Abstract
The purpose of this study was to characterize cell-specific expression patterns of Toll-like receptors (TLR) in non-human primate (NHP) neural retina tissue. TLR 4, 5, 6, and 7 proteins were detected by immunblotting of macaque retina tissue lysates and Quantitative PCR (qPCR) demonstrated TLRs 4-7 mRNA expression. Immunofluorescence (IF) microscopy detected TLRs 4-7 in multiple cell types in macaque neural retina including Muller, retinal ganglion cells (RGC), amacrine, and bipolar cells. These results demonstrate that TLRs 4-7 are constitutively expressed by neurons in the NHP retina raising the possibility that these cells could be involved in retinal innate inflammatory responses.
Keywords: Retina, Non-human primates, Innate immunity, Toll-Like Receptors, Inflammation
Graphical abstract
1. Introduction
Although the eye is considered an immunoprivileged site, this privilege can clearly be broken (Chang et al., 2004; Chang et al., 2006). Ocular inflammation can be the consequence of an immune response to an infectious agent, result from allergic or autoimmune responses, or be idiopathic (Chang et al., 2004; Chang et al., 2006). The resulting inflammation can affect any part of the eye and may threaten sight. Microbial infections of the eye, including herpes simplex virus keratitis, cytomegalovirus retinitis, bacterial keratitis, and bacterial endophthalmitis are quite common (Chang et al., 2004; Chang et al., 2006). In addition, viral gene delivery vectors can trigger ocular inflammation which may compromise transgene expression and preclude further vector administration (Bennett, 2003). Posterior uveitis, the inflammation of the posterior uveal tract (retina and choroid), can have infectious origins, which is more common in developing countries, non-infectious origins, or be part of a masquerade syndrome (Lee et al., 2017; Tsirouki et al., 2016). Acute retinal necrosis can be caused by herpes simplex virus or varicella zoster virus and viral retinitis can also occur as a consequence of intraocular corticosteroid injections (Lee et al., 2017).
Innate immune responses, which occur very early in inflammation, play an important role in direct anti-microbial responses, inducing inflammation, and modulating the adaptive immune response (Chang et al., 2004; Chang et al., 2006). One of the key groups of innate immune receptors that recognize and respond to microbes are the Toll-like receptors (TLRs), which are constitutively expressed in multiple cells types and up-regulated in inflammatory conditions (Akira et al., 2001). There are currently ten known mammalian TLRs which recognize conserved structural moieties called pathogen-associated molecular patterns (PAMPs) (Bowie and Haga, 2005; Pandey et al., 2013). PAMPs recognized by TLRs include microbial components such as lipoproteins (TLR1, TLR2, TLR6), glycoproteins (TLR2), lipopolysaccharide (TLR4), flagellin (TLR5), zymosan (TLR6), and viral and bacterial nucleic acids (TLR3, TLR7, TLR8, TLR9) (Pandey et al., 2013). The ligand specificity and function of TLR10 is still unknown, but its main function may be to modulation of the inflammatory response (Oosting et al., 2014).
In addition, molecules released from damaged cells, including damage associated molecular patterns (DAMPs), can activate pattern recognition receptors (PRRs), including TLRs, and exacerbate the inflammatory response (Wakefield et al., 2010). The recognition of PAMPs or DAMPs by TLRs initiates signal transduction pathways, involving the transcription factors NF-κB or IRF-3, leading to altered gene expression and the induction of inflammatory cytokines (Arancibia et al., 2007). Alterations in cytokine expression, including IL-1, IL-6, IL-17, and TNF have been associated with uveitis, which suggests the innate immune response may trigger the signaling events leading to ocular inflammation (Schwartzman, 2016).
Previous studies, using human retinal pigment epithelial (RPE) cell cultures, have detected expression of TLRs 1-7, 9, and 10 (Kumar et al., 2004), linked TLR3 signaling to an increase in inflammatory cytokine production (Brosig et al., 2015), and demonstrated that exposure to pro-inflammatory cytokines decreased the expression of genes critical for RPE function (Kutty et al., 2016). Recently, the expression pattern of TLRs in human retinal and choroidal vascular endothelial cells has been studied (Stewart et al., 2015). Muller glial cells have received the most attention to date, and have been shown to play a role in inflammatory responses during autoimmune uveitis (Hauck et al., 2007) and bacterial infection (Kumar et al., 2013; Kumar et al., 2010; Shamsuddin and Kumar, 2011; Singh et al., 2014). The human retinal Muller cell line MIO-M1 expresses TLRs 1-10 and TLR agonist or live pathogen challenge produces an increase in inflammatory mediators (Kumar and Shamsuddin, 2012). Murine Muller cells (Kumar and Shamsuddin, 2012; Lin et al., 2013) and a murine photoreceptor cell line (Singh and Kumar, 2015) also express TLRs and initiate innate responses following TLR ligand challenge (Gao et al., 2017; Singh and Kumar, 2015).
Studies with human ocular tissue identified TLR4 expression in iris/ciliary body, choroid, retina, sclera, and conjunctiva, however, TLR4 protein was only detected in uveal antigen presenting cells (Chang et al., 2004). Corneas from healthy patients expressed TLRs 1-10 mRNA and, following herpes simplex virus infection, the expression of all TLRs was upregulated (Jin et al., 2007). A direct correlation between TLR activation, cytokine production, and anterior uveitis has been established in mice (Allensworth et al., 2011). In addition, TLR3 activation has been linked to inflammation and photoreceptor cell death in the mouse model of dry age-related macular degeneration (Gao et al., 2017). There is a lack of data, however, on TLR expression in the non-human primate neural retina.
The importance of TLR signaling in viral gene delivery vector induced ocular inflammation cannot be overlooked. Secretion of IL-6 was detected following herpes simplex virus vector transduction of RPE cells (Cai and Brandt, 2008). Recent studies demonstrated that cytokines, including IL-6 and IL-10, as well as TLRs 6 and 7, were upregulated, following herpes simplex vector challenge of non-human primate neural retina tissue (Sauter and Brandt, 2016). To date, a detailed examination of which TLRs are expressed in NHP neural retina, and the cell types expressing them, has not been done. This study includes data only for TLRs 4, 5, 6, and 7, because we were able to source antibodies which recognized single bands of the appropriate size by immunoblotting of macaque retina lysates for these receptors.
2. Materials and Methods
2.1 Macaque retina tissue
Eyes from euthanized rhesus macaques (Macaca mulatta), or cynomolgus macaques (Macaca fascicularis), were obtained as they became available from the Wisconsin National Primate Research Center of the University of Wisconsin-Madison or Covance (Madison, WI). Animals were free of infectious agents at the time of sacrifice. No animals were deliberately sacrificed for these studies. Macaque eyes were kept on ice and dissected within one hour of sacrifice. Posterior eye cups were incubated with PBS/1 mM EDTA for 30 minutes at 37°C to loosen neural retina tissue and separate the retina from RPE cells. Neural retina tissue was rinsed in PBS before proceeding with further studies. All experiments adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2 RNA Isolation
Macaque neural retina tissue was rinsed in PBS prior to homogenization and RNA isolation using the TRIzol Reagent protocol (Ambion/Life Technologies, Grand Island, NY, #15596-026). DNase digestion (Qiagen, Valencia, CA, RNase-Free DNase Set, #79254) was completed prior to RNA cleanup on RNeasy spin columns (Qiagen, RNeasy Mini Kit, #74104). RNA was eluted in RNase-free H2O and quantitated on a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE, #ND-1000).
2.3 Quantitative PCR
PCR primers were designed for macaque TLR5 (Table 1). TLRs 4 and 7 primers were synthesized based on previously published human TLR primers (Table 1) (Kumar and Shamsuddin, 2012). A commercial primer pair was utilized for TLR6 (RT2 qPCR primer assay, Qiagen, PPQ002388). cDNA from cynomolgus and rhesus macaques was amplified with primers to TLRs 4, 5, 6 or 7 following the standard Qiagen RT2 qPCR primer assay protocol using RT2 SYBR Green ROX qPCR Mastermix (Qiagen, #330520) and an ABI 7300 cycler. The annealing temperature was reduced to 55°C for the TLR4 and TLR7 reactions. qPCR reactions were performed in triplicate and mean CT values were plotted versus the negative control (no primers) and positive control (β-actin primers, Qiagen, PPQ00182A).
Table 1.
Quantitative PCR primers for macaque Toll-like receptors
| Gene | Primer Sequence | Product Size |
|---|---|---|
| TLR4* | 5′ TGGATACGTTTCCTTATAAG 3′ 5′ GAAATGGAGGCACCCCTTC 3′ |
507 bp |
| TLR5 | 5′ ATTGCGTGTACCCTGACTCG 3′ 5′ TTGAACACCAGTCTCTGGGC 3′ |
214 bp |
| TLR6 | Qiagen RT2 qPCR primer assay PPQ00238B |
92 bp |
| TLR7* | 5′ TCTACCTGGGCCAAAACTGTT 3′ 5′ GGCACATGCTGAAGAGAGTTA 3′ |
388 bp |
Indicates that primer sequence was taken from human TLR primers designed by Kumar and Shamsuddin (Kumar and Shamsuddin, 2012).
2.4 Immunoblotting
Lysates were prepared from macaque neural retina tissue immediately post-sacrifice following the method of Gerhardinger et al., (Gerhardinger et al., 2001). The protein concentration was determined by Pierce BCA assay (Thermo Scientific, Rockford, IL #23225). Lysates were run on 4–15% Mini-PROTEAN TGX precast gels (BIORAD, Hercules, CA 4568084S) with BenchMark Pre-stained Protein Standard (Invitrogen, Carlsbad, CA 10748-010) as a molecular weight marker (MWM). Proteins were electrophorectically transferred to nitrocellulose prior to blocking with 5% non-fat dry milk in Genius Buffer I (100mM maleic acid, 150mM NaCl, pH 7.5) containing 0.3% v/v Tween-20. The primary antibodies were diluted in 5% non-fat dry milk in Genius Buffer I and incubated as noted in Table 2, followed by washing in Genius Buffer I containing 0.3% v/v Tween-20. HRP-conjugated secondary antibodies were diluted 1:5000 and applied for 1 hour at RT. After washing, the blots were developed using WesternSure PREMIUM chemilluminescent Substrate (LI-COR, Lincoln, NE, 926-95000).
Table 2.
Primary antibodies utilized for immunoblots and immunofluorescence
| Target | Species | Company | Catalog # | Immunoblot conditions | IF 1° antibody dilution |
|---|---|---|---|---|---|
| TLR4 | rabbit | Abcam | 13556 | NA | 1:50 ON |
| TLR4 | rabbit | SCBT | 10741 | 200 μg, 1:2000 ON | NA |
| TLR5 | mouse | Novus | NBP2-24787 | 200 μg, 1:500 ON | 1:50 ON |
| TLR6 | rabbit | Rockland | 600-401-FG3 | 300 μg, 1:500 ON | 1:100 ON |
| TLR7 | rabbit | Rockland | 200-401-A64 | 275 μg, 1:500 ON | 1:100 ON |
| Vimentin | rabbit | SCBT | 5565 | NA | 1:200 ON |
| Vimentin | mouse | SCBT | 373717 | NA | 1:200 ON |
| Calretinin | rabbit | SCBT | 50453 | NA | 1:50 ON |
| Calretinin | mouse | SCBT | 365956 | NA | 1:50 ON |
| Calbindin | mouse | SCBT | 74462 | NA | 1:50 ON |
| PKC-α | mouse | SCBT | 8393 | NA | 1:50 ON |
| CD11b | rabbit | Abcam | 133357 | NA | 1:200 ON |
| Iba 1 | goat | Abcam | 5076 | NA | 1:800 ON |
| GFAP | mouse | SCBT | 33673 | NA | 1:1000 ON |
| GFAP | rabbit | DAKO | Z0334 | NA | 1:1000 ON |
| RBPMS | mouse | Novus | NBP2-03905 | NA | 1:50 ON |
| RBPMS | rabbit | PhosphoSolutions | 1830-RBPMS | NA | 1:100 ON |
NA= not applicable, ON= overnight
2.5 Immunofluorescence
Neural retina tissue obtained from euthanized macaques was rinsed in PBS and fixed in 10% neutral buffered formalin (Thermo Fisher, Kalamazoo, MI, #305-510) for 24 hours before paraffin embedding and sectioning. Tissue sections were de-paraffinized and antigen retrieval was performed via heat treatment with pH 6 citrate buffer (10mM Citric acid/0.05% Tween, pH 6, 20 minutes 92°C). Slides were blocked with 5% fetal bovine serum (FBS) before incubation with the primary antibody diluted in 2.5% FBS/PBS. The secondary antibodies, diluted 1:400 in PBS containing 2.5% FBS, were applied for 1 hour at RT. Sections were stained with Hoechst (1 μg/ml) to visualize nuclei before mounting with Immu-mount (Thermo Scientific, Kalamazoo, MI, #9990402). Fluorescence images (40x objective) were taken with a Zeiss Axiocam 702 mono camera on a Zeiss Axio Imager.Z2 microscope with Zeiss ZEN 2.3 pro software (Zeiss Microimaging, Oberkochen, Germany). Scale bars represent 20 micrometers (μm).
The primary antibodies, as well as dilutions and incubation conditions, utilized for IF are listed in Table 2. The secondary antibodies, purchased from Life Technologies (Carlsbad, CA), included: goat anti-rabbit Alexa Fluor 594 (#A11012), goat anti-mouse Alexa Fluor 594 (#A11032), donkey anti-rabbit Alexa Fluor 594 (#A21207) donkey anti-goat Alexa Fluor 594 (#A11058), goat anti-mouse Alexa Fluor 488 (#A11029), goat anti-rabbit Alexa Fluor 488 (A11008) and donkey anti-rabbit Alexa Fluor 488 (#A21206).
3. Results
3.1 Expression of TLR proteins in neural retina lysates
To confirm that TLR protein was present in NHP retina, lysates of rhesus macaque neural retina tissue were screened by immunoblotting with antibodies to TLRs. Experimental conditions, including quantity of lysate loaded on the gel, dilution of primary antibody, and incubation conditions were optimized for each individual antibody (Table 2). The predicted molecular weights (MW) of TLR proteins range from 84 to 121 kDa. Post-translational modifications, such as glycosylation, or proteolytic processing, however, can increase or decrease the apparent molecular weights of TLR proteins (Leifer and Medvedev, 2016). Figure 1 demonstrates that we were able to detect single bands within the expected size range and consistent with published MWs for TLRs 4, 5, 6 and 7. These immunoblots confirmed that macaque neural retina tissue produced the TLR 4, 5, 6, and 7 proteins, identified antibodies useful for detecting TLRs by immunoblotting of macaque tissue, and demonstrated the migration patterns of macaque retina TLR 4-7 proteins for the first time.
Figure 1. TLRs 4-7 are detected in macaque neural retina tissue lysates by immunoblotting.
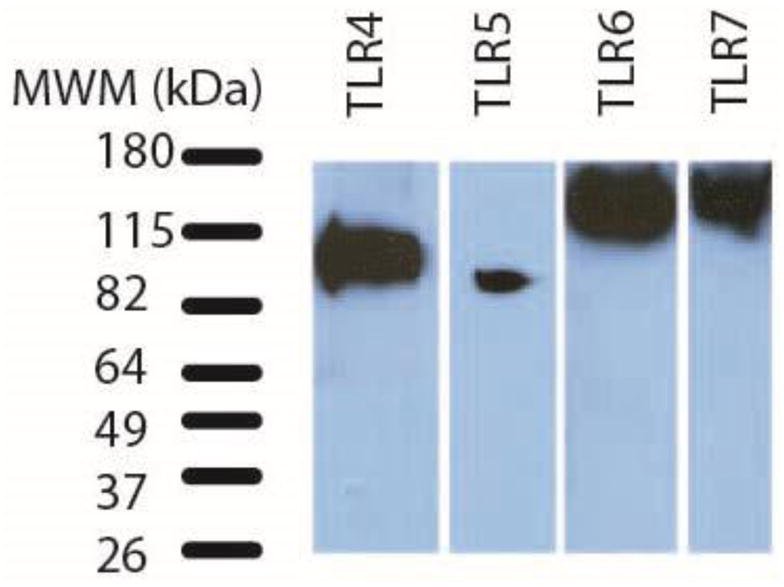
Immunblots of macaque retina tissue lysates demonstrate expression of TLR 4-7 proteins. Table 2 lists amount of retina lysate loaded and immunoblotting conditions for each antibody. This figure is a composite of 4 separate immunoblots. The bands in the MWM lane represent the migration of BenchMark Pre-Stained Protein Standard on a 4–15% Mini-PROTEAN TGX precast gel.
3.2 Quantitation of TLR 4-7 mRNA in macaque neural retina
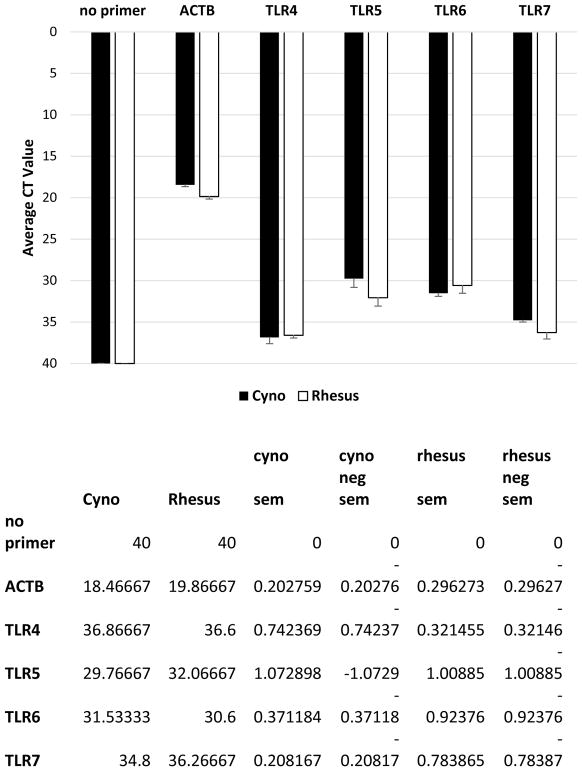
Using primers specific for TLRs 4-7 (Table 1), we utilized qPCR to detect mRNA expression in macaque neural retina tissue. Figure 2 demonstrates that similar levels of gene expression, above that of the no primer control, were detected for TLRs 4-7 in both cynomolgus and rhesus macaques. These data indicated that cynomolgus and rhesus macaque neural retina tissue expresses TLRs 4-7 mRNA.
Figure 2. Quantitative PCR detects TLRs 4-7 mRNAs in macaque neural retina tissue.
qPCR primer assays confirmed expression of TLRs 4-7 mRNA in cynomolgus and rhesus retina tissue compared to a no primer control. ACTB=Beta actin house-keeping gene control.
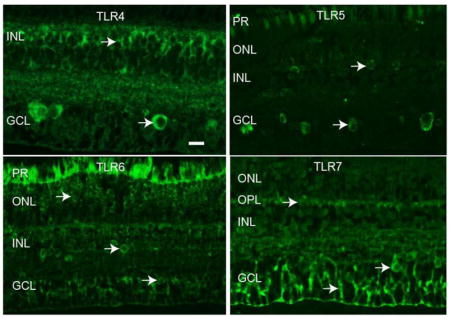
3.3 Localization of TLRs in macaque neural retina tissue
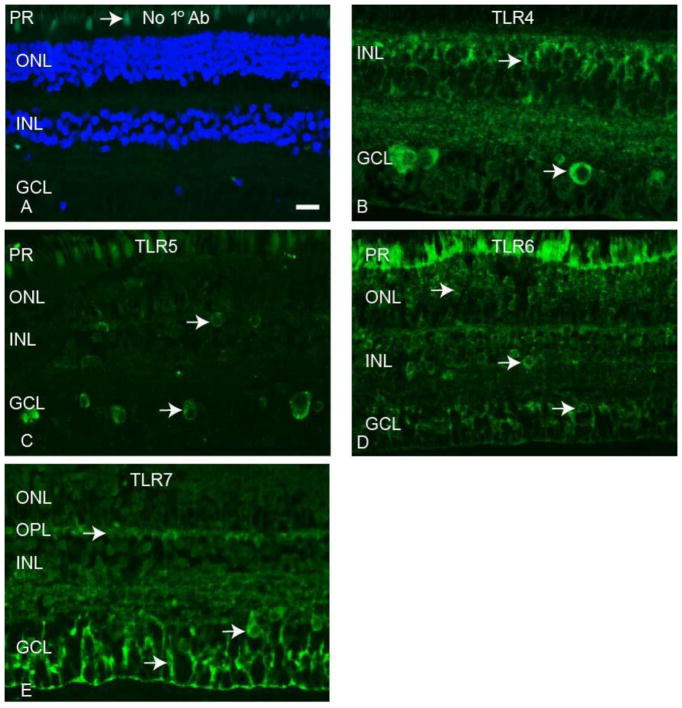
Immunofluorescence (IF) staining of rhesus macaque neural retina tissue was performed with antibodies to TLRs 4-7 to determine the localization of these innate immune receptors in the NHP retina. Staining was performed on neural retina tissue from a minimum of three animals to establish consistent staining patterns for each antibody. Figure 3 demonstrates the results of TLR 4-7 staining compared to a no primary antibody control (Fig 3A). Autofluorescence was present in the photoreceptor layer, as evident in panels A, C, and D of Figure 3. TLR4 staining was most prevalent in small, round cells in the inner nuclear layer (INL) and large, round cells in the ganglion cell layer (GCL) (Fig 3B). The TLR5 antibody stained large, round cells in the GCL and some smaller, scattered cells in the INL (Fig 3C). TLR6 staining was present in small, round cells in both the outer nuclear layer (ONL) and the INL, and also round cells in the GCL (Fig 3D). The TLR7 antibody identified filamentous cells predominantly in the GCL, as well as staining in the outer plexiform layer (OPL) and large, round cells in the GCL (Fig 3E). These data localize TLR 4-7 expression in the non-human primate neural retina and identify antibodies that function for staining of paraffin embedded macaque tissue.
Figure 3. Expression patterns of TLRs 4-7 in macaque neural retina tissue.
TLR 4-7 proteins were detected by immunofluorescence of macaque retina tissue. Arrow in panel A identifies autofluorescence in photoreceptor layer. Arrows in panels B–D identify TLR positive cells. Photoreceptor layer (PR), Outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), ganglion cell layer (GCL). Scale bar= 20 μm.
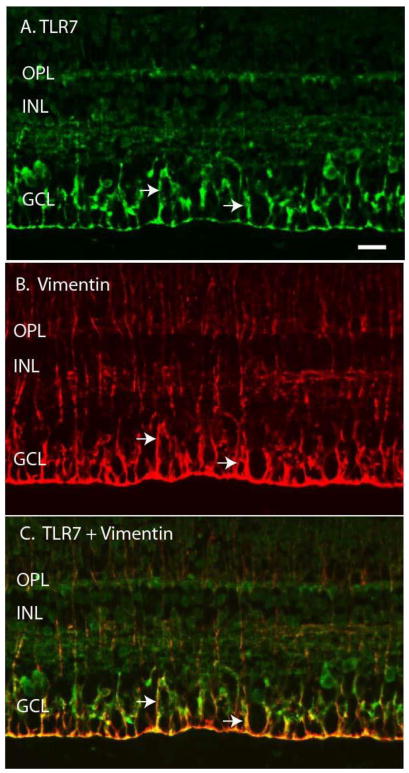
To identify the neural retina cell types that express TLRs 4-7, we performed double label IF with cell type specific marker antibodies. Representative examples for each cell marker are shown in Figures 4–9 and the results are summarized in Table 3. Some of the TLRs were present in filamentous cells, suggesting they may be expressed by Muller cells, the predominant glial cell type in the retina. To confirm this, we utilized vimentin antibodies as a Muller cell marker (Bhatia et al., 2009; Sauter and Brandt, 2016) and performed double label IF with antibodies to TLRs 4-7. Staining with the anti-vimentin antibody identified a filamentous pattern of Muller cells extending throughout the retina (Fig 4B). When IF images were merged (Fig 4C) it became evident that TLR7 and vimentin staining co-localized in some filamentous cells, especially in the Muller end feet, indicating that TLR7 was expressed in retinal Muller cells. Vimentin double label IF results indicated that TLR 4 was also expressed by Muller cells in macaque neural retina tissue (Table 3).
Figure 4. TLR7 co-localizes with the Muller cell marker vimentin.
A) Arrows indicate TLR7 staining of filamentous retinal cells. B) Arrows indicate vimentin staining of Muller cells. C) Merged image with arrows indicating co-localization of TLR7 and vimentin positive cells. Outer plexiform layer (OPL), inner nuclear layer (INL), ganglion cell layer (GCL). Scale bar= 20 μm.
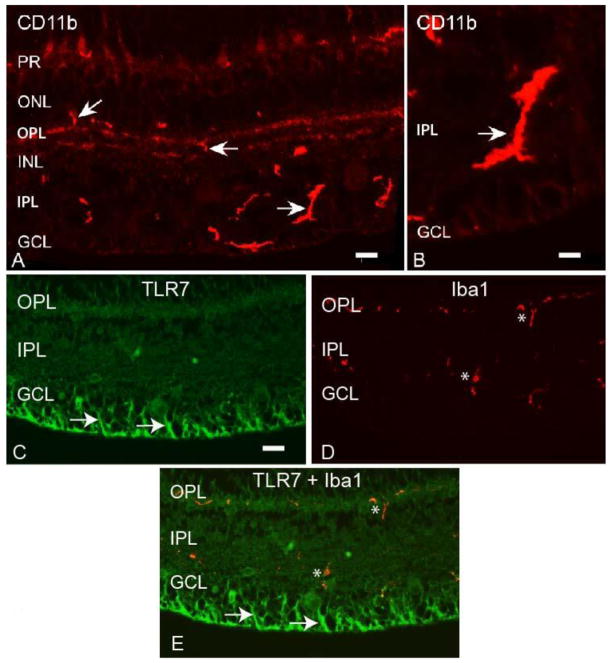
Figure 9. Macaque retinal microglial cells are identified by CD11b and Iba 1 antibodies.
A, B) Arrows indicate CD11b staining of microglial cells in macaque retina. Autofluorescence is present in the photoreceptor layer in panel A. C) Arrows indicates TLR7 positive retinal cells. D) Astericks indicate Iba 1 staining of retinal microglial cells. E) Merged image indicates no co-localization of TLR7 and Iba 1 staining. Photoreceptors (PR), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), ganglion cell layer (GCL). Scale bar= 20 μm.
Table 3.
Double Label Immunofluorescence with TLR and Cell Marker Antibodies
| Cell Type: | Muller | Astrocytes | RGC | Amacrine | Bipolar | Rod Bipolar | Microglial | Microglial |
|---|---|---|---|---|---|---|---|---|
| Co-localize w/ | Co-localize w/ | Co-localize w/ | Co-localize w/ | Co-localize w/ | Co-localize w/ | Co-localize w/ | Co-localize w/ |
| Antibody: | Vimentin | GFAP | RBPMS | Calretinin | Calbindin | PKC-α | Iba 1 | CD11b |
|---|---|---|---|---|---|---|---|---|
| TLR4 | yes | no | yes | yes | yes | yes | no | nd |
| TLR5 | no | no | yes | no | nd | nd | no | no |
| TLR6 | no | no | yes | yes | yes | yes | no | nd |
| TLR7 | yes | no | yes | yes | yes | yes | no | nd |
nd=not determined due to antibody incompatibility
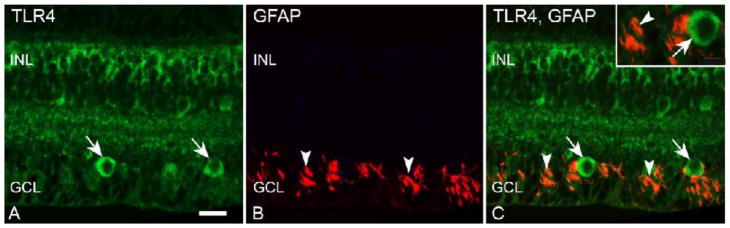
We next wanted to determine if TLRs 4-7 were expressed in astrocytes, the other type of macroglial cells in the mammalian retina. We utilized an antibody to glial fibrillary acidic protein (GFAP) (de Souza et al., 2016) to identify astrocytes in the GCL of macaque neural retina tissue (Figure 5B). Double label IF indicated that TLR4 staining (Fig 5A) did not co-localize with GFAP staining (Fig 5C). IF with antibodies to TLRs 5, 6, and 7 also did not indicate co-localization with GFAP-labeled astrocytes in the macaque neural retina (Table 3).
Figure 5. TLR4 does not co-localize with the astrocyte cell marker GFAP.
A) Long arrows indicate TLR4 staining in macaque retina GCL. B) Arrowheads indicate GFAP staining of astrocytes. C) Merged image indicates no co-localization of TLR4 (long arrows) and GFAP (arrowheads). Inner nuclear layer (INL), ganglion cell layer (GCL). Scale bar= 20 μm.
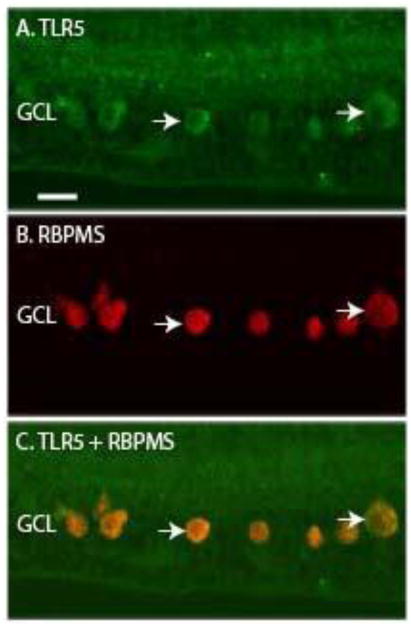
Several of the TLR antibodies labeled large, round cells in the GCL that were suggestive of retinal ganglion cell (RGC) morphology (Figure 3). To determine if TLRs 4-7 were expressed in RGCs, we utilized marker antibodies against RNA-binding protein with multiple splicing (RBPMS), which has been shown to be a marker of monkey retinal RGC (Rodriguez et al., 2014). The TLR5 antibody stained large, round cells in the GCL of macaque neural retina tissue (Fig 6A) and the anti-RBPMS antibody identified RGC in the GCL (Fig 6B). A merged image indicates that TLR5 was expressed by RBPMS-positive RGC in macaque neural retina (Fig 6C). TLRs 4, 6 and 7 were also expressed in macaque RGCs, as summarized in Table 3.
Figure 6. TLR5 co-localizes with the RGC marker RBPMS.
A) The arrows indicate TLR5 staining of large, round cells in the GCL. B) The arrows indicate RBPMS positive RGC. C) Merged image with arrows indicating TLR5 and RBPMS positive cells. Ganglion cell layer (GCL). Scale bar= 20 μm.
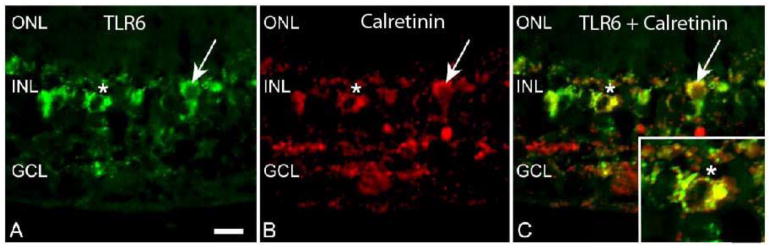
Some TLRs were expressed in small, round cells in the INL, suggestive of amacrine cell morphology (Fig 3). We performed double label IF with anti-calretinin antibodies, which label AII amacrine cells (de Souza et al., 2016; Kolb et al., 2002; Mills and Massey, 1999; Sauter and Brandt, 2016), and antibodies to TLRs 4-7 to determine if TLRs were expressed by calretinin positive amacrine cells. The staining pattern of the anti-TLR6 antibody clearly indicated TLR6 positive round cells in the INL (Fig 7A). Calretinin staining was also evident in round cells in the INL (Fig 7B) indicative of amacrine cell morphology (de Souza et al., 2016; Kolb et al., 2002; Mills and Massey, 1999), and when images were merged, co-localization of TLR6 and calretinin was evident (Fig 7C). TLRs 4 and 7 were also expressed in calretinin positive amacrine cells as determined by double label IF (Table 3).
Figure 7. TLR6 co-localizes with the amacrine cell marker calretinin.
A) The arrow and asterisk indicate TLR6 staining of round cells in INL of macaque retina tissue. B) The arrow and asterisk indicate staining of calretinin positive cells. C) Merged image with arrow and asterisk indicating co-localization of TLR6 and calretinin positive cells. Outer nuclear layer (ONL), inner nuclear layer (INL), ganglion cell layer (GCL). Scale bar= 20 μm.
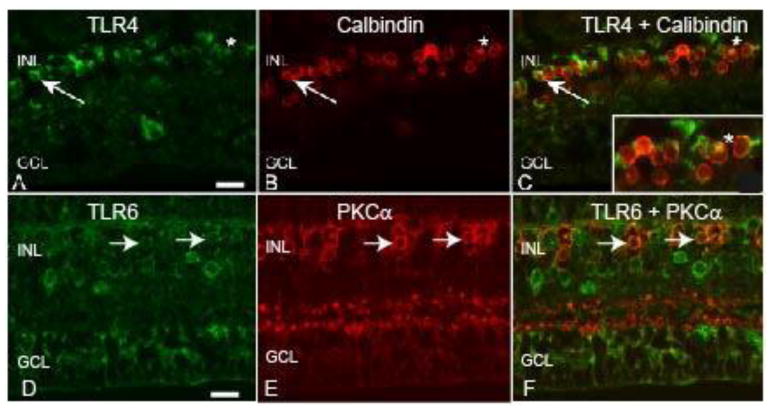
To determine whether TLRs 4-7 were expressed in bipolar cells we used an antibody to calbindin in double label IF experiments (de Souza et al., 2016). Staining with the calbindin antibody identified a row of small, round cells in the INL as bipolar cells (Fig 8B). When images for TLR4 and calbindin were merged, it became clear that TLR4 was expressed by some calbindin positive bipolar cells (Fig 8C). TLRs 6 and 7 were also expressed in calbindin positive bipolar cells (Table 3). We were not able to test if TLR5 was expressed in calbindin positive cells because both antibodies were of mouse origin (Table 3).
Figure 8. TLRs 4 and 6 are expressed by bipolar cells in the macaque retina.
A) The arrow and asterisk indicate TLR4 staining of round cells in the INL of macaque retina. B) The arrows and asterisk indicate calbindin positive OFF bipolar cells. C) The merged image indicates co-localization of TLR4 and calbindin positive cells. D) The arrows indicates TLR6 staining of round INL cells. E) The arrows indicates PKC-α positive rod bipolar cells. F) Merged image indicates co-localization of TLR6 and PKC-α stained cells. Inner nuclear layer (INL), ganglion cell layer (GCL). Scale bar= 20 μm.
An antibody to protein kinase C alpha (PKC-α), which reacts with rod bipolar cells, was also utilized in double label IF (de Souza et al., 2016). PKC-α staining was localized to round cells in the INL (Fig 8E), and, as an example, the TLR6 antibody stained small, round cells in the INL (Fig 8D). Merging of the TLR6 and PKC-α staining demonstrated that some of the cells were both TLR6 and PKC-α positive (Fig 8F). TLR4 and 7 were also expressed by PKC-α positive rod bipolar cells (Table 3). We were unable to determine whether TLR5 was expressed in PKC-α positive rod bipolar cells due to antibody incompatibility (Table 3).
We next wanted to determine whether TLRs 4-7 were expressed by retinal microglial cells. We tested numerous microglial marker antibodies, before finding two, goat anti-Iba 1 and rabbit anti-CD11b, that worked on paraffin embedded macaque neural retina tissue and gave the expected microglial cell morphology (Table 2). Staining with the CD11b antibody identified bright cells mainly in the OPL, inner plexiform layer (IPL), and GCL, with shapes ranging from small crescents to larger extended structures (Fig 9A, B). Staining in the photoreceptor layer was due to autofluorescence (Fig 9A). The Iba 1 antibody identified microglial cells mainly in the OPL and IPL (Fig 9D). The morphology and varied cell size we are seeing in these retina sections is likely the result of bisecting the microglial processes in a random manner during the sectioning process and is similar to the microglial staining pattern reported previously for vertical sections of macaque retina (Singaravelu et al., 2017).
To determine if the TLRs were expressed in microglial cells, double label IF was performed with anti-TLR 4-7 antibodies and the Iba 1 or CD11b antibody. The anti-TLR7 antibody stained filamentous cells in the retina (Fig 9C), and when this image was merged with Iba 1 staining of the same section, no co-localization of TLR7 and Iba 1 staining was observed (Fig 9E). In addition, no co-localization was observed for the Iba 1 antibody with TLR4 or TLR6, or the CD11b antibody with the anti-TLR5 antibody (Table 3).
4. Discussion
Although TLR expression has been documented in the human eye (Chang et al., 2006; Kumar and Yu, 2006), the majority of research has focused on the ocular surface (Redfern et al., 2015; Redfern and McDermott, 2010; Ueta and Kinoshita, 2010; Yamada et al., 2014), or retinal pigment epithelial cells (Kumar et al., 2004). One study of human retina detected TLRs 2, 4, 7, 8, and 10 by proteomic analysis and determined that several TLRs were upregulated in glaucomatous retina (Luo et al., 2010). This study also detected the expression of TLRs 2, 3 and 4 by immunofluorescence of human retina tissue (Luo et al., 2010). To our knowledge, similar studies have not been done for other TLRs in human neural retina tissue.
Non-human primates play an important role in drug development and testing, particularly for biologics, such as humanized monoclonal antibodies (mAbs), and gene delivery vectors. Inflammation, and the production of inflammatory cytokines, can occur after administration of humanized mAbs and following gene delivery with herpes simplex virus (HSV), adenovirus, or lentivirus vectors (Harding et al., 2010; Liu et al., 1999; Posarelli et al., 2011; Sauter and Brandt, 2016). Acute uveitis has also been linked to the innate immune response, in which the Toll-like receptors play a crucial role (Chang et al., 2006). The majority of studies on TLR expression in neural retina have been done in mice, but to date very little is known about expression in NHPs despite their importance in drug development. This study has determined that TLRs 4-7 are expressed in the macaque neural retina. In addition, we have identified TLR expression in Muller, RGC, amacrine, and bipolar cells raising the possibility that these cell types may play a role in retinal innate immunity.
To date, Muller cells and RPE have been the main focus of studies on the role of innate signaling in ocular inflammation. Muller cells are the principle glial cells in the retina, perform a variety of functions, and can act as modulators of inflammatory responses by producing cytokines (Zong et al., 2010). It was not surprising, therefore, that we detected expression of TLRs 4 and 7 in Muller cells (Table 3). Muller cells, whose end feet initiate at the ILM and processes span the retina, may play a key role in triggering innate immunity following viral vector or humanized mAb therapy. In fact, HSV gene delivery vector binding to the internal limiting membrane of macaque neural retina, and internalization of tegument and capsid proteins into cells with Muller morphology, was sufficient to induce expression of cytokines and TLRs (Sauter and Brandt, 2016).
Retinal astrocytes play important roles in retinal development and hemodynamics (de Souza et al., 2016). In response to certain stimuli, astrocytes become reactive and enlarge their soma, which increases their GFAP immunoreactivity (Vecino et al., 2016). Our studies indicated that TLRs 4-7 were not constitutively expressed in GFAP positive astrocytes in the macaque neural retina. Previous studies indicated that TLR4 could be detected on cultured murine retinal astrocytes (Jiang et al., 2009). Constitutive TLR3 expression has been detected in GFAP positive astrocytes in human retina, and this expression was increased in retina from glaucomatous donors (Luo et al., 2010). The poor quality of retinal architecture, low resolution IF images, and lack of TLR3 antibody validation in this study, however, makes this data hard to interpret (Luo et al., 2010). Our results probably differ from these studies because the macaque retina were not stimulated prior to fixation, or from glaucomatous animals. Alternately, species differences could account for the differences in our findings. Future studies will be needed to determine if stimulation of macaque neural retina tissue can induce TLR expression in macaque retinal astrocytes.
Macaque RGCs expressed TLR 4, 5, 6, and 7 proteins. The main function of ganglion cells is to receive synaptic inputs from bipolar and amacrine cells and transfer the signals to the lateral geniculate nucleus (de Souza et al., 2016). Previous studies have discovered a link between TLR4 activation, initiation of inflammatory pathways, and subsequent RGC degeneration (Kilic et al., 2008; Lee et al., 2015; Morzaev et al., 2015; Qi et al., 2014). In addition, an increase in TLR4 expression, along with a decrease in RGC viability, was noted after exposure to a high glucose environment (Zhao et al., 2016). These studies were performed in rodents, and our results indicating that non-human primate retina RGCs constitutively express TLR proteins, suggests that RGC TLR signaling, and subsequent inflammatory responses, may be detrimental to retinal function in NHPs.
One surprising finding of this study was that several neuronal cell types in the macaque retina express TLRs. Our data indicated that macaque calretinin-positive amacrine cells expressed TLRs 4, 6, and 7 (Table 3). The mammalian retina contains over 40 different types of amacrine cells, which make up approximately 40% of all neurons of the INL (Kolb et al., 2002; MacNeil and Masland, 1998; Majumdar et al., 2008; Ofri, 2013). Amacrine cell morphology varies, as does their contribution to retinal output, which is determined by their retinal synaptic partners and neurotransmitters (MacNeil and Masland, 1998). The presence of TLRs suggests that amacrine cells may play an as yet unexplored role in the innate immune response of the primate retina. In fact, we previously showed that macaque neural retina tissue responded to herpes simplex virus vector challenge with increased IL-10 expression in amacrine cells (Sauter and Brandt, 2016).
Bipolar cell bodies are also located in the INL and can synapse with rod or cone photoreceptors (Ofri, 2013). Our results indicated that TLR 4, 6, and 7 were expressed in calbindin positive bipolar cells and PKC-α positive rod bipolar cells (Table 3). These data are an indication that bipolar cells may respond to PAMPs or DAMPs and play a role in the innate immune response of the primate neural retina. Further studies will be required to determine the exact role that bipolar cells may play in retinal inflammation.
Microglial cells, which are scattered throughout the retina, play a crucial role in immunoregulation and defense against invading microorganisms including antigen presentation and phagocytosis (Chen et al., 2002; Singaravelu et al., 2017). Microglia are normally in a resting state, but can become activated by stimuli such as nerve damage, exposure to cytokines, or inflammation (Chen et al., 2002). Our findings that TLRs 4-7 do not seem to be constitutively expressed by macaque microglial cells was surprising, as this cell type plays a key role in immunoregulation and some retinal diseases. For example, a link between microglial antigen presentation and retinitis has been reported (Zhang et al., 1997). In addition, microglial cells have been shown to express cytokines which initiate uveoretinitis in an experimental autoimmune uveitis model (Gullapalli et al., 2000). TLRs 2, 3, and 4 were also detected by IF in microglial cells of normal human donors, and their expression was increased in glaucomatous retinas (Luo et al., 2010). These findings are questionable, however, due to poor retinal architecture, suboptimal quality of IF images, and lack of antibody validation on the part of these authors (Luo et al., 2010). Our results may differ from these studies because no stimulation of the macaque neural retina was performed prior to fixation and staining for microglial markers and TLRs. Studies with TLR ligands, ocular biologics, and gene delivery vectors are needed to determine if prior stimulation of macaque neural retina tissue can induce TLR expression in macaque microglial cells (Kochan et al., 2012).
5. Conclusions
Perhaps the most significant finding in this study was that multiple retinal neuronal cell types, including RGC, amacrine, and bipolar cells, constitutively express TLRs which suggests they may contribute to innate inflammatory responses in the NHP retina. Our findings have broad implications for the use of NHPs in drug development and testing, treatment of ocular diseases with viral gene delivery vectors or humanized mAbs, and understanding the triggers of uveitis and autoimmune disease. As the mammalian retina contains approximately 55 morphological types of neurons, (Masland, 2001) this study is by no means complete but is the beginning of a more detailed look into the role of neuronally expressed TLRs in retinal inflammation. Future studies will focus on finding validated antibodies for other TLRs to study their expression patterns in the NHP retina and examining the response of NHP retina to various pro-inflammatory stimuli including viral gene delivery vectors.
Highlights.
TLRs 4-7 protein expression was detected by immunoblotting of macaque retina tissue
qPCR detected TLRs 4-7 in cynomolgus and rhesus macaque retina tissue
TLRs are expressed in Muller, RGCs, amacrine and bipolar cells
TLR mediated innate inflammatory responses may occur in several retinal cell types
Acknowledgments
Inna Larsen for figure and manuscript preparation. Dr. Cassandra Schlamp for figure preparation. Dr. Robert Nickells for the GFAP antibody and comments on the manuscript. Dr. Gillian McLellan for the RBPMS antibody. Grant support: The Retina Research Foundation, Houston TX, Core Grant for Vision Research NIH P30 EY016665 to Curtis Brandt, NIH P51OD011106 to the Wisconsin National Primate Research Center, University of Wisconsin-Madison, and an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness, Inc.
Abbreviations
- TLR
Toll-like receptor
- NHP
Non-human primate
- IF
immunofluorescence
- qPCR
quantitative polymerase chain reaction
- PAMPs
pathogen-associated molecular patterns
- NF-κB
nuclear factor kappa-beta
- IL
Interleukin
- RPE
retina pigment epithelial
- PBS
phosphate buffered saline
- HRP
horseradish peroxidase
- FBS
fetal bovine serum
- RT
room temperature
- ON
over night
- INL
inner nuclear layer
- GCL
ganglion cell layer
- IPL
inner plexiform layer
- OPL
outer plexiform layer
- PR
photoreceptors
- RGC
retinal ganglion cells
- GFAP
glial fibrillary acidic protein
- RBPMS
RNA binding protein with multiple splicing
- PKC-α
protein kinase C alpha
- mAbs
monoclonal antibodies
- RNA
ribonucleic acid
- cDNA
complementary DNA
- EDTA
Ethylenediaminetetraacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Allensworth JJ, Planck SR, Rosenbaum JT, Rosenzweig HL. Investigation of the differential potentials of TLR agonists to elicit uveitis in mice. J Leukoc Biol. 2011;90:1159–1166. doi: 10.1189/jlb.0511249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia SA, Beltran CJ, Aguirre IM, Silva P, Peralta AL, Malinarich F, Hermoso MA. Toll-like receptors are key participants in innate immune responses. Biol Res. 2007;40:97–112. doi: 10.4067/s0716-97602007000200001. [DOI] [PubMed] [Google Scholar]
- Bennett J. Immune response following intraocular delivery of recombinant viral vectors. Gene Ther. 2003;10:977–982. doi: 10.1038/sj.gt.3302030. [DOI] [PubMed] [Google Scholar]
- Bhatia B, Singhal S, Lawrence JM, Khaw PT, Limb GA. Distribution of Müller stem cells within the neural retina: evidence for the existence of a ciliary margin-like zone in the adult human eye. Exp Eye Res. 2009;89:373–382. doi: 10.1016/j.exer.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Bowie AG, Haga IR. The role of Toll-like receptors in the host response to viruses. Mol Immunol. 2005;42:859–867. doi: 10.1016/j.molimm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Brosig A, Kuhrt H, Wiedemann P, Kohen L, Bringmann A, Hollborn M. Gene expression regulation in retinal pigment epithelial cells induced by viral RNA and viral/bacterial DNA. Mol Vis. 2015;21:1000–1016. [PMC free article] [PubMed] [Google Scholar]
- Cai S, Brandt CR. Induction of interleukin-6 in human retinal epithelial cells by an attenuated Herpes simplex virus vector requires viral replication and NFkappaB activation. Exp Eye Res. 2008;86:178–188. doi: 10.1016/j.exer.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, McCluskey P, Wakefield D. Expression of toll-like receptor 4 and its associated lipopolysaccharide receptor complex by resident antigen-presenting cells in the human uvea. Invest Ophthalmol Vis Sci. 2004;45:1871–1878. doi: 10.1167/iovs.03-1113. [DOI] [PubMed] [Google Scholar]
- Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 2006;90:103–108. doi: 10.1136/bjo.2005.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang P, Kijlstra A. Distribution, markers, and functions of retinal microglia. Ocul Immunol Inflamm. 2002;10:27–39. doi: 10.1076/ocii.10.1.27.10328. [DOI] [PubMed] [Google Scholar]
- de Souza CF, Nivison-Smith L, Christie DL, Polkinghorne P, McGhee C, Kalloniatis M, Acosta ML. Macromolecular markers in normal human retina and applications to human retinal disease. Exp Eye Res. 2016;150:135–148. doi: 10.1016/j.exer.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Gao ML, Wu KC, Deng WL, Lei XL, Xiang L, Zhou GH, Feng CY, Cheng XW, Zhang CJ, Gu F, Wu RH, Jin ZB. Toll-like receptor 3 activation initiates photoreceptor cell death in vivo and in vitro. Invest Ophthalmol Vis Sci. 2017;58:801–811. doi: 10.1167/iovs.16-20692. [DOI] [PubMed] [Google Scholar]
- Gerhardinger C, McClure KD, Romeo G, Podesta F, Lorenzi M. IGF-I mRNA and signaling in the diabetic retina. Diabetes. 2001;50:175–183. doi: 10.2337/diabetes.50.1.175. [DOI] [PubMed] [Google Scholar]
- Gullapalli VK, Zhang J, Pararajasegaram G, Rao NA. Hematopoietically derived retinal perivascular microglia initiate uveoretinitis in experimental autoimmune uveitis. Graefes Arch Clin Exp Ophthalmol. 2000;238:319–325. doi: 10.1007/s004170050359. [DOI] [PubMed] [Google Scholar]
- Harding FA, Stickler MM, Razo J, DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs. 2010;2:256–265. doi: 10.4161/mabs.2.3.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck SM, Schoeffmann S, Amann B, Stangassinger M, Gerhards H, Ueffing M, Deeg CA. Retinal Mueller glial cells trigger the hallmark inflammatory process in autoimmune uveitis. J Proteome Res. 2007;6:2121–2131. doi: 10.1021/pr060668y. [DOI] [PubMed] [Google Scholar]
- Jiang G, Ke Y, Sun D, Wang Y, Kaplan HJ, Shao H. Regulatory role of TLR ligands on the activation of autoreactive T cells by retinal astrocytes. Invest Ophthalmol Vis Sci. 2009;50:4769–4776. doi: 10.1167/iovs.08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Qin Q, Chen W, Qu J. Expression of toll-like receptiors in the healthy and herpes simples virus-infected cornia. Cornea. 2007;26:847–852. doi: 10.1097/ICO.0b013e318093de1f. [DOI] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Kochan T, Singla A, Tosi J, Kumar A. Toll-like receptor 2 ligand pretreatment attenuates retinal microglial inflammatory response but enhances phagocytic activity towards Staphylococcus aureus. Infect Immun. 2012;80:2076–2088. doi: 10.1128/IAI.00149-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Zhang L, Dekorver L, Cuenca N. A new look at calretinin - immunoreactive amacrine cell types in the monkey retina. J Comp Neurol. 2002;453:168–184. doi: 10.1002/cne.10405. [DOI] [PubMed] [Google Scholar]
- Kumar A, Pandey RK, Miller LJ, Singh PK, Kanwar M. Muller glia in retinal innate immunity: a perspective on their roles in endophthalmitis. Crit Rev Immunol. 2013;33:119–135. doi: 10.1615/critrevimmunol.2013006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Shamsuddin N. Retinal Muller glia initiate innate response to infectious stimuli via toll-like receptor signaling. PLoS One. 2012;7:e29830. doi: 10.1371/journal.pone.0029830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Singh CN, Glybina IV, Mahmoud TH, Yu FS. Toll-like receptor 2 ligand-induced protection against bacterial endophthalmitis. J Infect Dis. 2010;201:255–263. doi: 10.1086/649589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006;6:327–337. doi: 10.2174/156652406776894572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutty RK, Samuel W, Boyce K, Cherukuri A, Duncan T, Jaworski C, Nagineni CN, Redmond TM. Proinflammatory cytokines decrease the expression of genes critical for RPE function. Mol Vis. 2016;22:1156–1168. [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Agarwal A, Mahendradas P, Lee CS, Gupta V, Pavesio CE, Agarwal R. Viral posterior uveitis. Surv Ophthalmol. 2017;62:404–445. doi: 10.1016/j.survophthal.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Wang PW, Yang IH, Wu CL, Chuang JH. Amyloid-beta mediates the receptor of advanced glycation end product-induced pro-inflammatory response via toll-like receptor 4 signaling pathway in retinal ganglion cell line RGC-5. Int J Biochem Cell Biol. 2015;64:1–10. doi: 10.1016/j.biocel.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Leifer CA, Medvedev AE. Molecular mechanisms of regulation of Toll-like receptor signaling. J Leukoc Biol. 2016;100:927–941. doi: 10.1189/jlb.2MR0316-117RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Fang D, Zhou H, Su SB. The expression of Toll-like receptors in murine Muller cells, the glial cells in retina. Neurol Sci. 2013;34:1339–1346. doi: 10.1007/s10072-012-1236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Brandt CR, Gabelt BT, Bryar PJ, Smith ME, Kaufman PL. Herpes simplex virus mediated gene transfer to primate ocular tissues. Exp Eye Res. 1999;69:385–395. doi: 10.1006/exer.1999.0711. [DOI] [PubMed] [Google Scholar]
- Luo C, Yang X, Kain AD, Powell DW, Kuehn MH, Tezel G. Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling. Invest Ophthalmol Vis Sci. 2010;51:5697–5707. doi: 10.1167/iovs.10-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Wassle H, Jusuf PR, Haverkamp S. Mirror-symmetrical populations of wide-field amacrine cells of the macaque monkey retina. J Comp Neurol. 2008;508:13–27. doi: 10.1002/cne.21666. [DOI] [PubMed] [Google Scholar]
- Masland RH. Neuronal diversity in the retina. Curr Opin Neurobiol. 2001;11:431–436. doi: 10.1016/s0959-4388(00)00230-0. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. AII amacrine cells limit scotopic acuity in central macaque retina: A confocal analysis of calretinin labeling. J Comp Neurol. 1999;411:19–34. [PubMed] [Google Scholar]
- Morzaev D, Nicholson JD, Caspi T, Weiss S, Hochhauser E, Goldenberg-Cohen N. Toll-like receptor-4 knockout mice are more resistant to optic nerve crush damage than wild-type mice. Clin Exp Ophthalmol. 2015;43:655–665. doi: 10.1111/ceo.12521. [DOI] [PubMed] [Google Scholar]
- Ofri R. Optics and physiology of vision. In: Gelatt KN, Gilger BC, Kern TJ, editors. Veterinary Ophthalmology. John Wiley and Sons; New York: 2013. pp. 208–270. [Google Scholar]
- Oosting M, Cheng SC, Bolscher JM, Vestering-Stenger R, Plantinga TS, Verschueren IC, Arts P, Garritsen A, van Eenennaam H, Sturm P, Kullberg BJ, Hoischen A, Adema GJ, van der Meer JW, Netea MG, Joosten LA. Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc Natl Acad Sci U S A. 2014;111:E4478–4484. doi: 10.1073/pnas.1410293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey RK, Yu FS, Kumar A. Targeting toll-like receptor signaling as a novel approach to prevent ocular infectious diseases. Indian J Med Res. 2013;138:609–619. [PMC free article] [PubMed] [Google Scholar]
- Posarelli C, Arapi I, Figus M, Neri P. Biologic agents in inflammatory eye disease. J Ophthalmic Vis Res. 2011;6:309–316. [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Zhao M, Bai Y, Huang L, Yu W, Bian Z, Zhao M, Li X. Retinal ischemia/reperfusion injury is mediated by Toll-like receptor 4 activation of NLRP3 inflammasomes. Invest Ophthalmol Vis Sci. 2014;55:5466–5475. doi: 10.1167/iovs.14-14380. [DOI] [PubMed] [Google Scholar]
- Redfern RL, Barabino S, Baxter J, Lema C, McDermott AM. Dry eye modulates the expression of toll-like receptors on the ocular surface. Exp Eye Res. 2015;134:80–89. doi: 10.1016/j.exer.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern RL, McDermott AM. Toll-like receptors in ocular surface disease. Exp Eye Res. 2010;90:679–687. doi: 10.1016/j.exer.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AR, de Sevilla Muller LP, Brecha NC. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J Comp Neurol. 2014;522:1411–1443. doi: 10.1002/cne.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter MM, Brandt CR. Primate neural retina upregulates IL-6 and IL-10 in response to a herpes simplex vector suggesting the presence of a pro-/anti-inflammatory axis. Exp Eye Res. 2016;148:12–23. doi: 10.1016/j.exer.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman S. Advancements in the management of uveitis. Best Pract Res Clin Rheumatol. 2016;30:304–315. doi: 10.1016/j.berh.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Shamsuddin N, Kumar A. TLR2 mediates the innate response of retinal Muller glia to Staphylococcus aureus. J Immunol. 2011;186:7089–7097. doi: 10.4049/jimmunol.1100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaravelu J, Zhao L, Fariss RN, Nork TM, Wong WT. Microglia in the primate macula: specializations in microglial distribution and morphology with retinal position and with aging. Brain Struct Funct. 2017;222:2759–2771. doi: 10.1007/s00429-017-1370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Kumar A. Retinal photoreceptor expresses toll-like receptors (TLRs) and elicits innate responses following TLR ligand and bacterial challenge. PLoS One. 2015;10:e0119541. doi: 10.1371/journal.pone.0119541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Shiha MJ, Kumar A. Antibacterial responses of retinal Muller glia: production of antimicrobial peptides, oxidative burst and phagocytosis. J Neuroinflamm. 2014;11:33. doi: 10.1186/1742-2094-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart EA, Wei R, Branch MJ, Sidney LE, Amoaku WM. Expression of Toll-like receptors in human retinal and choroidal vascular endothelial cells. Exp Eye Res. 2015;138:114–123. doi: 10.1016/j.exer.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, Androudi S. A Focus on the Epidemiology of Uveitis. Ocul Immunol Inflamm. 2016:1–15. doi: 10.1080/09273948.2016.1196713. [DOI] [PubMed] [Google Scholar]
- Ueta M, Kinoshita S. Innate immunity of the ocular surface. Brain Res Bull. 2010;81:219–228. doi: 10.1016/j.brainresbull.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 2016;51:1–40. doi: 10.1016/j.preteyeres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Wakefield D, Gray P, Chang J, Di Girolamo N, McCluskey P. The role of PAMPs and DAMPs in the pathogenesis of acute and recurrent anterior uveitis. Br J Ophthalmol. 2010;94:271–274. doi: 10.1136/bjo.2008.146753. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ueta M, Sotozono C, Yokoi N, Inatomi T, Kinoshita S. Upregulation of Toll-like receptor 5 expression in the conjunctival epithelium of various human ocular surface diseases. Br J Ophthalmol. 2014;98:1116–1119. doi: 10.1136/bjophthalmol-2013-304645. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu GS, Ishimoto S, Pararajasegaram G, Rao NA. Expression of major histocompatibility complex molecules in rodent retina. Immunohistochemical study Invest Ophthalmol Vis Sci. 1997;38:1848–1857. [PubMed] [Google Scholar]
- Zhao M, Li CH, Liu YL. Toll-like receptor (TLR)-2/4 expression in retinal ganglion cells in a high-glucose environment and its implications. Gen Mol Res. 2016:15. doi: 10.4238/gmr.15026998. [DOI] [PubMed] [Google Scholar]
- Zong H, Ward M, Madden A, Yong PH, Limb GA, Curtis TM, Stitt AW. Hyperglycaemia-induced pro-inflammatory responses by retinal Muller glia are regulated by the receptor for advanced glycation end-products (RAGE) Diabetologia. 2010;53:2656–2666. doi: 10.1007/s00125-010-1900-z. [DOI] [PubMed] [Google Scholar]