 The organic alkylphenol 4-nonylphenol (NP) is regarded to be an endocrine disrupting chemical (EDC), one of the widely diffused and stable environmental contaminants.
The organic alkylphenol 4-nonylphenol (NP) is regarded to be an endocrine disrupting chemical (EDC), one of the widely diffused and stable environmental contaminants.
Abstract
The organic alkylphenol 4-nonylphenol (NP) is regarded to be an endocrine disrupting chemical (EDC), one of the widely diffused and stable environmental contaminants. Due to its hydrophobicity and long half-life, NP can easily accumulate in living organisms, including humans, where it displays a series of toxic effects. It has been widely reported that NP affects male reproduction. In addition, there is increasing evidence suggesting that NP is detrimental to various organs, including the pancreas. This study investigated the adverse effects of NP exposure on the pancreas. Sprague–Dawley rats were treated with different doses of NP for 90 consecutive days. The data suggested that the body weights of the rats treated with NP decreased, and the highest dose of NP treatment (180 mg kg–1) dramatically increased water consumption by rats. Meanwhile, H&E staining and immunohistochemistry indicated that islets in the pancreases shrunk when the rats were treated with the indicated doses of NP. TUNEL staining demonstrated that NP exposure up-regulated the level of apoptosis in the pancreases in a dose-dependent manner. Besides this, NP exposure inhibited the secretion of insulin and disrupted glucose tolerance. The levels of reactive oxygen species (ROS) and intracellular calcium ([Ca2+]i) in the islets were up-regulated in the groups of rats treated with NP, but the levels of Mitochondrial Membrane Potential (MMP) were down-regulated. These results suggest that NP-induced pancreatic damage in rats occurs through mitochondrial dysfunction and oxidative stress, which causes disruption of glucose tolerance and decrease in insulin secretion.
Introduction
The organic alkylphenol 4-nonylphenol (NP) is one of the widely diffused and stable environmental contaminants.1,2 It is the final degradation of alkyl phenol ethoxylates, which are the common surfactants used in several industrial, domestic, and agricultural applications.3–5 It has been reported that more than 50 000 tons of NP per year enter into the soil and water around the world.6 Although the use and production of NP-related compounds has been banned or strictly monitored in Europe and other parts of the world, such as Canada and Japan, NP could still be found at concentrations of 4.1 μg l–1 in the river water and 1 mg kg–1 in river sediments.7 In China, Zhengyan Li (2008)41 investigated the distribution of NP, octylphenol (OP) and bisphenol A (BPA) in Jiaozhou Bay and its adjacent rivers. The results showed that the concentration of NP exceeded the biologically effective threshold in more than 50% of the areas, which placed local organisms at potential risk. In more than 10% of the areas, the NP concentration exceeded the level leading to the feminization of male aquatic organisms. Due to its hydrophobicity and long half-life, NP can easily accumulate in living organisms, including humans, where it displays a series of toxic effects.8,9 Therefore, NP's contamination and toxicity poses a potential hazard to human health and development.
It has been widely shown that NP affects male reproduction.10–12 Previously, we also reported that 90 days exposure to NP has adverse effects on the spermatogenesis and sperm maturation of adult male rats.13 In addition, there is increasing evidence suggesting that NP is detrimental to various organs, including the pancreas. It was reported that NP affected the function of the adrenal gland in salamanders and inhibited the synthesis and secretion of glucocorticoids.14 It can inhibit luteinizing hormone (LH) secretion in ovariectomized rats by affecting the function of the anterior pituitary and cause thyroid hyperplasia.15,16 NP-induced toxicity could lead to apoptosis. In 2012, Jubendradass reported NP induced apoptosis via mitochondria- and Fas L-mediated pathways in the liver of adult male rats.17 Besides this, NP can cause neurotoxicity and weaken neurological function in Rana nigromaculata and impair immune responses.18–20 Recently, a series of animal studies suggested that NP could alter glucose metabolism and enhance the risk of diabetes. But the conclusions are not consistent. Jubendradass et al. reported that long-term NP exposure increased the levels of blood glucose and serum insulin.21 Meanwhile, short-term NP treatment decreased blood glucose levels and increased the serum insulin levels.22 Song and his partners showed that short-term exposure to NP lead to an increase in blood glucose and a decrease in serum insulin.23 Adachi et al. proposed that long-term exposure to NP increased insulin secretion, but acute exposure did not alter the secretion of insulin.24 Owing to its hydrophobicity and long half-life, NP can easily accumulate in living organisms and cause long-term toxic effects. But the effects of long-term NP exposure on the function of the pancreas remain obscure and there is a need to confirm its adverse effects on the secretion of insulin and the damage of the pancreas.
NP is regarded to be an endocrine disrupting chemical (EDC).25 A prominent effect of EDCs is the induction of oxidative stress.26–28 Moreover, enhanced oxidative stress results in the damage of DNA, protein, or lipid, which may induce apoptosis. NP has been reported to generate reactive oxygen species (ROS) and to induce oxidative damage in brain, testis, and neutrophils.25,29,30 However, its effects on pancreas are still not clarified. While NP has been reported that it could induce insulin secretion via intracellular estrogen of pancreas and hyperinsulinemia are one of the important leading factors for the development of insulin resistance and altered glucose metabolism.24,31,32 Disruption of insulin secretion could profoundly affect hepatic glucose metabolic enzymes and serum glucose levels.33 Additionally, the facilitative glucose transporter (GLUT-2), a glucose sensor, was decreased in a dose dependent manner in the rats exposed to NP for 7 consecutive days.22
In this study, we aimed to investigate the long-term NP exposure effects on the rat pancreas by focusing on the changes in insulin secretion and glucose tolerance, as well as any effects on oxidative stress.
Experimental
Chemicals
NP (4-nonylphenol; C15H24O), corn oil, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), mitochondrial membrane potential kit, Fluo-3/AM and collagenase V were purchased from Sigma-Aldrich. The Rat Insulin (INS) ELISA kit was purchased from Research & Diagnostics System, Inc. (R&D System). Pentobarbital sodium, eosin, hematoxylin were purchased from Thermo Scientific. The in situ cell death detection kit was purchased from Roche Diagnostics Limited (Roche). The primary antibody against insulin was purchased from Santa Cruz Biotechnology.
Animals and treatments
32 male rats (Sprague–Dawley) weighting 250–350 g were purchased from the National Institutes for Food and Drug Control. All animals were housed one per cage in a specific-pathogen-free environment under a light cycle of 12 h dark/12 h light with a controlled ambient temperature of 22 °C–24 °C and a humidity of approximately 10%–50%, and received water and food ad libitum. Animals received humane care in compliance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of the People's Republic of China. Experimental procedures were approved by the Animal Care and Use Committee of Soochow University. The rats were randomly allocated into 4 groups (8 rats per group): 0 mg kg–1 (corn oil), or NP (20 mg kg–1, 60 mg kg–1, 180 mg kg–1 in corn oil) and treated by gavage for 90 consecutive days. The dosages and the time used in this study were selected based on the previous studies.11,13,34 The body weights, food consumption, and water consumption of animals were monitored regularly until the end of treatment. Each rats’ food consumption was measured everyday and averaged for a week, including the loss of ground pellets in the litter. After 24 h of the last treatment, the rats were sacrificed and the blood and the pancreas were collected. The collected blood was centrifuged (2500 rpm × 25 min) and incubated for 30 min at room temperature. The serum was removed and stored at –80 °C. The pancreatic tissues were isolated from the indicated doses of NP treatment (Fig. 2A and B). The pancreas was cut into two parts: one part was used to analyze the ROS levels, MMP levels, and [Ca2+]i levels of islets in the pancreas with different doses of NP treatment. Briefly, the comparable amount of the pancreatic head from rats treated with indicated doses of NP was directly digested with collagenase V for 15 min and washed with PBS with 5% BSA three times, followed by re-suspending in 3 ml PBS, which was used to analyze insulin secretion, ROS levels, MMP levels, and [Ca2+]i levels. The other part was fixed in 10% buffered formalin and embedded in paraffin for histological analyses.
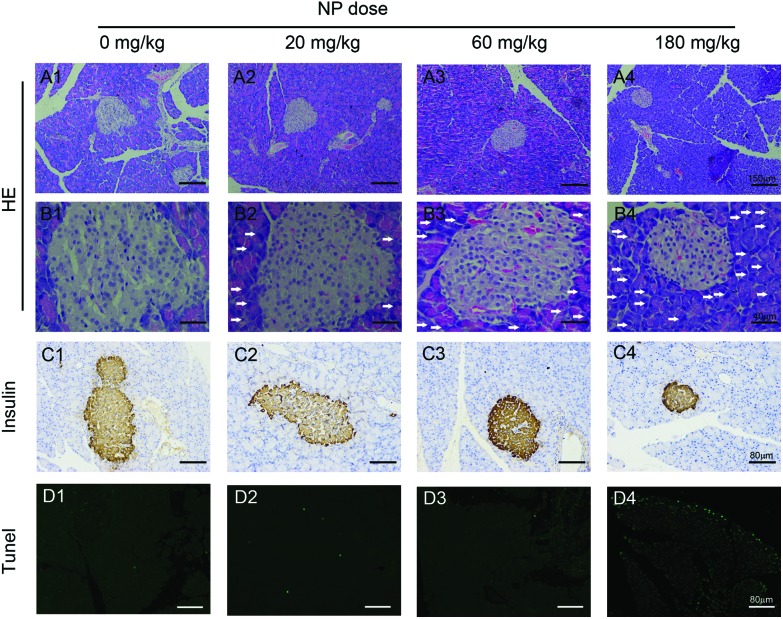
Fig. 2. Histopathology changes in the pancreas of rats treated with NP. Rats were treated with the indicated doses of NP for 90 consecutive days, after which their pancreases were harvested for histological analyses (n = 8 a representative image of the pancreas from each group is shown). A1–D1, 0 mg kg–1; A2–D2, 20 mg kg–1; A3–D3, 60 mg kg–1; A4–D4, 180 mg kg–1. A1–4, H&E staining of the pancreas with the indicated doses of NP treatment, 100×; B1–B4, H&E staining of the pancreas with the indicated doses of NP treatment, 400×; C1–4, the histopathology change of islets was analyzed by IHC staining with an insulin antibody, 200×; D1–4, cell death was analyzed using TUNEL staining, 200×. Scale bar for 100×: 150 μm; 200×: 80 μm; 400×: 40 μm. The white arrows indicated the infiltration of inflammatory cells in the pancreas of rats treated with NP.
Glucose tolerance tests
The oral glucose tolerance test outcomes of interest included basal (fasted) serum glucose concentration, or glucose and insulin concentrations at individual time points following glucose ingestion. At 20 h before performing the oral glucose tolerance test, animals were fasted overnight with water available. After the rats were weighed, a baseline blood sample was obtained from the tail vein, and additional blood samples were obtained at 30, 60, and 120 min following oral glucose administration at 3 g per kg body weight. The levels of blood glucose were measured with an automatic blood biochemical analyzer (Abbott Laboratories, US).
Detection of insulin levels in serum and secreted from islets
30 islets were randomly picked from the digested pancreatic mixture above and cultured with 500 μl PBS containing 16.7 mM glucose at 37 °C in a humidified incubator containing 5% CO2. After 2 h, the supernatant was harvested and insulin concentration was detected. The serum insulin levels and the insulin secreted from pancreatic islets were measured using the Rat Insulin ELISA kit. The assay was used according to the manufacturer's instructions. Briefly, the plate was coated with 100 μL capture antibody in coating buffer per well and incubated overnight at room temperature. The plate was washed with 250 μL washing buffer, blocked with 200 μL of the assay diluents, and incubated at room temperature for 1 h. A 100 μL of supernatant or serum was added and incubated at room temperature for 2 h. 100 μL detection antibody was then added to each well and incubated for 1 h at room temperature. Subsequently, 100 μL avidin–HRP was added and the plate was incubated for 30 min at room temperature. 100 μL of the substrate solution was added to each well and incubated for 15 min at room temperature and then 50 μL of the stop solution was added to stop the reaction. The plate was then read at 450 nm and analyzed. The ELISA was performed in triplicate. Serial dilutions of standards were also used to obtain a standard curve.
Tissue collection, hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC)
Formalin fixed, paraffin embedded tissues were cut into 5 μm sections and stained with H&E. For IHC analysis, the tissue sections were baked and deparaffinized. Antigen retrieval was performed by boiling in sodium citrate buffer. After blocking endogenous peroxidase and nonspecific binding, tissue sections were incubated with primary and then with secondary antibody. Staining was performed with the ABC kit (Vector Lab, Burlingame, CA, USA) and 3,3′-diaminobenzidine (DAKO, Carpinteria, CA, USA). The slides were then counterstained with hematoxylin.
Apoptotic cell death
An in situ cell death detection kit was used for detecting apoptotic cell death in the slides of the pancreas according to the manufacturer's instructions. Tissue sections were pretreated with proteinase K (15 μg mL–1 final concentration) in 10 mM Tris/HCl (pH 7.8) at 37 °C for 30 min. After washing 3 times with PBS, tissue sections were incubated with the TUNEL reaction mixture for 1 h at 37 °C in the dark. Tissue sections were then co-stained with Hoechst, and analyzed under a fluorescence microscope (Olympus, BX5). Excitation wavelength: 450–500 nm and the detection wavelength: 515–565 nm.
Detection of levels of ROS, MMP and [Ca2+]i
To detect the changes of ROS production and intracellular calcium ([Ca2+]i) in the islets when the rats were treated with NP with the indicated doses, 200 μL of the digested mixture was aliquoted, washed with PBS, and fresh medium containing H2DCFDA (10 μg mL–1 final concentration) or Fluo-3/AM (2 μM final concentration) was added according to the manufacturer's instructions. The plates were incubated for 60 minutes at 37 °C. Islets were washed twice with PBS and resuspended in 3 mL PBS. Fluorescence was measured using an Olympus microscope (BX5) with excitation at 488 nm and emission at 525 nm. All steps were handled in the dark.
To detect the MMP and monitor the apoptosis in the islets induced by NP treatment, the mitochondrial membrane potential kit was used according to the manufacturer's instructions (Sigma Aldrich). Briefly, to a 200 μL of the islets mixture mentioned above was added 2 μL of 200× JC-10. The plates were incubated for 60 min at 37 °C with 5% CO2, the fluorescence images were taken with a microscope (BX5) with excitation at 540 nm and emission at 590 nm for red and excitation at 490 nm and emission at 525 nm for green. All steps were handled in the dark. The images were analyzed using Image J online free software.
Statistics
The results are presented as fold changes to the NP untreated group. Data are all shown as mean ± SEM (n = 8). Statistical tests were performed using SPSS 20.0. Unpaired Student's t-tests were used to compare the means of the two groups. One-way analysis of variance was applied to compare the means of three or more groups. p < 0.05 was considered to be significant.
Results
Effects of NP on body weight, food consumption and water consumption
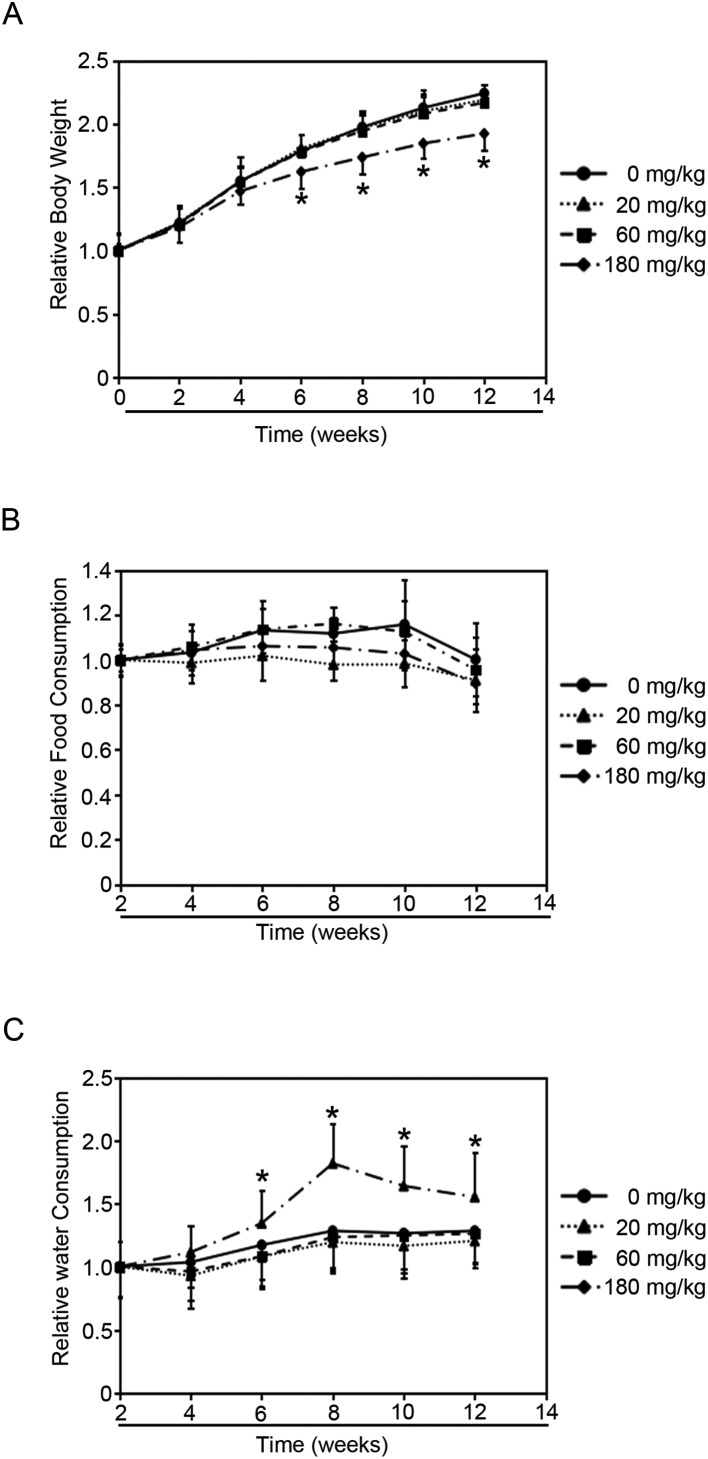
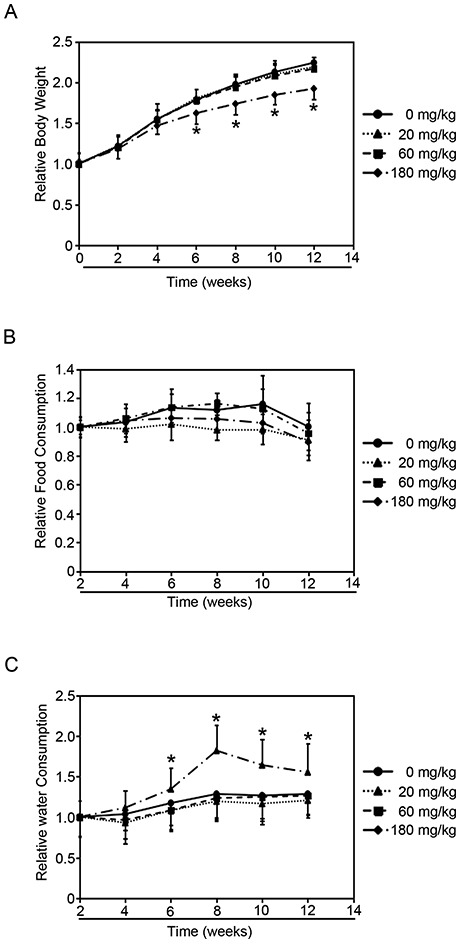
Rats were treated with either vehicle control or NP (20, 60, 180 mg per kg of body weight) by gavage for 90 days. NP treatments decreased the body weight in a dose-dependent manner (Fig. 1A). Rats treated with 20 mg kg–1 and 60 mg kg–1 NP had lower body weights than untreated rats (0 mg kg–1) starting on the 6th week, but did not show significant difference. Meanwhile, rats treated with 180 mg kg–1 NP dramatically decreased the body weight starting on the 2nd week, and showed significant difference on the 6th week. However, NP treatments did not alter the blood glucose levels when compared to the untreated group (Fig. S1†).
Fig. 1. Effects of NP treatment on rats. Rats were administered the indicated doses of NP for 90 consecutive days and the following parameters were recorded weekly. (A) Relative body weight. (B) Relative food consumption. (C) Relative water consumption. Results are expressed as mean ± SEM (n = 8) (*p < 0.05 indicated doses of NP treated groups vs. control group).
Since food consumption levels could affect the animals’ body weight, the amount of food consumed by each treated group was surveyed (Fig. 1B). The data showed that NP exposure did not affect the levels of food consumption compared to the untreated group. And interestingly, the levels of water consumed by the group of rats treated with 180 mg kg–1 NP began to show dramatic increase compared to the untreated group since the 4th week (Fig. 1C). However, lower doses of NP (20 mg kg–1 and 60 mg kg–1) did not affect the water-consumption levels (Fig. 1C). Since the disruption of glucose metabolism would cause the alternation of the body weight and the amount of water consumed, and the decrease in the body weight and increase in water consumption may be because NP exposure affects the glucose metabolism.
NP treatments lead to pathological pancreatic changes and induce apoptosis
To investigate the effects of NP-induced pancreatic injury, rats were exposed to the indicated doses of NP for 90 days. Pancreatic morphology was analyzed to assess pathological changes. H&E staining revealed the infiltration of inflammatory cells in the pancreas of rats in the NP treated groups compared to the pancreas of rats in the untreated groups (Fig. 2, A1–4, B1–4). Additionally, the size of islets in the pancreas of rats in the NP treated groups significantly decreased in a dose dependent manner (Fig. 2, A1–4, B1–4, C1–4; Fig. 3C). When the rats were treated with 60 mg kg–1 and 180 mg kg–1 NP by gavage, the islets in the pancreas decreased significantly compared to the untreated group (Fig. 3C). These results indicated that NP gavage caused a pronounced pancreatic inflammation, which is a process that leads to the dysfunction of pancreas.
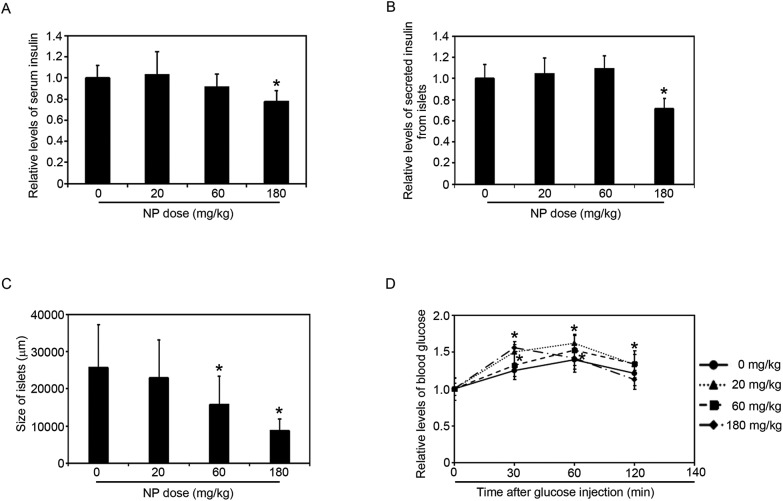
Fig. 3. NP exposure decreased the levels of insulin, the size of pancreatic islets, and impaired glucose tolerance. (A) Changes in serum insulin levels after NP exposure with the indicated doses for 90 consecutive days. (B) The relative levels of secreted insulin from islets with the indicated doses for 90 consecutive days. (C) The size of islets in the pancreas of rats with the indicated doses of NP treatment. (D) Changes in glucose tolerance after 90 days NP exposure. Results are expressed as mean ± SEM (n = 8) (*p < 0.05 indicated doses of NP treated groups vs. control group).
To further test the toxicity of NP to the pancreas, apoptotic cell death was measured by TUNEL labeling. NP induced pancreatic cell apoptosis in a dose dependent manner (Fig. 2, D1–4). There were no apoptotic cells observed in the untreated group (Fig. 2, D1), and when the rats were treated with 20 mg kg–1 NP, the level of the apoptotic cells began to increase (Fig. 2, D2). Consistently, the level of the apoptotic cells in the pancreas in the rats treated with 60 and 180 mg kg–1 NP was persistent and continuously increased (Fig. 2, D3 and 4). These results demonstrated that long-term NP treatment caused a dramatic increase in pancreatic cell apoptosis, which in turn decreased the size of the pancreatic islets.
NP treatments impaired glucose tolerance via down-regulation of insulin secretion from pancreatic islets
To further investigate whether the NP treatment affected the secretory function of the pancreas, the levels of serum insulin were measured (Fig. 3A). The data indicated that 20 mg kg–1 did not affect the levels of the serum insulin. When the rats treated with 60 mg kg–1 NP, the levels of serum insulin decreased, but there is no significant difference. However, in the rats exposed to 180 mg kg–1 NP for 90 days, the levels of serum insulin were dramatically decreased (Fig. 3A).
To test the hypothesis that the decrease of the serum insulin levels was caused by the inhibition of the insulin secretion from beta cells in the islets, the pancreatic islets were isolated. 180 mg kg–1 NP dramatically decreased the secretion of insulin from the islets, but 20 and 60 mg kg–1 NP had no effects (Fig. 3B).
Since pancreatic islets regulate blood glucose levels by secreting insulin into the bloodstream, the NP treatment could affect the insulin levels (Fig. S1†). The oral glucose tolerance test outcomes of interest included basal (fasted) serum glucose concentration, glucose and insulin concentrations at individual time points following glucose ingestion. The data indicated that NP-treated groups were less glucose tolerant than the untreated group, as measured by the oral glucose tolerance test (Fig. 3D), which shows that NP treatments inhibit the secretion of insulin from the pancreas. Taken together, these data demonstrated that NP treatments inhibited the secretion of insulin and impaired glucose tolerance.
NP-induced pancreatic damage through mitochondrial dysfunction and oxidative stress
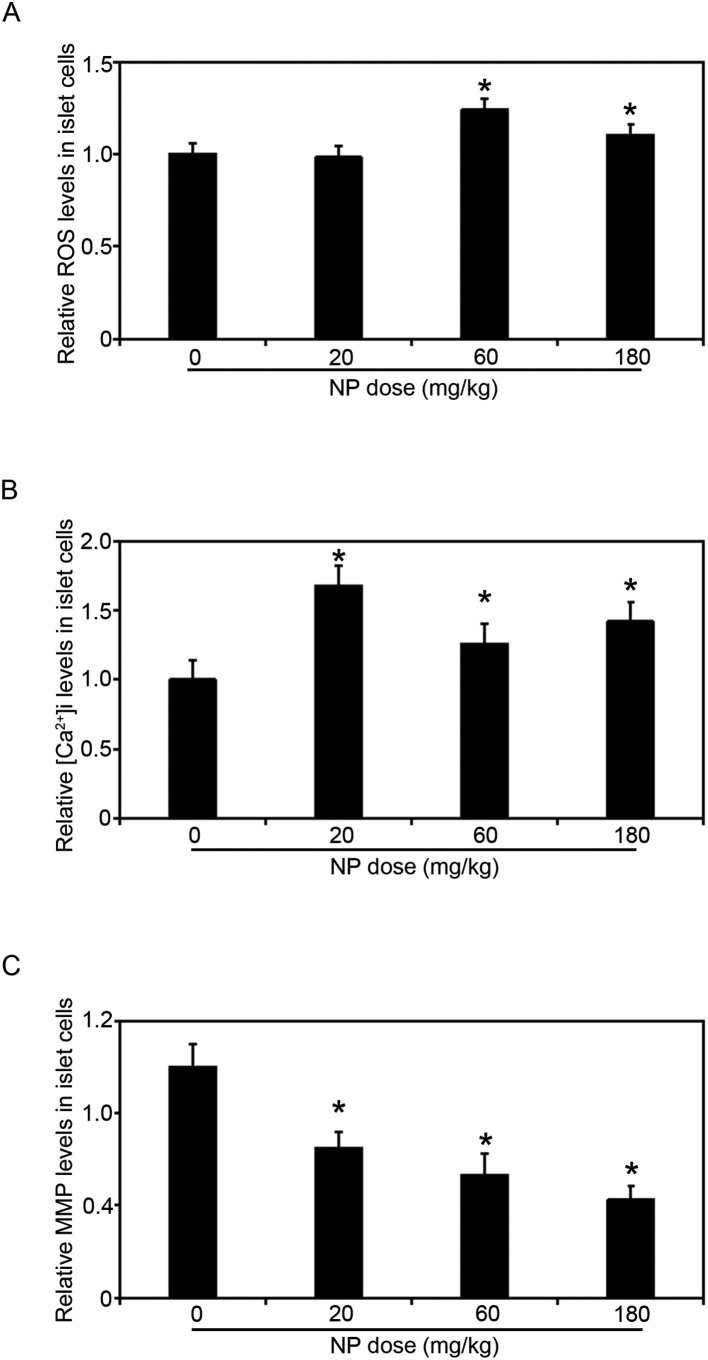
Since NP treatments decreased the size of islets in the pancreas and inhibited the secretion of insulin from the pancreas, we decided to look into the mechanisms of pancreatic damage induced by NP treatment. The islets in the pancreases were isolated from the rats with the indicated doses of NP treatments, and the levels of ROS, MMP and [Ca2+]i were measured (Fig. 4A–C). In the rats exposed to 20 mg kg–1 NP ROS levels did not change, but in the rats treated with 60 mg kg–1 and 180 mg kg–1 NP for 90 consecutive days the ROS levels in the islets were dramatically increased (Fig. 4A). Next, since the levels of [Ca2+]i are associated with the islets secretion of insulin, the levels of [Ca2+]i were determined. The [Ca2+]i levels in the islets increased when the rats were treated with 20 mg kg–1 NP (Fig. 4B). However, when the doses of NP increased, the levels of [Ca2+]i decreased compared to the 20 mg kg–1 NP treated group, but remained elevated compared to the control group.
Fig. 4. NP long term exposure caused mitochondrial dysfunction and oxidative stress of islet cells in the pancreas. (A) NP exposure increased the ROS levels of islet cells in the pancreas. (B) NP exposure up-regulated the levels of [Ca2+]i. (C) NP exposure decreased the levels of MMP in the islet cells in a dose-dependent manner. Results are expressed as mean ± SEM (n = 8) (*p < 0.05 indicated doses of NP treated groups vs. control group).
During apoptosis, a collapse of the mitochondrial membrane potential coincides with the opening of the mitochondrial permeability transition pores, leading to the release of cytochrome c into the cytosol, which in turn triggers other downstream events in the apoptotic cascade. Therefore, the MMP levels in the islets were next measured, and consistent with Fig. 2D, NP treatment decreased the MMP levels in a dose-dependent manner (Fig. 4C).
Discussion
The endocrine system is an important modulator and participates in the regulation of growth, development, reproduction and metabolism of organisms to maintain homeostasis.35 As one of the important endocrine glands, the pancreas synthesizes and secretes insulin. Insulin is the only hormone that controls blood glucose levels and the only determinant of diabetes. A series of evidence suggested that NP is detrimental to various organs, including pancreas.14–16,18–20
This study focused on the adverse effect of various doses of NP on the pancreatic function of male rats. Sprague–Dawley rats were treated with different doses of NP for consecutive 90 days. The data suggested that the body weight of the rats treated with NP decreased, and highest dose of NP treatment (180 mg kg–1) dramatically increased water consumption by the rats, which is consistent with the results reported by Korkmaz and Jubendradass.21,22,29,36 Since the disruption of glucose metabolism would cause the alternation of body weight and the water consumption, this result suggested that long-term NP exposure caused a decrease in the body weight and increase in water consumption may be because NP exposure affects the glucose metabolism.
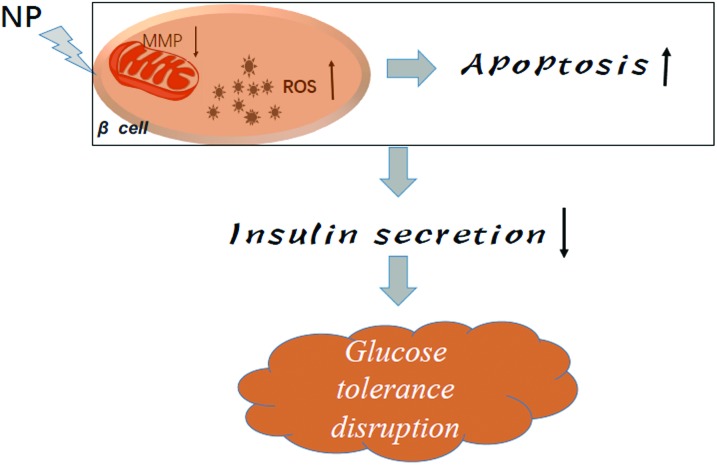
Many environmental contaminants have been reported to disrupt the balance of pro-oxidants/antioxidants in cells, inducing oxidative stress.37 Increased malondialdehyde (MDA) concentration and decreased glutathione (GSH) concentration indicated an increased generation of free oxygen radicals (ROS) which caused damage in various of organs.21,22,38 A series of studies have confirmed that NP exposure produces oxidative stress, enhances ROS level and inhibits the activity of antioxidant enzymes in testis, testicular cells and epididymal sperm.11,13 Our data supported that the levels of ROS increased in the groups of rats treated with high doses of NP, which is consistent with the changes of the [Ca2+]i levels in the islets. NP exposure with low dose (20 mg kg–1) increased the levels of [Ca2+]i, but as long as the NP exposure dose went up, this increase will go back to the basal level. These data demonstrated that low dose of NP treatment induce a compensatory increase of [Ca2+]i. Low dose of NP may induce the ERK1/2 signaling pathway by binding to ERα, which could increase the levels of [Ca2+]i. And the increasing levels of [Ca2+]i could enhance the secretion of insulin by up-regulating the pdx1 expression.39,40 However, high dose of NP treatment increased the levels of ROS (Fig. 4A), and enhanced oxidative stress results in the damage of DNA, protein or lipid, inducing apoptosis.13 Consistently, NP treatment decreases the MMP levels of the islets in the pancreas in a dose-dependent manner (Fig. 4C). During apoptosis, a collapse of the mitochondrial membrane potential coincides with the opening of the mitochondrial permeability transition pores, leading to the release of cytochrome c into the cytosol, which in turn triggers other downstream events in the apoptotic cascade. Consistent with Fig. 4, TUNEL staining demonstrated that NP exposure up-regulated the level of apoptosis in the pancreases in a dose dependent manner. The levels of apoptosis in the pancreases were dramatically increased when the rats were treated with 60 mg kg–1 and 180 mg kg–1 NP (Fig. 2, D1–4). Meanwhile, H&E staining and immunohistochemistry indicated that pancreatic islets were shrunk when the rats were treated with 60 mg kg–1 and 180 mg kg–1 NP (Fig. 2A–C). The increasing levels of apoptosis and shrunk islets would explain the decrease in the secretion of insulin and disruption of glucose tolerance.
GLUT2 is the principal transporter for the transfer of glucose between the liver and blood. And the levels of serum glucose did not get affected with NP treatment in this study. We measured the mRNA levels of GLUT2 and IR expression in the liver, which regulates the levels of glucose in the serum (Fig. 5). The data suggested that NP long-term exposure did not change the GLUT2 expression, but dramatically increased the IR expression in the liver, which could partially explain why there was no change of serum glucose levels with NP treatments (Fig. S1B and C†).
Fig. 5. A model depicted the action of NP to the pancreatic cells.
Conclusions
Our study provides proof-of-concept experimental evidence that NP induced pancreatic damage in rats through mitochondrial dysfunction, oxidative stress and the induction of islet cells apoptosis. In turn, the size of the islets in the pancreas of rats treated with NP decreased in a dose-dependent manner, and the decrease in the islet size caused a decrease in the insulin secretion and disruption of glucose tolerance. This study provides insights for the development of clinical therapeutic strategies to prevent NP-induced pancreatic damage. And for the next step of the study, we will focus on the effects of this damage induced by the NP exposure. In addition, the chemical protection against the damage will be tested.
Conflict of interest statement
No conflicts of interest to declare.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Scientific Foundation of China (Grant ref. 81673126), the Natural Science Research in Colleges and Universities of Jiangsu Province (Grant ref.: 16KJB330008), the Youth Foundation of Jiangsu Province (Grant ref.: BK20160333), China Postdoctoral Science Foundation (Grant ref.: 2016 M600440), the Postdoctoral Science Foundation of Jiangsu Province (Grant ref.: 1601079C). We thank Ms Montserrat Rojo de la Vega for editing this manuscript.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tx00450d
References
- Fairbairn D. J., Arnold W. A., Barber B. L., Kaufenberg E. F., Koskinen W. C., Novak P. J., Rice P. J., Swackhamer D. L. Environ. Sci. Technol. 2016;50:36–45. doi: 10.1021/acs.est.5b03109. [DOI] [PubMed] [Google Scholar]
- Soares A., Guieysse B., Jefferson B., Cartmell E., Lester J. N. Environ. Int. 2008;34:1033–1049. doi: 10.1016/j.envint.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Hoss S., Traunspurger W., Severin G. F., Juttner I., Pfister G., Schramm K. W. Environ. Toxicol. Chem. 2004;23:1268–1275. doi: 10.1897/03-226. [DOI] [PubMed] [Google Scholar]
- Capota C. D., Deventer B., Zimmermann R. D. Environ. Sci. Pollut. Res. 2004;11:121–125. doi: 10.1007/BF02979711. [DOI] [PubMed] [Google Scholar]
- Langdon K. A., Warne M. S., Smernik R. J., Shareef A., Kookana R. S. Chemosphere. 2012;86:1050–1058. doi: 10.1016/j.chemosphere.2011.11.057. [DOI] [PubMed] [Google Scholar]
- David A., Fenet H., Gomez E. Mar. Pollut. Bull. 2009;58:953–960. doi: 10.1016/j.marpolbul.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Ekelund R., Granmo A., Magnusson K., Berggren M., Bergman A. Environ. Pollut. 1993;79:59–61. doi: 10.1016/0269-7491(93)90178-q. [DOI] [PubMed] [Google Scholar]
- Ekelund R., Bergman A., Granmo A., Berggren M. Environ. Pollut. 1990;64:107–120. doi: 10.1016/0269-7491(90)90108-o. [DOI] [PubMed] [Google Scholar]
- Bjorklund K., Cousins A. P., Stromvall A. M., Malmqvist P. A. Sci. Total Environ. 2009;407:4665–4672. doi: 10.1016/j.scitotenv.2009.04.040. [DOI] [PubMed] [Google Scholar]
- Roy D., Colerangle J. B., Singh K. P. Front. Biosci. 1998;3:d913–d921. doi: 10.2741/a332. [DOI] [PubMed] [Google Scholar]
- Aly H. A., Domenech O., Banjar Z. M. Toxicol. Appl. Pharmacol. 2012;261:134–141. doi: 10.1016/j.taap.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Lewis C., Ford A. T. Aquat. Toxicol. 2012;120–121:79–89. doi: 10.1016/j.aquatox.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Lu W. C., Wang A. Q., Chen X. L., Yang G., Lin Y., Chen Y. O., Hong C. J., Tian H. L. Biomed. Environ. Sci. 2014;27:907–911. doi: 10.3967/bes2014.128. [DOI] [PubMed] [Google Scholar]
- Capaldo A., Gay F., Valiante S., De Falco M., Sciarrillo R., Maddaloni M., Laforgia V. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012;155:352–358. doi: 10.1016/j.cbpc.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Furuta M., Funabashi T., Kawaguchi M., Nakamura T. J., Mitsushima D., Kimura F. Neuroendocrinology. 2006;84:14–20. doi: 10.1159/000096093. [DOI] [PubMed] [Google Scholar]
- Xi Y., Li D., San W. Regul. Pept. 2013;185:52–56. doi: 10.1016/j.regpep.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Jubendradass R., D'Cruz S. C., Rani S. J., Mathur P. P. Regul. Toxicol. Pharmacol. 2012;62:405–411. doi: 10.1016/j.yrtph.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Jie Y., Xuefeng Y., Mengxue Y., Xuesong Y., Jing Y., Yin T., Jie X. Wien. Klin. Wochenschr. 2016;128:426–434. doi: 10.1007/s00508-016-0960-6. [DOI] [PubMed] [Google Scholar]
- Kim A., Jung B. H., Cadet P. Med. Sci. Monit. Basic Res. 2014;20:47–54. doi: 10.12659/MSMBR.890644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Yeon S. M., Kim H. G., Choi H. S., Kang H., Park H. D., Park T. W., Pack S. P., Lee E. H., Byun Y., Choi S. E., Lee K. S., Ha U. H., Jung Y. W. Inflammation. 2014;37:649–656. doi: 10.1007/s10753-013-9781-1. [DOI] [PubMed] [Google Scholar]
- Jubendradass R., D'Cruz S. C., Mathur P. P. Hum. Exp. Toxicol. 2012;31:868–876. doi: 10.1177/0960327111426587. [DOI] [PubMed] [Google Scholar]
- Jubendradass R., D'Cruz S. C., Mathur P. P. J. Biochem. Mol. Toxicol. 2011;25:77–83. doi: 10.1002/jbt.20361. [DOI] [PubMed] [Google Scholar]
- Song L., Xia W., Zhou Z., Li Y., Lin Y., Wei J., Wei Z., Xu B., Shen J., Li W., Xu S. J. Endocrinol. 2012;215:303–311. doi: 10.1530/JOE-12-0219. [DOI] [PubMed] [Google Scholar]
- Adachi T., Yasuda K., Mori C., Yoshinaga M., Aoki N., Tsujimoto G., Tsuda K. Food Chem. Toxicol. 2005;43:713–719. doi: 10.1016/j.fct.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Chitra K. C., Mathur P. P. Indian J. Exp. Biol. 2004;42:220–223. [PubMed] [Google Scholar]
- Qiu W., Chen J., Li Y., Chen Z., Jiang L., Yang M., Wu M. Ecotoxicol. Environ. Saf. 2016;130:93–102. doi: 10.1016/j.ecoenv.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Lin Y., Zeng X. G., Wu D. S., Wang X. Weisheng Yanjiu/J. Hyg. Res. 2006;35:419–422. [PubMed] [Google Scholar]
- Xu H., Yang M., Qiu W., Pan C., Wu M. Environ. Toxicol. Chem. 2013;32:1793–1799. doi: 10.1002/etc.2245. [DOI] [PubMed] [Google Scholar]
- Aydogan M., Korkmaz A., Barlas N., Kolankaya D. Toxicology. 2008;249:35–39. doi: 10.1016/j.tox.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Okai Y., Sato E. F., Higashi-Okai K., Inoue M. Environ. Health Perspect. 2004;112:553–556. doi: 10.1289/ehp.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prato S., Leonetti F., Simonson D. C., Sheehan P., Matsuda M., DeFronzo R. A. Diabetologia. 1994;37:1025–1035. doi: 10.1007/BF00400466. [DOI] [PubMed] [Google Scholar]
- Laurent G., Solari F., Mateescu B., Karaca M., Castel J., Bourachot B., Magnan C., Billaud M., Mechta-Grigoriou F. Cell Metab. 2008;7:113–124. doi: 10.1016/j.cmet.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Nordlie R. C., Foster J. D., Lange A. J. Annu. Rev. Nutr. 1999;19:379–406. doi: 10.1146/annurev.nutr.19.1.379. [DOI] [PubMed] [Google Scholar]
- Han X. D., Tu Z. G., Gong Y., Shen S. N., Wang X. Y., Kang L. N., Hou Y. Y., Chen J. X. Reprod. Toxicol. 2004;19:215–221. doi: 10.1016/j.reprotox.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Tsang A. H., Astiz M., Friedrichs M., Oster H. J. Endocrinol. 2016;230:R1–R11. doi: 10.1530/JOE-16-0051. [DOI] [PubMed] [Google Scholar]
- Aydogan M., Korkmaz A., Barlas N., Kolankaya D. Drug Chem. Toxicol. 2010;33:193–203. doi: 10.3109/01480540903286468. [DOI] [PubMed] [Google Scholar]
- Liu X., Nie S., Huang D., Xie M. Environ. Toxicol. Pharmacol. 2015;39:815–824. doi: 10.1016/j.etap.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Huang W., Quan C., Duan P., Tang S., Chen W., Yang K. Toxicology. 2016 doi: 10.1016/j.tox.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Wong W. P., Tiano J. P., Liu S., Hewitt S. C., Le May C., Dalle S., Katzenellenbogen J. A., Katzenellenbogen B. S., Korach K. S., Mauvais-Jarvis F. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13057–13062. doi: 10.1073/pnas.0914501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Lin Y., Li Y., Ying C., Chen J., Song L., Zhou Z., Lv Z., Xia W., Chen X., Xu S. Endocrinology. 2011;152:3049–3061. doi: 10.1210/en.2011-0045. [DOI] [PubMed] [Google Scholar]
- Zheng-Yan L., Ming-Zhu F., Dong W. Oceanol. Limnol. Sin. 2008;39(6):599–603. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.