 In recent years, pancreatic pathologies have become common problems and their etiology and pathogenesis are generally unknown.
In recent years, pancreatic pathologies have become common problems and their etiology and pathogenesis are generally unknown.
Abstract
In recent years, pancreatic pathologies have become common problems and their etiology and pathogenesis are generally unknown. Studies have shown that smoking may increase the risk of pancreatic disorders but very scant knowledge is available about the pathogenesis of cigarette induced pancreatic pathology. This study aimed to evaluate the oxidative stress status, biochemical, pathological and immunohistochemical findings of rats exposed to cigarette smoke, pathogenesis of smoking related pancreatic damage and usability of Alpha Lipoic Acid (ALA) for amelioration of cigarette smoking induced harmful effects on rat pancreas. Twenty eight female, Sprague Dawley rats were randomly distributed into three groups. The sham group (S) (n = 8), rats were given 0.1 ml of physiological serum by oral gavage for 8 weeks. The cigarette smoke exposed group (CSE) (n = 10), rats were exposed to successive periods of cigarette smoke for 2 hours per day per 8 weeks and given 0.1 ml of physiological serum orally during the study. The cigarette smoke exposed and ALA treated group (CSE + ALA) (n = 10), animals were exposed to cigarette smoke (2 hours per day per 8 weeks) and simultaneously treated with 100 mg per kg per day ALA orally during the study. At the end of the study, the serum samples were collected for insulin, glucagon, glucose and amylase analyses. Tissue samples were collected for biochemical, histopathological and immunohistochemical examinations. Total oxidant status (TOS), total antioxidant status (TAS) levels and oxidative stress index (OSI) were evaluated in the pancreas samples. Immunohistochemical analyses of insulin, glucagon, calcitonin gene related protein (CGRP), active caspase-3, hypoxia inducible factor-1 (Hif-1), Hif-2 and tumor necrosis factor (TNF-α) expressions of pancreas were examined. Cigarette smoke caused statistically significant increase in serum amylase and glucose but decreased insulin levels indicating both endocrine and exocrine cell damage. There were no statistically significant differences in serum glucagon levels between the groups. Histopathological examination of the pancreas exhibited generally normal tissue architecture but slightly degenerative and apoptotic cells were noticed both in the endocrine and exocrine part of the pancreas in the CSE group. Immunohistochemical analyses revealed marked increase in active caspase-3, Hif-1 and Hif-2, CGRP and TNF-α expressions with a slight increase in glucagon immunoreactivity in cells while a marked decrease was observed in insulin expression in some Langerhans islets in the CSE group. ALA ameliorated biochemical and pathological findings in the CSE + ALA group. These findings clearly demonstrated that cigarette smoke can cause damage in both endocrine and exocrine cells in rat pancreas and ALA has an ameliorative effect of cigarette induced lesions.
Introduction
In the modern world smoking is one of the major environmental health risk factors affecting almost all the organs or systems of human body.1 Smoking has become a common serious health and societal problem in the last century. It can cause numerous problems in organs and their function, and cause different diseases including respiratory, cardiovascular, cerebral, and peripheral vascular diseases and especially cancer.2,3 In relation to pancreatic pathology, smoking has been described as an important risk factor for endocrine and exocrine pancreatic functions and the most common environmental risk factor for pancreatic cancer.1,4
Recent human studies have reported that the possible increase of the risk of pancreatitis in a dose-dependent manner is due to smoking but in contrast some studies have reported no correlation with pancreatic lesions and smoking.5–8 It has been reported that smoking increases by approximately 2-fold the risk of non-gallstone related acute pancreatitis, but not for gallstone-related pancreatitis.7 Andriulli et al. reported that smoking increases 25% of the risk for chronic pancreatitis.6 But there is very little information about the pathogenesis of smoking-induced pancreatic pathology. Data from animal models suggest several potential mechanisms such as altered gene expression in the exocrine pancreas and activation of pancreatic enzymes with acinar cell damage. Nicotine modulated the oxidative stress and lipid peroxidation and these processes might be involved in the pathophysiology of acute and chronic pancreatitis.9
Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion or action.10 It is a life-long disease and swiftly increasing in all age groups and both genders. It causes problems in various physiological functions of organs or multiple systems,11 and is associated with wide ranging and devastating health complications.12 Although some authors report many relative effects of smoking and diabetes, little and contradictory knowledge is available about the effect of smoking on pancreatic endocrine cells and functions.13–15 The pathogenesis of the damage of smoking on pancreas is, however, not yet well understood, and it remains to be elucidated. Because of these reasons, new experimental studies are needed to explain the effect of cigarette smoke on pancreas. The aim of this study was to examine the pancreatic pathology by histopathological, immunohistochemical and biochemical methods in rats exposed to cigarette smoke for 8 weeks and effects of ALA against cellular damage.
Materials and methods
All experiments were performed in accordance with the guidelines for animal research from the National Institutes of Health and were approved by the Committee on Animal Research of Suleyman Demirel University, Isparta. Twenty eight female, Sprague Dawley skeletal development completed six-month old rats weighing 250–300 g were placed in a temperature (21–22 °C) and humidity (60 ± 5%) controlled room in which a 12 : 12 h light : dark cycle was maintained. All the rats were fed with standard commercial chow diet (Korkuteli Yem A.S.). Thioctacid 600 mg tablets (MEDA Pharma, Turkey) which are a commercial form of Alpha Lipoic Acid (ALA) were used for treatment. A single dose per day of 100 mg kg–1 for oral administration was prepared in a saline solution for the experiment.16
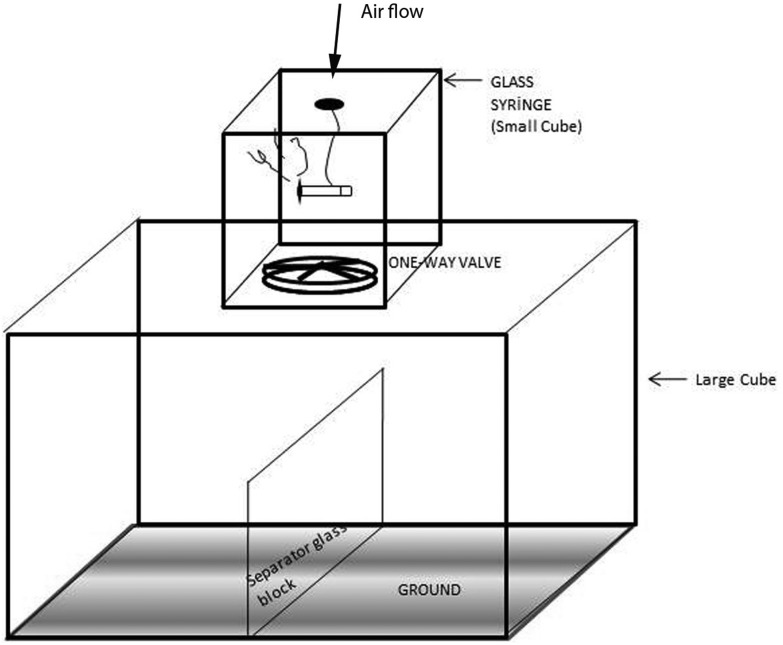
Cigarette smoke exposures were performed using commercially available filter cigarettes (Turkey Tobacco Industrial. Co., Ltd, Tekel 2000, Turkey). According to the product specifications, each cigarette contained 1 mg of nicotine, 10 mg of tar and 10 mg carbon monoxide. The smoking apparatus consists of three major parts, including a glass chamber with a glass door (large cube), a cigarette burner system with an inhalation apparatus (small cube), and a ventilation apparatus (one-way valve) on the top of the chamber.17,18 A 75 cm (length) × 75 cm (width) × 50 cm (height) glass chamber was separated into two layers with sufficient space for exposing 20 rats at a time (Fig. 1). The cigarette burner system (A 25 cm (length) × 15 cm (width) × 15 cm (height)) contains one cigarette holder and a 300 ml glass syringe which could burn up 1 cigarette in 10 minutes and inject cigarette smoke into the chamber (large cube) by manual control of one-way valves. After cigarette smoke exposure the ventilation apparatus would pump out all of the smoke in the chamber within 5 min after exposure. During the experiment, the temperature was maintained in the range of 21–23 °C in the apparatus and the carbon monoxide ratio was maintained in the range of 310–380 ppm.19 Animals were inserted into the apparatus and exposed to cigarette smoke in successive periods of 1 hour in the morning and 1 hour in the afternoon throughout 7 days a week for 8 weeks. According to ten minutes for burning time and five minutes for aeration of one cigarette smoke for controlling CO levels, the session completed in a total of 90 minutes for 6 cigarette smoke exposure. Increasing amount of smoke exposure is planned as follows: first day 3 cigarettes; second day 7 cigarettes; third day 5 up to 12 cigarettes per day (30 min) and 6th to 8th day 12 cigarettes until the end of the week (1 hour period at 2 times).
Fig. 1. The experimental setup of the CSE exposure system.
The rats were randomly divided into three groups:
(1) Sham (S) group (n = 8); animals were placed into the same type of apparatus as described in the cigarette smoke exposed group, but were exposed to fresh air instead of cigarette smoke and given 0.1 ml of physiological serum by gavage for 8 weeks.
(2) Cigarette smoke exposure (CSE) group (n = 10); animals were placed in the chamber and exposed to successive periods of cigarette smoke for 1 h in the morning and 1 h in the afternoon, 7 days for 8 weeks and given 0.1 ml of physiological serum orally for 8 weeks simultaneously.
(3) CSE + ALA group (n = 10); animals were placed in the chamber and exposed to successive periods of cigarette smoke for 1 h in the morning and 1 h in the afternoon, 7 days for 8 weeks and given 100 mg kg–1 d–1 orally for 8 weeks; simultaneously.
At the end of the experiment, rats were euthanized by ketamine (80 mg kg–1)/xylazine anaesthesia (10 mg kg–1) applied 24 hours after the last ALA administration. After the abdominal incision; blood samples were collected from vena cava inferior and extracted to determine the serum insulin, glucagon, glucose and amylase levels. An autoanalyser (Beckman Coulter AU680, Brea, California, USA) was used for analyzing the serum glucose and amylase levels. Serum insulin and glucagon levels were analyzed by a commercial ELISA kit purchased from Merck Millipore (Masseuses, USA) using an ultrasensitive rat/mouse insulin ELISA kit (EZRNI-13K) and a glucagon ELISA Kit, chemiluminescent (EZGLU-30K) respectively with a multiplate ELISA reader (EPOCH microplate reader; Bio-Tek, Inc., Vermont, USA).
Pancreases were quickly removed and divided equally into two longitudinal sections. One half of tissues were placed in a 10% neutral formaldehyde solution for routine histopathological and immunohistochemical examinations (caspase-3, CGRP, Hif-1, Hif-2, TNF-α, insulin and glucagon). The other half of the tissues were homogenized and kept at –80 °C for biochemical studies [total oxidant status (TOS), total antioxidant status (TAS) levels and oxidative stress index (OSI)]. For biochemical analyses, pancreatic tissue samples were collected and homogenized in a motor-driven tissue homogenizer (IKA Ultra-Turrax T25 Basic; Labortechnic, Staufen, Germany) and sonicator (UW-2070 Bandelin Electronic, Germany) with phosphate buffer (pH 7.4). Unbroken cells, cell debris, and nuclei were sedimented by centrifugation at 10 000g for 10 min. The levels of protein were determined in the supernatants. Protein levels in the homogenate were determined according to the method of Bradford et al.19 Determination of TOS and TAS; Rel Assay, a novel automated colorimetric kit which was developed by Erel, was used for determination of TOS and TAS of tissues samples.20,21 The color intensity was related to the total amount of oxidant molecules and the change of absorbance at 660 nm was related to the total antioxidant level of the sample which can be measured spectrophotometrically, as shown in the sample. The results are expressed in terms of mM hydrogen peroxide equivalent per g liter (mmol H2O2 equiv. l–1, mmol H2O2 equiv. per mg protein) for TOS levels and mmol Trolox eq. per mg protein for TAS levels. TAS and TOS were measured spectrophotometrically by an automated chemistry analyzer Beckman Coulter AU5800 (Tokyo, Japan). Determination of OSI which stands for an indicator parameter of the oxidative stress level, and the ratio of TOS to TAS were made using the following formula.22 OSI (arbitrary unit) = TOS/TAS × 100.
For histopathological examination, pancreas samples were collected during necropsy and fixed in 10% neutral formalin solution. After two days, the fixed samples were routinely processed and embedded in paraffin, and 5 μm sections were obtained using a Leica RM 2155 rotary microtome. Then the sections were stained with hematoxylin-eosin (HE) and examined under a light microscope. Histopathological changes were graded in a blinded manner and lesions scored for evaluation of the pathological findings by a specialized pathologist from another university who was unaware of the study design. Scores were made according to the numbers of the degenerative cells. To evaluate the percentage of degenerated cells, 10 different areas of both endocrine and exocrine parts were examined in each rat pancreas under the 40× objective of an Olympus CX41 light microscope. Morphometric evaluation was made by using the Database Manual Cell Sens Life Science Imaging Software System (Olympus Corporation, Tokyo, Japan). Pancreas samples were then immunostained with primary antibodies. All antibodies were purchased from the Abcam, Cambridge, UK. Selected tissue sections were immunostained by Calcitonin Gene Related Protein [Anti CGRP antibody [4901] (ab81887)]; caspase-3 [anti-caspase-3 antibody (ab4051)]; insulin [anti-insulin + proinsulin antibody, [D6D4] Abcam (ab8304)], glucagon [anti-glucagon antibody, Abcam (ab8055)], Hif-1 [anti-HIF-1-alpha (H1alpha67) antibody – ChIP Grade ab1], Hif-2 [anti-HIF-2-alpha [ep190b] antibody ab8365], and TNF [anti-TNF alpha antibody (ab6671)] antibodies according to the manufacturer's instructions. All the slides were analyzed for immunopositivity and a semiquantitative analysis was carried out. The samples were analyzed by examining five different sections in each sample, which were then scored from 0 to 3 according to the intensity of staining (0, absence of staining; 1, slight, 2, medium and 3, marked). Negative controls were incubated with a blocking solution without primary antibodies.
Variables were presented as frequencies, percentages, mean ± standard deviations, median or min–max. The Kolmogorov–Smirnov and Shapiro–Wilk tests were used to test for a normal distribution of continuous variables, and the Levene test was used for homogeneity of variance. Data characterized by a normal distribution were expressed as mean ± standard deviation. Parameters without such distribution were expressed as median with range. The groups were compared using a non-parametric Kruskal–Wallis test and a Mann–Whitney-U test. Biochemical parameters were shown to fit with the normal distribution and ANOVA and post hoc LSD tests were used to compare the groups. Calculations were made using the SPSS 15.0 program pack (SPSS Inc., Chicago, IL, USA). P < 0.05 was set as the value for significance.
Results
In this study, statistically significant increase in serum glucose and amylase levels were observed in the CSE group and ALA treatment decreased the levels in the CSE + ALA group. Results of serum samples are shown in Table 1. Serum insulin and glucagon analysis revealed statistically significant decrease in this hormone level but cigarette smoking did not significantly affect the serum glucagon level (Table 2). This study results indicated both endocrine and exocrine cell damage due to cigarette smoke exposure in pancreas.
Table 1. Serum glucose and amylase levels between the groups.
| Groups | Glucose (mg dl–1) | Amylase (U l–1) |
| Mean ± SD | Mean ± SD | |
| S | 238.16 ± 41.44 | 416.57 ± 37.29 |
| CSE | 280.60 ± 83.55 | 518.42 ± 64.33 |
| CSE + ALA | 206.42 ± 23.27 | 394.00 ± 52.99 |
| P value | S-CSE (0.05) | S-CSE (0.05) |
| S-CSE + ALA (NS) | S-CSE + ALA (NS) | |
| CSE-CSE + ALA (0.05) | CSE-CSE + ALA (0.001) |
Table 2. Serum insulin and glucagon levels between the groups.
| Groups | Insulin (ng ml–1) | Glucagon (pg ml–1) |
| Mean ± SD | Mean ± SD | |
| S | 0.65 ± 0.03 | 81.20 ± 5.84 |
| CSE | 0.46 ± 0.04 | 85.20 ± 1.98 |
| CSE + ALA | 0.54 ± 0.02 | 77.10 ± 4.55 |
| P value | S-CSE (0.05) | S-CSE (NS) |
| S-CSE + ALA (0.05) | S-CSE + ALA (NS) | |
| CSE-CSE + ALA (0.05) | CSE-CSE + ALA (NS) |
In the present study it was observed that cigarette smoke caused a statistical increase in TOS and OSI levels (p < 0.05 and p < 0.05; respectively), while causing a decrease in TAS levels (p < 0.001). In accordance with these parameters, TOS levels were decreased (p < 0.05) and TAS levels were increased (p < 0.05) in the ALA treated groups. Oxidative stress markers of pancreatic tissue are shown in Table 3. These results also supported cigarette smoke induced pancreatic cell damage.
Table 3. Oxidative stress markers status of pancreas.
| Groups | TAS (mmol Trolox equivalents per l) | TOS (μmol H2O2 equiv. l–1) | OSİ |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| S | 0.96 ± 0.44 | 11.99 ± 5.38 | 2.95 ± 1.50 |
| CSE | 0.19 ± 0.17 | 24.65 ± 9.95 | 6.05 ± 2.88 |
| CSE + ALA | 0.53 ± 0.21 | 12.83 ± 5.58 | 3.33 ± 0.83 |
| P value | S-CSE (0.05) | S-CSE (0.05) | S-CSE (0.05) |
| S-CSE + ALA (0.05) | S-CSE + ALA (NS) | S-CSE + ALA (NS) | |
| CSE-CSE + ALA (0.05) | CSE-CSE + ALA (0.05) | CSE-CSE + ALA (0.05) |
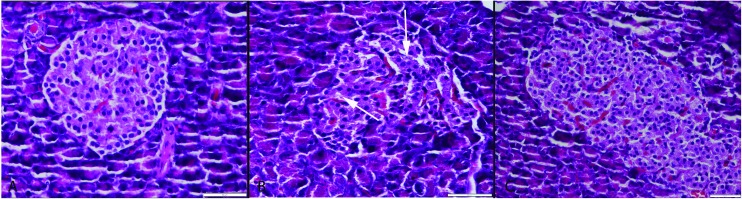
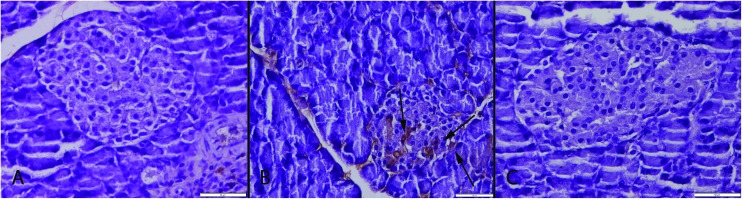
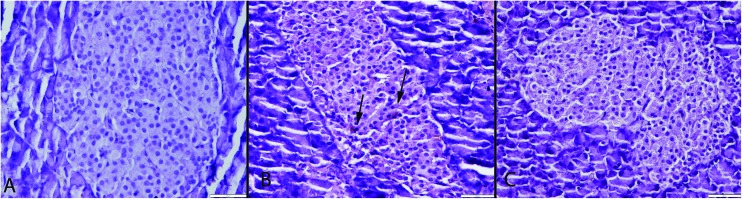
Histopathological examination of the pancreases revealed that they generally retained their normal tissue architecture but slightly degenerative cells were noticed in both endocrine and exocrine part of the pancreas in the CSE group. Most of the cells exhibited vacuolar and some of the cells exhibited hydropic degeneration (cell swelling). Very rarely cells with pyknotic and karyorrhectic nuclei were also seen. A small number of apoptotic cells were observed in pancreas of the CSE group. Histopathological evaluation showed no pancreatic inflammation in any group (Fig. 2). In the S group no pathological lesions were observed. ALA treatment caused marked amelioration in the CSE + ALA groups’ pancreatic cells.
Fig. 2. Histopathological appearance of the pancreas. (A) Normal tissue architecture in the S group. (B) Slight vacuolar degeneration in both endocrine and exocrine cells (arrows) in the CSE group, (C) normal histology in the CSE + ALA group; HE, bars = 50 μm.
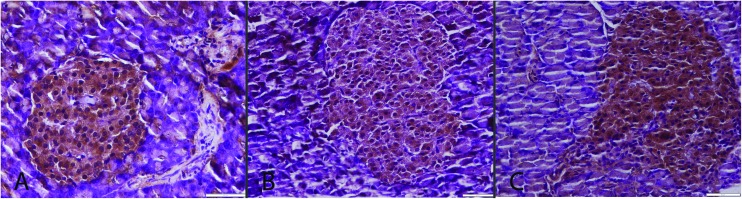
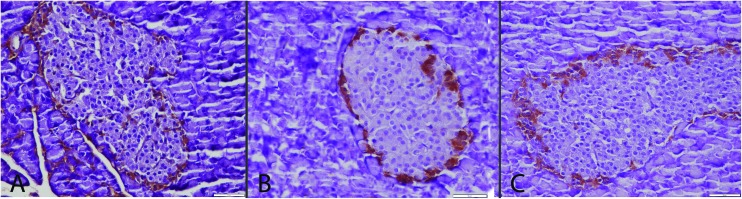
Immunohistochemistry revealed a decrease in insulin secreting cell numbers and severity of expression with a slight increase in glucagon secreting cells. In addition immunohistochemically marked increase in active caspase-3, Hif-1, Hif-2, CGRP and TNF-α expressions in both exocrine and Langerhans islets were noticed in the CSE group (Fig. 3–9). ALA treatment ameliorated the biochemical and pathological findings in the CSE + ALA group.
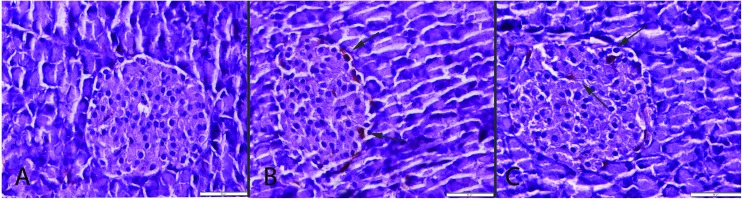
Fig. 3. Insulin immunoreaction between the groups. Normal expressions in S (A) and CSE + ALA (C) groups. Decrease in severity and insulin secreted cell numbers in Langerhans islet in the CSE group (B), streptavidin biotin peroxidase method, bars = 50 μm.
Fig. 4. Glucagon expressions between the groups. Normal expression in S (A) and CSE + ALA (C) groups. Increase in severity and glucagon secreted cell numbers in Langerhans islet in the CSE group (B), streptavidin biotin peroxidase method, bars = 50 μm.
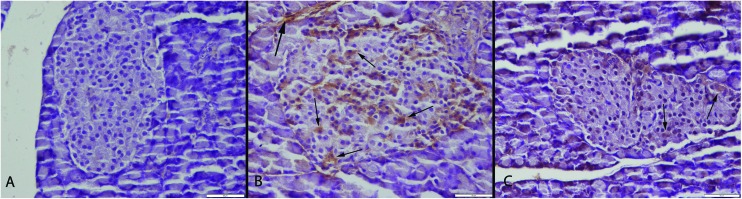
Fig. 5. Caspase-3 expressions of the groups. (A) Negative caspase-3 immunoreaction in pancreas in the S group. (B) Marked increase in endocrine and exocrine cells (arrows) in the CSE group. (C) Decreased caspase-3 expression in Langerhans islet cells (arrows) in the CSE + ALA group, streptavidin biotin peroxidase method, bars = 50 μm.
Fig. 6. Hif-1 expressions between the groups. Negative immunoreaction in S (A) and CSE + ALA (C) groups. Increased Hif-1 expression (arrows) in the CSE group (B), streptavidin biotin peroxidase method, bars = 50 μm.
Fig. 7. Hif-2 expressions between the groups. Negative immunoreaction in S (A) and CSE + ALA (C) groups. Increased Hif-2 expression (arrows) in both endocrine and exocrine cells of pancreas in the CSE group (B), streptavidin biotin peroxidase method, bars = 50 μm.
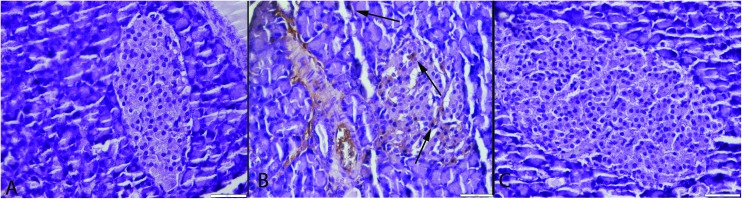
Fig. 8. CGRP immunoreaction between the groups. (A) Negative immunoreaction in the S group. (B) Marked increase in CGRP expression (arrows) in endocrine cells of pancreas in the CSE group (arrows), (C) decrease expression of CGRP (arrows) in the CSE + ALA group. Streptavidin biotin peroxidase method, bars = 50 μm.
Fig. 9. TNF-α expression between the groups. Negative immunoreaction in S (A) and CSE + ALA (C) groups. Increase TNF-α expression (arrows) in Langerhans islet cells of pancreas in the CSE group (B), streptavidin biotin peroxidase method, bars = 50 μm.
Discussion
In this study, the effects of cigarette smoke inhalation on pancreas were examined by biochemical and pathological methods. The oxidative stress markers, histopathological cell damage, active caspase-3, CGRP, TNF-α, Hif-1, Hif-2, insulin and glucagon expressions in the groups were evaluated. A marked increase was observed in active caspase-3, Hif-1, Hif-2 CGRP, TNF-α and relatively slight increase in glucagon expressions while a marked decrease was observed in insulin secretion in some Langerhans islets in the cigarette smoke exposed group related to pancreatic damage. In addition gene expressions except insulin and glucagon were also increased in exocrine cells of the pancreas in the CSE group.
In the present study, exposure to cigarette smoke increased serum glucose concentrations in rats. Immunohistochemical examination of the Langerhans islet revealed marked decrease in insulin expression in some islets. These biochemical and immunohistochemical results were parallel to each other. While the glucagon expression increased in the pancreas there were no statistically significant differences in serum glucagon levels. This result showed that increased immunohistochemical expression of glucagon may be a relative occurrence and insulin secreting cells may be more susceptible to cigarette smoke than glucagon secreting cells. Increased glucagon secreting cells and decreased insulin secreting cells show a possible diabetogenic effect of smoking. These findings were in agreement with previous studies showing alterations in glucose metabolism and sensitivity to insulin in smokers. Chronic cigarette users are generally hyperinsulinaemic and relatively intolerant to glucose when compared with non-smokers.23 Smoking has been established as a risk factor for incident type 2 DM.24
Acute pancreatitis is characterized by sudden inflammation of the pancreas due to injury from a variety of causes. Chronic pancreatitis is an inflammatory disease characterized by long-standing injury and irreversible structural and functional impairment of the pancreas.25,26 The cellular mechanisms through which smoking causes pancreatitis remain unknown.27,28 Cigarette smoking affects tissues, either directly, as is the case in the respiratory tract, or indirectly via the many circulating toxins and metabolic products of tobacco smoke.29 Nicotine is a significant constituent of tobacco and cigarettes and potentially mediates the development of pancreatic disease. A number of experimental studies exploring the effects of nicotine on the pancreas have been implemented. Nicotine exposure resulted in morphological changes in the exocrine pancreas including cytoplasmic swelling, vacuolization, pyknotic nuclei, and karyorrhexis.27,28 The pathological and biochemical changes observed in these studies reflect similar cellular reactions. But the severity of the inflammatory reaction was not prominent. A possible cause of slight finding may be related to the duration of the study. To explain the time related effect of smoking on pancreas, further studies are needed.
Apoptosis is a controlled active physiological process that removes unwanted or defective cells by intrinsically programmed cell suicide and characterized by some morphological changes and caused by an enzyme family of proteases named caspases. They are inactive proenzymes in cytosol that are activated when apoptosis is initiated; they play an essential role during various stages of apoptosis.30–32 In this study the most marked expression were observed at active caspase-3 expression. This result showed us that one of the most important mechanisms of cigarette induced cellular damage might be related to apoptosis.
Hypoxia is one of the other major reasons of degeneration in cells and they can respond to hypoxia with a series of events that include regulation of gene expression.33 The transcription factors activated during low oxygen conditions are called hypoxia-inducible factors (HIFs) and they play important roles in cell metabolism and damage.34 In this study smoke increased both Hif-1 and Hif-2 expressions in pancreas in the CSE group. Activation of the Hif-1 and -2 expression may be one of the other mechanisms of pancreatic injury that is triggered by cigarette smoke.
The TNF-α is a proinflammatory cytokine produced primarily by mononuclear phagocytes and numerous cells after stimulation by immune reactions.35 In this study, although there were no marked inflammatory cell infiltration in pancreas, increase in TNF-α immunoreaction showed that cigarette smoke can cause inflammation and proinflammatory cytokine secretion in pancreatic cells.
The increased formation of reactive oxygen species (ROS) or inefficiency of the antioxidant system,36,37 induces lipid peroxidation in cell structures and causes cellular damage.37,38 Chronic tobacco cigarette consumption is a source of oxidative stress.39 Our study findings were in agreement with a previous study and we observed one of the main pathways of the pancreatic cell damage induced by cigarette smoke via oxidative stress.
Some studies carried out have reported about ALA having a protective effect against free radicals and plays a protective role against oxidative stress in cells.39–41 Similarly ALA had a protective effect against pancreatic damage triggered by smoking in this study.
We investigated the association of cigarette smoke and pancreatic pathology in rats. These study findings clearly demonstrated that cigarette smoke can cause damage in both endocrine and exocrine cells of the rat pancreas and ALA has an ameliorative effect of cigarette induced findings. One of the possible mechanisms of the cell damage observed was oxidative stress that was characterized by high levels of oxidative stress markers. The other mechanism was inducing apoptotic activity that was related to increased caspase-3 immunoreaction in the cells. One of the other mechanisms was increased inflammatory process produced by cigarette smoke, TNF-α and CGRP which had an important role in cigarette related pancreatic damage. In summary, our results show that cigarette exposure leads to an increase in apoptotic activity, TNF-α and CGRP expression, glucagon immunoreaction and decrease in insulin expression which are associated with pancreatic damage.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Muscat J. E., Stellman S. D., Hoffmann D., Wynder E. L. Cancer Epidemiol. Biomarkers Prev. 1997;6:15–19. [PubMed] [Google Scholar]
- Peto R., Lopez A. D., Boreham J., Thun M., Heath Jr. C. Lancet. 1992;339:1268–1278. doi: 10.1016/0140-6736(92)91600-d. [DOI] [PubMed] [Google Scholar]
- Kosmider S., Hrycek A. Wiad. Lek. 1977;30:1023–1026. [PubMed] [Google Scholar]
- Boyle P., Maisonneuve P., Bueno de Mesquita B., Ghadirian P., Howe G. R., Zatonski W., Baghurst P., Moerman C. J., Simard A., Miller A. B., Przewoniak K., McMichael A. J., Hsieh C.-C., Walker A. M. Int. J. Cancer. 1996;67:63–71. doi: 10.1002/(SICI)1097-0215(19960703)67:1<63::AID-IJC12>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Tolstrup J. S., Kristiansen L., Becker U., Grønbæk M. Arch. Intern. Med. 2009;169:603–609. doi: 10.1001/archinternmed.2008.601. [DOI] [PubMed] [Google Scholar]
- Andriulli A., Botteri E., Almasio P. L., Vantini I., Uomo G., Maisonneuve P., ad hoc Committee of the Italian Association for the Study of the Pancreas Pancreas. 2010;39:1205–1210. doi: 10.1097/MPA.0b013e3181df27c0. [DOI] [PubMed] [Google Scholar]
- Sadr-Azodi O., Andrén-Sandberg Å., Orsini N., Wolk A. Gut. 2012;61:262–267. doi: 10.1136/gutjnl-2011-300566. [DOI] [PubMed] [Google Scholar]
- Luaces-Regueira M., Iglesias-García J., Lindkvist B., Castiñeira-Alvariño M., Nieto-García L., Lariño-Noia J., Domínguez-Muñoz J. E. Pancreas. 2014;43:275–280. doi: 10.1097/01.mpa.0000437324.52598.ee. [DOI] [PubMed] [Google Scholar]
- Alexandre M., Pandol S. J., Gorelick F. S., Thrower E. C. Pancreatology. 2011;11:469–474. doi: 10.1159/000332196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association (ADA) Diabetes Care. 2008;32:S30–S45. [Google Scholar]
- Vasilyeva O. N., Frisina S. T., Zhu X., Walton J. P., Frisina R. D. Hear. Res. 2009;249:44–53. doi: 10.1016/j.heares.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin J. R., Alberti K. G., Davidson M. B., Defronzo R. A., Drash A., Gabbe S. G., Genuth S., Harris M. I., Kahn R., Keen H., Knowler W. C., Lebovitz H., Maclaren N. K., Palmer J. P., Raskin P., Rizza R. A., Stern M. P. Diabetes Care. 2002;25:S5–S20. [Google Scholar]
- De Souza M. S. S., Sinzato Y. K., Lima P. H. O., Calderon I. M. P., Rudge M. V. C., Damasceno D. C. Reprod. BioMed. Online. 2010;20:547–552. doi: 10.1016/j.rbmo.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Wei X., Meng E., Yu S. Diabetes Res. Clin. Pract. 2015;107:9–14. doi: 10.1016/j.diabres.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Meo S. A., Memon A. N., Sheikh S. A., Rouq F. A., Mahmood Usmani A., Hassan A., Arain S. A. Eur. Rev. Med. Pharmacol. Sci. 2015;19:123–128. [PubMed] [Google Scholar]
- Bulut N. E., Ozkan E., Ekinci O., Dulundu E., Topaloglu U., Sehirli A. O., Ercan F., Sener G. Ulus. Travma. Acil. Cerrahi. Derg. 2011;17(5):383–389. [PubMed] [Google Scholar]
- Nie Y., Wu H., Li P., Luo Y., Long K., Xie L., Shen J., Su W. J. Med. Food. 2012;15(10):894–900. doi: 10.1089/jmf.2012.2251. [DOI] [PubMed] [Google Scholar]
- Sahin O. K., Aksoy M. C., Uz E., Dagdeviren B. H. SDU J. Health Sci. 2015;6(1):10–14. [Google Scholar]
- Bradford M. M. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Erel O. Clin. Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Erel O. Clin. Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Demirbag R., Gur M., Yilmaz R., Kunt A. S., Erel O., Andac M. H. Int. J. Cardiol. 2007;116(1):14–19. doi: 10.1016/j.ijcard.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Hirai N., Kawano H., Hirashima O., Motoyama T., Moriyama Y., Sakamoto T., Kugiyama K., Ogawa H., Nakao K., Yasue H. Am. J. Phys. Heart. Circ. Physiol. 2000;279:1172–1178. doi: 10.1152/ajpheart.2000.279.3.H1172. [DOI] [PubMed] [Google Scholar]
- Willi C., Bodenmann P., Ghali W. A., Faris P. D., Cornuz J. JAMA, J. Am. Med. Assoc. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- Peery A. F., Dellon E. S., Lund J., Crockett S. D., McGowan C. E., Bulsiewicz W. J., Gangarosa L. M., Thiny M. T., Stizenberg K., Morgan D. R., Ringel Y., Kim H. P., Dibonaventura M. D., Carroll C. F., Allen J. K., Cook S. F., Sandler R. S., Kappelman M. D., Shaheen N. J. Gastroenterology. 2012;143:1179–1187. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullady D. K., Yadav D., Amann S. T., O'Connell M. R., Barmada M. M., Elta G. H. Gut. 2011;60:77–84. doi: 10.1136/gut.2010.213835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury P., Rayford P. L., Chang L. W. Proc. Soc. Exp. Biol. Med. 1998;218:168–173. doi: 10.3181/00379727-218-44284. [DOI] [PubMed] [Google Scholar]
- Chowdhury P. Tob. Induced Dis. 2003;1:213–217. doi: 10.1186/1617-9625-1-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarini D. M. Mutat. Res. 2004;567:447–474. doi: 10.1016/j.mrrev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Harvey N. L., Kumar S. Adv. Biochem. Eng./Biotechnol. 1998;62:107–128. doi: 10.1007/BFb0102307. [DOI] [PubMed] [Google Scholar]
- Stegh A. H., Peter M. E. Cardiol. Clin. 2001;19:13–29. doi: 10.1016/s0733-8651(05)70192-2. [DOI] [PubMed] [Google Scholar]
- Zhang L. J. Soc. Gynecol. Invest. 2005;12(1):2–13. doi: 10.1016/j.jsgi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Crews S. T. Genes Dev. 1998;12:607–620. doi: 10.1101/gad.12.5.607. [DOI] [PubMed] [Google Scholar]
- Wang G. L., Jiang B. H., Rue E. A., Semenza G. L. Proc. Natl. Acad. Sci. U. S. A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horadagoda A., Eckersall P. D., Hodgson J. C., Gibbsh A., Moon G. M. Res. Vet. Sci. 1994;57:129–132. doi: 10.1016/0034-5288(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Bowen R. S., Moodley J., Dutton M. F., Theron A. J. Acta Obstet. Gynecol. Scand. 2001;80:719–725. doi: 10.1034/j.1600-0412.2001.080008719.x. [DOI] [PubMed] [Google Scholar]
- Griesmacher A., Kindhauser M., Andert E., Schreiner W., Toma C., Knoebl P., Pietschmann P., Prager R., Schnack C., Schernthaner G., Mueller M. M. Am. J. Med. 1995;98:469–475. doi: 10.1016/s0002-9343(99)80347-7. [DOI] [PubMed] [Google Scholar]
- Orhan H., Onderoglu L., Yucel A., Sahin G. Arch. Gynecol. Obstet. 2003;267:189–195. doi: 10.1007/s00404-002-0319-2. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Hong Y. C., Lee K. H., Park H. J., Park E. A., Moon H. S., Ha E. H. Reprod. Toxicol. 2005;19:487–492. doi: 10.1016/j.reprotox.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Cameron N. E., Cotter M. A., Horrobin D. H., Tritschler H. J. Diabetologia. 1998;41(4):390–399. doi: 10.1007/s001250050921. [DOI] [PubMed] [Google Scholar]
- Kozlov A. V., Gille L., Staniek K., Nohl H. Arch. Biochem. Biophys. 1999;363(1):148–154. doi: 10.1006/abbi.1998.1064. [DOI] [PubMed] [Google Scholar]