Abstract
Background
Dopamine receptors are implicated in cocaine reward and seeking. We hypothesize that (−)-stepholidine, a dopamine D1/D2/D3 multi-receptor agent, would be effective in reducing cocaine reward and seeking in an animal model. We investigated the effects of (−)-stepholidine in cue-induced reinstatement of cocaine seeking and cocaine self-administration (reward).
Methods
Cue-induced reinstatement experiment: Rats were trained to press a lever reinforced by cocaine (1 mg/kg/injection) for 15 consecutive daily sessions, after which the response was extinguished by withholding cocaine and cocaine-paired cues (light and pump activation). This was followed by a cue-induced reinstatement test where subjects were exposed to two cocaine cue presentations and presses on the active lever produced cues. Subjects were treated with one of four (−)-stepholidine doses prior to the reinstatement test. Cocaine self-administration (reward) experiment: Rats were trained to self-administer cocaine under a progressive ratio schedule of reinforcement. After stable breakpoints were established, rats were injected with four doses of (−)-stepholidine prior to testing; each dose was injected prior to a separate test session with no-treatment sessions intervening to re-establish break points.
Results
(−)-Stepholidine significantly reduced cue-induced reinstatement of cocaine seeking in a dose-related manner. Additionally, (−)-stepholidine significantly reduced break points for cocaine reward. (−)-Stepholidine did not significantly affect locomotor activity.
Conclusions
(−)-Stepholidine reduces cue-induced reinstatement of cocaine seeking and cocaine reward, suggesting that it may be useful in treating relapse in cocaine addiction.
Keywords: Cocaine, substance use disorder, (−)-stepholidine, drug addiction
1. Introduction
Drug use is a prevalent problem in the United States; in 2016 roughly 1.9 million people aged 12 or older were current users of cocaine (Center for Behavioral Health Statistics and Quality, 2017). Cocaine addiction is classified as a maladaptive cycle of use (often binge-like), abstinence and relapse. A major obstacle in the treatment of cocaine addiction is the prevention of relapse, itself caused by craving often induced by cocaine-associated cues (Childress et al., 1988; Tiffany, 1990; O’Brien et al., 1998).
Cocaine produces its rewarding effects through its capacity to increase dopamine neurotransmission in the mesolimbic system. It is thought that this mechanism plays a critical role in its abuse liability. Furthermore, cocaine associated cues, which instigate feelings of craving and increase the incentive motivation for drug, similarly increase dopamine neurotransmission in the mesolimbic system (Gratton and Wise, 1994; Kiyatkin, 1993). The increased dopamine transmission enhances stimulation of dopamine receptors in terminal regions that contribute to craving. Hence, from a pharmacological approach, effective cocaine addiction treatment could target mesolimbic dopamine receptors, disrupt cue-induced stimulation of these receptors and reduce cue-induced relapse.
Focus on individual dopamine receptor targets in treatment of cocaine addiction has yielded a range of results. Selective D1 receptor antagonists, although successful in reducing cue- and context-induced reinstatement of cocaine seeking (Alleweireldt et al., 2002; Crombag et al., 2002), have a limited therapeutic application in humans as their repeated administration can increase blood pressure and produce sedation, anhedonia and motor incapacitation (Haney et al., 2001). Focus on the D3 receptor has suggested its potential as a target in reducing cocaine reward: under low fixed ratio schedules (Gal and Gyertyan, 2003; Song et al., 2012) and under a progressive ratio (PR) schedule of reinforcement, D3 receptor antagonists have been shown to reduce cocaine self-administration (Galaj et al., 2014). Selective D3 antagonists have likewise been found to reduce cue-induced reinstatement of cocaine seeking (Gilbert et al., 2005; Galaj et al., 2014; Song et al., 2014). However, in early tests, currently available D3 antagonists have been shown to raise blood pressure (Appel et al., 2015) and potentiate the hypertensive effects of cocaine (Asico et al., 1998; Luippold et al., 2001). Taken together, these results show the utility of individual dopamine receptor therapy in their ability to reduce incentive motivational states integral to drug craving/seeking and to reduce drug reward, but also the limitations of currently available compounds. Thus, it is necessary to continue to search for dopamine receptor targeting compounds that might prove effective. One possible direction is toward compounds that can target multiple dopamine receptors.
Dopamine D1 and D3 receptors are co-localized to the same medium spiny neurons within the nucleus accumbens (Le Moine and Bloch, 1996; Ridray et al., 1998; Schwartz et al., 1998), a dopamine terminal region strongly implicated in cocaine reward and addiction-related behaviors. Interestingly, the anatomical and biochemical interplay of D1 and D3 receptors can result in the formation of complex heteromers producing functionally interactive states of these receptors (Fiorentini et al., 2008; Marcellino et al., 2008; Perreault et al., 2014). It might prove an interesting line of research to investigate compounds that can target these heteromers. We found that co-treatment with a D1 receptor partial agonist and D3 receptor antagonist produced effects on cocaine seeking and reward that far exceeded the therapeutic benefits of the individual compounds without reducing natural rewards or motoric ability (Galaj et al., 2016). These results suggest that compounds acting as both a D1 receptor partial agonist and a D3 receptor antagonist may hold potential as cocaine addiction treatments.
Tetrahydroprotoberberine (THPB) alkaloids adhere to a D1/D2/D3 multi-receptor pharmacological profile. They fall into two main subgroups that enact either similar action (e.g., tetrahydropalmatine, a D1/D2/D3 antagonist) or opposite action (e.g., isocorypalmine, a D1 partial agonist and D2/D3 antagonist). Tetrahydropalmatine and isocorypalmine have displayed efficacy in attenuating cocaine reward and reinstatement in cocaine addiction models (Mantsch et al., 2007; Xi et al., 2007). (−)-Stepholidine is a member of the THPB family that maintains similar dopamine receptor polypharmacology; its role in cocaine addiction constitutes the focus of this study.
(−)-Stepholidine is a naturally occurring compound in the Chinese herb Stephania intermedia that displays affinity for dopamine D1, D2 and D3 receptors. While (−)-stepholidine has been consistently reported to exhibit antagonist activity at D2 and D3 receptors in vitro, intrinsic activity at the D1 receptor appears to be more controversial; for example, being reported as a D1 partial agonist (Mo et al., 2007), D1 full agonist (Hicks et al., 2018) and D1 antagonist (Meade et al., 2015). Differences in the assay conditions (e.g., receptor reserve/level) and the transfected cells used may account for these differences in reported D1 intrinsic activity (Hicks et al., 2018). (−)-Stepholidine has been found to inhibit heroin-induced reinstatement (Ma et al., 2014), attenuate heroin self-administration and reduce cue-induced reinstatement of heroin seeking (Yue et al., 2014) with minimal effects on motoric activity. The effect of (−)-stepholidine on cocaine self-administration and reinstatement has not yet been investigated. In the present study we tested whether (−)-stepholidine would reduce cocaine self-administration/reward and cue-induced reinstatement of a cocaine seeking response.
2. Materials and methods
2.1 Subjects
The housing conditions and care of the animals were consistent with specifications in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 2011). All experiments were approved by the Queens College Institutional Animal Care and Use Committee.
Subjects consisted of male Long Evans rats, weighing 350–450 g at the start of the experiments, obtained from our in-house colony; male and female breeders were purchased from Charles River Laboratories (Kingston, NY, US). All animals were individually housed in a temperature-controlled environment (70 °F) and had free access to food and water under a 12 h light/dark cycle. All experiments were conducted during the animals’ active periods (dark cycle).
2.2 Surgery
Surgeries were conducted under atropine sulfate [0.54 μg/0.1 mL of distilled water, intraperitoneal (IP) injection] and sodium pentobarbital (65 mg/kg, IP) anesthesia. Surgical sites were cleaned and pruned of fur. An incision (2 cm) was made ventral to the right mandible. The jugular vein was isolated, cleaned and opened with a vessel dilator, allowing for insertion of the silastic catheter (Dow Corning, Midland, MI) to a position just short of the right atrium. The inserted catheter was secured to the vein with surgical sutures and the dorsal portion passed subcutaneously to the back of the neck and exited through an incision made on the scalp. A bent 22-gauge stainless-steel cannula (25 mm, 90° angle with plastic connector piece attached) was inserted into the exposed tip of the catheter such that 10 mm of the cannula was sheathed. Five stainless-steel screws were implanted in the skull around the cannula, secured in place by an arm of the stereotaxic apparatus, and submerged in dental acrylic until only the distal half of the plastic connector piece and tip of the cannula were exposed (attachment site for drug line). The rats were given an intravenous (IV) injection of Gentamicin (4.0 mg/kg/0.05 mL) after surgery and daily thereafter for one week, receiving an IV injection of heparin saline solution (200 U/mL) after surgery and daily thereafter. Animals were given 3 days to recover after surgery before initiating self-administration training.
2.3 Apparatus
2.3.1 Self-administration chambers
Intravenous self-administration chambers were equipped with two retractable levers (3.5 x 2.0 cm, 8 cm apart) with corresponding lights (2-W bulb, 10 cm above each lever). Chambers consisted of a transparent plastic door and opposing wall, left and right aluminum walls and a transparent plastic ceiling with a centered hole (3 cm diameter). The floors were made of aluminum rods, spaced 1.0 cm apart. Levers were embedded in the right aluminum wall (26 x 26 cm) and required a force of 0.09 N to register activity. Each chamber was encased within a sound-attenuating box equipped with a house light, ventilating fan and infusion pump (Razel, 3.33 rpm) that held a 20-mL syringe.
2.3.2 Food operant conditioning chambers
Food operant chambers were equipped with two levers (2.5 cm x 2.5 cm, 11.4 cm apart) with corresponding lights (2-W bulb, 5.0 cm above each lever). Chambers (20.3 cm x 30.5 cm x 20.3 cm) consisted of a transparent plastic door and opposing wall, left and right aluminum walls, and a transparent plastic ceiling. The floors were made of aluminum rods, spaced 2.0 cm apart. Levers were embedded in the right aluminum wall and connected to a food dispenser that dropped one food pellet into a square food dish (5.0 cm x 5.0 cm) centered between the two levers. Each chamber was encased within a sound-attenuating box (36.8 cm x 56.0 cm x 33.0 cm) equipped with a ventilating fan and food dispenser.
2.3.3 Locomotor activity chambers
Locomotor activity chambers measuring 43 x 43 x 30 cm (l x w x h) were used for the locomotor activity experiment. Each chamber was equipped with 32 photo-emitters, 16 positioned along the length and 16 along the width of the chamber, 4.0 cm above the floor. Each photo-emitter was paired directly opposite a photocell. Locomotor activity counts were registered when adjacent beams were broken consecutively.
2.4 Drugs
Cocaine (a gift from NIDA) was dissolved in 0.9% saline to achieve a dose of 1 mg/kg/injection for the self-administration experiment. (−)-Stepholidine, synthesized as described previously (Gadhiya et al., 2016), was dissolved in distilled water and 10% dimethyl sulfoxide (DMSO) to achieve doses of 2.5, 5.0 and 10 mg/kg and was administered by IP injection.
2.5 Procedures
2.5.1 Cue-induced reinstatement of cocaine seeking
This experiment entailed three phases: self-administration, extinction and the reinstatement test. Animals were trained to self-administer cocaine (1 mg/kg/injection) under a fixed ratio 1 (FR1) schedule of reinforcement over the course of daily three-hour sessions. Responding on the active lever initiated the drug injection pump for 4.5 s and the light above the active lever for 20 s, a period during which further lever pressing was registered but failed to activate the pump or cue light. Responding on the inactive lever was registered but had no consequences. The left/right positions of the active and inactive levers were counterbalanced across animals and remained constant for the duration of the experiment. Upon completing three consecutive sessions of stable responding, in which the total number of infusions did not vary by more than ±10% of the mean of the three sessions and showed no ascending or descending trends, animals continued on a FR1 schedule of reinforcement for five sessions followed by an additional 10 sessions under a FR3 schedule of reinforcement. This was followed by the extinction phase, in which responding on the active and inactive levers did not initiate the infusion pump or cue light. Upon meeting extinction criteria, defined as no more than seven presses per hour on either lever for three consecutive sessions, animals were put in the reinstatement test phase the following day. Animals were given an IP injection of (−)-stepholidine [vehicle (n=8), 2.5 (n=8), 5 (n=8) or 10 (n=7) mg/kg] 30 min prior to the reinstatement test. Each animal received only one of the doses. Non-contingent cocaine cues (20-s light and 4.5-s pump activation) were presented twice, 2 min apart, at the beginning of the reinstatement test session. Active lever presses were reinforced with the cocaine cues (light/pump) but cocaine was not delivered. Responding on the inactive lever produced no consequences. Responding on both levers was recorded for the duration of the 120-min reinstatement test.
To test the possibility that any observed effects of (−)-stepholidine on cue-induced reinstatement of lever pressing may be due to motoric effects, specifically on lever pressing, we evaluated the effects of (−)-stepholidine on lever pressing reinforced by food, a procedure that produces many times more lever pressing than the reinstatement procedure. These rats (N=8) were initially trained to self-administer cocaine (1 mg/kg/injection) under a FR1 schedule of reinforcement for eight days and subsequently under a FR3 schedule of reinforcement for 10 days. Then, they were trained to lever press reinforced by food pellets under a progressive ratio (PR) schedule of reinforcement using our standard procedure (see Galaj et al., 2014). After rats demonstrated stable responding, they were treated with the 5 mg/kg dose of (−)-stepholidine 30 mins prior to testing, in which the number of lever presses during 120 min, the same period of time as in the reinstatement test, was recorded for each rat. Thus, these rats were exposed to identical cocaine self-administration training and spent a similar amount of time in cocaine withdrawal (21 days) prior to the (−)-stepholidine test as those in the reinstatement experiment.
2.5.2 Cocaine self-administration under a PR schedule of reinforcement
In this experiment, we investigated the effects of (−)-stepholidine on lever pressing under a PR schedule of cocaine reinforcement. Animals (N=8) underwent self-administration training under a FR1 schedule of cocaine (1 mg/kg/injection) reinforcement in daily three-hour sessions. Once responding was stable for three consecutive sessions, the schedule was changed to a PR schedule of reinforcement (ratio steps 1, 3, 5, 8, 12, 16, 22, 29, 38, 50, 65, 84, 108, 139, 178, 228, 291, 371) in daily 5.5-hour sessions. Break point (BP) was operationally defined as the total number of cocaine infusions earned prior to a 60-min period during which no infusions were obtained. The rats were tested when they showed stable BPs, defined as three consecutive sessions where the BPs did not differ by more than 2 ratio steps and showed no upward or downward trends. The next day, animals were tested with one of four doses of (−)-stepholidine administered 30 min prior to the test session. BPs were subsequently re-stabilized across three sessions and the rats were retested with another randomly determined (−)-stepholidine dose. This continued until all rats were tested with all doses of (−)-stepholidine (vehicle, 2.5, 5.0 and 10 mg/kg).
2.5.3 Locomotor activity
Before receiving any drug, the animals were placed in locomotor activity chambers for habituation, for two hours per day on three consecutive days. Based on the ranked total locomotor activity counts observed during the third habituation session, animals were assigned to either vehicle (n=8) or 10 mg/kg (−)-stepholidine (n=7) treatment groups. The fourth session was the test session: all animals received an IP injection of their corresponding treatment and 30 min later placed in the locomotor activity chambers for two hours.
3. Results
3.1 Cocaine cue-induced reinstatement
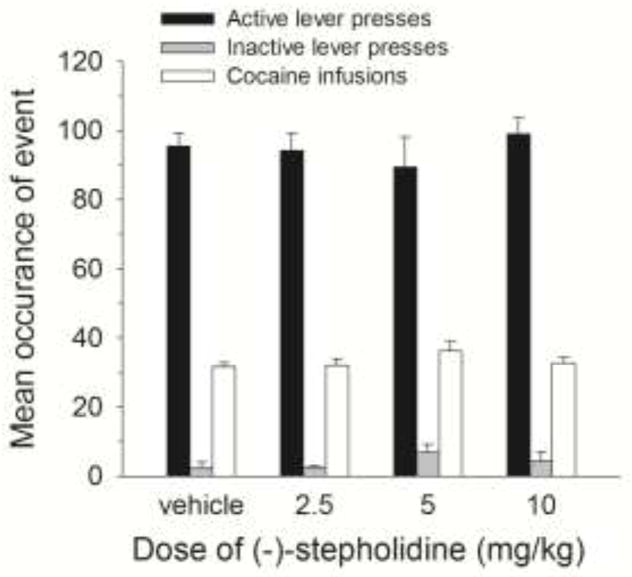
Figure 1 shows active lever presses, inactive lever presses and infusions averaged across the last five cocaine self-administration sessions, separated by group according to the (−)-stepholidine dose they would eventually be treated with prior to the reinstatement test. The groups showed similar amounts of drug intake as well as active and inactive lever presses during the self-administration phase. A two-way analysis of variance (ANOVA) with active and inactive lever as a repeated measures factor and group as a between groups factor showed a significant lever effect [F(1,27) = 736.996, P<.001] but no group effect or lever by group interaction. A one-way ANOVA on the infusions data showed no significant group effect (P>.05)
Figure 1.
Mean (± SEM) number of active lever presses, inactive lever presses and infusion average across the last five cocaine self-administration sessions for all rats separated into groups according to the dose of (−)-stepholidine they would later be treated with (during reinstatement test).
The left panel of Figure 2 illustrates average responding on the active and inactive levers across the first two hours of the last three extinction sessions in all groups separated by the future test doses of (−)-stepholidine. Responding on the active and inactive levers was similar across all groups. The right panel of Figure 2 depicts reinstatement test data and shows an increase in active lever responding compared to the extinction phase. Further, this increase in active lever pressing was smaller, in a dose-related manner, in groups treated with (−)-stepholidine compared to vehicle. Responding on the inactive lever in all dose groups was similar to levels seen during extinction. Statistical analyses with a three-way ANOVA (phase x lever x dose) revealed a significant interaction between phase (extinction and reinstatement), lever and dose [F(3,27) = 5.162, P< .006]. Lever by dose interaction comparisons at each level of phase revealed a significant interaction during the reinstatement phase [F(3,27) =9.92, P<.001] but not during extinction. Tests of simple effects of dose at each level of lever in the reinstatement phase revealed a significant dose effect at the active lever [F(3,27) = 24.07, P< .05] but not at the inactive lever. Tukey post hoc tests revealed a significant difference between the vehicle group and 5 mg/kg (−)-stepholidine group, and the vehicle group and 10 mg/kg (−)-stepholidine group (Ps<.025).
Figure 2.
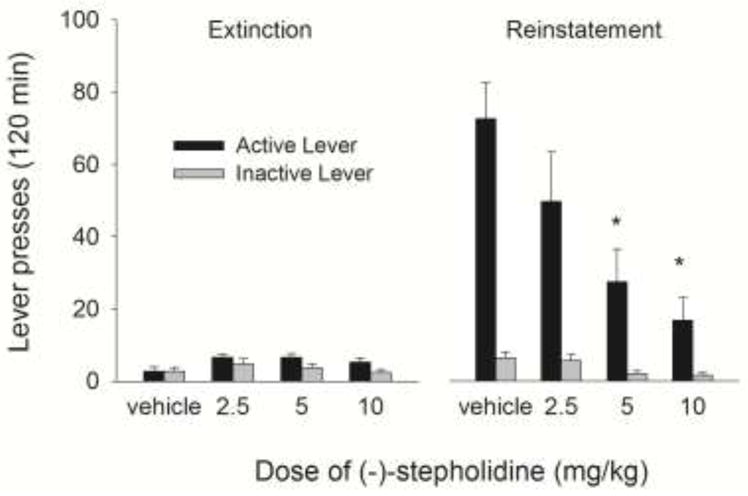
Mean (± SEM) number of presses on the active and inactive levers averaged across the last three extinction sessions in rats grouped by the (−)-stepholidine dose that they would eventually be treated with during the cue-induced reinstatement test (left panel) and during the cue-induced reinstatement test for all doses of (−)-stepholidine (right panel). * represents active lever pressing significantly different from vehicle at P < .05.
Rats treated with the 5 mg/kg dose of (−)-stepholidine and lever pressing for food under a PR schedule of reinforcement emitted on average 1,106.13 (± 155.59) lever presses, demonstrating that they could make at least as many, and in fact approximately 15 times more, lever presses than the vehicle-treated group (72.63 ± 9.99) in the reinstatement experiment during a similar 120-min period (data not shown). The numbers of lever presses for individual rats in the (−)-stepholidine-treated food reward group ranged from 625 to 1,952, all of which were higher than the numbers for any of the animals in the vehicle-treated reinstatement group.
3.2 Cocaine self-administration under a PR schedule
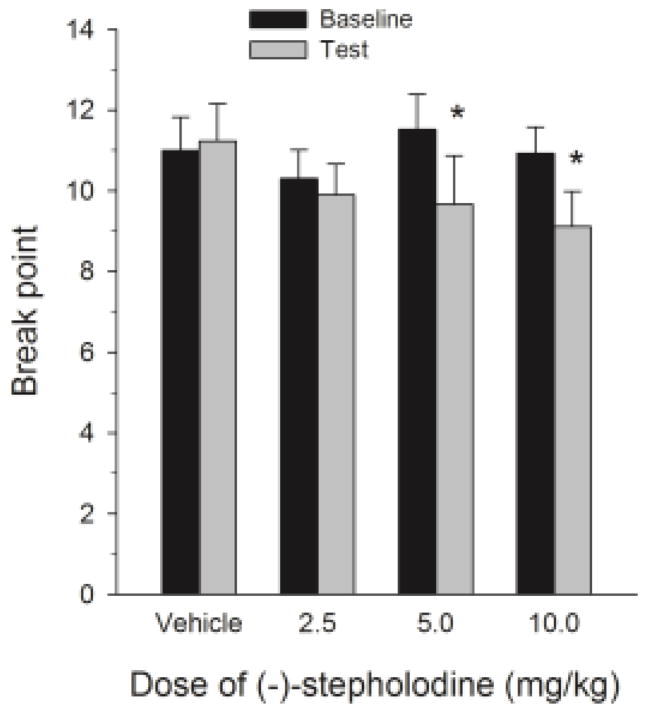
Figure 3 shows the mean baseline BPs compared to test day BPs. Statistical analyses utilizing a two-way ANOVA with repeated measures on dose and phase revealed a significant dose by phase interaction [F(3,24) = 4.719, P< 0.01]. Tests of simple effects of dose at each level of phase revealed a significant dose effect at the test phase [F(3,24) = 7.003, P< .01] but not at the baseline phase. Post hoc pairwise comparisons among doses in the test phase showed significantly smaller BPs in the 5 and 10 mg/kg (−)-stepholidine groups each compared to the vehicle group [Ps< .05].
Figure 3.
Mean (± SEM) BPs reached during the baseline and test sessions for all doses of (−)-stepholidine. * represents BPs significantly different from baseline at P < .05.
3.3 Locomotor activity
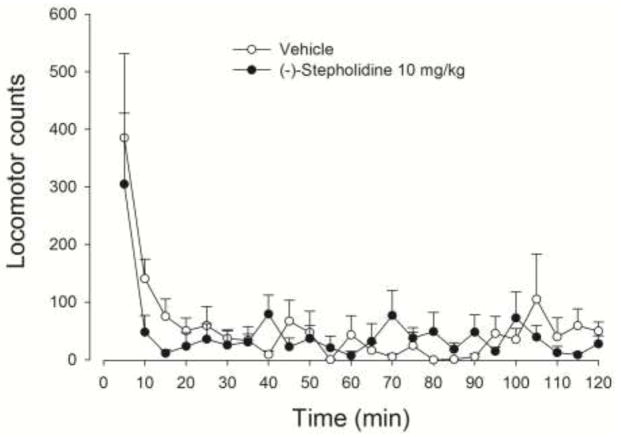
Figure 4 shows locomotor activity counts across 5-min time periods in a test session for rats treated with vehicle or 10 mg/kg (−)-stepholidine. Animals in both groups showed similar amounts of locomotor activity across the time periods. A two-way ANOVA with dose as a between groups factor and time as a repeated measures factor revealed a significant time effect [F(23, 253) = 7.158, P<.001] but no dose or dose by time interaction.
Figure 4.
Mean (± SEM) locomotor activity counts across 5-min time periods in a 2-h test session for rats receiving intraperitoneal injections of vehicle or 10 mg/kg (−)-stepholidine 30 min prior to the test.
4. Discussion
We found that (−)-stepholidine significantly reduced cue-induced reinstatement of responding on the active lever in a dose-related fashion, but not on the inactive lever. We also found that (−)-stepholidine significantly reduced cocaine self-administration under a PR schedule of reinforcement. To test whether or not lower responding under (−)-stepholidine was due to reduced capacity to emit the lever press response, we tested it in a group of animals pressing for food reward; these animals demonstrated that they could press the lever at least as often, and actually many times more, than the vehicle-treated reinstatement group. We also found that the highest dose of (−)-stepholidine – the 10 mg/kg dose – did not significantly affect locomotor activity.
Although it is possible that (−)-stepholidine may have reduced responding in reinstatement or self-administration through motoric effects, we believe this is not likely based on the following data: (1) (−)-stepholidine did not significantly reduce responding on the inactive lever, (2) rats treated with (−)-stepholidine and lever pressing for food pressed the lever approximately 15 times more than reinstatement rats treated with vehicle and (3) (−)-stepholidine did not significantly suppress locomotor activity. Furthermore, a recent article on the effects of the same dose of (−)-stepholidine as used here also reports no effects on locomotor activity (Hicks et al., 2018). Taken together, these results strongly suggest that (−)-stepholidine reduces cue-induced reinstatement of cocaine seeking and cocaine reward.
(−)-Stepholidine has been reported to inhibit heroin-induced reinstatement (Ma et al., 2014), reduce heroin self-administration and reduce cue-induced reinstatement of heroin seeking (Yue et al., 2014). Similarly, analogues of (−)-stepholidine, such as levo-tetrahydropalmatine (l-THP), can reduce cocaine self-administration under a PR schedule of reinforcement and cocaine discrimination in rodents (Mantsch et al., 2010) and reduce cocaine self-administration and reduce stress or cocaine-induced reinstatement in rats (Mantsch et al., 2007; Figueroa-Guzman et al., 2011).
Other multi-targeting treatments that act as D1 receptor partial agonists and D2–D3 receptor antagonists have also shown success in significantly reducing cocaine reward and cocaine seeking in rats that far outweighs the benefits achieved with single dopamine receptor targeting (Galaj et al., 2016; Xu et al., 2013). Recently, we have demonstrated that doses of SKF 77434, a selective D1 receptor partial agonist, and NGB 2904, a selective D3 receptor antagonist, that were ineffective at reducing cocaine cue-induced reinstatement, self-administration under a PR schedule of reinforcement or conditioned place preference, produced significant and robust reductions in all three when administered simultaneously (Galaj et al., 2016). This synergistic interaction is intriguing, for it suggests that significant effects on addiction-related behaviors can be achieved without resorting to administration of larger doses of dopaminergic agents that are typically accompanied by unwanted side effects. Furthermore, it suggests a close relationship between these two receptor subtypes in psychostimulant addiction. This idea is further supported by the observation of D1 receptor/D3 receptor heteromers that modulate intracellular signaling cascades in the striatum (Fiorentini et al., 2008) and may produce synergistic interactions between the two receptors. In addition to increased therapeutic benefits, D1 and D2 family receptor multitargeting treatments may likewise overcome substantial limitations of selective D1 partial agonists that increase hypotension and selective D3 antagonists that increase hypertension (Nichols et al., 1990; Oliver Jr. et al., 2006; Gonsai et al., 2017; Appel et al., 2015), but which, when administered together, could possibly cancel out their individual cardiovascular effects.
Data from the Psychoactive Drug Screening Program (https://pdspdb.unc.edu/pdspWeb/) indicates that (−)-stepholidine displays very good selectivity for dopamine receptors among several other CNS receptor sites. In that regard, (−)-stepholidine generally lacks any appreciable affinity (Ki >1000 nM) for serotonin receptors and transporters, adrenergic receptors and transporters, dopamine transporters, cannabinoid receptors, opioid receptors, cholinergic receptors and histamine receptors. Apart from dopamine D1, D2, D3 and D5 receptors (Ki values of 6, 974, 30 and 4 nM, respectively), the only other sites where any substantial affinity was observed was at the sigma-2 receptor (Ki = 53 nM), 5-HT1A receptor (Ki=143 nM) and the adrenergic alpha–2C receptor (Ki = 215 nM). Although the possibility of any contribution from these non-dopamine receptors in the activity observed herein cannot be ruled out at this time, the preference of (−)-stepholidine for dopamine D1 and D3 receptors in tandem with the known pharmacology of these receptors strongly suggests a role for D1 and D3 receptors in the behavioral effects exhibited by (−)-stepholidine in this study.
Other studies investigating the utility of (−)-stepholidine as an antipsychotic treatment have noted its limited extrapyramidal side effects and multiple potential therapeutic applications in humans (Mo et al., 2007). The consistent success of (−)-stepholidine in reducing drug reward, drug seeking and salience of drug associated cues suggests that it may be an interesting candidate for further testing as a potential treatment for substance use disorders. Moreover, given the dopamine multi-receptor targeting profile of (−)-stepholidine (and other THPBs that display anti-cocaine activity), further studies on compounds with D1 partial agonist/D2/D3 antagonist activity as well as compounds with D1/D2/D3 antagonist activity as anti-cocaine agents are warranted at this time.
Highlights.
(-)-stepholidine reduces cue-induced reinstatement of cocaine seeking
(-)-stepholidine reduces cocaine self-administration/reward
(-)-stepholidine may have potential as an anti-cocaine relapse treatment
Acknowledgments
Role of the Funding Source
This research was supported in part by NIH Grant SC1GM092282 (to WH) from the National Institute of General Medical Sciences.
Footnotes
Contributors
Monica Manuszak participated in the design and analysis of the study, was the principle collector of the data and wrote the complete first draft of the manuscript and all revisions. Satish Gadhiya and Wayne Harding synthesized (+)-stepholidine that was used in this study. Robert Ranaldi served as principle investigator, participated in the design and analysis of the study and assisted in writing all drafts of the manuscript. All authors have approved the final version of this manuscript.
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2002;159:284–93. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- Appel NM, Li SH, Holmes TH, Acri JB. Dopamine D3 receptor antagonist (GSK598809) potentiates the hypertensive effects of cocaine in conscious, freely-moving dogs. J Pharmacol Exp Ther. 2015;354:484–92. doi: 10.1124/jpet.115.224121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, Pocchiari F, Felder RA, Eisner GM, Jose PA. Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J Clin Invest. 1998;102:493–498. doi: 10.1172/JCI3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. 2016 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2017. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.pdf. [Google Scholar]
- Childress A, Ehrman R, McLellan AT, O’Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988;81:74–80. [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–15. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Figueroa-Guzman Y, Mueller C, Vranjkovic O, Wisniewski S, Yang Z, Li SJ, Bohr C, Graf EN, Baker DA, Mantsch JR. Oral administration of levo-tetrahydropalmatine attenuates reinstatement of extinguished cocaine seeking by cocaine, stress or drug-associated cues in rats. Drug Alcohol Depend. 2011;116:72–9. doi: 10.1016/j.drugalcdep.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol. 2008;74:59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- Gadhiya SV, Hu C, Harding WW. An alternative synthesis and x-ray crystallographic confirmation of (−)-stepholidine. Tetrahedron Lett. 2016;57:2090–2092. doi: 10.1016/j.tetlet.2016.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gál K, Gyertyán I. Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Res Bull. 2003;61:595–601. doi: 10.1016/s0361-9230(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Galaj E, Ananthan S, Saliba M, Ranaldi R. The effects of the novel DA D3 receptor antagonist SR 21502 on cocaine reward, cocaine seeking and cocaine-induced locomotor activity in rats. Psychopharmacology. 2014;231:501–10. doi: 10.1007/s00213-013-3254-y. [DOI] [PubMed] [Google Scholar]
- Galaj E, Harding W, Ranaldi R. Dopamine D1 and D3 receptor interactions in cocaine reward and seeking in rats. Psychopharmacology. 2016;233:3881–3890. doi: 10.1007/s00213-016-4420-9. [DOI] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, Peng XQ, Xi ZXb. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: Role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsai NH, Amin VH, Mendpara CG, Speth R, Hale GM. Effects of dopamine receptor antagonist antipsychotic therapy on blood pressure. J Clin Pharm Ther. 2017;43:1–7. doi: 10.1111/jcpt.12649. [DOI] [PubMed] [Google Scholar]
- Gratton A, Wise RA. Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous cocaine self-administration in rats. J Neurosci. 1994;14:4130–46. doi: 10.1523/JNEUROSCI.14-07-04130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology. 2001;155:330–7. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Hicks C, Huang P, Ramos L, Nayak SU, Caro Y, Reitz AB, Smith GR, Lee DY-W, Rawls SM, Liu-Chen L-Y. Dopamine D1-like receptor agonist and D2-like receptor antagonist (−)-stepholidine reduces reinstatement of drug-seeking behavior for 3,4-methylenedioxypyrovalerone (MDPV) in rats. ACS Chem Neurosci [Epub ahead of print] 2018 doi: 10.1021/acschemneuro.7b00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA. Cocaine enhances the changes in extracellular dopamine in nucleus accumbens associated with reinforcing stimuli: A high-speed chronoamperometric study in freely moving rats. Eur J Neurosci. 1993;5:284–91. doi: 10.1111/j.1460-9568.1993.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Bloch B. Expression of the D3 dopamine receptor in peptidergic neurons of the nucleus accumbens: Comparison with the D1 and D2 dopamine receptors. Neuroscience. 1996;73:131–143. doi: 10.1016/0306-4522(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Luippold G, Zimmermann C, Mai M, Kloor D, Starck D, Gross G, Muhlbauer B. Dopamine D3 receptors and salt dependent hypertension. J Am Soc Nephrol. 2001;12:2272–2279. doi: 10.1681/ASN.V12112272. [DOI] [PubMed] [Google Scholar]
- Ma B, Yue K, Chen L, Tian X, Ru Q, Gan Y, Wang D, Jin G, Li C. L-stepholidine, a natural dopamine receptor D1 agonist and D2 antagonist, inhibits heroin-induced reinstatement. Neurosci Lett. 2014;559:67–71. doi: 10.1016/j.neulet.2013.10.066. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Wisniewski S, Vranjkovic O, Peters C, Becker A, Valentine A, Li SJ, Baker DA, Yang Z. Levo-tetrahydropalmatine attenuates cocaine self-administration under a progressive-ratio schedule and cocaine discrimination in rats. Pharmacol Biochem Behav. 2010;97:310–6. doi: 10.1016/j.pbb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Li SJ, Risinger R, Awad S, Katz E, Baker DA, Yang Z. Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology. 2007;192:581–91. doi: 10.1007/s00213-007-0754-7. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Ferre S, Casado V, Cortes A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, Barnes C, Goldberg SR, Lluis C, Fuxe K, Franco R. Identification of dopamine D1–D3 receptor heteromers. Indications for a role of synergistic D1–D3 receptor interactions in the striatum. J Biol Chem. 2008;283:26016–25. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade JA, Free RB, Miller NR, Chun LS, Doyle TB, Moritz AE, Conroy JL, Watts VJ, Sibley DR. (−)-Stepholidine is a potent pan-dopamine receptor antagonist of both G protein- and β-arrestin-mediated signaling. Psychopharmacology. 2015;232:917–30. doi: 10.1007/s00213-014-3726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo J, Guo Y, Yang YS, Shen JS, Jin GZ, Zhen X. Recent developments in studies of l-stepholidine and its analogs: chemistry, pharmacology and clinical applications. Curr Med Chem. 2007;14:2996–3002. doi: 10.2174/092986707782794050. [DOI] [PubMed] [Google Scholar]
- Nichols AJ, Ruffolo RR, Jr, Brooks DP. The pharmacology of fenoldopam. Am J Hypertens. 1990;3:116S–119S. doi: 10.1093/ajh/3.6.116s. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Oliver WC, Jr, Nuttall GA, Cherry KJ, Decker PA, Bower T, Ereth MH. A comparison of fenoldopam with dopamine and sodium nitroprusside in patients undergoing cross-clamping of the abdominal aorta. Anesth Analg. 2006;103:833–40. doi: 10.1213/01.ane.0000237273.79553.9e. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O’Dowd BF, George SR. Heteromeric dopamine receptor signaling complexes: Emerging neurobiology and disease relevance. Neuropsychopharmacology. 2014;39:156–168. doi: 10.1038/npp.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridray S, Griffon N, Mignon V, Souil E, Carboni S, Diaz J, Schwartz JC, Sokoloff P. Coexpression of dopamine D1 and D3 receptors in islands of Calleja and 107 shell of nucleus accumbens of the rat: opposite and synergistic functional interactions. Eur J Neurosci. 1998;10:1676–86. doi: 10.1046/j.1460-9568.1998.00173.x. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Diaz J, Bordet R, Griffon N, Perachon S, Pilon C, Ridray S, Sokoloff P. Functional implications of multiple dopamine receptor subtypes: The D1/D3 receptor coexistence. Brain Res Rev. 1998;26:236–42. doi: 10.1016/s0165-0173(97)00046-5. [DOI] [PubMed] [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaál J, Xi ZX, Gardner EL. YQA14: A novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addict Biol. 2012;17:259–73. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Bi GH, Zhang HY, Yang RF, Gardner EL, Li J, Xi ZX. Blockade of D3 receptors by YQA14 inhibits cocaine’s rewarding effects and relapse to drug-seeking behavior in rats. Neuropharmacology. 2014;77:398–405. doi: 10.1016/j.neuropharm.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–68. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Wang JB, Mantsch JR. l-tetrahydropalamatine: A potential new medication for the treatment of cocaine addiction. Future Med Chem. 2012;4:177–86. doi: 10.4155/fmc.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Yang Z, Li SJ, Li X, Dillon C, Peng XQ, Spiller K, Gardner EL. Levo-tetrahydropalmatine inhibits cocaine’s rewarding effects: Experiments with self-administration and brain-stimulation reward in rats. Neuropharmacology. 2007;53:771–782. doi: 10.1016/j.neuropharm.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wang Y, Ma Z, Chiu YT, Huang P, Rasakham K, Unterwald E, Lee DY, Liu-Chen LY. L-isocorypalmine reduces behavioral sensitization and rewarding effects of cocaine in mice by acting on dopamine receptors. Drug Alcohol Depend. 2013;133:693–703. doi: 10.1016/j.drugalcdep.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue K, Ma B, Chen L, Tian X, Ru Q, Gan Y, Wang D, Jin G, Li C. L-Stepholidine, a naturally occurring dopamine D1 receptor agonist and D2 receptor antagonist, attenuates heroin self-administration and cue-induced reinstatement in rats. Neuroreport. 2014;25:7–11. doi: 10.1097/WNR.0000000000000012. [DOI] [PubMed] [Google Scholar]