Abstract
Reduced motivation is often noted as a consequence of cannabis use. However, prior studies examining this association have suboptimally operationalized motivation, and have yielded mixed findings. This review discusses motivation and the closely related construct of reward sensitivity. We summarize the available literature examining associations between motivation and cannabis use, addressing the following questions: (1) Is there evidence for decreased motivation among cannabis users? (2) Is there evidence that lack of motivation among cannabis users is specific to their use of cannabis (rather than to use of other addictive drugs)? (3) Is there evidence suggesting a causal relationship between cannabis use and motivation? We conducted a literature search using PubMed, PsycInfo, and WebofScience of studies examining non-acute effects of cannabis use on motivation, apathy, amotivation, effort, or reward sensitivity in humans. This search yielded 22 studies, which were reviewed in detail. We conclude that, although cross-sectional evidence of a cannabis-specific effect on motivation is equivocal, there is partial support from longitudinal studies for a causal link between cannabis use and reduced motivation. Additionally, we propose that reward sensitivity and motivation represent distinct yet related constructs, and reductions in one may not always lead to reductions in the other. Future work should longitudinally examine associations between cannabis use, motivation, and reward sensitivity, carefully define and operationalize these constructs, and control for the influence of potential confounding factors.
Keywords: motivation, cannabis use, apathy, reward sensitivity, amotivational syndrome
Introduction
Approximately 50 years ago, McGlothlin and West (1968) coined the term “amotivational syndrome” to describe the introversion, passivity, and lack of achievement-orientation observed among regular adult cannabis users. Prevalence estimates of “amotivational syndrome” have ranged from 5.2 to 6.3% among adult cannabis users (Duncan, 1987). The amotivated, “slacker” cannabis smoker has been a popular stereotype that has persisted in popular media for decades (Brownlee, 2011). Heavy chronic cannabis exposure has also been linked to less motivated responding in a progressive ratio reinforcement task among rhesus monkeys, as well as in a differential reinforcement of low-rate responding task among adult rats; in both studies, this “amotivational-like syndrome” resolved with prolonged abstinence (Paule et al., 1992; Stiglick & Kalant, 1983). Yet, until recently, empirical studies examining the relationship between cannabis use and motivation in human subjects have been sporadic.
With the recent proliferation of more permissive marijuana laws, growing interest in cannabis as medicine, and decreased perceived risk of use among adolescents, there has been renewed interest in this topic as researchers attempt to better characterize the potential adverse consequences of cannabis use. Indeed, motivation is identified as one of three high priority areas for research on adverse consequences of cannabis use in a recent, highly cited review (Volkow et al., 2016). Given that reduced motivation may have implications for academic achievement among adolescent cannabis users and other long-term outcomes, such as employment, quality of life, and treatment outcomes, we think it is timely to review the extant literature, identify challenges, and discuss possible future directions in this rapidly growing area. Specifically, in this review, we discuss the definition of motivation and its study in substance use research, summarize the available literature examining associations between motivation and cannabis use, and integrate findings from behavioral and neuroimaging studies broaching this topic. After reviewing the available literature, we grouped studies into one of four separate categories: self-report, performance-based, PET, and fMRI. Important confounding variables and gaps in our knowledge are discussed in order to inform future endeavors.
Motivation is a complex, multi-faceted construct that has proven difficult to operationalize (Kleinginna & Kleinginna, 1981). Broadly, motivation is defined as an umbrella term encompassing the cognitions, emotions, and behaviors involved in the activation, execution, and persistence of goal-directed behavior (Kleinginna & Kleinginna, 1981; Marin, Biedrzycki, & Firinciogullari, 1991). However, there are different types of motivation, such as extrinsic motivation, which refers to the performing of an action for its instrumental or material value, and intrinsic motivation, which refers to the performing of an action for its inherent satisfactions (Ryan & Deci, 2000). Also, motivation can be broken down into cognitive and behavioral components, such as self-efficacy (i.e. one’s belief in one’s ability to accomplish a task) and persistence (i.e. continuance in a course of action in spite of difficulty or obstacles), respectively (Liem & Martin, 2012).
Enhanced motivation to procure and consume drugs at the expense of other activities and in the face of adverse consequences is considered a hallmark of addiction (Robinson & Berridge, 2008). For instance, the incentive sensitization theory posits that drugs of abuse alter the brain’s mesocorticolimbic dopamine system, such that greater motivational salience is attributed to reward-associated stimuli (Robinson & Berridge, 2008). In other words, drug addiction, by definition, is characterized by greater motivation to obtain and consume drugs relative to other natural rewards. Although this usually results in increased incentive salience being attributed to drug cues, this can be generalized to other targets that often co-occur with drug use, such as food, gambling, and sex cues (Robinson & Berridge, 2008). Another relevant theory, the reward deficiency theory, posits that addiction may result, at least in part, from deficits in dopamine motivational circuitry in response to nondrug rewards, and only drugs of abuse are able to “normalize” dopaminergic signaling in the ventral striatum (Blum et al., 2000).
Indeed, dopamine is an important neurotransmitter in the context of motivation in addiction due to its role in reward-seeking behavior (Berridge, 2007). There has been considerable debate about the precise role of mesocorticolimbic dopamine in reward processes. Specifically, studies have examined whether dopamine mediates reward sensitivity or “liking” (i.e. perceptual vigilance, affective reactivity, and behavioral disposition towards rewarding stimuli), “wanting” (i.e. the pursuit of the reward), or reward learning (i.e. predicting future reinforcers; Berridge, 2007; Van den Berg, Ingmar Franken, & Muris, 2010). However, evidence suggests that dopamine is causally involved in “wanting,” and is neither required nor sufficient for either reward “liking” or learning (Berridge, 2007). Nevertheless, recent neuroimaging studies examining dopaminergic functioning and reward sensitivity among cannabis users posit that alterations in activation or dopamine functioning in mesocorticolimbic areas could constitute the biological basis for “amotivational syndrome.”
The preceding discussion on the definition of motivation and its broad role in addiction (beyond cannabis use disorder) is important in framing our understanding of the studies on cannabis use and motivation that are presented herein. Although several studies examine the effects of acute cannabis intoxication on motivation, this is beyond the scope of the current review, which aims to determine whether there is evidence of reduced motivation among chronic cannabis users lasting beyond transient periods of intoxication. It is important to note that although some studies provided definitions of motivation, most did not. Studies also varied widely in their operationalization of this construct. These studies are reviewed with the intent of arriving at conclusions based on the available literature on cannabis use and motivation to address the following questions: (1) Is there evidence for decreased motivation among cannabis users? (2) Is there evidence that lack of motivation among cannabis users is specific to their use of cannabis (rather than to use of other addictive drugs)? (3) Is there evidence suggesting a causal relationship between cannabis use and motivation?
Methods
Search Strategy
Searches were conducted on PubMed, PsycInfo, and WebofScience during March of 2018 using the terms (cannabis OR marijuana OR marihuana OR THC) AND (motivation OR amotivation OR apathy OR effort OR “reward sensitivity”.
Inclusion/Exclusion Criteria
We included peer-reviewed studies published since 1990 in the English language involving human participants, which examined the non-acute effects of cannabis use on motivation and/or reward sensitivity, as directly measured by a) interview or self-report measures of motivation or reward sensitivity, b) performance-based tasks measuring willingness to work for a reward, c) PET studies of dopaminergic functioning, and d) functional neuroimaging studies using the monetary incentive delay (MID) task or cue reactivity tasks.
We excluded non-peer reviewed articles, articles in foreign languages, animal studies, studies examining the effects of acute cannabis intoxication on motivation and/or reward sensitivity, studies examining motivation to quit or motives for use, studies using measures reward sensitivity to predict cannabis use, and structural neuroimaging studies. We also excluded studies that measured motivation indirectly through behavioral outcomes presumed to be related to motivation, such as academic achievement and employment trajectories, or neurocognitive measures designed to measure decision-making.
Results
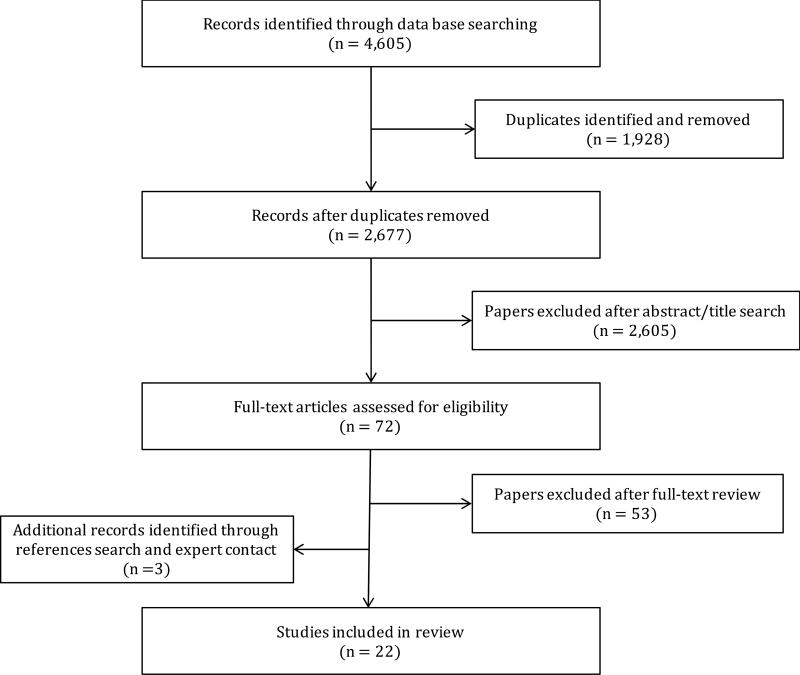
Figure 1 depicts the literature search process by which we identified 22 studies meeting our inclusion criteria, which are described in greater detail below.
Figure 1.
Literature search process.
Behavioral Studies Examining Cannabis Use and Motivation or Reward Sensitivity
The studies reviewed in this section examined the relationship between cannabis use and motivation by means of behavioral measures of motivation, including self-report measures, ranging from a single item to well-established questionnaires assessing motivation, as well as projective measures, and performance-based tasks.
Self-Report and Interview Measures of Motivation or Reward Sensitivity
Most of the studies that have examined direct associations between cannabis use and motivation have done so by means of self-report instruments. For instance, Kouri, Pope, Yurgelun-Todd, and Gruber (1995) examined an index of motivation as a function of cannabis use in a group of 45 heavy smokers, defined as users who smoked daily for 2 years or longer, and 44 occasional smokers, defined as users who had never smoked more than 10 times in a month at any time. These groups were matched on relevant demographic features (e.g. age, ethnicity), and did not differ significantly from each other on measures of mental health. Participants underwent a series of interviews, during which they were asked whether they thought that their cannabis use had impaired their motivation. Results revealed that heavy users were significantly more likely to agree that their cannabis use had impaired their motivation than occasional users. Although heavy smokers also reported significantly greater use of other substances, this variable was not controlled for in the analyses.
Looby and Earleywine (2007) surveyed a large sample of 2881 adult daily cannabis users about their substance use, motivation, and mental health. After responding to questions based on the DSM-IV-TR criteria for cannabis use dependence, 1111 were classified as dependent, and 1770 were classified as nondependent. Participants completed a subset of 12 items from the Apathy Evaluation Scale (AES), a measure defining apathy as a lack of motivation (Marin et al., 1991). Participants also completed several other measures assessing their substance use and life satisfaction. Results revealed that dependent users showed lower levels of motivation relative to nondependent users. Furthermore, the cannabis-dependent group reported significantly higher alcohol and other drug use as well as levels of depression. However, these variables were not controlled for in the analyses.
Recently, Lac and Luk (2018) collected substance use, personality, motivation, and demographics information from a sample of 505 college students at two time points one month apart. Approximately 27.5% of participants were cannabis users, suggesting that levels of cannabis use in the sample were low. Motivation was assessed through the General Self-Efficacy Scale, which contains 3 factors—initiative, effort, and persistence. Results from hierarchical regressions found that being a cannabis user at time 1 predicted lower initiative and persistence, but not effort, at time 2, even after accounting for covariates, including demographics, personality, alcohol and nicotine use, and self-efficacy at time 1. Similarly, results from cross-lagged models suggest that being a cannabis user, not an alcohol or nicotine user, longitudinally predicts lower initiative and persistence. The reverse was not true, as lower self-efficacy at time 1 did not predict cannabis use at time 2. Thus, this study supports a causal link between cannabis use and reduced motivation.
Several studies using self-report questionnaires have failed to find support for “amotivational syndrome” among cannabis users. Barnwell, Earleywine, and Wilcox (2006) surveyed an online sample of 487 adults. These participants completed a subset of items from the AES, with procedures identical to those of Looby and Earleywine (2007), as well as several other measures regarding their substance use and well-being. However, whereas Looby and Earleywine (2007) classified participants based on whether they met criteria for cannabis dependence, Barnwell et al.(2006) classified them based on patterns of use. Of the 487 adults, 243 were classified as current daily cannabis users, and 244 as nonusers, defined as those who had never tried cannabis. Results revealed no significant differences in apathy between current daily cannabis users and nonusers. Furthermore, effect size measures for motivation yielded a weak association, Cohen’s d = .06. The contrast between the findings of these two methodologically similar studies suggests that perhaps cannabis dependence, rather than cannabis use, is associated with greater apathy.
Similarly, Pacheco-Colón et al. (2017) examined associations between cannabis use and several indices of motivation in a sample of 79 adolescent cannabis users. Participants completed the AES, as well as the Motivation and Engagement Scale, which yields four main quotients of motivation measuring adaptive and maladaptive cognitions, and adaptive and maladaptive behaviors. After controlling for IQ, sex, depression, anxiety, ADHD symptoms, and use of alcohol and nicotine, there were no significant associations between any index of motivation and either lifetime or past 30-day amount of cannabis use. Based on their past 30-day frequency of cannabis use, participants were also divided into 36 recent regular users (used at least 10 days in past month), and 43 light users (used less than 10 days in past month and never used regularly). After controlling for covariates, there were no significant differences between recent regular users and light users on any index of motivation.
Other studies have found that the association between cannabis use and decreased motivation could be accounted for by coexisting depressive symptoms. Musty and Kaback (1995) found that the link between cannabis use and lower motivation could be accounted for by other factors. This study evaluated the associations between motivation and depression in a sample of 39 young adults, all of whom reported using cannabis at least several days over the past year. Participants completed a series of questionnaires assessing their patterns of alcohol and drug use and levels of depression, and a modified version of the Thematic Apperception Test, a projective picture interpretation test stemming from the psychoanalytic tradition, in which participants wrote five stories in response to a series of pictures. These were scored and used as indices of motivation across three dimensions: Need for Achievement, Need for Affiliation, and Need for Power. Participants were then divided into four groups based on their depression scores and frequency of cannabis use: heavy users with high depression, heavy users with low depression, light users with high depression, and light users with low depression. Analyses showed that heavy users with high depression had significantly lower Need for Achievement scores than heavy users without. There were no other significant between-group differences in any other motivational dimension. Notwithstanding concerns about the particular measure used to assess motivation, these results suggest that observed differences in motivation may be attributable to coexisting depressive symptoms.
Finally, Wright, Scerpella and Lisdahl (2016) examined the influence of cannabis use on self-reported apathy and reward sensitivity in a sample of 42 young adult cannabis users (> 10 times in past year and 500 times lifetime) relative to 42 controls. Reward sensitivity was measured through Behavioral Inhibition System and Behavioral Approach System (BIS/BAS). The subscales of the BAS include reward sensitivity, fun seeking, and drive. Apathy was assessed through a subscale of the Frontal Systems Behavioral Scale (FrSBe). After controlling for other substance use, results indicated that cannabis users demonstrated decreased BAS scores relative to controls, suggesting decreased sensitivity to reward. However, because cannabis users in this sample showed more symptoms of depression than controls, the authors suggested that these differences may reflect decreased mood. There were no significant group differences in apathy.
Performance-Based Measures of Motivation or Reward Sensitivity
Although self-report measures can shed light on participants’ perceptions of their own motivation, they are subjective by definition. Alternatively, performance-based tasks may serve as a more ecologically valid and objective measure of motivation, here operationalized as willingness to work for a reinforcer.
Only a handful of studies have examined the link between cannabis and motivation using performance-based measures. Lane, Cherek, Pietras, and Steinberg (2005) found that greater cannabis use was associated with performance patterns consistent with lower motivation. This study compared a group of 14 regular adolescent cannabis users, defined as using at least 4 days per week and meeting DSM-IV criteria for abuse or dependence, to a group of 20 current nonusers who served as a control group. The groups differed only on the number meeting criteria for conduct disorder, delinquency, and general cognitive ability. Participants who reported current use of drugs other than cannabis, past substance dependence for drugs other than cannabis were excluded from the study. Participants completed eight sessions of a behavioral motivation task over two days, consisting of two mutually exclusive response options: a progressive ratio reinforcement schedule, and a fixed time reinforcement schedule, both of which gave them the chance to earn money by pressing a button a certain amount of times in response to an on-screen stimulus. In the progressive ratio reinforcement schedule, each reinforcer was larger than the previous one but more responses were required to obtain it. In the fixed time reinforcement schedule, no responses were required, but monetary rewards were still provided. Although participants were allowed to switch to the fixed time or “nonwork” option at any time, remaining on the progressive ratio or “work” option yielded the most monetary earnings. Earlier switching to the nonwork option was considered an index of reduced motivation. Results revealed that regular adolescent cannabis users switched to the nonwork task significantly earlier than controls, and derived most of their monetary earnings from this task even though remaining on the work task would have resulted in greater earnings. These differences remained after controlling for cognitive aptitude, conduct disorder, and sex.
Martin-Soelch et al. (2009) compared behavioral and affective response to reward in 14 regular cannabis users, 20 tobacco smokers, and 19 nonsmokers. Participants performed a cognitive task at 3 levels of difficulty under 2 feedback conditions: rewarded and baseline. During the rewarded condition, participants could earn a monetary reward for every correct response. Results indicated a positive correlation between mood ratings and monetary wins during the rewarded condition among nonsmokers; this correlation was not significant among cannabis or tobacco users. Furthermore, cannabis users rated their mood as significantly worse during the rewarded condition relative to tobacco smokers and nonsmokers. There were no between-group differences in task performance. The authors concluded that, although both cannabis and nicotine led to similar motivational changes, cannabis may affect motivation to a greater extent than tobacco.
On the other hand, Lawn et al. (2016) used a similar experimental paradigm yet did not find significant associations between cannabis use and behavioral manifestations of motivation after controlling for confounding variables. They examined relationships between cannabis dependence, amotivation, and reward learning in a sample of 20 cannabis dependent individuals and 20 controls. Participants completed the effort expenditure for rewards task, assessing effort-related decision-making. Participants chose between a low-effort choice in which they won a small amount of money by pressing a button 30 times in 7 seconds, and a high-effort choice, in which they could win a larger amount by pressing a button 100 times in 21 seconds. Trials differed probabilistically as to whether participants actually won the money given task completion. Similar to Lane et al. (2005), more selections of the low-effort choice were thought to index reduced motivation. After controlling for depression and tobacco use, multiple regressions revealed no between-group differences in performance on the effort expenditure for rewards task, suggesting that cannabis dependent users did not show reduced motivation. Participants also completed a probabilistic reward task in which they had to quickly indicate whether the mouth in an abstract face was long or short, sometimes earning money when their answer was correct. One of the stimuli, however, was reinforced more often than the other (e.g. 30/50 trials were reinforced for long mouths vs. 10/50 for short). The main outcome of this task was response bias, a measure of reward learning indexing a person’s bias towards the more frequently reinforced stimulus. Results showed that although response bias increased across trials in controls, it did not increase in the cannabis group, suggesting impaired reward learning among cannabis dependent users. However, these differences were no longer present when depression and tobacco use were controlled. Together, these results suggest that cannabis dependent users do not show reduced motivation relative to controls. It is possible that they show impaired reward learning, but this may not necessarily be a result of their cannabis use, since this effect was no longer significant after controlling for use of other substances.
Neuroimaging Studies Examining Cannabis Use and Motivation or Reward Sensitivity
Recently, neuroimaging studies have advanced research on cannabis use and its effects on motivation. Although some of these studies link brain functioning to similar (and in few cases, the same) measures of motivation as those reported in the previously discussed behavioral studies, most focus on other constructs thought to be linked to motivation, such as dopaminergic functioning and reward sensitivity. The PET studies reviewed focus on dopamine receptor function. On the other hand, the fMRI studies reviewed focus on reward sensitivity, that is, perceptual vigilance, affective reactivity, and behavioral disposition towards rewarding stimuli (Van den Berg et al., 2010). These studies capture reward sensitivity by measuring neural response to reinforcers in areas of the brain’s mesocorticolimbic reward system. Furthermore, many of the following studies posit that alterations in dopaminergic functioning or reward sensitivity among cannabis users could constitute the biological basis for “amotivational syndrome.”
PET Studies of Dopaminergic Functioning
The link between cannabis use and altered motivation has been supported by several PET imaging studies, which correlate self-reported motivation with dopamine receptor function. Given dopamine’s role in reward-seeking behavior, it seems an ideal target for study as a mechanism for any cannabis-associated effects on motivation. For instance, a PET study conducted by Bloomfield, Morgan, Kapur, Curran, and Howes (2014) measured dopamine synthesis capacity in a sample of 14 regular adult cannabis users, defined as at least weekly use for more than one year, all of whom reported psychotic-like symptoms in response to smoking cannabis. Participants completed the AES, as well as detailed interviews assessing their drug use histories; participants reported no use of drugs other than cannabis, alcohol, and tobacco in the previous week. After being abstinent from cannabis use for at least 12 hours, participants underwent a scan, during which they were injected with [18F]-DOPA. Regions of interest (ROIs) consisted of the bilateral striatum (including its associative, limbic, and sensorimotor subdivisions), as well as the cerebellum. Dopamine synthesis capacity was indexed as the influx rate constant of [18F]-DOPA uptake in each ROI relative to the cerebellum. Results indicated that all participants reported significant levels of apathy on the AES, with no differences between users who met abuse or dependence criteria and those who did not. There was a significant inverse correlation between apathy and dopamine synthesis capacity in the whole striatum and its associative, but not limbic or sensorimotor, subdivisions. However, correlations between AES score and other variables, such as current cannabis consumption and age of first use were not significant. Thus, results suggest that reductions in striatal dopamine synthesis capacity among chronic cannabis users may be associated with increased apathy. However, it leaves open the question of whether disruptions in dopamine synthesis contributed to cannabis use in the sample or if they were a direct result of cannabis use.
Similarly, Volkow et al. (2014) used PET imaging to compare dopamine reactivity between 24 cannabis abusers and 24 controls when administered methylphenidate, a stimulant drug which blocks dopamine transporters and increases extracellular concentrations of dopamine. This allowed the investigators to assess dopamine reactivity to methylphenidate across several ROIs, including the striatum and thalamus. All cannabis abusers met DSM-IV criteria for cannabis abuse or dependence; those who had a history of abuse or dependence for drugs other than cannabis or nicotine were excluded, as were those who tested positive for psychoactive drugs other than cannabis. Controls were excluded if they reported smoking more than one joint of cannabis per month. Participants with a history of psychiatric conditions or current depression were also excluded. Participants also completed the Multidimensional Personality Questionnaire, which consists of 3 main factors—positive emotionality, negative emotionality, and constraint. Positive emotionality is, in turn, a combination of scores for well-being/reward sensitivity, social potency, achievement/motivation, and social closeness. Although there were no between-group differences in baseline striatal dopamine receptor availability, cannabis abusers showed significantly blunted responses (i.e. reduced decreases in distribution volumes of radioligand [C11]raclopride) when challenged with methylphenidate relative to controls as well as lower positive emotionality scores. Among cannabis abusers, positive emotionality scores were inversely correlated with methylphenidate-induced increases in midbrain dopamine. Because stimulation of midbrain dopamine receptors is thought to lead to decreased dopamine release in the striatum, which would result in decreased sensitivity to reward and reduced motivation, lower positive emotionality scores were indirectly associated with decreased dopamine release in reward areas of the brain. Nonetheless, the question of whether the observed alterations in dopaminergic signaling were due to cannabis use or may have been a cause of heavy cannabis use in the first place could not be addressed by this study.
fMRI Studies of Reward Sensitivity
Neuroimaging studies have also found significant associations between cannabis use and reduced reward sensitivity by using variations of the monetary incentive delay (MID) task. In this task, a cue is typically presented at the beginning of each trial, indicating a potentially rewarding or nonrewarding trial. Participants are then shown a visual target during which they have to press the button. After this, they receive feedback indicating whether they pressed the button quickly enough. Most studies using this task use reward anticipation activity, defined as activity during anticipation of reward versus anticipation of nonreward, as their measure of reward sensitivity. Some studies also examine reward outcome activity, defined as activity during feedback of rewarded targets versus feedback of missed targets.
One such study by van Hell et al. (2010) found that cannabis users showed reduced reward sensitivity to monetary rewards relative to controls. Participants were 14 cannabis users, 14 cigarette smokers, and 13 nonusers. Although cigarette smokers reported no cannabis use, cannabis users reported smoking cigarettes, but to a lesser extent. Levels of cannabis use varied widely within users. Groups were matched on all variables (e.g. hard drug use, mental health) other than cannabis and nicotine use. Cannabis users showed reduced activation during reward anticipation compared to nonsmoking controls in nucleus accumbens, caudate, left putamen, right inferior and medial frontal gyrus, superior frontal gyrus, and left cingulate. Relative to cigarette smokers, cannabis users also showed reduced activity during reward anticipation in some of these areas; however, there were no differences in activation of the nucleus accumbens, the left cingulate, or the right medial frontal gyrus. During reward outcome, cannabis users showed greater activation outcome relative to nonsmoking controls in the right caudate and bilateral putamen. Relative to cigarette smokers, cannabis users showed greater reward outcome activation only in the putamen, suggesting that alterations in this area are related to use of cannabis rather than nicotine. Together, these results suggest that, although cannabis use may be associated with reduced reward anticipation in the caudate and putamen, reduced activity in the nucleus accumbens may be linked to use of nicotine or other substances. Also, cannabis users showed reduced activity during reward anticipation but increased activity during reward outcome relative to both groups. This suggests that cannabis users may show impaired reward learning, with hypoactivation during anticipation reflecting an inability to predict upcoming rewards, and hyperactivity during outcome reflecting an unexpected reward (Luijten, Schellekens, Kühn, Machielse, & Sescousse, 2017).
Only one longitudinal study has examined the effects of cannabis use on neurobiological correlates of reward sensitivity. Martz et al. (2016) conducted an fMRI study examining the associations between cannabis use and ventral striatal response to reward in a sample of 108 young adults from the Michigan Longitudinal Study. Participants were assessed annually for substance use since age 11, and completed 3 consecutive fMRI scans at 2-year intervals starting at age 20. Participants performed a modified MID task Covariates included sex, age at fMRI baseline, parental history of substance use disorders, previous cannabis use and binge drinking, past year binge drinking, and previous and concurrent cigarette use. Results from cross-lagged models suggested that greater cannabis use was associated with later blunted activation in the nucleus accumbens during reward anticipation. Specifically, past year cannabis use at time 1 was negatively associated with activation of the nucleus accumbens at time 2; this association became marginally significant after controlling for cigarette use. Past year cannabis use at time 2 was also negatively associated with nucleus accumbens activation at time 3; covariates of previous cannabis use and binge drinking were significant. Of note, this association remained significant after controlling for cigarette use. Preexisting differences in nucleus accumbens activation were not associated with later cannabis use. Also, there was a marginally significant correlation between nucleus accumbens activation at time 1 and earlier onset of cannabis use, but not with amount of cannabis use, suggesting that greater activation of the reward system may be a risk factor for substance use rather than a consequence. Thus, cannabis use may lead to reduced reward sensitivity in the nucleus accumbens towards nondrug cues, making cannabis users more vulnerable to addiction.
On the other hand, several cross-sectional fMRI studies have found the opposite effect— hypersensitivity to rewards among cannabis users. For instance, a study by Nestor, Hester, and Garavan (2010) found that cannabis users showed hyper- rather than hyposensitivity to monetary reward. Participants were 14 regular cannabis users, defined as those using at least five days per week during the past two years, relative to 14 drug-naïve controls. During a modified MID task , cannabis users showed greater BOLD response in the ventral striatum during reward anticipation than nonusers. Furthermore, activity in the right ventral striatum was positively correlated with lifetime cannabis use amount and duration. This study suggests that chronic cannabis use is associated with ventral striatal hypersensitivity in anticipation to nondrug rewards, which is at odds with the view that cannabis use is associated with decreased reward sensitivity.
Similarly, a study by Filbey, Dunlop, and Myers (2013) found greater sensitivity to rewards among cannabis users during an MID task. Participants were 59 regular cannabis users, defined as 4 uses per week for at least 6 months, and 27 nonusers. Of the users, 6 met criteria for current cannabis abuse and 34 met criteria for current cannabis dependence. Participants in the control group reported no cannabis use within the past 9 months, with 3 meeting criteria for past cannabis abuse. No controls reported current cigarette use, with only 6 reporting past use. Groups differed in age, sex, years of education, and use of alcohol and other drugs. After controlling for age, sex, and education, group cannabis users showed greater activation relative to controls during gain versus loss trials in orbitofrontal cortex and cingulate gyrus, whereas controls had greater activation than cannabis users in loss compared to gain trials in orbitofrontal cortex. Additional analyses compared a subset of alcohol-matched cannabis users and controls, and found that cannabis users showed greater activation for gain versus loss trials in the orbitofrontal cortex relative to controls. There were no between-group differences in activation when comparing cannabis-dependent users and controls. These findings suggest that cannabis users show greater sensitivity to reward and reduced sensitivity to loss.
A study by Jager, Block, Luijten, and Ramsey (2013) found that adolescent cannabis users showed striatal hyperactivity during anticipation of both rewards and nonrewards. Participants were 21 frequent cannabis using adolescent males and 24 nonusing age-matched controls. Exclusion criteria included regular use of illegal drugs other than cannabis, and diagnosis of a psychiatric disorder other than conduct disorder. Cannabis users had lower IQ, and reported significantly higher past year alcohol and tobacco consumption than controls; these variables were not controlled for in analyses. Levels of cannabis use varied widely within users. Group analyses focused on two MID task contrasts: anticipation of reward trials versus neutral trials, and positive versus negative feedback. Results revealed that cannabis users showed marginally higher levels of brain activity in the caudate and putamen relative to controls during anticipation to both reward and neutral trials. This striatal hyperactivity may represent an overly sensitive motivational circuitry, such that frequent cannabis users show diminished ability to disengage this circuitry even though no reward can be obtained.
Another study conducted by Enzi et al. (2015) used fMRI to examine the neural correlates of reward and punishment processing among cannabis users. Participants were 15 male cannabis users (average of 13 joints/week) and 15 healthy controls with no history of psychiatric disorders. Groups were matched on age, sex, IQ, years of education, and cigarette smoking. The cannabis group reported a marginally greater number of drinks per week than controls, as well as more symptoms of depression. Participants completed the MID task in the scanner. Results revealed no between-group differences in brain activation during anticipation of rewards relative to neutral trials. During feedback, activation in the left caudate of healthy controls was able to differentiate between reward, punishment, and neutral conditions; for cannabis users, on the other hand, caudate activation only differentiated between reward and punishment feedback. Compared to healthy controls, cannabis users showed increased activation of the caudate for punishment and neutral conditions. Together, these results suggest impaired reward learning rather than disrupted reward sensitivity among cannabis users.
Other cross-sectional fMRI studies have failed to find significant relationships between cannabis use and reduced reward sensitivity. For example, a study conducted by Karoly et al. (2015) sought to examine neural response to reward among users of cannabis, tobacco, and alcohol during an MID task. Participants were 132 adolescents divided into 6 groups based on their past month substance use: 14 cannabis-only users (≤ 10 days in past month), 34 tobacco-only users (≤ 27 days in past month), 12 alcohol-only users (≤ 2 days in past month), 17 cannabis + tobacco users, 17 cannabis + tobacco + alcohol users, and 38 nonusers. Groups were matched on age, gender, and frequency of use of common substances (e.g. tobacco-only users and cannabis + tobacco users were matched on frequency of tobacco use). Group comparisons revealed no significant differences in nucleus accumbens activation between the cannabis-only group and any other group. However, the tobacco-only group showed reduced nucleus accumbens activation during reward versus neutral trials relative to controls, the alcohol-only group, and both polysubstance groups. Also, the alcohol-only group showed increased nucleus accumbens activation relative to the tobacco-only group at the highest level of reward. Overall, these findings do not support a link between cannabis use and disruptions in nucleus accumbens response to reward. Rather, they suggest that tobacco use may be associated with blunted nucleus accumbens response to nondrug rewards, while alcohol use may be associated with increased response to reward.
Another set of studies used visual cues to examine neural response to drug versus nondrug rewards. Cousijn et al (2013) examined neural response to cannabis cues relative to neutral cues among 31 frequent cannabis users (>10 days per month in last 2 years), 20 sporadic cannabis users (used 1–50 times), and 21 cannabis-naive controls. Groups were matched on alcohol use, whereas use of nicotine was included as a covariate in analyses. During the cue-reactivity task, participants viewed cannabis-related images, control images, and target images (e.g. animals) which they had to press a button in response to. Results indicated that frequent users showed greater activation of the ventral tegmental to cannabis cues than neutral cues relative to other groups. However, only a subset of frequent users showed greater activation for cannabis than neutral cues in orbitofrontal cortex, anterior cingulate, and striatum; this activation pattern was associated with cannabis problem severity. Amount of cannabis use, on the other hand, was not associated with fMRI activation. Thus, these results suggest that cannabis cue reactivity may be associated with addiction problem severity rather than cannabis use per se.
Additionally, a study conducted by Wetherill et al. (2014) found that cannabis users showed greater sensitivity in mesocorticolimbic reward areas of the brain in response to appetitive cues. This study examined the responses of cannabis users to subliminally presented cannabis-related cues (e.g. images of cannabis and cannabis paraphernalia, images of people smoking) and to compare these responses to those evoked by other emotionally evocative cues. Participants were 20 treatment-seeking, cannabis-dependent individuals who reported cannabis use on at least 10 days in the past month, and on more than 240 days in the past two years, with no use of drugs other than cannabis, alcohol, and tobacco. Using a backwards masking procedure, participants were subliminally presented with cannabis-related, sexual, aversive, and neutral cues. Participants showed increased activity in response to cannabis cues relative to neutral cues in the left anterior insula, left amygdala, and ventral striatum. Cannabis-dependent individuals also showed enhanced BOLD response to sexual cues relative to neutral cues in left striatum, anterior insula, right hippocampus/amygdala, and anterior cingulate, with no significant differences in activation between cannabis-related and sexual cues. These results indicate that cannabis dependence may be associated with enhanced reward-related responses to appetitive cues, rather than only drug-related cues. However, it is possible that sexual stimuli may have become secondary cues for cannabis use, since cannabis use has been previously linked to increased risky sexual behaviors (Ross, Coxe, Schuster, Rojas, & Gonzalez, 2015; Schuster, Crane, Mermelstein, & Gonzalez, 2012).
Similarly, Filbey et al. (2016) found that cannabis users showed enhanced reward responses towards cannabis-related cues. However, they found no evidence of reduced sensitivity towards nondrug rewards. This fMRI study examined BOLD response to cannabis-related cues relative to natural reward cues, such as fruit. Participants were 70 nonusers, and 59 adult regular cannabis users, defined as having a minimum of 5000 lifetime uses, and daily cannabis use in the preceding 60 days (with 3-day abstinence period). The fMRI task consisted of 2 runs of visual and tactile presentations of cannabis cues and natural reward cues, both tailored to match each participant’s preferences, and neutral cues. Results from group analyses revealed that regular cannabis users showed increased BOLD activation in frontal, cingulate, and midbrain areas, with peaks in the posterior cingulate and the medial frontal gyrus in response to cannabis cues relative to fruit cues, even after controlling for alcohol use and years of education. Thus, users showed enhanced response in areas of the mesocorticolimbic reward system that was specific to cannabis cues, rather than generalized hypersensitivity to reward. The lack of significant differences in activation during fruit cues relative to neutral cues between users and nonusers suggeststhat cannabis users did not show blunted activity towards nondrug cues., These results support the incentive sensitization theory, which posits that drugs of abuse sensitize the brain’s natural reward pathway, leading to greater attribution of incentive salience to drug-related cues (Robinson & Berridge, 2008).
Discussion
Over the course of several decades, a growing number of studies have examined the associations between cannabis use and motivation using a variety of methodologies, ranging from self-report instruments to behavioral neuroimaging protocols. The vast majority of these studies were published in the last decade, yet they have not been subject to a comprehensive review. We reviewed 22 of these studies, with some supporting a link between cannabis use and motivation and others finding no association. Results have also been mixed for reward sensitivity, with studies varying in terms of the direction of the observed effect. Importantly, studies have varied in the extent to which they controlled for confounding variables, as well as in their definition and operationalization of motivation, their assessment tools, and the levels of cannabis use in their sample. Furthermore, studies have failed to make a distinction between different types of motivation and have often equated the concepts of motivation and reward sensitivity. Thus, results have been equivocal. Below, we offer observations based on the findings of the reviewed studies to help answer the questions posed by this review.
We first answer the question, “Is there evidence for decreased motivation among cannabis users?” As seen in Table 1, of the 22 studies reviewed, 9 found that greater cannabis use was associated with decreased motivation. Of these, 6 studies controlled for the influence of at least some confounding variables by either including them as covariates in their analyses or having groups matched on these variables. However, the list of confounds that were controlled for differed across studies and, in most cases, was not exhaustive, with only 4 of the 7 studies that found an effect controlling for use of substances other than cannabis. Another potentially important confound that was not controlled in most studies was co-existing depression or depressive symptoms. Emerging evidence is showing a clear link between anhedonia in the context of depression and decreased motivation for rewards (Treadway & Zald, 2011). This is worth noting, particularly when considering that among the studies reviewed, two found additive effects of cannabis use and depression (Musty & Kaback, 1995; Wright, Scerpella, & Lisdahl, 2016) and another found that significant relationships between cannabis use and reward learning did not persist after controlling for depression (Lawn et al., 2016). Furthermore, definition and operationalization of the construct of motivation varied widely across studies. Whereas some self-report studies used the AES to measure apathy, or lack of motivation, others used less established measures of motivation, such as the positive emotionality factor of the Multidimensional Personality Questionnaire (in which motivation is one of several subcomponents), indices of the Thematic Apperception Test, and single-item measures. Studies using performance-based tasks, on the other hand, operationalized motivation as willingness to work for a reinforcer. Additionally, PET studies examined dopaminergic functioning as a marker of motivation, and fMRI studies measured reward sensitivity. When taking this heterogeneity into account, based on our review, we conclude that there is partial support for the link between cannabis use and decreased motivation. However, it is apparent that more work is needed. The field can benefit from a large-scale longitudinal study that assesses motivation in different ways (self-report, performance based, neuroimaging) and considers the effects of amount of cannabis use, whether a cannabis use disorder is present, and the impact of other drug use on results.
Table 1.
Summarized findings from reviewed studies.
| Study | Was cannabis use associated with reduced “motivation”? |
Controlled for other substance use? |
Possible evidence of causality? |
|---|---|---|---|
| Self-Report & Interview Studies | |||
| Kouri, Pope, Yurgelun-Todd & Gruber (1995) | + | − | − |
| Musty & Kaback (1995) | − | − | − |
| Barnwell, Earleywine, & Wilcox (2006) | − | − | − |
| Looby & Earleywine (2007) | + | − | − |
| Wright, Scerpella & Lisdahl (2016) | − | + | − |
| Pacheco-Colón et al. (2017) | − | + | − |
| Lac & Luk (2018) | + | + | + |
| Performance-Based Studies | |||
| Lane, Cherek, Pietras, & Steinberg (2005) | + | + | − |
| Martin-Soelch et al. (2009) | + | − | − |
| Lawn et al. (2016) | − | + | − |
| PET Studies | |||
| Bloomfield, Morgan, Kapur, Curran, & Howes (2014) | + | − | − |
| Volkow et al. (2014) | + | − | − |
| fMRI Studies | |||
| Van Hell et al. (2010) | + | + | − |
| Nestor, Hester, and Garavan (2010) | − | − | − |
| Cousijn et al. (2013) | − | + | − |
| Filbey, Dunlop, & Myers (2013) | − | + | − |
| Jager, Block, Luijten, & Ramsey (2013) | − | − | − |
| Wetherill et al. (2014) | − | − | − |
| Karoly et al. (2015) | − | + | − |
| Enzi et al. (2015) | − | + | − |
| Filbey et al. (2016) | − | + | − |
| Martz et al. (2016) | + | + | + |
We also reviewed evidence to answer the question, “Is there evidence that decreased motivation among cannabis users is unique to the use of cannabis (rather than to use of any addictive drug)?” As shown in Table 1, 12 of the reviewed studies accounted for the influence of use of substances other than cannabis, such as alcohol and nicotine. Several of these studies found significant associations between use of other substances and motivation. For instance, Lawn et al.(2016) found that impaired reward learning among cannabis users could be accounted for by use of other substances. Similarly, van Hell et al.(2010) and Karoly et al.(2015) found that reduced activity in the nucleus accumbens in response to rewards could be linked to nicotine rather than cannabis use. On the other hand, other studies continued to find a link between cannabis use and decreased motivation even after controlling for other substance use (Lac & Luk, 2018; Martz et al., 2016). Of the 9 studies that found significant associations between cannabis use and reduced motivation, 3 studies compared groups comprised of individuals with a cannabis use disorder to light users or non-using controls. Interestingly, the studies by Looby and Earleywine (2007) and Barnwell et al.(2006) employed identical assessment procedures, yet found different results. Specifically, the study by Looby and Earleywine (2007), which compared cannabis users who met dependence criteria to those who did not, found that dependent users were more apathetic, whereas the study by Barnwell et al. (2006), which compared daily users with nonusers, did not find between-group differences. Furthermore, a recent meta-analysis examining the effects of addiction (to cannabis, alcohol, nicotine, cocaine, and gambling) on reward processing concluded that addicts showed hypoactivation in striatal areas during reward anticipation, as well as increased activation during reward outcome regardless of drug choice (Luijten et al., 2017). This suggests the possibility that reduced motivation and/or reward sensitivity may be a common feature of addiction, rather than of cannabis use per se. Thus, evidence of a cannabis-specific effect on motivation, rather than a more broad effect of substance use in general, is equivocal.
Additionally, we reviewed findings regarding causality to answer the question, “Is there evidence suggesting a causal relationship between cannabis use and motivation?” As seen in Table 1, only two of the studies reviewed were longitudinal in design and thus better able to examine causal relationships. Martz et al.(2016) found that continued cannabis use was associated with blunted activity in the nucleus accumbens during reward anticipation even after controlling for an exhaustive set of confounds, including baseline nucleus accumbens activation. Pre-existing differences in accumbens functioning, on the other hand, did not predict later cannabis use. A longitudinal study by Lac and Luk (2018) found similar results: after controlling for confounds, being a cannabis user predicted lower self-efficacy at a later time point, whereas self-efficacy did not predict later cannabis use. Thus, there is evidence suggesting a causal relationship between cannabis use and reduced motivation and reward sensitivity.
Neuroimaging studies focusing on the effects of cannabis on reward sensitivity have consistently found differences in activation of reward areas of the brain in cannabis users relative to controls. Although studies vary as to whether they conducted ROI or whole-brain analyses, most found alterations among cannabis users in striatal areas, particularly ventral striatum, as well as frontal areas including the cingulate, thus implicating parts of the salience network. Alterations in this network among users suggest that cannabis use may alter how attention is allocated to rewarding stimuli and/or drug-related cues (Menon, 2011). However, as seen in Table 2, the direction of this effect varied across studies, with 3 studies reporting increased activation, and 2 reporting decreased activation during anticipation to monetary rewards among cannabis users relative to controls, and 2 reporting no between-group differences. These discrepancies may, at least in part, be due to the fact that the cross-sectional nature of most of these studies renders them unable to account for baseline levels of activation in reward areas of the brain. Furthermore, three studies examined activation in response to visual drug cues compared to nondrug reward cues. All found that cannabis users showed increased activation in reward regions in response to drug-related cues, and either increased or equivalent activation in response to nondrug reward cues. Taken together, these findings do not provide enough evidence to equivocally determine whether cannabis use is associated with a blunted neural response to nondrug rewards.
Table 2.
Summarized findings of neuroimaging studies examining the link between cannabis use and reward sensitivity.
| Study | Task | Direction of Finding for Cannabis Users |
|---|---|---|
| Van Hell et al. (2010) | Monetary Incentive Delay (MID) | Decreased activation during reward anticipation |
| Nestor, Hester, and Garavan (2010) | MID | Greater activation during reward anticipation |
| Cousijn et al. (2013) | Cue-reactivity | Increased activation in response to drug cues |
| Filbey, Dunlop, & Myers (2013) | MID | Greater activation during reward anticipation |
| Jager, Block, Luijten, & Ramsey (2013) | MID | Greater activation during reward anticipation |
| Wetherill et al. (2014) | Cue-reactivity | Increased activation in response to appetitive cues |
| Karoly et al. (2015) | MID | No effect of cannabis |
| Enzi et al. (2015) | MID | No difference during reward anticipation; activation pattern did not differentiate between reward and punishment trials |
| Filbey et al. (2016) | Cue-reactivity | Increased activation in response to drug cues; no differences in response to nondrug rewards |
| Martz et al. (2016) | MID | Decreased activation during reward anticipation |
In integrating these findings, it is important to consider the relationship between motivation and reward sensitivity. Although related, evidence from the Parkinson’s literature suggests that they are distinct constructs. Apathy, or lack of motivation, is frequently reported among nondepressed patients with Parkinson’s disease. Several studies have found that treatments involving L-dopa and high frequency stimulation of the subthalamic nucleus resulted in improved motivation but had limited or contrasting effects on reward sensitivity (Czernecki et al., 2002, 2005). Others found that insensitivity to reward was predictive of apathy severity (Muhammed et al., 2016). Thus, findings from this body of work suggest that reward sensitivity and motivation represent distinct yet interrelated constructs. Researchers examining the effects of cannabis on reward sensitivity should therefore consider that differences in reward sensitivity do not necessarily translate to problems in motivation.
In conclusion, studies examining the effects of cannabis use on motivation and reward sensitivity have yielded mixed findings. We conclude that cross-sectional evidence supporting the presence of “amotivational syndrome” among cannabis user or an adverse cannabis-specific effect on motivation is currently equivocal. Nevertheless, results from two longitudinal studies provide evidence of a causal relationship between cannabis use and reduced motivation and reward sensitivity. Future studies could help to fill existing gaps in our knowledge in several ways. First, studies should carefully control for the influence of potentially confounding variables. These include, but are not limited to, cognitive ability, sex, age, education, symptoms of psychiatric disorders known to affect motivation (such as depression and ADHD), and use of other substances, including alcohol and nicotine. This will allow us to better isolate the relationship of interest. Second, future studies should examine these associations longitudinally rather than cross-sectionally. This will enable us to examine how changes in cannabis use influence motivation over time and to infer causality. Third, future work would benefit from incorporating measures of both reward sensitivity and motivation in order to elucidate the potentially complex associations between these constructs. Fourth, studies assessing motivation should carefully define and operationalize this construct. Because motivation is a multi-faceted construct, studies should strive to incorporate measures assessing different types of motivation, as this will allow us to examine whether substances like cannabis selectively influence some types of motivation. We anticipate that such steps should help to improve our understanding of this important relationship, which may be a potential mechanism by which cannabis use is associated with poorer academic outcomes and employment trajectories (Arria, Caldeira, Bugbee, Vincent, & O’Grady, 2015; Lynskey & Hall, 2000).
Acknowledgments
This work was supported by grants R01 DA031176, R01 DA033156, and CNS-1532061 (PI: Gonzalez) from the National Institute of Drug Abuse, as well as the Presidential Fellowship at Florida International University (Recipient: Pacheco-Colón).
Contributor Information
Ileana Pacheco-Colón, Center for Children and Families, Department of Psychology, Florida International University
Jorge M. Limia, Department of Psychology, Florida International University
Raul Gonzalez, Center for Children and Families, Department of Psychology, Florida International University
References
- Arria AM, Caldeira KM, Bugbee BA, Vincent KB, O’Grady KE. The Academic Consequences of Marijuana Use during College. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors. 2015;29(3):564–575. doi: 10.1037/adb0000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnwell SS, Earleywine M, Wilcox R. Cannabis, motivation, and life satisfaction in an internet sample. Substance Abuse Treatment, Prevention, and Policy. 2006;1:2. doi: 10.1186/1747-597X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bloomfield MAP, Morgan CJA, Kapur S, Curran HV, Howes OD. The link between dopamine function and apathy in cannabis users: an [18F]-DOPA PET imaging study. Psychopharmacology. 2014;231(11):2251–2259. doi: 10.1007/s00213-014-3523-4. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Comings DE. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs. 2000;(32 Suppl):i–iv. 1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Brownlee N. This is Cannabis. Bobcat Books; 2011. [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Neural responses associated with cue-reactivity in frequent cannabis users. Addiction Biology. 2013;18(3):570–580. doi: 10.1111/j.1369-1600.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- Czernecki V, Pillon B, Houeto JL, Pochon JB, Levy R, Dubois B. Motivation, reward, and Parkinson’s disease: influence of dopatherapy. Neuropsychologia. 2002;40(13):2257–2267. doi: 10.1016/s0028-3932(02)00108-2. [DOI] [PubMed] [Google Scholar]
- Czernecki V, Pillon B, Houeto JL, Welter ML, Mesnage V, Agid Y, Dubois B. Does bilateral stimulation of the subthalamic nucleus aggravate apathy in Parkinson’s disease? Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(6):775–779. doi: 10.1136/jnnp.2003.033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan DF. Lifetime prevalence of “amotivational syndrome” among users and non-users of hashish. Psychology of Addictive Behaviors. 1987;1(2):114–119. [Google Scholar]
- Enzi B, Lissek S, Edel M-A, Tegenthoff M, Nicolas V, Scherbaum N, Roser P. Alterations of Monetary Reward and Punishment Processing in Chronic Cannabis Users: An fMRI Study. PLOS ONE. 2015;10(3):e0119150. doi: 10.1371/journal.pone.0119150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Dunlop J, Ketcherside A, Baine J, Rhinehardt T, Kuhn B, Alvi T. fMRI study of neural sensitization to hedonic stimuli in long-term, daily cannabis users. Human Brain Mapping. 2016;37(10):3431–3443. doi: 10.1002/hbm.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Dunlop J, Myers US. Neural Effects of Positive and Negative Incentives during Marijuana Withdrawal. PLOS ONE. 2013;8(5):e61470. doi: 10.1371/journal.pone.0061470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Tentative Evidence for Striatal Hyperactivity in Adolescent Cannabis Using Boys: A Cross-Sectional Multicenter fMRI Study. Journal of Psychoactive Drugs. 2013;45(2):156–167. doi: 10.1080/02791072.2013.785837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Bryan AD, Weiland BJ, Mayer A, Dodd A, Feldstein Ewing SW. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Developmental Cognitive Neuroscience. 2015;16(Supplement C):5–15. doi: 10.1016/j.dcn.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinginna PR, Kleinginna AM. A categorized list of motivation definitions, with a suggestion for a consensual definition. Motivation and Emotion. 1981;5(3):263–291. [Google Scholar]
- Kouri E, Pope HG, Yurgelun-Todd D, Gruber S. Attributes of heavy vs. occasional marijuana smokers in a college population. Biological Psychiatry. 1995;38(7):475–481. doi: 10.1016/0006-3223(94)00325-w. [DOI] [PubMed] [Google Scholar]
- Lac A, Luk JW. Testing the Amotivational Syndrome: Marijuana Use Longitudinally Predicts Lower Self-Efficacy Even After Controlling for Demographics, Personality, and Alcohol and Cigarette Use. Prevention Science. 2018;19(2):117–126. doi: 10.1007/s11121-017-0811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Pietras CJ, Steinberg JL. Performance of heavy marijuana-smoking adolescents on a laboratory measure of motivation. Addictive Behaviors. 2005;30(4):815–828. doi: 10.1016/j.addbeh.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Lawn W, Freeman TP, Pope RA, Joye A, Harvey L, Hindocha C, Curran HV. Acute and chronic effects of cannabinoids on effort-related decision-making and reward learning: an evaluation of the cannabis “amotivational” hypotheses. Psychopharmacology. 2016;233(19–20):3537–3552. doi: 10.1007/s00213-016-4383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem GAD, Martin AJ. The Motivation and Engagement Scale: Theoretical Framework, Psychometric Properties, and Applied Yields. Australian Psychologist. 2012;47(1):3–13. [Google Scholar]
- Looby A, Earleywine M. Negative consequences associated with dependence in daily cannabis users. Substance Abuse Treatment, Prevention, and Policy. 2007;2:3. doi: 10.1186/1747-597X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kühn S, Machielse MWJ, Sescousse G. Disruption of Reward Processing in Addiction : An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry. 2017;74(4):387–398. doi: 10.1001/jamapsychiatry.2016.3084. [DOI] [PubMed] [Google Scholar]
- Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000;95(11):1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Research. 1991;38(2):143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Kobel M, Stoecklin M, Michael T, Weber S, Krebs B, Opwis K. Reduced Response to Reward in Smokers and Cannabis Users. Neuropsychobiology. 2009;60(2):94–103. doi: 10.1159/000239685. [DOI] [PubMed] [Google Scholar]
- Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, Heitzeg MM. Association of Marijuana Use With Blunted Nucleus Accumbens Response to Reward Anticipation. JAMA Psychiatry. 2016;73(8):838–844. doi: 10.1001/jamapsychiatry.2016.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlothlin WH, West LJ. The Marihuana Problem: An Overview. American Journal of Psychiatry. 1968;125(3):370–378. [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Muhammed K, Manohar S, Ben Yehuda M, Chong TT-J, Tofaris G, Lennox G, Husain M. Reward sensitivity deficits modulated by dopamine are associated with apathy in Parkinson’s disease. Brain. 2016;139(10):2706–2721. doi: 10.1093/brain/aww188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musty RE, Kaback L. Relationships between motivation and depression in chronic marijuana users. Life Sciences. 1995;56(23):2151–2158. doi: 10.1016/0024-3205(95)00202-h. [DOI] [PubMed] [Google Scholar]
- Nestor L, Hester R, Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage. 2010;49(1):1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Colón I, Coxe S, Musser ED, Duperrouzel JC, Ross JM, Gonzalez R. Is Cannabis Use Associated with Various Indices of Motivation Among Adolescents? Substance Use & Misuse. 2017;0(0):1–12. doi: 10.1080/10826084.2017.1400566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule MG, Allen RR, Bailey JR, Scallet AC, Ali SF, Brown RM, Slikker W. Chronic marijuana smoke exposure in the rhesus monkey. II: Effects on progressive ratio and conditioned position responding. The Journal of Pharmacology and Experimental Therapeutics. 1992;260(1):210–222. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JM, Coxe S, Schuster RM, Rojas A, Gonzalez R. The moderating effects of cannabis use and decision making on the relationship between conduct disorder and risky sexual behavior. Journal of Clinical and Experimental Neuropsychology. 2015;37(3):303–315. doi: 10.1080/13803395.2015.1010489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. Intrinsic and Extrinsic Motivations: Classic Definitions and New Directions. Contemporary Educational Psychology. 2000;25(1):54–67. doi: 10.1006/ceps.1999.1020. [DOI] [PubMed] [Google Scholar]
- Schuster RM, Crane NA, Mermelstein R, Gonzalez R. The Influence of Inhibitory Control and Episodic Memory on the Risky Sexual Behavior of Young Adult Cannabis Users. Journal of the International Neuropsychological Society. 2012;18(5):827–833. doi: 10.1017/S1355617712000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Behavioral effects of prolonged administration of delta 9-tetrahydrocannabinol in the rat. Psychopharmacology. 1983;80(4):325–330. doi: 10.1007/BF00432114. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg I, Ingmar HA, Franken, Muris P. A New Scale for Measuring Reward Responsiveness. Frontiers in Psychology. 2010:1. doi: 10.3389/fpsyg.2010.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, Ramsey NF. Chronic effects of cannabis use on the human reward system: an fMRI study. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2010;20(3):153–163. doi: 10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, Baler R. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 2016;73(3):292–297. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Alexoff D, Logan J, Tomasi D. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proceedings of the National Academy of Sciences. 2014;111(30):E3149–E3156. doi: 10.1073/pnas.1411228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Childress AR, Jagannathan K, Bender J, Young KA, Suh JJ, Franklin TR. Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacology. 2014;231(7):1397–1407. doi: 10.1007/s00213-013-3342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NE, Scerpella D, Lisdahl KM. Marijuana Use Is Associated with Behavioral Approach and Depressive Symptoms in Adolescents and Emerging Adults. PLOS ONE. 2016;11(11):e0166005. doi: 10.1371/journal.pone.0166005. [DOI] [PMC free article] [PubMed] [Google Scholar]