Abstract
Early life stress (ELS) is associated with altered processing of threat signals, and increased lifetime risk of anxiety and affective pathology, disorders that disproportionately affect females. We tested the impact of a limited bedding paradigm of ELS (from P4–11) on contextual threat learning, context memory, foot shock sensitivity, and anxiety-like behavior, in adult male and female mice. To examine contextual threat learning, mice conditioned by context/foot-shock association were tested 24-hours later for the context memory. To determine the effect of ELS on foot-shock sensitivity, a separate cohort of mice were exposed to foot-shocks of increasing intensity (0.06 to 0.40mA) and behavioral responses (jump and audible vocalization) were assessed by observers blind to treatment condition, sex, and cycle stage. ELS impaired context memory in female, but not male, mice. ELS increased foot-shock induced threshold to vocalize, but not to jump, in both sexes. In female mice, this effect was most apparent during estrus. Decreased body weight, indicative of higher stress incurred by an individual mouse, correlated with increased threshold to jump in both sexes reared in ELS, and to audibly vocalize in ELS females. As ELS effects on shock sensitivity were present in both sexes, the contextual recall deficit in females was not likely driven by changes in the salience of aversive foot-shocks. No effects on anxiety-like behavior, as measured in the elevated plus maze, were observed. More work is needed to better understand the impact of ELS on both somatic and gonadal development, and their potential contribution to threat learning.
Keywords: early life stress, sex differences, foot-shock sensitivity, fear conditioning, context conditioning, threat-associated learning, anxiety, estrous cycle
Early life stress (ELS) is associated with an increased risk for developing stress-related psychopathologies (Agid et al., 1999; Draijer & Langeland, 1999; Heim & Nemeroff, 2001; Koenen & Widom, 2009; Widom, 1999). In humans, the lifetime risk of developing anxiety, depression and post-traumatic stress disorder is nearly two-fold higher in females compared with males (Altemus, Sarvaiya, & Neill Epperson, 2014; Donner & Lowry, 2013; Gater et al., 1998; McLean, Asnaani, Litz, & Hofmann, 2011; Weissman et al., 1993). However, the mechanisms underlying sex differences in risk is poorly understood.
While not meant to model pathology per se, foot-shock associated threat learning (previoulsy called fear conditioning- LeDoux & Pine, 2016) in rodents has been used to study the expression of negatively associated memories. Using this paradigm, multiple studies have attempted to elucidate the impact of ELS on threat learning and subsequent memory formation, often with mixed results. In models of maternal separation, some have found that stress increased contextual threat memory expression (Diehl et al., 2014), while others have observed a selective decrease in threat memory, either in females (Kosten, Lee, & Kim, 2006), or in males alone (Lehmann, Pryce, Bettschen, & Feldon, 1999). Handling stress and brief maternal separation in rats has also been associated with decreased contextual threat memory (Meerlo, Horvath, Nagy, Bohus, & Koolhaas, 1999). The mixed results make it difficult to determine whether different models of ELS impact contextual threat conditioning differentially, or if a third variable may be driving the observed differences. Given the lack of clarity, we sought to determine the effect of our limited bedding model of ELS on threat learning in male and female mice, and to further determine whether any observed differences could be attributed to effects on shock sensitivity or somatic changes.
Interpretation of the differences resulting from the various forms of ELS may be further complicated by differences in circulating gonadal hormones associated with reproduction. Previous work conducted in control reared rats have found that gonadal hormones significantly impacted the expression of threat associated learning (Cossio, Carreira, Vásquez, & Britton, 2016; Fenton, Halliday, Mason, Bredy, & Stevenson, 2016; Fenton et al., 2014; Gupta, Sen, Diepenhorst, Rudick, & Maren, 2001; Maren, De Oca, & Fanselow, 1994; Wiltgen, Sanders, Behne, & Fanselow, 2001). Further, studies have found that differences in gonadal hormones can impact the expression of auditory cued extinction learning (Fenton et al., 2016; Fenton et al., 2014; Milad, Igoe, Lebron-Milad, & Novales, 2009), and sensitivity to drugs that enhance threat extinction learning (Lebrón-Milad, Tsareva, Ahmed, & Milad, 2013). Thus, differences in estrous cycle stage must be taken into account when assessing learning and expression of a conditioned memory. However, few studies have focused on the interaction between ELS and estrous cycle on threat conditioning.
To test if ELS impacts the acquisition or recall of a conditioned threat memory, a limited bedding paradigm (Bath, Manzano-Nieves, & Goodwill, 2016; Walker et al., 2017), that results in fragmented maternal care, was used to model ELS. A two-day context/conditioning protocol was used to measure learning and recall of a threat memory. A separate group of mice was used to test the impact of ELS and estrous cycle stage on foot-shock sensitivity and anxiety-like behavior, all factors that can impact threat memory expression.
Here, ELS led to decreased freezing during contextual threat memory testing in female, but not male, mice. Further, ELS resulted in an increase in the threshold to vocalize in both male and female mice. In female mice, this effect was most apparent during the estrus phase of the estrous cycle. Although ELS did not alter the threshold to jump in either male or female mice, a negative correlation was found between body weight and threshold to jump in both male and female ELS mice, as well as a negative correlation between body weight and threshold to vocalize in ELS females only. These data suggest that the decreased freezing in ELS females during context recall testing, is not likely explained by ELS effects on shock sensitivity. However, the level of ELS (as indexed by body weight) negatively correlated with the intensity of foot-shock required to elicit a jump from mice. These data suggest that ELS decreases foot-shock sensitivity in mice. No changes in anxiety-like behavior were observed. Together these results indicate that ELS, in the form of limited bedding, alters contextual threat learning in females and can impact processing and response to startling or painful stimuli. In sum, greater attention should be paid to these important variables when assessing the impact of ELS on threat associated learning.
Methods
Subjects
A total of 250 C57BL/6N mice were used for this study. 40 mice (3–4 litters per group; postnatal age 75) were used for threat associated conditioning (Figure 1). A separate cohort of 140 mice (females = 15–17 litters per group, males = 7–9 litters per group; postnatal age 70–133) were used for the shock sensitivity assay (Figures 2,3,4). A separate group of 70 mice (6–7 litters per group; postnatal age 50) were used for testing in the elevated plus maze (Figure 5). The higher number of females relative to males was used to allow us to assess cycle dependent changes in behavior in females. All animals were housed on a 12-hour light:dark cycle and had ad libitum access to food and water throughout the study. All animal procedures were approved by the Brown University Institutional Animal Care and Use Committee and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Fig. 1. Impact of early life stress (ELS) on contextual threat conditioning in both males and females.
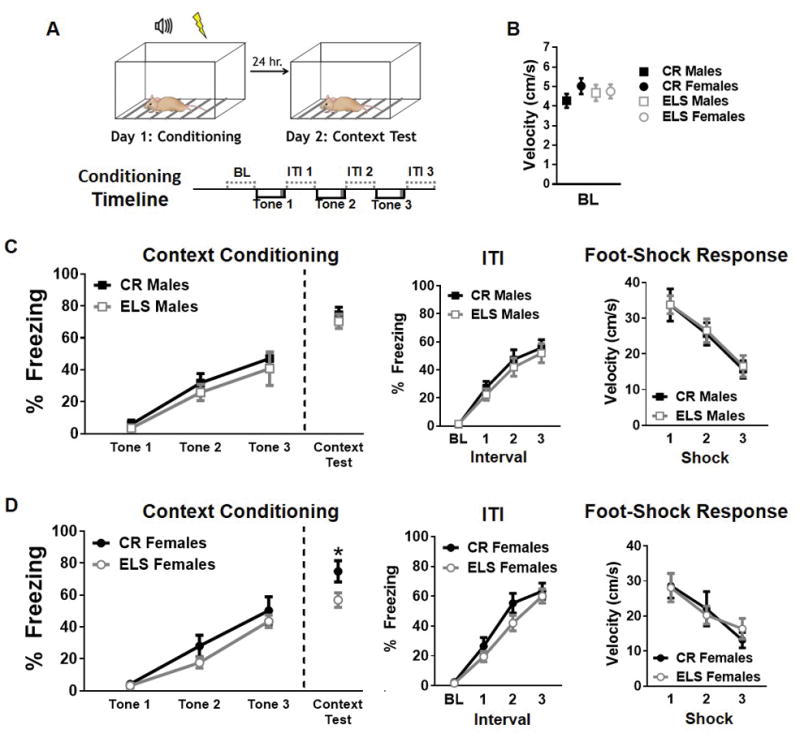
A) Schematic of contextual threat learning paradigm and description of day 1 protocol sequence. B) ELS does not affect baseline velocity in males or females. C) ELS in males does not alter freezing levels during learning or expression of contextual threat memories (left). Furthermore, freezing during ITIs (middle) and velocity during 1 second foot-shocks (right) remains unchanged. Black (CR males) and Gray (ELS males) dots and lines represent group means +/− SEM (CR males, n = 11; ELS males, n = 8). D) Unlike in males, ELS in females results in decreased freezing levels during contextual threat memory recall. However, ELS does not alter freezing during tones or during ITIs (middle). In addition, the mean velocity during the 1 second foot-shocks (right) remains unchanged. Black (CR females) and Gray (ELS females) dots and lines represent represents group means +/− SEM (CR females, n = 7; ELS females, n = 14). Two-way ANOVA and unpaired student t tests were used to assess statistical significance between groups during contextual memory expression* = p < 0.05.
Fig. 2. Comparison of shock reactivity threshold between control reared (CR) mice.
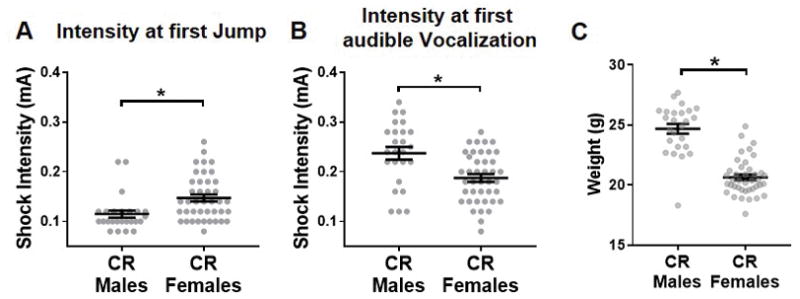
A) When compared to males, female mice have higher threshold sensitivity to jump but, B) lowered threshold to vocalize. C) Females were also found to have lower average body weight compared to males. Gray dots represent individual data, black line represents group means +/− SEM. Unpaired student t tests were used to assess statistical significance between groups (CR males, n = 25; CR females, n = 40–41) * = p < 0.05
Fig. 3. Comparison of shock reactivity between control reared (CR) and early life stressed (ELS) mice.
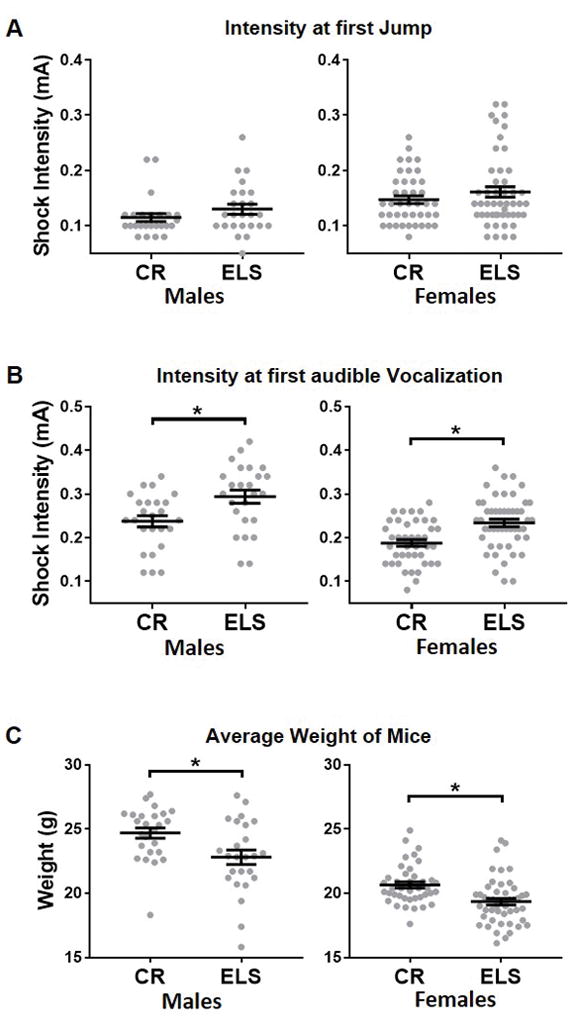
A) ELS does not affect the mice’s threshold to first jump but, B) it increased the mean threshold of onset to vocalize in both male (left) and female (right) mice. Although the mean age of all groups tested did not differ, C) ELS mice had lowered mean body weights than the CR mice. Gray dots represent individual data, black line represents group means +/− SEM. Unpaired student t test were used to assess statistical significance between CR and ELS mice of a given sex. (CR males, n = 25; ELS males, n = 25; CR females, n = 40–41; ELS females, n = 49). * = p < 0.05
Fig. 4. Emergence of weight correlation following ELS.
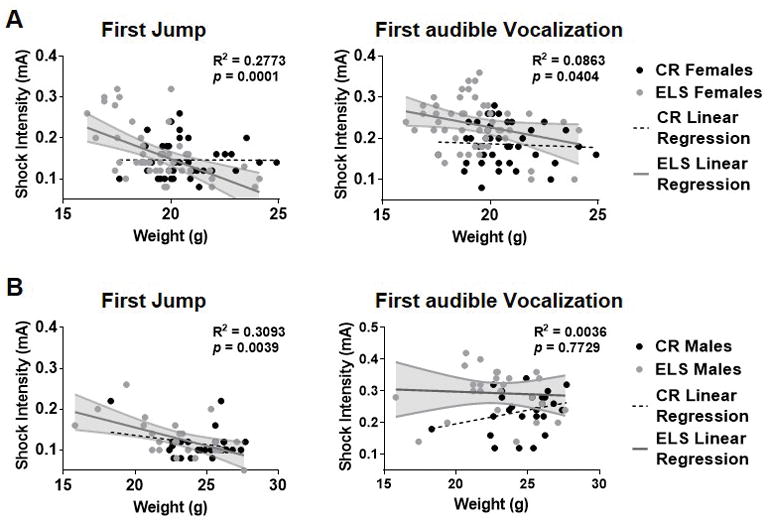
Previous studies have shown that weight is correlated with degree of ELS here we sought to assess whether ELS was associated with a correlation between weight and behavioral output. A) ELS led to the emergence of a negative correlation between the weight of female and the threshold at which they jump (left) and vocalize (right). B) In males, ELS leads to a negative correlation between the animal’s weight and their threshold to jump (left) but not their threshold to vocalize (right). Data is presented as individual values, where gray represents ELS animals and black represents CR animals. Gray line represents the linear regression for ELS animals, with shaded area corresponding to the 95% confidence interval. The dashed black line represents the linear regression for CR animals. For each graph R2 represents how well ELS data fits the curve, while the p value tests the linear regression’s departure from 0. The p value for all CR regressions are nonsignificant and presented in the results section. (CR males, n = 25; ELS males, n = 25; CR females, n = 40; ELS females, n = 49).
Fig. 5. Effect of estrous cycle on behavioral phenotypes.
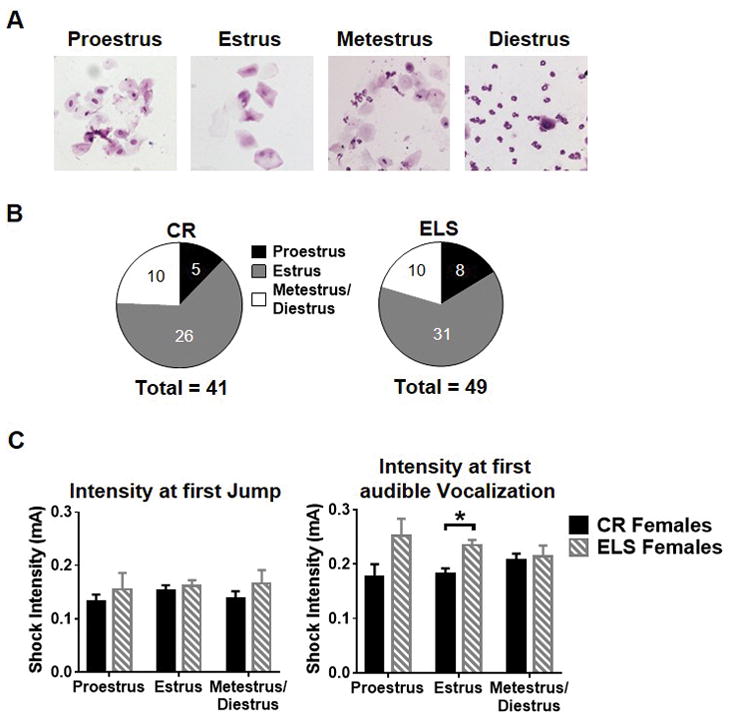
A) Representative images of each stage of the mouse estrous cycle. B) Graph depicting the distribution of cycle stages represented in each treatment group, control reared female mice (left) and early life stressed mice (right). C) Estrous cycle stage did not affect either CR nor ELS sensitivity threshold to jump (left) however it did significantly contribute to differences observed between the ELS and CR mice’s threshold to vocalize. Data is presented as group mean with SEM. A two-way ANOVA was used to assess main effects of treatment and cycle stage. Sidak’s multiple comparison analysis was used to assess differences between treatment groups at a given estrous cycle stage. * p < 0.05 (CR females, n = 41; ELS females, n = 49).
Limited Bedding Early Life Stress
ELS was modeled through a limited bedding paradigm which induces fragmented maternal care. In this paradigm, dam and pups were placed in low bedding conditions for 7 consecutive days (P4–P11), as previously described (Bath et al., 2016; Rice, Sandman, Lenjavi, & Baram, 2008). Four days after the birth of a litter (P4), the dam and pups were transferred from their standard home cage with cob bedding and a 4 x 4cm cotton nestlet to an ELS cage containing a wire mesh floor and only a 3 x 4cm cotton nestlet. The mice continued to have ad libitum access to food and water. Following one week (at P11), pups and dams were returned to their standard housing. The control reared mice (CR) were left undisturbed in a standard home cage. All pups were weaned and sex segregated at postnatal day 21.
This model of ELS is known to alter maternal behavior. Specifically, it increases the number of nest entries/departures and decreases the duration of care bouts between the dam and the pups (Rice et al., 2008). As a consequence of the limited bedding manipulation, ELS pups have decreased body weight and increased basal corticosterone levels compared with control reared pups, following the completion of the ELS manipulation (Bath et al., 2016).
Context Threat-Associated Conditioning
Mice were conditioned in a Med Associates (St. Albans City, VT) operant chamber. During the contextual conditioning session, mice were habituated to the conditioned context for 2 minutes. Following habituation, mice were administered 3 tones (30 second, 4 KHz, 80 dB), each co-terminating with a 1 second foot-shock (0.70 mA) and separated by a 30 second inter-trial interval (ITI). To assess contextual memory, mice were returned to the conditioning chamber 24 hours later and allowed to freely investigate for 5.5 minutes in the absence of further tone presentations. To insure no baseline differences in mobility were present between our groups, baseline (BL) freezing and velocity were assessed for the last 30 seconds of habituation. Freezing behavior was scored automatically using the activity tracker module in Noldus Ethovision XT11.0 and a subset of videos were hand scored to verify results.
Shock Sensitivity Assay
To determine if sex differences in shock sensitivity exist, a separate cohort of mice were tested in the same operant conditioning chamber as threat-associated conditioning (Med associates, Fairfax, VT, USA). Each mouse received multiple successive foot-shocks, beginning at 0.06 mA and increasing by 0.02 mA up to the minimum intensity at which both jumping and vocalization behaviors had been observed. The amplitude of the foot-shock at which each animal first jumped, and/or first audibly vocalized was recorded by two independent observers blind to sex and rearing condition to insure agreement on these measures. Jumping was defined as both hindpaws leaving the ground simultaneously directly following a foot-shock, vocalization was defined as the emittance of an audible sound (Quirk, Russo, Barron, & Lebron, 2000).
Elevated Plus Maze
To assess anxiety-like behavior, mice were tested on the elevated plus maze (EPM). The EPM apparatus was built in-house. It consisted of four 30 cm x 7.5 cm arms joined at a 7.5 cm x 7.5 cm center. Two of the arms were surrounded by black walls 25.5 cm tall. The floor of the maze was white to provide maximum contrast with C57BL/6N mice. The maze was elevated 80 cm from the ground.
To begin a trial a mouse was placed in the center of the arena and allowed to explore the maze for 7 minutes. The time spent in the closed versus open arms was assessed. All trials were conducted under normal room lighting conditions (~262 Lux). During the trial mice were recorded and behavior was tracked using Noldus Ethovision XT 10.0 software. Time spent in the open arms, visits to the open arms and distance walked in EPM were assessed using the Ethovision mouse tracking module. Trials in which a mouse fell off the arena were discarded, and each mouse was only tested once. Within an hour of completing the behavioral test, vaginal cytology samples were obtained.
Estrous Cycle Monitoring
Estrous cycle stage was determined by vaginal cytology in female mice immediately following testing. Vaginal cytology was assessed by dipping a sterile swab in water and gently swabbing the outer half of the vaginal canal. Vaginal samples were transferred to a microscope slide, air dried, stained using a Hema 3 staining kit (Fisher Scientific, Hampton, NH, USA), dehydrated, and then cover slipped prior to visualization on a light microscope at 20x magnification. Estrous cycle stage was assessed using previously established criteria (Aliagas et al., 2010; Bath et al., 2012) and were carried out by an observer blind to rearing condition and behavioral results.
Data Acquisition and Analysis
Videos were recorded and behavior was tracked using Noldus Ethovision XT 11.0 software. Percent time freezing was assessed using the activity tracker module in Noldus Ethovision XT11.0, and velocity was assessed through the mouse tracking module. To determine foot-shock sensitivity, mouse behavior was assessed during live video recording by two independent observers blind to the sex and rearing condition of the mouse. Vaginal cytology samples and mouse total bodyweight, in grams (g), were acquired within an hour of completing behavioral testing. Data was graphed and analyzed using Prism software (Prism, GraphPad Software, La Jolla, CA, USA). An unpaired two-tailed student t-test was used to assess group differences as indicated in the results section. Two-way ANOVA followed by either Sidak’s or Tukey’s post-hoc analysis was used to assess significant differences when more than two groups or timepoint were compared. Pearson’s correlation coefficient analysis was used to measure the level of correlation between whole body weight and the threshold to jump or audibly vocalize.
Results
Effects of ELS on Contextual Threat Conditioning
To assess the impact of ELS on contextual threat conditioning, male and female mice, reared under standard laboratory conditions (Control Reared; CR) and early life stress; ELS) conditions were used. Mice were conditioned to a context / shock association in a standard operant conditioning chamber (Figure 1A). On day one, mice were administered 3 tone / shock pairings. Inclusion of tones provided a more direct measure of threat-association learning in order to dissociate impairments in contextual memory from basic associative learning processes. On day two, mice were placed back in the conditioning chamber and their immobility (freezing) in the chamber was measured in the absence of further tone presentations. A two-tailed T-test between CR and ELS mice of the same sex did not show any significant differences in baseline velocity for males (t17 = 0.74, p = 0.46) or females (t19 = 0.4673, p = 0.64; Figure 1B).
Males
For male CR and ELS mice, a two-way ANOVA was used to test for differences in immobility (% freezing) during the acquisition (tone and ITI) and recall phases of context conditioning (Fig. 1C left). During acquisition a significant main effect of tone was found (F(2,51) = 25.94, p < 0.0001), with all groups of mice demonstrating an increase in the level of freezing across conditioning trials. During tones, no significant main effect of rearing condition was found (F(1,51) = 1.18, p = 0.28) or interaction between tone and rearing condition (F(2,51) = 0.077, p = 0.93), with ELS and CR mice showing a similar increase in levels of freezing across conditioning trials. For males, no main effect of group (CR versus ELS) was found for the context test (t-test: t17 = 0.63; p = 0.53), with ELS and CR male mice displaying similar levels of freezing during the recall of the context memory. In previous reports, ELS has been associated with increased freezing during the ITI’s (Arp et al., 2016) and decreased foot-shock sensitivity (Kosten, Miserendino, Bombace, Lee, & Kim, 2005). Here, immobility was assessed during the 30 second ITIs (Figure 1C middle). A main effect of ITI epoch was observed (F(3,68) = 40.52, p < 0.0001), indicating that the percent freezing increased as trials progressed even in the absence of a tone. As during the tone, no effect of rearing condition was found for freezing levels during ITIs (ANOVA; F(1,68) = 0.89, p = 0.34) and no significant interactions was found between rearing condition and ITI epoch (F(3,68) = 0.12, p = 0.94). To determine if ELS rearing impacted shock reactivity during conditioning, the mean velocity at the time of each 1-second 0.7mA foot-shock (Figure 1C right) was assessed. A main effect of trial (F(2,51) = 13.62, p < 0.0001) was found, with mean velocity decreasing over subsequent shock trials. However, no main effect of rearing condition (F(1,51) = 0.055, p = 0.82) or interaction between rearing condition and trial (F(2,51) = 0.010, p = 0.99) were observed for velocity during foot-shock delivery, indicating that ELS and CR males reacted similarly to the foot-shocks.
Females
To determine if ELS rearing had any impact on expression of a learned contextual threat, immobility (% freezing) was measured during the acquisition (tone and ITI) and recall phases of a context threat conditioning paradigm. During context recall, ELS females had significantly lower level of freezing when compared to control females (t-test: t19 = 2.26; p = 0.036). During the acquisition phase, no significant main effect of rearing condition (F(1,57) = 2.78; p = 0.10) or interaction between tone and rearing condition (F(2,57) = 0.58; p = 0.56) were observed, with ELS and CR mice showing near identical acquisition curves. As expected, a significant main effect of tone was found during acquisition (Figure 1D left), with mice showing increased freezing across tone-shock pairings (F(2,57) = 47.7; p < 0.0001), indicating that mice had learned the association. As with males, we also tested for group differences during the ITI phase (no tone) and for velocity in response to delivery of successive foot shocks. For females, no main effect of rearing condition was found for levels of freezing during the ITIs (F(1,76) = 3.28, p = 0.074; Figure 1D middle). Further, no interaction between rearing condition and ITI were found (F(3,76) = 0.66, p = 0.57), with both groups showing increased freezing across successive ITI’s (F(3,76) = 62.37, p < 0.0001). In response to foot-shock delivery (Figure 1D right), a significant main effect of trial was observed (F(2,57) = 6.61, p < 0.0026), with a decreasing reaction to successive foot-shocks in each group of mice. However, no main effect of rearing condition was found (F(1,57) = 0.0088, p = 0.93) nor was there a significant interaction between trial and rearing condition for velocity in response to foot shock delivery (F(2,51) = 0.25, p = 0.78). Together this data suggests that the significant decrease in contextual freezing observed during the context test cannot be explained by levels of freezing during acquisition or baseline locomotion. ELS females did not show a significant difference in within session learning when compared to controls, as measured by freezing during tones or ITIs. Furthermore, the decrease in contextual fear expression observed in ELS females was not the result of increased locomotion, as no differences in baseline velocity were observed (Figure 1B).
Sex Differences in Shock Sensitivity
To determine if sex differences in sensitivity to the aversive stimulus might drive sex differences in contextual threat recall, the behavioral response of CR male and female mice was assessed in response to foot-shocks of increasing intensity. CR female mice required a higher intensity foot-shock to elicit a jumping response compared to CR males (t-test: t64 = 2.94, p = 0.0046; Figure 2A). However, the intensity of foot-shock required to elicit an audible vocalization was significantly lower in CR females compared to CR males (t-test: t64 = 3.34, p = 0.0014; Figure 2B).
Impact of Early Life Stress on Foot-Shock Sensitivity
To determine if ELS alters the sensitivity to foot-shock in male or female CR and ELS reared mice, mice from each group were presented with foot-shocks of increasing intensity and the behavioral response to each foot-shock was recorded. ELS rearing did not impact the intensity of foot-shock required to elicit a jumping response in ether male (t-test: t48 = 1.38, p = 0.17) or female (t-test: t88 = 1.14, p = 0.26; Figure 3A) mice. However, a higher intensity foot-shock was required to elicit an audible vocalization in ELS reared male (t-test: t48 = 2.75, p = 0.0083) and female (t88 = 3.88, p = 0.0002; Figure 3B) mice compared to CR animals.
In previous work, ELS was found to significantly impact weight gain, with ELS reared animals weighing significantly less than control reared animals (Bath et al., 2016; Rice et al., 2008). Whole body weight of pups was shown to be correlated with the amount of bedding provided to dams, where less bedding resulted in decreased weight gain of pups (Rice et al., 2008). Thus, mice experiencing greater levels of stress present with a lower bodyweight. Here, we tested if body weight (as a proxy for level of stress) was related to shock sensitivity in either control reared or ELS mice, using Pearson correlation analysis. Within the control reared (CR) group, weight did not correlate with the animal’s thresholds to jump for male (r = −0.26, p = 0.22) or female (r = 0.003, p = 0.99) mice. Nor was a significant correlation found between body weight and shock intensity to elicit an audible vocalization in CR male (r = 0.28, p = 0.18) or CR female (r = −0.059, p = 0.72) mice. However, in ELS female mice, a significant negative correlation was observed between body weight and the foot-shock intensity at which mice first jumped (r = −0.53, p = 0.040; Figure 4A left) and first audibly vocalized (r = −0.29, p = 0.040; Figure 4A right). ELS female mice with lower body weight tended to require a higher intensity foot-shock to elicit a jump or vocalization. In ELS males, a negative correlation was found between body weight and the intensity of foot-shock at first jump (r = −0.56, p = 0.004; Figure 4B left) but not at first audible vocalization (r = −0.061, p = 0.77; Figure 4B right).
Contributions of Estrous Cycle Stage to Foot-Shock Sensitivity
To determine if estrous cycle stage impacted foot-shock sensitivity, vaginal cytology was used to determine the estrous cycle stage of mice immediately following foot-shock testing in both CR and ELS females (example swabs are shown in Figure 5A). The number of mice found at each stage of the estrous cycle is shown in Figure 5B. No significant main effect of estrous cycle stage (F(2.84) = 0.28, p = 0.76), rearing condition (F(2.84) = 1.58, p = 0.21), or interaction between rearing condition and cycle stage (F(2.84) = 0.16, p = 0.85) were found for mean foot-shock intensity at first jump (Figure 5C left). For vocalization, a significant main effect was found for rearing condition (F(1,84) = 9.57, p = 0.0026) but not cycle stage (F(2.84) = 0.052, p = 0.95) and no significant interaction between rearing condition and cycle stage (F(2,84) = 1.93, p = 0.15; Figure 5C right). A subsequent Sidak’s multiple comparison analysis was used to test for possible significant differences between ELS and CR female mice at each stage of the estrous cycle. Based on this analysis, female ELS reared mice required a higher intensity shock to vocalize during the estrus phase of the cycle (t84=3.50, p = 0.0024) compared with CR reared mice, with a trend in the same direction during proestrus (t84 = 2.36, p = 0.060), but no effect of rearing conditioning during metestrus/diestrus phase (t84 = 0.28, p = 0.99).
ELS Does Not Alter Anxiety-like Behavior
ELS is associated with increased risk for anxiety in humans (Hicks, DiRago, Iacono, & McGue, 2009; Kendler, Hettema, Butera, Gardner, & Prescott, 2003; Nugent, Tyrka, Carpenter, & Price, 2011) and anxiety-like behavior in rodent models (Lukkes, Mokin, Scholl, & Forster, 2009). Furthermore, increased anxiety has been shown to correlate with increased fear learning in both human (Lissek et al., 2014; Lissek et al., 2005) and rodents (Lukkes et al., 2009). However, studies in mouse models of ELS in the form of limited bedding have reported no effect (Naninck et al., 2015) or an increase (Wang et al., 2013) in anxiety-like behavior. Therefore, it remained unclear if ELS in the form of limited bedding and nesting material increases anxiety-like behavior in mice. To assess anxiety-like behavior, mice were placed in an elevated plus maze (EPM) for 7 minutes. Unpaired two-way t-test were used to test for differences between ELS and CR mice of a given sex. We found that ELS did not affect the percent of time spent in the open arms of the EPM for male (t28 = 1.586, p = 0.12; Figure 6A left) or female (t38 = 0.52, p = 0.60; Figure 6A right) mice. ELS did not affect the number of visits to the open arms in males (t28 = 0.15, p = 0.88; Figure 6B left) or females (t38 = 0.6366, p = 0.53; Figure 6B right). Since differences in locomotion could potentially impact the amount of time spent in the open arms, the total distance walked in the EPM was measured. There was no effect of rearing condition on the total distance traveled in the EPM for either males (t28 = 1.98, p = 0.057; Figure 6C left) or females (t38 = 0.91, p = 0.37; Figure 6C right). Together, this data suggests that ELS is the form of limited bedding does not alter anxiety-like behavior in mice.
Fig. 6. Elevated plus maze comparison of anxiety-like behavior between control reared (CR) and early life stressed (ELS) mice.
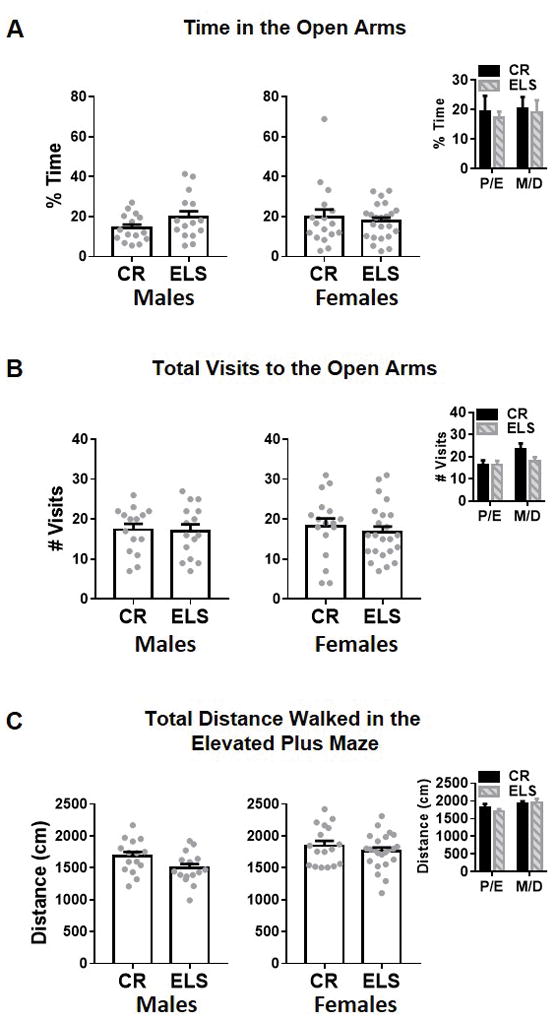
A) ELS does not affect the total percent time that mice spent on the open arms of the elevated plus mace for either males (left) or females (right), nor does estrous stage (right inset) effect this behavior. B) ELS and control male (left) and female (right) did not differ in the number of visits to the open arms. Estrous stage (right inset) did not affect the number of visits. C) ELS and CR mice walked the same total distance. Estrous stage did not affect the distance moved (right inset). Gray dots represent individual data, bars represent group means +/− SEM. Unpaired student t test were used to assess statistical significance between CR and ELS mice of a given sex, while a Two-Way ANOVA was used to assess differences in cycle stage between ELS and CR females. (CR males, n = 15; ELS males, n = 15; CR females, n = 17; ELS females, n = 23). * = p < 0.05
To assess if estrous cycle could be mediating anxiety-like behavior in females, we assessed differences in anxiety-like behavior during proestrus/estrus (P/E) and, metestrus/diestrus (M/D). A two-way ANOVA revealed no significant effect of cycle stage (F(1,36) = 0.082, p = 0.77) rearing condition (F(1,36) = 0.13, p = 0.72) or interaction (F(1,36) = 0.015, p = 0.90) on the percent time females spent in the open arms (Figure 6A right in set). Furthermore, no significant main effects were observed in visits to the open arms (cycle stage: F(1, 36) = 2.674, p = 0.11; rearing condition: F(1,36) = 0.88, p = 0.35; interaction: F(1, 36) = 1.052, p = 0.31; Figure 6B right in set) or total distance walked in the EPM (cycle stage: F(1,36) = 2.70, p = 0.10; rearing condition: F(1,36) = 0.094, p = 0.76; interaction: F(1,36) = 0.57; p = 0.45; Figure 6C right in set). Together this data suggests that estrus cycle stage does not mediate anxiety-like behavior in ELS reared female mice.
Discussion
In this study, we tested the effect of ELS and sex on contextual threat-associated learning and sensitivity to foot-shock. The response of mice to tones, ITI’s, foot-shocks, and the conditioning context were measured. In a separate group of mice, the foot-shock threshold at which mice first jumped and first exhibited an audible vocalization were also determined. Further, we tested whether estrous cycle phase or whole body weight impacted foot-shock sensitivity in either control or ELS reared mice. Here we found, that ELS selectively decreased the ability of female mice to recall and freeze to a threat associated context. However, no sex differences or effects of rearing condition were observed during the acquisition phase of testing. When assessing sex differences in the threshold of mice to jump and audibly vocalize, control females required a higher intensity foot-shock to elicit a jump, compared with males, but a lower foot-shock intensity to vocalize. Both male and female ELS reared mice had an increased threshold to vocalize, but not to jump, compared with control reared mice. Thus, the specific impairments in context recall observed in ELS female mice were not likely due to differences in sensitivity to the aversive stimulus (foot-shock), as both sexes showed similar effects of rearing condition on shock sensitivity. A significant correlation was also observed between body weight and sensitivity to foot-shock for ELS reared, but not control reared mice, indicating that the level of stress incurred by the mice may impact shock sensitivity. When assessing the impact of the estrous cycle on foot-shock sensitivity, we found that ELS females significantly differed from CR females only during the estrus stage of the estrous cycle.
Here, we found no sex differences in the levels of freezing of control reared mice during the acquisition or the recall of the contextual threat memory. These results are consistent with previous reports of some labs (Carreira, Cossio, & Britton, 2017; Graham, Yoon, Lee, & Kim, 2009), but conflict with the findings of others (Cossio et al., 2016; Gresack, Schafe, Orr, & Frick, 2009). Here, the intensity of the foot shock delivered to mice was higher (0.7 mA) than the observed threshold to jump or emit an audible vocalization in the shock sensitivity paradigm (~ 0.2 mA). It is possible that if a lower intensity foot shock were used during conditioning, sex differences may have emerged in the control reared group. Although no effect of sex was found for any phase of testing in control reared mice, ELS rearing had a significant effect on freezing during the contextual recall phase of testing in female, but not male mice. ELS reared female mice exhibited decreased freezing compared to sex matched control reared mice during the memory recall phase of testing. This finding is consistent with reports from female rats exposed to prolonged maternal separation during development (Kosten et al., 2005). However, in those reports, it was unclear if deficits in freezing in response to the conditioning context might be due to effects of ELS on either foot-shock sensitivity or differences in estrous cycle status of the female rodents.
To address these important points, a separate cohort of mice was tested in a foot-shock sensitivity paradigm to determine if sex or early life rearing conditions alter the response of mice to foot shocks. Previous work in rats have provided mixed results with some reports indicating that sex does not impact shock sensitivity (Day, Reed, & Stevenson, 2016), while several reports have found that females have a lower threshold to flinch, jump, and/or vocalize in response to a shock compared to males (Beatty & Beatty, 1970; Dalla & Shors, 2009; Kosten et al., 2005). In the current study, control reared female mice were found to have an elevated threshold to jump but a decreased threshold to vocalize when compared with control reared males. Interestingly, a high percentage of female mice (Control Females = 19.5% and ELS Females = 18.3%) vocalized at a lower threshold than the threshold required to elicit a jump. In males, a much lower percentage of mice (Control Males = 4% and ELS Males 8%), jumped at a higher threshold than that required to elicit a vocalization. Recent studies in rats have suggested that females have a more active, less stereotypical, response to foot-shock during threat conditioning (Gruene, Flick, Stefano, Shea, & Shansky, 2015). Here, we observed greater variability in the behavioral response of female mice to delivery of foot-shocks of increasing intensity, than that observed in males. Together, these results suggest that females may be more flexible in their behavioral response to foot-shock, whereas males may be more stereotyped in their behavioral response.
For ELS reared mice, we did not observe a significant effect of rearing condition on intensity at which first jump was observed, but found ELS to be associated with an increase in the threshold to audibly vocalize. This finding was surprising as previous reports that used neonatal isolation and maternal separation found a main effects of sex, but not stress, on threshold to jump and vocalize (Kosten et al., 2006; Kosten et al., 2005). Therefore, the current findings suggest that ELS in the form of maternal bedding restriction may impact behavioral response to painful stimuli in a way that is inconsistent with maternal separation or isolation. Alternatively, as previous work was carried out in rats, it is possible that the current result may reflect a species difference, with mice demonstrating a different behavioral response to pain following ELS rearing than what was observed in rats. However, more work, in which identical ELS models are employed across species, will be required to answer that question.
In previous work, bedding restriction has been associated with reduced weight across early development (Rice et al., 2008; Bath et al., 2016). Further, the degree of bedding restriction correlates with lower body weight in ELS mice (e.g. less nesting led to a lower weight) (Rice et al., 2008). Thus, the degree of weight restriction may serve as a proxy for the level of stress incurred by any individual animal. To determine if a relationship existed between stress and sensitivity to foot shock, we used whole body weight as a proxy for the level of stress and asked if a significant correlation existed between body weight and foot-shock sensitivity. We found a significant negative correlation in both male and female ELS reared mice between weight and the foot-shock intensity required to elicit a jumping response. In ELS females, but not ELS males, a significant negative correlation was also observed between the mouse’s weight and the foot-shock intensity required to elicit an audible vocalization. We did not find any significant correlation between CR animal’s whole body weight and foot-shock sensitivity. Based upon these results, ELS may in fact impact sensitivity to foot-shock, and the degree of impact may be predicted by the level of stress incurred by the animal. Alternatively, changes in body weight of the animal may impact motor function or gating of behavioral responses independent of prior stress status. However, additional studies exploring such hypotheses are needed.
To determine if estrous cycle stage contributed to differences in shock sensitivity, data on threshold to jump or audibly vocalize was separated based upon estrous cycle stage at the time of testing. While there was no main effect of cycle stage on threshold to jump or audibly vocalize, in ELS females, we observed a decrease in shock sensitivity selectively during the estrous phase of the cycle. Previous work in rats have found an increase in pain sensitivity during estrus and proestrus in control reared females (Moloney et al., 2016), indicating potential effects of circulating gonadal hormones on processing of pain signals. The current finding, that ELS decreases foot-shock sensitivity during estrus, could be indicative of disruptions in cycle associated changes in gonadal hormones that modify pain perception. However, more work would be required to test such a hypothesis.
Early life stress in the form of LBN has been reported to both increase (Dalle Molle et al., 2012) and not have effects on anxiety-like behavior (Molet et al., 2016) in rodents. Furthermore, increased anxiety-like behavior has been shown to correlate with increased fear learning in humans (Lissek et al., 2014; Lissek et al., 2005) and rodents (Lukkes et al., 2009). Therefore, we assessed if ELS modulated anxiety-like behavior in mice, potentially driving differences in contextual memory expression. Consistent with previously published work in mice (Naninck et al., 2015), we found that ELS in the form of limited bedding did not alter anxiety-like behavior in mice.
Given the constellation of results, impaired context memory in females and no effects on anxiety-like behavior, we hypothesize that deficits in fear learning are not the result of altered developmental of emotional centers, such as the amygdala, per se. Further, in prior work we found a selective deficit in spatial learning in ELS reared male, but not female, mice (Bath et al., 2017). Thus, we do not anticipate that the observed effects on contextual threat learning in females are due to deficits in hippocampal function. We instead hypothesize that ELS may be altering either prefrontal-amygdala connectivity or hippocampal to prefrontal projections. Recent studies have shown that prefrontal-amygdala connectivity is sexually dimorphic (Gruene, Roberts, Thomas, Ronzio, & Shansky, 2015). Furthermore, ELS in the form of maternal separation leads to increased dendritic arborization of the medial prefrontal cortex of female, but not male, rats (Farrell, Holland, Shansky, & Brenhouse, 2016). It is possible that ELS may be leading to hyper excitability of infralimbic-amygdala connectivity in females. Ongoing research in our lab is aimed at addressing this hypothesis.
Here, we found that ELS in female, but not male mice, led to decreased freezing in response to a context associated with threat. ELS rearing decreased sensitivity to foot shock, an effect that was present in both male and female mice. Thus, the selective decrease in freezing observed in female mice during the recall phase of testing is not likely explained by ELS effects on foot-shock sensitivity. We observed significant effects of both estrous cycle stage and body weight on shock sensitivity in ELS reared animals, indicating potential effects of these two key variables on behavioral output. No effects on anxiety-like behavior were observed. Future work will be needed to assess alternative variables that may be leading to the decrease in contextual memory expression seen in ELS females. Studies assessing changes in neuronal connectivity will be needed to fully understand the decreased contextual memory expression observed in ELS females. Additional work will also be needed to assess how ELS is affecting behaviors that are known to be modulated by the estrous cycle.
Acknowledgments
This work was supported by the Robert and Nancy Carney Fund for Scientific Innovation (KGB), the Norman Prince Neuroscience Institute Translational Research Award (KGB), National Institute of Health Grant R01MH115914 (KGB) and by the National Science Foundation Graduate Research Fellowships Program Award to GMN. Authors would also like to thank Dr. Burwell for the use of her lab’s microscopy equipment and the members of the Bath Lab for extensive reviews of the manuscript. All cartoon images are thanks to public domain clipart.
Footnotes
Disclosure statement
Dr. Bath serves as a consultant for Prothera Biologics. There are no other potential conflicts of interests to disclose.
References
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, … Lerer B. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4(2):163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Aliagas E, Torrejón-Escribano B, Lavoie EG, de Aranda IG, Sévigny J, Solsona C, Martín-Satué M. Changes in expression and activity levels of ecto-5′-nucleotidase/CD73 along the mouse female estrous cycle. Acta Physiol (Oxf) 2010;199(2):191–197. doi: 10.1111/j.1748-1716.2010.02095.x. [DOI] [PubMed] [Google Scholar]
- Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35(3):320–330. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp JM, Ter Horst JP, Loi M, den Blaauwen J, Bangert E, Fernández G, … Krugers HJ. Blocking glucocorticoid receptors at adolescent age prevents enhanced freezing between repeated cue-exposures after conditioned fear in adult mice raised under chronic early life stress. Neurobiol Learn Mem. 2016;133:30–38. doi: 10.1016/j.nlm.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Bath KG, Manzano-Nieves G, Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm Behav. 2016;82:64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, *, Chuang J, *, Spencer-Segal JL, Amso D, Altemus M, McEwen BS, Lee FS. Variant brain derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous-stage-specific expression of anxiety-like behavior in female mice. Biological Psychiatry. 2012;72(6):499–504. doi: 10.1016/j.biopsych.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Schilit Nitenson A, Lopez C, Lichtman E, Chen W, Gallo M, Goodwill H, Manzano-Nieves G. Early life stress leads to developmental and sex selective effects on performance in a novel object placement task. Neurobiology of Stress. 2017;24:57–67. doi: 10.1016/j.ynstr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Beatty PA. Hormonal determinants of sex differences in avoidance behavior and reactivity to electric shock in the rat. J Comp Physiol Psychol. 1970;73(3):446–455. doi: 10.1037/h0030216. [DOI] [PubMed] [Google Scholar]
- Carreira MB, Cossio R, Britton GB. Individual and sex differences in high and low responder phenotypes. Behav Processes. 2017;136:20–27. doi: 10.1016/j.beproc.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Cossio R, Carreira MB, Vásquez CE, Britton GB. Sex differences and estrous cycle effects on foreground contextual fear conditioning. Physiol Behav. 2016;163:305–311. doi: 10.1016/j.physbeh.2016.05.026. [DOI] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97(2):229–238. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Molle R, Portella AK, Goldani MZ, Kapczinski FP, Leistner-Segal S, Leistner-Segala S, … Silveira PP. Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl Psychiatry. 2012;2:e195. doi: 10.1038/tp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HL, Reed MM, Stevenson CW. Sex differences in discriminating between cues predicting threat and safety. Neurobiol Learn Mem. 2016;133:196–203. doi: 10.1016/j.nlm.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl LA, Pereira NeS, Laureano DP, Benitz AN, Noschang C, Ferreira AG, … Dalmaz C. Contextual fear conditioning in maternal separated rats: the amygdala as a site for alterations. Neurochem Res. 2014;39(2):384–393. doi: 10.1007/s11064-013-1230-x. [DOI] [PubMed] [Google Scholar]
- Donner NC, Lowry CA. Sex differences in anxiety and emotional behavior. Pflugers Arch. 2013;465(5):601–626. doi: 10.1007/s00424-013-1271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draijer N, Langeland W. Childhood trauma and perceived parental dysfunction in the etiology of dissociative symptoms in psychiatric inpatients. Am J Psychiatry. 1999;156(3):379–385. doi: 10.1176/ajp.156.3.379. [DOI] [PubMed] [Google Scholar]
- Farrell MR, Holland FH, Shansky RM, Brenhouse HC. Sex-specific effects of early life stress on social interaction and prefrontal cortex dendritic morphology in young rats. Behav Brain Res. 2016;310:119–125. doi: 10.1016/j.bbr.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Fenton GE, Halliday DM, Mason R, Bredy TW, Stevenson CW. Sex differences in learned fear expression and extinction involve altered gamma oscillations in medial prefrontal cortex. Neurobiol Learn Mem. 2016;135:66–72. doi: 10.1016/j.nlm.2016.06.019. [DOI] [PubMed] [Google Scholar]
- Fenton GE, Pollard AK, Halliday DM, Mason R, Bredy TW, Stevenson CW. Persistent prelimbic cortex activity contributes to enhanced learned fear expression in females. Learn Mem. 2014;21(2):55–60. doi: 10.1101/lm.033514.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry. 1998;55(5):405–413. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- Graham LK, Yoon T, Lee HJ, Kim JJ. Strain and sex differences in fear conditioning: 22 kHz ultrasonic vocalizations and freezing in rats. Psychology & Neuroscience. 2009;2:219–225. [Google Scholar]
- Gresack JE, Schafe GE, Orr PT, Frick KM. Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal-regulated kinase activation. Neuroscience. 2009;159(2):451–467. doi: 10.1016/j.neuroscience.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015;4 doi: 10.7554/eLife.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Roberts E, Thomas V, Ronzio A, Shansky RM. Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol Psychiatry. 2015;78(3):186–193. doi: 10.1016/j.biopsych.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Res. 2001;888(2):356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hicks BM, DiRago AC, Iacono WG, McGue M. Gene-environment interplay in internalizing disorders: consistent findings across six environmental risk factors. J Child Psychol Psychiatry. 2009;50(10):1309–1317. doi: 10.1111/j.1469-7610.2009.02100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiatry. 2003;60(8):789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Widom CS. A prospective study of sex differences in the lifetime risk of posttraumatic stress disorder among abused and neglected children grown up. J Trauma Stress. 2009;22(6):566–574. doi: 10.1002/jts.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Lee HJ, Kim JJ. Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006;1087(1):142–150. doi: 10.1016/j.brainres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Bombace JC, Lee HJ, Kim JJ. Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behav Brain Res. 2005;157(2):235–244. doi: 10.1016/j.bbr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Lebrón-Milad K, Tsareva A, Ahmed N, Milad MR. Sex differences and estrous cycle in female rats interact with the effects of fluoxetine treatment on fear extinction. Behav Brain Res. 2013;253:217–222. doi: 10.1016/j.bbr.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Pine DS. Using Neuroscience to Help Understand Fear and Anxiety: A Two-System Framework. Am J Psychiatry. 2016;173(11):1083–1093. doi: 10.1176/appi.ajp.2016.16030353. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav. 1999;64(4):705–715. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillon C. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol Psychiatry. 2014;75(11):909–915. doi: 10.1016/j.biopsych.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther. 2005;43(11):1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009;55(1):248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661(1–2):25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45(8):1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P, Horvath KM, Nagy GM, Bohus B, Koolhaas JM. The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. J Neuroendocrinol. 1999;11(12):925–933. doi: 10.1046/j.1365-2826.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164(3):887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, Stern H. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry. 2016;6:e702. doi: 10.1038/tp.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney RD, Sajjad J, Foley T, Felice VD, Dinan TG, Cryan JF, O’Mahony SM. Estrous cycle influences excitatory amino acid transport and visceral pain sensitivity in the rat: effects of early-life stress. Biol Sex Differ. 2016;7:33. doi: 10.1186/s13293-016-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naninck EF, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, Korosi A. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25(3):309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- Nugent NR, Tyrka AR, Carpenter LL, Price LH. Gene-environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology (Berl) 2011;214(1):175–196. doi: 10.1007/s00213-010-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20(16):6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149(10):4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, … Baram TZ. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress. 2017;20(5):421–448. doi: 10.1080/10253890.2017.1343296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Su YA, Wagner KV, Avrabos C, Scharf SH, Hartmann J, … Schmidt MV. Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nat Neurosci. 2013;16(6):706–713. doi: 10.1038/nn.3395. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU. Sex differences in rates of depression: cross-national perspectives. J Affect Disord. 1993;29(2–3):77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999;156(8):1223–1229. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Behne NS, Fanselow MS. Sex differences, context preexposure, and the immediate shock deficit in Pavlovian context conditioning with mice. Behav Neurosci. 2001;115(1):26–32. doi: 10.1037/0735-7044.115.1.26. [DOI] [PubMed] [Google Scholar]


