Abstract
Ongoing animal preclinical studies on transcutaneous bone-anchored prostheses have aimed to improve biomechanics of prosthetic locomotion in people with limb loss. It is much less common to translate successful developments in human biomechanics and prosthetic research to veterinary medicine to treat animals with limb loss. Current standard of care in veterinary medicine is amputation of the whole limb if a distal segment cannot be salvaged. Bone-anchored transcutaneous prostheses, developed for people with limb loss, could be beneficial for veterinary practice. The aim of this study was to examined if and how cats utilize the limb with a bone-anchored passive transtibial prosthesis during level and slope walking. Four cats were implanted with a porous titanium implant into the right distal tibia. Ground reaction forces and full-body kinematics were recorded during level and slope (±50%) walking before and 4–6 months after implantation and prosthesis attachment. The duty factor of the prosthetic limb exceeded zero in all cats and slope conditions (p < 0.05) and was in the range of 45.0–60.6%. Thus, cats utilized the prosthetic leg for locomotion instead of walking on three legs. Ground reaction forces, power and work of the prosthetic limb were reduced compared to intact locomotion, whereas those of the contralateral hind- and forelimbs increased (p < 0.05). This asymmetry was likely caused by insufficient energy generation for propulsion by the prosthetic leg, as no signs of pain or discomfort were observed in the animals. We concluded that cats could utilize a unilateral bone-anchored transtibial prosthesis for quadrupedal level and slope locomotion.
Keywords: Porous titanium implant, Bone-anchored prostheses, Prosthetic quadrupedal walking, Cat
1. Introduction
It is a common practice to use animal models to investigate biomechanical function, safety and efficacy of orthopedic and prosthetic implants, procedures and technologies for translation to human clinical practice. For example, ongoing animal studies on transcutaneous porous titanium bone implants (Farrell et al., 2014a,b; Fitzpatrick et al., 2011; Pitkin et al., 2009; Shelton et al., 2011) have aimed to reduce skin infection in individuals with bone-anchored lower limb prostheses (Branemark et al., 2014; Drygas et al., 2008; Tillander et al., 2010; Tsikandylakis et al., 2014), and ultimately to improve biomechanics of prosthetic locomotion. It is much less common, however, to translate successful developments in human biomechanics, orthopedic and prosthetic research to veterinary medicine to treat animals with limb loss. According to Mich (2014), the current dogma in veterinary medicine of quadrupedal pets (dogs and cats) is: ‘‘animals do great on 3 legs”. As a result, standard of care in veterinary medicine is amputation of the whole limb if a distal segment (e.g., foot) cannot be salvaged. This, in turn, leads to animal limited mobility, weight gain, break-down of a sound limb, chronic neck and back pain, and premature euthanasia (Mich, 2014; Mich et al., 2013). The method of direct attachment of a prosthesis to the residual limb, developed for people with limb loss, could be beneficial for veterinary practice.
Direct attachment of limb prosthesis to the residual bone using a transcutaneous solid titanium implant inside the medullary cavity has been used in individuals with limb loss since the 1990s (Branemark et al., 2001; Hagberg and Branemark, 2009; Jonsson et al., 2011; Van de Meent et al., 2013). Several advantages of bone-anchored limb prostheses over conventional socket-attached prostheses have been reported. Bone-anchored prostheses improve load transmission, eliminate skin problems caused by skin friction inside the socket (irritation, blisters, edema and dermatitis) (Hagberg and Branemark, 2009; Jonsson et al., 2011; Juhnke et al., 2015), and increase range of motion (Hagberg et al., 2005; Tranberg et al., 2011). Bone-anchored prostheses improve comfort and confidence of the users (Hagberg et al., 2008; Lundberg et al., 2011; Witso et al., 2006) and permit easier donning and doffing of the prosthesis (Jonsson et al., 2011). In addition, bone-anchored prostheses improve perception of prosthesis loading, defined as osseoperception (Haggstrom et al., 2013a; Jacobs et al., 2000; Lundborg et al., 2006), lead to fewer clinical visits to the prosthetist (Haggstrom et al., 2013b), and result in improvement of walking mechanics (Frossard et al., 2013; Hagberg et al., 2005; Tranberg et al., 2011).
All these advantages of bone-anchored prostheses would be beneficial to quadrupedal animals with limb loss if animals choose to utilize a prosthesis on one leg over locomoting on three sound legs, as currently assumed in veterinary medicine (Mich, 2014). Although few case report studies have suggested that quadrupedal animals might utilize a unilateral distal bone-anchored prosthesis for walking (Farrell et al., 2014a; Fitzpatrick et al., 2011), no studies have been published that rigorously document whether quadrupedal animals systematically utilize unilateral transtibial prostheses during locomotion and how prosthetic locomotion is performed. The use of the prosthetic limb during quadrupedal locomotion might depend on which limb is missing (forelimb versus hindlimb) and on loading demands on the prosthetic limb. For example, during downslope walking at grade 50%, peak loading on the hindlimbs is reduced by ~25% compared to 50%-upslope walking (Gregor et al., 2006; Prilutsky et al., 2011). Reduced loading on the prosthetic limb could prompt the animal not to utilize the prosthesis at all and to locomote on three legs instead.
Therefore, the aim of this study was to examined if and how cats utilize the limb with a bone-anchored passive transtibial pros-thesis during downslope, level and upslope walking. We judged whether the animal used the prosthetic limb for locomotion based on the duty factor (the ratio of the stance phase duration over the cycle duration). We hypothesized that the duty factor will not be zero, i.e. the stance phase of the prosthetic limb would be present. If the first hypothesis was confirmed, one would expect a reduced loading of the prosthetic leg during locomotion as observed in people walking with a unilateral passive transtibial prosthesis (Barr et al., 1992; Fey et al., 2011; Segal et al., 2006). Therefore, if the animals would utilize quadrupedal gait with the prosthesis, we would test a second hypothesis that the ground reaction forces and work done by the prosthetic limb would be reduced compared to those of the sound limbs.
2. Methods
Full descriptions of the surgical and rehabilitative procedures, prosthesis and implant design, and data acquisition have been published previously (Farrell et al., 2014a) and only briefly described here. All experimental procedures were in agreement with the US Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees at both Georgia Institute of Technology and St. Joseph’s Translational Research Institute (now known as T3 Labs).
The subjects were four adult purpose-bred cats (baseline mass range 3.0–3.2 kg, Table 1) from our ongoing translational study on integration of the titanium porous Skin- and Bone-Integrated Pylon (SBIP; Poly-Orth International; Sharon MA, USA) with the residual limb (Farrell et al., 2014a; Jarrell et al., 2016, 2017). The cats were trained (~2 h a day, 5 days a week for 3–4 weeks) to walk along an enclosed walkway with 3 embedded force platforms (Bertec Corporation, Columbus OH, USA). The walkway was set at three slopes: 0% (level), 50% (upslope), and −50% (downslope). At the end of the training period, full-body kinematics and ground reaction forces were recorded by a 6-camera motion capture system (Vicon, UK) and the force platforms during level and sloped walking.
Table 1.
Animal characteristics.
| Cat characteristics | QM04 | 09NHT4 | 11NLS4 | QMV5 | Mean ± SD |
|---|---|---|---|---|---|
| Baseline mass, kg | 3.2 | 3.2 | 3.2 | 3.0 | 3.15 ± 0.10 |
| Terminal mass, kg | 4.0 | 3.4 | 2.8 | 4.0 | 3.55 ± 0.57 |
| Estimated mass of the foot and distal third tibia, g | 54.1 | 52.5 | 49.5 | 51.2 | 51.8 ± 2.0 |
| Estimated moment of inertia of the foot and distal third tibia, g cm2 | 290 | 326 | 279 | 281 | 294 ± 22 |
| Prosthesis mass, g | 15.5 | 15.5 | 18.4 | 18.4 | 17.0 ± 1.8 |
| Prosthesis moment of inertia with respect to frontal axis through prosthesis center of mass, g cm2 | 172 | 172 | 157 | 157 | 164.5 ± 8.7 |
| Baseline walking speed, m/s | |||||
| Level | 0.67 ± 0.06 | 0.46 ± 0.02 | – | 0.54 ± 0.04 | 0.56 ± 0.10 |
| Downslope | 0.75 ± 0.14 | 0.55 ± 0.06 | 0.58 ± 0.10 | – | 0.61 ± 0.13 |
| Upslope | 0.56 ± 0.21 | 0.40 ± 0.07 | 0.71 ± 0.20 | – | 0.55 ± 0.20 |
| Terminal walking speed, m/s | |||||
| Level | 0.50 ± 0.07 | 0.39 ± 0.03 | – | 0.40 ± 0.03 | 0.44 ± 0.07* |
| Downslope | 0.61 ± 0.07 | 0.32 ± 0.08 | 0.59 ± 0.07 | – | 0.47 ± 0.16* |
| Upslope | 0.54 ± 0.14 | 0.41 ± 0.09 | 0.42 ± 0.04 | – | 0.47 ± 0.12* |
Notes: The term ‘Terminal’ designates measurements taken several days before euthanasia. Asterisks ‘*’ indicate significant difference (p < 0.05) between intact and prosthetic walking.
Prior to implantation, sagittal and frontal plane X-ray images of the right tibia of each cat were taken to evaluate the size and shape of the medullary canal. Porous titanium SBIP implants were obtained from Poly-Orth International (Sharon, MA, USA). Implants had a tapered design similar to the tibial marrow cavity, which was reamed to a press fit. The distinction of the SBIP from existing systems for direct skeletal attachment of limb prostheses (Pitkin, 2013) is its total permeability achieved in a structure consisting of porous cladding and perforated inserts (Pitkin et al., 2012). The SBIP material specification has a uniquely selected combination of four critical parameters: particle size, pore size, porosity and volume fraction (Pitkin and Raykhtsaum, 2012). This allows for deep ingrowth of the hosting tissues of bone and skin in combination with implant durability and resistance to fatigue (Farrell et al., 2014a,b; Pitkin et al., 2009).
After implantation surgery, performed on the right hindlimb in each cat under sterile conditions with isoflurane anesthesia, the residual right hindlimb with implant (prosthetic limb) was casted for 10 weeks to prevent premature loading (Farrell et al., 2014a). During weeks 6 through 10 after implantation, the distal end of the protruding implant was loaded for 15 min 3 times a week using a hand-held digital dynamometer (Accu-Force Cadet, Ametek, Largo, FL, USA) with gradually increased forces in each week from ~4% to 45% of body weight with a step of 10% (Farrell et al., 2014a). During this procedure, the animal, laying on the left side, was fed and petted by a researcher, while another one gently applied the specified load to the implant. This procedure aimed to strengthen bone-implant integration and was similar in terms of the loading initiation time, duration and magnitude to those used in individuals with press-fitted titanium implants for bone-anchored trans-femoral prostheses (Aschoff et al., 2010; Juhnke et al., 2015). Starting with week 11, the cast was removed and a prosthesis was attached to the SBIP.
The cat was trained to stand and walk on the prosthesis with food reward for 4–6 weeks (the same training protocol as before surgery). After the animal started walking on the J-shaped transtibial prosthesis, level and slope locomotion was recorded several days a week for at least 4 weeks.
Data for slope walking in the first studied cat (cat QMV5, Table 1) were not collected due to uncertainty about the ability of cats with a transtibial passive prosthesis to walk on slopes. Data of cat 11NLS4 for level intact walking were of poor quality and could not be analyzed. Since there were no intact control data for this cat during level walking, prosthetic level walking was not collected. Number of analyzed cycles per limb in each slope condition pre and post implantation are summarized in Table 2.
Table 2.
Number of cycles used for analysis of each limb.
| Limb Walking conditions | Prosthetic right hindlimb | Contralateral hindlimb | Ipsilateral forelimb | Contralateral forelimb |
|---|---|---|---|---|
| Pre implantation walking | ||||
| Level | 14 | 12 | 14 | 13 |
| Downslope | 12 | 17 | 14 | 19 |
| Upslope | 17 | 17 | 13 | 13 |
| Post implantation walking | ||||
| Level | 15 | 13 | 21 | 15 |
| Downslope | 14 | 11 | 15 | 13 |
| Upslope | 14 | 13 | 10 | 12 |
After locomotion data were collected, the animal was euthanized using deep anesthesia (an overdose of sodium pentobarbital, 120–180 mg/kg, IV) and the residual shank with the implant was harvested for histological analysis as described in (Farrell et al., 2014a).
A full-body inverse dynamics analysis in the sagittal plane was performed to determine the resultant moments at hindlimb and forelimb joints, and subsequently their negative and positive power and work (Prilutsky and Klishko, 2011; Prilutsky et al., 2005) before implantation and during prosthetic walking. Inertial properties of the prosthesis were determined using measurements of prosthesis weight, as well as suspension and geometric methods (Farrell et al., 2014a). Mass of the prosthesis was smaller than the estimated mass of the foot and distal third of the tibia that the prosthesis substituted (Table 1).
The limb duty factor and the mean walking speed in the cycle were calculated for each limb and cycle and averaged across cycles for each animal and slope condition and across animals. The time-dependent kinetic variables (tangential and normal ground reaction forces, GRFx and GRFz, respectively, and joint powers) were time-normalized to the duration of the stride of the corresponding limb. Powers were computed for individual joints of each limb: metatarsophalangeal, ankle, knee, and hip joints for hindlimbs and metacarpophalangeal, wrist, elbow and shoulder joints for forelimbs. Each time-normalized variable was averaged at each percent of the cycle across cycles of the corresponding limb for each cat and across cats. Total negative and positive work of each limb was obtained from the total limb power computed as the sum of powers in individual joints. All kinetic variables were also amplitude-normalized to subject’s body mass.
IBM SPSS Statistics software, v24 (IBM SPSS, Chicago IL, USA) was used to test hypotheses of the study. In these tests, cats served as their own controls. The one-sample T-test (or the one-sample Wilcoxon signed rank test when the variable was not normally distributed) was used to test the hypothesis that the duty factor of the prosthetic limb differed from zero, i.e. the animals utilized quadrupedal locomotion with the prosthesis. These tests were performed on individual animals and across all animals. To test if the duty factor, peak GRF, and work of the prosthetic limb pre and post implantation differed from those of the sound limbs, we used a mixed linear model analysis (Brown and Prescott, 2006; West et al., 2015). This analysis takes the advantage of a within-subject design and using all individual trials of each subject. That increases statistical power of the analysis and makes it suitable for analyzing small-sample data sets. Given a small number of subjects, the mixed linear model analysis was first performed on each cat. In this analysis, the fixed factors were walking condition (pre implantation, post implantation), limb (prosthetic right hindlimb, PH; ipsilateral forelimb, IF; contralateral hindlimb, CH; contralateral forelimb, CF), and walking slope (level, downslope, upslope). The dependent variables were the duty factor, peak of GRFz, and total positive work of the limb. The post hoc comparisons with Bonferroni adjustments were performed when a fixed factor was found to be significant. In addition, the same analysis was performed across all cats; to do that a random factor Cat was added. To account for a possible influence of the walking speed on kinetic variables (Lelas et al., 2003), the cycle time of the corresponding limb was used as a covariate in all mixed linear model analyses. The cycle time was considered a better covariate than walking speed due to interlimb variability of cycle time.
To compare patterns of kinetic variables within the walking cycle between pre and post implantation walking, the wavelet-based functional ANOVA (wfANOVA) analysis was used (McKay et al., 2013; Potocanac et al., 2016). This method reveals differences in the shape and magnitude of time-dependent variables with both high temporal resolution and high statistical power (McKay et al., 2013). Significance level in all statistical tests was set at 0.05.
3. Results
No signs of discomfort or pain were observed in the animals during the post-surgical pylon loading in weeks 6 through 10 (the absence of limb withdrawal) or during prosthetic use. Behavioral observations of the prosthesis use indicated that the cats engaged the prosthesis for standing, walking, and, occasionally, jumping.
3.1. Duty factor
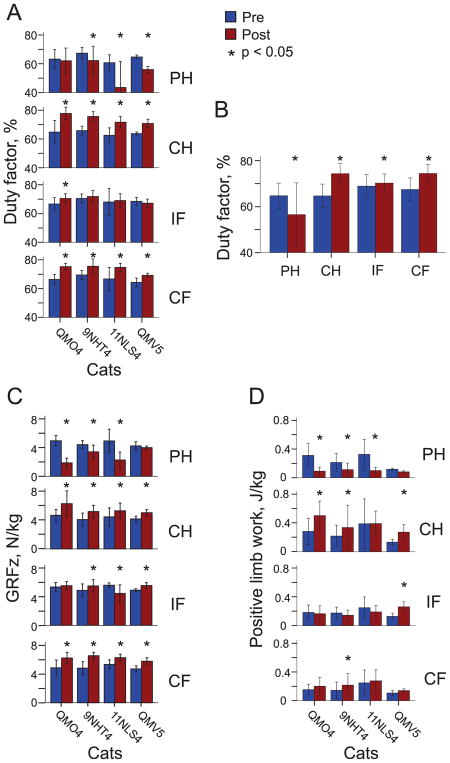
Since the independent fixed factor of slope did not significantly affect the duty factor (F2,268 = 1.913, p = 0.150), statistical tests on the duty factor were performed across the three slope conditions. The duty factor of the prosthetic hindlimb (PH) during walking was significantly different from zero for each of 4 animals (QM04: 0.62 ± 0.09, p = 0.001; 09NHT4: 0.62 ± 0.09, p < 0.001; 11NLS4: 0.44 ± 0.18, p < 0.003; QMV5: 0.56 ± 0.02, p < 0.001), see Fig. 1A. The duty factor for the prosthetic hindlimb was shorter in post implantation walking than in intact pre implantation walking in 3 out of 4 cats (F1,268 = 14.6–85.3, p ≤ 0.001; Fig. 1A). For the contralateral hindlimb (CH) and forelimb (CF), the duty factor was greater in post than in pre implantation walking in each animal (F1,268 = 4.8–84.0, p ≤ 0.001–0.029; Fig. 1A). The duty factor of the prosthetic hindlimb analyzed across all slopes and cats, was smaller in post implantation than in intact pre implantation walking (F1,268 = 84.4, p < 0.001), whereas the duty factor of the remaining 3 limbs was higher during post compared to pre walking (F1,268 = 4.2–180.1, p ≤ 0.001–0.041; Fig. 1B).
Fig. 1.
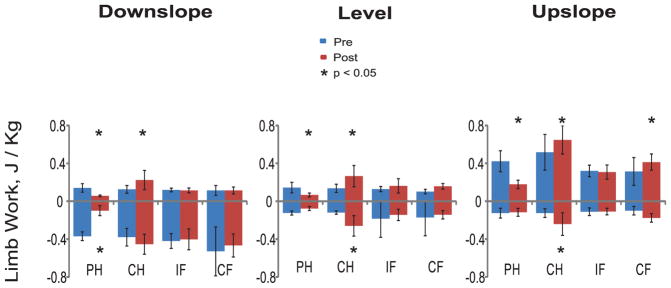
Major kinematic and kinetic variables (mean ± SD) during pre (Pre) and post implantation (Post) walking in individual cats and limbs. Asterisks indicate significant differences (p < 0.05) between pre and post implantation conditions. PH, right hindlimb (prosthetic hindlimb in Post condition); CH, contralateral hindlimb; IF, ipsilateral forelimb; CF, contralateral forelimb. (A) Duty factor of individual cats averaged across all slope conditions and walking cycles. (B) Duty factor averaged across all cats, slope conditions and walking cycles. (C) Peaks of normal ground reaction force (GRFz) of individual cats averaged across all slope conditions and walking cycles. (D) Positive limb work of individual cats averaged across all slope conditions and walking cycles.
3.2. Ground reaction forces
During the stance phase of post implantation walking, the prosthetic hindlimb of each cat exerted substantial peaks of the normal ground reaction force in all slope conditions (GRFz, ~2–4 N/kg) that, however, were lower than the GRFz peak values prior to surgery in 3 out of 4 cats (~4–5 N/kg, F1,268 = 17.7–207.4, p < 0.001; Fig. 1C, 2). The contralateral hind- and forelimb exerted larger peaks of GRFz during post than pre implantation walking in all cats, while the ipsilateral forelimb demonstrated higher peak forces in 3 out of 4 cats (F1,268 = 4.2–25.1, p < 0.041; Fig. 1C, 2).
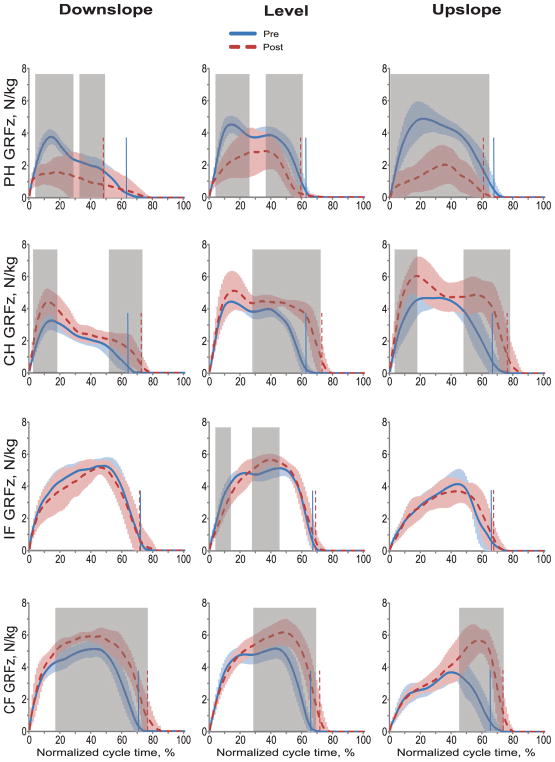
Fig. 2.
Normalized normal ground reaction forces (GRFz) during the cycle of pre implantation (Pre) and post implantation (Post) walking in downslope (–50%), level (0%), and upslope (+50%) conditions. Mean (±SD) data of 4 animals. The vertical dashed and solid lines separate stance and swing phases for post and pre implantation walking, respectively. The shaded areas in each panel indicate significant difference (p < 0.05) between the pre and post implantation walking determined using wfANOVA analysis. PH, right hindlimb (prosthetic hindlimb in Post condition); CH, contralateral hindlimb; IF, ipsilateral forelimb; CF, contralateral forelimb.
GRFz values of the prosthetic hindlimb post implantation were lower than those of the same limb before surgery in early and mid-stance of downslope walking, in early and late stance of level walking, and in the entire stance of upslope walking (wfANOVA, p < 0.05; Fig. 2, shaded areas). GRFz of the contralateral hindlimb and forelimb increased in late stance of post implantation walking in all slope conditions, as well as in early stance of downslope and level walking (wfANOVA, p < 0.05; Fig. 2). Peaks of GRFz of the prosthetic hindlimb post implantation decreased by 30%, 45%, and 46% during level, downslope, and upslope walking, respectively (F1,312 = 27.1–90.5, p < 0.001). The GRFz peak of contralateral hindlimb and forelimb increased during post implantation walking in all slope conditions in the range of 16%–60% (F1,312 = 11.1–68.0, p ≤ 0.001). A small significant increase in GRFz peak of ipsilateral forelimb occurred during post implantation level walking (10%, F1,313 = 4.5, p = 0.035).
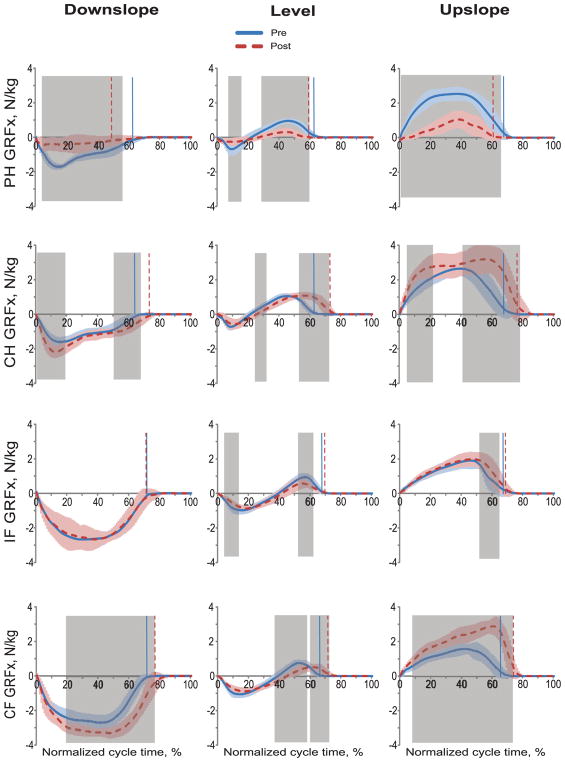
GRFx values of the prosthetic hindlimb were lower after implantation throughout most of the stance phase duration in all slope walking conditions (as revealed by the wfANOVA, p < 0.05, shaded areas in Fig. 3). GRFx values in the contralateral hindlimb and forelimb were higher in substantial portions of stance during post implantation walking (wfANOVA, p < 0.05). Compared to pre implantation walking, ipsilateral forelimb GRFx during prosthetic walking was slightly but significantly higher in the terminal period of stance in upslope condition, and it was lower in the initial and terminal periods of stance in level condition. No difference in ipsilateral forelimb GRFx between post and pre implantation walking was observed in downslope condition (p > 0.05; Fig. 3). GRFx peaks in the prosthetic hindlimb post implantation were lower than during pre implantation walking in level (acceleratory force by 59%: F1,316 = 25.7, p < 0.001; braking force by 52%: F1,316 = 14.9, p < 0.001), downslope (braking force by 66%: F1,316 = 133.3, p < 0.001), and upslope conditions (acceleratory force by 54%; F1,316 = 181.8, p < 0.001). GRFx peaks of the contralateral hindlimb and forelimb were higher in downslope (braking force by 22–33%: F1,316 = 31.2–47.7, p < 0.001) and upslope (acceleratory force by 21–85%: F1,316 = 29.4–143.8, p < 0.001) conditions of prosthetic walking (Fig. 3).
Fig. 3.
Normalized tangential ground reaction forces (GRFx) during the cycle of pre implantation (Pre) and post implantation (Post) walking in downslope (–50%), level (0%), and upslope (+50%) conditions. Mean (±SD) data of 4 animals. The vertical dashed and solid lines separate stance and swing phases for post and pre implantation, respectively. The shaded areas in each panel indicate significant difference (p < 0.05) between the pre and post implantation walking determined using wfANOVA analysis. PH, right hindlimb (prosthetic hindlimb in Post condition); CH, contralateral hindlimb; IF, ipsilateral forelimb; CF, contralateral forelimb.
3.3. Total limb power and work
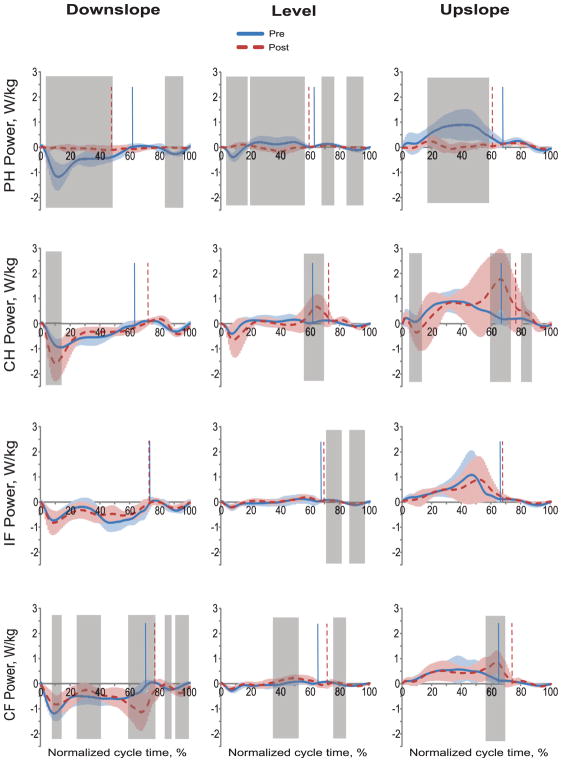
Little power and work was produced by the prosthetic hindlimb post implantation in all slope conditions (Figs. 4 and 5). During pre implantation walking, the same hindlimb produced negative power (absorbed mechanical energy) in the stance phase of down-slope walking and first third of stance of level walking; positive power (energy generation) was produced in last two thirds of the stance phase of level walking and during the entire stance of upslope walking. In three out of four cats, positive work done by the prosthetic limb post implantation was lower than that of the same limb before surgery (F1,268 = 12.6–78.0, p < 0.001; Fig. 1D). The contralateral hindlimb produced higher negative and positive power and work during post implantation walking in all slope conditions (wfANOVA, p = 0.05; Figs. 4 and 5). In three of four cats, positive work of the contralateral hindlimb was higher during post implantation than pre implantation walking (F1,268 = 4.9–84.6, p ≤ 0.001–0.027; Fig. 1D).
Fig. 4.
Normalized power of each limb during the cycle of pre implantation (Pre) and post implantation (Post) walking in downslope (–50%), level (0%), and upslope (+50%) conditions. Mean (±SD) data of 4 animals. The vertical dashed and solid lines separate stance and swing phases for post and pre walking, respectively. The shaded areas in each panel indicate significant difference (p < 0.05) between the pre and post implantation walking determined using wfANOVA analysis. PH, right hindlimb (prosthetic hindlimb in Post condition); CH, contralateral hindlimb; IF, ipsilateral forelimb; CF, contralateral forelimb.
Fig. 5.
Normalized negative and positive work done by four limbs during the cycle of pre (Pre) and post implantation (Post) walking in downslope (–50%), level (0%), and upslope (+50%) conditions. Mean (±SD) data of 4 animals. PH, right hindlimb (prosthetic hindlimb in Post condition); CH, contralateral hindlimb; IF, ipsilateral forelimb; CF, contralateral forelimb. Asterisks indicate significant differences (p<0.05) between pre and post implantation conditions.
Forelimbs produced primarily negative power and did negative work during pre implantation walking in downslope and level conditions. During post implantation level walking, both contralateral and ipsilateral forelimbs generated mostly positive power and work. During post implantation downslope walking, the contralateral forelimb produced more negative power in the end of stance (wfANOVA, p = 0.05; Fig. 4). There was less difference in power generation and work done between post and pre implantation walking in the ipsilateral and contralateral forelimbs in individual cats and across all cats (Figs. 1D, 4 and 5).
4. Discussion
The results of the study supported the hypothesis that cats with a SBIP-attached unilateral transtibial prosthesis would use it for support during quadrupedal locomotion – the duty factor of the prosthetic limb exceeded zero and was in the range of 45.0–60.6% for all cats. Additionally, the prosthetic limb generated substantial normal GRF during level and slope walking post implantation. The second hypothesis that the GRF and work values produced by the prosthetic limb would be lower compared to the sound limbs post implantation was also supported. Thus, the current standard of care in veterinary medicine, i.e. amputation of the whole limb if a distal limb segment cannot be salvaged, should be reexamined. In our study, all four cats utilized the unilateral transtibial prosthesis for walking rather than ambulating on three legs. Although quadrupedal prosthetic walking is still asymmetric (Figs. 1–5) and this may lead to secondary conditions in the sound limbs, back and neck (Mich, 2014; Mich et al., 2013), the extent of this asymmetry is certainly smaller than that occurring during 3–legged locomotion (Fuchs et al., 2014).
It is important to note that the locomotor asymmetry documented in this study was not likely pain related. There were no observed clinical signs of discomfort or pain while loading the implant and prosthesis (no limb withdrawal) and no signs of infection on X-ray and histological images at the end of the study. A possible explanation for the reduced loading of the prosthetic limb during walking might be the non-optimal length, alignment of the prosthesis and shape of the rocker bottom. These parameters were selected to approximately match the hindlimb length and orientation during the stance phase of normal cat walking (Farrell et al., 2014a). In addition, the decreased loading of the prosthetic limb may reflect the limited ability of the cat with the transtibial prosthesis, which lacks an active ankle joint, to generate a sufficient amount of mechanical energy for propulsion. Note that the intact ankle in the cat does approximately 35% of total hindlimb positive work during level and upslope walking (McFadyen et al., 1999; Prilutsky and Klishko, 2011).
There was much greater symmetry between the prosthetic and sound limbs in the normal GRFz than in the tangential GRFx during post implantation walking (Figs. 1C, 2 and 3). For example, the normal GRFz applied to the prosthesis during walking exceeded 50% of the pre implantation walking values on average across all cats (Figs. 1C and 2). Peak GRFx values, however, were only between 34% (braking force in downslope walking) and 48% (braking force in level walking) of the pre implantation values (Fig. 3). One possible explanation for the bigger decrease in GRFx compared to GRFz forces exerted by the prosthetic limb could be the reduced ability of the animal with a passive ankle to exert substantial tangential forces without slipping. The requirement to prevent slipping during stance might have forced the animal to reduce the ratio of the tangential to normal forces, known as the required coefficient of friction (Redfern et al., 2001).
The reduced loading of the prosthetic limb and greater loading of the sound contralateral limbs found in this study agreed well with previous results of dog locomotion with hindlimb lameness (Weishaupt et al., 2004) or hindlimb amputation (Fuchs et al., 2014), and of sheep prosthetic locomotion (Shelton et al., 2011). People with unilateral transtibial amputation also show reduced loading of the prosthetic leg and increased loading of the contralateral leg during prosthetic walking (Barr et al., 1992; Fey et al., 2011; Segal et al., 2006). As discussed above, these changes in prosthetic walking may be needed to compensate for the lack of energy generation by the passive prosthetic ankle joint. This suggestion is supported by the fact that during prosthetic walking in humans, power produced and work done at the contralateral leg increase (Beyaert et al., 2008). Cats apparently used a similar strategy to compensate for a reduced ability of the prosthetic limb to generate energy and do positive work (Figs. 1D, 5). Additional energy is generated by the contralateral hindlimb (Figs. 1D, 5) in late stance of level and upslope walking (Fig. 4). Note also that all cats increased the duty factor of the contralateral hind- and forelimb during prosthetic walking (Fig. 1A), which allowed relatively more time to generate mechanical energy.
Several limitations of this study should be mentioned. The limited number of subjects tested in this study was caused by the complexity of the procedures. The effects of this limitation was partially reduced by the statistical design we selected, i.e. the mixed linear model analysis (Brown and Prescott, 2006; West et al., 2015); see Methods for details. Although the small sample size limits our ability to generalize the results, the study nevertheless provides evidence that the titanium SBIP pylons with deep porosity can serve for anchoring transtibial limb prostheses and that the animals can adopt the prosthesis for walking. The observed large reduction in loading and utilization of the prosthetic limb during walking could be partially caused by a relatively short duration of the study. It is possible that if the study was longer, the loading and use of the prosthetic limb may have increased, and walking kinetics might have shifted more toward intact patterns as the cats became more familiar with the prostheses. There was uncertainty in the positioning of the markers on the prosthesis to specify the location of the metatarsophalangeal and ankle joints. This uncertainty should not have affected substantially the calculated power at the passive ankle.
In summary, the results of this study demonstrated that cats could utilize a unilateral bone-anchored transtibial prosthesis for quadrupedal level and slope locomotion. Although walking with the transtibial passive prosthesis was asymmetric, this asymmetry was lower than that reported for 3-legged locomotion. Thus, the current standard of care in veterinary medicine recommending amputation of the whole limb if a distal segment cannot be salvaged should be reexamined.
Acknowledgments
This study was supported in part by DOD grant W81XWH-16-1-0791, NIH grants T32HD055180, R44HD057492 and R44HD090768, and NSF grant 0946809. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Institutes of Health, National Science Foundation or Department of Defense. We would like to thank Dr. Ashley Strong, Dr. Evan Goldberg and staff of the T3 Labs for their excellent veterinary assistance, Mr. Nihir Patel for his help with data analysis, and Dr. J. Lucas McKay for providing assistance and Matlab code for the wfANOVA analysis.
Footnotes
Conflict of interest statement
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- Aschoff HH, Kennon RE, Keggi JM, Rubin LE. Transcutaneous, distal femoral, intramedullary attachment for above-the-knee prostheses: an endoexo device. J Bone Joint Surg-Am. 2010;92A:180–186. doi: 10.2106/JBJS.J.00806. [DOI] [PubMed] [Google Scholar]
- Barr AE, Siegel KL, Danoff JV, McGarvey CL, 3rd, Tomasko A, Sable I, Stanhope SJ. Biomechanical comparison of the energy-storing capabilities of SACH and Carbon Copy II prosthetic feet during the stance phase of gait in a person with below-knee amputation. Phys Ther. 1992;72:344–354. doi: 10.1093/ptj/72.5.344. [DOI] [PubMed] [Google Scholar]
- Beyaert C, Grumillier C, Martinet N, Paysant J, Andre JM. Compensatory mechanism involving the knee joint of the intact limb during gait in unilateral below-knee amputees. Gait Posture. 2008;28:278–284. doi: 10.1016/j.gaitpost.2007.12.073. [DOI] [PubMed] [Google Scholar]
- Branemark R, Berlin O, Hagberg K, Bergh P, Gunterberg B, Rydevik B. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: A prospective study of 51 patients. The Bone Joint J. 2014;96-B:106–113. doi: 10.1302/0301-620X.96B1.31905. [DOI] [PubMed] [Google Scholar]
- Branemark R, Branemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: A review. J Rehabil Res Dev. 2001;38:175–181. [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied Mixed Models in Medicine. John Wiley & Sons Ltd; Chichester, West Sussex, England: 2006. [Google Scholar]
- Drygas KA, Taylor R, Sidebotham CG, Hugate RR, Mcalexander H. Transcutaneous tibial implants: a surgical procedure for restoring ambulation after amputation of the distal aspect of the tibia in a dog. Vet Surg. 2008;37:322–327. doi: 10.1111/j.1532-950X.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- Farrell BJ, Prilutsky BI, Kistenberg RS, Dalton JFt, Pitkin M. An animal model to evaluate skin-implant-bone integration and gait with a prosthesis directly attached to the residual limb. Clin Biomech (Bristol, Avon) 2014a;29:336–349. doi: 10.1016/j.clinbiomech.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell BJ, Prilutsky BI, Ritter JM, Kelley S, Popat K, Pitkin M. Effects of pore size, implantation time, and nano-surface properties on rat skin ingrowth into percutaneous porous titanium implants. J Biomed Mater Res Part A. 2014b;102:1305–1315. doi: 10.1002/jbm.a.34807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey NP, Klute GK, Neptune RR. The influence of energy storage and return foot stiffness on walking mechanics and muscle activity in below-knee amputees. Clin Biomech (Bristol, Avon) 2011;26:1025–1032. doi: 10.1016/j.clinbiomech.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick N, Smith TJ, Pendegrass CJ, Yeadon R, Ring M, Goodship AE, Blunn GW. Intraosseous transcutaneous amputation prosthesis (ITAP) for limb salvage in 4 dogs. Veterinary Surgery: VS. 2011;40:909–925. doi: 10.1111/j.1532-950X.2011.00891.x. [DOI] [PubMed] [Google Scholar]
- Frossard L, Haggstrom E, Hagberg K, Branemark R. Load applied on bone-anchored transfemoral prosthesis: Characterization of a prosthesis-A pilot study. J Rehabil Res Dev. 2013;50:619–634. doi: 10.1682/jrrd.2012.04.0062. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Goldner B, Nolte I, Schilling N. Ground reaction force adaptations to tripedal locomotion in dogs. Veterinary J. 2014;201:307–315. doi: 10.1016/j.tvjl.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change, and ankle extensor EMG patterns. J Neurophysiol. 2006;95:1397–1409. doi: 10.1152/jn.01300.2004. [DOI] [PubMed] [Google Scholar]
- Hagberg K, Branemark R. One hundred patients treated with osseointegrated transfemoral amputation prostheses–rehabilitation perspective. J Rehabil Res Dev. 2009;46:331–344. [PubMed] [Google Scholar]
- Hagberg K, Branemark R, Gunterberg B, Rydevik B. Osseointegrated trans-femoral amputation prostheses: prospective results of general and condition-specific quality of life in 18 patients at 2-year follow-up. Prosthet Orthot Int. 2008;32:29–41. doi: 10.1080/03093640701553922. [DOI] [PubMed] [Google Scholar]
- Hagberg K, Häggström E, Uden M, Brånemark R. Socket versus bone-anchored trans-femoral prostheses: Hip range of motion and sitting comfort. Prosthet Orthot Int. 2005;29:153–163. doi: 10.1080/03093640500238014. [DOI] [PubMed] [Google Scholar]
- Haggstrom E, Hagberg K, Rydevik B, Branemark R. Vibrotactile evaluation: osseointegrated versus socket-suspended transfemoral prostheses. J Rehabil Res Dev. 2013a;50:1423–1434. doi: 10.1682/JRRD.2012.08.0135. [DOI] [PubMed] [Google Scholar]
- Haggstrom EE, Hansson E, Hagberg K. Comparison of prosthetic costs and service between osseointegrated and conventional suspended transfemoral prostheses. Prosthet Orthot Int. 2013b;37:152–160. doi: 10.1177/0309364612454160. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Branemark R, Olmarker K, Rydevik B, Van Steenberghe D, Branemark PI. Evaluation of the psychophysical detection threshold level for vibrotactile and pressure stimulation of prosthetic limbs using bone anchorage or soft tissue support. Prosthet Orthot Int. 2000;24:133–142. doi: 10.1080/03093640008726536. [DOI] [PubMed] [Google Scholar]
- Jarrell JR, Farrell BJ, Kistenberg RS, Dalton JF, IV, Pitkin M, Prilutsky BI. Cat level and slope walking with a transtibial osseointegrated prosthesis. First International Symposium on Innovations in Amputation Surgery and Prosthetic Technologies; Chicago, IL. 2016. [Google Scholar]
- Jarrell JR, Farrell BJ, Kistenberg RS, Dalton JF, IV, Pitkin M, Prilutsky BI. Kinetics of quadrupedal level and slope walking in the cat with a unilateral transtibial prosthesis anchored to the bone via a porous titanium pylon. 7th International Conference Advances in Orthopaedic Osseointegration; Coronado, CA. 2017. [Google Scholar]
- Jonsson S, Caine-Winterberger K, Branemark R. Osseointegration amputation prostheses on the upper limbs: methods, prosthetics and rehabilitation. Prosthet Orthot Int. 2011;35:190–200. doi: 10.1177/0309364611409003. [DOI] [PubMed] [Google Scholar]
- Juhnke DL, Beck JP, Jeyapalina S, Aschoff HH. Fifteen years of experience with Integral-Leg-Prosthesis: Cohort study of artificial limb attachment system. J Rehabil Res Dev. 2015;52:407–420. doi: 10.1682/JRRD.2014.11.0280. [DOI] [PubMed] [Google Scholar]
- Lelas JL, Merriman GJ, Riley PO, Kerrigan DC. Predicting peak kinematic and kinetic parameters from gait speed. Gait Posture. 2003;17:106–112. doi: 10.1016/s0966-6362(02)00060-7. [DOI] [PubMed] [Google Scholar]
- Lundberg M, Hagberg K, Bullington J. My prosthesis as a part of me: a qualitative analysis of living with an osseointegrated prosthetic limb. Prosthet Orthot Int. 2011;35:207–214. doi: 10.1177/0309364611409795. [DOI] [PubMed] [Google Scholar]
- Lundborg G, Waites A, Bjoerkman A, Rosen B, Larsson EM. Functional magnetic resonance imaging shows cortical activation on sensory stimulation of an osseointegrated prosthetic thumb. Scand J Plast Reconstr Surg Hand Surg. 2006;40:234–239. doi: 10.1080/02844310600787005. [DOI] [PubMed] [Google Scholar]
- McFadyen BJ, Lavoie S, Drew T. Kinetic and energetic patterns for hindlimb obstacle avoidance during cat locomotion. Exp Brain Res. 1999;125:502–510. doi: 10.1007/s002210050708. [DOI] [PubMed] [Google Scholar]
- McKay JL, Welch TD, Vidakovic B, Ting LH. Statistically significant contrasts between EMG waveforms revealed using wavelet-based functional ANOVA. J Neurophysiol. 2013;109:591–602. doi: 10.1152/jn.00447.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mich PM. The emerging role of veterinary orthotics and prosthetics (V-OP) in small animal rehabilitation and pain management. Top Companion Anim Med. 2014;29:10–19. doi: 10.1053/j.tcam.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Mich PM, Fair L, Borghese I. Assistive devices, orthotic, prosthetics, bandaging. In: Van Dyke J, Zink MC, editors. Canine Sports Medicine and Rehabilitation. Wiley Blackwell; Ames, IA: 2013. pp. 201–208. [Google Scholar]
- Pitkin M. Design features of implants for direct skeletal attachment of limb prostheses. J Biomed Mater Res Part A. 2013;101:3339–3348. doi: 10.1002/jbm.a.34606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkin M, Pilling J, Raykhtsaum G. Mechanical properties of totally permeable titanium composite pylon for direct skeletal attachment. Journal of biomedical materials research Part B, Appl Biomater. 2012;100:993–999. doi: 10.1002/jbm.b.32663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkin M, Raykhtsaum G. Skin integrated device. #8257435 US patent. 2012
- Pitkin M, Raykhtsaum G, Pilling J, Shukeylo Y, Moxson V, Duz V, Lewandowski J, Connolly R, Kistenberg RS, Dalton JFI, Prilutsky B, Jacobson S. Mathematical modeling and mechanical and histopathological testing of porous prosthetic pylon for direct skeletal attachment. J Rehabil Res Dev. 2009;46:315–330. doi: 10.1682/jrrd.2008.09.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocanac Z, Pijnappels M, Verschueren S, van Dieen J, Duysens J. Two-stage muscle activity responses in decisions about leg movement adjustments during trip recovery. J Neurophysiol. 2016;115:143–156. doi: 10.1152/jn.00263.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prilutsky BI, Klishko AN. Control of locomotion: Lessons from whole-body biomechanical analysis. In: Danion F, Latash ML, editors. Motor Control: Theories Experiments and Applications. Oxford University Press; Oxford: 2011. pp. 197–218. [Google Scholar]
- Prilutsky BI, Maas H, Bulgakova M, Hodson-Tole EF, Gregor RJ. Short-term motor compensations to denervation of feline soleus and lateral gastrocnemius result in preservation of ankle mechanical output during locomotion. Cells Tissues Organs. 2011;193:310–324. doi: 10.1159/000323678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prilutsky BI, Sirota MG, Gregor RJ, Beloozerova IN. Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J Neurophysiol. 2005;94:2959–2969. doi: 10.1152/jn.00704.2004. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Cham R, Gielo-Perczak K, Gronqvist R, Hirvonen M, Lanshammar H, Marpet M, Pai CY, Powers C. Biomechanics of slips. Ergonomics. 2001;44:1138–1166. doi: 10.1080/00140130110085547. [DOI] [PubMed] [Google Scholar]
- Segal AD, Orendurff MS, Klute GK, McDowell ML, Pecoraro JA, Shofer J, Czerniecki JM. Kinematic and kinetic comparisons of transfemoral amputee gait using C-Leg and Mauch SNS prosthetic knees. J Rehabil Res Dev. 2006;43:857–870. doi: 10.1682/jrrd.2005.09.0147. [DOI] [PubMed] [Google Scholar]
- Shelton TJ, Beck JP, Bloebaum RD, Bachus KN. Percutaneous osseointegrated prostheses for amputees: Limb compensation in a 12-month ovine model. J Biomech. 2011;44:2601–2606. doi: 10.1016/j.jbiomech.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillander J, Hagberg K, Hagberg L, Branemark R. Osseointegrated titanium implants for limb prostheses attachments: infectious complications. Clin Orthopaed Related Res. 2010;468:2781–2788. doi: 10.1007/s11999-010-1370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranberg R, Zugner R, Karrholm J. Improvements in hip- and pelvic motion for patients with osseointegrated trans-femoral prostheses. Gait Posture. 2011;33:165–168. doi: 10.1016/j.gaitpost.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Tsikandylakis G, Berlin O, Branemark R. Implant survival, adverse events, and bone remodeling of osseointegrated percutaneous implants for transhumeral amputees. Clin Orthopaed Related Res. 2014;472:2947–2956. doi: 10.1007/s11999-014-3695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Meent H, Hopman MT, Frolke JP. Walking ability and quality of life in subjects with transfemoral amputation: a comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil. 2013;94:2174–2178. doi: 10.1016/j.apmr.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Weishaupt MA, Wiestner T, Hogg HP, Jordan P, Auer JA. Compensatory load redistribution of horses with induced weightbearing hindlimb lameness trotting on a treadmill. Equine Vet J. 2004;36:727–733. doi: 10.2746/0425164044848244. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Gatecki AT. A Practical Guide using Statistical Software. CRC Press; Boca Raton, FL: 2015. Linear Mixed Models. [Google Scholar]
- Witso E, Kristensen T, Benum P, Sivertsen S, Persen L, Funderud A, Magne T, Aursand HP, Aamodt A. Improved comfort and function of arm prosthesis after implantation of a Humerus-T-Prosthesis in trans-humeral amputees. Prosthet Orthot Int. 2006;30:270–278. doi: 10.1080/03093640600605013. [DOI] [PubMed] [Google Scholar]