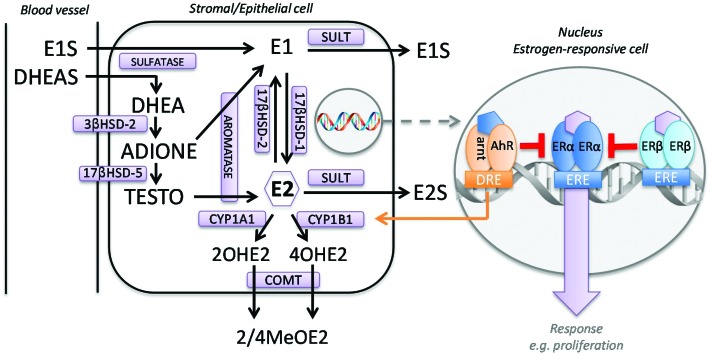
Tissue-specific expression of ERs, but also local regulation of estradiol levels, is crucial for the correct functioning of estrogen responsive tissues. In premenopausal non-pregnant women, estrogens are predominantly synthesized in the ovaries and, to a lesser extent, in peripheral tissues. In postmenopausal women, the source of estrogen production shifts toward the peripheral tissues, which is reflected in lower plasma, but not tissue estrogen levels.68,69 Estrogens can be produced via de novo synthesis from cholesterol or via local desulfatation of estrogen precursors. De novo synthesis includes several rate-limiting steps, which makes fine-regulation of sex steroid levels possible. These rate-limiting steps include cholesterol transport to the inner mitochondrial membrane by steroidogenic acute regulatory protein (StAR) followed by conversion into pregnenolone by cytochrome P450 side chain cleavage (P450scc, CYP11A1).70 Next, androgens are produced by cytochrome P450 17 (CYP17)-mediated activity.71,72 The final and rate-liming step in estrogen synthesis is the aromatization of androgens to estrogens by cytochrome P450 19 (CYP19A1, aromatase).73,74 In women, the main sites of androgen production, predominantly dehydroepiandrosterone (DHEA) and its sulfated conjugate DHEA sulfate (DHEAS), are the adrenal cortex and the ovaries.75 Plasma levels of these estrogen precursors are higher than the level of circulating estradiol in pre- and postmenopausal women.76,77 In peripheral tissues, desulfatation of DHEAS occurs and estrone can be formed through the activities of 3-beta-hydrosteroid dehydrogenase (HSD), 17-beta-HSD and aromatase (Fig. 1). Estrone can be conjugated to estrone sulfate (E1S) to form a reservoir of estrone in blood and tissue.78 Alternatively, estrone can be converted by 17-beta-HSD, ultimately leading to local formation of freely available E2.79,80 The major route of estrogen metabolism is via hydroxylation to form the catechol estrogens (CEs) 2- and 4-hydroxyestrogen, catalyzed by CYP1A1 and CYP1B1, respectively.81–83 CEs may be further converted by catechol-O-methyltransferase (COMT) to the more stable methoxy derivatives.84–87
Fig. 1. Local estrogen homeostasis in an estrogen-responsive cell. Unlike ovarian and breast tissue, the endometrium does not possess enzymes to transform DHEA into estrogens and it is generally acknowledged that aromatase expression and activity in the endometrium is restricted to women with endometrial diseases.97 2OHE2 = 2-hydroxyestradiol; 3β-HSD-2 = 3β-hydroxysteroid dehydrogenase type 2; 4OHE2 = 4-hydroxyestradiol; 2/4MeOE2 = 2-/4-methoxyestradiol; 17β-HSD = 17β-hydroxysteroid dehydrogenase; ADIONE = androstenedione; AhR = aryl hydrocarbon receptor; arnt = AhR nuclear transferase; COMT = catechol-O-ethyltransferase; CYP1A1 = cytochrome P450 1A1; CYP1B1 = cytochrome P450 1B1; DHEA = dehydroepiandosterone; DHEAS = dehydroepiandosterone sulfate; DRE = dioxin responsive element; E2 = estradiol; E1 = estrone; E1S = estrone-3-sulfate; E2S = estradiol-3-sulfate; ERE = estrogen responsive element; SULT = sulfotransferase; TESTO = testosterone.
Several in vitro studies describe the effects of phytoestrogens on enzymes involved in estrogen synthesis and metabolism. An overview of the commonly used phytoestrogens, i.e. 8-PN, biochanin A, daidzein, genistein, naringenin, resveratrol and quercetin, on specific enzyme activities is given below (summarized in Table 3). Next, the effect concentrations of the in vitro studies will be compared with blood concentrations that can be found after intake of these phytoestrogens followed by an assessment of the potential implications for estrogen levels (summarized in Table 4).
Table 3. In vitro effects of selected phytoestrogens on the activity of enzymes involved in estrogen synthesis or metabolism. The degree and direction of effect are expressed with arrows to indicate arbitrary potency values, being low (↑/↓ IC50 value >10 μM), moderate (↑↑/↓↓ IC50 value 1–10 μM) and strong (↑↑↑/↓↓↓ IC50 value <1 μM).
| Target enzyme | Cellular origin (tissue) | Cell type | Degree and direction of effect | IC50 (μM) | Additional relevant information regarding effect on activity and/or gene expression, when assessed | Ref. |
| 8-Prenylnaringenin | ||||||
| Aromatase | Ovary | KGN | ↓↓↓ | 0.008 μM | Gene expression was increased 2.3-fold at 3 μM a | 107 |
| Adrenal | H295R | ↓↓↓ | 0.1 μM | 14 | ||
| CYP1A1 | Breast | MCF-7 | ↑ | ±5-Fold induction of 2-MeOE1 formation at 1 μM. 90-fold increase of CYP1A1 gene expression | 179 | |
| Recombinant | ↓↓↓ | 0.38 μM | 179 | |||
| CYP1B1 | Breast | MCF-7 | ↑ | ±4.5-Fold induction of 4-MeOE1 formation at 1 μM. 20-fold increase of CYP1B1 gene expression | 179 | |
| Recombinant | ↓↓↓ | 0.41 μM | 179 | |||
| Biochanin A | ||||||
| Aromatase | Breast | Primary human breast adipose fibroblasts | ↓ | 25 μM | IC50 between 10 and 50 μM. No effect on protein expression | 55 |
| Ovary | Primary human granulosa cells | ↓ | 135 | |||
| 3β-HSD | Ovary | Primary human granulosa cells | ↓ | 10 μM | No effect on protein level | 135 |
| Placenta | Placental microsomes | ↓ | 10 μM | 137 | ||
| 3β-HSD2 | Adrenal | H295R microsomes | ↓↓↓ | 0.5 μM | 136 | |
| Recombinant | ↓↓↓ | 0.94 μM | 134 | |||
| 17β-HSD | Placenta | Recombinant | ↓↓ | 8–14 μM | 145 | |
| Placental microsomes | ↓↓ | 4.9 μM | 137 | |||
| CYP1A1 | Breast | MCF-7 | ↑ | 4-Fold induction at 10 μM (lowest concentration tested) | 161 | |
| Recombinant | ↓↓ | 4 μM | 171 | |||
| CYP1B1 | Recombinant | ↓↓↓ | 0.7 μM | 171 | ||
| Daidzein | ||||||
| CYP17 | Adrenal | H295R | ↑ | Concentration-dependent increase (no EC50 value given). 2-fold increase at 10 μM | 128 | |
| Adrenal | H295R | ↑ | 5-Fold increase at 10 μM | 130 | ||
| STS | Liver | Hepatic microsomes | ↓↓↓ | 0.8 μM | Effect of daidzein 4,7-bisulfate, a trace metabolite of daidzein | 114 |
| 3β-HSD | Placenta | Placental microsomes | ↓ | 10 μM | 137 | |
| Adrenal | H295R microsomes | ↓↓ | 2 μM | 136 | ||
| 3β-HSD2 | Recombinant | ↓↓ | 3.2 μM | 134 | ||
| 17β-HSD | Recombinant | ↓ | >20 μM | 145 | ||
| Placenta | Placental microsomes | ↓ | 10 μM | 137 | ||
| SULT1A1 | Blood | Human platelet | ↓↓↓ | 0.6 μM | 114 | |
| SULT1E1 | Liver | Human liver cytosol | ↓↓ | K i value 2.0 μM | 114 | |
| Recombinant | ↓ | K i value 14 μM | 147 | |||
| CYP1A1 | Breast | MCF-7 | ↓ | EROD activity. 50.6% of control activity at 10 μM | 164 | |
| COMT | Breast | Cytosol from MCF-7 cells | ↓ | 54% of control activity at 1 μM | 182 | |
| Genistein | ||||||
| Aromatase | Adrenal | H295R | ↑↑ | ±6 μM | 2.5-Fold induction at 10 μM.Increased pII and I.3-specific transcript about 2.3-fold and 1.8-fold | 103 |
| Breast | Primary human breast adipose fibroblasts | ↓↓ | 3.6 μM | 55 | ||
| MCF-7 | ↓ | Decreased estrone production from androstenedione by 70% at 10 μM. No effect at 5 μM | 104 | |||
| Ovary | KGN | NE | Increased gene expression by 1.9-fold at 10 μM a | 107 | ||
| Ovary | Primary human granulosa cells | ↓ | IC50 between 10 and 50 μM. No effect on protein expression | 135 | ||
| Endometrium | Primary human endometrial stromal cells derived from women with endometriosis | ↑ | 1.5-Fold at 1 nM–1 μM. No concentration-dependent effect | 108 | ||
| CYP17 | Adrenal | H295R | ↑↑ | 7.71 μM | 129 | |
| Adrenal | H295R | ↑ | 2-Fold increase at 10 μM | 128 | ||
| 3β-HSD | Ovary | Primary human granulosa cells | ↓ | Only effect at highest concentration tested (100 μM). No effect on protein expression | 135 | |
| Placenta | Placental microsomes | ↓↓ | 2.9 μM | 137 | ||
| Adrenal | H295R microsomes | ↓ | 1 μM | 136 | ||
| 3β-HSD2 | Recombinant | ↓↓↓ | 0.25 μM | 134 | ||
| 17β-HSD | Recombinant | ↓ | >20 μM | 145 | ||
| Placenta | Placental microsomes | ↓↓ | 1 μM | 137 | ||
| Breast | MCF-7 | ↓ | Inhibition of E1 > E2 conversion (17β-HSD type 1) of approximately 20%, 25% and 55% at 5, 10 and 50 μM, respectively. | 104 | ||
| SULT1A1 | Blood | Human platelet | ↓↓↓ | 0.5 μM | 114 | |
| SULT1A1 | Liver | HepG2 cells | ↑ | 1–5 μM | Increased activity after 4 and 7 days of exposure. Increased gene and protein expression was found at 0.2 μM and 5 μM, respectively, after 7 days. | 149 |
| SULT1E1 | Liver | Human liver cytosol | ↓↓↓ | KI value 0.5 μM | 114 | |
| Recombinant | ↓↓ | KI value 7 μM | 147 | |||
| CYP1A1 | Breast | MCF-7 | ↓↓ | EROD activity. 44% of control activity at 1 μM | 164 | |
| Recombinant | ↓ | 25 μM | 171 | |||
| Recombinant | ↓ | 23 μM | 174 | |||
| CYP1B1 | Recombinant | ↓↓ | 3 μM | 171 | ||
| COMT | Breast | Cytosol from MCF-7 cells | ↓ | 68% of control activity at 1 μM | 182 | |
| Naringenin | ||||||
| Aromatase | Adrenal | H295R | ↓ | 85 μM | 103 | |
| Ovary | KGN | ↑↑ | 1.3 μM | 155% at 10 μM. No effect on gene expression at 3 μM | 107 | |
| Breast | Primary human breast adipose fibroblasts | ↓↓ | 2.2 μM | 55 | ||
| Recombinant | ↓↓↓ | 0.3 μM | 108 | |||
| CYP17 | Adrenal | H295R | ↑ | 1.35-Fold increase at 10 μM | 128 | |
| STS | Liver | Hepatic microsomes | ↓↓ | <10 μM | Activity ±45% of control at 10 μM | 116 |
| 17β-HSD | Recombinant | ↓ | 10–33 μM | 145 | ||
| Placenta | Placental microsomes | ↓ | 15 μM | 137 | ||
| CYP1A1 | Recombinant | ↓ | 15.2 μM | 170 | ||
| Recombinant | ↓ | 55 μM | 171 | |||
| CYP1B1 | Recombinant | ↓↓ | 3.7 μM | 170 | ||
| Recombinant | ↓↓ | 1.3 μM | 171 | |||
| Resveratrol | ||||||
| Aromatase | Breast | SK-BR-3 | ↓ | 43 μM | Lower gene expression IC50 ±25 μM, protein >25 μM | 106 |
| Breast | MCF-7aro | ↓ | 25 μM | 106 | ||
| Ovary | KGN | NE | Up to 10 μM | 107 | ||
| CYP17 | Adrenal | H295R | ↓↓ | 4 μM | 130 | |
| STS | Liver | Hepatic microsomes | ↓↓ | <10 μM | Inhibition in MCF-7 cells was only 10% at 10 μM compared to 50% in liver microsomes. | 116 |
| SULT | Breast | Primary human mammary epithelial cells | ↓↓ | 1.3 μM | Type unspecified. | 148 |
| Recombinant | ↓↓ | 1.6 μM | Type unspecified. | 148 | ||
| CYP1A1 | Recombinant | ↓↓ | 4.8 μM | 177 | ||
| Recombinant | ↓↓ | 2 μM | 173 | |||
| Recombinant | ↓ | 23 μM | 174 | |||
| CYP1B1 | Recombinant | ↓ | 25 μM | 173 | ||
| Quercetin | ||||||
| Aromatase | Adrenal | H295R | ↑↑ | ±5 μM | 4-Fold at 10 μM. About 2.6-fold and 2-fold increased pII and I.3-specific transcript respectively. | 103 |
| Breast | Primary human breast adipose fibroblasts | ↓ | 30 μM | 55 | ||
| Ovary | Primary human granulosa cells | ↓ | Only inhibition at highest concentration tested (100 μM). No effect on protein expression | 135 | ||
| Ovary | KGN | ↑↑ | 4.7 μM | 2.1-Fold maximum induction of activity at 10 μM, which sharply declined at 30 μM (not due to cytotoxicity). 3.2-fold increased gene expression at 10 μM a | 107 | |
| STS | Liver | Hepatic microsomes | ↓↓ | <10 μM | 116 | |
| Breast | MCF-7 | ↓ | 10% inhibition at 10 μM | 116 | ||
| 3β-HSD | Ovary | Primary human granulosa cells | ↓ | 10 μM | No effect on protein level | 135 |
| 3β-HSD2 | Recombinant | ↓ | 83.5 μM | 134 | ||
| 17β-HSD | Recombinant | ↓↓ | 5–9 μM | 145 | ||
| SULT | Breast | Primary human mammary epithelial cells | ↓↓↓ | 0.13 μM | Type unspecified. | 148 |
| Recombinant | ↓↓ | 1.4 μM | Type unspecified. | 148 | ||
| SULT1A1 | Liver | Human platelets | ↓↓↓ | 0.06 μM | 114 | |
| CYP1A1 | Recombinant | ↓↓ | 3.2 μM | 177 | ||
| Recombinant | ↓↓ | 1.2 μM | 170 | |||
| Recombinant | ↓↓ | 6.0 μM | 172 | |||
| Recombinant | ↓↓ | 2 μM | 171 | |||
| CYP1B1 | Recombinant | ↓↓↓ | 0.08 μM | 170 | ||
| Recombinant | ↓↓↓ | 0.55 μM | 172 | |||
| Recombinant | ↓↓↓ | 1 μM | 171 | |||
| COMT | Breast | Primary human mammary cytosol | ↓↓↓ | 0.5 μM | 180 | |
| Breast | Primary human mammary cytosol | ↓↓ | 8.5 μM | 181 | ||
aNo effect on protein levels.
Table 4. Phytoestrogens, their potential target enzymes and subsequent effect on estrogen levels. Enzymes involved in estrogen synthesis and metabolism are considered potential target enzymes when in vitro effect concentrations (Table 3) are equal to or lower than reported plasma concentrations for a specific phytoestrogen (Table 2). Enzymes for which whole cell data are available are printed in bold. Potential effect on estrogen levels are hypothetical effects based on the physiological roles of the enzymes in estrogen homeostasis (see Fig. 1).
| Phytoestrogen | Target enzymes involved in estrogen synthesis and metabolism (IC50 < Cmax) | Potential effect on estrogen levels |
| 8-PN | Aromatase | Lower estrogen production |
| Biochanin A | 3β-HSD2, CYP1B1 | Not clear a |
| Daidzein | 3β-HSD, SULT1A1 | Not clear |
| Genistein | 3β-HSD, 17β-HSD, SULT1E1, SULT1A1, CYP1B1 | Increased intracellular E1, lower circulating E1/E2S |
| Naringenin | Aromatase, STS, CYP1B1 | Altered estrogen production in ovary (↓) and breast (↑) |
| Resveratrol | CYP17, SULT, CYP1A1 | Decreased androgen production, lower circulating E1/E2S |
| Quercetin | Aromatase, 17β-HSD, SULT, CYP1A1, CYP1B1, COMT | Increased estrogen (ovary) |
a In vivo, effects of biochanin A might be similar to genistein due to metabolic conversion.
Aromatase
Aromatase (CYP19A1) catalyzes the conversion of androgens to estrogens. It is bound to the membrane of the endoplasmatic reticulum and is exclusively expressed in estrogen producing cells.88 Expression of the aromatase gene is regulated in a species- and tissue-specific manner.89,90 In humans, tissue-specific promoters in the 5′-untranslated region are linked to different signal transduction pathways.88,91 Although aromatase expression is driven by tissue-specific promoters, the aromatase protein encoded in each of these tissues is identical. The highest levels of aromatase can be found in the ovarian granulosa cells of premenopausal women, but after menopause adipose tissue becomes the major aromatase-expressing body site.92 Ovarian aromatase activity and local estradiol production, together with pituitary gonadotropins, ensure successful folliculogenesis and regulation of the menstrual cycle.93 In ovarian granulosa cells, aromatase is regulated via a gonad-specific promoter PII.94 In healthy breast tissue, aromatase is mainly located in adipose stromal cells (fibroblasts) and gene transcription is mainly regulated by a glucocorticoid-dependent pathway using the promoter region I.4.95,96 In breast tumors, aromatase transcripts derived from promoter regions I.3 and pII become more prevalent. It is generally accepted that aromatase expression and activity in the endometrium is restricted to women with endometriosis, leiomyomas and adenomyosis,97 although some studies show aromatase activity and/or expression in the normal endometrium throughout the entire menstrual cycle.98,99 Local uterine estrogen levels are found to be higher in endometrial cancers than in healthy endometrial tissue, which indicates an important role for local aromatase in the progression, but also in the etiology of endometrial malignancies.100
Many studies have shown that phytoestrogens can interact with aromatase activity and/or expression (reviewed in ref. 101). These include human cells that are derived from female hormone-responsive tissues, e.g. KGN granulosa-like cells, MCF-7 and SK-BR-3 breast cancer cells and primary mammary fibroblasts, placental tissues and placental microsomes. Also, the human adrenocortical carcinoma cell line H295R is often used to study in vitro interactions with aromatase activity.102 H295R cells contain high constitutive and inducible aromatase activity and expression, using promoter-regions PII and I.3. Several phytochemicals have been found to interact in H295R cells with the aromatase enzyme as inhibitors or inducers in the micromolar range.103 Quercetin and genistein, for example, induced aromatase activity 2- and 4.5-fold, respectively, at 10 μM.14,103 In contrast, genistein and quercetin showed to be inhibitors of aromatase activity in MCF-7 cells104 and in healthy primary human mammary fibroblasts derived from reduction mammoplastic surgery.55 Most likely, the tissue-specific regulation of aromatase expression can explain the differences in response. Several studies have shown that resveratrol inhibits aromatase activity in breast (cancer) cells. For example, in T47D breast cancer cells co-cultured with primary fibroblasts resveratrol (20 μM) inhibited CYP19 gene expression and E2 formation.105 Resveratrol suppressed promoter I.3/II-driven aromatase activity in SK-BR-3 breast cancer cells and inhibited aromatase activity in MCF-7 cells transfected with aromatase.106 In human KGN granulosa-like tumor cells, 8-PN, genistein and quercetin increased CYP19A1 mRNA levels via the activation of PII and I.3 promoters.107 Yet only naringenin and quercetin also statistically significantly induced aromatase activity at concentrations >1 μM. Interestingly, 8-PN acted as a potent inhibitor of aromatase activity in KGN cells with an apparent IC50 value of 8 nM, despite an increase in CYP19A1 mRNA expression. Also in H295R cells, aromatase activity was inhibited by 8-PN with an IC50 value of 0.1 μM (no gene expression was determined).14 One study with human primary endometrial stromal cells (ESC) derived from patients with endometriosis showed an increase in aromatase activity by genistein to approximately 150%, but not by naringenin and daidzein.108 However, this increase was consistent at all concentrations tested (1 nM–1 μM) and no concentration-dependent effect was observed in this study.
Steroid sulfatase
Steroid sulfatase (STS) is responsible for the hydrolysis of the major circulating inactive steroids DHEAS and E1S into their unconjugated, active forms. Unlike ovarian and breast tissue, the endometrium does not possess enzymes to transform DHEA into estrogens, which makes STS an important regulator of endometrial estrogen levels.97 This specific steroidogenic enzyme expression in the endometrium is thought to protect the endometrium against excess estrogenic stimulation, which is considered a risk factor in failure to establish a pregnancy.109 While endometrial STS shows little variation in expression throughout the menstrual cycle,110 changes in STS activity are seen in gynecological diseases, such as endometriosis.111 Moreover, STS has been described to be a major regulator of in situ estrogen activity in ovarian, breast and endometrial carcinomas.112,113
Despite the importance of STS in hormonal tissues, relatively few studies have investigated the effects of phytoestrogens on STS activity and/or expression. Harris et al. found daidzein 4,7-bisulfate, a trace metabolite of daidzein in humans, to be a potent inhibitor of estrogen sulfatase activity with an IC50 of 0.8 μM.114 Genistein had no significant effect on STS activity up to 10 μM in MDA-MB-321 and MCF-7 breast cancer cells.115 An early study showed that also quercetin and naringenin were inhibitors of human estrone sulfatase activity in human hepatic microsomes.116 However, when tested in MCF-7 cells, this inhibition by quercetin was much less effective with only 10% inhibition of STS activity in MCF-7 cells compared to 50% at 10 μM in liver microsomes.
Cytochrome P450c17 (CYP17)
CYP17 mediates several steps in androgen formation in adrenal cells and ovarian theca cells. CYP17 displays both 17 alpha-hydroxylase and 17,20-lyase activity. This dual function of CYP17 allows the adrenal glands and gonads to synthesize cortisol (17 alpha-hydroxylase activity) and sex steroid precursors (17,20-lyase activity).71,72 CYP17 mediates sex steroidogenesis by the conversion of pregnenolone to 17α-hydroxypregnenolone and then to DHEA. Several studies have shown that this pathway is favored in sex steroidogenesis by human CYP17.117,118 Alternatively, androgens can be produced by CYP17 via the conversion of 17α-hydroxyprogesterone to androstenedione, which is subsequently converted to testosterone by 17β-HSD.117,119 Changes in CYP17 expression and activity have been associated with increased circulating estrogen levels,120,121 modulation of breast cancer risk factors, such as BRCA status, menstrual cycle length122–126 and polycystic ovarian syndrome (PCOS).127
No studies were found that investigated the effects of 8-PN, biochanin A or quercetin on CYP17 activity. Genistein and daidzein concentration-dependently increased DHEA secretion in H295R cells, suggesting an increase in CYP17 activity.128–130 Only one study reported an EC50 value of 7.71 μM for the induction of CYP17 activity by genistein.129 Others reported an increased production of 2- to 5-fold at 10 μM daidzein128,130 or genistein.128 Also naringenin increased DHEA production in H295R cells to 135% of vehicle control levels at 10 μM.128 Resveratrol was shown to concentration-dependently inhibit CYP17 activity in H295R cells with an IC50 value of 4 μM.130
3β-Hydroxysteroid dehydrogenase
There are two 3β-HSD isoenzymes, both of which are essential for androgen production, as it catalyzes the conversion from DHEA to androstenedione. In women, 3β-HSD Type 1 is predominant in the placenta and the mammary gland, while Type 2 is almost exclusively expressed in adrenals and in ovarian theca cells.131–133
Several phytoestrogens have been shown to be moderate/strong inhibitors of 3β-HSD activity. A study with human recombinant 3β-HSD Type 2 showed that genistein and biochanin A inhibited the conversion of DHEA to androstenedione with IC50 values of 0.25 and 0.94 μM, respectively, while daidzein and quercetin were less potent with IC50 values of 3.2 and 85.5 μM, respectively.134 Biochanin A was also shown to cause a concentration-dependent inhibition of 3β-HSD Type 2 activity in primary human granulosa cells with an IC50 value of 10 μM.135 In contrast, quercetin, genistein and daidzein did not inhibit 3β-HSD Type 2 activity up to 100 μM in the same study. In microsomes from H295R cells, genistein, daidzein, quercetin and biochanin A all inhibited 3β-HSD activity with IC50 values between 1.3 and 10 μM.136 Also in placental microsomes, genistein, daidzein and biochanin A have been shown to inhibit 3β-HSD activity.137 Here, genistein was the most potent phytoestrogen tested with an IC50 value of 2.9 μM. In the same study, no effect on 3β-HSD activity was detected for naringenin up to 50 μM.
17β-Hydroxysteroid dehydrogenase
17β-Hydroxysteroid dehydrogenases (17β-HSD) play a role in regulating estrogen and androgen levels via the reduction or oxidation of steroids at position 17. Several types have been described with varying substrate specificity and tissue distribution.138 Most relevant for local estrogen homeostasis are Types 1, 2 and 5. Type 1 is involved in conversion from estrone to estradiol, while 17β-HSD Type 2 catalyses conversion from E2 to E1.139 In the endometrium, mid-luteal ovarian estrogen production is balanced, among others, by progesterone-dependent increases in 17β-HSD Type 2 levels.110 Type 5 is involved in conversion from androstenedione into testosterone and can be found in breast tissue,140 but it is highly expressed in theca cells.141 17β-HSD Type 5 also possesses 20alpha-HSD activity that inactivates progesterone. Therefore, the role of 17β-HSD Type 5 in theca cells appears to be dual, namely ovarian production of estradiol precursors as well as protection of theca cells against progesterone produced by granulosa cells.
No effects on 17β-HSD Type 1 were found for quercetin and biochanin A in primary human granulosa-luteal cells.142 The authors concluded that the absence of effect was due to rapid metabolism of the phytoestrogens in their experimental model. In MCF-7 cells, genistein decreased estradiol formation from estrone, suggesting inhibition of 17β-HSD Type 1 activity.104 Structural features, especially the position of (methylation of) hydroxyl groups, appear to be important in determining the potential of phytoestrogens to inhibit 17β-HSD Type 1 and Type 5 activity.143,144 Using recombinant 17β-HSD, one study described the inhibitory potencies of 17β-HSD Type 5 with respect to quercetin (5–9 μM), biochanin A (8–14 μM), naringenin (10–33 μM), genistein and daidzein (>20 μM).145 The IC50 values for 17β-HSD Type 5 inhibition were somewhat different for the conversion of androstenedione into testosterone or androstanediol to androsterone. However, only for naringenin did this result in a 3-fold difference, with IC50 values of 33 μM for the inhibition of androstenedione to testosterone conversion and 10 μM for the inhibition of androstanediol to androsterone conversion. In placental microsomes, genistein, daidzein, biochanin A and naringenin inhibited 17β-HSD activity (type not specified) with IC50 values of 1, 10, 4.9 and 15 μM, respectively.137
Sulfotransferase
Estrogen sulfotransferases catalyze the transfer of a sulfonate group to estrone and estradiol thereby reducing its biological activity. In humans, three families and 8 subfamilies of sulfotransferases (SULT) have been characterized, of which SULT1E1 (estrogen sulfotransferase) has the most marked affinity for estrogens. However, sulfotransferase 1A1 (SULT1A1) expression is even higher than that of SULT1E1 in premenopausal endometrium and may also be involved in estrogen sulfoconjugation.110,146
Harris et al. reported that SULT1E1 was less sensitive to inhibition by phytoestrogens than SULT1A1.114 Quercetin was found to be the most potent inhibitor of human platelet-derived SULT1A1 of the flavonoids tested with an IC50 value of 60 nM, while genistein and daidzein displayed IC50 values of 500 and 600 nM, respectively.114 With respect to SULT1E1, the authors found that daidzein was less active than genistein and its metabolite equol as inhibitors of SULT1E1 activity derived from human liver cytosol. For these phytoestrogens, only inhibitory constants (Ki values) were given, being 2.0, 0.5 and 0.4 μM for daidzein, genistein and equol, respectively. Based on that, the authors concluded that it was unlikely that SULT1E1 inhibition would occur in vivo.114 In contrast, Nishiyama et al. concluded that genistein and daidzein were sulfated by SULT1E1 but predominantly by SULT1A1.147 Also, the authors found that SULT1E1-mediated sulfation of E2 may be strongly inhibited by genistein and daidzein at tissue concentrations around Ki, which were 7 and 14 μM, respectively. Quercetin and resveratrol inhibited estradiol sulfation in recombinant sulfotransferase (subtype not specified) with IC50 values of 1.4 and 1.6 μM, respectively.148 The same study showed a much lower IC50 value for the inhibition of estradiol sulfation by quercetin in human mammary epithelial cells with IC50 values of 0.13 μM, while the IC50 value for resveratrol was similar at 1.3 μM. Although all these studies show an inhibition of SULT1A1/1E1 activity by phytoestrogens, a study with hepatic HepG2 cells showed that genistein can induce SULT1A1 protein expression and activity.149 Interestingly, a statistically significant induction of SULT1A1 activity was observed after 4 days at 5 μM, but after 7 days of exposure already at 0.2 μM. The same study also found a concentration- and time-dependent increase in gene and protein expression of SULT1A1 in intestinal Caco-2 cells, but this was not accompanied by increased SULT1A1 activity.
Phase I metabolism
Oxidative A-ring metabolism of estrogens is the most important metabolic route of estrogen metabolism and yields the catechol estrogens 2-hydroxy- and 4-hydroxyestrogen. A-Ring metabolism in extrahepatic tissues is predominantly mediated by cytochrome P450 1A1 (CYP1A1) and 1B1 (CYP1B1).81–83 CYP1A1 mainly converts estrogens to 2-hydroxyestrogens but, due to a lack of specificity of the enzyme, about 15–20% to 4-hydroxyestrogens.150 CYP1B1, on the other hand, is a catalytically highly efficient and specific estrogen 4-hydroxylase, which is predominantly responsible for the extrahepatic 4-hydroxylation of estrogens.151 Expression of CYP1A1 and CYP1B1 is regulated by the vertebrate aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor that belongs to a protein family characterized by the presence of a basic helix–loop–helix/Per–ARNT–Sim (bHLH/PAS) domain.152 In addition, CYP1B1 expression is also regulated via ERα.153 AhR was originally discovered to mediate the toxic responses of dioxins, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), but nowadays its role in various endogenous cellular processes is widely acknowledged.152 Of specific interest here is the crosstalk between AhR- and ER-mediated pathways, among others via the AhR-mediated induction of CYP1A1 and CYP1B1-mediated metabolism of estradiol. Indeed, activation of the AhR by TCDD has been shown to increase estrogen metabolism in MCF-7 and non-tumorigenic breast epithelial MCF-10A cells.154
Several phytoestrogens have been shown to interact with AhR either as agonists or antagonists.155 For example, biochanin A and formononetin were activators of AhR with EC50 values of 0.25 μM and 0.13 μM, respectively, in a yeast AhR-reporter assay.156 Quercetin and resveratrol are often considered antagonists of AhR, but at least some of their actions appear to be indirectly via inhibition of the formation of the endogenous AhR ligand 6-formylindolo[3,2-b]carbazole (FICZ).157 The AhR-mediated actions of phytoestrogens appear to be cell context dependent, which is a commonly observed feature for AhR-mediated effects. For example in MCF-7 cells, but not in hepatic HepG2 cells, quercetin inhibited the TCDD-induced transactivation of AhR.158
Several studies have shown that quercetin, biochanin A, naringenin, 8-PN, resveratrol, genistein and daidzein can induce CYP1A1 and 1B1 gene expression in mammary malignant MCF-7 and/or non-tumorigenic MCF-10 cells.159–162 In MCF-7 cells at 1 μM, genistein, daidzein and equol were approximately 2-fold more potent in inducing CYP1A1 than CYP1B1 gene expression.162 Here, IC50 values for the induction of CYP1A1 gene expression were 2.2 μM, 2.0 μM and 3.5 μM for genistein, daidzein and equol, respectively. However, in another study, the apparent induction of CYP1B1 gene expression by genistein (10 μM) was somewhat higher than CYP1A1 induction in MCF-7 cells (apparent 5- vs. 8-fold for CYP1A1 and CYP1B1, respectively).163 The authors speculate that this was due to both ER-mediated as well as AhR-mediated regulation of CYP1B1 gene expression. In contrast, another study showed that genistein and daidzein inhibited CYP1A1 gene expression in MCF-7 cells.164 This study also described a decrease in CYP1A1 activity by genistein and daidzein by assessing ethoxyresorufin-O-deethylase (EROD) activity. EROD activity is commonly used as a proxy for the activity of CYP1A1, and to a lesser extent CYP1B1. This is especially relevant when assessing EROD activity in whole cell systems, where both CYP1A1 and CYP1B1 are typically present. CYP1A1/1B1-mediated estrogen metabolism yields catechol estrogens that can undergo oxidation to reactive quinones thereby forming potentially harmful reactive oxygen species (ROS).165 Moreover, quinones of predominantly CYP1A1-derived 2-hydroxyestrogens (2-OHE1/2) can form stable DNA adducts that remain in the DNA unless repaired, but quinones of the CYP1B1-derived 4-hydroxyestrogens (4-OHE1/2) can form depurinating DNA adducts, a potential tumor-initiating event in human cancers.165–169 Several studies have shown a preferential inhibition of CYP1B1 by genistein, naringenin, quercetin and biochanin A over CYP1A1 inhibition in recombinant human enzymes with an order of magnitude difference in IC50 values (see Table 3).170–172 This is not the case for resveratrol. For resveratrol, IC50 values for CYP1A1- and CYP1B1-mediated inhibition of estrogen metabolism were 2 and 25 μM, respectively, in recombinant human CYPs.173 Chun et al. described a similar IC50 value for inhibition of EROD activity by resveratrol in human liver microsomes (IC50 of 1.1 mM), but a higher IC50 value (23 μM) in recombinant CYP1A1.174 Some studies describe an induction of CYP1A1/1B1 gene expression by resveratrol in mammary non-malignant MCF-10A and MCF10F cells,175,176 but a decrease in CYP1B1-mediated estrogen metabolism leading to decreased DNA adduct formation.175 This shows the importance of assessing catalytic activity when evaluating the effects of phytoestrogens on CYP1A1/CYP1B1. Schwarz et al. described the inhibition of recombinant CYP1A1-mediated estradiol 2-hydroxylation by quercetin and resveratrol with IC50 values of 3.2 μM and 4.8 μM, respectively.177 However, because both Lu et al.175 and Schwarz et al.177 only determined one estradiol metabolite, no conclusion can be drawn regarding relative CYP1A1/CYP1B1 inhibition. A study with MCF-10A cells showed that hop extract was able to reduce E2-induced CYP1A1/CYP1B1 gene expression and estrogen metabolite formation in MCF-10A cells after 6 days.178 The authors describe that 8-PN (0.5 μM) was able to reduce E2 metabolite formation after 6 days of exposure to 8-PN and 1 μM E2.178 In a later study, the same authors describe that 8-PN (1 μM) induces CYP1A1/1B1 gene expression in MCF-7 cells, but not in MCF-10A cells.179 Analysis of methoxyestrogen formation after 2 days of exposure to 1 μM 8-PN and 1 μM E2 showed that 2- and, to a lesser extent, 4-methoxyestradiol levels were increased. While the same study also showed that 8-PN inhibited the activity of recombinant CYP1A1 and CYP1B1 with IC50 values of 0.38 and 0.41 μM, respectively, the authors conclude that induction of enzyme levels overcomes the direct catalytic inhibition by 8-PN in whole cell systems.179
Phase II metabolism
A major route of catechol estrogen detoxification is via methylation by catechol-O-methyltransferase (COMT).84–87 COMT is involved in the inactivation of endogenous catecholamines and catechol estrogens, by transferring a methyl group from S-adenosyl-l-methionine (SAM) to the substrate and thus converting them into methoxy derivatives.87
Phytoestrogens with a catechol group, such as tea catechins, their metabolites and quercetin have been shown to inhibit COMT activity. For example, quercetin inhibits COMT activity in the cytosol of healthy mammary tissue180 and human liver cytosol181 with IC50 values of 0.5 μM and 8.5 μM, respectively. Although genistein did not show inhibitory activity against COMT in human liver cytosol,181 another study showed that genistein reduced COMT activity and inhibited 4-OHE2 methylation in MCF-7 cells.182 A study with MCF-7 cells showed that genistein and daidzein decreased CYP1A1 and COMT activity and expression at micromolar concentrations, suggesting an inhibited detoxification of catechol estrogens.164 A study with MCF10F cells showed no change in COMT protein expression and activity by resveratrol.183
Comparison of in vitro findings and systemic concentrations in humans
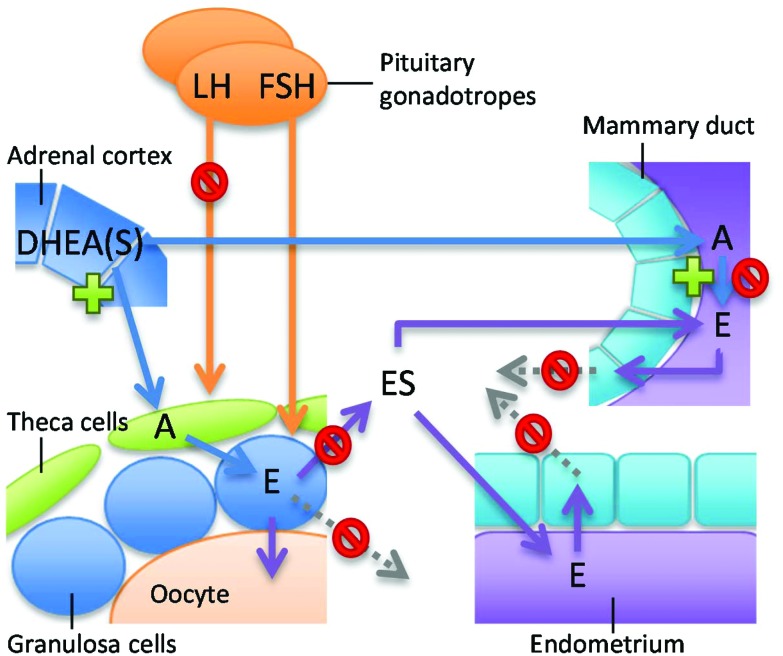
As described above, many phytoestrogens can modulate the activity of a wide variety of enzymes involved in estrogen synthesis and metabolism in vitro. But how relevant are these findings for women who use dietary supplements containing these phytoestrogens? One way to approach this question is to compare in vitro effect concentrations with phytoestrogen concentrations in plasma or, even better, target tissues. However, only two studies were found that provided tissue concentrations upon phytoestrogen intake, namely breast tissue concentrations after soy intake (genistein and daidzein) and 8-PN supplement intake.17,31 For 8-PN, breast tissue levels between 0.8 and 4.8 nM† were found after intake of low levels of 8-PN (0.1 mg, three times per day for 5 days). These breast tissue levels are lower than in vitro concentrations where induction of CYP-mediated E2 metabolism in breast cells is observed (Table 3). However, these breast tissue levels are in the same order of magnitude where 8-PN was found to inhibit aromatase activity in vitro in ovarian KGN (IC50 8 nM)107 and adrenal H295R cells (IC50 0.1 μM).14 Also, a high single oral dose of 750 mg 8-PN can lead to higher plasma levels, up to 0.2 μM.25 Considering that supplements can contain up to 1000 mg 8-PN13 and are typically taken daily for a longer period of time, inhibition of aromatase activity potentially leading to decreased estrogen production in women taking these supplements is highly likely. High estrogen levels are associated with increased breast cancer risk in postmenopausal women184 and aromatase inhibitors reduce the incidence of breast cancer.185 Therefore, the aromatase inhibitory effects of 8-PN could reduce breast cancer risk in postmenopausal women. However, these favourable effects might be overcome by the potent ERα-mediated actions of 8-PN.53 Genistein and daidzein concentrations attainable in breast tissue (0.09–0.5 μM genistein and 0.02–0.7 μM daidzein) and plasma (up to 2.8 μM, see Table 2) are similar to concentrations at which modulation of the activity of several estrogen synthesizing/metabolising enzymes can occur (Table 3). The in vitro studies point toward a decreased estradiol level in healthy breast tissue due to the inhibition of aromatase and 17β-HSD type 1 activity. This suggests a beneficial effect of genistein on breast health. However, the activity of breast cancer-associated aromatase, which is driven by promoters II and I.3, is increased by genistein.92 This might have implications for the risk of genistein in postmenopausal women with breast cancer undergoing adjuvant endocrine therapies. This will be addressed in more detail below (see: Phytoestrogens and women's health: epidemiological data). Besides aromatase, the in vitro effects of genistein point toward a decreased E1 to E2 conversion via inhibition of 17β-HSD, but also decreased inactivation of estrogens via SULT1E1 and CYP1B1. Together, this may result in increased intracellular E1 levels and lower circulating E1/E2S (Fig. 2). Modulation of estrogen metabolism by genistein in ovaria might affect menstrual cycling, possibly also via regulatory feedback mechanisms on the hypothalamus–pituitary–gonad (HPG) axis. Indeed, consumption of phytoestrogen-rich soy foods increases menstrual cycle length in healthy premenopausal women.186,187 Typically, studies show moderate effects of soy phytoestrogens on circulating estrogen levels, but consistently report suppressed circulating LH and/or FSH concentrations.187 A reduction in LH concentrations was also found in healthy premenopausal women after a single 750 mg dose of 8-PN.25 An effect on menstrual cycling poses a real problem for women attempting to become pregnant. Some studies suggest that soy food intake and soy supplements improve fertility treatment outcomes with assisted reproductive technologies.188,189 However, several in vitro and in vivo experimental studies clearly show that genistein, but also 8-PN and naringenin, can inhibit cumulus cell expansion, oocyte maturation and developmental competence of mouse and porcine oocytes.190–192 Considering that a predominantly intrafollicular estrogenic environment is essential for good follicle growth and maturation,193,194 it is highly plausible that high intake of phytoestrogens can affect the ovarian hormonal environment thereby leading to a profound effect on oocyte quality. In addition, lower circulating E1/E2S may subsequently lead to lower estrogen levels in peripheral tissues (Fig. 2). This is especially relevant for the endometrium, considering that the endometrium, unlike ovarian and breast tissue, does not possess enzymes to transform DHEA into estrogens and is strongly dependent on circulating E1S for local estrogen synthesis (Fig. 1).109 Changes in the endometrial hormonal environment by phytoestrogens could affect the process of embryonic implantation. On the other hand, lower estrogen is associated with lower risk for hormonal cancers. Lower circulating estrogen levels could therefore be a beneficial effect, especially relevant for postmenopausal women.
Fig. 2. Graphical overview of the potential effects of genistein in specific tissues via modulation of estrogen synthesis and metabolism. Upon soy-rich food or supplement intake, genistein might reach sufficient levels to decrease estrogen (E) levels in healthy breast tissue due to the inhibition of aromatase, but increase the activity of breast cancer-associated aromatase. Genistein may affect ovarian and endometrial functions by the inhibition of 17β-HSD, SULT1E1 and CYP1B1 activity, leading to increased intracellular E levels and lower circulating levels of estrogen sulfates (ES). In premenstrual women, genistein suppresses circulating LH and/or FSH concentrations.186,187 A = androgen; DHEA(S) = dehydroepiandosterone (sulfate); E = estrogen; FSH = follicle stimulating hormone; LH = luteinizing hormone.
As said, data on tissue levels for other phytoestrogens other than genistein and 8-PN are lacking, but some data are available on plasma levels of phytoestrogens (Table 2). It is clear that the plasma levels of many phytoestrogens are in the same order of magnitude as concentrations that have been shown to affect estrogen synthesis or metabolism in vitro (summarized in Table 4). Biochanin A concentrations in blood seem to be too low to affect estrogen synthesizing or metabolizing enzymes, although effect concentrations for the inhibition of 3β-HSD and CYP1B1 in purified enzymes are within the range. It is worth noting here that biochanin A is metabolised into genistein, which may in turn potentially lead to in vivo effects. For naringenin, the increase in aromatase activity in ovarian cells and decrease in breast cells suggest a tissue-specific effect on estrogen synthesis. As described above, this might lead to effects on fertility and breast health. For resveratrol, plasma concentrations in the low micromolar range have been described upon supplement intake.26,36,37 Based on the in vitro data in Table 3, these resveratrol concentrations could decrease DHEA production by CYP17 subsequently leading to lowered estrogen levels (see Table 3). Altered ovarian hormone production will lead to modulation of the menstrual cycle, which can affect fertility in young women. In contrast, a decreased androgen production might be favorable for women with PCOS, which is characterized by hyperandrogenism.195 Also, decreased sulfatation of estradiol by resveratrol via effects on SULT would also decrease circulating estrogen sulfate levels. As described above, this might be beneficial for postmenopausal women, but might give rise to fertility problems in premenopausal women. For quercetin, the increased aromatase activity and decreased sulfation activity point toward an increase in local estrogen levels, for example in ovarian cells, which may lead to changes in menstrual cycle, and endometrial and breast health. Moreover, virtually all effects described for quercetin in Table 3 appear to be in the range of quercetin plasma concentrations, at least when looking at the study of Kaushik et al.38 However, most of the effects for quercetin are based on studies using recombinant or purified enzymes. Considering that in these types of test systems a potential increase in enzyme levels and activity cannot be established, effect concentrations observed in recombinant enzymes or microsomes should be interpreted with caution. Moreover, whole cell systems certainly affect the actual concentration of a test compound that can reach a target, e.g. a receptor, due to membrane barriers, cell-type specific metabolism and compensatory effects. Therefore, the effect of a phytoestrogen in whole cell systems should be deemed more relevant for hazard and risk assessment. The data in Table 3 also clearly show the importance of having whole cell in vitro models that represent specific tissues. This is especially apparent for aromatase, which is regulated by tissue-specific promoters, but also holds true for enzymes that have clear tissue-specific isoforms such as 17β-HSD. While aromatase is considered a rate-limiting step in estrogen synthesis, clearly other enzymes that modulate estrogen levels are also important to maintain correct hormonal levels, as described above. Unfortunately, for most phytoestrogens and/or estrogen synthesizing/metabolising enzymes relevant in vitro data from relevant, whole cell systems are lacking, which clearly hampers the risk assessment of these compounds. Specifically, more data are needed on effects in ovarian and endometrial cells. With respect to specific enzymes, it would be relevant to establish the net effect of a phytoestrogen on estrogen production and/or secretion in multiple cell types, considering that the phytoestrogens typically affect multiple enzymes in a tissue-specific manner. Here, steroid profiling using a metabolomics approach196 could provide a useful tool that can help better risk assessment of phytoestrogens based on in vitro effects on estrogen synthesis and metabolism.
Phytoestrogens and women's health: epidemiological data
Considering that local sex steroidogenesis is crucial for normal physiology, it is not surprising that altered local hormonal regulation is associated with pathogenesis. For example, local uterine estrogen levels are found to be higher in endometrial cancers than in healthy tissues, which indicates an important role for local aromatase.100 Disruption of local hormonal regulation in the endometrium is known to affect the process of implantation and the reproductive potential and contributes to infertility.197 Changes in STS and SULT enzymes are seen in a variety of gynecological diseases, such as endometriosis and ovarian tumors.111 In addition, local estrogen modulating enzymes are used as therapeutic targets in the treatment of hormone-dependent diseases, such as tumors of the breast,198 endometrium199 and ovary.200 As summarized in Table 4, phytoestrogens can modulate the activity of enzymes known to affect female reproductive health at concentrations that can easily be reached in the blood of women using phytoestrogen-containing supplements. However, clear epidemiological evidence to establish that these phytoestrogens in fact cause (adverse) health effects in women is scarce. Especially in women, such studies are complicated due to variations in menstrual phase (background levels of estrogens modulate phytoestrogenic effects) and menopausal status (natural changes in local enzyme expression and background steroid levels). But also interaction with therapeutic drugs (e.g. breast cancer treatment) or other phytoestrogens (mixtures in plant extracts and dietary interaction) can modulate the effects of specific phytoestrogens in women. Most available epidemiological studies describe the potential association with breast cancer risk in peri- and postmenopausal women who use soy isoflavones to alleviate menopausal symptoms. Menopause arises due to a natural decline in estrogen levels or can occur suddenly as the result of cancer treatment with ER antagonists and/or aromatase inhibitors. Yet, the evidence about the efficacy of phytoestrogens is rather limited in randomized clinical trials. A recent meta-analysis concludes that there is a statistically significant and clinically relevant benefit for using a specific standardized extract of red clover isoflavones (Promensil) at 80 mg day–1 for treating hot flushes in menopausal women.201 No increase in reported side effects was noted for a duration of 3 months. However, the conclusions were based on only three studies that met the inclusion criteria of the total of 20 relevant papers identified. Another meta-analysis of side effects comparing phytoestrogen treatment with placebo or no treatment in randomized controlled trials found no significant increase in the occurrence of adverse side effects, i.e. vaginal bleeding, endometrial hyperplasia, endometrial cancer and breast cancer among postmenopausal women.202 However, in this study no specification of specific type of phytoestrogen was considered. Also, the studies included in the meta-analysis varied greatly in study duration, which reportedly varied between 1 day and 5 years (median 6.2 months). It could be argued that it takes some time for side effects to develop, which makes it highly questionable whether for example an effect on cancer risk can indeed be assessed in these studies. In a three-year randomized, double-blind, placebo-controlled trial among 350 postmenopausal women, isoflavone soy protein supplementation showed no effect on endometrial thickness or on the rates of endometrial hyperplasia and cancer.203 Also, in a recent evaluation, the European Food Safety Authority concluded that isoflavones do not adversely affect the breast, thyroid or uterus of postmenopausal women.204 Yet remarkably, all epidemiological studies conclude that there is not enough data to determine the safety of long-term use of high doses of phytoestrogens. This is clearly a reason for concern, considering that cases of postmenopausal bleeding in women using hop- and soy-based supplements are regularly being reported.205,206 Also, little is known about the potential interaction of phytoestrogens with therapeutic treatments. Some describe that soy foods consumed at levels comparable to those in Asian populations do not appear to interfere with breast cancer treatment in human populations.207 Yet, in vitro studies have shown that genistein and 8-PN, but also extracts of commercially available menopausal supplements, can overcome the inhibitory actions of the ER antagonist tamoxifen and aromatase inhibitor letrozole on MCF-7 tumor cell proliferation at human-relevant dose levels.14 In addition, in vivo experimental studies show that at high intake levels genistein can overcome the breast tumor growth inhibitory effect of tamoxifen208 and letrozole.209
Strikingly, little attention is focused on the potential health effects of phytoestrogen intake in premenopausal women. This is of great concern considering that several phytoestrogen-containing supplements are specifically marketed for young women in the reproductive age, for example for breast enhancement13 or reduction of premenstrual symptoms.210,211 As described above, several phytoestrogens have been shown to affect menstrual cycle length in healthy premenopausal women,186,187 possibly via modulation of local steroid hormone production and/or altered pituitary gonadotropin release via modulation of the HPG axis.25,187 In addition, potential effects on oocyte quality and endometrial receptivity by phytoestrogens via alterations of ovarian and endometrial hormonal environments could seriously affect the ability of a woman to become pregnant. This potential effect of phytoestrogens on fertility clearly needs to be addressed in future research studies.
Are supplements with phytoestrogens safe for women?
While many studies have addressed the potential health effects of soy and soy-derived phytoestrogens in postmenopausal women, there still remains a lot of controversy regarding the safety of soy-derived phytoestrogens.212 Even less is known about the potential adverse or beneficial effects of other phytoestrogens on the health of premenopausal women. It is important to distinguish here regular dietary intake of phytoestrogens and intake of supplements with high levels of phytoestrogens. Normal dietary intake of phytoestrogens is generally regarded as safe, but more data are needed for a better understanding of risks upon repeated intake of high levels of phytoestrogen for a longer period of time. Particularly, data on tissue levels of phytoestrogens and effect data from dedicated, tissue-specific assays that are relevant for premenopausal women are largely lacking. However, based on in vitro data it is clear that many phytoestrogens are potent modulators of enzymes that are known to play important roles in estrogen-producing and -responsive tissues. In some cases, in vitro effect concentrations of phytoestrogens lie in the range of in vivo obtainable plasma or even tissue levels, which suggest that modulation of estrogen levels in women might occur. Hormonal modulation by phytoestrogens may lead to fertility problems in premenopausal women due to effects on the menstrual cycle, oocyte quality and endometrial receptivity. It can be concluded that until more certainty is obtained regarding the safety, especially young women should better avoid long-term use of supplements with high phytoestrogen content.
Conflicts of interest
There are no conflicts of interest to declare.
Biography

Majorie B. M. van Duursen
Majorie van Duursen is Associate Professor and Endocrine Toxicologist at the Institute for Risk Assessment Sciences (IRAS) at Utrecht University (the Netherlands) with over 15 years' experience in toxicological research and human risk assessment. As principal investigator, she has been involved in various (inter)national projects on the endocrine-modulating effects of chemicals and natural compounds with special emphasis on female health and the developing child. In her research, she develops and applies in vitro models, using 3D cultured (primary) human cells in a relevant tissue microenvironment to increase mechanistic understanding on how (phyto)chemicals can modulate biological pathways and affect women's health.
Footnotes
†Assuming that 1 g of breast tissue is 1 mL.
References
- War A. R., Paulraj M. G., Ahmad T., Buhroo A. A., Hussain B., Ignacimuthu S., Sharma H. C. Plant Signaling Behav. 2012;7:1306–1320. doi: 10.4161/psb.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz B. M., Hajirahimkhan A., Dunlap T. L., Bolton J. L. Pharmacol. Rev. 2016;68:1026–1073. doi: 10.1124/pr.115.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell T., Cohick W., Raskin I. Phytochemistry. 2004;65:995–1016. doi: 10.1016/j.phytochem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Touillaud M. S., Pillow P. C., Jakovljevic J., Bondy M. L., Singletary S. E., Li D., Chang S. Nutr. Cancer. 2005;51:162–169. doi: 10.1207/s15327914nc5102_6. [DOI] [PubMed] [Google Scholar]
- Sirtori C. R., Arnoldi A., Johnson S. K. Ann. Med. 2005;37:423–438. doi: 10.1080/07853890510044586. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H. Lancet Oncol. 2002;3:364–373. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- Posadzki P., Lee M. S., Moon T. W., Choi T. Y., Park T. Y., Ernst E. Maturitas. 2013;75:34–43. doi: 10.1016/j.maturitas.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Yazbek P. B., Tezoto J., Cassas F., Rodrigues E. J. Ethnopharmacol. 2016;179:310–331. doi: 10.1016/j.jep.2015.12.054. [DOI] [PubMed] [Google Scholar]
- Garcia-Alvarez A., Egan B., de Klein S., Dima L., Maggi F. M., Isoniemi M., Ribas-Barba L., Raats M. M., Meissner E. M., Badea M., Bruno F., Salmenhaara M., Mila-Villarroel R., Knaze V., Hodgkins C., Marculescu A., Uusitalo L., Restani P., Serra-Majem L. PLoS One. 2014;9:e92265. doi: 10.1371/journal.pone.0092265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T., Kawa K., Eckl V., Johnson J. S. HerbalGram. 2016;111:67–73. [Google Scholar]
- Jiang B., Ma C., Motley T., Kronenberg F., Kennelly E. J. Phytochem. Anal. 2011;22:339–351. doi: 10.1002/pca.1285. [DOI] [PubMed] [Google Scholar]
- Wang L. Q. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2002;777:289–309. doi: 10.1016/s1570-0232(02)00281-7. [DOI] [PubMed] [Google Scholar]
- Coldham N. G., Sauer M. J. Food Chem. Toxicol. 2001;39:1211–1224. doi: 10.1016/s0278-6915(01)00081-3. [DOI] [PubMed] [Google Scholar]
- van Duursen M. B., Smeets E. E., Rijk J. C., Nijmeijer S. M., van den Berg M. Toxicol. Appl. Pharmacol. 2013;269:132–140. doi: 10.1016/j.taap.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Imhof M., Molzer S., Imhof M. Toxicol. in Vitro. 2008;22:1452–1460. doi: 10.1016/j.tiv.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Patisaul H. B., Jefferson W. Front. Neuroendocrinol. 2010;31:400–419. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolca S., Urpi-Sarda M., Blondeel P., Roche N., Vanhaecke L., Possemiers S., Al-Maharik N., Botting N., De Keukeleire D., Bracke M., Heyerick A., Manach C., Depypere H. Am. J. Clin. Nutr. 2010;91:976–984. doi: 10.3945/ajcn.2009.28854. [DOI] [PubMed] [Google Scholar]
- Moon Y. J., Wang L., DiCenzo R., Morris M. E. Biopharm. Drug Dispos. 2008;29:205–217. doi: 10.1002/bdd.605. [DOI] [PubMed] [Google Scholar]
- de Cremoux P., This P., Leclercq G., Jacquot Y. Maturitas. 2010;65:334–339. doi: 10.1016/j.maturitas.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Ozdal T., Sela D. A., Xiao J., Boyacioglu D., Chen F., Capanoglu E. Nutrients. 2016;8:78. doi: 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell K. D., Brown N. M., Zimmer-Nechemias L., Brashear W. T., Wolfe B. E., Kirschner A. S., Heubi J. E. Am. J. Clin. Nutr. 2002;76:447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- Rowland I. R., Wiseman H., Sanders T. A., Adlercreutz H., Bowey E. A. Nutr. Cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- Setchell K. D., Brown N. M., Desai P., Zimmer-Nechemias L., Wolfe B. E., Brashear W. T., Kirschner A. S., Cassidy A., Heubi J. E. J. Nutr. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- Boonpawa R., Moradi N., Spenkelink A., Rietjens I. M., Punt A. Biochem. Pharmacol. 2015;98:690–702. doi: 10.1016/j.bcp.2015.09.022. [DOI] [PubMed] [Google Scholar]
- Rad M., Humpel M., Schaefer O., Schoemaker R. C., Schleuning W.-D., Cohen A. F., Burggraaf J. Br. J. Clin. Pharmacol. 2006;62:288–296. doi: 10.1111/j.1365-2125.2006.02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida L., Vaz-da-Silva M., Falcao A., Soares E., Costa R., Loureiro A. I., Fernandes-Lopes C., Rocha J. F., Nunes T., Wright L., Soares-da-Silva P. Mol. Nutr. Food Res. 2009;53(Suppl 1):S7–15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- Yang Z., Kulkarni K., Zhu W., Hu M. Anticancer Agents Med. Chem. 2012;12:1264–1280. doi: 10.2174/187152012803833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maubach J., Depypere H. T., Goeman J., Van der Eycken J., Heyerick A., Bracke M. E., Blondeel P., De Keukeleire D. Obstet. Gynecol. 2004;103:892–898. doi: 10.1097/01.AOG.0000124983.66521.6a. [DOI] [PubMed] [Google Scholar]
- Enokizono J., Kusuhara H., Sugiyama Y. Mol. Pharmacol. 2007;72:967–975. doi: 10.1124/mol.107.034751. [DOI] [PubMed] [Google Scholar]
- Tan K. W., Cooney J., Jensen D., Li Y., Paxton J. W., Birch N. P., Scheepens A. Mol. Nutr. Food Res. 2014;58:2099–2110. doi: 10.1002/mnfr.201400288. [DOI] [PubMed] [Google Scholar]
- Bolca S., Li J., Nikolic D., Roche N., Blondeel P., Possemiers S., De Keukeleire D., Bracke M., Heyerick A., van Breemen R. B., Depypere H. Mol. Nutr. Food Res. 2010;54(Suppl 2):S284–S294. doi: 10.1002/mnfr.200900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhang W., Liu J., Sun Y., Li Y., Li H., Xiao S., Shen X. Toxicol. Appl. Pharmacol. 2013;269:280–289. doi: 10.1016/j.taap.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Howes J., Waring M., Huang L., Howes L. G. J. Altern. Complement. Med. 2002;8:135–142. doi: 10.1089/107555302317371424. [DOI] [PubMed] [Google Scholar]
- Erlund I., Meririnne E., Alfthan G., Aro A. J. Nutr. 2001;131:235–241. doi: 10.1093/jn/131.2.235. [DOI] [PubMed] [Google Scholar]
- Kanaze F. I., Bounartzi M. I., Georgarakis M., Niopas I. Eur. J. Clin. Nutr. 2007;61:472–477. doi: 10.1038/sj.ejcn.1602543. [DOI] [PubMed] [Google Scholar]
- Sergides C., Chirila M., Silvestro L., Pitta D., Pittas A. Exp. Ther. Med. 2016;11:164–170. doi: 10.3892/etm.2015.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkon A., Somoza V. Mol. Nutr. Food Res. 2008;52:549–557. doi: 10.1002/mnfr.200700290. [DOI] [PubMed] [Google Scholar]
- Kaushik D., O'Fallon K., Clarkson P. M., Dunne C. P., Conca K. R., Michniak-Kohn B. J. Food Sci. 2012;77:H231–H238. doi: 10.1111/j.1750-3841.2012.02934.x. [DOI] [PubMed] [Google Scholar]
- Csiszar A. Ann. N. Y. Acad. Sci. 2011;1215:117–122. doi: 10.1111/j.1749-6632.2010.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Larrea M. B., Mohan A. R., Paganga G., Miller N. J., Bolwell G. P., Rice-Evans C. A. Free Radical Res. 1997;26:63–70. doi: 10.3109/10715769709097785. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Herceg Z. Cancer Lett. 2014;342:275–284. doi: 10.1016/j.canlet.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Huang Y. W., Kuo C. T., Stoner K., Huang T. H., Wang L. S. FEBS Lett. 2011;585:2129–2136. doi: 10.1016/j.febslet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G. R., Xiao C. L., He G. W., Yin X. F., Chen N. P., Cao Y., He Q. Y. Proteomics. 2010;10:976–986. doi: 10.1002/pmic.200900662. [DOI] [PubMed] [Google Scholar]
- Toft D., Gorski J. Proc. Natl. Acad. Sci. U. S. A. 1966;55:1574–1581. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper G. G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J. A. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Gustafsson J. A. Nat. Rev. Cancer. 2011;11:597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- Cowley S. M., Parker M. G. J. Steroid Biochem. Mol. Biol. 1999;69:165–175. doi: 10.1016/s0960-0760(99)00055-2. [DOI] [PubMed] [Google Scholar]
- Kuiper G. G., Lemmen J. G., Carlsson B., Corton J. C., Safe S. H., van der Saag P. T., van der Burg B., Gustafsson J. A. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Rietjens I. M., Louisse J., Beekmann K. Br. J. Pharmacol. 2016;174:1263–1280. doi: 10.1111/bph.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovee T. F., Helsdingen R. J., Rietjens I. M., Keijer J., Hoogenboom R. L. J. Steroid Biochem. Mol. Biol. 2004;91:99–109. doi: 10.1016/j.jsbmb.2004.03.118. [DOI] [PubMed] [Google Scholar]
- Reiter E., Reiter E., Beck V., Medjakovic S., Jungbauer A. Gynecol. Endocrinol. 2009;25:554–580. doi: 10.1080/09513590802596461. [DOI] [PubMed] [Google Scholar]
- Roelens F., Heldring N., Dhooge W., Bengtsson M., Comhaire F., Gustafsson J. A., Treuter E., De Keukeleire D. J. Med. Chem. 2006;49:7357–7365. doi: 10.1021/jm060692n. [DOI] [PubMed] [Google Scholar]
- Milligan S., Kalita J., Pocock V., Heyerick A., De Cooman L., Rong H., De Keukeleire D. Reproduction. 2002;123:235–242. [PubMed] [Google Scholar]
- Schaefer O., Humpel M., Fritzemeier K. H., Bohlmann R., Schleuning W. D. J. Steroid Biochem. Mol. Biol. 2003;84:359–360. doi: 10.1016/s0960-0760(03)00050-5. [DOI] [PubMed] [Google Scholar]
- van Meeuwen J. A., Korthagen N., de Jong P. C., Piersma A. H., van den Berg M. Toxicol. Appl. Pharmacol. 2007;221:372–383. doi: 10.1016/j.taap.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Shang Y., Brown M. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- van Meeuwen J. A., Ter Burg W., Piersma A. H., van den Berg M., Sanderson J. T. Food Chem. Toxicol. 2007;45:2319–2330. doi: 10.1016/j.fct.2007.06.011. [DOI] [PubMed] [Google Scholar]
- van Meeuwen J. A., van den Berg M., Sanderson J. T., Verhoef A., Piersma A. H. Toxicol. Lett. 2007;170:165–176. doi: 10.1016/j.toxlet.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Filardo E. J., Thomas P. Endocrinology. 2012;153:2953–2962. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz E. R., Barton M. Nat. Rev. Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz E. R., Arterburn J. B. Pharmacol. Rev. 2015;67:505–540. doi: 10.1124/pr.114.009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filigheddu N., Sampietro S., Chianale F., Porporato P. E., Gaggianesi M., Gregnanin I., Rainero E., Ferrara M., Perego B., Riboni F., Baldanzi G., Graziani A., Surico N. Cell. Signalling. 2011;23:1988–1996. doi: 10.1016/j.cellsig.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Wang C., Lv X., He C., Hua G., Tsai M. Y., Davis J. S. Cell Death Dis. 2013;4:e869. doi: 10.1038/cddis.2013.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariazi E. A., Brailoiu E., Yerrum S., Shupp H. A., Slifker M. J., Cunliffe H. E., Black M. A., Donato A. L., Arterburn J. B., Oprea T. I., Prossnitz E. R., Dun N. J., Jordan V. C. Cancer Res. 2010;70:1184–1194. doi: 10.1158/0008-5472.CAN-09-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Chen Z. J., Zhang K. S., Yang X. L., Wu Y. M., Chen X. H., Huang H. B., Liu H. L., Cai S. H., Du J., Wang H. S. Cell Death Dis. 2014;5:e1428. doi: 10.1038/cddis.2014.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Zhang Q., McDonald T., Davidoff M. J., Bailey W., Bai C., Liu Q., Caskey C. T. Gene. 1999;228:101–109. doi: 10.1016/s0378-1119(98)00619-2. [DOI] [PubMed] [Google Scholar]
- Kiyama R., Wada-Kiyama Y. Environ. Int. 2015;83:11–40. doi: 10.1016/j.envint.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Jameson J. L., De Groot L. J., de Kretser D., Giudice L. C., Grossman A., Melmed S., Potts J. T. and Weir G. C., Endocrinology: Adult and Pediatric, Elsevier Saunders, Philadelphia, PA, 7th edn, 2016. [Google Scholar]
- van Landeghem A. A., Poortman J., Nabuurs M., Thijssen J. H. Cancer Res. 1985;45:2900–2906. [PubMed] [Google Scholar]
- Miller W. L., Auchus R. J. Endocr. Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B. C., Picado-Leonard J., Haniu M., Bienkowski M., Hall P. F., Shively J. E., Miller W. L. Proc. Natl. Acad. Sci. U. S. A. 1987;84:407–411. doi: 10.1073/pnas.84.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagimoto M., Winter J. S., Kagimoto K., Simpson E. R., Waterman M. R. Mol. Endocrinol. 1988;2:564–570. doi: 10.1210/mend-2-6-564. [DOI] [PubMed] [Google Scholar]
- Tilson-Mallett N., Santner S. J., Feil P. D., Santen R. J. J. Clin. Endocrinol. Metab. 1983;57:1125–1128. doi: 10.1210/jcem-57-6-1125. [DOI] [PubMed] [Google Scholar]
- Adams J. B., Li K. Br. J. Cancer. 1975;31:429–433. doi: 10.1038/bjc.1975.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A. D., Conley A. J. Endocr. Dev. 2008;13:33–54. doi: 10.1159/000134765. [DOI] [PubMed] [Google Scholar]
- Simpson E. R. J. Steroid Biochem. Mol. Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- Pasqualini J., Chetrite G., Nguyen B., Maloche C., Delalonde L., Talbi M., Feinstein M., Blacker C., Botella J., Paris J. J. Steroid Biochem. Mol. Biol. 1995;53:407–412. doi: 10.1016/0960-0760(95)00116-h. [DOI] [PubMed] [Google Scholar]
- Raftogianis R., Creveling C., Weinshilboum R., Weisz J. J. Natl. Cancer Inst. Monogr. 2000;27:113–124. doi: 10.1093/oxfordjournals.jncimonographs.a024234. [DOI] [PubMed] [Google Scholar]
- Tseng L., Mazella J., Lee L. Y., Stone M. L. J. Steroid Biochem. 1983;19:1413–1417. doi: 10.1016/0022-4731(83)91116-0. [DOI] [PubMed] [Google Scholar]
- Santner S. J., Leszczynski D., Wright C., Manni A., Feil P. D., Santen R. J. Breast Cancer Res. Treat. 1986;7:35–44. doi: 10.1007/BF01886734. [DOI] [PubMed] [Google Scholar]
- Martucci C. P., Fishman J. Pharmacol. Ther. 1993;57:237–257. doi: 10.1016/0163-7258(93)90057-k. [DOI] [PubMed] [Google Scholar]
- Badawi A. F., Cavalieri E. L., Rogan E. G. Metabolism. 2001;50:1001–1003. doi: 10.1053/meta.2001.25592. [DOI] [PubMed] [Google Scholar]
- Ziegler R. G., Rossi S. C., Fears T. R., Bradlow H. L., Adlercreutz H., Sepkovic D., Kiuru P., Wahala K., Vaught J. B., Donaldson J. L., Falk R. T., Fillmore C. M., Siiteri P. K., Hoover R. N., Gail M. H. Environ. Health Perspect. 1997;105:607–614. doi: 10.1289/ehp.97105s3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz J., Clawson G. A., Crevelingen C. R. Adv. Pharmacol. 1998;42:828–833. doi: 10.1016/s1054-3589(08)60874-1. [DOI] [PubMed] [Google Scholar]
- Borchardt R. T. Methods Enzymol. 1981;77:267–272. doi: 10.1016/s0076-6879(81)77036-8. [DOI] [PubMed] [Google Scholar]
- Dawling S., Roodi N., Mernaugh R. L., Wang X., Parl F. F. Cancer Res. 2001;61:6716–6722. [PubMed] [Google Scholar]
- Männistö P. T., Kaakkola S. Pharmacol. Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- Kamat A., Hinshelwood M. M., Murry B. A., Mendelson C. R. Trends Endocrinol. Metab. 2002;13:122–128. doi: 10.1016/s1043-2760(02)00567-2. [DOI] [PubMed] [Google Scholar]
- Chen S. A., Besman M. J., Sparkes R. S., Zollman S., Klisak I., Mohandas T., Hall P. F., Shively J. E. DNA. 1988;7:27–38. doi: 10.1089/dna.1988.7.27. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Clyne C., Rubin G., Boon W. C., Robertson K., Britt K., Speed C., Jones M. Annu. Rev. Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Michael M. D., Agarwal V. R., Hinshelwood M. M., Bulun S. E., Zhao Y. FASEB J. 1997;11:29–36. doi: 10.1096/fasebj.11.1.9034163. [DOI] [PubMed] [Google Scholar]
- Bulun S. E., Lin Z., Imir G., Amin S., Demura M., Yilmaz B., Martin R., Utsunomiya H., Thung S., Gurates B., Tamura M., Langoi D., Deb S. Pharmacol. Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- Stocco C. Steroids. 2008;73:473–487. doi: 10.1016/j.steroids.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Wu Y., Li R., Hu Y. Oncogene. 2005;24:2236–2246. doi: 10.1038/sj.onc.1208415. [DOI] [PubMed] [Google Scholar]
- Agarwal V. R., Bulun S. E., Leitch M., Rohrich R., Simpson E. R. J. Clin. Endocrinol. Metab. 1996;81:3843–3849. doi: 10.1210/jcem.81.11.8923826. [DOI] [PubMed] [Google Scholar]
- Zhou C., Zhou D., Esteban J., Murai J., Siiteri P. K., Wilczynski S., Chen S. J. Steroid Biochem. Mol. Biol. 1996;59:163–171. doi: 10.1016/s0960-0760(96)00100-8. [DOI] [PubMed] [Google Scholar]
- Kitawaki J., Noguchi T., Amatsu T., Maeda K., Tsukamoto K., Yamamoto T., Fushiki S., Osawa Y., Honjo H. Biol. Reprod. 1997;57:514–519. doi: 10.1095/biolreprod57.3.514. [DOI] [PubMed] [Google Scholar]
- Taga S., Yoshida N., Sekiba K. J. Steroid Biochem. Mol. Biol. 1990;37:741–745. doi: 10.1016/0960-0760(90)90359-s. [DOI] [PubMed] [Google Scholar]
- Brosens J., Verhoeven H., Campo R., Gianaroli L., Gordts S., Hazekamp J., Hägglund L., Mardesic T., Varila E., Zech J., Brosens I. Hum. Reprod. 2004;19:352–356. doi: 10.1093/humrep/deh075. [DOI] [PubMed] [Google Scholar]
- Bulun S. E., Lin Z., Imir G., Amin S., Demura M., Yilmaz B., Martin R., Utsunomiya H., Thung S., Gurates B., Tamura M., Langoi D., Deb S. Pharmacol. Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- Lephart E. D., Enzyme Res., 2015, 2015 , , 594656 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia T., Hilscherova K., Jones P. D., Newsted J. L., Zhang X., Hecker M., Higley E. B., Sanderson J. T., Yu R. M. K., Wu R. S. S., Giesy J. P. Ecotoxicol. Environ. Saf. 2006;65:293–305. doi: 10.1016/j.ecoenv.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanderson J. T., Hordijk J., Denison M. S., Springsteel M. F., Nantz M. H., van den Berg M. Toxicol. Sci. 2004;82:70–79. doi: 10.1093/toxsci/kfh257. [DOI] [PubMed] [Google Scholar]
- Brooks J. D., Thompson L. U. J. Steroid Biochem. Mol. Biol. 2005;94:461–467. doi: 10.1016/j.jsbmb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Chottanapund S., Van Duursen M. B., Navasumrit P., Hunsonti P., Timtavorn S., Ruchirawat M., Van den Berg M. Toxicol. in Vitro. 2014;28:1215–1221. doi: 10.1016/j.tiv.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Wang Y., Lee K. W., Chan F. L., Chen S., Leung L. K. Toxicol. Sci. 2006;92:71–77. doi: 10.1093/toxsci/kfj190. [DOI] [PubMed] [Google Scholar]
- Solak K. A., Wijnolts F., Nijmeijer S. M., Blaauboer B. J., van den Berg M., van Duursen M. B. M. Toxicol. Rep. 2014;1:360–372. doi: 10.1016/j.toxrep.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds K. M., Holloway A. C., Crankshaw D. J., Agarwal S. K., Foster W. G. Reprod., Nutr., Dev. 2005;45:709–720. doi: 10.1051/rnd:2005055. [DOI] [PubMed] [Google Scholar]
- Sterzik K., Dallenbach C., Schneider V., Sasse V., Dallenbach-Hellweg G. Fertil. Steril. 1988;50:457–462. doi: 10.1016/s0015-0282(16)60132-2. [DOI] [PubMed] [Google Scholar]
- Rubin G. L., Harrold A. J., Mills J. A., Falany C. N., Coughtrie M. W. Mol. Hum. Reprod. 1999;5:995–1002. doi: 10.1093/molehr/5.11.995. [DOI] [PubMed] [Google Scholar]
- Rizner T. L. Front. Pharmacol. 2016;7:30. doi: 10.3389/fphar.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Nakata T., Miki Y., Kaneko C., Moriya T., Ishida T., Akinaga S., Hirakawa H., Kimura M., Sasano H. Cancer Res. 2003;63:2762–2770. [PubMed] [Google Scholar]
- Utsunomiya H., Ito K., Suzuki T., Kitamura T., Kaneko C., Nakata T., Niikura H., Okamura K., Yaegashi N., Sasano H. Clin. Cancer Res. 2004;10:5850–5856. doi: 10.1158/1078-0432.CCR-04-0040. [DOI] [PubMed] [Google Scholar]
- Harris R. M., Wood D. M., Bottomley L., Blagg S., Owen K., Hughes P. J., Waring R. H., Kirk C. J. J. Clin. Endocrinol. Metab. 2004;89:1779–1787. doi: 10.1210/jc.2003-031631. [DOI] [PubMed] [Google Scholar]
- Rice S., Amon A., Whitehead S. A. Maturitas. 2007;56:359–367. doi: 10.1016/j.maturitas.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Huang Z., Fasco M. J., Kaminsky L. S. J. Steroid Biochem. Mol. Biol. 1997;63:9–15. doi: 10.1016/s0960-0760(97)00065-4. [DOI] [PubMed] [Google Scholar]
- Fluck C. E., Miller W. L., Auchus R. J. J. Clin. Endocrinol. Metab. 2003;88:3762–3766. doi: 10.1210/jc.2003-030143. [DOI] [PubMed] [Google Scholar]
- Lee-Robichaud P., Wright J. N., Akhtar M. E., Akhtar M. Biochem. J. 1995;308(Pt 3):901–908. doi: 10.1042/bj3080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne A. H., Hales D. B. Endocr. Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Carey A. H., Waterworth D., Patel K., White D., Little J., Novelli P., Franks S., Williamson R. Hum. Mol. Genet. 1994;3:1873–1876. doi: 10.1093/hmg/3.10.1873. [DOI] [PubMed] [Google Scholar]
- Feigelson H. S., Shames L. S., Pike M. C., Coetzee G. A., Stanczyk F. Z., Henderson B. E. Cancer Res. 1998;58:585–587. [PubMed] [Google Scholar]
- Chakraborty A., Murthy N. S., Chintamani C., Bhatnagar D., Mohil R. S., Sharma P. C., Saxena S. J. Hum. Genet. 2007;52:159–165. doi: 10.1007/s10038-006-0095-0. [DOI] [PubMed] [Google Scholar]
- Verla-Tebit E., Wang-Gohrke S., Chang-Claude J. Breast Cancer Res. 2005;7(4):R455–R464. doi: 10.1186/bcr1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningson M., Johansson U., Borg A., Olsson H., Jernstro?m H. Mol. Hum. Reprod. 2007;13:231–236. doi: 10.1093/molehr/gam004. [DOI] [PubMed] [Google Scholar]
- Little J., Simard J. Breast Cancer Res. 2005;7:238–242. doi: 10.1186/bcr1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y., Ando A., Ooka M., Shiba E., Taguchi T., Tamaki Y., Noguchi S. Cancer Lett. 2003;195:81–86. doi: 10.1016/s0304-3835(02)00211-2. [DOI] [PubMed] [Google Scholar]
- Wickenheisser J. K., Nelson-DeGrave V. L., Hendricks K. L., Legro R. S., Strauss 3rd J. F., McAllister J. M. J. Clin. Endocrinol. Metab. 2005;90:4858–4865. doi: 10.1210/jc.2005-0330. [DOI] [PubMed] [Google Scholar]
- Hasegawa E., Nakagawa S., Sato M., Tachikawa E., Yamato S. Biol. Pharm. Bull. 2013;36:228–237. doi: 10.1248/bpb.b12-00627. [DOI] [PubMed] [Google Scholar]
- Nielsen F. K., Hansen C. H., Fey J. A., Hansen M., Jacobsen N. W., Halling-Sorensen B., Bjorklund E., Styrishave B. Toxicol. in Vitro. 2012;26:343–350. doi: 10.1016/j.tiv.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Lin C. J., Cheng L. C., Lin T. C., Wang C. J., Li L. A. Biochem. Pharmacol. 2014;90:288–296. doi: 10.1016/j.bcp.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Gingras S., Moriggl R., Groner B., Simard J. Mol. Endocrinol. 1999;13:66–81. doi: 10.1210/mend.13.1.0221. [DOI] [PubMed] [Google Scholar]
- Rheaume E., Lachance Y., Zhao H. F., Breton N., Dumont M., de Launoit Y., Trudel C., Luu-The V., Simard J., Labrie F. Mol. Endocrinol. 1991;5:1147–1157. doi: 10.1210/mend-5-8-1147. [DOI] [PubMed] [Google Scholar]
- Marti N., Galvan J. A., Pandey A. V., Trippel M., Tapia C., Muller M., Perren A., Fluck C. E. Mol. Cell. Endocrinol. 2017;441:116–123. doi: 10.1016/j.mce.2016.07.029. [DOI] [PubMed] [Google Scholar]
- Ohno S., Matsumoto N., Watanabe M., Nakajin S. J. Steroid Biochem. Mol. Biol. 2004;88:175–182. doi: 10.1016/j.jsbmb.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Lacey M., Bohday J., Fonseka S. M., Ullah A. I., Whitehead S. A. J. Steroid Biochem. Mol. Biol. 2005;96:279–286. doi: 10.1016/j.jsbmb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Ohno S., Shinoda S., Toyoshima S., Nakazawa H., Makino T., Nakajin S. J. Steroid Biochem. Mol. Biol. 2002;80:355–363. doi: 10.1016/s0960-0760(02)00021-3. [DOI] [PubMed] [Google Scholar]
- Le Bail J. C., Champavier Y., Chulia A. J., Habrioux G. Life Sci. 2000;66:1281–1291. doi: 10.1016/s0024-3205(00)00435-5. [DOI] [PubMed] [Google Scholar]
- Peltoketo H., Luu-The V., Simard J., Adamski J. J. Mol. Endocrinol. 1999;23:1–11. doi: 10.1677/jme.0.0230001. [DOI] [PubMed] [Google Scholar]
- Labrie F., Luu-The V., Lin S. X., Labrie C., Simard J., Breton R., Belanger A. Steroids. 1997;62:148–158. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- Han B., Li S., Song D., Poisson-Pare D., Liu G., Luu-The V., Ouellet J., Li S., Labrie F., Pelletier G. J. Steroid Biochem. Mol. Biol. 2008;112:194–200. doi: 10.1016/j.jsbmb.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Luu-The V., Dufort I., Pelletier G., Labrie F. Mol. Cell. Endocrinol. 2001;171:77–82. doi: 10.1016/s0303-7207(00)00425-1. [DOI] [PubMed] [Google Scholar]
- Whitehead S. A., Lacey M. Hum. Reprod. 2003;18:487–494. doi: 10.1093/humrep/deg125. [DOI] [PubMed] [Google Scholar]
- Makela S., Poutanen M., Kostian M. L., Lehtimaki N., Strauss L., Santti R., Vihko R. Proc. Soc. Exp. Biol. Med. 1998;217:310–316. doi: 10.3181/00379727-217-44237. [DOI] [PubMed] [Google Scholar]
- Deluca D., Krazeisen A., Breitling R., Prehn C., Moller G., Adamski J. J. Steroid Biochem. Mol. Biol. 2005;93:285–292. doi: 10.1016/j.jsbmb.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Krazeisen A., Breitling R., Moller G., Adamski J. Mol. Cell. Endocrinol. 2001;171:151–162. doi: 10.1016/s0303-7207(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Pasqualini J. R. Ann. N. Y. Acad. Sci. 2009;1155:88–98. doi: 10.1111/j.1749-6632.2009.04113.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama T., Ogura K., Nakano H., Kaku T., Takahashi E., Ohkubo Y., Sekine K., Hiratsuka A., Kadota S., Watabe T. Drug Metab. Pharmacokinet. 2002;17:221–228. doi: 10.2133/dmpk.17.221. [DOI] [PubMed] [Google Scholar]
- Otake Y., Nolan A. L., Walle U. K., Walle T. J. Steroid Biochem. Mol. Biol. 2000;73:265–270. doi: 10.1016/s0960-0760(00)00073-x. [DOI] [PubMed] [Google Scholar]
- Chen Y., Huang C., Zhou T., Chen G. Basic Clin. Pharmacol. Toxicol. 2008;103:553–559. doi: 10.1111/j.1742-7843.2008.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefcoate C. R., Liehr J. G., Santen R. J., Sutter T. R., Yager J. D., Yue W., Santner S. J., Tekmal R., Demers L., Pauley R., Naftolin F., Mor G., Berstein L. J. Natl. Cancer Inst. Monogr. 2000;27:95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. [DOI] [PubMed] [Google Scholar]
- Hayes C. L., Spink D. C., Spink B. C., Cao J. Q., Walker N., Sutter T. R. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M. S., Soshilov A. A., He G., DeGroot D. E., Zhao B. Toxicol. Sci. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y., Nakajima M., Kyo S., Kanaya T., Inoue M., Yokoi T. Cancer Res. 2004;64:3119–3125. doi: 10.1158/0008-5472.can-04-0166. [DOI] [PubMed] [Google Scholar]
- van Duursen M. B., Sanderson J. T., van der Bruggen M., van der Linden J., van den Berg M. Toxicol. Appl. Pharmacol. 2003;190:241–250. doi: 10.1016/s0041-008x(03)00166-2. [DOI] [PubMed] [Google Scholar]
- Jeuken A., Keser B. J. G., Khan E., Brouwer A., Koeman J., Denison M. S. J. Agric. Food Chem. 2003;51:5478–5487. doi: 10.1021/jf030252u. [DOI] [PubMed] [Google Scholar]
- Medjakovic S., Jungbauer A. J. Steroid Biochem. Mol. Biol. 2008;108:171–177. doi: 10.1016/j.jsbmb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Mohammadi-Bardbori A., Bengtsson J., Rannug U., Rannug A., Wincent E. Chem. Res. Toxicol. 2012;25:1878–1884. doi: 10.1021/tx300169e. [DOI] [PubMed] [Google Scholar]
- Zhang S., Qin C., Safe S. H. Environ. Health Perspect. 2003;111:1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. M., Chhabra J., Bhat H. K. J. Steroid Biochem. Mol. Biol. 2008;110:157–162. doi: 10.1016/j.jsbmb.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolino H. P., Daschner P. J., Yeh G. C. Biochem. J. 1999;340(Pt 3):715–722. [PMC free article] [PubMed] [Google Scholar]
- Han E. H., Kim J. Y., Jeong H. G. Arch. Pharmacal Res. 2006;29:570–576. doi: 10.1007/BF02969267. [DOI] [PubMed] [Google Scholar]
- Gong P., Madak-Erdogan Z., Flaws J. A., Shapiro D. J., Katzenellenbogen J. A., Katzenellenbogen B. S. Mol. Cell. Endocrinol. 2016;437:190–200. doi: 10.1016/j.mce.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnow P., Tralau T., Hunecke D., Luch A. Toxicol. in Vitro. 2013;27:1467–1475. doi: 10.1016/j.tiv.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Wagner J., Jiang L., Lehmann L. Adv. Exp. Med. Biol. 2008;617:625–632. doi: 10.1007/978-0-387-69080-3_65. [DOI] [PubMed] [Google Scholar]
- Cavalieri E. L., Stack D. E., Devanesan P. D., Todorovic R., Dwivedy I., Higginbotham S., Johansson S. L., Patil K. D., Gross M. L., Gooden J. K., Ramanathan R., Cerny R. L., Rogan E. G. Proc. Natl. Acad. Sci. U. S. A. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri E., Frenkel K., Liehr J. G., Rogan E., Roy D. J. Natl. Cancer Inst. Monogr. 2000;27:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- Yager J. D. J. Natl. Cancer Inst. Monogr. 2000;27:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- Liehr J. G. Eur. J. Cancer Prev. 1997;6:3–10. doi: 10.1097/00008469-199702000-00002. [DOI] [PubMed] [Google Scholar]
- Cao K., Stack D. E., Ramanathan R., Gross M. L., Rogan E. G., Cavalieri E. L. Chem. Res. Toxicol. 1998;11:909–916. doi: 10.1021/tx9702291. [DOI] [PubMed] [Google Scholar]
- Takemura H., Itoh T., Yamamoto K., Sakakibara H., Shimoi K. Bioorg. Med. Chem. 2010;18:6310–6315. doi: 10.1016/j.bmc.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Shimada T., Tanaka K., Takenaka S., Murayama N., Martin M. V., Foroozesh M. K., Yamazaki H., Guengerich F. P., Komori M. Chem. Res. Toxicol. 2010;23:1921–1935. doi: 10.1021/tx100286d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary A., Willett K. L. Toxicology. 2006;217:194–205. doi: 10.1016/j.tox.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Chen Z. H., Hurh Y. J., Na H. K., Kim J. H., Chun Y. J., Kim D. H., Kang K. S., Cho M. H., Surh Y. J. Carcinogenesis. 2004;25:2005–2013. doi: 10.1093/carcin/bgh183. [DOI] [PubMed] [Google Scholar]
- Chun Y. J., Kim M. Y., Guengerich F. P. Biochem. Biophys. Res. Commun. 1999;262:20–24. doi: 10.1006/bbrc.1999.1152. [DOI] [PubMed] [Google Scholar]
- Lu F., Zahid M., Wang C., Saeed M., Cavalieri E. L., Rogan E. G. Cancer Prev. Res. 2008;1:135–145. doi: 10.1158/1940-6207.CAPR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licznerska B., Szaefer H., Wierzchowski M., Sobierajska H., Baer-Dubowska W. Mol. Cell. Biochem. 2016;425:169–179. doi: 10.1007/s11010-016-2871-2. [DOI] [PubMed] [Google Scholar]
- Schwarz D., Kisselev P., Schunck W. H., Roots I. Biochim. Biophys. Acta. 2011;1814:168–174. doi: 10.1016/j.bbapap.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Madhubhani P., Hemachandra L. P., Esala P., Chandrasena R., Chen S. N., Main M., Lankin D. C., Scism R. A., Dietz B. M., Pauli G. F., Thatcher G. R., Bolton J. L. Cancer Prev. Res. 2012;5:73–81. doi: 10.1158/1940-6207.CAPR-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Dunlap T. L., Howell C. E., Mbachu O. C., Rue E. A., Phansalkar R., Chen S. N., Pauli G. F., Dietz B. M., Bolton J. L. Chem. Res. Toxicol. 2016;29:1142–1150. doi: 10.1021/acs.chemrestox.6b00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duursen M. B. M., Sanderson J. T., de Jong P., Kraaij M., van den Berg M. Toxicol. Sci. 2004;81:316–324. doi: 10.1093/toxsci/kfh216. [DOI] [PubMed] [Google Scholar]
- Chen D., Wang C. Y., Lambert J. D., Ai N., Welsh W. J., Yang C. S. Biochem. Pharmacol. 2005;69:1523–1531. doi: 10.1016/j.bcp.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Lehmann L., Jiang L., Wagner J. Carcinogenesis. 2008;29:363–370. doi: 10.1093/carcin/bgm235. [DOI] [PubMed] [Google Scholar]
- Zahid M., Gaikwad N. W., Ali M. F., Lu F., Saeed M., Yang L., Rogan E. G., Cavalieri E. L. Free Radical Biol. Med. 2008;45:136–145. doi: 10.1016/j.freeradbiomed.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key T., Appleby P., Barnes I., Reeves G., Endogenous H., G. Breast Cancer Collaborative J. Natl. Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- Ingle J. N. Clin. Cancer Res. 2005;11:900s–905s. [PubMed] [Google Scholar]
- Kumar N. B., Cantor A., Allen K., Riccardi D., Cox C. E. Cancer. 2002;94:1166–1174. doi: 10.1002/cncr.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L., Ryder J. J., Kurzer M. S., Lampe J. W., Messina M. J., Phipps W. R., Cassidy A. Hum. Reprod. Update. 2009;15:423–440. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas J. C., Afeiche M. C., Gaskins A. J., Minguez-Alarcon L., Williams P. L., Wright D. L., Toth T. L., Hauser R., Chavarro J. E. Fertil. Steril. 2015;103:749–755 e742. doi: 10.1016/j.fertnstert.2014.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unfer V., Casini M. L., Gerli S., Costabile L., Mignosa M., Di Renzo G. C. Fertil. Steril. 2004;82:1509–1513. doi: 10.1016/j.fertnstert.2004.07.934. [DOI] [PubMed] [Google Scholar]
- Solak K. A., Santos R. R., van den Berg M., Blaauboer B. J., Roelen B. A., van Duursen M. B. Reprod. Toxicol. 2014;49:1–11. doi: 10.1016/j.reprotox.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Chan W. H. Reprod. Toxicol. 2009;28:52–58. doi: 10.1016/j.reprotox.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Mahalingam S., Gao L., Gonnering M., Helferich W., Flaws J. A. Toxicol. Appl. Pharmacol. 2016;295:47–55. doi: 10.1016/j.taap.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelli A., Delle Piane L., Casano S., Molinari E., Massobrio M., Rinaudo P. Reprod. Biol. Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Spiessens C., D'Hooghe T., Peeraer K., Carpentier S. Proteome Sci. 2016;14:17. doi: 10.1186/s12953-016-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson V. L., Legro R. S., Strauss 3rd J. F., McAllister J. M. Mol. Endocrinol. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- Rijk J. C., Peijnenburg A. A., Blokland M. H., Lommen A., Hoogenboom R. L., Bovee T. F. Chem. Res. Toxicol. 2012;25:1720–1731. doi: 10.1021/tx3001779. [DOI] [PubMed] [Google Scholar]
- Strowitzki T., Germeyer A., Popovici R., von Wolff M. Hum. Reprod. Update. 2006;12:617–630. doi: 10.1093/humupd/dml033. [DOI] [PubMed] [Google Scholar]
- Sasano H., Suzuki T., Nakata T., Moriya T. Breast Cancer. 2006;13:129–136. doi: 10.2325/jbcs.13.129. [DOI] [PubMed] [Google Scholar]
- Piccinato C. A., Neme R. M., Torres N., Sanches L. R., Derogis P. B., Brudniewski H. F., Rosa e Silva J. C., Ferriani R. A. J. Steroid Biochem. Mol. Biol. 2016;158:117–126. doi: 10.1016/j.jsbmb.2015.12.025. [DOI] [PubMed] [Google Scholar]
- Ren X., Wu X., Hillier S. G., Fegan K. S., Critchley H. O., Mason J. I., Sarvi S., Harlow C. R. J. Steroid Biochem. Mol. Biol. 2015;150:54–63. doi: 10.1016/j.jsbmb.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers S. P., Vigar V. Phytomedicine. 2017;24:141–147. doi: 10.1016/j.phymed.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Tempfer C. B., Froese G., Heinze G., Bentz E. K., Hefler L. A., Huber J. C. Am. J. Med. 2009;122:939–946 e939. doi: 10.1016/j.amjmed.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Quaas A. M., Kono N., Mack W. J., Hodis H. N., Felix J. C., Paulson R. J., Shoupe D. Menopause. 2013;20:840–844. doi: 10.1097/GME.0b013e3182804353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA EFSA J. 2015;13:4246. [Google Scholar]
- van Hunsel F. P., Kampschoer P. Ned. Tijdschr. Geneeskd. 2012;156:A5095. [PubMed] [Google Scholar]
- Chandrareddy A., Muneyyirci-Delale O., McFarlane S. I., Murad O. M. Complement. Ther. Clin. Pract. 2008;14:132–135. doi: 10.1016/j.ctcp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Magee P. J., Rowland I. Curr. Opin. Clin. Nutr. Metab. Care. 2012;15:586–591. doi: 10.1097/MCO.0b013e328359156f. [DOI] [PubMed] [Google Scholar]
- Du M., Yang X., Hartman J. A., Cooke P. S., Doerge D. R., Ju Y. H., Helferich W. G. Carcinogenesis. 2012;33:895–901. doi: 10.1093/carcin/bgs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y. H., Doerge D. R., Woodling K. A., Hartman J. A., Kwak J., Helferich W. G. Carcinogenesis. 2008;29:2162–2168. doi: 10.1093/carcin/bgn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M., Cassidy A., Hill C., Powell J., Talbot D., Dye L. Br. J. Nutr. 2005;93:731–739. doi: 10.1079/bjn20041396. [DOI] [PubMed] [Google Scholar]
- Burke B. E., Olson R. D., Cusack B. J. Biomed. Pharmacother. 2002;56:283–288. doi: 10.1016/s0753-3322(02)00181-6. [DOI] [PubMed] [Google Scholar]
- Messina M. Nutrients. 2016;8(12):pii: E754. [Google Scholar]