 A novel hybrid able to protect organophosphate-inhibited AChE as well as to reactivate it.
A novel hybrid able to protect organophosphate-inhibited AChE as well as to reactivate it.
Abstract
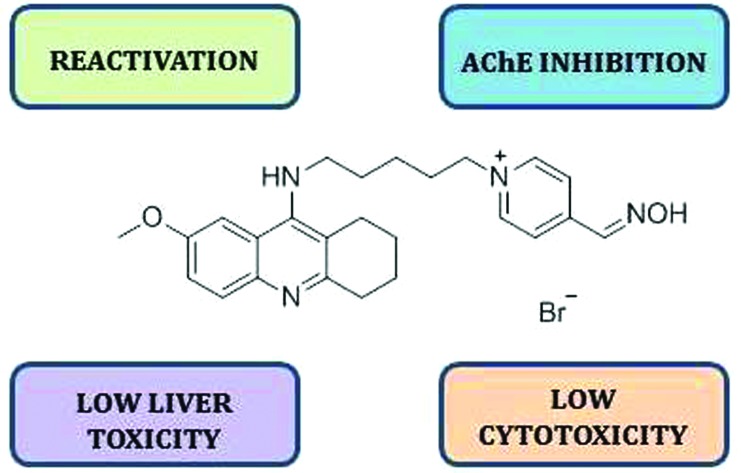
Chemical warfare agents constitute an increasing threat to both military and civilian populations. Therefore, effective prophylactic approaches are urgently needed. Herein, we present a novel hybrid compound which is able not only to keep acetylcholinesterase resistant to organophosphate (OP) inhibitors, but also to serve as an enzyme reactivator in the case of OP intoxication.
Until the nineties of the 20th century, the awareness of the threat of organophosphate nerve agents had been mainly confined to the military sector, however their extension to the terrorist field together with current globalization of terrorist attacks suggests that these chemical warfare (CW) agents are becoming an increasing threat to the entire world.1,2 The main toxic mechanism of such compounds consists of covalent binding to the active site of acetylcholinesterase (AChE, E.C. 3.1.1.7).3 Current post-exposure therapy involves respiratory support and combined administration of an anticholinergic drug, atropine, AChE reactivator and anticonvulsant.1,3 For the situations when OP poisoning is expected, there are also several prophylactic countermeasures which are based on the following principles: (1) use of reversible cholinesterase inhibitors; (2) administration of enzymes diminishing the concentration of OP in the bloodstream and; (3) application of standard post-exposure antidotes.4 The latter approach can be considered as “treatment in advance”.1
Our research group has an almost 20-year long scientific history in design, synthesis and biological evaluation of modulators of cholinesterases. The former efforts have been dedicated to searching for novel reactivators with improved pharmacological properties. These endeavours bore fruit in the form of oximes K027 and K203.5,6 More recently, we have started to implement our knowledge about cholinesterases into a new field, Alzheimer's disease. The aim of such research is to develop multipotent compounds bearing a reversible AChE inhibitor – 7-methoxytacrine (7-MEOTA) – as a core scaffold.7–9 Tacrine (9-amino-1,2,3,4-tetrahydroacridine) was launched in 1993 as the first drug for the symptomatic treatment of Alzheimer's disease (AD). Although it proved its efficacy in delaying the symptoms of AD, it was also confirmed that tacrine may cause serious adverse effects consisting mainly of hepatotoxicity and dose-dependent peripheral cholinergic effects.7 7-Methoxytacrine, originally introduced to the Armed Forces of the Czech Republic as an antidote against the incapacitating agent 3-quinuclidinyl benzilate (QNB, BZ compound), does not exert the aforementioned side effects presumably due to a different metabolic fate of the compound.10 Moreover, 7-MEOTA was also tested as a prophylactic agent against OP poisoning.11 For design of the hybrid 5 (Scheme 1) we have utilized the best of our knowledge in both fields of interest and synthesized a novel prophylactic agent with multiple modes of action in addition to reversible inhibition of AChE.
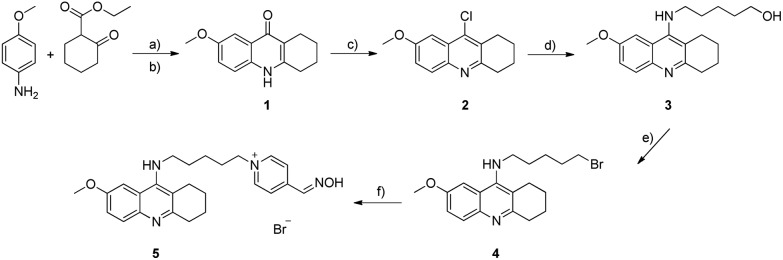
Scheme 1. Synthesis of hybrid 5. Conditions and reagents: (a) p-TSA, toluene, 120 °C, 8 h; (b) diphenylether, 220 °C, 2 h, after two steps 98%; (c) POCl3, 110 °C, 2 h, 100%; (d) NH2(CH2)5OH, pentan-1-ol, 150 °C, 24 h, 59%; (e) CBr4, PPh3, CH2Cl2, 0 °C – rt, 24 h, 41%; (f) 4-pyridinealdoxime, NaI, EtOH, 80 °C, 48 h, UHPLC, 14%.
In rational design of the hybrid 5 we have combined common structural features of commercially available reactivators (pralidoxime, obidoxime, trimedoxime, methoxime and asoxime) with the requirements for effective dual-binding site inhibitors of AChE (donepezil, bis(7)-tacrine) in a single molecule.7,8,12,13 Coupling of a reversible inhibitor with a reactivator by an appropriate linker seems to be crucial not only for a multipotent therapeutic effect but also for the enhancement of inhibitory and reactivation capabilities of the hybrid compound. Classical aldoxime reactivators share several common characteristics, i.e. a permanent positive charge on the pyridinium ring and the oxime group at position C2 or C4.3 Based on these precedents we have selected 4-pyridinealdoxime (4-PA) as a building block more frequently occurring in conventional reactivators. Additionally, in order to maximize the inhibitory and reactivation potencies of the hybrid compound 5in silico optimization of the spacer-length was carried out, suggesting that a 5-carbon linker is the optimum. The presumed mechanism of action of such a hybrid drug would primarily consist of reversible inhibition of AChE, due to the occupation of the catalytic active site (CAS) by the 7-MEOTA moiety, making the enzyme intact for OP inhibitors. Secondly, in case intoxication occurs it would serve as an immediate causal antidote to restore the function of phosphylated AChE via the 4-pyridiniumaldoxime fragment.
To validate the proof of concept molecular docking simulation on AChE and VX-inhibited AChE was performed prior to synthesis and in vitro evaluation. The binding mode of 5 into the human AChE (hAChE) active site was explored taking advantage of the X-ray crystallographic structure of hAChE complexed with donepezil (PDB ID: ; 4EY7).14 The binding pattern clearly showed that ligand 5 (–13.1 kcal mol–1) spanned the cavity gorge of the native enzyme occupying the peripheral anionic site (PAS), mid-gorge region and CAS (Fig. 1, see the ESI†). Such ligand accommodation is in agreement with the kinetic analysis of hAChE inhibition described below. In line with our previous observation15, the 7-MEOTA moiety was sandwiched between Trp86 and Tyr337 by π–π and/or cation–π interactions in the CAS of the enzyme. The binding of this moiety was also assisted by distorted T-shaped aryl–aryl as well as hydrogen bond interactions with His447, an important amino acid residue of the catalytic triad. At the cavity entrance, the 4-pyridiniumaldoxime scaffold was engaged in parallel π–π/cation–π interactions between Tyr124 and Trp286 being stacked between them. Additionally, the aliphatic linker exhibited the formation of weak hydrophobic interactions with several aromatic residues (e.g. Tyr341, Phe297 and Phe338). Based on the aforementioned results, it can be concluded that ligand 5 acts as a dual-binding site inhibitor of hAChE.
The crystallographic structure of non-aged VX-inhibited mouse AChE (mAChE; PDB ID: ; 2Y2U) was used to explore the plausible reactivation process induced by the ligand 5 (Fig. 2, see the ESI†). The source of the enzyme has been appropriately selected based upon high sequence identity (100% for the active site residues and 88% in total) as well as structural 3D similarity (RMSD(Cα) = 0.63 Å for 529 residues matched in an alignment) to hAChE.16 As expected, the 7-MEOTA moiety protruded out of the enzyme gorge forming disordered π–π interactions with Phe338, Tyr337 and Phe297 with an overall estimated binding energy of –11.3 kcal mol–1. Importantly, a flexible aliphatic linker enabled ligand packing in the enzyme cavity. In this context, Tyr337 seems to be a “dual-binder”, since it provides favourable hydrophobic interactions with both scaffolds of the target hybrid 5. The most remarkable finding concerning the electrostatic interactions formed between the oximate and VX-Ser203 at the distance of 4.0 Å is that the oximate is capable of nucleophilic attack to cleave the P–O bond thus restoring the enzyme activity.
The synthesis of the novel compound of interest 5 followed the strategy depicted in Scheme 1. The first step consisted of cyclocondensation of p-anisidine with ethyl 2-oxocyclohexanecarboxylate to give compound 1 in excellent yield. Subsequent treatment and reflux of 1 with phosphoryl chloride afforded intermediate 2 in quantitative yield. Incorporation of an α,ω-aminohydroxyalkane chain into 2 by nucleophilic substitution with 5-amino-1-pentanol provided compound 3 in 59% yield. Further, the conversion of the terminal alcohol to the corresponding halide 4 was carried out by the Appel reaction. Finally, the last step involved alkylation of 4-PA by 4 to obtain the desirable product 5. As the product contained several contaminants, it was obvious that classical column chromatography was not efficient enough to eliminate all the impurities. Therefore, it turned out to be necessary to purify 5 additionally by preparative ultra-high-performance liquid chromatography (UHPLC), which is described in the ESI.†
The ability of the newly prepared compound 5 to reactivate human red blood cell AChE (RBC-AChE) inhibited by tabun (GA), sarin (GB), VX and paraoxon (POX) was evaluated by the modified protocol formerly described by Ellman et al.17 Pralidoxime and obidoxime were used as the reference compounds. 4-PA, the fragment responsible for an assumed reactivation potency of 5, was tested as well. The measurements were performed at two concentrations of 5 (100 μM and 10 μM). The reactivation results are listed in Table 1. Reactivation of tabun-inhibited AChE by commercial reactivators was formerly found to be difficult.1 Pralidoxime (1%) and 5 (3%) proved to be weak reactivators of GA-inhibited AChE at concentration 100 μM, whereas obidoxime (22%) demonstrated relatively satisfactory reactivation ability at the same concentration. These results are in strong agreement with the general structural requirements for GA-reactivators, i.e. the hydroxyiminomethyl group in position C4 of the pyridinium ring and the presence of two heteroaromatic rings with quaternary nitrogen atoms linked by a spacer of three or four carbons.1 Although monoquaternary compounds such as pralidoxime (52%) exhibit only moderate reactivation potency compared to obidoxime (80%) to split the GB–AChE complex, they are still used worldwide against sarin threats as a part of antidotal or prophylactic therapy.4,18 Pralidoxime was almost twice as potent as the novel hybrid 5 (25%) at 100 μM concentration. The situation dramatically changed at a concentration relevant for in vivo use, i.e. 10 μM,19 when 5 (15%) turned out to possess a better reactivation profile than pralidoxime (10%), however, not surpassing obidoxime (42%) at the same concentration. The most potent reactivator of VX-inhibited AChE among the tested compounds seemed to be pralidoxime (43%) at concentration 100 μM. Nevertheless, at a therapeutically more relevant concentration (10 μM), the novel hybrid 5 (10%) surpassed all the tested compounds for VX-inhibited AChE. The reactivation in vitro should exceed 10% to warrant further in vivo investigations.20 With regard to POX-induced AChE inhibition, not all the tested compounds were able to fulfil this criterion. At 10 μM concentration 5 (15%) was three times as strong as pralidoxime (5%), exceeding the limit of 10% only slightly. Regardless of this, obidoxime (43%) proved to be the most potent POX-reactivator at the same concentration. Pursuant to the above mentioned results it is evident that the superior in vitro reactivation potency of 5 was achieved against VX, and the inferior against tabun. Additionally, the uncharged building block – 4-PA – exerted only minimal reactivation potency in the case of GB and POX, whilst it was unable to reactivate VX- and GA-inhibited AChE at all.
Table 1. Reactivation potencies of the tested compounds.
| Compound | Reactivation ± SD
a
(%) |
|||||||
| Tabun |
Sarin |
VX |
Paraoxon |
|||||
| 100 μM | 10 μM | 100 μM | 10 μM | 100 μM | 10 μM | 100 μM | 10 μM | |
| 5 | 2.7 ± 0.9 | 0 | 24.7 ± 1.2 | 14.6 ± 0.5 | 27.7 ± 0.6 | 10.0 ± 0.6 | 16.5 ± 0.2 | 14.6 ± 0.2 |
| Pralidoxime | 1.1 ± 0.7 | 0 | 52.2 ± 0.5 | 10.4 ± 2.7 | 43.3 ± 3.0 | 8.0 ± 1.0 | 30.4 ± 1.3 | 5.2 ± 0.5 |
| Obidoxime | 21.7 ± 0.8 | 3.4 ± 0.4 | 80.0 ± 0.3 | 41.7± 0.8 | 16.7 ± 0.1 | 2.9 ± 0.5 | 84.9 ± 0.4 | 42.6 ± 0.8 |
| 4-PA | 0 | 0 | 2.2 ± 0.3 | 1.7 ± 0.0 | 0.3 ± 0.2 | 0.2 ± 0.2 | 2.4 ± 0.3 | 1.7 ± 0.5 |
aResults are expressed as the mean of at least three independent experiments; time of inhibition – 1 h; time of reactivation – 10 min; pH 7.4; temperature – 25 °C.
One of the most relevant properties of promising prophylactic agents in OP intoxications is the capacity to reversibly inhibit cholinesterase activity. For this purpose, affinities of the compounds of interest to RBC-AChE were determined. As reported in Table 2, conventional reactivators (pralidoxime and obidoxime) hardly inhibited RBC-AChE, which is reflected by high IC50 values. On the other hand, 5 proved to be an even stronger inhibitor than its parent compound, 7-MEOTA. Such inhibitory activity may also serve as an explanation of why the novel hybrid 5 exerted better reactivation capability at the lower concentration (10 μM) than at the higher concentration (100 μM).20 A lower IC50 value indicates increased affinity of the compound for the target enzyme, therefore, a chance of 5 to protect RBC-AChE, by preventing its phosphylation by OP, is higher compared to 7-MEOTA.
Table 2. Synopsis of in vitro biological data and calculated octanol/water partition coefficients (log P).
| Compound | IC50 RBC-AChE ± SEM a (μM) | log P | IC50 CHO-K1 ± SEM a (mM) | IC50 HepG2 ± SEM a (mM) |
| 5 | 2.47 ± 0.13 | –1.65 | 5.0 ± 0.7 | 1.21 ± 0.08 |
| Pralidoxime | 217 ± 17 | –3.26 | 32.0 ± 3.4 | 23.59 ± 0.52 |
| Obidoxime | 197 ± 8 | –6.93 | 12.0 ± 1.6 | 4.02 ± 0.28 |
| 7-MEOTA | 10.0 ± 1.0 | 0.66 | 0.063 ± 0.004 | 0.0115 ± 0.0003 |
| 4-PA | >100 | –0.51 | 15.8 ± 1.6 | 13.19 ± 1.47 |
aResults are expressed as the mean of at least two independent experiments.
To gain insight into the interactions of 5 with RBC-AChE, a kinetic study was conducted. The graphical analysis of Lineweaver–Burk reciprocal plots (Fig. 3, see the ESI†) showed intersection of lines just above the x-axis. Km increased very slightly, whereas Vmax decreased with the increasing concentration of 5. Such a pattern indicates mixed type inhibition. Intersection of the lines just above the x-axis points out the prevailing interactions with the allosteric site of the enzyme, PAS. Such interaction causes conformational changes of the enzyme as a whole as well as of its active site. This is highly desirable from the prophylactic and from the consecutive therapeutic point of view, too. In the case of prophylaxis, 5 would change the conformation of AChE thereby complicating the binding of OP to the enzyme. On the other hand, from the perspective of OP intoxication treatment, the 7-MEOTA moiety would serve as the PAS ligand orientating the functional oxime group to a closer proximity to the CAS thus facilitating the process of reactivation. Replots of the slope versus the concentration of 5 gave an estimate of the competitive inhibition constants, Ki = 2.35 ± 0.54 μM and Ki′ = 3.73 ± 0.76 μM, which are consistent with the IC50(RBC-AChE) value of the novel hybrid mentioned above.
It is generally accepted that reactivators with a permanent positive charge on the heteroaromatic ring cannot diffuse freely across the BBB and therefore their access to the CNS is severely restricted.1 Due to their hydrophilic structure such compounds exhibit large negative octanol/water partition coefficients (log P). To elicit whether the novel hybrid 5 possesses better physicochemical properties compared to conventional reactivators (pralidoxime and obidoxime) as well as to its building blocks (7-MEOTA and 4-PA), its lipophilicity value was estimated using Marvin 14.9.8.0, 2014, ChemAxon (; http://www.chemaxon.com). The data are summarized in Table 2. Pursuant to the obtained results it is obvious that insertion of the PAS ligand (7-MEOTA) into the target molecule was not sufficient enough to increase the log P up to the positive values. However, these calculations are merely tentative. Sakurada et al.21 disproved an assumption that pyridinium aldoximes cannot pass through the BBB using the in vivo rat brain microdialysis technique and HPLC. He found out that pralidoxime, demonstrating the log P value of –3.26, penetrates into the CNS in an amount of 10% of the plasma level. This phenomenon was explained by a participation of one of the amino acid/ion transporters. Accordingly, neither the use of an artificial membrane nor the calculation of the log P can be a substitute for a properly performed in vivo experiment that will reveal the real degree of permeation across the BBB.
Evaluation of cell viability was performed in order to establish the potential cytotoxic effect of the novel prophylactic agent 5, its parent compounds (7-MEOTA and 4-PA) as well as reference compounds (pralidoxime and obidoxime) on Chinese hamster ovary cells (CHO-K1, ECACC, Salisbury, UK). The results, expressed as IC50 values, are listed in Table 2. In conformity with the obtained data the cytotoxic impact of the novel hybrid 5 was approximately similar to that observed with reference compounds, ranging in millimolar concentration. While the effect of 7-MEOTA was completely different, exerting the IC50 value in micromolar concentration. Such observation may be elucidated by an increased lipophilic character of 7-MEOTA. Altogether, the findings indicate an acceptable cytotoxic profile of 5 on CHO-K1 cells, since the concentration attainable in vivo is one or two orders of magnitude lower than that inducing the decline in viability of the cells.19
As reported previously, long-term administration of tacrine to patients with AD apart from the significant therapeutic effect also caused hepatotoxicity, which is likely associated with the ability of tacrine to induce lipid peroxidation in hepatocytes. 7-MEOTA did not exert any sign of hepatotoxicity even in a chronic toxicity experiment. Conversely, it demonstrated the capability to reduce lipid peroxidation, ascribing it a huge advantage from the clinical point of view.10 Such a difference in induction of the hepatotoxic effect is mainly explained by a tiny distinction in their metabolic transformation process.10 For this reason, incorporation of the tacrine-like moiety in the target molecule usually requires an assessment of hepatotoxicity to get a deeper look into this issue. The results of the MTT assay on the human hepatoma cell line (HepG2, ATCC, Virginia, USA) expressed as IC50 values are summarized in Table 2. A very similar pattern to that in the general cytotoxicity assay was also observed in the in vitro hepatotoxicity test. The toxicity of compounds was analogous, i.e. pralidoxime > 4-PA > obidoxime > 5 > 7-MEOTA, where pralidoxime represents the less toxic agent. It is evident that if in the case of 7-MEOTA it does not exert any sign of hepatotoxicity in vivo, the same effect could also be anticipated in the novel prophylactic agent 5, exceeding the IC50 value of 7-MEOTA 100-times.
In summary, we described a novel promising prophylactic approach exploitable in the case of OP intoxication. Encouraging in vitro results should, however, be approved by in vivo tests prior to the determination of its real therapeutic value.
This work was supported by the Czech Science Foundation (no. GA15-16701S), MH CZ-DRO (University Hospital Hradec Kralove, no. 00179906) and the grant of Ministry of Defense (Long Term Organization Development Plan – 1011).
The experiments were performed in compliance with the Czech legislation and institutional guidelines of the Faculty of Military Health Sciences, University of Defence and University Hospital Hradec Kralove. The institutional ethical committees of the Faculty of Military Health Sciences, University of Defence and University Hospital Hradec Kralove approved the experiments. Informed consent was obtained from all human subjects from whom the blood samples were drawn.
Supplementary Material
Footnotes
†Electronic supplementary information (ESI) available: Experimental procedures and characterization of all compounds. See DOI: 10.1039/c6tx00130k
References
- Kuca K., Jun D., Musilek K., Pohanka M., Karasova J. Z., Soukup O. Mini–Rev. Med. Chem. 2013;13:2102–2115. doi: 10.2174/13895575113136660108. [DOI] [PubMed] [Google Scholar]
- Jang Y. J., Kim K., Tsay O. G., Atwood D. A., Churchill D. G. Chem. Rev. 2015;115:PR1–P76. doi: 10.1021/acs.chemrev.5b00402. [DOI] [PubMed] [Google Scholar]
- Kuca K., Juna D., Musilek K. Mini–Rev. Med. Chem. 2006;6:269–277. doi: 10.2174/138955706776073510. [DOI] [PubMed] [Google Scholar]
- Bajgar J., Fusek J., Kassa J., Kuca K., Jun D. Curr. Med. Chem. 2009;16:2977–2986. doi: 10.2174/092986709788803088. [DOI] [PubMed] [Google Scholar]
- Kuca K., Musilek K., Jun D., Pohanka M., Ghosh K. K., Hrabinova M. J. Enzyme Inhib. Med. Chem. 2010;25:509–512. doi: 10.3109/14756360903357569. [DOI] [PubMed] [Google Scholar]
- Musilek K., Jun D., Cabal J., Kassa J., Gunn-Moore F., Kuca K. J. Med. Chem. 2007;50:5514–5518. doi: 10.1021/jm070653r. [DOI] [PubMed] [Google Scholar]
- Nepovimova E., Korabecny J., Dolezal R., Babkova K., Ondrejicek A., Jun D., Sepsova V., Horova A., Hrabinova M., Soukup O., Bukum N., Jost P., Muckova L., Kassa J., Malinak D., Andrs M., Kuca K. J. Med. Chem. 2015;58:8985–9003. doi: 10.1021/acs.jmedchem.5b01325. [DOI] [PubMed] [Google Scholar]
- Korabecny J., Dolezal R., Cabelova P., Horova A., Hruba E., Ricny J., Sedlacek L., Nepovimova E., Spilovska K., Andrs M., Musilek K., Opletalova V., Sepsova V., Ripova D., Kuca K. Eur. J. Med. Chem. 2014;82:426–438. doi: 10.1016/j.ejmech.2014.05.066. [DOI] [PubMed] [Google Scholar]
- Korabecny J., Andrs M., Nepovimova E., Dolezal R., Babkova K., Horova A., Malinak D., Mezeiova E., Gorecki L., Sepsova V., Hrabinova M., Soukup O., Jun D., Kuca K. Molecules. 2015;20:22084–22101. doi: 10.3390/molecules201219836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup O., Jun D., Zdarova-Karasova J., Patocka J., Musilek K., Korabecny J., Krusek J., Kaniakova M., Sepsova V., Mandikova J., Trejtnar F., Pohanka M., Drtinova L., Pavlik M., Tobin G., Kuca K. Curr. Alzheimer Res. 2013;10:893–906. doi: 10.2174/1567205011310080011. [DOI] [PubMed] [Google Scholar]
- Bajgar J., Fusek J., Patocka J., Hrdina V. Arch. Toxicol. 1983;54:163–166. doi: 10.1007/BF01261385. [DOI] [PubMed] [Google Scholar]
- Worek F., Thiermann H. Pharmacol. Ther. 2013;139:249–259. doi: 10.1016/j.pharmthera.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Korabecny J., Soukup O., Dolezal R., Spilovska K., Nepovimova E., Andrs M., Nguyen T. D., Jun D., Musilek K., Kucerova-Chlupacova M., Kuca K. Mini–Rev. Med. Chem. 2014;14:215–221. doi: 10.2174/1389557514666140219103138. [DOI] [PubMed] [Google Scholar]
- Cheung J., Rudolph M. J., Burshteyn F., Cassidy M. S., Gary E. N., Love J., Franklin M. C., Height J. J. J. Med. Chem. 2012;55:10282–10286. doi: 10.1021/jm300871x. [DOI] [PubMed] [Google Scholar]
- Spilovska K., Korabecny J., Horova A., Musilek K., Nepovimova E., Drtinova L., Gazova Z., Siposova K., Dolezal R., Jun D., Kuca K. Med. Chem. Res. 2015;24:2645–2655. [Google Scholar]
- Wiesner J., Kriz Z., Kuca K., Jun D., Koca J. J. Enzyme Inhib. Med. Chem. 2007;22:417–424. doi: 10.1080/14756360701421294. [DOI] [PubMed] [Google Scholar]
- Ellman G. L., Courtney K. D., Andres V., Feather-Stone R. M. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Kuca K., Hrabinova M., Soukup O., Tobin G., Karasova J., Pohanka M. Bratisl. Lek. Listy. 2010;111:502–504. [PubMed] [Google Scholar]
- Thiermann H., Eyer F., Felgenhauer N., Pfab R., Zilker T., Eyer P., Worek F. Toxicol. Lett. 2010;197:236–242. doi: 10.1016/j.toxlet.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Musilek K., Komloova M., Holas O., Horova A., Pohanka M., Gunn-Moore F., Dohnal V., Dolezal M., Kuca K. Bioorg. Med. Chem. 2011;19:754–762. doi: 10.1016/j.bmc.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Sakurada K., Matsubara K., Shimizu K., Shiono H., Seto Y., Tsuge K., Yoshino M., Sakai I., Mukoyama H., Takatori T. Neurochem. Res. 2003;28:1401–1407. doi: 10.1023/a:1024960819430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.