Abstract
Cognitive deficits are predictive of long-term social and occupational functional deficits in schizophrenia, but are currently without gold-standard treatments. In particular, augmentation of auditory cortical neuroplasticity may represent a rate-limiting first step prior to addressing higher-order cognitive deficits. We review the rationale for N-methyl-d-aspartate-type glutamate receptor (NMDAR) modulators as treatments for auditory plasticity deficits in schizophrenia, along with potential serum and electroencephalographic (EEG) target engagement biomarkers for NMDAR function. Several recently published NMDAR modulating treatment studies are covered, involving d-serine, memantine, and transcranial direct current stimulation (tDCS). While all three interventions appear to modulate auditory plasticity, direct agonists (d-serine) appear to have the largest and most consistent effects on plasticity, at least acutely. We hypothesize that there may be synergistic effects of combining pro-cognitive NMDAR modulating approaches with auditory cortical neuroplasticity cognitive training interventions. Future studies should assess biomarkers for target engagement and patient stratification, along with head to head studies comparing putative interventions and potential long term vs. acute effects.
Keywords: plasticity, schizophrenia, treatment, MMN, cognition, biomarker, NMDA
Introduction
In addition to positive and negative symptoms (1), schizophrenia is associated with deficits in neurocognition (2–5) that represent a core feature of the disorder (6, 7). Schizophrenia patients show impairments across a large variety of cognitive domains; they also show related deficits in perceptual (auditory and visual) learning, sometimes termed ‘cortical neuroplasticity’ (8, 9), during training on exercises that place implicit, increasing demands on early perceptual processing (10). Remediating or ameliorating these impaired cortical neuroplasticity processes represents a rate-limiting first step prior to addressing higher-order cognitive domains that are more proximal to occupational or social functioning (e.g., verbal or working memory, executive functioning) (11, 12). Examples of available neuroplasticity programs include adaptive tone-matching (13)—a repeated series of tone pairs in which subjects respond whether the second tone is higher or lower in frequency. Other programs, developed by Posit science, train to detect brief, high frequency-modulated auditory transient sweeps. However, ~45% of schizophrenia patients demonstrate minimal improvement when remediation is used adjunctive to antipsychotics (14), suggesting that further refinement is needed.
Similarly, previous efforts to develop procognitive/plasticity-enhancing agents as adjuncts to antipsychotics have been mixed (15–20), potentially based on two important weaknesses. First, the medication trials were not conducted in the context of active cognitive interventions. Simply adding a putative procognitive drug to a daily antipsychotic regimen may not provide a sensitive test of its activity: drugs that enhance specific neurocognitive processes, e.g. working memory capacity, might not yield clinical benefits unless paired with interventions that access those domains, i.e. utilize/place demands on working memory. Second, schizophrenia is highly heterogeneous, and pro-cognitive medication trials in schizophrenia have suffered from the absence of target engagement biomarkers that could either identify "sensitive" clinical subgroups (21) or refine dosing and intervention strategies.
What measures could potentially serve as useful biomarkers for engagement of a meaningful cognitive target in schizophrenia? And what is the evidence to support such an approach? We first briefly review the rationale for focusing on N-methyl-d-aspartate-type glutamate receptor (NMDAR) function as a target for enhancing cognition in schizophrenia. We also discuss emerging evidence of putative electroencephalographic (EEG) target engagement biomarkers for NMDAR function. We then focus on a promising cognitive enhancing strategy in schizophrenia-- the augmentation of auditory cortical neuroplasticity via modulation of NMDAR. Finally, we review evidence suggesting that while using NMDAR modulators or plasticity training alone is of interest, there may be synergistic effects of combining pro-cognitive NMDAR agents or neuromodulation methods with well-defined cognitive training interventions that target auditory cortical neuroplasticity, as well as patient stratification via the use of reliable biomarkers—a true personalized psychiatry approach.
Rationale and mechanism for NMDAR as a target for cognitive enhancement
Recent studies of NMDAR modulators have shown the ability to enhance neuroplasticity both in specific patient populations (22–26) and healthy volunteers (13, 27)—suggesting a potentially useful role in addressing cognitive deficits in schizophrenia, yet one which has not yet been translated into clinical practice. The use of NMDAR modulators stems from the well-characterized role of brain NMDAR function in both schizophrenia pathophysiology (28), and in learning and neuroplasticity (22–26), including acquisition, consolidation, and retrieval of perceptual information (29). NMDAR serve as critical triggers for long-term potentiation (LTP) and depression (LTD) (30–33). LTP occurs in two different phases – an initial NMDAR-induced increase in AMPAR trafficking that occurs over minutes (acquisition phase), followed by a delayed increase in NMDAR trafficking that occurs over hours (consolidation phase) (34).
Furthermore, in animals (35), intensive cognitive activity increases brain levels of d-serine, an endogenous, direct agonist at the NMDAR’s glycine modulatory site (36). Furthermore, administration of low-dose d-serine improves recognition and working memory function (35). In humans, serum d-serine levels are generally found to be lower at baseline in people with schizophrenia compared to controls (37). In a recent study (Panizzutti et al, under review), mean serum d-serine and l-serine levels were found to be significantly lower in schizophrenia subjects (N=90) prior to intensive cognitive training of auditory processing, as compared to healthy control subjects (N=53). Schizophrenia subjects were then assigned 50 hours of either auditory training (N=47) or a computer games control condition (N=43), followed by repeat assessment of cognition and serum amino acids. While there were no significant changes in these measures at a group level after the intervention, in the active training group increased d-serine was significantly and positively correlated with improvements in global cognition and in verbal learning and memory. No such associations were observed after the control condition. Consistent with the animal literature, these results suggest that d-serine—and hence NMDAR function—is involved in the plasticity processes induced by intensive cognitive training in schizophrenia. The significant association between change in serum d-serine levels and training-induced cognitive gains indicates the presence of inter-individual variation.
Together, these results suggest two important directions for the field: 1) Pharmacologic strategies that modulate NMDAR functioning may provide a mechanism for enhancing the behavioral effects of intensive cognitive training; 2) The identification of target engagement biomarkers will support a precision psychiatry approach for identifying those individuals most likely to respond to this method of cognitive enhancement.
EEG Target Engagement Biomarkers for NMDAR Function
Event-related potential (ERP) and event-related oscillation (ERO or time-frequency) EEG responses hold particular promise as biomarkers for NMDAR function. ERP and ERO approaches are standardized reactions of the brain to a particular stimulus. Mismatch negativity (MMN) (38, 39) is an ERP elicited when a sequence of repetitive standard tones are interrupted infrequently by a physically different “oddball” stimulus, that differs in frequency, duration or location (40–42). The oddball typically produces a more neagtive signal on the ERP, and the difference between standard and oddball response is termed mismatch negativity.
Deficits in auditory MMN generation in schizophrenia were first demonstrated in the early 1990’s and have been extensively replicated (43–46). These deficits correlate extensively with poor premorbid function and impaired psychosocial outcome even following covariation for more general demographic and neurocognitive factors (43, 46–51). In addition, test-retest reliability of auditory MMN is high, showing an ICC of 0.9 (52), further encouraging its use as a neurophysiological biomarker (45, 53).
Links between NMDAR dysfunction and MMN is supported by a series of studies in which ketamine, an NMDAR antagonist, produces schizophrenia like deficits in MMN across non-human (54–56) and human (57–62) investigations. This was confirmed in a recent meta-analysis (62), and in studies showing that MMN deficits predict parallel deficits in proton magnetic resonance spectroscopy (1H MRS) measured glutamate (63, 64). By contrast, MMN is relatively unaffected by treatment with antipsychotics (46, 65, 66), suggesting minimal impact of dopamine antagonism on MMN.
Pertinent to the present review, auditory MMN is increasingly conceptualized as reflecting the “prediction error” when the deviant differs from the standard stimulus (67), and is highly predictive of response to auditory cognitive training (68, 69), and thus can be considered a neurophysiological proxy of both target engagement for NMDAR-associated cortical plasticity and the integrity of early auditory information processing. This association permits some interesting predictions on the expected benefit of interventions that improve early auditory information processing as indexed by MMN response. Using structural equation modeling in 1415 schizophrenia patients, a recent study (10) determined that measures of early auditory information processing, particularly MMN, had a direct (mediating) effect on cognition (p<0.001), that cognition had a direct effect on negative symptoms (p<0.001), and that both cognition (p<0.001) and negative symptoms (p<0.001) had direct effects on functional outcome. Overall, early auditory information processing had a fully mediated effect on functional outcome, engaging general rather than modality-specific cognition. Explicitly, this model predicted that a 1 µV change in early auditory information processing (including MMN) will result in improvements of approximately d=0.78 for cognition and d=0.28 for psychosocial functioning (10).
ERO activity complements ERP data by assessing circuit-level functions. ERO activity is divided conventionally into discrete (e.g. θ (4–7 Hz), α (7–12 Hz) and β (12–30 Hz) bands, which reflect differential underlying local-circuit processes (21, 70–72). Within these bands, ERO is further differentiated into those that reflect alterations in phase reset mechanisms, as reflected in intertrial coherence (ITC), vs. those that reflect alterations in single-trial power (e.g. 71, 73). ERO measurements complement early auditory processing ERP, such as MMN (74), which are typically associated with increases in both θ-ITC and power (75). By contrast, suppression of ongoing α and β activity (e.g., event-related desynchronization, ERD) has been tied to bringing regions “on-line” during cognitive processing (76–78). In this review, we will also discuss other measures of early auditory information processing, such as steady-state (ASSR) responses (21) and prepulse inhibition of startle (PPI). Although literature linking these measures to NMDAR dysfunction is less robust than for MMN, they are also widely used measures (21, 52) with potential clinical utility. Enhancing visual plasticity and visual ERP biomarkers (79–82) are also under active study, but will not be specifically covered.
The NMDAR agonist d-serine as a means of enhancing auditory cortical neuroplasticity
Two recently published studies investigated whether novel NMDAR agonists can differentially modify neurophysiological plasticity (auditory MMN), and whether changes in this neurophysiological measure has predictive value for efficacy. Studies utilized d-serine at a dose of 60 mg/kg/d, which may be more effective than earlier studies at 30 mg/kg (83, 84), along with a glycine type I (GlyT1) transport inhibitor (bitopertin). Bitopertin showed promise as a stand-alone treatment in an initial study (85), but subsequent studies for bitopertin (86, 87) and other selective GlyT1 inhibitors (88) were negative.
Subchronic d-serine administration
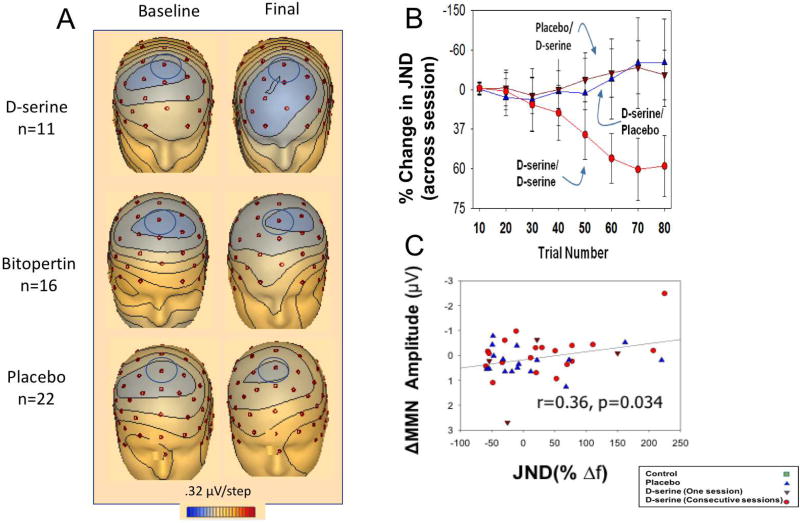
In this study (89), 16 schizophrenia patients were treated with d-serine or placebo daily for 6 weeks. Significant, large effect size improvement in auditory MMN (d=2.3) was seen as compared to placebo (Fig. 1A), along with a significant treatment effect for PANSS total (d=0.80) and negative symptoms (d=0.88). Specificity of d-serine’s effect on auditory MMN was suggested by a parallel study (86) of bitopertin. Using an identical paradigm to the d-serine study, no significant effect was seen for auditory MMN, nor for any other behavioral outcome. Moreover, in the d-serine study, baseline auditory MMN predicted change in total PANSS, whereas no relationship between outcome and MMN was seen in the bitopertin study. This suggests that bitopertin failed because of inadequate target engagement (as measured by the auditory MMN).
Figure 1.
A. Voltage topography maps for d-serine (top), bitopertin (middle) and placebo (bottom) subjects for MMN, shown at peak latencies. Analyzed electrode noted by blue circle (Fz). B. Line graph of % change in behavioral plasticity on the adaptive tone-matching task across sessions for the indicated treatment orders. C. Scatter plot for % change in behavioral plasticity on the adaptive tone-matching task vs. change in MMN amplitude to the trained tone. E. Modified from (8, 89). Error bars indicate standard error of the mean; ***p<0.001
Acute d-serine administration
Following up on these findings of sub-chronic, daily treatment with d-serine, a recent study (8) utilized a well validated, brief neuroplasticity-based adaptive tone-matching program (auditory training) (13) to assess the efficacy of intermittent, weekly d-serine treatment. In addition to behavioral effects, participants were assessed on neurophysiological cortical plasticity biomarkers, including MMN and θ-ITC. 21 schizophrenia patients received three auditory training sessions separated by 1-week, paired with either d-serine 60 mg/kg or placebo. Analyses focused on effects of both initial and repeated d-serine administration, given 30 minutes before sessions to allow for assessment at peak serum d-serine levels (84).
While there were no significant treatment effects after the 1st session, over subsequent sessions, a highly significant improvement in cortical plasticity, as assessed by a smaller tone-matching threshold, was seen in subjects who received d-serine in two consecutive sessions (d=1.03, Fig. 1B). By contrast, subjects showed non-significant worsening in the second session if they received either placebo followed by d-serine (d=−0.36) or d-serine followed by placebo (d=−0.30).
As expected, schizophrenia patients had significant baseline deficits in auditory MMN generation compared to healthy controls. Similar to the behavioral results, schizophrenia patients receiving two consecutive sessions of d-serine+audtitory training had a significant larger pre-post change in auditory MMN (d=0.7), demonstrating both target engagement of the NMDAR by d-serine and improvement in neurophysiological plasticity. By contrast, and consistent with prior studies (68), groups receiving placebo+auditory tarining showed a tendency toward worsening of the auditory MMN response. Across schizophrenia patients, a relationship between plasticity and functional target engagement was demonstrated by a significant correlation between changes in MMN and plasticity improvements (tone-matching threshold: r=−0.34, Fig. 1C).
In addition, significant overall statistical effects were observed for θ-ITC and β-power, driven primarily by significant differences between the consecutive session d-serine and the placebo groups. Similar to auditory MMN, θ-ITC during the motor-preparation interval correlated significantly with plasticity thresholds. Correlations remained significant after control for group status (r=−0.32).
A noteworthy result of this study is the observed large behavioral improvement following only 2 sessions of auditory training paired with d-serine, as opposed to following ~50 hours of training (90, 91). We hypothesize that two consecutive treatments allow for action during two phases of NMDAR-induced plasticity: the acquisition phase and the consolidation phase. Overall, these studies suggest that repeated administration of d-serine led to intercorrelated improvements in auditory plasticity as assessed by a behavioral measure and by auditory MMN generation.
The NMDAR uncompetitive antagonist memantine as a means of enhancing auditory cortical neuroplasticity
In contrast to d-serine, memantine is an uncompetitive NMDAR partial antagonist of low affinity (92), producing only 30% NMDAR occupancy (93). Along with its NMDAR modulating effects, memantine also activates dopaminergic (94) receptors. By contrast to the detrimental effects of ketamine, a non-competitive antagonist of the NMDAR, previous studies in healthy subjects have reported that acute administration of memantine significantly increases auditory MMN (d=0.87 for 30 mg) (95), as well as PPI (96).
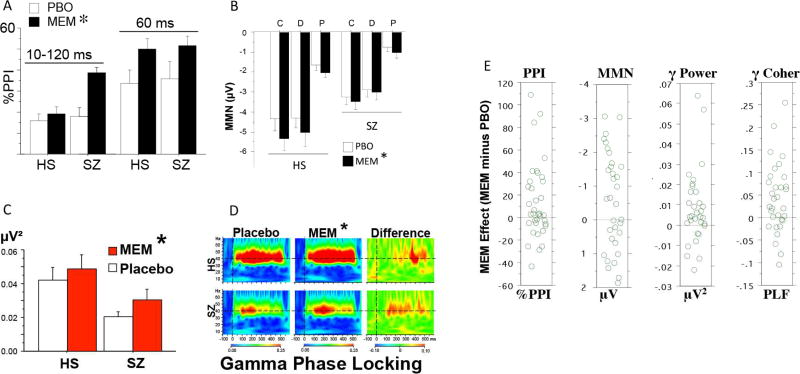
In a series of recent studies using a placebo-controlled, within-subject cross-over design, statistically significant positive effects of memantine (20 mg) on auditory MMN, PPI and ASSR were detected (97, 98) in schizophrenia and healthy controls. In each case, one 20 mg pill of memantine significantly “moved” these measures in schizophrenia patients towards “normal” values. These changes could not be explained on the basis of antipsychotic medication interactions, or other artifacts related to illness treatment or chronicity, as qualitatively similar changes were also detected in healthy controls. For PPI, these memantine-induced changes were somewhat less robust in healthy controls; for MMN, they were somewhat more robust in healthy controls; and for ASSR, they were roughly comparable in healthy controls and patients (Fig. 2A–D).
Figure 2.
Effects of memantine on A: PPI; B: MMN; C, D: ASSR in healthy subjects or schizophrenia patients. Patients and HS (n’s=42 & 42) were tested after placebo or memantine (10 or 20 mg p.m.; 20 mg shown here). Compared to healthy subjects, patients had deficits in MMN and ASSR. Memantine (20 mg) significantly enhanced PPI (A; p<0.04 for 10–120 ms; p<0.01 for 60 ms), MMN (B; p<0.014; Duration; Pitch; Combined) and ASSR (C: evoked power, 40 Hz *p<0.025; D: gamma phase locking, *p<0.002). E. Distributions of “memantine effect” (memantine (20 mg) minus placebo) on early auditory processing performance, for (left to right): PPI (60 ms), MMN, gamma power and gamma coherence, in healthy subjects or schizophrenia patients (pooled, since there were no group differences in memantine effects) Modified from (97, 98).
Recent meta-analyses (99–101), suggest that subchronic use of adjunctive memantine offers modest but statistically significant symptomatic benefits, along with cognitive benefits. One recent study did not demonstrate improvement in cognition after a single dose of memantine (102). Importantly, none of these previous studies utilized memantine in concert with a systematic cognitive training intervention, nor were any specific biomarkers used to stratify the cohort into memantine-sensitive and memantine-insensitive patient subgroups.
Plasticity as a biomarker for individualized treatment
Conceivably, the magnitude of the early auditory information processing response to memantine challenge might serve as such a “biomarker” of memantine sensitivity in schizophrenia patients. While statistically significant increases in early auditory processing measures were detected after memantine in both healthy controls and schizophrenia patients, there was heterogeneity in this memantine response, with some healthy controls and patients exhibiting robust increases after memantine, and others showing little change, or even reductions (Fig. 2E). This heterogeneity of response might provide a basis for stratifying patients into subgroups based on predicted treatment effects, and may be more specific than stratifying based on clinician rated symptom scales.
We propose that, by using a memantine “challenge” test, in the span of two office visits separated by one week, individuals with schizophrenia might be defined as “memantine-sensitive” vs. “memantine-insensitive”, based on their change in early auditory processing measures such as MMN, ASSR and PPI after a single dose of memantine vs. placebo. Although data is limited, similar challenge tests could be used for other NMAR modulating agents. It is also conceivable that such patient subgroups might differ in cognition-relevant NMDAR circuitry, and hence represent neurobiologically distinct forms of this disorder, shedding important light on pathophysiologic mechanisms.
Transcranial Direct Current Stimulation effects on NMDAR function as a means of enhancing auditory cortical neuroplasticity
Transcranial direct current stimulation (tDCS) is a non-invasive neuromodulation technique that applies low direct electrical current on the scalp to target underlying cortical regions (103–105). tDCS effects may be due in part to modifications of synaptic strength, mediated by NMDAR activity (106–108). The effects of tDCS are divided into two phases: online, when the current is active, and offline, during the post-stimulation period. During the online phase, the current is proposed to preferentially polarize pyramidal neurons resulting in intracellular voltage shifts. Cortical regions under the anodal or positive electrode undergo a slight depolarization where neurons are brought closer to their action potential threshold. This depolarization is not sufficient to cause neuronal firing, but increases the probability of an action potential to occur. Conversely, cathodal stimulation hyperpolarizes the cell thereby decreasing the probability of neuronal firing (109–111). Once the tDCS current is turned off, intracellular voltages return to their baseline state but the effects of tDCS persist (103).
tDCS and NMDAR Function
tDCS appears to enhance plasticity through NMDAR mechanisms, as shown through studies that have focused on motor plasticty. tDCS of the motor cortex induces offline effects lasting 60–90 min after a single stimulation (112, 113). After anodal tDCS, amplitudes of motor evoked potential (MEP) are increased compared to pre-tDCS. Following cathodal tDCS, there is a decrease in MEP amplitudes (109, 114). In human studies, NMDAR antagonists abolish tDCS induced MEP changes (112, 113). A recent mouse study suggested that tDCS induces neuroplastic changes through modulation of NMDAR activity (115, 116). Given the putative association between NMDAR dysfunction and symptoms of schizophrenia, tDCS may have a role in helping to address aspects of the cognitive impairments seen in the illness (36, 73, 117). Indeed, a recent pilot study demonstrated that tDCS combined with working memory training in schizophrenia enhanced the behavioral gains, as compared to training alone (118).
tDCS and Auditory Mismatch Negativity
The neural effects of tDCS applied alone in non-motor systems such as auditory processes are less well characterized (119–121). In an initial study, tDCS was applied to the dorsal lateral prefrontal cortex of schizophrenia participants in a parallel, sham-controlled design. There was a large effect size (d=0.95) decrease in auditory MMN amplitude with anodal tDCS compared to sham (control condition) (122). This suggests that anodal tDCS facilitated neuroplastic changes as reflected by modulation of MMN amplitude. The decrease in amplitude was unexpected, as although polarity-specific effects in non-motor systems are less consistent (121), anodal tDCS has been associated with increased cortical activity during stimulation of the motor cortex. This tDCS-induced decrease in MMN does not apppear to be a schizophrenia specific finding, as anodal tDCS of prefrontal regions in healthy controls also decreased MMN amplitude (123).
Based on findings that reduced MMN amplitudes in schizophrenia are associated with poorer cognitive status and greater functional impairments, we hypothesize that improved plasticity should be associated with increased MMN. However, these studies typically compare MMN deficits with chronic and stable functional impairments (68, 124, 125), and it may be possible that acute reduction in MMN represents increased neural efficiency.
tDCS and Tone Matching Task
In addition to neurophysiological measures such as MMN, auditory discrimination tasks may also corroborate auditory system target engagement by tDCS. In a static version of the adaptive tone-matching program (13), the Tone Matching Task (TMT) (126), subjects are presented with pairs of auditory tones and asked to determine if they are identical or differing in frequency. The early auditory processing deficits in schizophrenia contribute to impairments in tone discrimination and patients perform worse than healthy controls (127–129).
In a within subject cross-over study (130), a single session of cathodal tDCS targeting the primary auditory cortex demonstrated significant improvement in TMT performance compared to sham. Anodal stimulation also improved TMT performance at a trend level. The improvement in tone discrimination suggested that tDCS is able to engage and modulate auditory processing. However, the improvement after both cathodal and anodal tDCS was unexpected. A post-hoc analysis revealed an interaction between negative symptoms and stimulation condition where TMT performance declined with greater negative symptom severity after cathodal stimulation. In contrast, higher negative symptom burden was associated with improved TMT performance after anodal stimulation (130). NMDAR dysfunction has been implicated in both auditory processing deficits and negative symptoms (131, 132). Therefore, a possible explanation of these results is that tDCS modulates NMDAR activity and produces variable effects that reflect individual patient variability in NMDAR dysfunction, consistent with the picture emerging from the other studies reviewed here.
Auditory Plasticity Training alone and NMDAR functioning
Recent studies examining the effects of auditory training interventions alone on ERP biomarkers have yielded mixed results (68, 124, 125). In a recent study by Perez et al., schizophrenia participants engaged in one hour of auditory perceptual training (68). Consistent with previous findings, training improved task performance (133, 134). However, there was a small, but statistically significant decrease in auditory MMN amplitude assessed immediately after the one-hour training. This decrease in MMN is similar to the acute effects seen after tDCS (122) and to placebo findings in the d-serine study (8).
Few studies have assessed plasticity intervention effects on MMN beyond the acute post-intervention period, so the longer-term effects on MMN are unknown. One possible consideration is that training induced changes to neural substrates continue to evolve beyond the acute time frame. Eventually, steady state is achieved at which time MMN amplitudes may look very different from the acute post-intervention period.
Menning et al. conducted a study of healthy controls undergoing multiple sessions of auditory training over three weeks and assessed mismatch field amplitudes (MMF, the neuromagnetic counterpart to MMN) during and post training. There was a significant increase in MMF amplitude midway through the three weeks of training. MMF was then assessed immediately at the conclusion of the three-week training, and three weeks after the conclusion of training. Illustrating the evolving nature of the mismatch response, the MMF at these two later time points had decreased compared to the midway findings and were no longer statistically different from baseline amplitudes (125).
Thus, there appear to be some interesting contrasts between the increases in auditory MMN observed after acute or sustained d-serine or memantine treatment, which act directly on NMDAR function, vs. the decreases that are observed immediately after tDCS and single session cognitive training which act on distributed neural system function. Furthermore, findings from multiple training sessions suggests a dynamic pattern of change rather than a single static shift of amplitude in response to training. These findings deserve closer investigation, as they are likely to yield insights into the nature and timing of differing mechanisms of action that can be brought to bear on auditory cortical plasticity.
Conclusions
In this review, we have demonstrated that that deficient auditory plasticity, as measured by behavioral tone matching paradigms and neurophysiological measures (MMN) in schizophrenia patients – widely viewed to reflect fixed, heritable abnormalities in brain mechanisms regulating auditory information processing – can be significantly enhanced (i.e. brought significantly closer to normal values) via interventions targeting the NMDAR receptor in chronically ill schizophrenia patients. These findings demonstrate significant plasticity in brain mechanisms that are thought to contribute to “core” neurocognitive deficits in schizophrenia.
While d-serine, memantine and tDCS all appear to modulate auditory plasticity, the degree of modulation varies across studies. Agents that work directly on NMDAR functioning, particularly d-serine or glycine (135), appear to have the largest, most consistent and sustained effects in schizophrenia, while those that work at the circuit level (tDCS, auditory training) appear to decrease MMN (at least acutely). Head to head studies are warranted to assess potential differences between d-serine and memantine on plasticity, potential synergistic effects of pharmacological, brain stimulation and auditory training and potential long term vs. acute effects. In addition, the refinement of target engagement biomarkers such as MMN will support a precision psychiatry approach for patient stratification most likely to respond to this method of cognitive enhancement (see Fig. 2E). Finally, the auditory plasticity paradigms described appear to be well suited to be used as a “screening” paradigm for assessing the efficacy of a putative pharmacologic cognitive enhancers. For example, the effects of d-serine or memantine on the adaptive tone-matching task could be used as the “gold standard,” and used to test novel compounds such as non-selective inhibitors of glycine transport (136, 137), d-amino acid oxidase (DAAO) inhibitors (136, 138) or phosphodiesterase inhibitors (139, 140).
Acknowledgments
This work was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 RR024156 and the Dr. Joseph E. And Lillian Pisetsky Young Investigator Award for Clinical Research in Serious Mental Illness to JTK; MH59803, MH94320 and the Brain & Behavior Research Foundation to NRS; and MH068725 to SV.
Dr. Kantrowitz reports having received consulting payments within the last 24 months from Krog & Partners Incorporated, Kinetix Group, Annenberg Center for Health Sciences at Eisenhower, Semantics MR LTD, Transperfect, and Cowen and Company. He has conducted clinical research supported by the NIMH, the Stanley Foundation, Taisho, Lundbeck, Boehringer Ingelheim, NeuroRX, Merck, and Lilly within the last 24 months. He owns a small number of shares of common stock in GlaxoSmithKline. Dr. Vinogradov is a site Investigator on an SBIR grant to Positscience Inc., a company with a commercial interest in cognitive training software. She is on the Scientific Advisory Board of Mindstrong and Alkermes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This review was previously presented in part as a symposium entitled “Neural Substrates of Auditory Plasticity” at the Society of Biological Psychiatry's 72nd Annual Scientific Meeting, May 18–20, 2017.
Financial Disclosures:
All other authors report no biomedical financial interests or potential conflicts of interest.
Literature cited
- 1.Kantrowitz JT. Managing Negative Symptoms of Schizophrenia: How Far Have We Come? CNS Drugs. 2017;31:373–388. doi: 10.1007/s40263-017-0428-x. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz MM, Moberg JP, Ragland JD, Gur RC, Gur RE. Symptoms versus neurocognitive test performance as predictors of psychosocial status in schizophrenia: a 1- and 4-year prospective study. Schizophrenia bulletin. 2005;31 doi: 10.1093/schbul/sbi004. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophrenia bulletin. 2007;33:1013–1022. doi: 10.1093/schbul/sbl057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. The American journal of psychiatry. 1994;151:351–356. doi: 10.1176/ajp.151.3.351. [DOI] [PubMed] [Google Scholar]
- 5.Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr A separate disease within the syndrome of schizophrenia. Archives of general psychiatry. 2001;58:165–171. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- 6.Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Archives of general psychiatry. 2012;69:1216–1224. doi: 10.1001/archgenpsychiatry.2012.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nature reviews Neuroscience. 2015;16:620–631. doi: 10.1038/nrn4005. [DOI] [PubMed] [Google Scholar]
- 8.Kantrowitz JT, Epstein ML, Beggel O, Rohrig S, Lehrfeld JM, Revheim N, et al. Neurophysiological mechanisms of cortical plasticity impairments in schizophrenia and modulation by the NMDA receptor agonist D-serine. Brain : a journal of neurology. 2016;139:3281–3295. doi: 10.1093/brain/aww262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher M, Mellon SH, Wolkowitz O, Vinogradov S. Neuroscience-informed Auditory Training in Schizophrenia: A Final Report of the Effects on Cognition and Serum Brain-Derived Neurotrophic Factor. Schizophrenia research Cognition. 2016;3:1–7. doi: 10.1016/j.scog.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas ML, Green MF, Hellemann G, Sugar CA, Tarasenko M, Calkins ME, et al. Modeling Deficits From Early Auditory Information Processing to Psychosocial Functioning in Schizophrenia. JAMA psychiatry. 2017;74:37–46. doi: 10.1001/jamapsychiatry.2016.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medalia A, Erlich M. Why Cognitive Health Matters. American journal of public health. 2017;107:45–47. doi: 10.2105/AJPH.2016.303544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medalia A, Saperstein AM, Hansen MC, Lee S. Personalised treatment for cognitive dysfunction in individuals with schizophrenia spectrum disorders. Neuropsychological rehabilitation. 2016:1–12. doi: 10.1080/09602011.2016.1189341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahissar M, Lubin Y, Putter-Katz H, Banai K. Dyslexia and the failure to form a perceptual anchor. Nature neuroscience. 2006;9:1558–1564. doi: 10.1038/nn1800. [DOI] [PubMed] [Google Scholar]
- 14.Murthy NV, Mahncke H, Wexler BE, Maruff P, Inamdar A, Zucchetto M, et al. Computerized cognitive remediation training for schizophrenia: an open label, multi-site, multinational methodology study. Schizophrenia research. 2012;139:87–91. doi: 10.1016/j.schres.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 15.Barch DM. There are currently no proven pharmacological or psychological treatments for the core cognitive deficits of schizophrenia. Biological psychiatry. 2011;69 [Google Scholar]
- 16.Barch DM. Pharmacological strategies for enhancing cognition in schizophrenia. Current topics in behavioral neurosciences. 2010;4:43–96. doi: 10.1007/7854_2010_39. [DOI] [PubMed] [Google Scholar]
- 17.Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. The American journal of psychiatry. 2007;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- 18.Green MF. Cognition, drug treatment, and functional outcome in schizophrenia: a tale of two transitions. The American journal of psychiatry. 2007;164:992–994. doi: 10.1176/ajp.2007.164.7.992. [DOI] [PubMed] [Google Scholar]
- 19.Goff DC. Once-weekly D-cycloserine effects on negative symptoms and cognition in schizophrenia: an exploratory study. Schizophrenia research. 2008;106:320–327. doi: 10.1016/j.schres.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goff DC, Keefe R, Citrome L, Davy K, Krystal JH, Large C, et al. Lamotrigine as add-on therapy in schizophrenia: results of 2 placebo-controlled trials. Journal of clinical psychopharmacology. 2007;27:582–589. doi: 10.1097/jcp.0b013e31815abf34. [DOI] [PubMed] [Google Scholar]
- 21.Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heresco-Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar G, et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biological psychiatry. 2005;57:577–585. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 23.Goff DC. D-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophrenia bulletin. 2012;38:936–941. doi: 10.1093/schbul/sbs012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javitt DC. Harnessing N-methyl-d-aspartate receptors for new treatment development in psychiatry: positive lessons from negative studies. The American journal of psychiatry. 2013;170:699–702. doi: 10.1176/appi.ajp.2013.13040503. [DOI] [PubMed] [Google Scholar]
- 25.Cain CK, McCue M, Bello I, Creedon T, Tang DI, Laska E, et al. d-Cycloserine augmentation of cognitive remediation in schizophrenia. Schizophrenia research. 2014;153:177–183. doi: 10.1016/j.schres.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ori R, Amos T, Bergman H, Soares-Weiser K, Ipser JC, Stein DJ. Augmentation of cognitive and behavioural therapies (CBT) with d-cycloserine for anxiety and related disorders. The Cochrane database of systematic reviews. 2015;5:Cd007803. doi: 10.1002/14651858.CD007803.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahissar M, Nahum M, Nelken I, Hochstein S. Reverse hierarchies and sensory learning. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2009;364:285–299. doi: 10.1098/rstb.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clinical schizophrenia & related psychoses. 2010;4:189–200. doi: 10.3371/CSRP.4.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin R, Dor-Abarbanel AE, Edelman S, Durrant AR, Hashimoto K, Javitt DC, et al. Behavioral and cognitive effects of the N-methyl-D-aspartate receptor co-agonist D-serine in healthy humans: initial findings. Journal of psychiatric research. 2015;61:188–195. doi: 10.1016/j.jpsychires.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. The American journal of psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 31.Hunt DL, Castillo PE. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Current opinion in neurobiology. 2012;22:496–508. doi: 10.1016/j.conb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe Y, Saito H, Abe K. Effects of glycine and structurally related amino acids on generation of long-term potentiation in rat hippocampal slices. European journal of pharmacology. 1992;223:179–184. doi: 10.1016/0014-2999(92)94837-l. [DOI] [PubMed] [Google Scholar]
- 34.Watt AJ, Sjostrom PJ, Hausser M, Nelson SB, Turrigiano GG. A proportional but slower NMDA potentiation follows AMPA potentiation in LTP. Nature neuroscience. 2004;7:518–524. doi: 10.1038/nn1220. [DOI] [PubMed] [Google Scholar]
- 35.Bado P, Madeira C, Vargas-Lopes C, Moulin TC, Wasilewska-Sampaio AP, Maretti L, et al. Effects of low-dose D: -serine on recognition and working memory in mice. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2330-4. [DOI] [PubMed] [Google Scholar]
- 36.Balu DT, Coyle JT. The NMDA receptor 'glycine modulatory site' in schizophrenia: D-serine, glycine, and beyond. Current opinion in pharmacology. 2015;20:109–115. doi: 10.1016/j.coph.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho SE, Na KS, Cho SJ, Kang SG. Low d-serine levels in schizophrenia: A systematic review and meta-analysis. Neuroscience letters. 2016;634:42–51. doi: 10.1016/j.neulet.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Naatanen R, Sussman ES, Salisbury D, Shafer VL. Mismatch negativity (MMN) as an index of cognitive dysfunction. Brain Topogr. 2014;27:451–466. doi: 10.1007/s10548-014-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naatanen R, Todd J, Schall U. Mismatch negativity (MMN) as biomarker predicting psychosis in clinically at-risk individuals. Biological psychology. 2016;116:36–40. doi: 10.1016/j.biopsycho.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Mantysalo S, Naatanen R. The duration of a neuronal trace of an auditory stimulus as indicated by event-related potentials. Biol Psychol. 1987;24:183–195. doi: 10.1016/0301-0511(87)90001-9. [DOI] [PubMed] [Google Scholar]
- 41.Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000;5:207–215. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- 42.Perrin MA, Kantrowitz JT, Silipo G, Dias E, Jabado O, Javitt DC. Mismatch negativity (MMN) to spatial deviants and behavioral spatial discrimination ability in the etiology of auditory verbal hallucinations and thought disorder in schizophrenia. Schizophrenia research. 2018;191:140–147. doi: 10.1016/j.schres.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophrenia research. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Erickson MA, Ruffle A, Gold JM. A Meta-Analysis of Mismatch Negativity in Schizophrenia: From Clinical Risk to Disease Specificity and Progression. Biological psychiatry. 2016;79:980–987. doi: 10.1016/j.biopsych.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Light GA, Swerdlow NR, Thomas ML, Calkins ME, Green MF, Greenwood TA, et al. Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophrenia research. 2015;163:63–72. doi: 10.1016/j.schres.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman T, Sehatpour P, Dias E, Perrin M, Javitt DC. Differential relationships of mismatch negativity and visual p1 deficits to premorbid characteristics and functional outcome in schizophrenia. Biological psychiatry. 2012;71:521–529. doi: 10.1016/j.biopsych.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:822–829. doi: 10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. Journal of cognitive neuroscience. 2007;19:1624–1632. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wynn JK, Sugar C, Horan WP, Kern R, Green MF. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biological psychiatry. 2010;67:940–947. doi: 10.1016/j.biopsych.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jahshan C, Wynn JK, Green MF. Relationship between auditory processing and affective prosody in schizophrenia. Schizophrenia research. 2013;143:348–353. doi: 10.1016/j.schres.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kantrowitz JT, Hoptman MJ, Leitman DI, Moreno-Ortega M, Lehrfeld JM, Dias E, et al. Neural Substrates of Auditory Emotion Recognition Deficits in Schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:14909–14921. doi: 10.1523/JNEUROSCI.4603-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Light GA, Swerdlow NR, Rissling AJ, Radant A, Sugar CA, Sprock J, et al. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PloS one. 2012;7:e39434. doi: 10.1371/journal.pone.0039434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amann LC, Gandal MJ, Halene TB, Ehrlichman RS, White SL, McCarren HS, et al. Mouse behavioral endophenotypes for schizophrenia. Brain research bulletin. 2010;83:147–161. doi: 10.1016/j.brainresbull.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Ehrlichman RS, Maxwell CR, Majumdar S, Siegel SJ. Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. Journal of cognitive neuroscience. 2008;20:1403–1414. doi: 10.1162/jocn.2008.20097. [DOI] [PubMed] [Google Scholar]
- 56.Gil-da-Costa R, Stoner GR, Fung R, Albright TD. Nonhuman primate model of schizophrenia using a noninvasive EEG method. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15425–15430. doi: 10.1073/pnas.1312264110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate C, Merchant K, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3:102mr102. doi: 10.1126/scitranslmed.3002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Archives of general psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 59.Gunduz-Bruce H, Reinhart RM, Roach BJ, Gueorguieva R, Oliver S, D'Souza DC, et al. Glutamatergic modulation of auditory information processing in the human brain. Biological psychiatry. 2012;71:969–977. doi: 10.1016/j.biopsych.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heekeren K, Daumann J, Neukirch A, Stock C, Kawohl W, Norra C, et al. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology. 2008;199:77–88. doi: 10.1007/s00213-008-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Catts VS, Lai YL, Weickert CS, Weickert TW, Catts SV. A quantitative review of the postmortem evidence for decreased cortical N-methyl-d-aspartate receptor expression levels in schizophrenia: How can we link molecular abnormalities to mismatch negativity deficits? Biological psychology. 2015 doi: 10.1016/j.biopsycho.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 62.Rosburg T, Kreitschmann-Andermahr I. The effects of ketamine on the mismatch negativity (MMN) in humans - A meta-analysis. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2016;127:1387–1394. doi: 10.1016/j.clinph.2015.10.062. [DOI] [PubMed] [Google Scholar]
- 63.Rowland LM, Summerfelt A, Wijtenburg SA, Du X, Chiappelli JJ, Krishna N, et al. Frontal Glutamate and gamma-Aminobutyric Acid Levels and Their Associations With Mismatch Negativity and Digit Sequencing Task Performance in Schizophrenia. JAMA psychiatry. 2016;73:166–174. doi: 10.1001/jamapsychiatry.2015.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagai T, Kirihara K, Tada M, Koshiyama D, Koike S, Suga M, et al. Reduced Mismatch Negativity is Associated with Increased Plasma Level of Glutamate in First-episode Psychosis. Scientific reports. 2017;7:2258. doi: 10.1038/s41598-017-02267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Umbricht D, Javitt D, Novak G, Bates J, Pollack S, Lieberman J, et al. Effects of risperidone on auditory event-related potentials in schizophrenia. Int J Neuropsychopharmacol. 1999;2:299–304. doi: 10.1017/S1461145799001595. [DOI] [PubMed] [Google Scholar]
- 66.Umbricht D, Javitt D, Novak G, Bates J, Pollack S, Lieberman J, et al. Effects of clozapine on auditory event-related potentials in schizophrenia. Biological psychiatry. 1998;44:716–725. doi: 10.1016/s0006-3223(97)00524-6. [DOI] [PubMed] [Google Scholar]
- 67.Friston K. A theory of cortical responses. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perez VB, Tarasenko M, Miyakoshi M, Pianka ST, Makeig SD, Braff DL, et al. Mismatch Negativity is a Sensitive and Predictive Biomarker of Perceptual Learning During Auditory Cognitive Training in Schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biagianti B, Roach BJ, Fisher M, Loewy R, Ford JM, Vinogradov S, et al. Trait aspects of auditory mismatch negativity predict response to auditory training in individuals with early illness schizophrenia. Neuropsychiatric electrophysiology. 2017;3 doi: 10.1186/s40810-017-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends in cognitive sciences. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Lakatos P, Schroeder CE, Leitman DI, Javitt DC. Predictive suppression of cortical excitability and its deficit in schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:11692–11702. doi: 10.1523/JNEUROSCI.0010-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Womelsdorf T, Valiante TA, Sahin NT, Miller KJ, Tiesinga P. Dynamic circuit motifs underlying rhythmic gain control, gating and integration. Nature neuroscience. 2014;17:1031–1039. doi: 10.1038/nn.3764. [DOI] [PubMed] [Google Scholar]
- 73.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in neurosciences. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Javitt DC, Lee M, Kantrowitz JT, Martinez A. Mismatch negativity as a biomarker of theta band oscillatory dysfunction in schizophrenia. Schizophrenia research. 2018;191:51–60. doi: 10.1016/j.schres.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 75.Lee M, Hoptman MJ, Lakatos P, Kantrowitz JT, Dias E, Martinez AM, et al. Neural basis of mismatch negativity (MMN) dysfunction in schizophrenia: circuit and cellular level of analysis. Molecular psychiatry. 2017 doi: 10.1038/mp.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bickel S, Dias EC, Epstein ML, Javitt DC. Expectancy-related modulations of neural oscillations in continuous performance tasks. NeuroImage. 2012;62:1867–1876. doi: 10.1016/j.neuroimage.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dias EC, Bickel S, Epstein ML, Sehatpour P, Javitt DC. Abnormal task modulation of oscillatory neural activity in schizophrenia. Frontiers in psychology. 2013;4:540. doi: 10.3389/fpsyg.2013.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Capotosto P, Baldassarre A, Sestieri C, Spadone S, Romani GL, Corbetta M. Task and Regions Specific Top-Down Modulation of Alpha Rhythms in Parietal Cortex. Cerebral cortex (New York, NY : 1991) 2016 doi: 10.1093/cercor/bhw278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jahshan C, Wynn JK, Mathalon DH, Green MF. Cognitive correlates of visual neural plasticity in schizophrenia. Schizophrenia research. 2017 doi: 10.1016/j.schres.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 80.Kantrowitz JT, Revheim N, Pasternak R, Silipo G, Javitt DC. It's all in the cards: effect of stimulus manipulation on Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry research. 2009;168:198–204. doi: 10.1016/j.psychres.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Contreras NA, Tan EJ, Lee SJ, Castle DJ, Rossell SL. Using visual processing training to enhance standard cognitive remediation outcomes in schizophrenia: a pilot study. Psychiatry research. 2017 doi: 10.1016/j.psychres.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 82.Forsyth JK, Bachman P, Mathalon DH, Roach BJ, Ye E, Asarnow RF. Effects of Augmenting N-Methyl-D-Aspartate Receptor Signaling on Working Memory and Experience-Dependent Plasticity in Schizophrenia: An Exploratory Study Using Acute d-cycloserine. Schizophrenia bulletin. 2017;43:1123–1133. doi: 10.1093/schbul/sbw193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D'Souza DC, Radhakrishnan R, Perry E, Bhakta S, Singh NM, Yadav R, et al. Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:492–503. doi: 10.1038/npp.2012.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kantrowitz JT, Malhotra AK, Cornblatt B, Silipo G, Balla A, Suckow RF, et al. High dose D-serine in the treatment of schizophrenia. Schizophrenia research. 2010;121:125–130. doi: 10.1016/j.schres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Umbricht D, Alberati D, Martin-Facklam M, Borroni E, Youssef EA, Ostland M, et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA psychiatry. 2014;71:637–646. doi: 10.1001/jamapsychiatry.2014.163. [DOI] [PubMed] [Google Scholar]
- 86.Kantrowitz JT, Nolan KA, Epstein ML, Lehrfeld N, Shope C, Petkova E, et al. Neurophysiological Effects of Bitopertin in Schizophrenia. Journal of clinical psychopharmacology. 2017;37:447–451. doi: 10.1097/JCP.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bugarski-Kirola D, Iwata N, Sameljak S, Reid C, Blaettler T, Millar L, et al. Efficacy and safety of adjunctive bitopertin versus placebo in patients with suboptimally controlled symptoms of schizophrenia treated with antipsychotics: results from three phase 3, randomised, double-blind, parallel-group, placebo-controlled, multicentre studies in the SearchLyte clinical trial programme. The lancet Psychiatry. 2016;3:1115–1128. doi: 10.1016/S2215-0366(16)30344-3. [DOI] [PubMed] [Google Scholar]
- 88.Schoemaker JH, Jansen WT, Schipper J, Szegedi A. The selective glycine uptake inhibitor org 25935 as an adjunctive treatment to atypical antipsychotics in predominant persistent negative symptoms of schizophrenia: results from the GIANT trial. Journal of clinical psychopharmacology. 2014;34:190–198. doi: 10.1097/JCP.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 89.Kantrowitz JT, Epstein ML, Lee M, Lehrfeld N, Nolan KA, Shope C, et al. Improvement in mismatch negativity generation during d-serine treatment in schizophrenia: Correlation with symptoms. Schizophrenia research. 2018;191:70–79. doi: 10.1016/j.schres.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 90.Fisher M, Herman A, Stephens DB, Vinogradov S. Neuroscience-informed computer-assisted cognitive training in schizophrenia. Annals of the New York Academy of Sciences. 2016;1366:90–114. doi: 10.1111/nyas.13042. [DOI] [PubMed] [Google Scholar]
- 91.Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophrenia bulletin. 2010;36:869–879. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gardoni F, Di Luca M. New targets for pharmacological intervention in the glutamatergic synapse. European journal of pharmacology. 2006;545:2–10. doi: 10.1016/j.ejphar.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 93.More L, Gravius A, Nagel J, Valastro B, Greco S, Danysz W. Therapeutically relevant plasma concentrations of memantine produce significant L-N-methyl-D-aspartate receptor occupation and do not impair learning in rats. Behavioural pharmacology. 2008;19:724–734. doi: 10.1097/FBP.0b013e3283123cad. [DOI] [PubMed] [Google Scholar]
- 94.Mancini M, Ghiglieri V, Bagetta V, Pendolino V, Vannelli A, Cacace F, et al. Memantine alters striatal plasticity inducing a shift of synaptic responses toward long-term depression. Neuropharmacology. 2016;101:341–350. doi: 10.1016/j.neuropharm.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 95.Korostenskaja M, Nikulin VV, Kicic D, Nikulina AV, Kahkonen S. Effects of NMDA receptor antagonist memantine on mismatch negativity. Brain research bulletin. 2007;72:275–283. doi: 10.1016/j.brainresbull.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 96.Swerdlow NR, van Bergeijk DP, Bergsma F, Weber E, Talledo J. The effects of memantine on prepulse inhibition. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1854–1864. doi: 10.1038/npp.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Light GA, Zhang W, Joshi YB, Bhakta S, Talledo JA, Swerdlow NR. Single-Dose Memantine Improves Cortical Oscillatory Response Dynamics in Patients with Schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Swerdlow NR, Bhakta S, Chou HH, Talledo JA, Balvaneda B, Light GA. Memantine Effects On Sensorimotor Gating and Mismatch Negativity in Patients with Chronic Psychosis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:419–430. doi: 10.1038/npp.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kishi T, Matsuda Y, Iwata N. Memantine add-on to antipsychotic treatment for residual negative and cognitive symptoms of schizophrenia: a meta-analysis. Psychopharmacology. 2017;234:2113–2125. doi: 10.1007/s00213-017-4616-7. [DOI] [PubMed] [Google Scholar]
- 100.Matsuda Y, Kishi T, Iwata N. Efficacy and safety of NMDA receptor antagonists augmentation therapy for schizophrenia: an updated meta-analysis of randomized placebo-controlled trials. Journal of psychiatric research. 2013;47:2018–2020. doi: 10.1016/j.jpsychires.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Zheng W, Li XH, Yang XH, Cai DB, Ungvari GS, Ng CH, et al. Adjunctive memantine for schizophrenia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Psychological medicine. 2017;1–10 doi: 10.1017/S0033291717001271. [DOI] [PubMed] [Google Scholar]
- 102.Bhakta SG, Chou HH, Rana B, Talledo JA, Balvaneda B, Gaddis L, et al. Effects of acute memantine administration on MATRICS Consensus Cognitive Battery performance in psychosis: Testing an experimental medicine strategy. Psychopharmacology. 2016;233:2399–2410. doi: 10.1007/s00213-016-4291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, et al. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. Journal of Physiology-London. 2004;557:175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain stimulation. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 105.Nitsche MA, Muller-Dahlhaus F, Paulus W, Ziemann U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: building models for the clinical use of CNS active drugs. J Physiol. 2012;590:4641–4662. doi: 10.1113/jphysiol.2012.232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marquez-Ruiz J, Leal-Campanario R, Sanchez-Campusano R, Molaee-Ardekani B, Wendling F, Miranda PC, et al. Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6710–6715. doi: 10.1073/pnas.1121147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rohan JG, Carhuatanta KA, McInturf SM, Miklasevich MK, Jankord R. Modulating Hippocampal Plasticity with In Vivo Brain Stimulation. J Neurosci. 2015;35:12824–12832. doi: 10.1523/JNEUROSCI.2376-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 110.Nitsche MA, Paulus W. Noninvasive Brain Stimulation Protocols in the Treatment of Epilepsy: Current State and Perspectives. Neurotherapeutics. 2009;6:244–250. doi: 10.1016/j.nurt.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9:2257–2260. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- 112.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 113.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology-London. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Monai H, Hirase H. Astrocytic calcium activation in a mouse model of tDCS-Extended discussion. Neurogenesis (Austin) 2016;3:e1240055. doi: 10.1080/23262133.2016.1240055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Monai H, Ohkura M, Tanaka M, Oe Y, Konno A, Hirai H, et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun. 2016;7:11100. doi: 10.1038/ncomms11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. The American journal of psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 118.Nienow TM, MacDonald AW, 3rd, Lim KO. TDCS produces incremental gain when combined with working memory training in patients with schizophrenia: A proof of concept pilot study. Schizophrenia research. 2016;172:218–219. doi: 10.1016/j.schres.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 119.Horvath JC, Forte JD, Carter O. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-session Transcranial Direct Current Stimulation (tDCS) Brain stimulation. 2015;8:535–550. doi: 10.1016/j.brs.2015.01.400. [DOI] [PubMed] [Google Scholar]
- 120.Horvath JC, Forte JD, Carter O. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia. 2015;66:213–236. doi: 10.1016/j.neuropsychologia.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 121.Hsu TY, Juan CH, Tseng P. Individual Differences and State-Dependent Responses in Transcranial Direct Current Stimulation. Front Hum Neurosci. 2016;10:643. doi: 10.3389/fnhum.2016.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dunn W, Rassovsky Y, Wynn JK, Wu AD, Iacoboni M, Hellemann G, et al. Modulation of neurophysiological auditory processing measures by bilateral transcranial direct current stimulation in schizophrenia. Schizophrenia research. 2016;174:189–191. doi: 10.1016/j.schres.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 123.Chen JC, Hammerer D, Strigaro G, Liou LM, Tsai CH, Rothwell JC, et al. Domain-specific suppression of auditory mismatch negativity with transcranial direct current stimulation. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2014;125:585–592. doi: 10.1016/j.clinph.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 124.Lovio R, Halttunen A, Lyytinen H, Naatanen R, Kujala T. Reading skill and neural processing accuracy improvement after a 3-hour intervention in preschoolers with difficulties in reading-related skills. Brain research. 2012;1448:42–55. doi: 10.1016/j.brainres.2012.01.071. [DOI] [PubMed] [Google Scholar]
- 125.Menning H, Roberts LE, Pantev C. Plastic changes in the auditory cortex induced by intensive frequency discrimination training. Neuroreport. 2000;11:817–822. doi: 10.1097/00001756-200003200-00032. [DOI] [PubMed] [Google Scholar]
- 126.Kantrowitz JT, Hoptman MJ, Leitman DI, Silipo G, Javitt DC. The 5% difference: early sensory processing predicts sarcasm perception in schizophrenia and schizo-affective disorder. Psychological medicine. 2014;44:25–36. doi: 10.1017/S0033291713000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jahshan C, Wynn JK, Green MF. Relationship between auditory processing and affective prosody in schizophrenia. Schizophrenia research. 2013;143:348–353. doi: 10.1016/j.schres.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2000;111:1733–1737. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- 129.Strous RD, Cowan N, Ritter W, Javitt DC. Auditory sensory ("echoic") memory dysfunction in schizophrenia. The American journal of psychiatry. 1995;152:1517–1519. doi: 10.1176/ajp.152.10.1517. [DOI] [PubMed] [Google Scholar]
- 130.Dunn W, Rassovsky Y, Wynn J, Wu AD, Iacoboni M, Hellemann G, et al. The effect of bilateral transcranial direct current stimulation on early auditory processing in schizophrenia: a preliminary study. J Neural Transm (Vienna) 2017 doi: 10.1007/s00702-017-1752-5. [DOI] [PubMed] [Google Scholar]
- 131.Lodge D, Mercier MS. Ketamine and phencyclidine: the good, the bad and the unexpected. British journal of pharmacology. 2015;172:4254–4276. doi: 10.1111/bph.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sanchez JT, Ghelani S, Otto-Meyer S. From development to disease: diverse functions of NMDA-type glutamate receptors in the lower auditory pathway. Neuroscience. 2015;285:248–259. doi: 10.1016/j.neuroscience.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 133.Fisher M, Loewy R, Carter C, Lee A, Ragland JD, Niendam T, et al. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophrenia bulletin. 2015;41:250–258. doi: 10.1093/schbul/sbt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nahum M, Lee H, Merzenich MM. Principles of neuroplasticity-based rehabilitation. Prog Brain Res. 2013;207:141–171. doi: 10.1016/B978-0-444-63327-9.00009-6. [DOI] [PubMed] [Google Scholar]
- 135.Greenwood LM, Leung S, Michie PT, Green A, Nathan PJ, Fitzgerald P, et al. The effects of glycine on auditory mismatch negativity in schizophrenia. Schizophrenia research. 2017 doi: 10.1016/j.schres.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 136.Lin CY, Liang SY, Chang YC, Ting SY, Kao CL, Wu YH, et al. Adjunctive sarcosine plus benzoate improved cognitive function in chronic schizophrenia patients with constant clinical symptoms: A randomised, double-blind, placebo-controlled trial. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2017;18:357–368. doi: 10.3109/15622975.2015.1117654. [DOI] [PubMed] [Google Scholar]
- 137.Amiaz R, Kent I, Rubinstein K, Sela BA, Javitt D, Weiser M. Safety, tolerability and pharmacokinetics of open label sarcosine added on to anti-psychotic treatment in schizophrenia - preliminary study. The Israel journal of psychiatry and related sciences. 2015;52:12–15. [PubMed] [Google Scholar]
- 138.Lin C-H, Lin C-H, Chang Y-C, Huang Y-J, Chen P-W, Yang H-T, et al. Sodium Benzoate, a D-amino Acid Oxidase Inhibitor, Added to Clozapine for the Treatment of Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Biological psychiatry. 2017 doi: 10.1016/j.biopsych.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 139.Boland K, Moschetti V, Dansirikul C, Pichereau S, Gheyle L, Runge F, et al. A phase I, randomized, proof-of-clinical-mechanism study assessing the pharmacokinetics and pharmacodynamics of the oral PDE9A inhibitor BI 409306 in healthy male volunteers. Human psychopharmacology. 2017;32 doi: 10.1002/hup.2569. [DOI] [PubMed] [Google Scholar]
- 140.Duinen MV, Reneerkens OA, Lambrecht L, Sambeth A, Rutten BP, Os JV, et al. Treatment of Cognitive Impairment in Schizophrenia: Potential Value of Phosphodiesterase Inhibitors in Prefrontal Dysfunction. Current pharmaceutical design. 2015;21:3813–3828. doi: 10.2174/1381612821666150605110941. [DOI] [PubMed] [Google Scholar]