Abstract
Background:
Paradoxically, elderly persons with type 2 diabetes mellitus (T2DM) fracture despite having higher bone density than nondiabetics. Systemic factors associated with aging and T2DM may have detrimental, local effects on the skeleton. One such factor could be by altering the microenvironment of the mesenchymal stem-cells (MSCs), multipotent progenitors capable of differentiating into adipocytes or osteoblasts.
Methods
Sera were obtained from four participant groups (n=40 total, 10 per group): 1) young women with normal glucose tolerance (NGTY), 2) post-menopausal women with NGT) 3) post-menopausal women with impaired glucose tolerance (IGT) and 4) post-menopausal women with T2DM. Sera were incubated with human MSCs for 14 days. Cell proliferation and apoptosis were measured using EdU and TUNEL labeling assays, respectively. MSC differentiation for each group were determined using osteogenic and adipogenic gene expression markers quantified by qRT-PCR, as well as Alizarin Red and Oil Red O staining.
Results:
Expression of adipogenic genes was greater than 2 fold higher (P<0.05) in MSCs cultured with T2DM sera compared to those incubated with NGTY, NGT or IGT sera. The increase in adipogenic gene expression corresponded with increased Oil Red O staining. Despite the increased adipogenic differentiation of MSCs exposed to T2DM sera, cell proliferation and apoptosis rates as well as osteoblastic activity were not significantly different amongst the four conditions.
Conclusions:
Systemic, circulating factors in the serum of older women with T2DM may promote MSC differentiation into adipocytes versus osteoblasts. Increased differentiation of MSCs into adipocytes is one possible mechanism by which T2DM increases fracture risk.
Keywords: Type 2 Diabetes Mellitus, Aging, Mesenchymal stem cell, Fracture, Bone density
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a state of accelerated aging (1, 2). It is well recognized that T2DM prematurely hastens cardiovascular, renal and ocular decline (3). Over the last decade, the skeleton has been added to the expanding list of organs subject to diabetic complications (4). Paradoxically, individuals with T2DM are at higher risk for fractures despite having higher bone mineral density than those without diabetes (5–9). The pathophysiology underlying this incongruity between bone quantity, as measured by dual x-ray absorptiometry (DXA), and bone strength with progressive glycemic derangement is unknown.
Using diabetes as a model for premature aging, there may be lessons to learn from mechanisms underlying bone fragility in diabetes that parallel normal human aging. In the older skeleton, the etiology of bone loss is multifactorial. Some pathways are clear, exemplified by declines in renal function, the menopausal transition, inactivity or sarcopenia (10–13). Others are not as apparent and may be attributed to systemic changes such as increased inflammation, hormonal dysregulation or a more oxidized bone microenvironment (14–19). Such alterations in systemic factors observed with aging may have adverse effects on the bone microenvironment and its resident mesenchymal stem cells (MSCs).
Lineage commitment of MSCs is an important determinant of bone density and strength given the MSC’s capacity to differentiate into osteoblasts versus adipocytes (20). The proliferation and differentiation of the MSC cells are dependent on extrinsic and intrinsic factors. Locally, osteocyte signaling, inflammatory cytokines, growth factors, the oxidizing microenvironment and other signaling molecules contribute to MSC activity (17, 21–24). The MSC is also continually bathed in systemic, circulating factors which flow freely into the bone microenvironment through the sinusoidal circulation. Fluctuations in systemic factors including anabolic hormones, advanced glycation end-products (AGEs), inflammatory cytokines and oxidative stressors have the potential to directly and adversely influence MSC activity with downstream effects on skeletal fragility (25–27). Though many of the alterations in systemic factors present in aging are also present in T2DM, their contribution to the bone microenvironment, MSC activity and overall fragility in the diabetic skeleton is unknown.
In this study, we investigate the impact of extrinsic, circulating factors on a well-characterized human MSC (hMSC) cell line exposed to sera from young women with normal glucose tolerance (NGTY) versus postmenopausal women with normal glucose tolerance (NGT), impaired glucose tolerance (IGT) and frank T2DM with respect to MSC proliferation, apoptosis, differentiation and matrix mineralization. Given what is known about skeletal fragility in T2DM, we hypothesized that those MSCs exposed to diabetic serum would proliferate less, exhibit more apoptosis, differentiate into adipocytes rather than osteoblasts, and exhibit less mineralization when compared to MSCs exposed to the serum of normoglycemic individuals. Furthermore, we hypothesized that IGT, or the pre-diabetic state, would also adversely impact these same indices in hMSCs, although not to the degree of frank T2DM.
MATERIALS AND METHODS
Study population and design
A total of 40 women were recruited from the Baltimore community for the cross-sectional study. Three comparison groups of older postmenopausal women with NGT, IGT and T2DM (N=10 each group, age 60–75 years) were recruited in addition to 10 younger women (30–45 years) to serve as control subjects. Young women had serum drawn during menses to minimize variation in sex hormone levels. Exclusion criteria included pregnancy, current tobacco use, alcohol use greater than three units daily, current use of hormone replacement therapy, type 1 diabetes mellitus, creatinine clearance < 60 ml/min./1.73 m2 by Cockcroft-Gault, hemoglobin A1c (HbA1c) > 10%, BMI < 18 kg/m2, thiazolidinedione use within the last year, current use of heparin, proteinuria or microalbuminuria, uncontrolled thyroid disease, or other significant medical illness. Possible participants were additionally excluded for the use of medications known to impact bone and mineral metabolism, including use of a bisphosphonate in the last 6 months or greater than 6 months total, past or present use of teriparatide or denosumab, use of calcitonin, selective estrogen receptor modulators (SERMs), prednisone > 5mg for over 10 days in the previous three months, anti-epileptic medications, anti-estrogen medications. Participants were excluded for known metabolic bone disease (e.g. Paget’s disease, hyperparathyroidism, multiple myeloma, sarcoid or other granulomatous disease, celiac disease) other than low bone density.
At the single study visit, fasting blood and urine samples were collected from women following informed consent. Women with T2DM were asked to hold their morning dose of insulin until after the blood draw, to minimize the known influence of insulin on adipogenesis. For human serum (HuS) preparation, blood samples were allowed to sit for 20 minutes, followed by centrifugation and HuS removal with storage at −80 degrees. Participants who were not on medications for diabetes (oral or insulin) were asked to undergo a 2-hour, 75 gram oral glucose tolerance test (OGTT) in the Clinical and Translational Science Award-sponsored John Hopkins Bayview Clinical Research Unit. NGT was defined by a 2-hour glucose less than 140 mg/dL; the IGT group was defined by a glucose of 140 to 199 mg/dL at 2 hours; T2DM was defined as a 2-hour glucose of ≥ 200 mg/dL or if subjects were on T2DM pharmacotherapy. Note that only subjects on pharmacotherapy for T2DM (oral, insulin or both) were included in this study. Participants additionally underwent anthropometric and DXA evaluation.
Cell line, culture and expansion
Primary human mesenchymal stem cells (hMSCs) were obtained from the Institute for Regenerative Medicine at Texas A&M Health Science Center. Derived from adult human bone marrow, the hMSCs have been well characterized. hMSCs were cultured in a complete culture medium (CCM) consisting of modified essential medium (MEM, Invitrogen), supplemented with fetal bovine serum (FBS, Atlanta Biologicals), L-glutamine (Life Technologies) and penicillin/streptomycin (Life Technologies). Cells from passage three were used in all experiments, and all experiments were done in triplicate.
Cell proliferation and apoptosis assays
Cells were plated at a density of 4000 cells/cm2 on chamber slides (Lab Tek, 0.5-1mL volume) and cultured in the presence of a modified growth medium consisting of MEM and L-glutamine plus 5% FBS (control) or MEM plus 5% HuS from each of the four study groups. Growth medium was replaced every three days. On day 14 of culture, cells were fixed and processed according to manufacturers’ instructions using 5-ethynyl-2-deoxyuridine (Click-It EDU, Invitrogen) or terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL, Invitrogen) assays to quantify proliferation and apoptosis, respectively(28)[28], Counterstaining with DAPI (Vector Lab) allowed for cell counting and quantification of proliferation and apoptosis indices.
Osteoblast and adipocyte differentiation assays
hMSCs were plated in 6-well plates prepared with 0.5×105 cells/well using CCM. CCM was replaced every three days until cells reached 80% confluence. To measure osteogenic differentiation, hMSCs were then treated with osteogenic induction medium comprised of MEM and supplemented with L-glutamine (2mM, Life Technologies), β-glycerophosphate (10mM, Sigma), 2-phosphate ascorbate (50μM, Sigma), dexamethasone (1nM, Alfa Aesar), and vitamin D3 (Sigma) 1 uL, in addition to either 15% FBS (control) or 15% pooled HuS from individuals from one of each of the four donor groups. The osteogenic induction medium was replaced every three days. To measure adipogenic differentiation, hMSCs were treated with an adipogenic induction medium comprised of MEM and supplemented with L-glutamine (2mM, Life Technologies), dexamethasone (0.5μM, Alfa Aesar), biotin (Sigma), calcium pantothenate (Sigma), recombinant human insulin (Sigma) and 1-methyl-3 isobutylxanthine (IBMX, 0.5μM, Sigma) in addition to either 15% FBS (control) or 15% pooled HuS from each of the four groups. The adipogenic medium was replaced every three days.
RNA extraction and quantitative reverse transcriptase real-time polymerase chain reaction (RT-PCR)
qRT-PCR was used to quantify mRNA markers of osteoblasts and adipocyte differentiation. On day 14, cells were fixed in 4% paraformaldehyde and DNA-free RNA extracted using Trizol (Fisher Scientific) following the manufacturer’s protocol. RNA (1 μg) was reverse transcribed using Superscript Reverse Transcriptase III kit (Fisher Scientific) according to manufacturer’s instructions. cDNA was obtained from total RNA using the cDNA Archive Kit according to the manufacturer’s protocol (Applied Biosystems, Foster City, CA). To characterize osteoblast differentiation RUNX2, Type 1 Collagen (COL1A1) and Osteocalcin (OCN) transcripts were quantified. Probes and primers for osteogenic differentiation were designed and synthesized by Applied Biosystems and all PCR amplifications were carried out in triplicate on an ABI Prism® 7300 Sequence Detection System, using a fluorogenic 5’ nuclease assay (TaqMan® probes). Early adipocyte differentiation primers included PPARy, LPL, ADD1; both adiponectin, aP2 were used as primers for late adipocyte differentiation. Primers for osteogenic differentiation were synthesized by Qiagen and all PCR amplifications were carried out in triplicate on an ABI Prism® 7300 Sequence Detection System, using SYBR® green PCR core reagents (PE Applied Biosystems). Each SYBR green reaction mixture consisted of 10ng RTcDNA and 200nM each primer (forward and reverse). Relative gene expressions were calculated by using the 2-ΔΔCt method (29). The ΔCt value of each sample was calculated using huGAPDH.
Assays for matrix mineralization and adipose formation
To quantify hMSC allocation to the adipogenic or osteogenic lineage in when exposed to sera from participants with different degrees of glucose intolerance, hMSCs were cultured in 6-well plates with either osteogenic or adipogenic media, as above. To determine in-vitro matrix mineralization by osteoblasts, hMSCs treated with osteogenic media (+15% FBS or HuS) were fixed in 4% paraformaldehyde at 14 days. They were stained with 40 mM alizarin red S (AR-S; Sigma), pH 4.2 for 10 minutes at room temperature and counterstained with hematoxylin (Sigma). hMSCs differentiation into adipocytes was measured using Oil Red O. Cells cultured in adipogenic media supplemented with either 15% FBS or 15% HuS for 14 days were fixed in 4% paraformaldehyde and stained using Oil Red O according to manufacturer’s protocol (Polyscience).
Statistical analysis
All data analyses were performed using STATA 10. Unless otherwise noted, statistical significance was assessed using as p≤0.05. To study the differences in outcomes among different groups, one-factor analysis of variance (ANOVA) were performed. Post-hoc tests adjusting for multiple comparisons were used for pair-wise comparisons between specific groups. In order to adjust for potential risk factors such as age, we performed multivariate linear regression to assess the relationship between group indicator and the outcomes. For analyses involving RT-PCR, relative gene expression between two groups (“fold change”) and corresponding p-values were calculated by using the 2”ΔΔCt method (29). The three technical replicate Ct values for each gene and sample were averaged and then ΔCt values were obtained using GAPDH as the normalizing gene. GAPDH Ct values were obtained using SYBR green for samples for which genes were assayed with SYBR green, and via TaqMan for samples for which genes were assayed using TaqMan.
RESULTS
Study participant characteristics, anthropometries, biochemical parameters and skeletal measures
Women participating in this study differed across few baseline parameters (Table 1). While women in the NGTY group were expectedly younger than those in the older cohorts, there were no significant age differences amongst the older women of differing glycemic status categories. There was no difference in BMI amongst the four groups despite their measured differences in glucose tolerance. This finding paralleled serum leptin and adiponectin levels that were not significantly different in the four groups. Biochemically, there were no statistically significant differences in serum calcium or 25-hydroxy vitamin D levels amongst the four study groups; renal function, as measured by glomerular filtration rate (MDRD), was better in young controls with normal glucose tolerance (121.4 ± 28.9 mL/min) and not notably different amongst older participants. At the spine, total hip and femoral neck, there were no significant differences in bone mineral density (BMD) amongst the NGTY, NGT, older IGT and older T2DM groups. Similarly, serum markers of bone resorption (C-telopeptides) and bone formation (bonespecific alkaline phosphatase, total procollagen type 1 N-terminal propeptide) were similar amongst the four groups. Considering phosphaturic hormone production (FGF-23) and osteocyte function (sclerostin), there were also no measurable differences in these serum studies no matter the participant age or glucose status. While growth hormone levels (IGF-1) were higher in NGTY controls, there were no marked differences in the inflammatory marker, C-reactive protein (CRP).
Table 1:
Characteristics of study participants
| NGTY (n=10) | NGT (n=10) | IGT (n=10) | T2DM (n=10) | |
| Age (years) | 37.1 ± 4.3* | 66 ± 3.1 | 68.9 ± 4.1 | 68 ± 4.0 |
| BMI (kg/m2) | 25.8 ± 7.3 | 31.3 ± 4.5 | 29.1 ± 5.1 | 33.0 ± 8.2 |
| Vitamin D (ng/mL) | 32 ± 11.7 | 33.9 ± 10.0 | 36.3 ± 13.6 | 25.9 ± 12.6 |
| Calcium (mg/dL) | 9.3 ± 0.2 | 9.4 ± 0.3 | 9.6 ± 0.3 | 9.5 ± 0.4 |
| GFR (mL/min) | 121.4 ± 28.9* | 85.7 ± 21.5 | 82.7 ± 28.5 | 81.7 ± 20.8 |
| Fasting glucose (mg/dL) | 87.3 ± 6.3 | 93.8 ± 9.2 | 99.4 ± 10.8 | 167.7 ± 62.3* |
| 2h glucose (mg/dL) | 97 ± 25.2 | 99.9 ± 19.0 | 170.3 ± 24.6* | - |
| HbA1c (%) | 5.4 ± 0.2 | 5.6 ± 0.4 | 6.0 ± 0.3 | 8.1 ± 1.9* |
| HOMA-IR | 1.5 ± 1.8 | 1.5 ± 1.6 | 1.6 ± 1.5 | 21.1 ± 20.0* |
| Insulin (μU/mL) | 6.9 ± 5.9 | 6.4 ± 5.3 | 6.2 ± 5.1 | 54.2 ± 14.0* |
| BMD spine (g/cm2) | 1.3 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.3 | 1.3 ± 0.2 |
| BMD total hip (g/cm2) | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| BMD femoral neck(g/cm2) | 1.1 ± 0.1* | 0.9 ± 0.07 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Leptin (ng/mL) | 24.8 ± 33.7 | 28.7 ± 21.4 | 34.7 ± 12.7 | 34.7 ± 31.2 |
| Adiponectin (ug/mL) | 9215.1 ± 6906.5 | 9588.8 ± 5750.3 | 6361.0 ± 3995.2 | 11497.0 ± 7938.2 |
| Sclerostin (ng/mL) | 0.8 ± 0.4 | 0.7 ± 0.2 | 0.8 ± 0.3 | 0.8 ± 0.2 |
| Undercarboxylated OC (ng/mL) | 4.2 ± 2.0 | 6.0 ± 4.2 | 6.3 ± 4.7 | 7.8 ± 5.7 |
| C-telopeptides (ng/mL) | 0.6 ± 0.3 | 0.5 ± 0.2 | 0.7 ± 0.4 | 0.4 ± 0.2 |
| P1NP (ng/mL) | 87.9 ± 37.7 | 67.4 ± 29.1 | 82.9 ± 56.9 | 51.5 ± 16.8 |
| BAP (ug/L) | 16.9 ± 10.1 | 20.8 ± 7.9 | 20.4 ± 10.0 | 13.6 ± 4.7 |
| FGF-23 (pg/mL) | 3.5 ± 0.1 | 4.7 ± 3.0 | 5.6 ± 6.0 | 4.8 ± 6.1 |
| IGF-1 (ng/mL) | 157.9 ± 44.5* | 109 ± 36.5 | 119.9 ± 20.1 | 108.8 ± 26.5 |
| CRP (mg/L) | 1.4 ± 3.4 | 2.1 ± 2.6 | 2.0 ± 2.0 | 3.4 ± 3.2 |
Values are reported as the mean ± SD, HOMA index (IR) = (fasting glucose mg/dL × fasting insulin (mU/L) /405) Body mass index (BMI); Glomerular filtration rate (GFR); homeostatic model assessment of insulin resistance (HOMA-IR); Bone mineral density (BMD); osteocalcin (OC); total procollagen type 1 N-terminal propeptide (P1NP); Bone-specific alkaline phosphatase (BAP); fibroblast growth factor (FGF-23); Insulin-like growth factor 1 (IGF-1); C-reactive protein (CRP)
significantly different from all other groups, p ≤ 0.05
Study participant diabetes status
Consistent with their designation to the T2DM group, participants with insulin-dependent diabetes had significantly higher homeostatic model assessment of insulin resistance (HOMA-IR) measures (21.1 ± 20.0) and fasting insulin levels (54.2 ± 14.0 μU/mL) pre-exogenous insulin administration than NGTY controls and older participants with NGT and IGT. Study participants with T2DM had significantly higher HbA1c levels, though control was fair (HbA1c 8.1 ± 1.9%) overall. The average duration of diabetes in the 10 participants with T2DM was 13.3 ± 6.4 years. Regarding diabetic medication use in those with T2DM, 100% were on insulin (required for study inclusion) alone or in combination with metformin (80%), sulfonylureas (50%), or GLP-1 agonists (10%). No participants were on thiazolidinediones (exclusion criteria) or sodium-glucose co-transporter 2 (SGLT2) inhibitors.
Effect of participant glucose status on hMSC proliferation and apoptosis
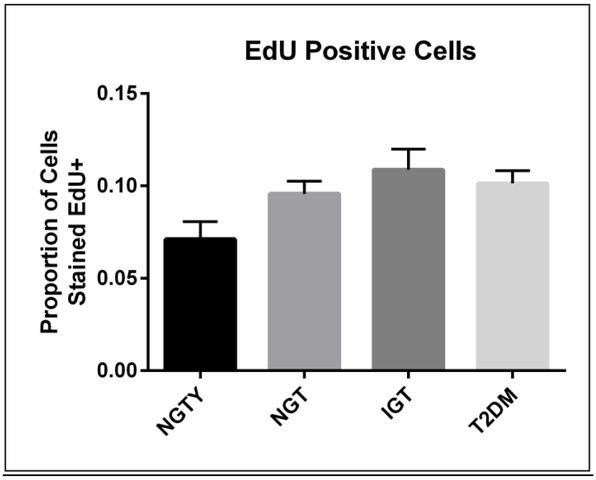
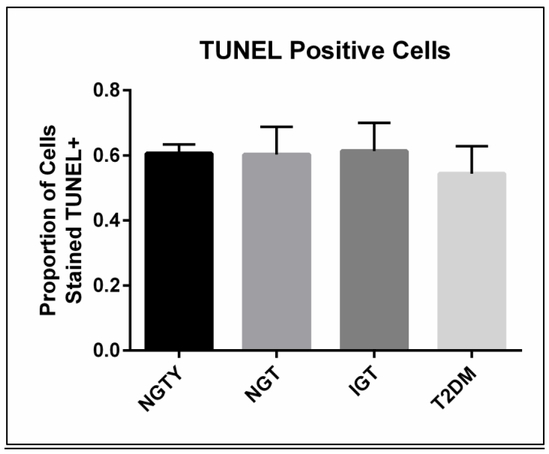
To quantify the effects of participant age and glycemic status on hMSC proliferation and apoptosis, we incubated HuS pooled separately from each group (NGTY, NGT, IGT, T2DM) with the common line of hMSCs. We then quantified changes in proliferation and apoptosis in undifferentiated hMSCs incubated with the pooled HuS using EdU incorporation and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays, respectively. As shown (Figure 1), cell proliferation rates were not significantly different amongst the four conditions, though there was a trend towards diminished cellular proliferation in young controls with normoglycemia (p = 0.14). Neither participant age nor glycemic status significantly affected hMSC apoptosis following incubation with HuS (p=0.41).
Figure 1: Proliferation and apoptosis in MSC cultured with serum from normal and glucose intolerant women.
A) Unadjusted median values and range of proportion of total cells that incorporated the thymidine analog EdU (proliferation marker), averaged over three experiments.
*all p>0.05
B) Unadjusted median values and range of proportion of cells that were labeled with TUNEL (apoptosis marker), averaged over three experiments.
*all p>0.05
Effect of participant glucose tolerance status on hMSC osteoblast differentiation and matrix mineralization
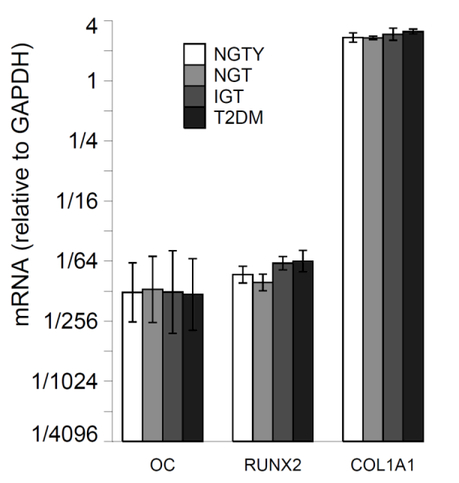
The effects of age and glycemic status (NTGY, NGT, IGT, T2DM) on hMSC differentiation into the osteoblast lineage were evaluated through the quantification of the mRNA expression of early (RUNX2), mid (COL1a1) and late (OCN) differentiation markers for osteoblast lineages. hMSCs were incubated with osteogenic media and pooled HuS from each of the 4 groups (NGTY, NGT, IGT, T2DM) for a total of 14 days, and constitutive levels of mRNA of osteoblastic marker genes were measured using qRT-PCR. Osteoblast gene expression for all stages of osteoblastic development was similar in cultures incubated with sera from all donor groups (Figure 2A). hMSCs were incubated with adipogenic media and pooled HuS from each of the 4 groups (NGTY, NGT, IGT, T2DM) for a total of 14 days, and constitutive levels of mRNA of osteoblastic marker genes were measured using qRT-PCR. We investigated the influence of pooled serum from young controls with normoglycemia vs. older participants with different degrees of glucose intolerance on osteoblast function and in vitro matrix mineralization using Alizarin Red cytochemical staining. Osteoblast-mediated calcium deposition demonstrated by the degree and intensity of staining were similar in cultures incubated with sera from all donor groups (Figure 2B).
Figure 2.
a: Expression of osteoblastic gene markers
For each group, plot mRNA levels relative to the housekeeping gene (GAPDH). Error bars are from +/− the SEM about the mean delta Ct.
Horizontal brackets indicate p <= 0.05 for the comparison of mRNA values (relative to GAPDH) between the two groups pointed to by the ends of each bracket.
Young normal glucose tolerant (NGTY); post-menopausal normal glucose tolerant (NGT); post-menopausal impaired glucose tolerant (IGT); post-menopausal type 2 diabetes mellitus (T2DM); osteocalcin (OC); runt-related transcription factor 2 (RUNX2); collagen type I alpha 1 chain (COL1A1)
b: Alizarin Red staining
Note: well plates photographed at 10× magnification
Effect of participant glucose status on hMSC adipocyte differentiation
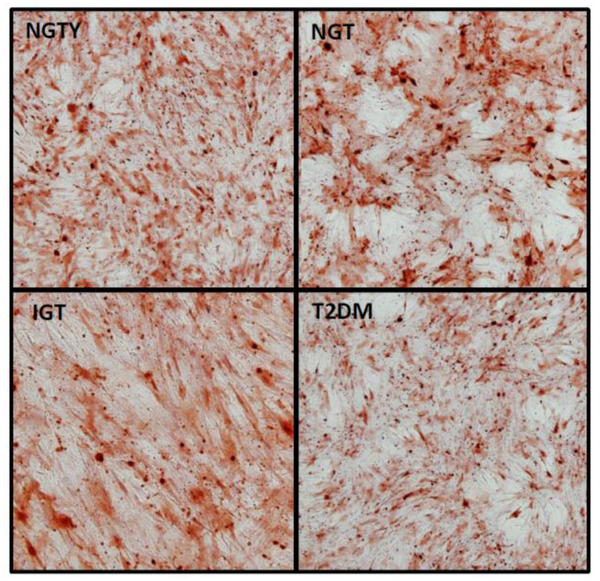
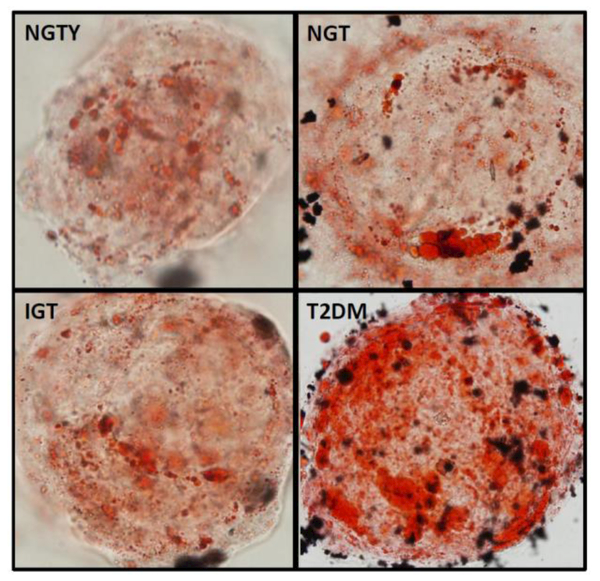
The effects of both age (young vs. older) and glycemic status (NGT, IGT, T2DM) on hMSC differentiation into the adipocyte lineage were measured via quantification of mRNA expression of early, mid and late differentiation markers for adipocyte lineages. For 14-days, hMSCs were incubated with adipogenic media and pooled HuS from each of the four participant groups, followed by mRNA extraction and qRT-PCR. Adipocyte markers included adiponectin (ADIPOQ), adipocyte protein 2 (aP2), lipoprotein lipase (LPL) and Peroxisome proliferator-activated receptor gamma (PPARγ). There were significant differences in the constitutive expression of all adipogenic markers in hMSCs incubated with sera from subjects with T2DM compared with those incubated with sera from NGTY women, or NGT or IGT participants. Specifically, expression of adipogenic genes aP2, LPL, and PPARγwere greater than 2 fold higher (P<0.05) in MSCs cultured with T2DM sera compared to those incubated with NGTY, NGT or IGT sera (Figure 3A). Further, expression of the adipogenic gene ADIPOQ was over 2 fold higher (P<0.05) in hMSCs cultured with T2DM sera compared to those incubated with IGT sera. We additionally qualified histochemical markers of lineage allocation into adipocytes in hMSCs incubated with HuS using oil Red O cytochemical staining (adipocyte lineage). The increase in adipogenic gene expression corresponded with increased lipid accumulation demonstrated by the intensity of Oil Red O staining in MSCs cultured with T2DM sera (Figure 3B).
Figure 3.
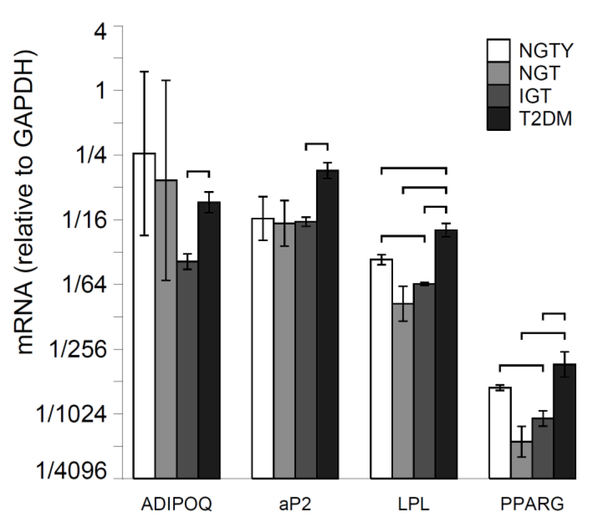
a: Expression of adipogenic gene markers
For each group, plot mRNA levels relative to the housekeeping gene (GAPDH). Error bars are +/− SEM about the mean delta Ct.
Horizontal brackets indicate p <= 0.05 for the comparison of mRNA values (relative to GAPDH) between the two groups pointed to by the ends of each bracket.
Young normal glucose tolerant (NGTY); post-menopausal normal glucose tolerant (NGT); post-menopausal impaired glucose tolerant (IGT); post-menopausal type 2 diabetes mellitus (T2DM); adiponectin (ADIPOQ); adipocyte protein 2 (aP2); lipoprotein lipase (LPL); peroxisome proliferator activated receptor gamma (PPARγ)
b: Oil Red O staining
Note: well plates photographed at 40× magnification
DISCUSSION
Here, we report that hMSCs exposed to the sera of individuals with T2DM demonstrate a significantly greater adipogenic differentiation pattern compared to hMSCs exposed to the serum of individuals with NGT (young and older) or IGT. Conversely, glucose status appeared to play no role in hMSC proliferation, apoptosis, matrix mineralization or the osteoblastic gene expression. Participant age was also not a strong determinant of osteoblast differentiation. Thus, our research enhances the understanding of the mechanisms by which progressive glycemic derangement and its systemic sequelae potentially lead to skeletal fragility via promotion of increased MSC differentiation to adipocytes rather than osteoblasts in the marrow microenvironment.
Despite the significant physiologic overlap in T2DM and aging, there has been little investigation into how common circulating factors thought to contribute to multi-system aging have detrimental, local effects on the diabetic skeleton. It is known that despite normal to high bone density which should portend skeletal integrity, older individuals with T2DM are at increased risk of fracture (30). To date, the explanation for this discrepancy between bone quantity, measured by dual x-ray absorptiometry (DXA), and bone strength in states of altered glucose homeostasis is unknown. There is speculation that end-organ damage from diabetic complications, medication side effects, increased fall risk and/or alterations in body composition may be culprit in diabetic skeletal fragility (4, 31). However, in longitudinal analyses of large populations that control for these factors, the increased fracture risk remains significant (5, 5, 6, 32).
As such, there have been additional investigations bone microarchitecture, material properties and remodeling parameters in T2DM that may lead to increased skeletal weakness. Individuals with T2DM may have increased cortical porosity, unfavorable bone material properties and a reduced rate of skeletal turnover compared to healthy controls (33–36, 36). However, the mechanistic underpinnings of these differences remain largely unexplored, presenting a significant gap in the literature. Ours is the first study to examine the possible interaction between extrinsic factors present in diabetic vs. nondiabetic patient serum and a simulated bone microenvironment employing hMSC cultures. The MSC is an essential constituent of the marrow microenvironment, serving as the progenitor cell for osteoblasts. With capacity to differentiate into a number of different tissues including chondrocytes, myocytes, adipocytes and osteoblasts, processes that shift differentiation to other tissue types rather than the osteoblastic lineage have the potential to have detrimental effects on the skeleton (37, 38).
There are some data suggesting that systemic circulating factors influence hMSC differentiation in older individuals. For example, compared with MSCs from young donors, the MSCs isolated from older individuals demonstrate a phenotype consistent with cellular aging. Specifically, these osteoprogenitor cells exhibit decreased lifespan, proliferation, colony numbers and growth rate (38–41). MSCs from elderly subjects also show greater expression of key senescence pathway genes p53 and p21, decreased differentiation and selective lineage allocation into adipocytes compared to young controls (40, 41). As osteoblast precursors, decreased numbers of MSCs result in lower numbers of osteogenic cells and diminished bone with age. Preferential differentiation into adipocytes fills the marrow with adipose, further compromising skeletal integrity (42).
MSCs undergo intrinsic, involutional aging over time, but this appears to be closely tied to the extrinsic, circulating environment of its host. That is, MSCs may be capable of “escaping” an aging pathway when exposed to different environmental factors in a younger host. In skeletal muscle, progenitor cell rejuvenation occurs following exposure to young sera in parabiotic pairing model (23, 43, 44). The same may be true for bone. There is evidence that MSCs taken from the bone marrow of elderly subjects maintain osteoblastic and adipogenic differentiation potential compared to young controls when removed from the aging host and cultured in a favorable environment (23, 45). With these data suggesting that MSC proliferation, differentiation and capacity for matrix mineralization may be dependent on factors associated with the host, we queried the effects human sera from across the spectrum of systemic glucose derangement on a hMSC cell line.
There have been few studies examining skeletal progenitor cells in individuals with diabetes compared to non-diabetic controls. Cramer and colleagues studied, adipose-derived progenitor cells from nine middle-aged, diabetic subjects and demonstrated reduced proliferation and enhanced adipogenic potential compared to controls (46)[46]. This study suggests that the diabetic state has a direct effect on progenitor cell behavior in the hyperglycemic host. Our study supports the argument that the diabetic milieu has direct and adverse effects on progenitor cells. Focusing on osteogenic potential, we showed that exposure to diabetic serum was strongly associated with significant differences in hMSC differentiation patterns compared with young and older NGT controls as well as “pre-diabetic” serum. Incubation with T2DM serum enhanced adipogenic differentiation of hMSCs compared with non-diabetic or IGT serum. Interestingly, there was not a difference in proliferation or apoptosis patterns in hMSCs cultured with T2DM serum as we had hypothesized. This may suggest that the skeletal fragility observed in T2DM is more a product of bone progenitor cell lineage allocation rather than number or lifespan.
Clinically, the bone marrow of individuals with T2DM has increased adiposity compared to non-diabetic controls (47). The significance of the marrow fat is not entirely known, though it is associated with lower BMD and fracture (48). Progenitor cells that preferentially differentiate to adipocytes reduce osteogenic potential, leading to less skeletal formation. This is thought to be one mechanism underlying the increased fracture risk seen with thiazolidinedione use, whereby upregulation of PPARγ preferentially drives MSCs to adipocytes (49–51). Marrow adipocytes may also inhibit bone formation through the production of TNF-alpha and IL-6 which impair osteoblastogenesis (52). This could potentially explain why the bone in T2DM is considered to be in a blunted remodeling state. Bone turnover, as measured by histomorphometry and bone turnover markers, appears to be lower in T2DM compared to non-diabetic states (34, 35). Without the capacity to renew itself, the diabetic skeleton may become fragile and susceptible to fracture. Our findings demonstrating that T2DM serum promotes adipogenic differentiation in hMSCs support these clinical observations.
CONCLUSION
In sum, our research is significant because it enhances our appreciation that factors present in the circulation of individuals with T2DM may have local, detrimental effects on the skeleton. We found no significant differences in growth hormone, adipokines or inflammation amongst the four groups that might explain the underlying pathophysiology of increased adipogenic differentiation of the hMSCs exposed to serum of women with T2DM. Future studies will be needed to determine the specific factors present in the systemic circulation of those with T2DM that may alter the bone microenvironment and adversely influence hMSC differentiation. Such studies may require isolation and culture of bone marrow derived hMSCs from donors with NGT, IGT and T2DM to determine In vivo differences in differentiation and proliferation capacity, matrix mineralization, and other determinants of bone remodeling. Serum fractionation and proteomic analysis may also assist in identifying the specific factors contributing to changes in hMSC differentiation based on glucose status. Greater knowledge of the mechanisms contributing to skeletal fragility in T2DM will translate into early interventions and targeted therapeutics to prevent future disability, morbidity and mortality following fracture.
Acknowledgments
This work was supported by the National Institutes of Health / National Institute on Aging, R03AG040695; Clinical research was conducted in the Johns Hopkins Bayview Clinical Research Unit, funded by the Institute for Clinical and Translational Research, UL1TR001079.
Footnotes
Authors’ roles: KFM designed the project with additional contributions by SJ and MD. KFM and MD performed data collection with additional contributions by SJ. KFM and MD performed data interpretation, and KM and MD wrote the manuscript with input from SJ.
The authors of this paper have no conflicts of interest.
REFERENCES
- 1.Toperoff G, Kark JD, Aran D, Nassar H, Ahmad WA, Sinnreich R, Azaiza D, Glaser B, Hellman A. Premature aging of leukocyte DNA methylation is associated with type 2 diabetes prevalence. Clin Epigenetics. 2015. March 28;7:35,015-0069-1. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mooradian AD. Tissue specificity of premature aging in diabetes mellitus. the role of cellular replicative capacity. J Am Geriatr Soc. 1988. September;36(9):831–9. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017. June 3;389(10085):2239–51. [DOI] [PubMed] [Google Scholar]
- 4.Dhaliwal R, Rosen CJ. Type 2 diabetes and aging: A not so sweet scenario for bone. Horm Metab Res. 2016. November;48(11):771–8. [DOI] [PubMed] [Google Scholar]
- 5.Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL. Risk of fracture in women with type 2 diabetes: The women’s health initiative observational study. J Clin Endocrinol Metab. 2006. September;91(9):3404–10. [DOI] [PubMed] [Google Scholar]
- 6.de L II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: The rotterdam study. Osteoporos Int. 2005. December;16(12):1713–20. [DOI] [PubMed] [Google Scholar]
- 7.Petit MA, Paudel ML, Taylor BC, Hughes JM, Strotmeyer ES, Schwartz AV, Cauley JA, Zmuda JM, Hoffman AR, Ensrud KE, for the Osteoporotic Fractures in Men (MrOs) Study Group. Bone mass and strength in older men with type 2 diabetes: The osteoporotic fractures in men study. J Bone Miner Res. 2009. July 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: The health, aging, and body composition study. Arch Intern Med. 2005. July 25;165(14):1612–7. [DOI] [PubMed] [Google Scholar]
- 9.Vestergaard P Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007. April;18(4):427–44. [DOI] [PubMed] [Google Scholar]
- 10.Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the united states. J Am Soc Nephrol. 2006. November;17(11):3223–32. [DOI] [PubMed] [Google Scholar]
- 11.Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR, Osteoporotic Fractures Research Group. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007. January 22;167(2):133–9. [DOI] [PubMed] [Google Scholar]
- 12.Heinemann DF. Osteoporosis. an overview of the national osteoporosis foundation clinical practice guide. Geriatrics 2000. May;55(5):31,6; quiz 39. [PubMed] [Google Scholar]
- 13.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006. October;61(10):1059–64. [DOI] [PubMed] [Google Scholar]
- 14.Cauley JA, Danielson ME, Boudreau RM, Forrest KY, Zmuda JM, Pahor M, Tylavsky FA, Cummings SR, Harris TB, Newman AB, for the Health ABC Study. Inflammatory markers and incident fracture risk in older men and women: The health aging and body composition study. J Bone Miner Res. 2007. July;22(7):1088–95. [DOI] [PubMed] [Google Scholar]
- 15.Joseph C, Kenny AM, Taxel P, Lorenzo JA, Duque G, Kuchel GA. Role of endocrine-immune dysregulation in osteoporosis, sarcopenia, frailty and fracture risk. Mol Aspects Med. 2005. June;26(3):181–201. [DOI] [PubMed] [Google Scholar]
- 16.Khosla S, Peterson JM, Egan K, Jones JD, Riggs BL. Circulating cytokine levels in osteoporotic and normal women. J Clin Endocrinol Metab. 1994. September;79(3):707–11. [DOI] [PubMed] [Google Scholar]
- 17.Lacey DC, Simmons PJ, Graves SE, Hamilton JA. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: Implications for bone repair during inflammation. Osteoarthritis Cartilage. 2009. June;17(6):735–42. [DOI] [PubMed] [Google Scholar]
- 18.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005. February 25;120(4):483–95. [DOI] [PubMed] [Google Scholar]
- 19.Beausejour C. Bone marrow-derived cells: The influence of aging and cellular senescence. Handb Exp Pharmacol. 2007;(180)(180):67–88. [DOI] [PubMed] [Google Scholar]
- 20.Stenderup K, Justesen J, Eriksen EF, Rattan SI, Kassem M. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J Bone Miner Res. 2001. June;16(6):1120–9. [DOI] [PubMed] [Google Scholar]
- 21.Ben David D, Reznick AZ, Srouji S, Livne E. Exposure to pro-inflammatory cytokines upregulates MMP-9 synthesis by mesenchymal stem cells-derived osteoprogenitors. Histochem Cell Biol. 2008. May;129(5):589–97. [DOI] [PubMed] [Google Scholar]
- 22.DIppolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999. July;14(7):1115–22. [DOI] [PubMed] [Google Scholar]
- 23.Justesen J, Stenderup K, Eriksen EF, Kassem M. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int. 2002. July;71(1):36–44. [DOI] [PubMed] [Google Scholar]
- 24.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004. December;3(6):379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takada I, Kouzmenko AP, Kato S. Molecular switching of osteoblastogenesis versus adipogenesis: Implications for targeted therapies. Expert Opin Ther Targets. 2009. May;13(5):593–603. [DOI] [PubMed] [Google Scholar]
- 26.Kume S, Kato S, Yamagishi S, Inagaki Y, Ueda S, Arima N, Okawa T, Kojiro M, Nagata K. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res. 2005. September;20(9):1647–58. [DOI] [PubMed] [Google Scholar]
- 27.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010. September;65(9):963–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doyle ME, McConville P, Theodorakis MJ, Goetschkes MM, Bernier M, Spencer RG, Holloway HW, Greig NH, Egan JM. In vivo biological activity of exendin (1–30). Endocrine. 2005. June;27(1):1–9. [DOI] [PubMed] [Google Scholar]
- 29.Yuan JS, Reed A, Chen F, Stewart CN Jr., Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006. February 22;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moseley KF. Type 2 diabetes and bone fractures. Curr Opin Endocrinol Diabetes Obes. 2012. January 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz AV, Sellmeyer DE. Diabetes, fracture, and bone fragility. Curr Osteoporos Rep. 2007. September;5(3):105–11. [DOI] [PubMed] [Google Scholar]
- 32.Botushanov NP, Orbetzova MM. Bone mineral density and fracture risk in patients with type 1 and type 2 diabetes mellitus. Folia Med (Plovdiv). 2009. Oct-Dec;51(4):12–7. [PubMed] [Google Scholar]
- 33.Farr JN, Drake MT, Amin S, Melton LJ 3rd McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2013. October 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shu A, Yin MT, Stein E, Cremers S, Dworakowski E, Ives R, Rubin MR. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int. 2011. March 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen A, Dempster DW, Muller R, Guo XE, Nickolas TL, Liu XS, Zhang XH, Wirth AJ, van Lenthe GH, Kohler T, McMahon DJ, Zhou H, Rubin MR, Bilezikian JP, Lappe JM, Recker RR, Shane E. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: Comparison with transiliac bone biopsy. Osteoporos Int. 2010. February;21(2):263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, Link TM. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013. February;28(2):313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. April 2;284(5411):143–7. [DOI] [PubMed] [Google Scholar]
- 38.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006. February;5(1):91–116. [DOI] [PubMed] [Google Scholar]
- 39.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003. December;33(6):919–26. [DOI] [PubMed] [Google Scholar]
- 40.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008. June;7(3):335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Y, Mishima H, Sakai S, Liu YK, Ohyabu Y, Uemura T. Gene expression analysis of major lineagedefining factors in human bone marrow cells: Effect of aging, gender, and age-related disorders. J Orthop Res. 2008. July;26(7):910–7. [DOI] [PubMed] [Google Scholar]
- 42.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–71. [DOI] [PubMed] [Google Scholar]
- 43.Abdallah BM, Haack-Sorensen M, Fink T, Kassem M. Inhibition of osteoblast differentiation but not adipocyte differentiation of mesenchymal stem cells by sera obtained from aged females. Bone. 2006. July;39(1):181–8. [DOI] [PubMed] [Google Scholar]
- 44.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005. February 17;433(7027):760–4. [DOI] [PubMed] [Google Scholar]
- 45.Leskela HV, Risteli J, Niskanen S, Koivunen J, Ivaska KK, Lehenkari P. Osteoblast recruitment from stem cells does not decrease by age at late adulthood. Biochem Biophys Res Commun. 2003. November 28;311(4):1008–13. [DOI] [PubMed] [Google Scholar]
- 46.Cramer C, Freisinger E, Jones RK, Slakey DP, Dupin CL, Newsome ER, Alt EU, Izadpanah R. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev. 2010. September 11 [DOI] [PubMed] [Google Scholar]
- 47.de Paula FJ, Horowitz MC, Rosen CJ. Novel insights into the relationship between diabetes and osteoporosis. Diabetes Metab Res Rev. 2010. October 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim TY, Schafer AL. Diabetes and bone marrow adiposity. Curr Osteoporos Rep. 2016. December;14(6):337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, Strotmeyer ES, Resnick HE, Carbone L, Beamer BA, Park SW, Lane NE, Harris TB, Cummings SR. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006. September;91(9):3349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vestergaard P Bone metabolism in type 2 diabetes and role of thiazolidinediones. Curr Opin Endocrinol Diabetes Obes. 2009. April;16(2):125–31. [DOI] [PubMed] [Google Scholar]
- 51.Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: A randomized, controlled trial. J Clin Endocrinol Metab. 2007. April;92(4):1305–10. [DOI] [PubMed] [Google Scholar]
- 52.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995. February 2;332(5):305–11. [DOI] [PubMed] [Google Scholar]