Abstract
BACKGROUND
Trait anger or the dispositional tendency to experience a wide range of situations as annoying or frustrating is associated with negative mental and physical health outcomes. The experience of adversity in childhood is one risk factor for the later emergence of high trait anger. This association has been hypothesized to reflect alterations in neural circuits supporting bottom-up threat processing and top-down executive control.
METHODS
Here, using functional magnetic resonance imaging and self-report questionnaire data from 220 volunteers, we examined how individual differences in top-down prefrontal executive control and bottom-up amygdala threat activity modulate the association between childhood adversity and trait anger in young adulthood.
RESULTS
We report that the association between childhood adversity and trait anger is attenuated specifically in young adults who have both relatively low threat-related amygdala activity and high executive control-related dorsolateral prefrontal cortex activity.
CONCLUSIONS
These brain activity patterns suggest that simultaneous consideration of their underlying cognitive processes – namely, threat processing and executive control – may be useful in strategies designed to mitigate the negative mental health consequences of childhood adversity.
Keywords: childhood adversity, trait anger, prefrontal cortex, amygdala, fMRI, executive control
Introduction
Trait anger refers to the dispositional tendency to perceive a wide range of situations as annoying or frustrating, resulting in a low threshold for feeling angry (1). High trait anger is associated with both physical health, including increased risk for coronary heart disease (2), and mental health, including higher reactive aggression and violence (3). These health-related features of high trait anger may reflect an increased attentional and neural bias towards signals of interpersonal threat such as angry facial expressions (4). For example, higher reactive aggression has been associated with higher amygdala activity to angry facial expressions, especially in men who also report higher threat-sensitivity as indexed by trait anxiety (5). Moreover, higher reactive aggression is associated with relatively poor behavioral control and lower dorsolateral prefrontal cortex (dlPFC) executive function (6).
Prior research suggests that an important factor in the development of trait anger is the experience of adversity during childhood (7). Interestingly, such childhood adversity is also associated with higher amygdala activity to threat-related facial expressions (8) as well as lower dlPFC executive function (9). These independent findings suggest that high trait anger may be particularly present in individuals with both increased threat- and decreased executive control-related brain function, which are both linked with experience of childhood adversity. However, how these two neural circuits jointly modulate the link between childhood adversity and trait anger has not been examined.
Here, we ask if individual differences in threat-related amygdala and executive control-related dlPFC activity modulate the expression of trait anger as a function of childhood adversity. We focused on amygdala activity to threat-related facial expressions because it has been consistently associated with individual differences in personality traits (10), and the evaluation of environmental threat (11). Similarly, we focus on dlPFC activity during a working memory task because it is associated with the typical use of cognitive reappraisal as a strategy for regulating negative emotions as well as the experience of stress-related mood and anxiety (12). Specifically, we chose a computational component within a working memory task to assay individual differences in dlPFC activity. This particular task was designed to identify and probe the prefrontal executive control system, which was recently found to have implications for cognition-emotion interactions, as it operated in conjunction with other neural circuits to predict emotion regulation and risk for anxiety (12,13). Based on the patterns noted above, we hypothesized that the association between adversity in childhood and trait anger later in young adulthood would be most pronounced in individuals with the combination of relatively higher threat-related amygdala and lower executive control-related dlPFC activity.
Methods and Materials
Participants
Data were available from 220 undergraduate students (123 women, age range 18–22 years, mean age = 19.87 years) who successfully completed the Duke Neurogenetics Study (DNS) between September 30th, 2014 and November 21st, 2016 including functional magnetic resonance imaging (fMRI) tasks measuring threat-related amygdala and executive control-related dlPFC activity as well as self-reported childhood adversity and trait anger. These participants were free of past or current diagnosis of a DSM-IV Axis I or select Axis II (borderline and antisocial personality) disorder. Categorical diagnosis was assessed with the electronic Mini International Neuropsychiatric Interview (14) and Structured Clinical Interview for the DSM-IV subtests (15).
All participants provided written informed consent according to the Duke University Medical Center Institutional Review Board. To be eligible for the DNS, participants were required to be free of the following conditions: 1) medical diagnoses of cancer, stroke, head injury with loss of consciousness, untreated migraine headaches, diabetes requiring insulin treatment, chronic kidney, or liver disease; 2) use of psychotropic, glucocorticoid, or hypolipidemic medication; and 3) conditions affecting cerebral blood flow and metabolism (e.g., hypertension).
Self-Report Questionnaires
The Childhood Trauma Questionnaire (CTQ) was used to assess exposure to childhood adversity in five categories: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect (16). The State-Trait Anger Expression Inventory (STAXI) was used to index trait anger (1). The two subscales of STAXI – angry temperament (the propensity to experience anger without being provoked) and angry reaction (the propensity to experience anger in response to negative events) – were also calculated. Additionally, the State-Trait Anxiety Inventory-Trait Version (STAI-T) was used to assess trait anxiety (17) for use as a covariate as previous studies have found significant positive correlations between trait anger and trait anxiety (4,5).
Rationale for the Selection of Threat-Related Amygdala and Executive Control-Related dlPFC Activity
The DNS targeted a number of discrete neural circuit hubs while participants engaged in tasks associated with specific psychological processes, including the threat (focused on the amygdala) and executive control (focused on the dlPFC). This design allows for the isolation of individual differences in brain function associated with these psychological processes. Here, we were specifically interested in examining how an interaction between individual differences in amygdala activity supporting threat processing and dlPFC activity supporting executive control shape the emergence of trait anger associated with early adversity. We were not, in contrast, interested in directly capturing individual differences in brain function during explicit emotion regulation as this approach would not readily afford opportunities to isolate threat-related from executive control-related signals. Our rationale for further isolating the computational component of working memory is based on recent studies that demonstrate this dlPFC activity also interacts with threat processing-related amygdala activity to predict emotion regulation and future negative affect (12,13).
Amygdala Paradigm
An emotional face matching paradigm was used to elicit robust amygdala activity. The paradigm version used in the DNS consisted of four task blocks interleaved with five control blocks. A total of four emotion categories were used for each task block: fearful (F), angry (A), surprised (S), and neutral (N), taken from a standardized facial expression set (18). Participants viewed the task blocks in one of four randomly assigned orders as determined by a Latin Square (i.e., FNAS, NFSA, ASFN, SANF). During task blocks, participants viewed a trio of faces and matched one of two faces identical to a target face. Each trial in the task blocks lasted for 4 s with a variable interstimulus interval of 2–6 s (mean = 4 s), for a total block length of 48 s. The control blocks consisted of six geometric shape trios, which were presented for 4 s with a fixed interstimulus interval of 2 s for a total block length of 36 s. Each block was preceded by a brief instruction (“Match faces” or “Match shapes”) lasting 2 s. Total task time was 390 s.
dlPFC Paradigm
An event-related paradigm was used to elicit dlPFC activity during the manipulation of information in working memory (19). Briefly, the paradigm included 10 trials for each of 6 different trial types, including 3 control conditions consisting only of a 3 s response phase, and 3 working memory (WM) conditions consisting of a 0.5 s encoding phase followed by a 4 s maintenance interval and a 3s response phase. For the present study, we focused on the two types of WM conditions: maintenance and manipulation (Figure S1). In the maintenance (EC_RJ) condition, participants performed subtraction of 2 or 3 from one of 2 numbers during the brief encoding phase, then recalled the resulting two numbers and performed a numerical size judgment as instructed during the response phase after the maintenance interval. In the working memory manipulation (E_RCJ) condition, the participants performed subtraction of 2 or 3 from one of the remembered numbers after a delay, and then made the numerical size judgment. Control conditions were also included in which participants performed 1) a simple motor task (M) in response to a prompt, 2) a numerical size judgment (J), and 3) a numerical computation and size judgment task (CJ) in which they performed a numerical subtraction before size judgment. In each trial, numbers were single digits from 0 to 9; were equally balanced across 0 to 9, and equally likely to differ by either 2 or 3 units. Numerical computation and correct responses were counterbalanced for each trial type. Trials were interleaved with jittered rest intervals lasting 4 s to 8.5 s for a total scan length of 11 m 48 s. Additional details are provided the Supplemental Methods section in the Supplemental Material available online.
fMRI Data Acquisition and Preprocessing
Each participant was scanned using one of the two identical research-dedicated GE MR750 3T scanner equipped with high-power high-duty-cycle 50-mT/m gradients at 200 T/m/s slew rate, and an eight-channel head coil for parallel imaging at high bandwidth up to 1 MHz at the Duke-UNC Brain Imaging and Analysis Center. BOLD time series for each participant were processed in AFNI (20). Additional details regarding image acquisition parameters and preprocessing steps are provided in the Supplemental Methods available online.
fMRI Data Analysis
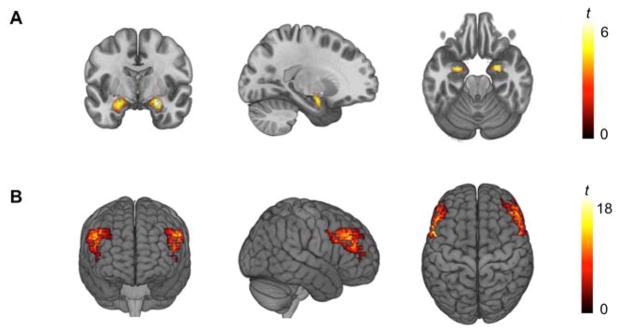
The AFNI program 3dREMLfit (http://afni.nimh.nih.gov/) was used to fit a general linear model for first-level fMRI data analyses. To obtain expression-specific parameter estimates, we explicitly modeled each respective task block (convolved with the canonical hemodynamic response function) along with the adjacent half of the preceding and following control blocks, and a first order polynomial regressor to account for low frequency noise. This allowed for the estimation of the individual task block parameters while minimizing the influence of adjacent task blocks as well as low frequency noise across the entire run. The resulting parameter estimates for the Angry + Fearful and Neutral blocks were then subtracted to obtain the Angry + Fearful > Neutral contrast. This contrast allows to index general threat-related amygdala activity, by combining mean BOLD signals to explicit, interpersonal threat (i.e., angry faces) and implicit, environmental threat (i.e., fearful faces)(21). Individual contrast images were then used in second-level random effects models in SPM12 (http://www.fil.ion.ucl.ac.uk/spm) accounting for scan-to-scan and participant-to-participant variability to determine mean condition-specific regional responses using one-sample t-tests. A statistical threshold of p < 0.05, family-wise error (FWE)-corrected across our amygdala regions of interest (ROIs; defined using a high-resolution template generated from 168 Human Connectome Project datasets)(22), and ≥ 10 contiguous voxels was applied to the Angry + Fearful blocks > Neutral block contrast. Mean parameter estimates from supra-threshold clusters within the anatomical ROIs (MNI −25, −3, −19, k = 151 and MNI 25, −5, −17, k = 151; Figure 1A) were extracted, averaged across hemispheres, and entered into our primary statistical analyses.
Figure 1.
(A) Significant bilateral amygdala activity during the perceptual processing of threat-related facial expressions (left: MNI −25, −3, −19, t = 5.23, p < 0.001, k = 151 voxels; right: MNI 25, −5, −17, t = 3.94, p < 0.001, k = 151). (B) Significant bilateral dlPFC activity during the computation of information maintained in working memory (left: MNI −45, 5, 37, t = 16.07, p < 0.001, k = 933 voxels; right: MNI 49, 9, 33, t = 15.12, p < 0.001, k = 1053 voxels).
For the dlPFC paradigm, following preprocessing, events were modeled for correctly performed trials for the response phase for each of the six trial types, and the maintenance and encoding (with and without computation modeled separately) phases for working memory trials. Incorrect responses were also modeled as regressors of no interest. A linear contrast employing the canonical hemodynamic response function was used to estimate main effects for the specific contrast of E_RCJ > EC_RJ for each individual, in order to isolate the core dlPFC executive control function of manipulation of information in working memory above and beyond basic computation and maintenance of information across a delay. Individual contrast images for E_RCJ > EC_RJ were then used in second-level random effects models accounting for scan-to-scan and participant-to-participant variability to determine mean condition-specific regional responses using one-sample t-tests. An a priori dlPFC ROI analysis was conducted using bilateral Brodmann Areas 9 & 46 generated in the WFU Pick Atlas toolbox (23). These ROIs were used to investigate dlPFC activity specifically during our contrast of interest, using an FWE threshold of p < 0.05 across the ROIs. Mean parameter estimates from the primary activation clusters (MNI −45, 5, 37, k = 933 and MNI 49, 9, 33, k = 1053; Figure 1B) within these ROIs surviving FWE-correction were extracted, averaged across hemispheres, and entered into our primary statistical analyses.
Statistical Analysis
PROCESS for SPSS (24) was utilized within SPSS 21 (IBM Corp., Armonk, NY, USA) to test whether an interaction between amygdala and dlPFC activity moderated the association between CTQ and STAXI scores (independent and dependent variables, respectively). Furthermore, we verified that resulting interactions were not confounded by age, sex, STAI-T, and task performance (mean accuracy across trials for the amygdala paradigm and dlPFC paradigm, respectively) when including them as covariates.
Results
Behavioral Results
Means and standard deviations for the self-report measures were as follows: STAXI (15.44 ± 4.45); CTQ (32.51 ± 8.27); STAI-T (36.93 ± 8.94, n = 218; STAI-T data from two participants were missing). Scores for the STAXI subscales were as follows: anger temperament (5.07 ± 1.81) and anger reaction (7.74 ± 2.54). Scores for the CTQ subscales were as follows: emotional abuse (6.9 ± 2.7), physical abuse (5.9 ± 1.9), sexual abuse (5.25 ± 1.64), emotional neglect (8.07 ± 3.36), and physical neglect (6.39 ± 2.03). STAXI scores were positively correlated with both CTQ (r = 0.4, p < 0.001) and STAI-T scores (r = 0.45, p < 0.001). CTQ and STAI-T scores were also positively correlated (r = 0.4, p < 0.001). Mean accuracy across all task blocks for the amygdala paradigm was 99.4% (± 1.7%, range = 92–100%). Mean accuracy across all conditions for the dlPFC paradigm was 96.2% (± 3.8%, range = 82–100%). There were no significant sex differences in any of these measures, except for the CTQ physical abuse score, which was higher for men compared to women (men: 6.27 ± 2.39, women: 5.62 ± 1.35, t = 2.55, p = 0.018).
fMRI Results
As expected, there was robust bilateral threat-related amygdala activity associated with the perceptual processing of angry and fearful facial expressions in comparison with neutral facial expressions (Figure 1A). There was similarly robust bilateral dlPFC activity during the computation of information maintained in working memory (Figure 1B).
Moderation Analysis
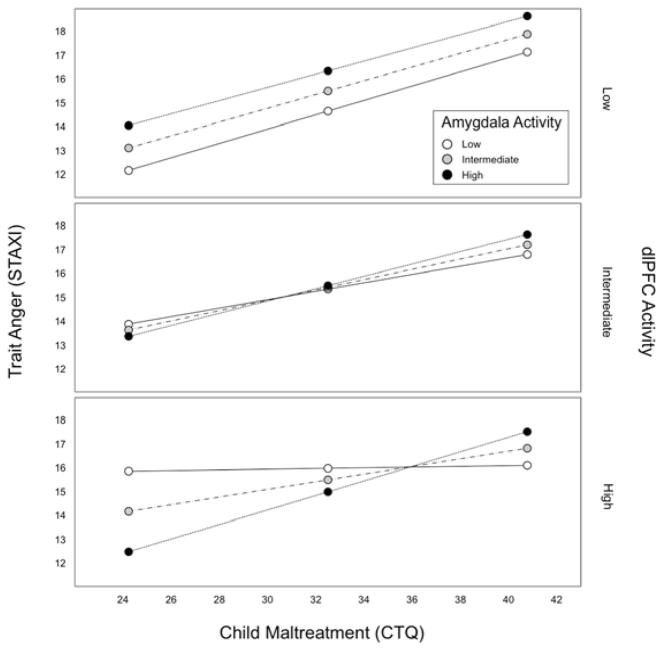
The overall model was significant in predicting trait anger (R2 = 0.25, F(7, 212) = 9.85, p < 0.0001). Importantly, a significant three-way interaction between amygdala activity, dlPFC activity, and CTQ predicted STAXI total scores (b = 1.08, 95% confidence interval (CI) = [0.22, 1.94], ΔR2 = 0.02, p = 0.0139). Follow up simple slopes analysis showed that the interaction was primarily driven by individuals with relatively low amygdala and high dlPFC activity, for whom the association between CTQ and STAXI total scores was attenuated (b = 0.001, CI = [−1.27, 0.13], p = 0.994; Figure 2). Specifically, the Johnson-Neyman technique indicated that the interaction between childhood adversity and amygdala activity was significantly associated with trait anger only when dlPFC activity was less than 0.24 standard deviations below the mean. Conditional effects of CTQ on STAXI total scores at below -1 SD, between -1 SD and +1 SD, and above +1 SD of amygdala and dlPFC activity are summarized in Table S1 in the Supplemental Material available online. Notably, this interaction was robust to the inclusion of age, sex, STAI-T, and task performance as covariates in the model (b = 0.93, CI = [0.11, 1.75], ΔR2 = 0.02, p = 0.027). For completeness, moderation analysis results using the CTQ and STAXI subscales are summarized in the Supplemental Results section in the Supplemental Material available online.
Figure 2.
The interaction of threat-related amygdala and executive control-related dlPFC activity moderates the association between childhood adversity and trait anger. Specifically, the positive association between adversity and trait anger is attenuated in individuals with relatively high dlPFC and low amygdala activity (bottom panel).
Discussion
Here we provide initial evidence that the association between childhood adversity and later trait anger is moderated by the individual differences in bottom-up threat and top-down executive control activity of the amygdala and dlPFC, respectively. Specifically, we found that a combination of relatively low threat-related amygdala and high executive control-related dlPFC activity buffered individuals against the association of higher adversity in childhood and later trait anger. Higher threat-related amygdala activity is associated with negative behavioral traits or internalizing symptoms, particularly those related to mood and anxiety disorders (10), whereas lower dlPFC activity supporting executive control is associated with poor behavioral control including regulation of negative emotions (12). In this context, the present data suggest a possibility that relatively high dlPFC and low amygdala activity may buffer against the negative impact of childhood adversity on trait anger by creating a neural platform for better cognitive control of negative emotions (25).
It is important to consider an alternative explanation to the potential buffering role of these neural activation patterns. When the degree of experienced childhood adversity was low, individuals with low amygdala activity and high dlPFC activity showed average, not low, trait anger. It is possible that individuals showing such neural activation patterns tend to have average trait anger, regardless of childhood adversity. It is unclear as to why lower levels of childhood adversity do not correspond to low trait anger in these particular individuals; this apparent complexity should be addressed in future investigations using a longitudinal design and greater sample size.
Some caveats of the present study should be addressed. First, the present data are cross-sectional and thus causal inference is inherently limited. In addition, childhood adversity was self-reported in retrospect. Since the causal relationship across variables is uncertain, we cannot rule out the possibility that individuals who showed lower amygdala activity and higher dlPFC activity may have experienced qualitatively different childhood adversity compared to others, which may have had weaker influence on brain activity and trait anger. Second, as the present data were sampled from college students, the current study cannot directly comment on the generalizability of the present findings to individuals with more severe experiences of childhood adversity such as institutionalization. Finally, as we utilized two independent fMRI tasks specifically designed to evoke neural activity in either the amygdala or dlPFC respectively, connectivity analyses that could examine how functional coupling between the amygdala and dlPFC impact the link between childhood adversity and trait anger were not possible in a meaningful way. Future studies applying functional connectivity analyses through the use of a single experimental task could help unpack the extent to which dynamic interactions between the amygdala and dlPFC activity further influence the link between childhood adversity and trait anger.
Our data expand the well-documented association between childhood abuse and personality development (7,26), by offering novel evidence showing that a specific pattern of individual differences in brain function may buffer against the detrimental effects of childhood adversity. Our findings further support efforts to understand the role of top-down prefrontal executive control in processing emotion and affect (12,27). While the cross-sectional nature of our analyses and the retrospective reporting of childhood adversity limit our ability to make causal inferences, our results nevertheless provide initial evidence that distinct patterns of brain function can moderate associations between childhood adversity and trait anger. If the patterns we observe herein are replicated in studies utilizing longitudinal designs, simultaneous consideration of threat processing and executive control may become useful in strategies designed to mitigate the negative mental health consequences of childhood adversity.
Supplementary Material
Supplemental Fig. S1. Sample trials for the E_RJ and E_RCJ conditions. E_RCJ > E_RJ contrast was used to isolate the computational component of working memory.
Supplemental Table S1. Conditional effects of childhood adversity on trait anger at low, intermediate, and high activity of the dlPFC and amygdala.
Acknowledgments
We thank the Duke Neurogenetics Study participants and the staff of the Laboratory of NeuroGenetics. The Duke Neurogenetics Study received support from Duke University as well as US-National Institute on Drug Abuse under Grant R01DA033369 and R01DA031579 to A. R. Hariri. M. J. Kim received further support from US-National Institute on Aging under Grant R01AG049789. M. A. Scult is supported by an NSF Graduate Research Fellowship. In addition, the Brain Imaging and Analysis Center received support from the Office of the Director, National Institutes of Health under Award Number S10OD021480.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spielberger CD. State-Trait Anger Expression Inventory. Odessa, FL: Psychological Assessment Resources Inc; 1991. [Google Scholar]
- 2.Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. J Am Coll Cardiol. 2009;53:936–946. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Bettencourt BA, Talley A, Benjamin AJ, Valentine J. Personality and aggressive behavior under provoking and neutral conditions: a meta-analytic review. Psychol Bull. 2006;132:751–777. doi: 10.1037/0033-2909.132.5.751. [DOI] [PubMed] [Google Scholar]
- 4.van Honk J, Tuiten A, de Haan E, van den Hou M, Stam H. Attentional biases for angry faces: relationships to trait anger and anxiety. Cogn Emot. 2001;15:279–297. [Google Scholar]
- 5.Carré JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala activity to angry facial expressions in men but not women. Soc Cogn Affect Neurosci. 2012;7:213–221. doi: 10.1093/scan/nsq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coccaro EF, Sripada CS, Yanowitch RN, Phan KL. Corticolimbic function in impulsive aggressive behavior. Biol Psychiatry. 2011;69:1153–1159. doi: 10.1016/j.biopsych.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Springer KW, Sheridan J, Kuo D, Carnes M. Long-term physical and mental health consequences of childhood physical abuse: results from a large population-based sample of men and women. Child Abuse Negl. 2007;31:517–530. doi: 10.1016/j.chiabu.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 12.Scult MA, Knodt AR, Swartz JR, Brigidi BD, Hariri AR. Thinking and feeling: Individual differences in habitual emotion regulation and stress-related mood are associated with prefrontal executive control. Clin Psychol Sci. 2017;5:150–157. doi: 10.1177/2167702616654688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scult MA, Knodt AR, Radtke SR, Brigidi BD, Hariri AR. Prefrontal executive control rescues risk for anxiety associated with high threat and low reward brain function. Cereb Cortex. 2017 doi: 10.1093/cercor/bhx304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry. 1997;12:224–231. [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JBM. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Nonpatient Edition. New York: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- 16.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Spielberger CD, Gorsuch RL, Lushene RE. STAI-Manual for the State Trait Anxiety Inventory. 3. Palo Alto, CA: Consulting Psychologists Press; 1988. [Google Scholar]
- 18.Ekman PF, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- 19.Tan H-Y, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, et al. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 2007;27:13393–13401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 21.Hariri AR, Whalen PJ. The amygdala: inside and out. F1000 Biol Rep. 2011;3:2. doi: 10.3410/B3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyszka JM, Pauli WM. In vivo delineation of subdivisions of the human amygdaloid complex in a high-resolution group template. Hum Brain Mapp. 2016;37:3979–3998. doi: 10.1002/hbm.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 24.Hayes AF. Introduction to Mediation, Moderation and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- 25.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Sudbrack R, Manfro PH, Kuhn IM, de Carvalho HW, Lara DR. What doesn’t kill you makes you stronger and weaker: How childhood trauma relates to temperament traits. J Psychiatr Res. 2015;62:123–129. doi: 10.1016/j.jpsychires.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Evans LD, Kouros CD, Samanez-Larkin S, Garber J. Concurrent and short-term prospective relations among neurocognitive functioning, coping, and depressive symptoms in youth. J Clin Child Asolesc Psychol. 2016;45:6–20. doi: 10.1080/15374416.2014.982282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1. Sample trials for the E_RJ and E_RCJ conditions. E_RCJ > E_RJ contrast was used to isolate the computational component of working memory.
Supplemental Table S1. Conditional effects of childhood adversity on trait anger at low, intermediate, and high activity of the dlPFC and amygdala.