Abstract
Background
Bitter tasting drugs represent a large portion of active pharmaceutical ingredients. Mini-tablets are specifically designed for patients with difficulty in swallowing particular in young children up to 10 years of age, geriatric patients and patients with esophagitis.
Objective
The present study was aimed to prepare, taste-masked mini-tablets, which are easily swallowed dosage forms, primarily to be used by pediatric and geriatric patients.
Methods
Ketoprofen (10%–50% w/w) and Eudragit® EPO were blended and extruded with a 5-mm strand die and cut into consistent mini-tablets by using an adapted downstream pelletizer.
Results
Differential scanning calorimetry and polarized light microscopy-hot stage microscopy studies confirmed that the binary mixtures were miscible under the employed extrusion temperatures. In-vitro release studies showed that drug release was less than 0.5% within the first 2 min in simulated salivary fluid (pH 6.8) and more than 90% in the first 20 min in gastric media (pH 1.0). The results of the electronic tongue analysis were well correlated with the drug release profile of the mini-tablets in the artificial saliva. Scanning electron microscopy revealed no cracks on the surface of the mini-tablets, confirming that the mini-tablets were compact solids. Chemical imaging confirmed the uniform distribution of ketoprofen inside the polymer matrices.
Conclusion
Eudragit® EPO containing ketoprofen at various drug loads were successfully melt extruded into tasted-masked mini-tablets. The reduced drug release at salivary pH correlated well with Astree e-Tongue studies for taste masking efficiency.
Keywords: Eudragit® E PO, FTIR chemical imaging, Hot-melt extrusion, Ketoprofen, Mini-tablets, Taste masking
1. INTRODUCTION
The perceived taste of an oral dosage form is one of the primary factors influencing patient compliance. Among the different taste sensations, bitterness is the most repellent [1]. However, bitter tasting drugs represent a large portion of active pharmaceutical ingredients (APIs). Therefore, masking the taste of bitter APIs can achieve improved patient compliance, particularly in the case of pediatric patients, who are mostly unwilling to ingest bitter tasting drugs, and geriatric patients, who may exhibit altered taste perception [2].
To mask bitter tastes, the receptors of the taste buds must be sequestered from he bitter APIs, which can be achieved by several different approaches. Bitter tastes can be masked by applying a polymeric coating layer to create a physical barrier around the drug [3, 4] or by the use of complexing agents to form inclusion complexes or resonates [5]. Recently, taste suppressant molecules have been used to block the gap junction channels and hemichannels in taste bud cells to suppress taste [6, 7]. However, there is a need for an approach to taste masking that offers robustness and cost-effectiveness while being simple to scale up. Hot-melt extrusion (HME) satisfies these criteria and can be used to efficiently produce taste-masked dosage forms [8].
HME is a continuous and solvent-free process for the manufacture of solid dispersions of APIs for various applications [9, 10]. The HME technique can be used to enhance the solubility of drugs with poor water solubility, as well as to manufacture modified/extended release and targeted drug delivery systems. Gue et al. (2013) have used HME to enhance apparent aqueous solubility of ketoprofen (KPR) by producing solid dispersion with Eudragit® E PO polymer. They found that the drug release is slow in demineralised water whereas fast at pH 1.0 [11]. In recent years, HME has gained interest as a method for masking the taste of bitter APIs by producing solid dispersions, which prevents direct contact of bitter drugs with the taste buds [12]. Using HME, extruded APIs can be molecularly dispersed in various polymer and/or lipid matrices [13, 14]. Several studies have demonstrated the use of HME as a viable tool to mask the bitter taste of the APIs. Morott et al. (2015) used HME to embed a bitter taste drug sildenafil citrate in ethyl cellulose along with calcium carbonate as a pH-dependent pore former. This study also found that taste-masking efficiency is affected by using different screw configurations which change the physical state of the API [15]. Pimparade et al. (2015) have also demonstrated HME as an effective tool to mask the bitter taste of caffeine citrate. The drug was embedded in ethyl cellulose matrix along with different pore formers such as calcium phosphate and magnesium oxide to enhance drug release. The crystallinity of the API was preserved by modifying the screw configuration [16]. Alshehri et al. (2015) and Singh et al. (2013) have also used a pH-dependent polymer Eudragit® E PO to mask the bitter taste of mefenamic acid and efavirenz, respectively [17, 18]. Maniruzzaman et al. (2015) have used Eudragit® L100 and Eudragit® L100-55 anionic polymers to mask the taste of the cationic drug propranolol HCL and evaluated the taste using two different electronic tongues (Astree e-tongue and TS5000Z) along with the in-vivo taste evaluation study on healthy human volunteers. They found that both E-tongues studies were able to detect the taste and were in good agreement with the in-vivo study [19]. A wide range of drug dosage forms have been produced using HME [20–22]. For example, HME has been used to prepare matrix mini-tablets for ibuprofen using ethyl cellulose as a sustained release agent. To prepare the ibuprofen mini-tablets, the researchers used a twin-screw extruder to prepare extrudates, which were manually cut into 2-mm mini-matrices [23].
Mini-tablets are a compact dosage form with a diameter of 2–5 mm or smaller [24]. The conventional method of preparing mini-tablets involves an ordinary reciprocating rotary tableting machine [25]. The mini-tablet dosage form offers several advantages over conventional solid dosage forms such as tablet or capsules. Generally, mini-tablets are specifically designed for patients with difficulty in swallowing, in particular young children (up to 10 years), geriatric patients, and patients with esophagitis. Mini-tablets are particularly useful in pediatric patients under 10 years who may not have the skills to swallow conventional dosage forms [26]. In a previous study by Thomson et al. (2009), it was shown that children aged between 2 and 6 years had high acceptance towards mini-tablets [24]. Additionally, mini-tablets serve as a multi-particulate drug delivery system, allowing fine-tuning of individual administration, reducing inter- and intra-subject variability, and dose dumping while maintaining accurate dosing [23, 27].
Content uniformity of mini-tablets is critical for accurate dosing. Fourier transform infrared (FTIR) imaging is a well-established biomedical spectroscopy technique for visually estimating content uniformity. FTIR imaging provides information regarding drug distribution, as well as concentrations of chemical components, according to the unique spectroscopic signature of each component. Analysis of FTIR images is based on selection of the unique spectroscopic wavelength(s) from which the image was obtained.
Ketoprofen (KPR) and Eudragit® E PO were selected as materials for the manufacture of mini-tablets in this study. KPR is a biopharmaceutical classification system (BCS) class II drug for which dissolution is the rate-limiting step for oral bioavailability [28]. It is a bitter tasting, thermally stable drug that is practically insoluble in water at 20 °C and has low solubility in pH 1.2 buffer (0.06 μg/mL) [29]. Furthermore, it is an anionic drug, which could facilitate drug–polymer intermolecular interactions with cationic-based polymers such as Eudragit® E PO [11]. Eudragit® E PO is a dimethylaminoethyl methacrylate-, butyl methacrylate-, and methyl methacrylate-based cationic copolymer that is thermally stable. It is insoluble and swellable in saliva (pH 6.8); however, it is soluble in gastric fluid at pH < 5.0 [30]. All of the above characteristics make KPR and Eudragit® E PO suitable choices as an API and taste-masking polymer, respectively, for this study.
The objective of the present study was to prepare novel taste-masked mini-tablets of KPR with the taste-masking carrier Eudragit® E PO by HME and to evaluate the effectiveness of the taste masking with multiple in vitro methods. In addition, FTIR imaging was used to elucidate the nature of the interactions between KPR and Eudragit® E PO.
2. MATERIALS AND METHODS
2.1. Materials
Ketoprofen USP was purchased from Letco Medical (Decatur, AL, USA). Eudragit® E PO was received as a gift sample from Evonik Degussa Corporation (Parsippany, NJ, USA). Calcium chloride dihydrate, magnesium chloride hexahydrate, sodium chloride, potassium carbonate, sodium phosphate dibasic heptahydrate, sodium phosphate monobasic monohydrate, and all other chemicals used in this study were of analytical grade and purchased from Fisher Scientific (Norcross, GA, USA).
2.2. Methods
2.2.1. Thermogravimetric Analysis (TGA)
The thermal stability of the starting materials (pure KPR, Pure Eudragit® E PO and physical mixtures which contains KPR ranged from 10–50% drug load) was studied using a PerkinElmer Pyris™ 1 TGA system. The Pyris™ Manager software (PerkinElmer Life and Analytical Sciences, Waltham, MA, USA) was used to operate the instrument and analyze the data. Under an inert nitrogen atmosphere at a flow rate of 20 mL/min, 3–4 mg of the sample was weighed and heated from 25 °C to 220 °C at 10 °C/min. Percent weight loss was plotted against temperature to determine the relationship between weight loss and temperature.
2.2.2. Differential Scanning Calorimetry (DSC)
A Perkin Elmer Diamond DSC (Perkin Elmer Life and Analytical Sciences, Waltham, MA, USA) was used to study polymer-drug miscibility and to assess the physical state of KPR inside the mini-tablets. Polymer-drug miscibility studies were performed on the physical binary mixtures of Eudragit® E PO and KPR. Under an inert nitrogen atmosphere at a flow rate of 20 mL/min, 3–4 mg of the sample was weighed in an aluminum pan and heated from 30°C to 150°C at a constant heating rate of 10°C/min. Endothermic onset and peak temperature of melting were calculated from the obtained thermogram using Pyris™ Manager software. The crystallinity of KPR inside the mini-tablets was evaluated similarly.
2.2.3. Hot Stage and Polarized Light Microscopy (HSM-PLM)
The miscibility of the binary mixtures and changes in the crystalline shape of KPR and Eudragit® E PO were evaluated using HSM-PLM. A glass slide with a small amount of the sample was inserted into a hot-stage system (FTIR 600, Linkam Scientific Instruments, Surrey, UK). The sample was heated from 35 °C to 150 °C at a constant rate of 10 °C/min. A camera-mounted optical microscope (Cary 620 IR, Agilent Technologies, Santa Clara, CA, USA) equipped with a hot-stage and a crossed polarizer was used to capture images at different stages of the transformation.
2.2.4. HME Processing
KPR (10%, 20%, 30%, 40%, and 50%) was blended with Eudragit® E PO using a V-shell blender (Maxiblend®, GlobePharma, New Brunswick, NJ, USA) at 25 rpm for 10 min and analyzed for drug content and blend uniformity. The drug-polymer blends were melt-extruded using a co-rotating twin-screw extruder (Process 11 mm, ThermoScientific, Waltham, MA, USA) at processing temperatures of 100 to 120 °C, a screw speed of 100 rpm, and a feeding rate of 0.7 kg/h (Table 1). The die plate was attached to a circular nozzle insert (length: 15 mm; diameter: 5 mm) at a temperature range of 70 to 90 °C. Increasing the nozzle length and decreasing the die temperature prevents the die swell phenomena associated with polymer melt and thus maintains the strand shape [31, 32]. A conveyor belt was used to transfer the strands toward the cutting apparatus. The cutting length was set at 2 mm from the pelletizer. The resulting strands were cut into mini-tablets using an adapted pelletizer (Type L-001-9482, ThermoScientific, Stone, UK). HPLC analysis was used to evaluate post-extrusion drug content.
Table 1.
Ketoprofen formulations and hot-melt extrusion processing parameters.
| Formulations | Ketoprofen % (w/w) | Eudragit® E PO % (w/w) | Zone temp. (°C) | Die temp. (°C) | Screw speed (rpm) | Feeding rate (kg/h) |
|---|---|---|---|---|---|---|
|
| ||||||
| KPR1 | 10 | 90 | 120 | 90 | 100 | 0.7 |
| KPR2 | 20 | 80 | 110 | 80 | ||
| KPR3 | 30 | 70 | 110 | 80 | ||
| KPR4 | 40 | 60 | 100 | 70 | ||
| KPR5 | 50 | 50 | 100 | 70 | ||
2.2.5. Powder X-Ray Diffraction (PXRD)
PXRD was performed using a Bruker D8 Advance system with a Cu-source theta-2theta diffractometer equipped with a Lynx-eye PSD detector. The generator was set at a voltage of 40 kV and a current of 30 mA. The samples were dispersed on a low-background Si sample holder and compacted gently with the back of a metal spatula. The scan ran from 10°–40° with 2θ values at 0.05° step size and 3 s/step.
2.2.6. HPLC Method
A Waters 600 binary pump, Waters 2489 UV/detector, and Waters 717 plus autosampler (Waters Technologies Corporation, Milford, MA, USA) were the components of the HPLC system. The stationary phase of the column was a Waters Symmetry shield C18 (250 × 4.6 mm, 5 μm particle size, reversed phase). The mobile phase was acetonitrile: 20 mmol phosphate buffer (pH 4.0) (55:45, v/v) at a flow rate of 1 mL/min [33]. KPR was analyzed at a wavelength of 256 nm and retention time was 5.2 min. Linearity was determined from 1 to 100 μg/ml and the regression coefficient obtained is ≥ 0.998. The powder of the physical mixtures was analyzed (n=5) by dissolving weighed samples in 20 mL of acetonitrile. The contents of the mini-tablets were analyzed by dissolving the tablets (n=10) in the mobile phase and pre-filtering the samples through a 0.45-μm membrane to extract the drug prior to HPLC injection. Similarly, the samples obtained from the dissolution studies were filtered and injected at a volume of 20 μL. The data were acquired and processed using the Waters Empower 3 software suite.
2.2.7. In Vitro Drug Release Studies
Taste-masking ability was assessed by measuring the in vitro release of KPR in 2 dissolution media to mimic in vivo salivary (pH 6.8) and gastric (pH 1.0) conditions. The taste-masking study was performed in 200 mL of simulated saliva (pH 6.8) with a USP Dissolution Apparatus I (Hanson SR8, Hanson Research, Chatsworth, CA, USA), maintained at (37±0.5) °C with a shaft rotation speed of 50 rpm (n = 3) and equipped with UV/Vis probes (Pion Rainbow® instrument, Pion Inc., Billerica, MA, USA). Samples were collected every 5 sec for 2 min. The composition of the simulated salivary media (pH 6.8) is CaCl2·2H2O (0.228 g/l), MgCl2·6H2O (0.061 g/l), NaCl (1.017 g/l), K2CO3·1.5H2O (0.603 g/l), Na2HPO4·7H2O (0.204 g/l) and NaH2PO4·H2O (0.273 g/l) [34].
For the in vitro release studies at gastric pH, a USP Dissolution Apparatus I was used with 900 mL of 0.1N HCl maintained at (37±0.5) °C with a shaft rotation speed of 50 rpm (n = 3). Samples were collected at 10, 20, 30, 45, 60, 90, and 120 min, filtered, and analyzed using a Waters HPLC-UV system. Fresh dissolution medium was added to the dissolution vessel to replace the volume of the sample withdrawn at each time point. The cumulative of drug release was plotted against time as a percentage.
2.2.8. Evaluation of Taste Using an Electronic Tongue
An Astree Liquid and Taste Analyzer with an electronic tongue (E-tongue) (Alfa MOS, Toulouse, France) was used to evaluate the taste of the formulations. The Astree E-tongue system was equipped with Alfa MOS sensor set #2 (pharmaceutical analysis), which was composed of 7 specific sensors (ZZ, AB, BA, BB, CA, DA, and JE) on a 48-position auto sampler (Alfa MOS, Toulouse, France). The sensors on the E-tongue imitate the taste buds on the human tongue by initiating changes in electrical potentials that can be compared to physiological action potentials to allow estimation of taste. Pure unformulated KPR and mini-tablets approximately equivalent to 75 mg of KPR were added to 50 mL of buffer solution (pH 6.8) and shaken at 50 rpm for 60 sec at 37 °C using a shaking bath (Thermo Fisher Scientific, Stone, UK). The solution with KPR and without (placebo buffer) was filtered with a 0.45-micron syringe filter (nylon membrane) into a 25-mL beaker (120 sec acquisition time, n = 5). The data generated using the E-tongue were analyzed using principle component analysis with the AlphaSoft V12.3 software suite (Mathworks Inc., Natick, MA, USA).
2.2.9. Scanning Electron Microscopy
Scanning electron microscopy (SEM) was used to study the surface morphology of the mini-tablets. Samples were mounted on adhesive carbon pads placed on aluminum stubs. A Hummer® 6.2 sputtering system was used to coat the samples in gold in a high vacuum evaporator (Anatech Ltd., Battle Creek, MI, USA). A scanning electron microscope operating at an accelerating voltage of 5 kV was used for imaging (JEOL JSM-5600, JEOL Ltd., Tokyo, Japan).
2.2.10. Chemical Imaging and FTIR Analysis
Mid-IR FTIR analysis was conducted in the spectral range of 4000–650 cm−1 using Cary 660 and Cary 620 FTIR Microscopes (Agilent Technologies, Santa Clara, CA, USA). The bench was equipped with a MIRacle ATR (Pike Technologies, Fitchburg, WI, USA), that was fitted with a single-bounce, diamond-coated ZnSe internal reflection element. Chemical imaging was conducted using Cary 620 FTIR Microscope (Agilent Technologies, Santa Clara, CA, USA) equipped with a 64 × 64 pixel focal plane array (FPA) with and without a germanium micro-ATR. FTIR samples were studied before and after physical blending and melt extrusion to study intermolecular interactions before and after applying high shear forces and elevated temperatures. The chemical imaging samples were embedded in melted paraffin (Paraplast X-TRA®, Sigma-Aldrich, St. Louis, MO, USA) and reduced to 5-μm thickness using a microtome (Olympus America Inc., Center Valley, PA, USA) to allow IR light to penetrate it and reflect out. Chemical imaging was utilized to visually approximate drug homogeneity and interactions.
2.2.11. Mini-tablet Properties
A Vanderkamp friabilator (Model 47-0100, VanKel Industries, Edison, NJ, USA) was used to study friability and determine weight loss. Twenty mini-tablets from each batch were uniformly tumbled for 4 min at 25 rpm. The tested tablets were gently tapped on ASTM #40 mesh and carefully collected. The weight loss was measured to determine the friability of the mini-tablets.
The hardness of the mini-tablets was measured using a VanKel hardness tester (Model VK 200, VanKel Industries, Edison, NJ, USA). Twenty mini-tablets from each batch were randomly chosen for hardness testing. Each tablet was placed in the center of the apparatus against the face plate of the sensing jaw. The force applied was continuously measured and recorded until initial fracturing of the mini-tablet occurred.
2.2.12. Stability Studies
To study the stability of the mini-tablets, samples were stored in open glass vials for a period of 3 months in ambient conditions (25 °C/60% relative humidity). After the 3-month stability study, the samples were tested for physical and chemical stability utilizing DSC, PXRD, and chemical assays. Chemical assays was performed by dissolving the stored tablets (n=5) in the mobile phase and pre-filtering the samples through a 0.45-μm membrane to extract the drug prior to HPLC injection. In addition, in vitro release studies were performed using the stored mini-tablets. The release profiles of the stored mini-tablets were compared with the fresh ones by using the similarity factor (f2 value).
3. RESULTS AND DISCUSSION
3.1. Thermal Analysis
TGA studies showed that the drug was thermally stable. When heated from 25 °C to 220 °C at a heating rate of 10 °C/min under an inert nitrogen atmosphere at a flow rate of 20 mL/min, KPR began to degrade at approximately 200 °C (data not shown). Eudragit® E PO showed no degradation in the thermal range, which was used. The polymer matrix shows no effect on thermal stability of the drug and the weight loss was observed at around around 200°C. Therefore, the temperatures in the extrusion process were kept well below the degradation temperature of KPR to maintain its stability in the binary mixtures.
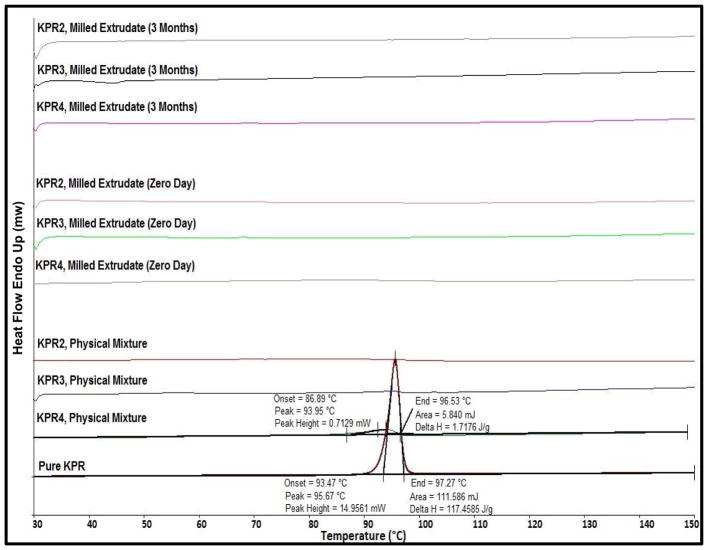
In the DSC studies, a pure KPR melting endothermic peak was observed at 97 °C that was not detected in the second round of heating, which confirmed the conversion of KPR into an amorphous form. KPR in physical mixtures was found to be miscible with Eudragit® E PO in all tested formulations (Fig. 1). During the heating step, the endothermic melting peak and onset temperature were lower than those of pure KPR in the physical mixtures containing 40% drug load [35]. The endothermic melting peak of KPR was comparatively lower to that of pure KPR. This indicates that the polymer matrix solubilised a portion of the KPR. However, the physical mixture containing 20% and 30% drug loading showed no crystalline peak and melting point value which indicates the complete miscibility in this binary mixtures. The melting peak of KPR was not identified in the final milled mini-tablets, confirming the amorphous state of the KPR inside the mini-tablets (Fig. 1).
Fig. (1).
Thermogram of pure ketoprofen (KPR), physical mixtures, and milled extrudates (0-day and 3-month stability samples) utilizing Eudragit® E PO matrices.
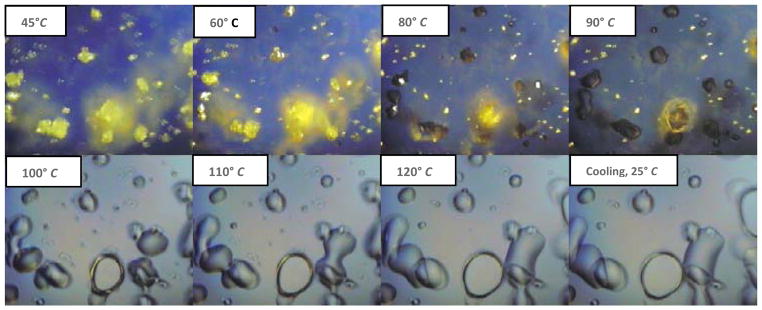
Polarized light can be used to detect the conversion of a crystalline form of an API into a glassy/amorphous form under different temperatures on a hot-stage system [36, 37]. This technique was utilized in this study to investigate the changes in the crystallinity of KPR under the temperatures applied in the extruder. All blended binary mixtures were studied using HSM. Based on the HSM results, the binary mixture containing 40% (w/w) KPR was chosen for further study due to the maximum birefringence obtained from its high drug loading capacity. At the beginning of the heating cycle, KPR crystals were clearly visible, whereas Eudragit® E PO particles were not visible, as they did not exhibit birefringence. At approximately 80 °C, the KPR crystals began solubilizing in the polymer matrix. However, the KPR crystals that were not in contact with Eudragit® E PO were not solubilized. The complete conversion of KPR to a glassy form was observed at 97 °C, at which temperature the drug formed round-shaped droplets. During cooling, the glassy/amorphous KPR stayed in a miscible state and did not recrystallize back into its original physical form. The binary mixtures were found to be miscible with each other at the temperatures employed during extrusion (Fig. 2). All of the KPR–Eudragit® E PO binary mixtures exhibited markedly similar results.
Fig. (2).
HSM-PLM images of the ketoprofen (KPR4) formulation. The polymer matrix began to solubilize the KPR crystals (yellow-golden color) at around 80 °C. Complete conversion of crystalline KPR into a molten form was observed at 97 °C. KPR in a glassy/amorphous form did not recrystallize during the cooling step. The field of view is 250 μm × 250 μm (15X).
3.2. HME Processing
Disconnection between the glass transition temperature (Tg) of Eudragit® E PO and its melt extrusion processing temperature has been reported in the literature. Sathagiri et al. (2012) extruded Eudragit® E PO at a high temperature of 120 °C, even after adding 50% drug load [38]. Wu et al. (2003) found that the torque of the extruded formulation was an important parameter to consider while setting the processing temperature [39]. Pure Eudragit® E PO was extruded first at 120°C to investigate its ability to form mini-tablets. The resulting mini-tablets from the polymer had sharp edges only. KPR, which melts at 97 °C, has been shown to provide a plasticizing effect in the melt extrudate [33]. Therefore, in the presence of KPR, extrusion could be carried out at a lower temperature and a higher screw speed. Measurements of drug loading demonstrated a significant effect of temperature on the extrudability of the formulations. HME conditions, including barrel temperature, screw speed, feeding rate, and die temperature were optimized to maximize the integrity of the resulting strand. The shape of the strand was rounded due to the shape of the nozzle used. After a short cooling phase on the conveyor belt, the strands entered the adapted cutting pelletizer. The speed of the conveyor belt was slightly adjusted manually for each formulation to yield 2 mm mini-tablets based on the extruder output. The resulting mini-tablets were 5 mm in diameter and 2 mm in thickness (Fig. 3). The addition of KPR smoothed the sharp edges of the mini-tablets. The edges of the mini-tablets with 10% KPR were smoother than the pure Eudragit® E PO tablets, but required further smoothing. The edges of the mini-tablets with 20%, 30%, and 40% KPR were relatively smooth in comparison with those of the mini-tablets with a 10% drug load. Drug loading in the range from 20% to 40% resulted in clear and smooth KPR extrudate strands, which suggested the formation of a single-phase system. On melting, KPR exhibits a tacky nature and cannot be extruded without a polymer matrix [33]. At 50% drug loading, KPR-Eudragit® E PO began to form elastic strands, which presented an obstacle for the pelletization process. In addition, due to the relatively high drug loading of the 50% KPR mini-tablets, the mini-tablets stuck together and exhibited surface deformation upon storage. Twenty mini-tablets from the various batches were selected randomly for study of weight variation. The average weights of the KPR2, KPR3, and KPR4 formulations were (63.02 mg ± 1.18 mg), (61.65 mg ± 1.07 mg), and (60.90 mg ± 1.11 mg), respectively. The drug content of the mini-tablets was found to be (100.35 % ± 2.58 %), (98.08 % ± 3.50%) and (95.56 % ± 1.7%) for KPR2, KPR3 and KPR4 formulations, respectively as well as the relative standard deviation was not more than 4%.
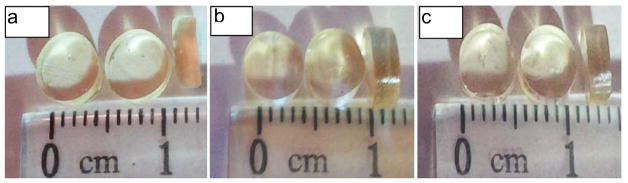
Fig. (3).
Images of the ketoprofen mini-tablets with a) 20%, b) 30%, and c) 40% drug loading.
3.3. PXRD
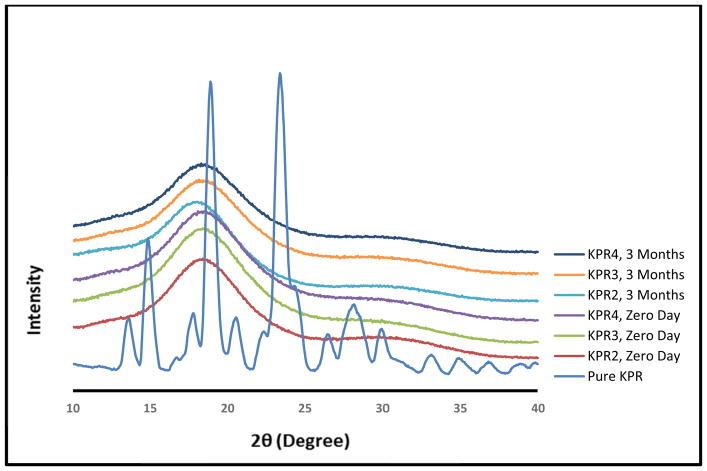
PXRD studies were performed to investigate the crystallinity and physical stability of KPR in the milled mini-tablets. The XRD data of KPR exhibited characteristic sharp peaks at 2θ = 14.2, 18.4, and 22.9° [33], which were not observed in the XRD data of the milled extrudates (Fig. 4), suggesting that KPR was converted into an amorphous form during extrusion processing, as the exrudates showed a halo pattern with no intense peaks. The conversion of KPR into an amorphous form was confirmed by DSC and XRD.
Fig. (4).
Powder X-ray diffraction (PXRD) data for pure ketoprofen (KPR) and milled extrudates (0-day and 3-month stability samples) utilizing Eudragit® E PO matrices.
3.4. In Vitro Drug Release Studies
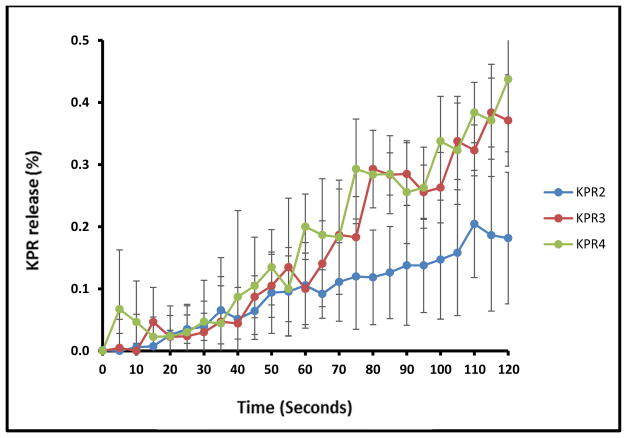
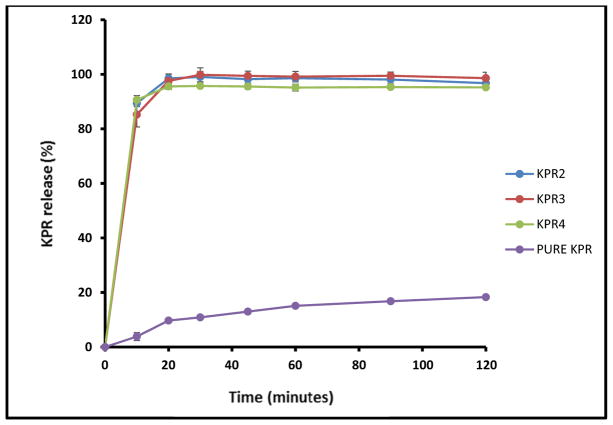
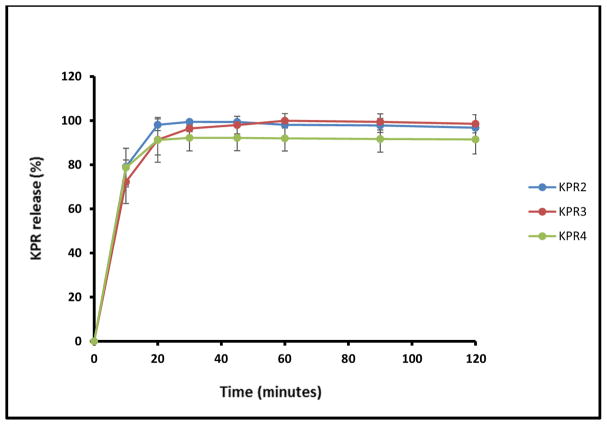
Taste masking was assessed by measuring the in vitro release of KPR in 2 dissolution media designed to mimic in vivo salivary (pH 6.8) and gastric (pH 1.0) conditions. In the simulated salivary medium (pH 6.8), drug release was found to be less than 0.5% within the first 2 min for all tested formulations (Fig. 5), which was attributed to the strong inhibition of drug release in the oral cavity by Eudragit® E PO. In contrast, formulations KPR2, KPR3, and KPR4 showed complete release of the drug within the first 20 min in the gastric fluid medium (pH 1.0), perhaps due to the rapid erosion of Eudragit® E PO at the relatively low pH of the medium. The super-saturated solutions were stable during the 2 h dissolution interval (Fig. 6). The concentration higher than the solubility of KPR in gastric medium (pH 1.0) was maintained by the salt formation and solubilization effect of Eudragit® E PO. All extruded formulations demonstrated significantly increased drug release in comparison with that of pure KPR due to electrostatic interactions and salt formation between the -COOH groups of KPR and the tertiary ammonium groups of Eudragit® E PO. The –COOH/ammonium group interaction can be formed during the extrusion process and/or during drug release in 0.1N HCl. Quinteros et al. (2008) and Kindermann et al. (2011) have previously demonstrated such molecular interactions between acidic drugs and basic Eudragit® E PO [40, 41].
Fig. (5).
In vitro release profiles of ketoprofen mini-tablets in simulated saliva (pH 6.8).
Fig. (6).
In vitro release profiles of ketoprofen (KPR) mini-tablets in 0.1N HCl.
The in vitro results in media mimicking artificial saliva and gastric fluid indicated that incorporation into the KPR mini-tablets masked the taste of KPR in the oral cavity and facilitated rapid drug release in the stomach, owing to the formation of a single-phase system, in which electrostatic interactions and the formation of salts by acidic KPR and basic Eudragit® E PO were of crucial importance.
3.5. Evaluation Using the E-tongue System
The taste-masking efficiency of the mini-tablets was evaluated by E-tongue analysis. The selected data set was analyzed by PCA using 2 principal components (PC). PC1 explained 99.6% of the variance in the data, whereas only 0.4% of the variance was explained by PC2. All formulations were located very close to the buffer (pH 6.8 phosphate buffer) on the PC1 axis (Table 2), indicating that the taste of each formulation, as measured by the E-tongue, was very similar to that of the buffer solution and not to that of the bitter drug. The great distance between pure KPR and the buffer on the PC1 plot clearly demonstrates the high degree of bitterness of KPR. The small distances between the mini-tablet formulations could be attributed to the differences in release rates during the first 60 sec, which correspond to the concentration differences. Indeed, these results were well correlated with the results for drug release from the mini-tablets in artificial saliva, where the drug release for all mini-tablet formulations was less than 0.5% in 120 sec (Fig. 5). The results for the KPR formulations, which were far from the drug PC axis and near the buffer PC axis, indicated that using Eudragit® E PO in the extruded formulation suppressed the bitter taste of KPR. This finding is important because it demonstrates that mini-tablets prepared by the HME process reduce exposure of the drug surface area to the taste buds.
Table 2.
E-Tongue distance of the mini-tablets from the buffer.
| Formulation | Reference samples | Distance | Pattern discrimination index (%) |
|---|---|---|---|
| Pure KPR | pH 6.8 buffer | 3361 | 98.56 |
| KPR2 | pH 6.8 buffer | 43 | 1.26 |
| KPR3 | pH 6.8 buffer | 173 | 17.82 |
| KPR4 | pH 6.8 buffer | 203 | 25.11 |
3.6. Chemical Imaging and FTIR Analysis
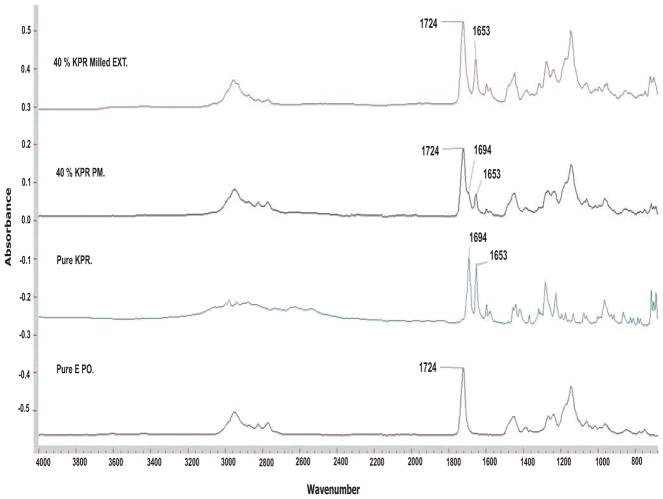
Intermolecular forces between oppositely charged compounds, such as drugs and polymers, result in masking of the bitter tastes of drugs [30]. To assess this effect, intermolecular interactions between KPR (an anionic drug) and Eudragit® E PO (a cationic polymer) were investigated by FTIR. The infrared spectrum of KPR showed 2 carbonyl peaks located at 1694 cm−1 and 1653 cm−1, which have been reported previously as a dimeric carboxylic acid and a ketonic carbonyl group, respectively [42, 43]. Extruded formulations containing 40% w/w KPR were studied for clarity and showed stronger absorption arising from a higher concentration of KPR, which correlated with a higher concentration of the carbonyls under investigation. The positions of the carbonyls corresponding to the ester group in the polymeric carrier and the ketone carbonyl in KPR, which were centered at 1724 cm−1 and 1653 cm−1, respectively, did not change from their pre-processing spectral positions [44]. However, the peak corresponding to the carboxylic acid carbonyl in KPR at 1694 cm−1 was not distinguishable in the extruded samples, while it was clearly visible in the physical mixtures. It is likely that the carboxylic acid group in KPR had a molecular interaction with the carrier, leading to disruption of the carboxylic acid dimer in the crystalline KPR, which results in stretching of the carboxylic acid bond and subsequently results in a somewhat distorted spectral appearance at higher wavenumbers. The appearance of the carboxylic acid overlapped with, and thus was indistinguishable from, the strong ester vibrations associated with the carrier (Fig. 7). The KPR carbonyl peak located at 1653 cm−1 was clearly visible and was chosen for chemical imaging. Fig. (8) shows an infrared image of KPR in the mini-tablets taken at 5.5 μm spatial resolution in transmission mode with a total field of view (FOV) of 300 × 300 μm. The intensity of the chosen peak significantly affects the color represented in the images. The light blue area represents low peak intensity, which correlates to a lower concentration of API relative to areas of higher peak intensity, which are represented by orange to red coloration. As the concentration of KPR inside the matrices increases, the intensity of the carbonyl peak at 1653 cm−1 becomes higher and the colors in the images become intensified. Homogeneous distribution of the drug in the mini-tablets was observed at different levels of drug loading. The chemical imaging results were in agreement with the content uniformity test, which was performed by conventional chemical analysis.
Fig. (7).
FTIR analysis of pure ketoprofen (KPR), pure Eudragit® E PO, the physical mixture of 40% KPR/Eudragit® E PO, and the milled extrudate containing 40% KPR/Eudragit® E PO.
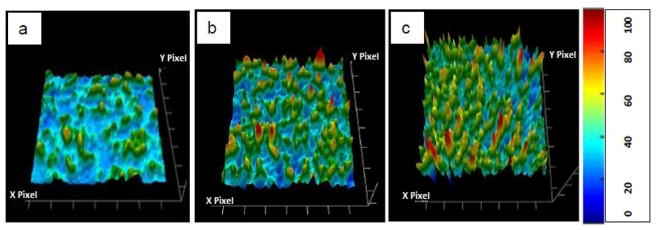
Fig. (8).
FTIR chemical images illustrating the distribution of ketoprofen (KPR) in the mini-tablets. Homogeneous drug distribution was observed in the mini-tablets with a) 20%, b) 30%, and c) 40% drug loading.
3.7. SEM
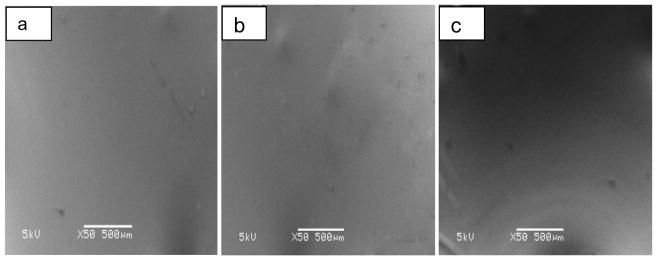
SEM was used to examine the surface morphology of the mini-tablets. SEM images were taken of the cross-sections of the extrudate strands to allow examination of the mini-tablet surface. The mini-tablets surfaces of KPR2, KPR3 and KPR4 formulations were found to be clear and homogenous at a range of drug loads and processing temperatures. No pores or cracks were detected on the mini-tablet surface, confirming that the mini-tablets were compact solids (Fig. 9).
Fig. (9).
SEM images of the surface of the ketoprofen (KPR) mini-tablets with a) 20%, b) 30%, and c) 40% drug loading.
3.8. Mini-tablet Properties
A friability testing apparatus was used to determine the weight loss of the mini-tablets (as a percentage). In friability testing, no weight loss was observed and all tested mini-tablets were found to be hard. These results were correlated well with the SEM findings, in which no pores and cracks were detected on the surface of the mini-tablets. These findings confirm the excellent mechanical properties of the mini-tablets prepared by the continuous HME process.
The hardness of the mini-tablets was measured using a hardness tester. The forces applied to fracture the mini-tablets KPR2, KPR3, and KPR4 were (82.67±2.15) N, (79.63±1.85) N, and (77.06±1.46) N, respectively. The slight variation in the forces required to fracture the mini-tablets was due to the differences in the percentage of KPR inside the mini-tablets. The addition of the plasticizer (KPR) to the polymer matrix reduced the number of entanglements [45], disrupting the forces holding the chains together and decreasing the force required to fracture the mini-tablets.
3.9. Stability Studies
Physical and chemical analyses were performed on the mini-tablets following storage at ambient conditions (25 °C/60% relative humidity) in open glass vials for 3 months. The lack of a crystalline melting peak in DSC (Fig. 1) and the characteristic peaks in the PXRD studies (Fig. 4) for all formulations after 3 months of storage confirmed that the amorphous form of the drug was maintained. The drug content of stored mini-tablets containing 20%, 30% and 40% was (98.10±1.15) %, (97.97±2.37) % and (92.15±2.56) %, respectively. The similarity value (f2) was used to compare the degree of similarity between in vitro dissolution profiles of freshly prepared mini-tablets to the stored ones. The two release profiles are considered similar, if the similarity factor is between 50 and 100 [46]. The similarity factor of mini-tablets containing 20%, 30% and 40% is 64, 56, and 56, respectively. The drug release was not significantly affected by 3 months of storage (Fig. 10). The drug exhibited excellent stability inside the matrix over the storage period of 3 months. This result corroborates the previous findings, which indicated that significant drug-polymer interactions enhance the stability of the prepared formulations [11].
Fig. (10).
In vitro release profiles of ketoprofen (KPR) mini-tablets in 0.1N HCl after 3 months of storage in open vials at ambient conditions (25 °C/60% relative humidity).
4. CONCLUSION
Mini-tablets containing various amounts of KPR with the taste-masking polymer Eudragit® E PO were successfully produced via a continuous process of pelletization with an adapted pelletizer directly connected to a hot-melt extruder. In the previous study by De Brabander et al. (2003), the twin screw extruder was used to prepare the extrudates of ibuprofen-ethyl cellulose, which were manually cut into 2-mm mini-matrices [23]. In this study, the authors have used continuous pelletization process to prepare mini-tablets. The manufactured mini-tablets were compact, with smooth surfaces and excellent uniformity. KPR bitterness was reduced due to the formation of a physical barrier layer of the polymer around the drug. Intermolecular interactions between oppositely charged compounds may have reduced the bitter taste of the drug and increased the stability of the formulation. These results confirmed the promise of Eudragit® E PO in the preparation of novel pediatric and geriatric mini-tablet dosage formulations as a means of masking drug taste and increasing patient compliance.
Acknowledgments
This project was partially supported by Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of NIH. The authors would also like to thank Dr Vijayasankar Raman of the National Center for Natural Products Research, School of Pharmacy, The University of Mississippi, for his valuable assistance with the SEM imaging studies.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
PATIENT CONSENT
Declared none.
References
- 1.Joshi S, Petereit HU. Film coatings for taste masking and moisture protection. Int J Pharm. 2013;457(2):395–406. doi: 10.1016/j.ijpharm.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Vummaneni V, Nagpal D. Taste masking technologies: an overview and recent updates. Int J Pharm Biomed Res. 2012;3(2):510–524. [Google Scholar]
- 3.Douroumis D. Orally disintegrating dosage forms and taste-masking technologies; 2010. Expert Opin Drug Deliv. 2011;8(5):665–675. doi: 10.1517/17425247.2011.566553. [DOI] [PubMed] [Google Scholar]
- 4.Douroumis D. Practical approaches of taste masking technologies in oral solid forms. Expert Opin Drug Deliv. 2007;4(4):417. doi: 10.1517/17425247.4.4.417. [DOI] [PubMed] [Google Scholar]
- 5.Woertz K, Tissen C, Kleinebudde P, Breitkreutz J. Rational development of taste masked oral liquids guided by an electronic tongue. Int J Pharm. 2010;400(1–2):114–1123. doi: 10.1016/j.ijpharm.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 6.Sato T, Nishishita K, Okada Y, Toda K. Effect of gap junction blocker beta-glycyrrhetinic acid on taste disk cells in frog. Cell Mol Neurobiol. 2009;29(4):503–512. doi: 10.1007/s10571-008-9342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyall V, Phan TH, Ren Z, Mummalaneni S, Melone P, Mahavadi S, Murthy KS, DeSimone JA. Regulation of the putative TRPV1t salt taste receptor by phosphatidylinositol 4,5-bisphosphate. J Neurophysiol. 2010;103(3):1337–1349. doi: 10.1152/jn.00883.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patil H, Tiwari RV, Repka MA. Hot-melt extrusion: from theory to application in pharmaceutical formulation. AAPS PharmSciTech. 2015:1–23. doi: 10.1208/s12249-015-0360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, Martin C, McGinity JW. Pharmaceutical applications of hot-melt extrusion: Part II. Drug Dev Ind Pharm. 2007;33(10):1043–1057. doi: 10.1080/03639040701525627. [DOI] [PubMed] [Google Scholar]
- 10.Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, McGinity JW, Martin C. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33(9):909–926. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]
- 11.Gue E, Willart JF, Muschert S, Danede F, Delcourt E, Descamps M, Siepmann J. Accelerated ketoprofen release from polymeric matrices: Importance of the homogeneity/heterogeneity of excipient distribution. Int J Pharm. 2013;457(1):298–307. doi: 10.1016/j.ijpharm.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Maniruzzaman M, Boateng JS, Chowdhry BZ, Snowden MJ, Douroumis D. A review on the taste masking of bitter APIs: hot-melt extrusion(HME) evaluation. Drug Dev Ind Pharm. 2014;40(2):145–156. doi: 10.3109/03639045.2013.804833. [DOI] [PubMed] [Google Scholar]
- 13.Maniruzzaman M, Rana MM, Boateng JS, Mitchell JC, Douroumis D. Dissolution enhancement of poorly water-soluble APIs processed by hot-melt extrusion using hydrophilic polymers. Drug Dev Ind Pharm. 2013;39(2):218–227. doi: 10.3109/03639045.2012.670642. [DOI] [PubMed] [Google Scholar]
- 14.Maniruzzaman M, Boateng JS, Snowden MJ, Douroumis D. A Review of Hot-Melt Extrusion: Process Technology to Pharmaceutical Products. ISRN Pharm. 2012;2012:9. doi: 10.5402/2012/436763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morott JT, Pimparade MPJ-B, Worley CP, Majumdar S, Lian Z, Pinto E, Bi Y, Durig T, Repka MA. The effects of screw configuration and polymeric carriers on hot-melt extruded taste-masked formulations incorporated into orally disintegrating tablets. J Pharm Sci. 2015;104(1):124–134. doi: 10.1002/jps.24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pimparade MB, Morott JT, Park JB, Kulkarni VI, Majumdar S, Murthy SN, Lian Z, Pinto E, Bi V, Durig T, Murthy R, HNS, Vanaja K, Kumar PC, Repka MA. Development of taste masked caffeine citrate formulations utilizing hot melt extrusion technology and in vitro–in vivo evaluations. Int J Pharm. 2015;487(1–2):167–176. doi: 10.1016/j.ijpharm.2015.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alshehri SM, Park JB, Alsulays BB, Tiwari RV, Almutairy B, Alshetaili AS, Morott J, Shah S, Kulkarni V, Majumdar S, Martin ST, Mishra S, Wang L, Repka MA. Mefenamic acid taste-masked oral disintegrating tablets with enhanced solubility via molecular interaction produced by hot melt extrusion technology. J Drug Deliv Sci Technol. 2015;27:18–27. doi: 10.1016/j.jddst.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh A, Majumdar S, Deng W, Mohammed NN, Chittiboyina AG, Raman V, Shah S, Repka MA. Development and characterization of taste masked Efavirenz pellets utilizing hot melt extrusion. J Drug Deliv Sci Technol. 2013;23(2):157–163. [Google Scholar]
- 19.Maniruzzaman M, Douroumis D. An in-vitro–in-vivo taste assessment of bitter drug: comparative electronic tongues study. J Pharm Pharmacol. 2015;67(1):43–55. doi: 10.1111/jphp.12319. [DOI] [PubMed] [Google Scholar]
- 20.Breitenbach J. Melt extrusion: from process to drug delivery technology. Eur J Pharm Biopharm. 2002;54(2):107–117. doi: 10.1016/s0939-6411(02)00061-9. [DOI] [PubMed] [Google Scholar]
- 21.Mehuys E, Remon JP, Vervaet C. Production of enteric capsules by means of hot-melt extrusion. Eur J Pharm Biopharm. 2005;24(2–3):207–212. doi: 10.1016/j.ejps.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Andrews GP, Jones DS, Diak OA, McCoy CP, Watts AB, McGinity JW. The manufacture and characterisation of hot-melt extruded enteric tablets. Eur J Pharm Biopharm. 2008;69(1):264–273. doi: 10.1016/j.ejpb.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 23.De Brabander C, Vervaet C, Remon JP. Development and evaluation of sustained release mini-matrices prepared via hot melt extrusion. J Control Release. 2003;89(2):235–247. doi: 10.1016/s0168-3659(03)00075-0. [DOI] [PubMed] [Google Scholar]
- 24.Thomson SA, Tuleu C, Wong IC, Keady S, Pitt KG, Sutcliffe AG. Minitablets: new modality to deliver medicines to preschool-aged children. Pediatrics. 2009;123(2):e235–8. doi: 10.1542/peds.2008-2059. [DOI] [PubMed] [Google Scholar]
- 25.Lennartz P, Mielck JB. Minitabletting: improving the compactability of paracetamol powder mixtures. Int J Pharm. 1998;173(1–2):75–85. [Google Scholar]
- 26.Lou H, Liu M, Wang L, Mishra SR, Qu W, Johnson J, Brunson E, Almoazen H. Development of a mini-tablet of co-grinded prednisone-Neusilin complex for pediatric use. AAPS PharmSciTech. 2013;14(3):950–958. doi: 10.1208/s12249-013-9981-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoltenberg I, Breitkreutz J. Orally disintegrating mini-tablets(ODMTs)--a novel solid oral dosage form for paediatric use. Eur J Pharm Biopharm. 2011;78(3):462–469. doi: 10.1016/j.ejpb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 29.Mohammed NN, Majumdar S, Singh A, Deng W, Murthy NS, Pinto E, Tewari D, Durig T, Repka MA. Klucel™ EF and ELF polymers for immediate-release oral dosage forms prepared by melt extrusion technology. AAPS PharmSciTech. 2012;13(4):1158–1169. doi: 10.1208/s12249-012-9834-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gryczke A, Schminke S, Maniruzzaman M, Beck J, Douroumis D. Development and evaluation of orally disintegrating tablets(ODTs) containing Ibuprofen granules prepared by hot melt extrusion. Colloids Surf B Biointerfaces. 2011;86(2):275–284. doi: 10.1016/j.colsurfb.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Gavis J, Modan M. Expansion and contraction of jets of Newtonian liquids in air: Effect of tube length. Phys Fluids (1958–1988) 1967;10(3):487–497. [Google Scholar]
- 32.Hiemenz PC, Lodge TP. Polymer chemistry. CRC press; 2007. [Google Scholar]
- 33.Crowley MM, Fredersdorf A, Schroeder B, Kucera S, Prodduturi S, Repka MA, McGinity JW. The influence of guaifenesin and ketoprofen on the properties of hot-melt extruded polyethylene oxide films. Eur J Pharm Sci. 2004;22(5):409–418. doi: 10.1016/j.ejps.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Azarmi S, Roa W, Löbenberg R. Current perspectives in dissolution testing of conventional and novel dosage forms. Int J Pharm. 2007;328(1):12–21. doi: 10.1016/j.ijpharm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Palmieri GF, Cantalamessa F, Di Martino P, Nasuti C, Martelli S. Lonidamine solid dispersions: in vitro and in vivo evaluation. Drug Dev Ind Pharm. 2002;28(10):1241–1250. doi: 10.1081/ddc-120015357. [DOI] [PubMed] [Google Scholar]
- 36.Lopes Jesus A, Nunes SC, Ramos Silva M, Matos Beja A, Redinha J. Erythritol: Crystal growth from the melt. Int J Pharm. 2010;388(1):129–135. doi: 10.1016/j.ijpharm.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 37.Lakshman JP, Cao Y, Kowalski J, Serajuddin AT. Application of melt extrusion in the development of a physically and chemically stable high-energy amorphous solid dispersion of a poorly water-soluble drug. Mol Pharm. 2008;5(6):994–1002. doi: 10.1021/mp8001073. [DOI] [PubMed] [Google Scholar]
- 38.Sathigari SK, Radhakrishnan VK, Davis VA, Parsons DL, Babu RJ. Amorphous-state characterization of efavirenz—polymer hot-melt extrusion systems for dissolution enhancement. J Pharm Sci. 2012;101(9):3456–3464. doi: 10.1002/jps.23125. [DOI] [PubMed] [Google Scholar]
- 39.Wu C, McGinity JW. Influence of methylparaben as a solid-state plasticizer on the physicochemical properties of Eudragit® RS PO hot-melt extrudates. Eur J Pharm Biopharm. 2003;56(1):95–100. doi: 10.1016/s0939-6411(03)00035-3. [DOI] [PubMed] [Google Scholar]
- 40.Quinteros DA, Rigo VR, Kairuz AFJ, Olivera ME, Manzo RH, Allemandi DA. Interaction between a cationic polymethacrylate (Eudragit E100) and anionic drugs. Eur J Pharm Sci. 2008;33(1):72–79. doi: 10.1016/j.ejps.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Kindermann C, Matthée K, Strohmeyer J, Sievert F, Breitkreutz J. Tailor-made release triggering from hot-melt extruded complexes of basic polyelectrolyte and poorly water-soluble drugs. Eur J Pharm Biopharm. 2011;79(2):372–381. doi: 10.1016/j.ejpb.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Sancin P, Caputo O, Cavallari C, Passerini N, Rodriguez L, Cini M, Fini A. Effects of ultrasound-assisted compaction on Ketoprofen/Eudragit® S100 Mixtures. Eur J Pharm Sci. 1999;7(3):207–213. doi: 10.1016/s0928-0987(98)00022-0. [DOI] [PubMed] [Google Scholar]
- 43.Mura P, Faucci M, Parrini P, Furlanetto S, Pinzauti S. Influence of the preparation method on the physicochemical properties of ketoprofen–cyclodextrin binary systems. Int J Pharm. 1999;179(1):117–128. doi: 10.1016/s0378-5173(98)00390-1. [DOI] [PubMed] [Google Scholar]
- 44.Kojima T, Higashi K, Suzuki T, Tomono K, Moribe K, Yamamoto K. Stabilization of a supersaturated solution of mefenamic acid from a solid dispersion with Eudragit® EPO. Pharm Res. 2012;29(10):2777–2791. doi: 10.1007/s11095-011-0655-7. [DOI] [PubMed] [Google Scholar]
- 45.Swallowe GM. Mechanical properties and testing of polymers: an AZ reference. Vol. 3 Springer; 1999. [Google Scholar]
- 46.Kim JY, Kim DW, Kuk YM, Park CW, Rhee YS, Oh TO, Weon KY, Park ES. Investigation of an active film coating to prepare new fixed-dose combination tablets for treatment of diabetes. Int J Pharm. 2012;427(2):201–208. doi: 10.1016/j.ijpharm.2012.01.057. [DOI] [PubMed] [Google Scholar]