 The results confirm that the circulating levels of miR-21, miR-145, miR-155, and miR-191 are increased in patients with arsenism caused by coal-burning and these miRNAs inhibit the target genes of pathways related to immune inflammation, oxidative stress, and DNA damage repair.
The results confirm that the circulating levels of miR-21, miR-145, miR-155, and miR-191 are increased in patients with arsenism caused by coal-burning and these miRNAs inhibit the target genes of pathways related to immune inflammation, oxidative stress, and DNA damage repair.
Abstract
Endemic arsenism, caused by burning coal containing high levels of arsenic, is found only in the Guizhou and Shanxi Provinces of China. Dysregulated microRNAs (miRNAs), detected in the blood, are emerging as promising biomarkers. At present, little is known about the change and clinical efficacy of circulating miRNAs in patients with endemic arsenism produced by burning of coal. Here, we determined, by using TaqMan Human miRNA Array Chips, the differential expression of plasma miRNAs between patients with arsenism caused by coal-burning and a control group. Four increased miRNAs (miR-21, miR-145, miR-155, and miR-191) were verified in a larger sample by quantitative real-time PCR. Furthermore, bioinformatics and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were used to associate changes in plasma levels of the miRNAs with their functions and their effects on various pathways. The results of chip array assays show that the levels of miR-21, miR-141, miR-148a, miR-145, miR-155, miR-191, miR-218, and miR-491 were most prominently increased and that the levels of miR-200b, miR-200c, miR-26, and miR-34c were decreased. The qRT-PCR results confirm that the circulating levels of miR-21, miR-145, miR-155, and miR-191 are increased in patients with arsenism caused by coal-burning. KEGG analyses show that these miRNAs inhibit the target genes of pathways related to immune inflammation, oxidative stress, and DNA damage repair. Therefore, the four miRNAs may be biomarkers of endemic arsenism caused by coal-burning. Further studies with larger samples should be performed to confirm these findings and to elucidate the underlying mechanisms.
Introduction
Arsenic, a naturally occurring metalloid with distinct physical characteristics and toxicity, is widely distributed in the environment. Exposure to arsenic commonly results from either oral consumption through arsenic-contaminated ground water, soil, or food, or arsenic inhalation of air in an industrial work setting.1 Currently, endemic arsenicosis, a public health concern worldwide, affects tens of millions of people,2 particularly in China. Epidemiological evidence shows that there is an association between arsenic exposure and increased incidences of skin, liver, lung, and bladder cancers.3 Due to the unclear mechanism of arsenic toxicity and the lack of early diagnostic indicators, endemic arsenicosis has not yet been controlled. To date, considerable progress has been made in relation to arsenic toxicity, and various hypotheses have been formed relative to its toxic mechanism of action,4,5 including oxidative stress, DNA damage, chromosomal abnormalities, and epigenetic changes.
MicroRNAs (miRNAs) may mediate toxicity and carcinogenicity resulting from arsenic exposure.1,6 miRNAs are a class of highly conserved, 18–25 nucleotide non-coding RNAs, that post-transcriptionally regulate gene expression by complementary pairing of bases.7 miRNAs have been used to study and to explain the development and diagnosis of diseases. The use of miRNA arrays has become a widely used scientific method. miRNA dysregulation has been associated with biological processes, such as cell development, differentiation, apoptosis, and proliferation.8 miR-191, miR-21, miR-190, and miR-200b, with their target genes, are implicated in the development and progression of arsenic toxicity and carcinogenesis.9–12 Moreover, miRNAs can be used as biomarkers of arsenic exposure, and can account for disease etiology.13 Regarding target populations, however, little is known about the changes of miRNA expression profiles related to arsenic-induced toxicity. The regulatory networks of miRNAs and their targets are complicated, as each individual miRNA may target hundreds of genes, and an mRNA can be the target of multiple miRNAs.14 Thus, it is appropriate to characterize the key miRNAs and their targets in arsenic toxicity.
miRNAs are present in body fluids, including plasma, serum, urine, and saliva.15 With regard to their molecular mechanism, miRNAs are secreted as microvesicles or exosomes, which may be involved in their release into circulation.16,17 miRNAs are stable in the plasma and serum,18 and thus can be obtained by non-invasive means. Since they serve as mediators of cell communication, immune regulation, and tumor invasion, they have potential to serve as non-invasive biomarkers for diseases.
In the present study, to identify the circulating miRNAs and their functions involved in arsenic toxicity and carcinogenesis, plasma miRNA expression profiles were screened through miRNA arrays, and the results were validated by quantitative real-time PCR (qRT-PCR) assays. Based on these results, the levels of these dysregulated miRNAs were measured in paired plasma samples from 50 patients and 50 controls. The results showed that, compared to the control group, four miRNAs were upregulated in the arsenism group. Bioinformatics tools were used to search for the miRNA-targeted signaling pathways of immune inflammation, oxidative stress and DNA damage repair in relation to endemic arsenism caused by coal-burning. The results revealed distinct plasma miRNA expression patterns and relative pathways that may provide new targets for diagnosis in arsenism.
Materials and methods
Study design, patients and control volunteers
A database maintained by the Guizhou Provincial Office of Endemic Disease was used to identify populations exposed to arsenic.19 In 2014, 100 villagers agreeing to participate were enrolled, 50 of which were from the target population in the village of Jiaole, and were designated as the arsenic exposure group according to the Chinese National Arsenicosis Diagnosis Standard Protocol.20 The others were healthy controls (living approximately 13 km away from Jiaole) not exposed to arsenic and with no symptoms of arsenicosis (Table 1). All participants gave written informed consent. Their participation was approved by our institutional review board. A multi-stage case–control study was designed to identify plasma miRNA profiles. In the screening stage, plasma samples from 10 patients and 10 matched controls (based on age and gender) were used in a miRNA microarray to identify miRNAs that were differentially expressed between the case group and control group. Subsequently, qRT-PCR assays were performed to filter signals of the screened miRNAs. Then, several plasma miRNAs were established as the signature of the case group by validation with 50 cases and 50 controls. This study was reviewed and approved by the Ethical Committee of Guizhou Medical University.
Table 1. Demographic features of the villagers of the Guizhou Province in the case and control groups.
| Screening cohort |
Validation cohort |
|||||
| Case group (n = 10) | Control group (n = 10) | P-value | Case group (n = 50) | Control group (n = 50) | P-value | |
| Sex | 1.00 b | 0.27 b | ||||
| Male | 6 (60) | 6 (60) | 30 (60) | 25 (49) | ||
| Female | 4 (40) | 4 (40) | 20 (40) | 26 (51) | ||
| Age | 53.90 ± 4.70 | 54.30 ± 4.24 | 0.84 a | 56.16 ± 6.02 | 52.12 ± 8.14 | 0.34 a |
aStudent's t-test.
bTwo-sided chi-square test.
miRNA microarray
In the screening stage, plasma samples from 10 patients and 10 controls were pooled separately to establish the signatures of plasma miRNAs. TaqMan Human miRNA Arrays (Life Technologies) were used to screen differentially expressed miRNAs in the two pooled samples. An equal volume of the Trizol reagent (Invitrogen) was used for plasma denaturation, according to the manufacturer's protocol. Megaplex RT reactions and pre-amplification reactions were run according to the manufacturer's protocol, in which 75 μl 0.1 of Tris-EDTA buffer (pH 8.0) was added to the PreAmp product, and 9 μl of the diluted PreAmp product was used for the RT-PCR reactions by dispensing 100 μl of the PCR reaction mix into each port of the TaqMan MicroRNA Array. The default PCR procedure was used, and the analysis was performed by using RQ manager software (Life Technologies). Differentially expressed miRNAs were identified through fold-change filtering (fold change >2).
Plasma RNA extraction and quantitative real-time PCR
The quantification of plasma miRNAs was described earlier.21 The total RNA isolation was performed with the mirVana PARIS Kit (Life Technologies; final elution volume: 60 μl), according to the manufacturer's instructions.22 To detect miRNAs, 1 μg of total RNA and the HiScript II Q Select RT Supermix (Vazyme Biotech) were used in reverse transcription according to the manufacturer's protocol. U6 snRNA was used as the control. Quantitative real-time PCR was performed with the Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) and an Applied Biosystems 7300HT machine. The primers are listed in ESI Table 1.† The fold changes in the expression of each gene were calculated by a comparative threshold cycle (Ct) method using the formula 2–(ΔΔCt).23
Bioinformatics analysis
The targets of differentially expressed miRNAs were identified using TargetScan (http://www.targetscan.org/), miRanda (; http://www.microrna.org/), miRDB (; http://www.mirdb.org/), and miRNAMap (; http://mirnamap.mbc.nctu.edu.tw/) algorithms. To reduce the false positive rate, genes predicted by using the four algorithms together as high-degree confident target genes of miRNA were chosen for subsequent analyses. DAVID (; http://david.ncifcrf.gov/) was used to identify significantly enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. A P-value < 0.05 and an FDR < 0.05 were chosen as thresholds. Protein–protein interactions (PPIs) were completed with STRING (; http://www.string-db.org/) and Cytoscape software.
Statistical analyses
Chi-squared tests and Student's t-tests were used to evaluate statistical differences in demographic and clinical characteristics. The non-parametric Mann–Whitney U-test was used for statistical analyses of plasma miRNA levels between the case and control groups. All statistical analyses were accomplished with SPSS software (Version 16.0), and values of P < 0.05 were considered to be significant.
Results
Overview of the design strategy and differentially expressed miRNAs in the screening phase
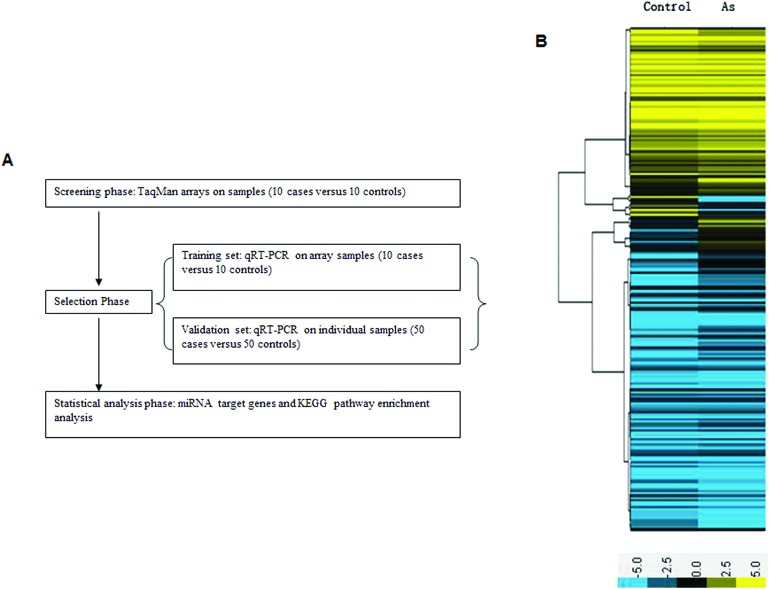
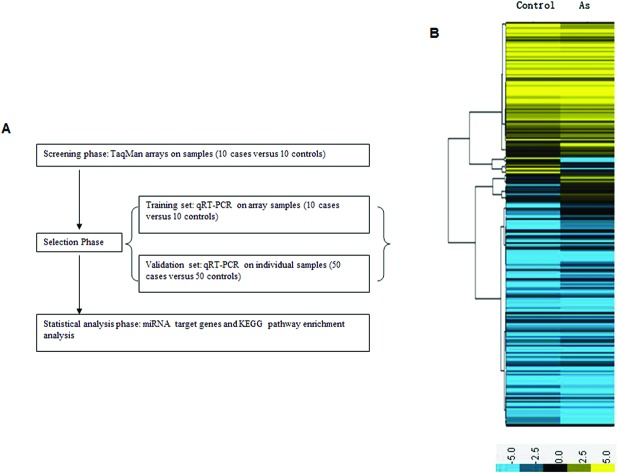
A multi-stage case–control study was designed to establish plasma miRNA profiles. A miRNA array was used as the screening phase and qRT-PCR to verify the results (Fig. 1A). Demographic characteristics of the persons providing the screening and validation samples are included in Table 1. Among the groups, there was no significant difference in the distributions of age and sex. The severity of the poisoning was evaluated by the Chinese National Arsenicosis Diagnosis Standard Protocol. In the screening phase, 7 intermediate and 3 severe individuals were included in the case group. In the control group there were 10 non-exposed individuals who lived 13 km away from the arseniasis area. In the validation phase, the case group included 32 intermediate and 18 severe cases; the control group included 50 non-exposed individuals. In the initial screening phase, the TaqMan Human miRNA Array (754 miRNAs) was used to identify the differentially expressed miRNAs in 10 cases and 10 controls to gain an expression profile of miRNAs in the plasma that is specific for patients exposed to arsenic. The data indicated that 74 miRNAs presented a fold change >2, including 56 miRNAs that were upregulated and 18 that were downregulated in the case group compared with the control group (Fig. 1B). Among these, the levels of miR-21, miR-141, miR-148a, miR-145, miR-155, miR-191, miR-218, and miR-491 were most significantly increased, while the levels of miR-200b, miR-200c, miR-26, and miR-34c were remarkably decreased (Table 2). The results indicate that arsenism caused by coal-burning induces changes in the miRNA profile.
Fig. 1. (A) Overview of the design strategy. A multi-stage, case–control study was performed to identify plasma miRNA profiles. (B) Profiles of plasma miRNAs in the case group compared with controls. Total RNA was separately extracted from the case group and the control group. Significant changes in the miRNA profiles were analyzed by pairwise comparisons between the groups. Only miRNAs showing 2-fold changes or greater between the two groups were selected. (Yellow denotes increased expression, blue denotes reduced expression, and a median expression value equal to 1 is denoted by black.).
Table 2. Results of array analysis of plasma miRNAs differentially expressed in exposed cases compared with controls.
| miRNA | Fold change |
| Hsa-miR-21 | 5.56 |
| Hsa-miR-191 | 4.68 |
| Hsa-miR-148a | 4.59 |
| Hsa-miR-155 | 4.01 |
| Hsa-miR-141 | 3.81 |
| Hsa-miR-491 | 3.51 |
| Hsa-miR-145 | 3.34 |
| Hsa-miR-218 | 2.25 |
| Hsa-miR-200c | 0.28 |
| Hsa-miR-200b | 0.32 |
| Hsa-miR-34c | 0.36 |
| Hsa-miR-26 | 0.45 |
Aberrantly expressed miRNAs in the training phase
qRT-PCR was used to examine the twelve dysregulated miRNAs as shown in Table 3. The results showed that the levels of miR-21, miR-145, miR-155, and miR-191 were higher in the cases of arsenism than in controls. Moreover, the level of miR-200b was lower in the cases of arsenism than in controls. There were no significant differences of miR-26, miR-141, miR-148a, miR-200c, miR-218, miR-34c, and miR-491 levels between cases and controls (Table 3). Thus, arsenism caused by coal-burning induces increases of the miR-21, miR-145, miR-155, and miR-191 levels and decreases of the miR-200b level. The miR-200b expression levels were also examined through individual samples (50 cases versus 50 controls), but the aberrant expression did not reach statistical significance between cases and controls (ESI Fig. 1†).
Table 3. Plasma miRNAs differentially expressed in 10 cases compared with 10 controls.
| miRNA | Controls | Cases | Fold change |
| (Mean ± SD, n = 10) | (Mean ± SD, n = 10) | ||
| Hsa-miR-21 | 0.97 ± 0.03 | 3.39 ± 0.97** | 3.40 |
| Hsa-miR-191 | 1.02 ± 0.05 | 2.85 ± 0.63** | 2.84 |
| Hsa-miR-148a | 0.97 ± 0.03 | 1.27 ± 0.43 | 1.28 |
| Hsa-miR-155 | 0.99 ± 0.03 | 3.14 ± 0.60** | 3.14 |
| Hsa-miR-141 | 0.99 ± 0.03 | 1.26 ± 0.45 | 1.26 |
| Hsa-miR-491 | 1.01 ± 0.03 | 1.20 ± 0.26 | 1.20 |
| Hsa-miR-145 | 1.01 ± 0.04 | 2.28 ± 0.93* | 2.28 |
| Hsa-miR-218 | 1.01 ± 0.04 | 1.26 ± 0.08 | 1.25 |
| Hsa-miR-200c | 1.00 ± 0.03 | 0.61 ± 0.19 | 0.61 |
| Hsa-miR-200b | 1.00 ± 0.04 | 0.37 ± 0.13** | 0.37 |
| Hsa-miR-34c | 1.02 ± 0.06 | 0.67 ± 0.31 | 0.68 |
| Hsa-miR-26 | 1.01 ± 0.04 | 0.76 ± 0.26 | 0.76 |
Increases of miR-21 in arsenism cases and analysis of its target genes
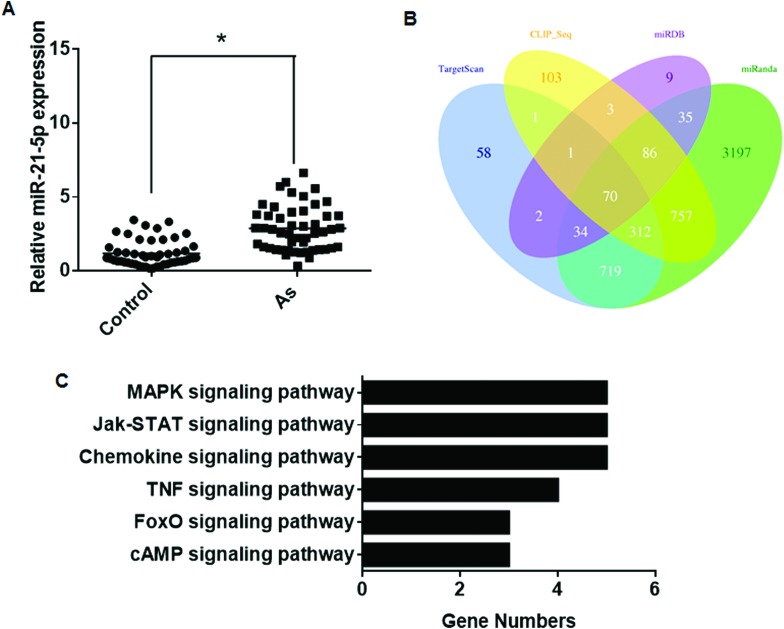
Because miRNAs negatively regulate the expression of their target genes,24 we aimed to compute all possible interactions of each miRNA with all predicted target genes. A list of predicted target genes were obtained by using the TargetScan, miRanda, miRDB, and miRNAMap algorithms (ESI†), and genes predicted by these algorithms were selected for further analysis to reduce redundancy. KEGG pathway analysis was used to find the target genes regulated by miR-21, miR-145, miR-155, and miR-191. Further, the expression levels of miR-21 were examined through individual samples (50 cases versus 50 controls). The miR-21 levels in the plasma of arsenism cases were increased relative to controls (Fig. 2A), which was consistent with the results of the array and qRT-PCR results in the training set. A total of 70 target genes of miR-21 were predicted by using all four algorithms (Fig. 2B). The DAVID online analysis tool was used to analyze the KEGG database. KEGG pathway analysis for the target genes showed that they were mainly involved in six pathways (Table 4). There were more target genes in the MAPK, Jak-STAT, and chemokine pathways than in the other pathways (Fig. 2C). Thus, the MAPK, Jak-STAT, and chemokine pathways may be involved in arsenism via miR-21 regulation.
Fig. 2. Aberrantly expressed miR-21 between the case group and the control group and its target gene analysis. (A) The expression levels of miR-21 were determined by performing qRT-PCR assays (means ± SD, n = 3) in the case group (As, n = 50) and control group (n = 50). *P < 0.05 different from the control group. (B) Target genes predicted by four computer-aided algorithms. (C) The results of the functional enrichment of target gene expression analysis in the KEGG databases.
Table 4. Enriched KEGG pathways of miR-21 target genes.
| Signaling pathway | Target genes |
| MAPK | DUSP8, MEF2C, MAP3 K1, MAP2 K3, SOS2 |
| Jak-STAT | SPRY2, IL12A, PIK3R1, SOS2, STAT3 |
| FoxO | PIK3R1, SOS2, STAT3 |
| Chemokine | PIK3R1, CCL1, STAT3, TIAM1 |
| TNF | DNM1L, JAG1, PIK3R1, MAP2 K3 |
| cAMP | ATP2B4, PIK3R1, TIAM1 |
Aberrantly expressed miR-145 between cases and controls and its target gene analysis
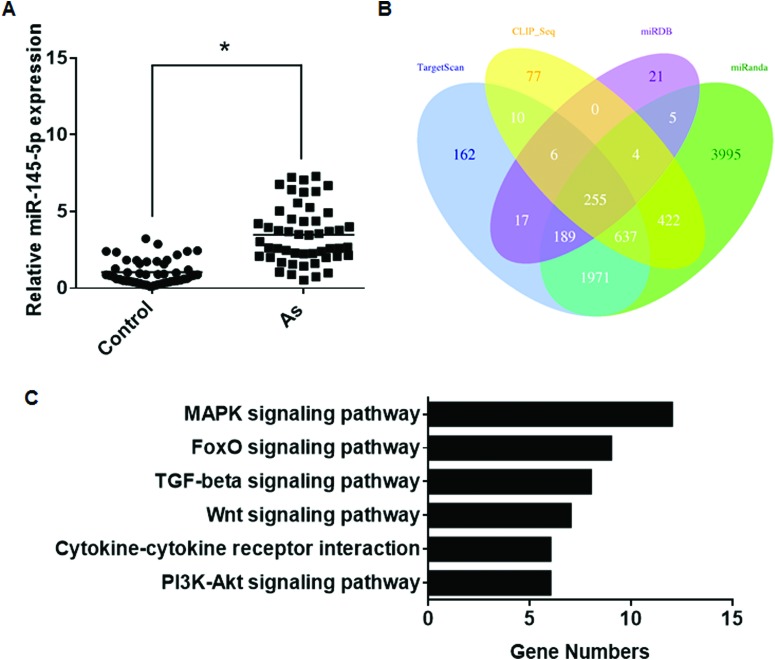
MAPK signaling is associated with oxidative stress,25 and the FoxO signaling pathway is involved in various types of diseases.26 The up-regulation of miR-145 in arsenism cases was determined by qRT-PCR (Fig. 3A). The four algorithms predicted 255 target genes of miR-145 (Fig. 3B). KEGG pathway analysis showed that five signaling pathways were related to miR-145 target genes (Table 5). Several target genes were in the MAPK and FoxO signaling pathways (Fig. 3C), indicating that, in arsenism caused by coal-burning, miR-145 is involved in these pathways.
Fig. 3. Aberrantly expressed miR-145 between the case group and control group and its target gene analysis. (A) The expression levels of miR-145 were determined by performing qRT-PCR assays (means ± SD, n = 3) in the case group (As, n = 50) and control group (n = 50). *P < 0.05 different from the control group. (B) Target genes predicted by four computer-aided algorithms. (C) The results of the functional enrichment of target gene expression analysis in the KEGG databases.
Table 5. Enriched KEGG pathways of miR-145 target genes.
| Signaling pathway | Target genes |
| TGF-beta | SMAD3, SMAD5, RPS6KB1, SKP1, TGFBR2, ACVR1B, ACVR2A, ZFYVE9 |
| MAPK | CRKL, DUSP6, FLNB, MAP3 K3, NRAS, PPP3CA, PRKX, TAOK1, RASA1, TGFBR2, MAP4 K4, RAPGEF2 |
| PI3K-Akt | IRS1, ITGB8, MDM2, NRAS, RPS6KB1, CREB3L2 |
| FoxO | GABARAPL2, FOXO1, GABARAPL1, IRS1, SMAD3, MDM2, NRAS, BNIP3, TGFBR2 |
| Cytokine–cytokine | IFNGR2, TNFRSF11B, TGFBR2, TNFRSF10A, ACVR1B, ACVR2A |
| Wnt | SMAD3, PPP3CA, PRKX, CTNNBIP1, SENP2, SKP1, FZD7 |
Increases of miR-155 in arsenism cases and analysis of its target genes
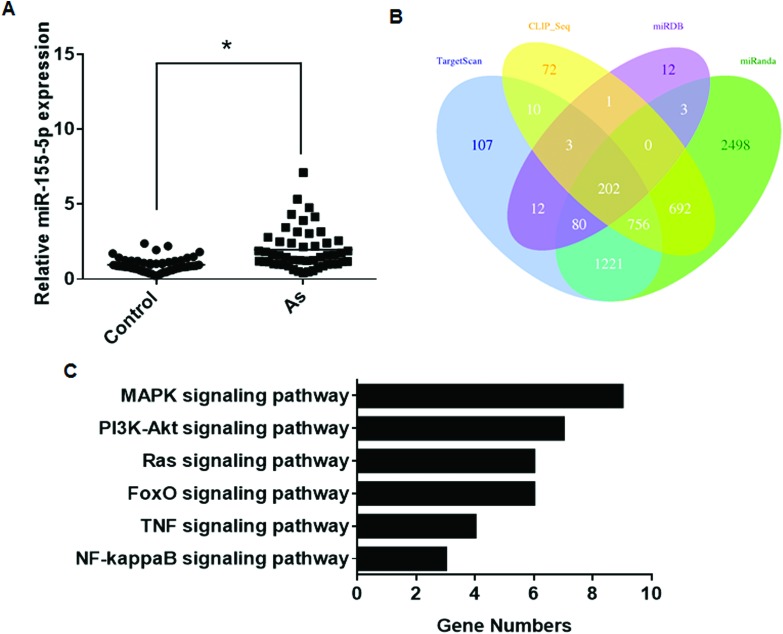
The levels of miR-155, a regulator of immune cell development, function, and disease,27 were up-regulated in arsenism patients (Fig. 4A). The four algorithms predicted 202 target genes (Fig. 4B). KEGG pathway analysis for the target genes showed that they were mainly associated with the MAPK signaling pathway (Table 6 and Fig. 4C). Thus, in arsenism caused by coal-burning, miR-155 is involved in the changes in the MAPK pathway.
Fig. 4. Aberrantly expressed miR-155 between the case group and the control group and its target gene analysis. (A) The expression levels of miR-155 were determined by performing qRT-PCR assays (means ± SD, n = 3) in the case group (As, n = 50) and controls (n = 50). *P < 0.05 different from the control group. (B) Target genes predicted by using four computer-aided algorithms. (C) The results of the functional enrichment of target gene expression analysis in the KEGG databases.
Table 6. Enriched KEGG pathways of miR-155 target genes.
| Signaling pathway | Target genes |
| MAPK | EGFR, TAB2, FOS, KRAS, RAC1, RPS6KA3, MAP4 K3, MAP3 K14, RAPGEF2 |
| PI3K-Akt | EGFR, SGK3, KRAS, PKN2, RAC1, RPS6KB1, YWHAE |
| FoxO | S1PR1, EGFR, SGK3, GABARAPL1, KRAS, SMAD2 |
| Ras | EGFR, ETS1, RGL1, KRAS, RAB5C, RAC1 |
| NF-κB | TAB2, XIAP, MAP3 K14 |
| TNF | CEBPB, TAB2, FOS, MAP3 K14 |
Increases of miR-191 in arsenism cases and analysis of its target genes
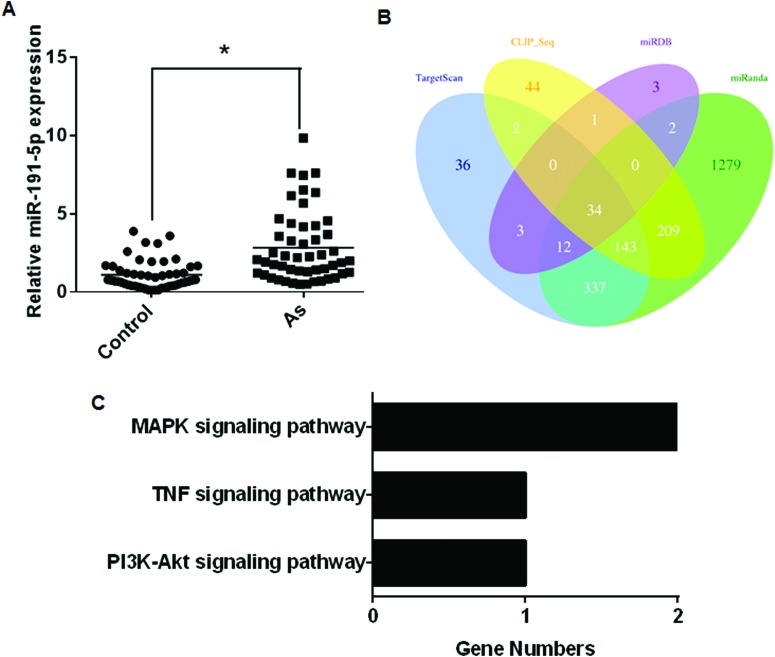
The up-regulation of miR-191 was validated in patients exposed to arsenic (Fig. 5A). The four algorithms predicted 34 target genes (Fig. 5B). The target genes were determined by KEGG pathway analysis (Table 7). Only a few were related to signaling pathways (Fig. 5C). The role of miR-191 in arsenism caused by coal-burning remains unknown.
Fig. 5. Aberrantly expressed miR-191 between the case and control groups and its target gene analysis. (A) The expression levels of miR-191 were determined by performing qRT-PCR assays (means ± SD, n = 3) in the case group (n = 50) and controls (n = 50). *P < 0.05 different from the control group. (B) Target genes predicted by using four computer-aided algorithms. (C) The results of the functional enrichment of target gene expression analysis in the KEGG databases.
Table 7. Enriched KEGG pathways of miR-191 target genes.
| Signaling pathway | Target genes |
| MAPK | miR-191: CRKL, MAP3 K1 |
| PI3K-Akt | miR-191: CCND2 |
| TNF | miR-191: CEBPB |
Pathway and protein–protein interaction (PPI) network analysis
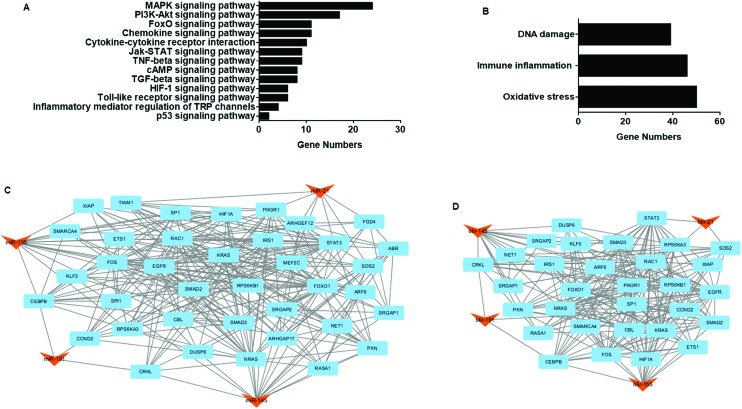
To evaluate the possible regulation mechanisms of miR-21, miR-145, miR-155, and miR-191, as well as their relationships in the process of endemic arsenism, KEGG pathway analyses for target genes were performed. The results showed that the genes were largely involved in five signaling pathways: MAPK, PI3K-Akt, Jak-STAT, FoxO, and the cytokine–cytokine receptor interaction (Table 8 and Fig. 6A). Also, the pathways were classified by considering three toxicities: immune inflammation, oxidative stress and DNA damage repair. Most signaling pathways and genes were related to oxidative stress and immune inflammation (Table 9 and Fig. 6B), revealing that endemic arsenism caused by coal-burning had a high correlation with these processes. Considering the interactions between target genes, the PPI network between target genes of the four miRNAs was analyzed based on the information in the STRING database. A total of 428 nodes and 1380 edges were analyzed using the plug-in MCODE, and the top two significant modules were selected (Fig. 6C and D). The network indicated a complicated regulation system in coal-burning endemic arsenism. Taken together, the results show that the signaling pathways of immune inflammation, oxidative stress and DNA damage repair regulated by miR-21, miR-145, miR-155, and miR-191 are associated with endemic arsenism caused by coal-burning.
Table 8. Enriched KEGG pathways of arsenite-upregulated miRNA target genes.
| Signaling pathway | Target genes |
| MAPK | DUSP8, MEF2C, MAP3 K1, MAP2 K3, SOS2, CRKL, DUSP6, FLNB, MAP3 K3, NRAS, PPP3CA, PRKX, TAOK1, RASA1, TGFBR2, MAP4 K4, RAPGEF2, EGFR, TAB2, FOS, KRAS, RAC1, RPS6KA3, MAP3 K14 |
| PI3K-Akt | PIK3R1, SOS2, IRS1, ITGB8, MDM2, NRAS, RPS6KB1, CREB3L2, EGFR, SGK3, KRAS, PKN2, RAC1, YWHAE, CCND2 |
| Jak-STAT | SPRY2, IL12A, PIK3R1, SOS2, STAT3, IFNGR2, CBL, SOCS5, CCND2 |
| FoxO | PIK3R1, SOS2, STAT3, MDM2, S1PR1, EGFR, SGK3, GABARAPL1, KRAS, SMAD2, CCND2 |
| Cytokine–cytokine | IL12A, CCL1, IFNGR2, TNFRSF11B, TGFBR2, TNFRSF10A, ACVR1B, ACVR2A, EGFR, IL17RB |
| Chemokine | PIK3R1, CCL1, SOS2, STAT3, TIAM1, CRKL, NRAS, PRKX, PXN, KRAS, RAC1 |
| TNF | DNM1L, JAG1, PIK3R1, MAP2 K3, CREB3L2; CEBPB, TAB2, FOS, MAP3 K14 |
| cAMP | ATP2B4, PIK3R1, TIAM1, PRKX, CREB3L2, FOS, GNAS, RAC1 |
| TGF-beta | SMAD3, SMAD5, RPS6KB1, SKP1, TGFBR2, ACVR1B, ACVR2A, ZFYVE9 |
| HIF-1α | PIK3R1, STAT3, IFNGR2, RPS6KB1, EGFR, HIF1A |
| Toll-like receptor | IL12A, PIK3R1, MAP2 K3, TAB2, FOS, RAC1 |
| Inflammatory mediator regulation of TRP channels | GNAS, PRKX, PIK3R1, MAP2 K3 |
| p53 | CCND2, MDM2 |
Fig. 6. Pathway and PPI network analysis of target genes. (A) The top 13 pathways significantly influenced by up-regulated miR-21, miR-145, miR-155, and miR-191. (B) Relative functions of the target genes in various signaling pathways. (C) and (D) The top 2 modules from the target gene network including miRNA regulation.
Table 9. Relative functional effects of various signaling pathways.
| Functions | Signaling pathways |
| Oxidative stress | MAPK, cAMP, HIF-1, FoxO, PI3K-Akt, Jak-STAT and p53 signaling pathways |
| Immune inflammation | Cytokine–cytokine receptor interaction, chemokine, HIF-1, FoxO, toll-like receptor, Jak-STAT, inflammatory mediator regulation, TGF-beta and TNF signaling pathways |
| DNA damage repair | MAPK, FoxO, PI3K-Akt, p53 signaling pathways |
Discussion
Previous studies demonstrated the role of circulating miRNAs in the plasma as biomarkers for molecular diagnosis of various diseases.28 In the present study, we first identified the miRNA expression profiles in the plasma between patients with endemic arsenism caused by coal-burning and a control group. Among 754 miRNAs, there were profound alterations of four miRNAs (miR-21, miR-145, miR-155 and miR-191) in response to arsenic exposure. These miRNAs were associated with immune inflammation, oxidative stress and DNA damage repair as determined by bioinformatics tools and KEGG pathway enrichment analysis.
To date, relevant investigations have largely focused on miRNAs that mediate the biological functions of inorganic arsenic.29 There is evidence that circulating miRNAs may serve as biomarkers for disease diagnosis, prognosis, and treatment.30–32 It was unclear, however, if miRNA expression is altered in response to chronic exposure to arsenic. In the present study, miRNA expression profiles were analyzed with miRNA chips. Four miRNAs (miR-21, miR-145, miR-155 and miR-191) were upregulated in the case group compared to the control group. Plasma miR-21 is a predictive biomarker for chemo-resistance in esophageal squamous cell carcinoma and a potential diagnostic marker for primary intrahepatic cholangiocarcinoma.33,34 Moreover, plasma miR-145, miR-155, and miR-191 are increased in aggressive prostate cancer and abdominal aortic aneurysms and serve as potential biomarkers for these diseases.35–37 Therefore, miR-21, miR-145, miR-155, and miR-191 may be potential biomarkers for endemic arsenism caused by coal-burning.
Since exposure to inorganic arsenic correlates with the generation of ROS, DNA damage repair, inflammation, and tumor promotion,38,39 the biological functions of miR-21, miR-145, miR-155, and miR-191 were analyzed by bioinformatics techniques. This analysis found that miR-21, miR-145, and miR-155 have the capacity to target the MAPK, Akt, FoxO, Jak-STAT, chemokine, TNF, TGF-beta, and toll-like receptor pathways, which are related to immune inflammation, oxidative stress, DNA damage repair, and tumor promotion. The FoxO family members support chronic inflammation by promoting the survival of inflammatory leukocytes acting as mediators in the regulation of oxidative stress.40,41 The MAPK pathway is related to low-density lipoprotein-derived oxidative stress and to pathways for repair of DNA double-strand breaks.42–44 The Jak/STAT pathway is associated with immune inflammation and with regulation of DNA damage repair.45,46 However, the targets of miR-191 showed little evidence of this relationship. Therefore, miR-21, miR-145, and miR-155 may mediate arsenic-induced cytotoxicity.
miR-21 is frequently overexpressed in peripheral blood and tumor tissues of patients with malignant tumors.47,48 Plasma miR-21 has been evaluated as a marker of inflammation, since it is related to chronic inflammatory diseases.28,49 Moreover, the transformation of HELF cells induced by chronic exposure to arsenite (a reactive form of arsenic) is mediated by increased miR-21 expression, which, in turn, is mediated by activation of reactive oxygen species by the ERK/NF-κB pathway.50 miR-155 is a regulatory factor participating in various physiological and pathological processes, including malignant growth and cardiovascular diseases.51,52 Furthermore, for acute ischemic stroke patients, circulating miR-145 is related to the plasma levels of high-sensitivity C-reactive protein.53
In the present study, four out of the twelve miRNAs analyzed by using microarray chips showed the same tendency in response to arsenic exposure as determined by qRT-PCR. As determined by qRT-PCR, the four upregulated miRNAs were significantly altered in the case group compared to the control group. They may be involved in the disease progression of endemic arsenism caused by coal-burning. These differentially expressed miRNAs may contribute to arsenic-induced toxicity, in particular oxidative stress and immune inflammation. Furthermore, circulating miRNAs can be used as biomarkers for the grading of various diseases,54 and plasma miRNAs as biomarkers for diagnosis of diseases.55,56 Therefore, the present results may facilitate the diagnosis of endemic arsenism caused by coal-burning.
In conclusion, we have provided plasma miRNA profiles for patients with endemic arsenism caused by coal-burning and for controls, and we have identified unique miRNA signatures (miR-21, miR-145, miR-155, and miR-191). Bioinformatics analyses of miR-21, miR-145, and miR-155 show potential roles of these three in regulating immune inflammation, oxidative stress and DNA damage repair. Further, the circulating levels of miR-21, miR-145, miR-155, and miR-191 are increased in arsenism, and they inhibit their target genes in signaling pathways related to oxidative stress and immune inflammation. These findings reveal that these miRNAs may function as biomarkers for endemic arsenism caused by coal-burning and that they may be targets for therapeutic intervention. Further studies with larger samples are warranted to verify these findings and to establish the underlying mechanisms.
Conflict of interest statement
The authors declare that they have no competing financial interest.
Funding
This work was supported by the Natural Science Foundations of China (81430077 and 81172603), and the foundation at Guizhou Province for 2011 Collaborative Innovation Project ([2014]06).
Supplementary Material
Acknowledgments
The authors wish to thank Donald L. Hill (University of Alabama at Birmingham, USA), an experienced, English-speaking scientific editor for editing.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tx00428h
References
- Ren X. F., McHale C. M., Skibola C. F., Smith A. H., Smith M. T., Zhang L. P. Environ. Health Perspect. 2011;119:11–19. doi: 10.1289/ehp.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y. S., Song K. H., Chung J. Y. J. Prev. Med. Public Health. 2014;47:245–252. doi: 10.3961/jpmph.14.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Lu Y., Stemmer P. M., Chen F. Oncotarget. 2015;6:12009–12019. doi: 10.18632/oncotarget.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustaffa E., Stoccoro A., Bianchi F., Migliore L. Arch. Toxicol. 2014;88:1043–1067. doi: 10.1007/s00204-014-1233-7. [DOI] [PubMed] [Google Scholar]
- Stueckle T. A., Lu Y., Davis M. E., Wang L., Jiang B. H., Holaskova I., Schafer R., Barnett J. B., Rojanasakul Y. Toxicol. Appl. Pharmacol. 2012;261:204–216. doi: 10.1016/j.taap.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchio E., Colombo T., Boccia P., Carucci N., Meconi C., Minoia C., Macino G. Sci. Total Environ. 2014;472:672–680. doi: 10.1016/j.scitotenv.2013.11.092. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Sontheimer E. J. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leva G., Croce C. M. Curr. Opin. Genet. Dev. 2013;23:3–11. doi: 10.1016/j.gde.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beezhold K., Liu J., Kan H., Meighan T., Castranova V., Shi X. L., Chen F. Toxicol. Sci. 2011;123:411–420. doi: 10.1093/toxsci/kfr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. S., Zhao Y., Smith E., Goodall G. J., Drew P. A., Brabletz T., Yang C. F. Toxicol. Sci. 2011;121:110–122. doi: 10.1093/toxsci/kfr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. C., Luo F., Sun B. F., Ye H., Li J., Shi L., Liu Y., Lu X. L., Wang B. R., Wang Q. L., Liu Q. Z., Zhang A. H. Toxicol. Res. 2016;5:66–78. doi: 10.1039/c5tx00225g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling M., Li Y., Xu Y., Pang Y., Shen L., Jiang R. R., Zhao Y., Yang X. J., Zhang J. P., Zhou J. W., Wang X. R., Liu Q. Z. Free Radical Biol. Med. 2012;52:1508–1518. doi: 10.1016/j.freeradbiomed.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Sharma D., Tiwari M., Lakhwani D., Tripathi R. D., Trivedi P. K. Metallomics. 2015;7:174–187. doi: 10.1039/c4mt00264d. [DOI] [PubMed] [Google Scholar]
- Di Leva G., Garofalo M., Croce C. M. Annu. Rev. Phytopathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. A., Baxter D. H., Zhang S. L., Huang D. Y., Huang K. H., Lee M. J., Galas D. J., Wang K. Clin. Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akao Y., Iio A., Itoh T., Noguchi S., Itoh Y., Ohtsuki Y., Naoe T. Mol. Ther. 2011;19:395–399. doi: 10.1038/mt.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Liang H. W., Zhang J. F., Zen K., Zhang C. Y. Trends Cell Biol. 2012;22:125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Cortez M. A., Bueso-Ramos C., Ferdin J., Lopez-Berestein G., Sood A. K., Calin G. A. Nat. Rev. Clin Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A. H., Feng H., Yang G. H., Pan X. L., Jiang X. Y., Huang X. X., Dong X. X., Yang D. P., Xie Y. X., Peng L., Jun L., Hu C., Jian L., Wang X. L. Environ. Health Perspect. 2007;115:653–658. doi: 10.1289/ehp.9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. Q., Sun D. J., Zheng Y. Environ. Health Perspect. 2007;115:636–642. doi: 10.1289/ehp.9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfken L. M., Moritz R., Ohlmann C., Holdenrieder S., Jung V., Becker F., Herrmann E., Walgenbach-Brunagel G., von Ruecker A., Muller S. C., Ellinger J. PLoS One. 2011;6:e25787. doi: 10.1371/journal.pone.0025787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourvinou I. S., Markou A., Lianidou E. S. J. Mol. Diagn. 2013;15:827–834. doi: 10.1016/j.jmoldx.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Pinweha P., Rattanapornsompong K., Charoensawan V., Jitrapakdee S. Comput. Struct. Biotechnol. J. 2016;14:223–233. doi: 10.1016/j.csbj.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Y., Huang S. S., Lee M. M., Deng J. S., Huang G. J. Int. Immunopharmacol. 2015;25:332–339. doi: 10.1016/j.intimp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Smit L., Berns K., Spence K., Ryder W. D., Zeps N., Madiredjo M., Beijersbergen R., Bernards R., Clarke R. B. Oncotarget. 2016;7:2596–2610. doi: 10.18632/oncotarget.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Boldin M. P., O'Connell R. M., Rao D. S., Taganov K. D. Nat. Immunol. 2008;9:839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- Jiang C. S., Yu H. C., Sun Q. Z., Zhu W. H., Xu J., Gao N., Zhang R., Liu L., Wu X. Y., Yang X. D., Meng L. S., Lu S. M. BMC Pulm. Med. 2016;16:50. doi: 10.1186/s12890-016-0216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Giri A. K. Environ. Int. 2015;81:8–17. doi: 10.1016/j.envint.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y. C., Xu Z., Zhang T. F., Wang Y. L. World J. Gastroenterol. 2015;21:9853–9862. doi: 10.3748/wjg.v21.i34.9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P., Pandey R. K., Prajapati P., Prajapati V. K. Front. Microbiol. 2016;7:1274. doi: 10.3389/fmicb.2016.01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis M., Nair K., Vyas A., Chaturvedi L. S., Gambhir S., Vyas D. World J. Gastroenterol. 2015;21:8284–8292. doi: 10.3748/wjg.v21.i27.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S., Ichikawa D., Kawaguchi T., Miyamae M., Okajima W., Ohashi T., Imamura T., Kiuchi J., Konishi H., Shiozaki A., Fujiwara H., Okamoto K., Otsuji E. Am. J. Cancer Res. 2016;6:1511–1523. [PMC free article] [PubMed] [Google Scholar]
- Correa-Gallego C., Maddalo D., Doussot A., Kemeny N., Kingham T. P., Allen P. J., D'Angelica M. I., DeMatteo R. P., Betel D., Klimstra D., Jarnagin W. R., Ventura A. PLoS One. 2016;11:e0163699. doi: 10.1371/journal.pone.0163699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Hruby G. W., McKiernan J. M., Gurvich I., Lipsky M. J., Benson M. C., Santella R. M. Prostate. 2012;72:1469–1477. doi: 10.1002/pros.22499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., Wang X., Yang D., Shi W. J. Yonsei Med. J. 2016;57:298–305. doi: 10.3349/ymj.2016.57.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. W., Shang T., Huang C., Yu T., Liu C., Qiao T., Huang D., Liu Z., Liu C. J. Clin. Biochem. 2015;48:988–992. doi: 10.1016/j.clinbiochem.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Choudhury S., Ghosh S., Mukherjee S., Gupta P., Bhattacharya S., Adhikary A., Chattopadhyay S. J. Nutr. Biochem. 2016;38:25–40. doi: 10.1016/j.jnutbio.2016.09.001. [DOI] [PubMed] [Google Scholar]
- van Breda S. G., Claessen S. M., Lo K., van Herwijnen M., Brauers K. J., Lisanti S., Theunissen D. H., Jennen D. G., Gaj S., de Kok T. M., Kleinjans J. C. Arch. Toxicol. 2015;89:1959–1969. doi: 10.1007/s00204-014-1351-2. [DOI] [PubMed] [Google Scholar]
- Peng S. L. Int. J. Biochem. Cell Biol. 2010;42:482–485. doi: 10.1016/j.biocel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M., Lin F., Zhang J. Q., Tang Y. T., Chen W. K., Liu H. L. J. Biol. Chem. 2012;287:25727–25740. doi: 10.1074/jbc.M112.349902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhang H., Li X., Jia B., Yang Y., Zhou P., Li P., Chen J. Phytomedicine. 2016;23:1806–1813. doi: 10.1016/j.phymed.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Estrada-Bernal A., Chatterjee M., Haque S. J., Yang L. L., Morgan M. A., Kotian S., Morrell D., Chakravarti A., Williams T. M. Cell Cycle. 2015;14:3713–3724. doi: 10.1080/15384101.2015.1104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J. Y., Eom H. J., Choi J. Toxicol. Res. 2012;28:19–24. doi: 10.5487/TR.2012.28.1.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T. J., Cho B. J., Choi E. J., Kim D. H., Song S. H., Paek S. H., Kim I. A. J. Neuro-Oncol. 2016;130:89–98. doi: 10.1007/s11060-016-2231-9. [DOI] [PubMed] [Google Scholar]
- Villarino A. V., Kanno Y., Ferdinand J. R., O'Shea J. J. J. Immunol. 2015;194:21–27. doi: 10.4049/jimmunol.1401867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churov A. V., Oleinik E. K., Knip M. Autoimmun. Rev. 2015;14:1029–1037. doi: 10.1016/j.autrev.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Liu J., Zhu H. C., Yang X., Ge Y. Y., Zhang C., Qin Q., Lu J., Zhan L. L., Cheng H. Y., Sun X. C. Tumor Biol. 2014;35:3975–3979. doi: 10.1007/s13277-014-1623-8. [DOI] [PubMed] [Google Scholar]
- Olivieri F., Spazzafumo L., Santini G., Lazzarini R., Albertini M. C., Rippo M. R., Galeazzi R., Abbatecola A. M., Marcheselli F., Monti D., Ostan R., Cevenini E., Antonicelli R., Franceschi C., Procopio A. D. Mech. Ageing Dev. 2012;133:675–685. doi: 10.1016/j.mad.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Wei C., Li L., Kim I. K., Sun P., Gupta S. Free Radical Res. 2014;48:282–291. doi: 10.3109/10715762.2013.865839. [DOI] [PubMed] [Google Scholar]
- Wan J. H., Xia L., Xu W. T., Lu N. H. Int. J. Mol. Sci. 2016;17:709. doi: 10.3390/ijms17050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S. D., Karimpour-Fard A., Peterson V., Auerbach S. R., Stenmark K. R., Stauffer B. L., Sucharov C. C. J. Heart Lung Transpl. 2015;34:724–733. doi: 10.1016/j.healun.2015.01.979. [DOI] [PubMed] [Google Scholar]
- Jia L. H., Hao F., Wang W. H., Qu Y. Cell Biochem. Funct. 2015;33:314–319. doi: 10.1002/cbf.3116. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Toyoda H., Tanahashi T., Tanaka J., Kumada T., Yoshioka Y., Kosaka N., Ochiya T., Taguchi Y. PLoS One. 2012;7:48366. doi: 10.1371/journal.pone.0048366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi F., Shao N. Y., Li B. W., Xue L., Deng D. N., Xu Y., Lan Q., Peng Y., Yang Y. L. Sci. Rep. 2016;6:32067. doi: 10.1038/srep32067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amr K. S., Ezzat W. M., Elhosary Y. A., Hegazy A. E., Fahim H. H., Kamel R. R. Gene. 2016;575:66–70. doi: 10.1016/j.gene.2015.08.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.