 We investigated the question of whether the brain can be adversely affected after 4 weeks by whole-body exposure with different doses of 56Fe ion irradiation and the experiments showed that exposure resulted in significant impairment of cognitive performance.
We investigated the question of whether the brain can be adversely affected after 4 weeks by whole-body exposure with different doses of 56Fe ion irradiation and the experiments showed that exposure resulted in significant impairment of cognitive performance.
Abstract
The rapid growth of manned space flight results in more concerns about health risks and an urgent need for health assessment for space travel. The cosmic environment is complicated and full of radiation. Because of their strong biological effects, heavy ions such as 56Fe ions are considered to be an important component of these lethal galactic rays. Due to the importance of brain function to astronauts, we explored the long-term effects and potential mechanisms of 56Fe ion radiation on mice brains containing the hippocampus. In our study, radiation doses were carried out with 0.5 Gy, 1 Gy or 2 Gy. One month after whole-body 56Fe ion exposure, the Morris water maze test was performed to assess the ability of spatial learning and memory. A histological study was used for pathology analysis of the hippocampus. Alteration of oxidative stress was reflected by MDA and GSH and oxidative DNA damage marked by 8-OHdG was detected by biochemical and immunofluorescence methods. In our results, irradiated groups exhibited significant changes in behavioral performance and also showed loose and edematous arrangement in the pathological characteristics. Furthermore, whole brain levels of MDA, GSH and 8-OHdG increased in the irradiated groups. In addition, increased expression of 8-OHdG can also be detected by immunofluorescence in the hippocampus. Our findings revealed a linkage between radiation-induced oxidative stress and behavioral deficits. This may suggest an underlying mechanism of brain tissue protection and risk assessment in manned space flight.
Introduction
As the development of manned space flight continues, the duration and distance of shuttle missions extend from those in past years. However, it has also increased the risks of central nervous system (CNS) damage which is attributed to exposure to solar particles and cosmic rays. In general, these solar particles and cosmic rays mainly consist of high linear energy transfer (LET) ions such as photons and high (H) atomic number (Z) and high-energy (E) ions.1 Although HZE particles are a small part of cosmic rays, these highly diverse charged ions contribute a dominant share of the effective dose and they also possess a strong ability for oxidative damage2–4 which induces impairment of DNA and some other biological molecules.5 In space, Fe ions are likely the most important component of cosmic rays as they provide the largest contribution to the equivalent dose in the radiation spectrum.6 For these reasons, Fe ions are ideal for ground-based research into space radiation. Studies have said that there is a high uncertainty between 400%–600% in the radiation assessment for travel to Mars.7 In large part, this is because of the lack of knowledge about the biological response to HZE particles. Therefore, understanding the acute and long-term effects of the oxidative stress of response to HZE particles provides a theoretical basis to evaluate the risks of space travel and radiation protection. Here we use an 56Fe ion beam to simulate HZE particles in the cosmic environment to detect the underlying mechanisms of the radiation-induced long-term effects on the CNS.
It is known that whole-body exposure of mice to HZE particles may induce significant deficits in the CNS.8–10 Although the CNS is the most important system in the body and has been fully researched, it remained uncertain how ionizing radiation exposure affected the CNS. The underlying mechanisms will certainly be multifaceted and several mechanisms are considered to play a role in the radiation-induced deficits,11,12 but oxidative stress may represent the direct and most important mechanistic explanation for it since studies shows that oxidative damage of nucleic acids, proteins, and lipids is directly correlated with neurodegeneration, aging, cardiovascular diseases and pathologies of some carcinomas.13–17 Interactions between DNA and reactive oxygen species (ROS) induced by radiation in damaged cells lead to DNA strand-breaks and base modification which can be quantitatively estimated with 8-hydroxy-2′-deoxyguanosine (8-OHdG) produced by the reaction of ROS on guanine ordinarily18,19 in animal organs and in human samples.20,21
In this study, we investigated the question of whether the brain can be adversely affected after 4 weeks by whole-body exposure to different doses (600 MeV u–1, 0.5, 1 and 2 Gy) of 56Fe ion irradiation. Experiments show that exposure to 56Fe beam resulted in significant impairment of cognitive performance. To further study the causes of cognitive impairment, oxidative stress and DNA damage, which may relate to cognitive deficits, were evaluated by the levels of malondialdehyde (MDA), glutathione (GSH) and 8-OHdG. In addition, some other histological and biochemical experiments were carried out. Finally, the studies imply that these doses of 56Fe ion irradiation compromise cognitive performance through mechanisms involving changes of oxidative stress and oxidative DNA damage in brain tissue.
Materials and methods
Animals
SPF-class young adult male Kunming mice (6–8 weeks old, weighing 24–28 g) obtained from the Gansu University of Chinese Medicine (Gansu Province, China) were used for the study. The mice were maintained in 12 h light/dark cycles and had free access to a certified rodent diet and filtered water. All experiments of this study were carried out in accordance with the principles of Laboratory Animal Care and approved by the Institutional Animal Care and Use Committee of Gansu University of Chinese Medicine (Permit number: 2015-051). Furthermore, we also tried to minimize animal suffering.
Irradiation procedure
Mice were divided into four groups: a sham and three test (0.5 Gy, 1 Gy, 2 Gy) groups (total 32, n = 8 each group). The three test groups were exposed to a single dose of total body 56Fe beam (600 MeV u–1) irradiation without anesthesia at the Heavy Ion Research Facility in Lanzhou (HIRFL, Institute of Modern Physics, Chinese Academy of Sciences, Lanzhou, China) with a dose rate of approximately 1 Gy min–1.
Behavioral testing
Morris water maze (MWM) apparatus (ZS Dichuang New Technological Development Limited Liability Company, Beijing, China) which consists of a circular metal pool (120 cm in diameter, 40 cm in height, and filled to a depth of 21 cm with water at 21 ± 1 °C) and a video-capturing system were used to estimate the spatial learning and memory ability of mice.
The MWM was virtually divided into four equivalent quadrants. 4 weeks after irradiation, mice were trained to locate a small round hidden platform which was submerged 2.0 cm below the surface of the water in the 2nd quadrant. There were 2 trials per session (15 min intertrial interval) and two sessions (3 h apart) per day on five consecutive days. For each trial, the mice were moved to a new quadrant to locate the platform. The mice have 120 s to find the hidden platform by themselves. After successfully reaching the platform, the mice need to remain on the platform for 10 s to finish the trial. If the mice fail in 120 s, the trial is also ended but the experimenter needs to place the mice on the hidden platform for 10 s. After removal from the MWM, the mice were towel-dried and returned to their home cages.
At day 6 mice were placed in the 4th quadrant to locate the hidden platform. The movement of mice in the MWM was recorded for further studying. All tests were conducted in the morning and early afternoon (beginning at approximately 9:00 a.m. and 2:30 p.m., respectively).
Tissue preparation
24 hours after the last MWM experiment, mice were sacrificed by cervical dislocation. Brain tissue of each animal was dissected and quickly removed to cold physiological saline on ice to wash away the remaining blood. Then part of the tissue was placed and fixed in 4% paraformaldehyde (4 g per 100 ml) with 0.01 mol L–1 phosphate buffer solution (pH 7.4) for analysis by hematoxylin and eosin staining (HE) and immunofluorescence. Remaining tissue was soon stored at –80 °C for biochemical analysis.
Biochemical analysis
Brain tissues of one group, which were stored at –80 °C, were mixed and homogenized in ice cold phosphate buffer solution (pH 7.4) by a mechanical homogenizer. Then the homogenate was centrifuged for 10 minutes at 2500 rpm. After that, the supernatant was used for analysis of the levels of GSH, MDA and 8-OHdG with commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China; Shanghai Enzyme-linked Biotechnology, Shanghai, China). In these experiments, a Microplate Reader (infinite M200, TECAN, Switzerland) was used for detection.
Histological study
Brain tissues which were fixed in 4% paraformaldehyde were washed, dehydrated in ethanol with different concentrations from 50% to 100%, cleared in xylene, and embedded in paraffin at 55 °C for 3 h. Paraffin blocks were cut with coronal sections by a microtome (Jung SM 2000R, Leica, Nussloch, Germany). Sections cut to 4 μm in thickness were stained with hematoxylin and eosin (HE). Histopathological examinations were carried out under a microscope with a camera and an experienced observer to avoid any bias.
Immunofluorescence analysis
Immunofluorescence analysis was utilized to locate and determine 8-OHdG in brain tissue sections. In brief, these sections were de-paraffinized, immerged in citrate solution for antigen retrieval in an environment of high temperature and pressure, and treated with 0.2% Triton X-100 for 15 min at room temperature. Afterwards, sections were incubated with 1% bovine serum albumin (BSA) for 1 h and incubated with the primary antibody to 8-OHdG (Beijing Biosynthesis Biotechnology, Beijing, China) (1 : 200 in PBS) overnight at 4 °C in PBS. Then paraffin sections were exposed to Alexa Fluor-488 goat anti-rabbit fluorochrome-conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA) with a concentration of 0.2% in Tris buffered saline (TBS) and maintained for 1 h in the dark. Slides were washed three times in PBS and a medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA) was used for mounting for 5 minutes at room temperature. Finally, a cover slip was placed on the surface of the slide. The expression and location of 8-OHdG were observed using a laser scanning confocal microscope with a digital camera (LSM700, Carl Zeiss).
Statistical analysis
There were at least three repetitions of each experiment. Data were presented as the mean ± the standard error of the mean (SEM). Two tailed t-tests were carried out for single comparisons. Analysis of variance (ANOVA) followed by Student Neuman and Keuls (S–N–K) post-hoc comparisons was used for statistical comparison between different groups. A p-value of less than 0.05 was selected as a principle for a statistically significant difference.
Results
Morris water maze
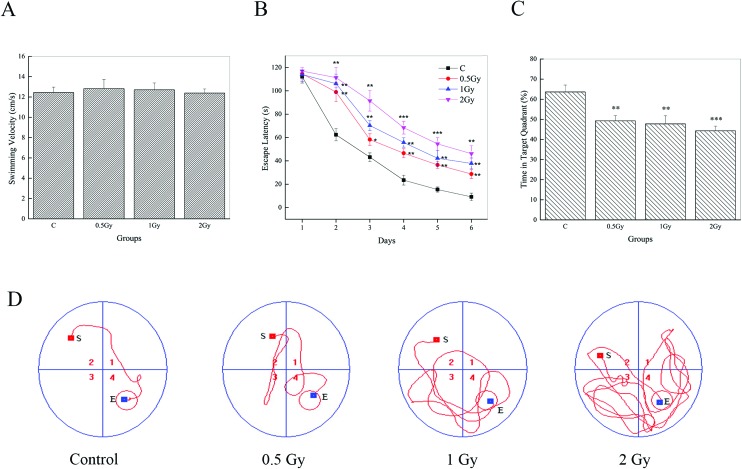
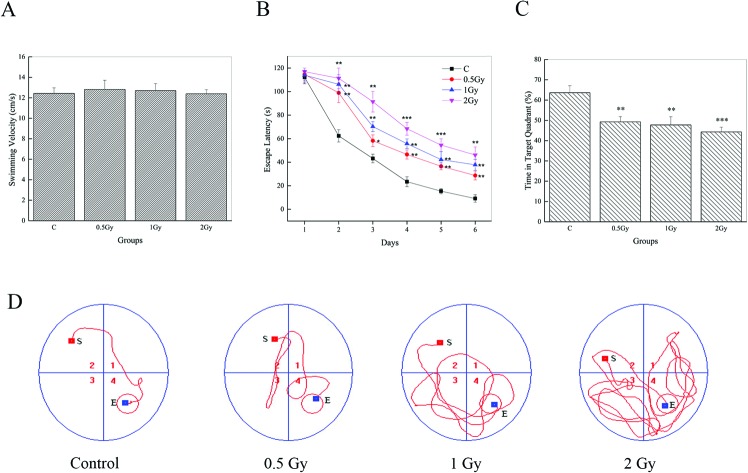
MWM data of the visible platform for 6 consecutive days are represented in Fig. 1. It can be observed that there were no effects on swim velocity of test groups post-irradiation Fig. 1A (0.5 Gy, P = 0.70; 1 Gy, P = 0.25; 2 Gy, P = 0.95). Hence, escape latency can be used as a measurement of performance. During trial sessions, all group animals showed an experimental effect by the decrease in escape latency to reach the immersed platform (Fig. 1B). However, the escape latencies of the 56Fe ion exposed groups showed significant increases (P < 0.05) compared to the controls. The evidence can be found to be more conspicuous on day 2. On this day, the mean escape latency of the control, which decreased significantly compared to that on day 1, was found to be ∼60 s. But in the tests groups, it was found to be ∼100 s or ∼110 s, similar to that on day 1. Repeated measures of one way ANOVA analysis and post-hoc comparisons confirmed a significant prolonging of escape latency between radiation groups and the control in the last 5 days of the MWM trials. In addition, our results indicated that the radiation groups showed a slight but significant abatement in the percentage of time spent in the target quadrant (Fig. 1C) (63.61 ± 3.43, control; 49.25 ± 2.56, 0.5 Gy; 47.74 ± 4.02, 1 Gy; 44.29 ± 2.35, 2 Gy) and repeated measures of one way ANOVA with post-hoc comparisons also confirmed this (P < 0.05). We also found that the animals in the test groups found it hard to reach the platform and an arbitrary swimming pattern can be seen (Fig. 1D). Furthermore, an interesting phenomenon can be seen during the experiment process. Some irradiated animals, especially in the group of 2 Gy, were just floating in the water and there was no evident movement. Although these data were not included, that may indicate a mood disturbance.
Fig. 1. 56Fe ion radiation induced deficits of spatial learning and memory, one month after exposure. (A) All groups exhibited similar swimming speeds in the MWM tests. (B) Mice in the irradiated groups showed a significant increment of escape latency on days 2–6 compared to the control group (*P < 0.05, **P < 0.01 and ***P < 0.001 vs. control). (C) Compared to the control, the percentage of time spent in the target quadrant was conspicuously lower in the irradiated groups (**P < 0.01 and ***P < 0.001 vs. control). (D) Representative tracing images of the test trials on day 6.
MDA and GSH activity analysis
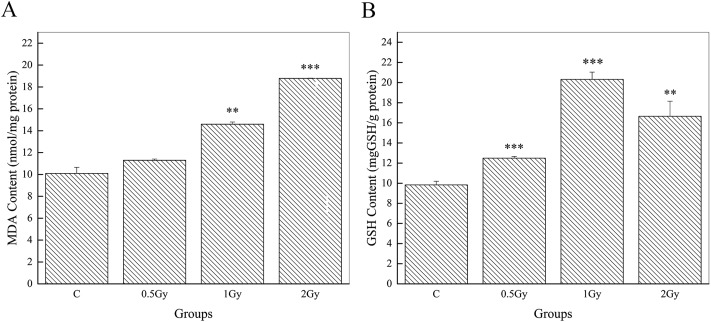
The levels of MDA and GSH in brain tissues are exhibited in Fig. 2. Compared to the controls (10.10 ± 0.55), our results showed a significant increase in MDA content (14.60 ± 0.55, P < 0.01, 1 Gy; 18.78 ± 0.03, P < 0.001, 2 Gy) in animals exposed to a high dose (>0.5 Gy). However, a slight but not conspicuous increment can be seen in the group of 0.5 Gy (11.30 ± 0.11, P = 0.10) (Fig. 2A). The GSH concentration of mice in the irradiated groups (12.50 ± 0.17, P < 0.001, 0.5 Gy; 20.32 ± 0.72, P < 0.001, 1 Gy; 16.65 ± 1.51, P < 0.01, 2 Gy) was found to be significantly higher than that of the control group mice (9.83 ± 0.36) (Fig. 2B). Interestingly, a slight abatement of the GSH concentration was found in the irradiated groups when comparing between 1 Gy and 2 Gy. MDA and GSH are important markers of oxidative stress. Their increment indicates a high degree of oxidative stress. Hence, the results showed oxidative damage after 56Fe ion exposure.
Fig. 2. 56Fe ion radiation induced long term effects on oxidative stress. Graphs showing whole brain levels of MDA (A) and GSH (B) in the groups of control, 0.5 Gy, 1 Gy and 2 Gy (**P < 0.01 and ***P < 0.001 vs. control).
Histopathological evaluations
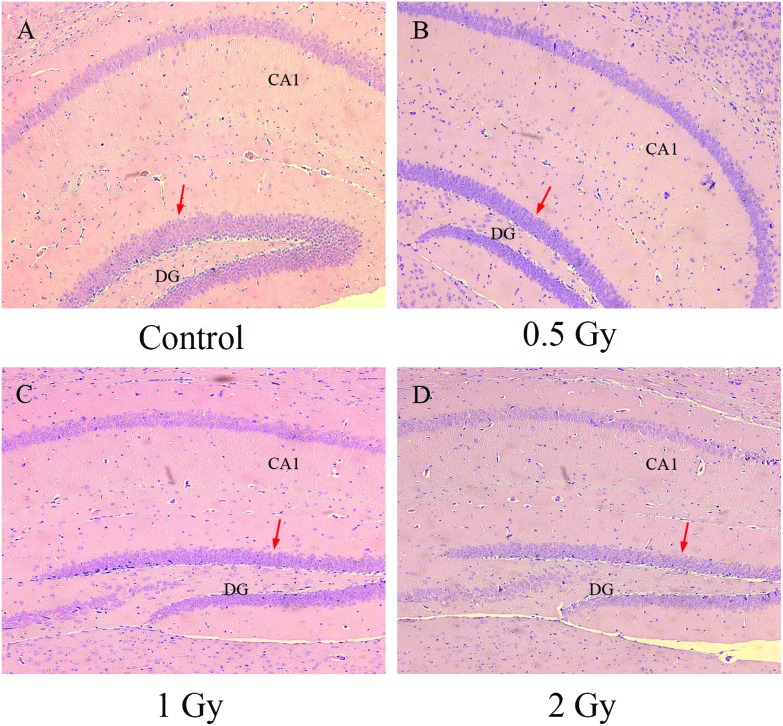
Results of the MWM have indicated an impairment of cognition and memory. To further detect the radiation effects on this, our research focused on the hippocampus and serial sections with HE staining were taken for histopathological analysis. In the group of the control, neurons were arranged tightly and displayed distinct cell structure with a clear nucleus, nucleolus and edges (Fig. 3A). By contrast, the hippocampus in the groups with 56Fe ion exposure showed a conspicuous decrease in the number of cells especially in the edge of the DG division (Fig. 3B–D). In addition, we also observed that edema and loose arrangement occurred in the hippocampus of the irradiated groups. These histopathological changes can be seen to be deteriorative as the dose of the 56Fe ion exposure was increased.
Fig. 3. 56Fe ion radiation induced histopathological alteration of the hippocampus. Groups of the control (A), 0.5 Gy (B), 1 Gy (C) and 2 Gy (D) are displayed. Locations of cell decrease are showed with a red arrow.
DNA damage analysis
As shown in Fig. 2, 56Fe ion radiation significantly increased the accumulated oxidative stress one month after exposure. Hence, we wanted to determine whether radiation induced oxidative DNA damage in brain tissue. 8-OHdG, which is predominantly free radical-induced, is a widely used biomarker of oxidative lesions.22 Here we used a commercial enzyme-linked immunosorbent assay (ELISA) kit to quantify the concentration of 8-OHdG in brain tissue and further detect its relative expression in the hippocampus by immunofluorescence analysis.
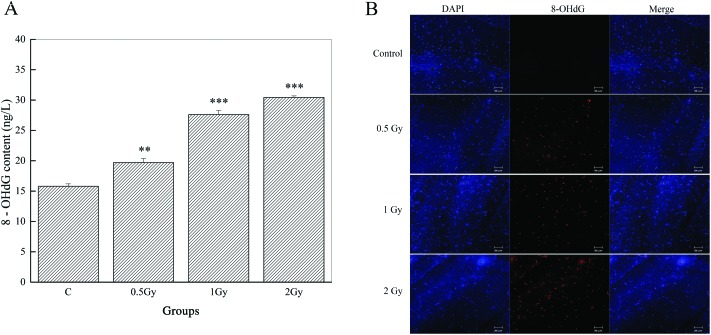
We observed significant increases of 8-OHdG in both of the irradiated groups (98.47 ± 3.35, P < 0.01, 0.5 Gy; 138.04 ± 3.48, P < 0.001, 1 Gy; 152.23 ± 1.2, P < 0.001, 2 Gy) and nearly double the level in the group of 2 Gy, compared to the control (79.02 ± 1.96) (Fig. 4A). Repeated measures of one way ANOVA and S–N–K post-hoc comparisons were used to confirm this.
Fig. 4. Graphs exhibiting the assessment of DNA oxidative damage induced by 56Fe ion radiation. (A) Whole brain levels of 8-OHdG (**P < 0.01 and ***P < 0.001 vs. control). (B) Representative immunofluorescence analysis shows an increase of the 8-OHdG fluorescence (red) signal compared to that of the control. Nuclei were stained with DAPI (blue).
As shown by immunofluorescence microscopy (Fig. 4B), we also found that radiation induced noticeable DNA damage performed by 8-OHdG and this was located in the CA1 area of the hippocampus. In the control group, nearly invisible immunoreactivities of 8-OHdG can be observed in the CA1 division. However, the expression levels and the intensity of 8-OHdG immunoreactivities were gradually increased as the radiation dose escalated. These data indicate that radiation caused serious consequences of DNA damage.
Discussion
Studies have shown that HZE particles, which are abundant in the cosmic environment, induce various brain impairments in executive function, spatial learning and memory.23–25 But most articles focused on the acute-term and single dose effects of HZE particle radiation. As the duration of manned space flight increases, there is an urgent need to maintain and even improve the astronaut's performance in cognition, memory and response. Hence, more attention should be paid to gradient-dose irradiation and the long term effects of HZE particles. Our study was designed to detect the long-term effects on cognitive impairment after exposure. An 56Fe ion beam was used to simulate cosmic irradiation and gradient doses of 0.5 Gy, 1 Gy, 2 Gy were used. Afterwards, we performed behavioral, biochemical, and histological analysis on the brain tissue of mice.
Our results displayed conspicuous radiation effects on behavior which may be associated with changes on a physiological and cellular level. As a classic instrument of behavioral analysis, the MWM was selected in our research for measuring spatial learning and memory retention, which depend on functions of an intact hippocampus (Fig. 5).26,27
Fig. 5. A diagrammatic representation of the experimental design and conclusion.
In the MWM test, both groups showed an experimental effect with a decrease of escape latency during the training trials. We found that there were no significant changes in swimming velocity. This indicates that radiation with 56Fe ions has no effect on the motor ability of mice, which makes escape latency and some other parameters of the MWM usable. Animals in the irradiated groups showed a significant prolonging of escape latency (at least 3 times) in the test session (day 6) compared to the controls. In the training trials of day 2, a significant decrease of escape latency can be seen in the control group while a slight decline of escape latency was seen in the irradiated groups. Theoretically, mice in the control group have an intact ability for learning and memory. Therefore, they should spend more time in the target quadrant to find it. The percentage of time spent in the target quadrant proved this inference. It is significant that mice in the irradiated groups spent less time in the target quadrant. Tracing images also verified that the irradiated groups had a worse memory of the location of the platform. Based on these results, we suggested that 56Fe ion radiation induces significant effects on spatial learning and memory and the consequences of radiation worsen as the exposure dose increases.
In addition, we also observed some freezing behavior in the irradiated groups although it is a small probability event and our data did not cover it. Mice were found simply floating and showing no movement in the water, especially in the high dose irradiation (2 Gy) group. Studies have shown that this phenomenon may correlate with mood disturbance.28–30 But little research has been carried out on 56Fe ion beam radiation and this phenomenon may need to be further studied.
The behavioral effects induced by 56Fe ion radiation can be explained well by the results of the histopathological analysis. The brain tissues of mice in the irradiated groups indicate a significant decrease and loose arrangement of cells, especially in the edge of the dentate gyrus (DG). The edge of the DG consists of the granule cell layer (GCL) and the subgranular zone (SGZ). These cells and structures are critical to brain functions. Studies have shown that granule cells in the DG are critical to spatial memory formation.31,32 In fact adult-generated granule cells are incorporated preferentially in the process of spatial memory and loss of them brings about deficits in spatial memory.33 The function of the SGZ is related to neurogenesis and it has also been reported that there is a correlation between neurogenesis in the SGZ and spatial memory.34,35 On the other hand, the control group showed more cells that were arranged tightly in this area. CA1 also plays an important role in spatial learning. Other researchers have shown that CA1 is critical for processing temporal information of visible objects.36,37 However, edemas were found in this critical area unlike in the control. Therefore, we suggest that 56Fe ions severely damaged the hippocampus and these aberrant performances in pathology resulted in impairment of spatial memory.
To detect whether changes in oxidative stress were caused by 56Fe ion exposure, here we carried out biochemical analysis with biomarkers for antioxidation and lipid peroxidation. In the process of radiation exposure, unpaired electrons cause various ROS which are potentially highly damaging to cells.3 On the other hand, this process induces production of antioxidants and normally forms a dynamic balance. Studies have shown that there is a correlation between high oxidative stress, which is induced by a decrease in the non-enzymatic antioxidant GSH, and neurodegeneration.38 The results showed that there were significant changes in GSH after exposure. Compared with the control, the GSH level of the irradiated groups showed a conspicuous increase. But in the groups of 2 Gy, the GSH level showed a slight decrease in comparison with the group of 1 Gy. We suggest that this phenomenon may be caused by over-consumption of GSH, which is induced by the higher oxidative stress of 2 Gy radiation. In addition, it is also suggested that the GSH tolerance of 56Fe ion radiation (600 MeV u–1) may be in the dose range of 1 Gy to 2 Gy. However, the underlying mechanisms need to be studied further. Researchers have shown that brain tissue has a high content of lipid39 and MDA is one of the essential products of lipid peroxidation.40 Hence, we decided to use MDA, a widely used marker of oxidative stress, as a parameter to assess oxidative damage in the brain. In our results, the MDA concentration is significantly increased in the test groups of 1 Gy and 2 Gy. However, there were no significant changes compared to the control that can be seen in the group of 0.5 Gy, although a slight increment on average exists. In this study, we affirmed that 56Fe ion radiation negatively affected oxidative stress in brain tissue. But the effects of lipid peroxidation which was induced by low dose radiation can be eliminated with personal antioxidants.
Oxidative lesions in DNA are thought to be the main reason for cell damage.41 Oxidative destruction of guanine is a considerable reason for the transversion of guanine to thymine42,43 and results in the accumulation of 8-OHdG subsequently in DNA.44 Therefore, the nucleoside 8-OHdG can be utilized for estimating DNA damage.45 Our ELISA analysis of 8-OHdG showed a significant increase in the irradiated groups. It is suggested that 56Fe ion radiation has caused grave damage to DNA in brain cells and it can be detected even one month after exposure. We also suggested that even if lipid peroxidation can be eliminated after one-month post-irradiation, the DNA damage cannot be cleared up in the group of 0.5 Gy.
CA1 is a subfield in the hippocampus, which has been demonstrated as critical to spatial learning and vulnerable to free radicals.36,37,46 Hence, our work focused on detecting oxidative DNA damage in the CA1 area of the hippocampus. The results of immunofluorescence exhibited noteworthy expression of 8-OHdG in irradiated groups compared to in the control. Therefore, this indicates that DNA oxidative damage induced by 56Fe ion radiation in CA1 of the hippocampus is one of the reasons for impairment of spatial learning and memory.
Conclusions
In conclusion, we demonstrated radiation induced increased expression of 8-OHdG in the hippocampus with immunofluorescence after one-month exposure and also demonstrated that 56Fe ions have a long-term effect on impairment of spatial learning and memory. These cognitive deficits were shown clearly to correlate with radiation-induced changes in pathology, an increase of oxidative stress and oxidative DNA damage, which were detected in the brain tissue and the hippocampus of mice. In addition, we also found that lipid peroxidation induced by low dose exposure can be eliminated in the brain tissue, but DNA damage and behavioral deficits induced by changes of oxidative stress are still existent and the underlying mechanisms need to be further studied. Overall, our research proved a correlation between long-term oxidative stress and behavioral deficits following exposure to a high-LET space radiation ray. Our findings revealed a linkage between Fe ion radiation-induced oxidative stress and behavioral deficits and this may provide an experimental basis and ideas for further research on CNS protection and risk assessment.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
This work was supported by grants from the Key Program of National Natural Science Foundation of China (U1432248), National Key Projects of Research and Development (2016YFC0904600), National Natural Science Foundation of China (no. 11305224; no. 11675234), and the Western Talent Program of Chinese Academy of Sciences (Y260230XB0).
References
- Zeitlin C., Hassler D., Cucinotta F., Ehresmann B., Wimmer-Schweingruber R., Brinza D., Kang S., Weigle G., Böttcher S., Böhm E. Science. 2013;340:1080–1084. doi: 10.1126/science.1235989. [DOI] [PubMed] [Google Scholar]
- Schimmerling W. Radiat. Environ. Biophys. 1995;34:133–137. doi: 10.1007/BF01211538. [DOI] [PubMed] [Google Scholar]
- Riley P. Int. J. Radiat. Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- Sun J., Chen Y., Li M., Ge Z. Free Radicals Biol. Med. 1998;24:586–593. doi: 10.1016/s0891-5849(97)00291-8. [DOI] [PubMed] [Google Scholar]
- Durante M., Warren K. Eighth. Health Phys. 2012;103:532–539. doi: 10.1097/HP.0b013e318264b4b6. [DOI] [PubMed] [Google Scholar]
- Durante M., Kronenberg A. Adv. Space Res. 2005;35:180–184. doi: 10.1016/j.asr.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Cucinotta F. A., Schimmerling W., Wilson J. W., Peterson L. E., Badhwar G. D., Saganti P. B., Dicello J. F. Radiat. Res. 2001;156:682–688. doi: 10.1667/0033-7587(2001)156[0682:srcrau]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Joseph J., Erat S., Rabin B. Adv. Space Res. 1998;22:209–216. doi: 10.1016/s0273-1177(98)80012-4. [DOI] [PubMed] [Google Scholar]
- Cucinotta F. A., Kim M.-H. Y. and Chappell L. J., Space radiation cancer risk projections and uncertainties-2010, NASA Technical Paper 2011-216155, NASA Scientific and Technical Information (STI) Program, Hampton, VA, 2011.
- Parihar V. K., Allen B., Tran K. K., Macaraeg T. G., Chu E. M., Kwok S. F., Chmielewski N. N., Craver B. M., Baulch J. E., Acharya M. M., Cucinotta F. A., Limoli C. L. Sci. Adv. 2015;1:e1400256. doi: 10.1126/sciadv.1400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Tong F., Cai Q., Chen L.-j., Dong J.-h., Wu G., Dong X.-r. Acta Pharmacol. Sin. 2015;36:1288–1299. doi: 10.1038/aps.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J., Olsen R. H., Su W., Foster S., Xing R., Acevedo S. F., Sherman L. S. Behav. Brain Res. 2014;275:146–149. doi: 10.1016/j.bbr.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld S. J., Bhatti P., Brown E. E., Linet M. S., Simon S. L., Weinstock R. M., Hutchinson A. A., Stovall M., Preston D. L., Alexander B. H. Cancer Causes & Control. 2010;21:1857–1866. doi: 10.1007/s10552-010-9613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Robbins M. E. Curr. Med. Chem. 2009;16:130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- Finkel T., Holbrook N. J. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Spitz D. R., Azzam E. I., Li J. J., Gius D. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- Hao L., Huang H., Gao J., Marshall C., Chen Y., Xiao M. Neurosci. Lett. 2014;571:45–49. doi: 10.1016/j.neulet.2014.04.038. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J.-W., Degan P., Ames B. N. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A., Vlachogianni T., Fiotakis C. J. Environ. Sci. Health, Part C. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- Collins A. Free Radical Res. 2000;32:333–341. doi: 10.1080/10715760000300331. [DOI] [PubMed] [Google Scholar]
- Lodovici M., Casalini C., Cariaggi R., Michelucci L., Dolara P. Free Radicals Biol. Med. 2000;28:13–17. doi: 10.1016/s0891-5849(99)00194-x. [DOI] [PubMed] [Google Scholar]
- Fischer-Nielsen A., Jeding I. B., Loft S. Carcinogenesis. 1994;15:1609–1612. doi: 10.1093/carcin/15.8.1609. [DOI] [PubMed] [Google Scholar]
- Cherry J. D., Liu B., Frost J. L., Lemere C. A., Williams J. P., Olschowka J. A., O'Banion M. K. PLoS One. 2012;7:e53275. doi: 10.1371/journal.pone.0053275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. A., Davis L. K., Johnson A. M., Keeney S., Siegel A., Sanford L. D., Singletary S. J., Lonart G. Radiat. Res. 2011;177:146–151. doi: 10.1667/rr2637.1. [DOI] [PubMed] [Google Scholar]
- Lonart G., Parris B., Johnson A. M., Miles S., Sanford L. D., Singletary S. J., Britten R. A. Radiat. Res. 2012;178:289–294. doi: 10.1667/rr2862.1. [DOI] [PubMed] [Google Scholar]
- Morris R. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Vorhees C. V., Williams M. T. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D. J., Klemenhagen K. C., Holick K. A., Saxe M. D., Mendez I., Santarelli L., Craig D., Zhong H., Swanson C., Hegde L. J. Pharmacol. Exp. Ther. 2007;321:237–248. doi: 10.1124/jpet.106.109678. [DOI] [PubMed] [Google Scholar]
- Daniels W. M., Pitout I. L., Afullo T. J., Mabandla M. V. Metab. Brain Dis. 2009;24:629–641. doi: 10.1007/s11011-009-9164-3. [DOI] [PubMed] [Google Scholar]
- Saikhedkar N., Bhatnagar M., Jain A., Sukhwal P., Sharma C., Jaiswal N. Neurol. Res. 2014;36:1072–1079. doi: 10.1179/1743132814Y.0000000392. [DOI] [PubMed] [Google Scholar]
- McNaughton B., Barnes C., Meltzer J., Sutherland R. Exp. Brain Res. 1989;76:485–496. doi: 10.1007/BF00248904. [DOI] [PubMed] [Google Scholar]
- Clark R. E., Broadbent N. J., Squire L. R. Hippocampus. 2005;15:260–272. doi: 10.1002/hipo.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicos M. A., Dash P. K. Brain Res. 1996;739:120–131. doi: 10.1016/s0006-8993(96)00824-4. [DOI] [PubMed] [Google Scholar]
- Abrous D. N., Wojtowicz J. M. Cold Spring Harbor Monograph Archive. 2008;52:445–461. [Google Scholar]
- Mandyam C. D. Brain Reward & Stress Systems Addiction. 2015:96. [Google Scholar]
- Hoge J., Kesner R. P. Neurobiol. Learn. Mem. 2007;88:225–231. doi: 10.1016/j.nlm.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman D. M., Sprengel R., Sanderson D. J., McHugh S. B., Rawlins J. N. P., Monyer H., Seeburg P. H. Nat. Rev. Neurosci. 2014;15:181–192. doi: 10.1038/nrn3677. [DOI] [PubMed] [Google Scholar]
- Schulz J. B., Lindenau J., Seyfried J., Dichgans J. Eur. J. Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- White D. Form Function Phospholipids. 1973;3:441–482. [Google Scholar]
- Yurekli A. I., Ozkan M., Kalkan T., Saybasili H., Tuncel H., Atukeren P., Gumustas K., Seker S. Electromagn. Biol. Med. 2006;25:177–188. doi: 10.1080/15368370600875042. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Free Radical Res. Commun. 1989;7:121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. J. Biol. Chem. 1992;267:166–172. [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K., Ohtsuka E., Nishimura S. Nature. 1987;327:77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Won M. H., Kang T.-C., Jeon G.-S., Lee J.-C., Kim D.-Y., Choi E.-M., Lee K. H., Do Choi C., Chung M.-H., Cho S. S. Brain Res. 1999;836:70–78. doi: 10.1016/s0006-8993(99)01611-x. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab B. A., Salama R. H. Pharmacol., Biochem. Behav. 2011;100:59–65. doi: 10.1016/j.pbb.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Wilde G., Pringle A., Wright P., Iannotti F. J. Neurochem. 1997;69:883–886. doi: 10.1046/j.1471-4159.1997.69020883.x. [DOI] [PubMed] [Google Scholar]